Loading AI tools
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95 (the number of protons in the nucleus of the americium atom). Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
Eighteen radioisotopes of americium, ranging from 229Am to 247Am with the exception of 231Am, have been characterized; another isotope, 223Am, has also been reported but is unconfirmed. The most stable isotopes are 243Am with a half-life of 7,370 years and 241Am with a half-life of 432.2 years. All of the remaining radioactive isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. This element also has 8 meta states, with the most stable being 242m1Am (t1/2 = 141 years). This isomer is unusual in that its half-life is far longer than that of the ground state of the same isotope.
| Nuclide [n 1] |
Z | N | Isotopic mass (Da) [n 2][n 3] |
Half-life[1] |
Decay mode[1] [n 4] |
Daughter isotope |
Spin and parity[1] [n 5][n 6] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy[n 6] | |||||||||||||||||||
| 223Am[n 7] | 95 | 128 | 223.04584(32)# | 10(9) ms | α | 219Np | 9/2–# | ||||||||||||
| 229Am | 95 | 134 | 229.04528(11) | 1.8(15) s | α | 225Np | 5/2–# | ||||||||||||
| 230Am | 95 | 135 | 230.04603(15)# | 40(9) s | β+ (<70%) | 230Pu | 1–# | ||||||||||||
| β+SF (>30%) | (various) | ||||||||||||||||||
| 232Am | 95 | 137 | 232.04661(32)# | 1.31(4) min | β+ (97%) | 232Pu | 1–# | ||||||||||||
| α (3%) | 228Np | ||||||||||||||||||
| β+SF (0.069%) | (various) | ||||||||||||||||||
| 233Am | 95 | 138 | 233.04647(12)# | 3.2(8) min | β+ (95.5%) | 233Pu | 5/2–# | ||||||||||||
| α (4.5%) | 229Np | ||||||||||||||||||
| 234Am | 95 | 139 | 234.04773(17)# | 2.32(8) min | β+ (99.95%) | 234Pu | 0–# | ||||||||||||
| α (0.039%) | 230Np | ||||||||||||||||||
| β+, SF (0.0066%) | (various) | ||||||||||||||||||
| 235Am | 95 | 140 | 235.047906(57) | 10.3(6) min | β+ (99.60%) | 235Pu | 5/2−# | ||||||||||||
| α (0.40%) | 231Np | ||||||||||||||||||
| 236Am | 95 | 141 | 236.04943(13)# | 3.6(1) min | β+ | 236Pu | 5− | ||||||||||||
| α (4×10−3%) | 232Np | ||||||||||||||||||
| 236mAm | 50(50)# keV | 2.9(2) min | β+ | 236Pu | (1−) | ||||||||||||||
| α ? | 232Np | ||||||||||||||||||
| 237Am | 95 | 142 | 237.049995(64)# | 73.6(8) min | β+ (99.975%) | 237Pu | 5/2− | ||||||||||||
| α (.025%) | 233Np | ||||||||||||||||||
| 238Am | 95 | 143 | 238.051983(63) | 98(3) min | β+ | 238Pu | 1+ | ||||||||||||
| α (1.0×10−4%) | 234Np | ||||||||||||||||||
| 238mAm | 2500(200)# keV | 35(18) μs | SF | (various) | |||||||||||||||
| IT ? | 238Am | ||||||||||||||||||
| 239Am | 95 | 144 | 239.0530227(21) | 11.9(1) h | EC (99.99%) | 239Pu | 5/2− | ||||||||||||
| α (0.01%) | 235Np | ||||||||||||||||||
| 239mAm | 2500(200) keV | 163(12) ns | SF | (various) | (7/2+) | ||||||||||||||
| IT ? | 239Am | ||||||||||||||||||
| 240Am | 95 | 145 | 240.055298(15) | 50.8(3) h | β+ | 240Pu | (3−) | ||||||||||||
| α (1.9×10−4%) | 236Np | ||||||||||||||||||
| 240mAm | 3000(200) keV | 940(40) μs | SF | (various) | |||||||||||||||
| IT ? | 240Am | ||||||||||||||||||
| 241Am | 95 | 146 | 241.0568273(12) | 432.6(6) y | α | 237Np | 5/2− | ||||||||||||
| SF (3.6×10−10%) | (various) | ||||||||||||||||||
| 241mAm | 2200(200) keV | 1.2(3) μs | SF | (various) | |||||||||||||||
| 242Am | 95 | 147 | 242.0595474(12) | 16.02(2) h | β− (82.7%) | 242Cm | 1− | ||||||||||||
