Heart failure (HF) is a shared chronic phase of many cardiac diseases, including ischaemic heart disease, chronic obstructive pulmonary disease and hypertension. It is characterised by structural or functional impairment of ventricular filling or ejection of blood from the heart.1–3 It is estimated that there are more than 37.7 million cases of HF globally and its prevalence is on the rise.2 HF can be categorised into two subtypes: HF with reduced ejection fraction (HFrEF), defined by guidelines as an ejection fraction ≤40%, and HF with preserved ejection fraction (HFpEF), defined as an ejection fraction ≥50%, with those falling between these ranges considered to have borderline HFpEF or mid-range ejection fraction.3,4
Treatment options for HFrEF include combining angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) with mineralocorticoid receptor antagonists (MRAs), beta-blockers or diuretics as needed.3 The evidence for the efficacy of these treatments in HFrEF was published in the early 1990s and 2000s; since this time, the only treatment to demonstrate a benefit for patients with HFrEF has been the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril–valsartan, which reported superiority to ACE inhibition in reducing the risks of death and hospitalisation for HF in 2014.5 Disappointingly, the vast majority of trials in patients with HFpEF have failed to demonstrate robust efficacy. The much-anticipated Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF) trial of sacubitril–valsartan in patients with HFpEF missed its primary endpoint, although there were some indicators of efficacy in specific patient subgroups, meaning that patients with HFpEF still have very few treatment options with demonstrated benefit in hospitalisation and mortality.6
The US Food and Drug Administration (FDA) has recently approved the sodium–glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin for the treatment of patients with HFrEF.7 Originally developed to aid glycaemic control in type 2 diabetes (T2D), dapagliflozin has the potential to substantially improve outcomes for HFrEF patients. This approval follows the release of data from the groundbreaking Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure (DAPA-HF) trial, which was the first large-scale trial to demonstrate the efficacy of an SGLT2 inhibitor in a patient population that included both those with and without T2D.8 More recently, the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trial investigating another SGLT2 inhibitor, empagliflozin, in patients with HFrEF also reported reductions in hospitalisation for HF, but failed to demonstrate benefit in terms of mortality.9
This article aims to discuss the evidence supporting the use of dapagliflozin and empagliflozin in patients with HFrEF with and without T2D and the optimal place for SGLT2 inhibitors in HF therapy.
The Impact of SGLT2 Inhibition on Cardiovascular Outcomes in Type 2 Diabetes Patients
T2D is a major cardiovascular (CV) risk factor; CV disease (CVD) affects around one-third of people with T2D and is a major cause of mortality.10 Amid this background of increased CV risk, and following concerns surrounding the CV safety profile of the thiazolidinedione rosiglitazone, the FDA and European Medicines Agency issued guidance requiring sponsors to investigate the CV safety profiles of new glucose-lowering drugs through CV outcomes trials (CVOTs).11 Unexpectedly, rather than simply providing assurance surrounding the CV safety of SGLT2 inhibitors, results from three large-scale trial programmes in T2D suggested that these therapies could prevent serious CV events.12
