Loading AI tools
Toxic gaseous compound (COCl2) From Wikipedia, the free encyclopedia
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass.[7] It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.
 | |
 | |
 A sample case of toxic gases used in chemical warfare; the leftmost contains phosgene in a sealed capillary | |
| Names | |
|---|---|
| Preferred IUPAC name
Carbonyl dichloride[2] | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.792 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1076 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| COCl2 | |
| Molar mass | 98.91 g·mol−1 |
| Appearance | Colorless gas |
| Odor | Suffocating, like musty hay or grass[3] |
| Density | 4.248 g/L (15 °C, gas) 1.432 g/cm3 (0 °C, liquid) |
| Melting point | −118 °C (−180 °F; 155 K) |
| Boiling point | 8.3 °C (46.9 °F; 281.4 K) |
| Insoluble, reacts[4] | |
| Solubility | Soluble in benzene, toluene, acetic acid Decomposes in alcohol and acid |
| Vapor pressure | 1.6 atm (20°C)[3] |
| −48·10−6 cm3/mol | |
| Structure | |
| Trigonal planar | |
| 1.17 D | |
| Hazards | |
| GHS labelling: | |
  [5] [5] | |
| Danger | |
| H314, H330[5] | |
| P260, P280, P303+P361+P353+P315, P304+P340+P315, P305+P351+P338+P315, P403, P405[5] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
Threshold limit value (TLV) |
0.1 ppm (1 ppm = 4 mg/m3) |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
|
LCLo (lowest published) |
|
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.1 ppm (0.4 mg/m3)[3] |
REL (Recommended) |
TWA 0.1 ppm (0.4 mg/m3) C 0.2 ppm (0.8 mg/m3) [15-minute][3] |
IDLH (Immediate danger) |
2 ppm[3] 1 ppm = 4 mg/m3 |
| Safety data sheet (SDS) | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It is a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas.
It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform.[8]
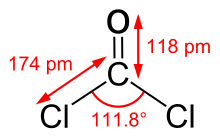
Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl angle is 111.8°.[9] Phosgene is a carbon oxohalide and it can be considered one of the simplest acyl chlorides, being formally derived from carbonic acid.
Industrially, phosgene is produced by passing purified carbon monoxide and chlorine gas through a bed of porous activated carbon, which serves as a catalyst:[8]
This reaction is exothermic and is typically performed between 50 and 150 °C. Above 200 °C, phosgene reverts to carbon monoxide and chlorine, Keq(300 K) = 0.05. World production of this compound was estimated to be 2.74 million tonnes in 1989.[8]
Phosgene is fairly simple to produce, but is listed as a Schedule 3 substance under the Chemical Weapons Convention. As such, it is usually considered too dangerous to transport in bulk quantities. Instead, phosgene is usually produced and consumed within the same plant, as part of an "on demand" process. This involves maintaining equivalent rates of production and consumption, which keeps the amount of phosgene in the system at any one time fairly low, reducing the risks in the event of an accident. Some batch production does still take place, but efforts are made to reduce the amount of phosgene stored.[10]
Simple organochlorides slowly convert into phosgene when exposed to ultraviolet (UV) irradiation in the presence of oxygen.[11] Before the discovery of the ozone hole in the late 1970s large quantities of organochlorides were routinely used by industry, which inevitably led to them entering the atmosphere. In the 1970-80s phosgene levels in the troposphere were around 20-30 pptv (peak 60 pptv).[11] These levels had not decreased significantly nearly 30 years later,[12] despite organochloride production becoming restricted under the Montreal Protocol.
Phosgene in the troposphere can last up to about 70 days and is removed primarily by hydrolysis with ambient humidity or cloudwater.[13] Less than 1% makes it to the stratosphere, where it is expected to have a lifetime of several years, since this layer is much drier and phosgene decomposes slowly through UV photolysis. It plays a minor part in ozone depletion.
Carbon tetrachloride (CCl4) can turn into phosgene when exposed to heat in air. This was a problem as carbon tetrachloride is an effective fire suppressant and was formerly in widespread use in fire extinguishers.[14] There are reports of fatalities caused by its use to fight fires in confined spaces.[15] Carbon tetrachloride's generation of phosgene and its own toxicity mean it is no longer used for this purpose.[14]
Phosgene is also formed as a metabolite of chloroform, likely via the action of cytochrome P-450.[16]
Phosgene was synthesized by the Cornish chemist John Davy (1790–1868) in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight. He named it "phosgene" from Greek φῶς (phos, light) and γεννάω (gennaō, to give birth) in reference of the use of light to promote the reaction.[17] It gradually became important in the chemical industry as the 19th century progressed, particularly in dye manufacturing.
The reaction of an organic substrate with phosgene is called phosgenation.[8] Phosgenation of diols give carbonates (R = H, alkyl, aryl), which can be either linear or cyclic:
An example is the reaction of phosgene with bisphenol A to form polycarbonates.[8] Phosgenation of diamines gives di-isocyanates, like toluene diisocyanate (TDI), methylene diphenyl diisocyanate (MDI), hexamethylene diisocyanate (HDI), and isophorone diisocyanate (IPDI). In these conversions, phosgene is used in excess to increase yield and minimize side reactions. The phosgene excess is separated during the work-up of resulting end products and recycled into the process, with any remaining phosgene decomposed in water using activated carbon as the catalyst. Diisocyanates are precursors to polyurethanes. More than 90% of the phosgene is used in these processes, with the biggest production units located in the United States (Texas and Louisiana), Germany, Shanghai, Japan, and South Korea. The most important producers are Dow Chemical, Covestro, and BASF. Phosgene is also used to produce monoisocyanates, used as pesticide precursors (e.g. methyl isocyanate (MIC).
