Loading AI tools
This page provides supplementary chemical data on tetrahydrofuran.
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions. MSDS is available at Mallinckrodt Baker.
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4040 at 25°C |
| Abbe number | ? |
| Dielectric constant, εr[1] | 7.52 ε0 at 22 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Viscosity[1] | 0.456 mPa·s at 25°C |
| Phase behavior | |
|---|---|
| Triple point | 164.76 K (−108.39 °C), ? Pa |
| Critical point | 541 K (268 °C), 5190 kPa |
| Std enthalpy change of fusion, ΔfusH |
8.540 kJ/mol |
| Std entropy change of fusion, ΔfusS |
51.8 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
32 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
51.8 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp[2] | 81.65 J/(mol K) at −108.39°C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
203.8 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−2501.2 kJ/mol |
| Heat capacity, cp[2] | 107.4 J/(mol K) at −108.39 °C
123.9 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−184.2 kJ/mol |
| Standard molar entropy, S |
301.7 J/(mol K) |
| Heat capacity, cp | 76.6 J/(mol K) at 25°C |
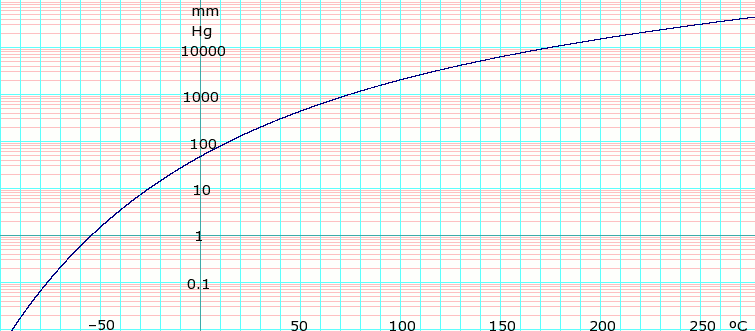
Vapor pressure 143 mm Hg at 20°C[1]
| P in mBar[3] | 9.9 | 19.5 | 36.3 | 63.9 | 107 | 173 | 268 | 402 | 586 | 831 | 1013 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T in °C | −30 | −20 | −10 | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 66 | |
| Vapor-Liquid Equilibrium of Tetrahydrofuran/Ethanol[5] P = 100 kPa | ||
| BP Temp. °C |
% by mole THF | |
|---|---|---|
| liquid | vapor | |
| 78. | 0.00 | 0.00 |
| 77.4 | 1.72 | 3.69 |
| 76.2 | 5.36 | 11.5 |
| 73.8 | 13.9 | 26.4 |
| 71.0 | 26.3 | 42.8 |
| 67.7 | 49.7 | 62.8 |
| 67.2 | 54.3 | 65.4 |
| 65.9 | 71.5 | 76.2 |
| 65.4 | 85.7 | 86.5 |
| 65.2 | 90.8 | 90.8 |
| 65.4 | 91.8 | 91.48 |
| 65.4 | 94.99 | 94.46 |
| 65.5 | 98.15 | 97.9 |
| 65.6 | 100.0 | 100.0 |
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.