In 2015, the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) was the first trial to demonstrate that treatment with an SGLT2 inhibitor significantly reduced the rate of CV events compared with placebo in patients with T2D and established CVD.13 The trial reported a significant reduction in the primary outcome, a composite of death from CV causes, non-fatal MI or non-fatal stroke in the empagliflozin group compared with placebo (10.5% versus 12.1%; HR 0.86; 95% CI [0.74–0.99]; p<0.001 for non-inferiority and p=0.04 for superiority).13 The outcome was primarily driven by CV death, with rates of 3.7% in the empagliflozin versus 5.9% in the placebo groups (p<0.001). Rates of all MI (4.8% versus 5.4%; p=0.23) and all stroke (3.5% versus 3.0%; p=0.26) did not differ significantly between treatment groups. Subsequently, the Canagliflozin Cardiovascular Assessment Study (CANVAS) programme, consisting of sister randomised controlled trials CANVAS and CANVAS-Renal (CANVAS-R), demonstrated similar outcomes in terms of CV events, also in patients with T2D, the majority of whom had established CVD.14 Both trials demonstrated substantial improvements in the rates of hospitalisation for HF for the investigational treatment versus placebo (35% reduction compared with placebo, HR 0.65; 95% CI [0.50–0.85]; p=0.002 in EMPA-REG OUTCOME, and 33% reduction compared with placebo, HR 0.67; 95% CI [0.52–0.91] in the CANVAS programme).13,14
Following the disclosure of these trial results, the protocol of the Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI 58) trial was amended to include a composite of hospitalisation for HF and CV death as a co-primary endpoint. DECLARE-TIMI 58 reported a significant reduction in this co-primary endpoint (HR 0.83; 95% CI [0.73–0.95]; p=0.005), driven primarily by a lower rate in hospitalisation for HF (27% reduction compared with placebo, HR 0.73; 95% CI [0.61–0.88]).15 There was a non-significant numerical reduction in the second primary endpoint (CV death, MI or stroke).15 This was speculated to be due to the lower overall rate of CV events in the study population, the majority of whom (59.5%) had multiple CVD risk factors but not established CVD, when compared with the populations recruited for the other SGLT2 inhibitor CVOTs.15
In addition to the CVOTs, the renal outcomes trial Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE), investigating the efficacy of canagliflozin in patients with diabetic nephropathy, also reported a substantial reduction in terms of a composite of hospitalisation for HF and CV death (HR 0.69; 95% CI [0.57–0.83]; p<0.001) and hospitalisation for HF (HR 0.61; 95% CI [0.47–0.80]; p<0.001) for patients treated with canagliflozin compared with those receiving placebo.16
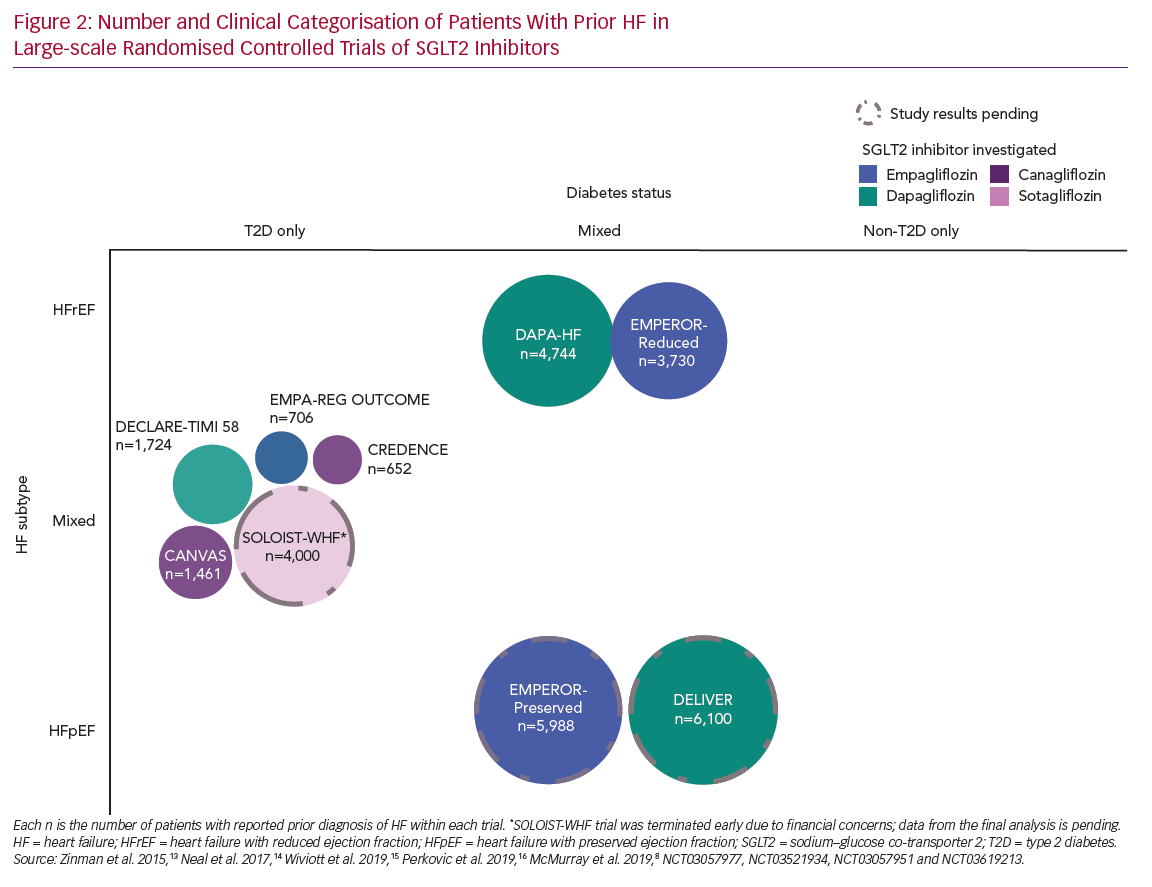
Meta-analyses of CVOTs reported that SGLT2 inhibitors, as a class, reduced the risk of hospitalisation for HF by 31–32% in patients with T2D, and that this risk reduction was consistent in patients with and without recognised CVD (~30% reduction in risk of hospitalisation for HF in both subgroups).12,17 Furthermore, they demonstrated that the event reductions were similar in comparable patient populations (Figure 1).