| EC (17.3%) | 242Pu | ||||||||||||||||||
| 242m1Am | 48.60(5) keV | 141(2) y | IT (99.54%) | 242Am | 5− | ||||||||||||||
| α (.46%) | 238Np | ||||||||||||||||||
| SF ? | (various) | ||||||||||||||||||
| 242m2Am | 2200(80) keV | 14.0(10) ms | SF | (various) | (2+, 3−) | ||||||||||||||
| IT ? | 242Am | ||||||||||||||||||
| 243Am | 95 | 148 | 243.0613799(15) | 7,350(9) y | α | 239Np | 5/2− | ||||||||||||
| SF (3.7×10−9%) | (various) | ||||||||||||||||||
| 243mAm | 2300(200) keV | 5.5(5) μs | SF | (various) | |||||||||||||||
| IT ? | 243Am | ||||||||||||||||||
| 244Am | 95 | 149 | 244.0642829(16) | 10.01(3) h | β− | 244Cm | (6−) | ||||||||||||
| 244m1Am | 89.3(16) keV | 26.13(43) min | β− (99.96%) | 244Cm | 1+ | ||||||||||||||
| EC (0.0364%) | 244Pu | ||||||||||||||||||
| 244m2Am | 2000(200)# | 900(150) μs | SF | (various) | |||||||||||||||
| IT ? | 244Am | ||||||||||||||||||
| 244m3Am | 2200(200)# | ~6.5 μs | SF | (various) | |||||||||||||||
| IT ? | 244Am | ||||||||||||||||||
| 245Am | 95 | 150 | 245.0664528(20) | 2.05(1) h | β− | 245Cm | 5/2+ | ||||||||||||
| 245mAm | 2400(400)# | 640(60) ns | SF | (various) | |||||||||||||||
| IT ? | 245Am | ||||||||||||||||||
| 246Am | 95 | 151 | 246.069774(19)# | 39(3) min | β− | 246Cm | (7−) | ||||||||||||
| 246m1Am | 30(10)# keV | 25.0(2) min | β− | 246Cm | 2(−) | ||||||||||||||
| IT ? | 246Am | ||||||||||||||||||
| 246m2Am | 2000(800)# keV | 73(10) μs | SF | (various) | |||||||||||||||
| IT ? | 246Am | ||||||||||||||||||
| 247Am | 95 | 152 | 247.07209(11)# | 23.0(13) min | β− | 247Cm | 5/2# | ||||||||||||
| This table header & footer: | |||||||||||||||||||
- mAm – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- Modes of decay:
CD: Cluster decay EC: Electron capture IT: Isomeric transition SF: Spontaneous fission - ( ) spin value – Indicates spin with weak assignment arguments.
- # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
| Actinides[3] by decay chain | Half-life range (a) |
Fission products of 235U by yield[4] | ||||||
|---|---|---|---|---|---|---|---|---|
| 4n | 4n + 1 | 4n + 2 | 4n + 3 | 4.5–7% | 0.04–1.25% | <0.001% | ||
| 228Ra№ | 4–6 a | 155Euþ | ||||||
| 248Bk[5] | > 9 a | |||||||
| 244Cmƒ | 241Puƒ | 250Cf | 227Ac№ | 10–29 a | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Puƒ | 243Cmƒ | 29–97 a | 137Cs | 151Smþ | 121mSn | ||
| 249Cfƒ | 242mAmƒ | 141–351 a |
No fission products have a half-life | |||||
| 241Amƒ | 251Cfƒ[6] | 430–900 a | ||||||
| 226Ra№ | 247Bk | 1.3–1.6 ka | ||||||
| 240Pu | 229Th | 246Cmƒ | 243Amƒ | 4.7–7.4 ka | ||||
| 245Cmƒ | 250Cm | 8.3–8.5 ka | ||||||
| 239Puƒ | 24.1 ka | |||||||
| 230Th№ | 231Pa№ | 32–76 ka | ||||||
| 236Npƒ | 233Uƒ | 234U№ | 150–250 ka | 99Tc₡ | 126Sn | |||
| 248Cm | 242Pu | 327–375 ka | 79Se₡ | |||||
| 1.33 Ma | 135Cs₡ | |||||||
| 237Npƒ | 1.61–6.5 Ma | 93Zr | 107Pd | |||||
| 236U | 247Cmƒ | 15–24 Ma | 129I₡ | |||||
| 244Pu | 80 Ma |
... nor beyond 15.7 Ma[7] | ||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7–14.1 Ga | |||||
| ||||||||
Americium-241

Americium-241 is the most common isotope of americium in nuclear waste.[8] It is the isotope used in an americium smoke detector based on an ionization chamber. It is a potential fuel for long-lifetime radioisotope thermoelectric generators.
