Transferring H7N9 Into Vials – Credit CDC
# 8959
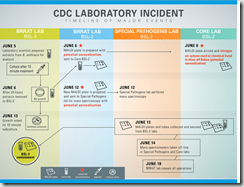
In the wake of two serious lapses in biosecurity at CDC labs involving both anthrax and H5N1 avian flu CDC Director Dr. Thomas Frieden promised a complete review of their safety procedures. One of his first actions was to halt the transfer of highly infectious agents from high containment labs until a safety review could be conducted.
In late July, the CDC issued a Statement On Formation Of An External Lab Safety Workgroup, and on the same day announced New safety protocols in place, first CDC lab resumes transfer of inactivated materials out of high-containment laboratory.
Over the past month seen a good deal of editorializing on the problems of lab safety at the CDC and elsewhere, including in The Journal Nature Weighs In On Lab Accidents & Biosafety & The Laboratory Bio-Safety Backlash Continues, and in testimony before a House Subcommittee.
Today the CDC has released a progress report on their efforts to get to the bottom of these incidents and improve laboratory safety, along with an internal review of the H5N1 cross-contamination.
For Immediate Release: Friday, August 15, 2014
Contact: Media Relations
(404) 639-3286CDC Progress on Laboratory Safety
The Centers for Disease Control and Prevention today released a series of updates and actions taken showing its progress in laboratory safety. CDC reported on its response to the Animal and Plant Health Inspection Service’s (APHIS) Agricultural Select Agent Program’s (ASAP), part of the U.S. Department of Agriculture, summary of findings on the anthrax incident. CDC also reported findings of an internal CDC investigation on the H5N1 flu lab incident, and actions taken in response to findings of both the APHIS and CDC investigations into the H5N1 incident.
“CDC is working intensively to make our labs to be models not only of scientific rigor but also of safety," said CDC Director Tom Frieden, M.D., M.P.H. “We will implement changes identified in these reviews – and more – so that we can continue the critical laboratory science needed to protect health in the US and around the world.”
Internal report on H5N1 flu lab incident
This from the H5N1 lab incident executive summary.
What Happened
CDC’s Influenza Division (ID) laboratories had received the H9N2 and H5N1 virus samples as part of ongoing surveillance of human and animal influenza viruses. The virus samples were grown in cell culture and stored for future use. In response to a request from the U.S. Department of Agriculture (USDA), Southeast Poultry Research Laboratory (SEPRL), an aliquot of the H9N2 virus was sent from an ID laboratory to SEPRL on March 12, 2014. Since the H9N2 strain is not a select agent and the ID laboratory was unaware that it had been contaminated, select agent transfer procedures were not followed. On May 23, 2014, SEPRL notified CDC that it had identified an HPAI H5N1 virus (a select agent) in the H9N2 sample. The ID laboratory subsequently confirmed the contamination but did not notify the supervisory chain of command, including branch, division, center, and CDC leadership. The incident was reported to the CDC internal select agent program and to CDC management on July 9, 2014.
Why the Incident HappenedContamination of the LPAI H9N2 virus culture with the HPAI H5N1 virus occurred at a CDC BSL3-E influenza laboratory. The contamination most likely happened due to the failure of a laboratory scientist to adhere to established best practices and the absence of an approved laboratory team-specific standard operating procedure (SOP) for the work being done. Although several factors contributed to the delay in reporting the incident, the primary factors were 1) a lack of sound professional judgment by those aware of the contamination; and 2) insufficient or ambiguous select agent and institutional reporting requirements. For example, guidance documents from the Federal Select Agent Program and from the CDC internal select agent program do not describe reporting of an unauthorized transfer of a select agent as required reporting of a release.
What Has CDC Done Since the Incident Occurred
In response to this specific incident, CDC has taken the following steps:
- Conducted an internal review (described in this report)
- Closed the laboratory involved until enhancements to safety and security can be implemented
- Included this laboratory in the CDC-wide moratorium on any biological material leaving any CDC BSL-3 or BSL-4 laboratory until adequate, additional approved safety measures are shown to be in place
- Notified USDA’s Animal and Plant Health Inspection Service (APHIS), which has since conducted an investigation and issued a report on the incident
These specific actions are in addition to broader actions recently undertaken by CDC to improve overall lab safety and security agency-wide. These broad actions include
- The appointment of a CDC Director of Laboratory Safety to serve as the single point of accountability to improve all laboratory safety protocols, practices, and procedures
- The establishment of an internal Biosafety Working Group under the direction of the CDC Director of Laboratory Safety
- The establishment of an external advisory group on biosafety comprising leading scientists and biosafety experts, which will serve as a work group of the Advisory Committee to the CDC Director
- A review of policies and procedures for laboratory safety and security in all CDC BSL-3 and BSL-4 laboratories
What Additional Steps Are Recommended Moving Forward
Additional recommendations include the following:
- Reassess all CDC laboratory procedures related to H5N1 and other HPAI viruses, including how and where this work is done at CDC and how it relates to CDC’s mission
- Develop written, approved policies and procedures to ensure that cross contamination of influenza viruses does not occur in the future
- Institute comprehensive quality control measures across all CDC laboratories through the performance of exclusivity testing of materials (i.e., testing to exclude the presence of other organisms) before transfer to internal and external laboratories. Materials should be accompanied by a written certificate of analysis that describes the tests and methods used.
- Broaden exclusivity testing of incoming samples to ensure the safety of laboratory scientists who work with the samples and their derivatives. For the near term, due to technologic constraints, such testing will likely be limited to agents of highest concern based on the likelihood or seriousness of their presence.
- Ensure that all ID staff are appropriately trained to understand when biosafety events are reportable and to whom they should be reported (both for select agents and non-select agents), and, more broadly, ensure that all CDC laboratory scientists receive such training. A site-specific SOP for event notification should be in place in each laboratory.
- Institute personnel actions as appropriate
Despite an impressively detailed `after-action’ review of the events leading up to biosafety lapse, due to a variety of factors, the exact point of cross-contamination has not been conclusively established.
The entire 16 page report is quite illuminating, shows just how complex the issues are when working in high containment facilities, and is well worth reading in its entirety.