Subgroup analyses of EMPA-REG OUTCOME and DECLARE-TIMI 58 demonstrated consistent benefit on the composite of hospitalisation for HF or CV death, irrespective of baseline HF status.15,18 Results from an equivalent analysis of the CANVAS programme suggested that the benefit of canagliflozin on the composite of hospitalisation for HF and CV death may have been more pronounced in those with a prior history of HF compared with those without (p interaction = 0.021). However, all other outcomes were similar between the subgroups (Figure 2).19 A meta-analysis of EMPA-REG OUTCOME, DECLARE-TIMI 58 and the CANVAS programme reported similar benefits for patients with and without a history of HF, with low heterogeneity between interventions.12
SGLT2 Inhibitors in Patients with HFrEF
Limited additional post-hoc analyses provided more insights on the impact of SGLT2 inhibitors by HF subtype. An analysis of the CANVAS programme found that canagliflozin reduced the overall risk of HFrEF (ejection fraction <50%) events leading to hospitalisation or death (HR 0.69; 95% CI [0.48–1.00]).20 Ejection fraction classification for patients with a history of HF was not required at baseline within the CANVAS programme, and this analysis was not restricted to those with a history of HF, resulting in a limited application of these results for those with a history of HF specifically.20 A post-hoc analysis of DECLARE-TIMI 58, which collected more complete baseline data, found a 36% risk reduction for hospitalisation for HF, a 45% risk reduction for CV death and a 51% risk reduction in all-cause death in patients with a history of HFrEF (known ejection fraction <45%).21 These subgroup analyses, although suggestive of the potential benefits of SGLT2 inhibitors for patients with HFrEF, are difficult to interpret owing to low patient numbers: only 10–14% of patients reported prior HF at baseline across the SGLT2 inhibitor CVOTs.13–15 Additionally, as well as the limitation of incomplete classification of HF subtypes, these trials only included patients with T2D (Figure 2).20,21 Dedicated studies were needed to confirm the signals observed in SGLT2 inhibitor CVOTs and to investigate whether these benefits extend to patients with HF without T2D.
The DAPA-HF Trial
DAPA-HF was the first outcomes trial of an SGLT2 inhibitor to investigate the treatment of HF in patients with HFrEF with and without T2D.8 DAPA-HF was a multicentre Phase III placebo-controlled clinical trial in 4,744 patients with New York Heart Association class II, III or IV HF and ejection fraction ≤40%. Patients were required to have a plasma level of N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥600 pg/ml (≥400 pg/ml if they had been hospitalised for HF within the previous 12 months and ≥900 pg/ml if they had AF or atrial flutter on baseline ECG). Exclusion criteria were recent treatment with, or intolerance to, an SGLT2 inhibitor, type 1 diabetes, systolic blood pressure <95 mmHg, an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73m2, or rapidly declining renal function. Patients received dapagliflozin 10 mg once daily or placebo, and were required to receive standard best-practice HF device and drug therapy, including an ACE inhibitor, an ARB, or sacubitril–valsartan plus a beta-blocker, unless contraindicated.8
At baseline, 45% of patients had T2D and 55% did not. Baseline therapies were similar between groups, with 93.4% and 93.5% receiving a diuretic, 71.5% and 70.6% receiving MRAs, 56.1% receiving an ACE inhibitor, 28.4% and 26.7% receiving an ARB, 10.5% and 10.9% receiving sacubitril–valsartan, and 96.0% and 96.2% receiving a beta-blocker, for dapagliflozin and placebo groups, respectively. The majority of patients were men (76.2% in the dapagliflozin group and 77% in the placebo group).8 Mean eGFR was 66.0 ml/min/1.73m2 in the dapagliflozin group and 65.5 ml/min/1.73m2 in the placebo group at baseline.