| Parameter | Value |
|---|---|
| Atomic mass | 241.056829 u |
| Mass excess | 52930 keV |
| Beta decay energy | −767 keV |
| Spin | 5/2− |
| Half-life | 432.6 years |
| Spontaneous fissions | 1200 per kg s |
| Decay heat | 114 watts/kg |
Possible parent nuclides: beta from 241Pu, electron capture from 241Cm, alpha from 245Bk.
241Am alpha decays, with a by-product of gamma rays. Its presence in plutonium is determined by the original concentration of 241Pu and the sample age. Due to the low penetration of alpha radiation, 241Am only poses a health risk when ingested or inhaled. Older samples of plutonium containing plutonium-241 contain a buildup of 241Am. A chemical removal of americium from reworked plutonium (e.g. during reworking of plutonium pits) may be required.
Americium-242m
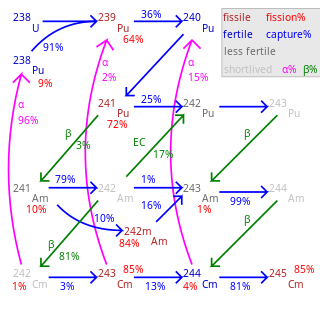
Fission percentage is 100 minus shown percentages.
Total rate of transmutation varies greatly by nuclide.
245Cm–248Cm are long-lived with negligible decay.
| Probability | Decay mode | Decay energy | Decay product |
|---|---|---|---|
| 99.54% | isomeric transition | 0.05 MeV | 242Am |
| 0.46% | alpha decay | 5.64 MeV | 238Np |
| (1.5±0.6) × 10−10 [10] | spontaneous fission | ~200 MeV | fission products |
Americium-242m has a mass of 242.0595492 g/mol. It is one of the rare cases, like 108mAg, 166mHo, 180mTa, 186mRe, 192mIr, 210mBi, 212mPo and others, where a higher-energy nuclear isomer is more stable than the ground state, americium-242.[11]
242mAm is fissile with a low critical mass, comparable to that of 239Pu.[12] It has a very high fission cross section, and is quickly destroyed if it is produced in a nuclear reactor. It has been investigated whether this isotope could be used for a novel type of nuclear rocket.[13][14]
| Probability | Decay mode | Decay energy | Decay product |
|---|---|---|---|
| 82.70% | beta decay | 0.665 MeV | 242Cm |
| 17.30% | electron capture | 0.751 MeV | 242Pu |
Americium-243

Americium-243 has a mass of 243.06138 g/mol and a half-life of 7,370 years, the longest lasting of all americium isotopes. It is formed in the nuclear fuel cycle by neutron capture on plutonium-242 followed by beta decay.[15] Production increases exponentially with increasing burnup as a total of 5 neutron captures on 238U are required. If MOX-fuel is used, particularly MOX-fuel high in 241
Pu and 242
Pu, more americium overall and more 243
Am will be produced.
It decays by either emitting an alpha particle (with a decay energy of 5.27 MeV)[15] to become 239Np, which then quickly decays to 239Pu, or rarely, by spontaneous fission.[16]
As for the other americium isotopes, and more generally for all alpha emitters, 243Am is carcinogenic in case of internal contamination after being inhaled or ingested. 243Am also presents a risk of external irradiation associated with the gamma ray emitted by its short-lived decay product 239Np. The external irradiation risk for the other two americium isotopes (241Am and 242mAm) is less than 10% of that for americium-243.[8]
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.