Disulfur monoxide
Chemical compound with sulfur and oxygen From Wikipedia, the free encyclopedia
Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature.
 | |
 | |
| Names | |
|---|---|
| Other names
sulfur suboxide; sulfuroxide; | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| S2O | |
| Molar mass | 80.1294 g/mol[1] |
| Appearance | colourless gas or dark red solid[2] |
| Structure | |
| bent | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
toxic |
| Related compounds | |
Related compounds |
Trisulfur SO Ozone SO2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
S
2O occurs rarely in natural atmospheres, but can be made by a variety of laboratory procedures. For this reason, its spectroscopic signature is very well understood.
Structure and spectrum
Like sulfur dioxide (and, indeed, most molecules) but unlike sulfur monoxide, disulfur, or dioxygen, the ground state of disulfur monoxide is a singlet.[3][4]
Condensed solid S2O absorbs at 420 nm (roughly indigo) and 530 nm (roughly lime). These bands have been assigned to decomposition products S3 and S4.[5]
In the ultraviolet, S2O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm.[6] The band in the 315–340 nm range is due to the C1A′–X1A′ (π* ← π) transition.[7]
Gaseous disulfur monoxide does not absorb light in the visible spectrum.
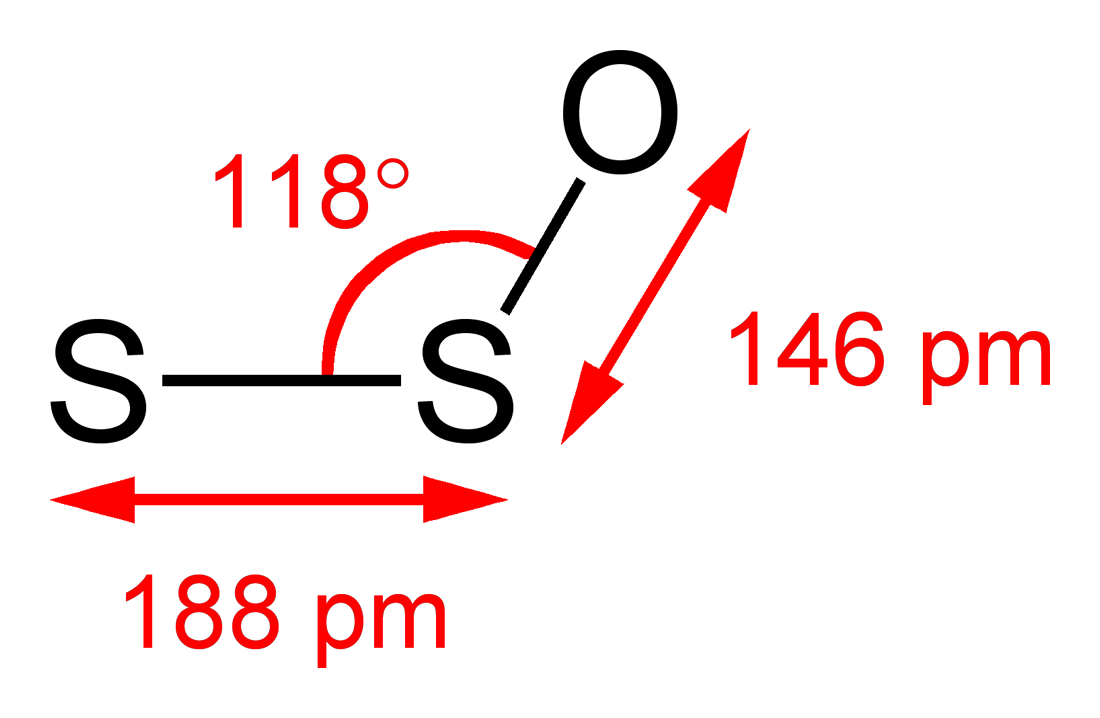
The microwave spectrum of S2O has the following rotational parameters: A = 41915.44 MHz, B = 5059.07 MHz, and C = 4507.19 MHz.[8] Moreover, the microwave spectrum suggests the S−S−O angle is 117.88° with S−S and S−O bond lengths of 188.4 and 146.5 pm, respectively.[9] In the 327.8 nm excited state, the central angle tightens to 109°.[6]
The harmonic frequency for S−S stretching is 415.2 cm−1.[7]
Synthesis
Historical
Disulfur monoxide was discovered by Peter W. Schenk in 1933[10] with a glow discharge though sulfur vapour and sulfur dioxide. He discovered that the gas could survive for hours at single digit pressures of mercury in clean glass, but it decomposed near 30 mmHg (4 kPa). Schenk assigned the formula as SO and called it sulfur monoxide. In 1956, D. J. Meschi and R. J. Myers established the formula as S2O.[11]
Preparation
Oxidizing sulfur with copper(II) oxide:[12][13]
- 3 S8 + 12 CuO → 12 CuS + 4 S2O + 4 SO2
A relatively pure generator is the reaction of thionyl chloride with silver(I) sulfide:[14]
- SOCl2 + Ag2S → 2 AgCl + S2O
Also 5,6-di-tert-butyl-2,3,7-trithiabicyclo[2.2.1]hept-5-ene 2-endo-7-endo-dioxide decomposes upon heating with release of S2O:[15]

Triphenylphosphine sulfide reacts with sulfinyltosylimide to give S2O and tosyltriphenylphosphinylamide:[16]
- TsNSO + SPPh3 → TsNPPh3 + S2O
Occurrence
Volcanism
Volcanoes on Io produce substantial quantities of S
2O. It can form between 1% and 6% when hot 100-bar S2 and SO2 gas erupts from volcanoes. It is believed that Pele on Io is surrounded by solid S2O.[17]
Terran atmosphere
Disulfur monoxide is too unstable to survive at standard conditions,[10] but transient sources include incomplete combustion of sulfur vapor[18] and thermal decomposition of sulfur dioxide in a glow discharge.[19]
As a ligand
Disulfur monoxide occurs as a ligand bound to transition metals, typically with hapticity 2.[20] Examples include OsCl(NO)(PPh3)2(S2O);[21] [Ir(PPh2)2(S2O)]+; and MeCpMn(CO2)(S2O).[20] These complexes are closely related to transition metal sulfur dioxide complexes.
Reactions
On decomposition at room temperature it forms SO2 via the formation of polysulfur oxides:[19]
- 2 S2O → "S3" + SO2
S
2O reacts with diazoalkanes to form dithiirane 1-oxides.[22]
Further reading
- Possible biological occurrence: Iverson, W. P. (26 May 1967). "Disulfur monoxide: production by Desulfovibrio". Science. 156 (3778): 1112–1114. Bibcode:1967Sci...156.1112I. doi:10.1126/science.156.3778.1112. PMID 6024190. S2CID 3058359. Archived from the original on September 26, 2017.
- Cyclic disulfur monoxide: Lo, Wen-Jui; Wu, Yu-Jong; Lee, Yuan-Pern (September 2003). "Ultraviolet Absorption Spectrum of Cyclic S2O in Solid Ar". The Journal of Physical Chemistry A. 107 (36): 6944–6947. Bibcode:2003JPCA..107.6944L. doi:10.1021/jp034563j.
- Discovery of S2O: Schenk, Peter W. (18 March 1933). "Über das Schwefelmonoxyd" [On sulfur monoxide]. Zeitschrift für Anorganische und Allgemeine Chemie (in German). 211 (1–2): 150–160. doi:10.1002/zaac.19332110117.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.