The primary outcome was a composite of worsening HF (hospitalisation or an urgent visit resulting in IV therapy for HF) or CV death. Over a median of 18.2 months, the primary outcome occurred in 386 out of 2,373 patients (16.3%) in the dapagliflozin group and in 502 out of 2,371 patients (21.2%) in the placebo group (HR 0.74; 95% CI [0.65–0.85]; p<0.001). Death from CV causes occurred in 9.6% of the dapagliflozin group and in 11.5% of the placebo group (HR 0.82; 95% CI [0.69–0.98]). During the trial period, the number of patients needed to treat to prevent one primary event was 21 (95% CI [15–38]). Hospitalisation for HF occurred in 9.7% of the dapagliflozin group and in 13.4% of the placebo group (HR 0.70; 95% CI [0.59–0.83]; p<0.001; Table 1).8 Both first and recurrent hospitalisations for HF were significantly reduced in the dapagliflozin group compared with placebo.22 The incidence of the secondary composite outcome of hospitalisation for HF or death from CV causes was also lower in the dapagliflozin group than in the placebo group (567 events versus 742 events, HR 0.74; 95% CI [0.65–0.85]; p<0.001; Table 1). In addition, there was a 17% reduction in all-cause mortality (HR 0.83; 95% CI [0.71–0.97]) in the dapagliflozin arm of DAPA-HF compared to placebo.8 A 29% reduction in the worsening of renal function, which was not statistically significant (HR 0.71; 95% CI [0.44–1.16]), was also observed in the dapagliflozin arm.8 Overall numbers of renal progression events were low in DAPA-HF, with 40.6% of patients having impaired renal function (eGFR between ≥30 ml/min/1.73m2 and <60 ml/min/1.73m2), but the relative reduction observed in the dapagliflozin arm was similar to renal composite results for patients with T2D from DECLARE-TIMI 58.15
Moreover, recent results from DAPA-CKD, the first dedicated renal outcomes trial assessing the efficacy and safety of an SGLT2 inhibitor in patients with chronic kidney disease with and without T2D, supported the renoprotective effects of dapagliflozin in patients with impaired renal function (eGFR ≥25 ml/min/1.73 m2 and ≤75 ml/min/1.73 m2). Dapagliflozin treatment was associated with a 39% reduction in renal function decline (HR 0.61; 95% CI [0.51–0.72]; p<0.001) and a 31% reduction in all-cause death (HR 0.69; 95% CI [0.53–0.88]; p=0.004) compared to placebo in this patient population.23
Subgroup analyses from DAPA-HF, both prespecified and post-hoc, demonstrated similar effects of dapagliflozin compared with placebo regardless of diuretic, MRA or ARNI use in patients who received ≥50% of target ACE inhibitor/ARB or beta-blocker dose as well as those who did not, suggesting that treatment with dapagliflozin is beneficial regardless of baseline HFrEF therapy.24 Similar treatment benefits of dapagliflozin over placebo were also observed, irrespective of the underlying cause of HF, baseline renal function (eGFR <60 ml/min/1.73m2 versus eGFR ≥60 ml/min/1.73 m2),8 systolic blood pressure,8,25 BMI,8,26 or NT-pro-BNP concentration.27 Dapagliflozin was also found to reduce the risk of death and worsening HF and to improve symptoms across a broad spectrum of age (range 22–94 years; mean age 66.3 years [SD 10.9]).28
In addition to major clinical events, DAPA-HF also used the Kansas City Cardiomyopathy Questionnaire (KCCQ) patient-reported outcome measure to assess HF symptom burden from the patients’ perspective. A clinically meaningful ≥5-point improvement from baseline to 8 months was reported in 58.3% of dapagliflozin-treated patients versus 50.9% of placebo-treated patients (OR 1.15; 95% CI [1.08–1.23]; p<0.001). The number needed to treat for one patient experiencing a ≥5-point KCCQ improvement was 14.8 Moderate (≥10 points) and large (≥15 points) improvements were also more likely in the dapagliflozin group compared with placebo (OR 1.15; 95% CI [1.08–1.22] and OR 1.14; 95% CI [1.08–1.22], respectively).29 There was also less deterioration in KCCQ score from baseline to 8 months in the dapagliflozin group than in the placebo group (25.3% and 32.9%, respectively; OR 0.84; 95% CI [0.78–0.90]; p<0.001).8 A recent post-hoc analysis also demonstrated that dapagliflozin reduced CV death and worsening HF across the range of baseline KCCQ scores (p heterogeneity = 0.52).29
Dapagliflozin was well tolerated and the rate of treatment discontinuation was low. The rates of serious adverse events related to volume depletion were slightly lower in the dapagliflozin group compared with placebo (1.2% and 1.7%, respectively; p=0.23), and the rate of serious renal adverse events was significantly lower in dapagliflozin-treated patients than those receiving placebo (1.6% and 2.7%, respectively; p=0.009).8 There had been concern that the use of dapagliflozin might lead to hypoglycaemia in patients without T2D. However, major hypoglycaemic episodes were extremely rare and equal (0.2%) in both the dapagliflozin and placebo groups.8 There were no issues with ketoacidosis and no other significant safety concerns were reported,8 even in elderly individuals.28 Unlike other SGLT2 inhibitors, dapagliflozin did not increase the risk of fractures or amputations.