Aside from the widely used reactions described above, phosgene is also used to produce acyl chlorides from carboxylic acids:
For this application, thionyl chloride is commonly used instead of phosgene.
The synthesis of isocyanates from amines illustrates the electrophilic character of this reagent and its use in introducing the equivalent synthon "CO2+":[18]
Such reactions are conducted on laboratory scale in the presence of a base such as pyridine that neutralizes the hydrogen chloride side-product.
Phosgene is used to produce chloroformates such as benzyl chloroformate:
In these syntheses, phosgene is used in excess to prevent formation of the corresponding carbonate ester.
With amino acids, phosgene (or its trimer) reacts to give amino acid N-carboxyanhydrides. More generally, phosgene acts to link two nucleophiles by a carbonyl group. For this purpose, alternatives to phosgene such as carbonyldiimidazole (CDI) are safer, albeit expensive.[19] CDI itself is prepared by reacting phosgene with imidazole.
Phosgene is stored in metal cylinders. In the US, the cylinder valve outlet is a tapered thread known as "CGA 160" that is used only for phosgene.
In the research laboratory, due to safety concerns phosgene nowadays finds limited use in organic synthesis. A variety of substitutes have been developed, notably trichloromethyl chloroformate ("diphosgene"), a liquid at room temperature, and bis(trichloromethyl) carbonate ("triphosgene"), a crystalline substance.[20]
Phosgene reacts with water to release hydrogen chloride and carbon dioxide:
Analogously, upon contact with ammonia, it converts to urea:
Halide exchange with nitrogen trifluoride and aluminium tribromide gives COF2 and COBr2, respectively.[8]

It is listed on Schedule 3 of the Chemical Weapons Convention: All production sites manufacturing more than 30 tonnes per year must be declared to the OPCW.[21] Although less toxic than many other chemical weapons such as sarin, phosgene is still regarded as a viable chemical warfare agent because of its simpler manufacturing requirements when compared to that of more technically advanced chemical weapons such as tabun, a first-generation nerve agent.[22]
Phosgene was first deployed as a chemical weapon by the French in 1915 in World War I.[23] It was also used in a mixture with an equal volume of chlorine, with the chlorine helping to spread the denser phosgene.[24][25] Phosgene was more potent than chlorine, though some symptoms took 24 hours or more to manifest.
Following the extensive use of phosgene during World War I, it was stockpiled by various countries.[26][27][28]
Phosgene was then only infrequently used by the Imperial Japanese Army against the Chinese during the Second Sino-Japanese War.[29] Gas weapons, such as phosgene, were produced by the IJA's Unit 731.
Phosgene is an insidious poison as the odor may not be noticed and symptoms may be slow to appear.[30]
Phosgene at low concentrations, may have a pleasant odor of freshly mown hay or green corn,[31] but has also been described as sweet, like rotten banana peels. The odor detection threshold for phosgene is 0.4 ppm, four times the threshold limit value (time weighted average). Its high toxicity arises from the action of the phosgene on the −OH, −NH2 and −SH groups of the proteins in pulmonary alveoli (the site of gas exchange), respectively forming ester, amide and thioester functional groups in accord with the reactions discussed above. This results in disruption of the blood–air barrier, eventually causing pulmonary edema. The extent of damage in the alveoli does not primarily depend on phosgene concentration in the inhaled air, with the dose (amount of inhaled phosgene) being the critical factor.[32] Dose can be approximately calculated as "concentration" × "duration of exposure".[32][33] Therefore, persons in workplaces where there exists risk of accidental phosgene release usually wear indicator badges close to the nose and mouth.[34] Such badges indicate the approximate inhaled dose, which allows for immediate treatment if the monitored dose rises above safe limits.[34]
In case of low or moderate quantities of inhaled phosgene, the exposed person is to be monitored and subjected to precautionary therapy, then released after several hours. For higher doses of inhaled phosgene (above 150 ppm × min) a pulmonary edema often develops which can be detected by X-ray imaging and regressive blood oxygen concentration. Inhalation of such high doses can eventually result in fatality within hours up to 2–3 days of the exposure.
The risk connected to a phosgene inhalation is based not so much on its toxicity (which is much lower in comparison to modern chemical weapons like sarin or tabun) but rather on its typical effects: the affected person may not develop any symptoms for hours until an edema appears, at which point it could be too late for medical treatment to assist.[35] Nearly all fatalities as a result of accidental releases from the industrial handling of phosgene occurred in this fashion. On the other hand, pulmonary edemas treated in a timely manner usually heal in the mid- and longterm, without major consequences once some days or weeks after exposure have passed.[36][37] Nonetheless, the detrimental health effects on pulmonary function from untreated, chronic low-level exposure to phosgene should not be ignored; although not exposed to concentrations high enough to immediately cause an edema, many synthetic chemists (e.g. Leonidas Zervas) working with the compound were reported to experience chronic respiratory health issues and eventual respiratory failure from continuous low-level exposure.
If accidental release of phosgene occurs in an industrial or laboratory setting, it can be mitigated with ammonia gas; in the case of liquid spills (e.g. of diphosgene or phosgene solutions) an absorbent and sodium carbonate can be applied.[38]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.