Effect of Dapagliflozin in HFrEF Patients with and without Type 2 Diabetes
At baseline, 42% of patients in DAPA-HF had T2D, and an additional 3% received a new diagnosis of T2D during the course of the trial, resulting in a total of 2,139 (45%) patients with T2D.8 The reduction in the rate of the primary outcome was very similar between patients with T2D at baseline (HR 0.75; 95% CI [0.63–0.90]) and those without T2D at baseline (HR 0.73; 95% CI [0.60–0.88]), although the overall risk of events was higher in the T2D group, as expected.8,30 Similar benefits were observed across secondary outcomes, including risk reductions of total hospitalisation for HF and CV death of 23% (HR 0.77; 95% CI [0.63–0.94]) for patients with baseline T2D, and 27% (HR 0.73; 95% CI [0.59–0.91]) for those without T2D at baseline for dapagliflozin compared with placebo.30
The DEFINE-HF Trial
The Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE-HF) was a small trial of 263 patients with HFrEF, eGFR ≥30 ml/min/1.73m2 and elevated natriuretic peptides.31 Patients were randomised to receive either 10 mg dapagliflozin or placebo in addition to optimal medical therapy for 12 weeks. The dual primary outcomes were mean NT-proBNP and the proportion of patients with ≥5-point increase in the KCCQ Overall Summary Score (KCCQ-OSS) or a ≥20% decrease in NT-proBNP. Although there was no significant difference in average-adjusted NT-proBNP at 6 and 12 weeks, more patients in the dapagliflozin group than in the placebo group met the second dual primary outcome of clinically meaningful improvement in quality of life as measured by KCCQ-OSS or a reduction of ≥20% in NT-proBNP (61.5% versus 50.4%; p=0.039). This was attributable to both higher proportions of patients with a ≥5-point improvement in KCCQ-OSS (42.9% versus 32.5%; adjusted OR 1.73; 95% CI [0.98–3.05]; p=not significant) and with a ≥20% reduction in NT-proBNP (44.0% versus 29.4%; adjusted OR 1.9; 95% CI [1.1–3.3]; p=not significant) by 12 weeks. The results were consistent in patients with and without T2D, and across other prespecified subgroups including gender, baseline left ventricular ejection fraction and AF.31
The EMPEROR-Reduced Trial
Results from the EMPEROR-Reduced Phase III, placebo-controlled study (NCT03057977), which involved 3,730 patients with class II, III or IV HF with ejection fraction ≤40% randomised to placebo or empagliflozin 10 mg daily, added to guideline-directed medical therapy (ACE inhibitors/ARBs/ARNIs, beta‐blockers and MRAs), were recently published.9 Plasma NT-proBNP level required for enrolment was dependent on ejection fraction at baseline; ≥600 pg/ml for patients with ejection fraction ≤30%, ≥1,000 pg/ml for patients with ejection fraction 31–35%, and ≥2,500 pg/ml for patients with ejection fraction 36–<40% (NT-proBNP threshold was doubled for patients with AF). Exclusion criteria included recent treatment with, or intolerance to, an SGLT2 inhibitor, systolic blood pressure ≥180 mmHg or <100 mmHg, eGFR <20 ml/min/1.73m2, and impaired renal function requiring dialysis.
Approximately half of patients had diabetes at baseline (49.8%), and the majority of patients were men (76.5% and 75.6% in empagliflozin and placebo groups, respectively). Baseline therapies were similar between treatment groups; 70.5% and 68.0% received an ACE inhibitor or ARB, 18.3% and 20.7% received sacubitril–valsartan in the empagliflozin and placebo groups, respectively, while 94.7% in each group received a beta-blocker.9
The primary endpoint was a time-to-first-event analysis of the combined risk of CV death and hospitalisation for HF. After a median of 16 months, the primary outcome occurred in 361 of 1,863 patients (19.4%) in the empagliflozin group and in 462 of 1,867 patients (24.7%) in the placebo group (HR 0.75; 95% CI [0.65–0.86]; p<0.001; Table 1).9 This was primarily driven by reduced rates of hospitalisation for HF in the empagliflozin group (HR 0.70; 95% CI [0.58–0.85]; p<0.001; Table 1) and the trial failed to demonstrate a significant reduction in CV death (HR 0.92; 95% CI [0.75–1.12]) compared to placebo.9 There was also a significant reduction in the rate of renal disease progression, as measured by eGFR slope over time, in the empagliflozin group compared to patients receiving placebo (–0.55 versus –2.28 ml/min/1.73m2 per year; absolute difference 1.73 ml/min/1.73m2 per year; 95% CI [1.10–2.37]; p<0.001).9 Unlike dapagliflozin in DAPA-HF, empagliflozin failed to demonstrate efficacy in terms of quality of life as measured using KCCQ.9 Also, empagliflozin did not affect all-cause mortality (HR 0.92; 95% CI [0.77–1.10]).9,13
Subgroup analyses demonstrated similar effects of empagliflozin on the primary endpoint irrespective of baseline diabetes status (HR 0.72; 95% CI [0.60–0.87] with diabetes; HR 0.78; 95% CI [0.64–0.97] without diabetes).9 Comparable treatment benefits of empagliflozin over placebo were also observed regardless of MRA or ARNI use, underlying cause of HF, or baseline renal function.9
The adverse events profile for empagliflozin was similar to that reported in previous studies.9,13 The overall rates of adverse events and serious adverse events were lower in the empagliflozin group compared to the placebo group, with only genital infections reported substantially more frequently in the empagliflozin group (1.7%) compared to the placebo group (0.6%).9 As in DAPA-HF, hypoglycaemic episodes of any severity were infrequent and rates were similar between treatment groups (1.4% versus 1.5% in empagliflozin and placebo groups, respectively).9 No cases of ketoacidosis were recorded. Although rates of fractures and amputations were slightly more frequent in the empagliflozin group compared to placebo (2.4% versus 2.3% and 0.7% versus 0.5%, respectively), the differences were not statistically significant.9
The EMPERIAL-Reduced Trial
The Empagliflozin Compared With Placebo On Exercise Ability and Heart Failure Symptoms, in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPERIAL-Reduced; NCT03448419) study investigated the impact of empagliflozin on exercise capacity in 312 patients with HFrEF over 12 weeks.32 The trial did not meet its primary endpoint, the 6-minute walk test, with no significant differences reported between the empagliflozin and placebo arms. Initial reports outlined substantial improvements in KCCQ total symptom score in the empagliflozin group compared to placebo.33 However, the results are yet to be published in full.
Recent Trials of SGLT2 Inhibitors in HFrEF with Results Pending
Data are still awaited from some recently completed trials of SGLT2 inhibitors treating patients with HFrEF. Dapagliflozin Effect on Exercise Capacity Using a 6-minute Walk Test in Patients With Heart Failure With Reduced Ejection Fraction (DETERMINE-reduced; NCT03877237), investigating the impact of dapagliflozin compared to placebo on exercise capacity and quality of life in 313 patients with HFrEF, completed in March 2020 with results pending. The co-primary endpoints of this study were KCCQ total symptom score, the KCCQ physical limitation score and 6-minute walk test following 16 weeks of treatment. In addition, the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF; NCT03521934) trial targeted 4,000 patients with T2D and HFrEF or HFpEF in the immediate post-hospitalisation setting. In May 2020, the study was discontinued early because of financial concerns. Sotagliflozin is a dual SGLT1/SGLT2 inhibitor and thus differs slightly from the SGLT2 inhibitors studied to date.34 Despite the early closure, it is still anticipated that results from the trial will be made available, and it will be interesting to see whether the safety and efficacy of this drug replicate those observed for dapagliflozin and empagliflozin.
SGLT2 Inhibitors in Patients with HFpEF
A post-hoc subgroup analysis of the CANVAS programme suggested that canagliflozin may reduce the rates of HFpEF (ejection fraction ≥50%) hospitalisation or mortality events (HR 0.83; 95% CI [0.55–1.25]).20 However, the subgroup analysis did not achieve statistical significance because the study was not powered to detect such a difference in this small subpopulation (<1% of the overall study population).20 A post-hoc subgroup analysis of patients with known HFpEF at baseline in the DECLARE-TIMI 58 study also found a signal for reduced risk of hospitalisation for HF in this study population (HR 0.74; 95% CI [0.48–1.14]), but not for CV death (HR 1.44; 95% CI [0.83–2.49]).21 Again, only 4.7% of the total trial population of DECLARE-TIMI 58 had a documented history of HFpEF; therefore, these results should be interpreted with caution.21 The Empagliflozin Compared With Placebo on Exercise Ability and Heart Failure Symptoms, In Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPERIAL-Preserved; NCT03448406) trial investigated the impact of empagliflozin on exercise capacity in 312 patients with HFpEF, however, found no significant difference between the empagliflozin and placebo groups in terms of 6-minute walk test or KCCQ total symptom scores after 12 weeks.33
Two major trials of SGLT2 inhibitors in patients with HFpEF are currently on-going (Figure 2). The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved; NCT03057951) trial will follow a similar study design to the EMPEROR-Reduced trial and has randomised 5,988 patients with HFpEF to empagliflozin or placebo.35 The study is due to be completed in 2020. The Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER; NCT03619213) trial is aiming to recruit 6,100 patients with HFpEF, who will be randomised to dapagliflozin or placebo in addition to current standard therapy. The study is due to be completed in mid-2021. In addition, Dapagliflozin Effect on Exercise Capacity Using a 6-minute Walk Test in Patients With Heart Failure With Preserved Ejection Fraction (DETERMINE-Preserved; NCT03877224), a randomised controlled trial investigating the impact of dapagliflozin compared with placebo on exercise capacity and quality of life in 504 patients with HFpEF, is anticipated to complete in 2020, and the recently discontinued SOLOIST-WHF trial of the dual SGLT1/SGLT2 inhibitor sotagliflozin included both patients with HFpEF and those with HFrEF, and may still provide some insights if sufficient data were collected.
Many aspects of HFpEF diagnosis and treatment remain unresolved. The HFpEF umbrella describes a population of patients that are very heterogeneous, and though there is overlap between the causes and risk factors for HFrEF and HFpEF, the pathophysiologies of these two subtypes are very distinct.36 It is hoped that the SGLT2 inhibitors will succeed where other drugs have failed, and the on-going trial results are widely anticipated.
Mechanism of SGLT2 Inhibitors in CVD
Although originally investigated as agents for glucose management, SGLT2 inhibitors are now recognised to impact a wider range of systems, primarily in the cardio–renal axis, many of which are independent of glycaemic control.37–39 The SGLT2 channel is primarily located in the proximal tubule of the kidney, where the majority of glucose reabsorption takes place.40 It has been postulated that the mechanisms underlying SGLT2 inhibitor-associated CV benefits could include improvements in ventricular load through the effect on natriuresis and osmotic diuresis (Figure 3).37–39 SGLT2 inhibitors have a profound effect on haemodynamics. Unlike diuretics, they do not deplete intravascular volume, but instead reduce interstitial volume.40 Optimised ventricular loading conditions, through reduction in preload and afterload, result in a lowering of blood pressure, improved endothelial function, and reduced vascular stiffness.40
It has also been suggested that SGLT2 inhibitors may improve cardiac metabolism and bioenergetics. The utilisation of both glucose and fatty acids is believed to be inefficient in the hearts of patients with HF and T2D. SGLT2 inhibition shifts metabolism towards the oxidation of ketone bodies, which has been shown to be associated with myocardial benefits.41 Preliminary in-vitro studies have also explored the role of SGLT2 inhibitors in ion exchange, cardiac remodelling and their influence on adipokine expression, cytokine production, and epicardial adipose tissue mass.40 Downstream myocardial Na+/H+ exchange inhibition has been shown to lead to lower levels of sodium and thus lower levels of calcium in cardiomyocytes, which improve contractility and mitochondrial function.40,42
The efficacy of dapagliflozin and empagliflozin in patients with HFrEF irrespective of T2D status has challenged our assumptions about the dominant mechanisms of action of SGLT2 inhibitors.43 Several relevant studies investigating the impact of SGLT2 inhibitors are currently underway or have recently been completed, including mechanistic studies of empagliflozin and dapagliflozin. The empagliflozin studies include: Empagliflozin in Heart Failure Patients With Reduced Ejection Fraction (EMPIRE-HF; NCT03198585),44 A Study That Looks at the Function of the Heart in Patients With Heart Failure Who Take Empagliflozin (EMPA-VISION; NCT03332212), and Empagliflozin Impact on Hemodynamics in Patients With Heart Failure (EMBRACE-HF; NCT03030222). For dapagliflozin the studies include: Dapagliflozin in PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF; NCT03030235), A Clinical Study to Investigate the Effects of Dapagliflozin on Heart Work, Heart Nutrient Uptake, and Heart Muscle Efficiency in Type 2 Diabetes Patients (DAPACARD; NCT03387683),45 Study to Evaluate Average 24-hr Sodium Excretion During Dapagliflozin Treatment in Patients With Type 2 Diabetes Mellitus With Preserved or Impaired Renal Function or Non-diabetics With Impaired Renal Function (DAPASALT; NCT03152084) and Effects of 5 Weeks Treatment With Dapagliflozin in Type 2 Diabetes Patients on How the Hormone Insulin Acts on Sugar Uptake in Muscles (DAPAMAAST; NCT03338855). These studies are investigating the effect of SGLT2 inhibition on cardiac biomarkers, cardiac function, cardiac haemodynamics and metabolism.44 The outcomes of this research will enable us to better understand how the mechanism of action of SGLT2 inhibitors directly influences HF outcomes in the clinical setting.
Updating Clinical Guidelines
The importance of prevention of symptomatic HF will be a key consideration in current guidelines. There has also been recognition of the unmet need for disease‐modifying therapies that have an immediate impact on patient well‐being without dose‐limiting side-effects in both HFrEF and HFpEF patients. SGLT2 inhibitors have been confirmed as a new disease-modifying class of drug for the prevention of HF.12 Accumulating evidence also suggests that SGLT2 inhibitors induce combined cardiac and renal beneficial effects in patients with HFrEF, and potentially HFpEF.8,9,12,23,37–41
The 2016 European Society of Cardiology (ESC) guidelines stated that empagliflozin should be considered in patients with T2D in order to prevent or delay the onset of HF (class of recommendation IIa, level of evidence B).46 In 2019, prior to the availability of results from DAPA-HF, the ESC updated its guidelines on diabetes, prediabetes and CVD, and recommended that canagliflozin, dapagliflozin or empagliflozin should be considered in patients with T2D and CVD, or those at very high/high CV risk, to reduce CV events (class of recommendation Ia).47 Empagliflozin was also recommended in patients with T2D and CVD to reduce the risk of death (class of recommendation Ia) and all SGLT2 inhibitors were recommended in patients with T2D to lower the risk of hospitalisation for HF (class of recommendation Ia).47 An ESC/Heart Failure Association (HFA) position paper published in 2019 stated that, because the three SGLT2 inhibitors (empagliflozin, canagliflozin, and dapagliflozin) have consistently demonstrated a substantial reduction in the risk of hospitalisation for HF across the spectrum of CV risk and regardless of a history of HF, they should be recommended to prevent hospitalisation for HF in patients with T2D and high CV risk.48
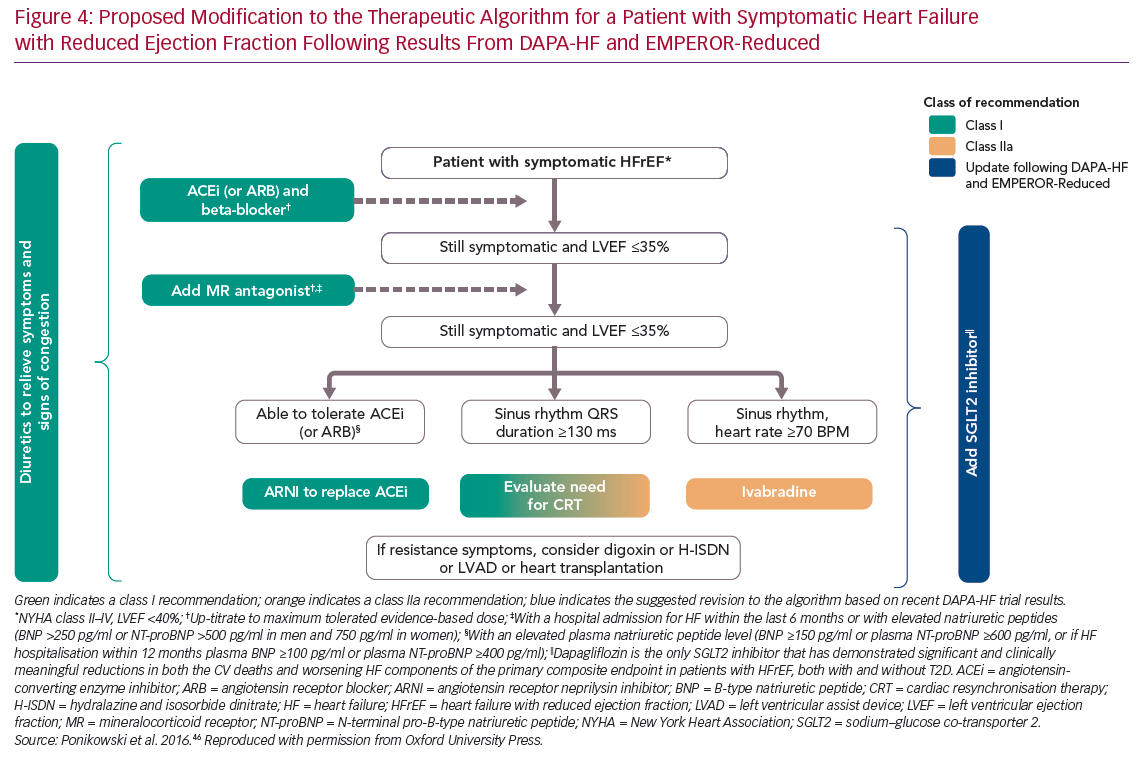
More recently, an ESC/HFA position paper on the role of SGLT2 inhibitor in the treatment of HF has clearly stated that these drugs should be used in all patients with HFrEF as soon as possible on top or together with class Ia recommended medications (beta-blockers, ACE inhibitors/ARBs and MRAs) and also on top of sacubitril/valsartan. The clear and significant impact of dapagliflozin on cardiovascular and total mortality in addition to the significant reduction in hospitalisation for HF suggest this drug is the SGLT2 inhibitor of choice in patients with HFrEF. Therefore, the initiation of dapagliflozin should be pursued in all patients with HFrEF regardless of their background therapy at any stage of the disease (Figure 4).49
Conclusion
The results from the DAPA-HF and EMPEROR-Reduced trials represent a completely new approach to HF management, strengthening the rationale for the use of SGLT2 inhibitors in patients with HFrEF, which will impact future clinical practice. These results establish a new standard of care in HFrEF consisting of four branches: ACE inhibitors/ARBs/ARNIs, beta-blockers, MRAs, and SGLT2 inhibitors, with these four agents being used together to reduce mortality and morbidity and to slow the progression of the disease.
Dapagliflozin is the only SGLT2 inhibitor to demonstrate a significant and clinically meaningful reduction in both the CV death and worsening HF components of the primary composite endpoint in patients with HFrEF, both with and without T2D. Therefore, dapagliflozin should be the agent of choice in all patients with HFrEF, irrespective of their current HFrEF treatment, as well as in those with T2D at increased risk of developing HF. Subgroup analyses suggest the addition of dapagliflozin following recommended first-line ACE inhibitors/ARBs is beneficial whether ACE inhibitor/ARB dosing is optimal or not, therefore dapagliflozin can be added to therapy at any point following HFrEF diagnosis.
The reported renoprotective impact of dapagliflozin extends its use to the many patients with HFrEF who have impaired renal function, patients with chronic kidney disease with HFrEF and those at risk of developing it. Further on-going studies will also determine whether these results can be observed in patients with HFpEF, for whom there are currently no therapies that clearly improve outcomes.