Preprint
Article
Important Role and Properties of Granular Nanocellulose Particle in the In Vitro Simulated Gastrointestinal System and Lipid Digestibility and Permeability
Altmetrics
Downloads
105
Views
72
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
17 August 2023
Posted:
22 August 2023
You are already at the latest version
Alerts
Abstract
This research evaluated the role and feasibility of the granular nanocellulose particles (GNC) from sugarcane bagasse obtained from enzymatic hydrolysis in reducing lipid digestibility and permeability in the in vitro simulated gastrointestinal (GI) system. GNC concentration (0.02%, w/v) had significantly affected the released free fatty acid (FFA) reduction of approximately 20%. The Pick-ring emulsion of the GNC and olive oil simulation mixture revealed higher oil droplet size distribution and stability in the initial stage than the vortexed mixture formation. The difference in particle size distribution and zeta potential of the ingested GNC suspension and GNC-olive oil emulsion were displayed during the in vitro gastrointestinal simulation. GNC particles interacted and distributed surrounding the oil droplet leading to the interfacial emulsion. The GNC concentration (0.01-0.10%, w/v) showed low toxicity on the HIEC-6 cell, ranging from 80.0 to 99% of cell viability. The release of FFA containing the ingested GNC suspension and GNC-olive oil emulsion was about 30% reduction compared to without GNC digest solution. The FFA and triglyceride permeability through the HIEC-6 intestinal epithelium monolayer were deceased in the digesta containing the ingested GNC and emulsion. This work indicated that GNC represented a significantly critical role and properties in the GI tract and reduced lipid digestion and absorption. This GNC could be utilized as an alternative food additive or supplement in fatty food for weight control due to their inhibiting lipid digestibility and assimilation.

Keywords:
Subject: Chemistry and Materials Science - Biomaterials
1. Introduction
Obesity is a severe health problem worldwide, with continuously increasing morbidity and mortality of non-communicable chronic diseases caused by multiple factors. One of the factors is that excessive fatty food consumption leads to the accumulation of excess body fat and suffering from being overweight. The direct up-take of dietary fat in the digestion system occurs as pancreatic lipase is secreted into the small intestine and hydrolyzes triglycerides into monoglycerides and free fatty ac-ids prior to absorption in the small intestinal [1]. The primary approach for preventing and treating obesity was lifestyle modification, including diet and exercise. Unfortunately, lifestyle modifications are often unsuccessful [2]. Consequently, medications have been developed to address overweight and obesity, with the FDA approving five drugs-namely orlistat (Xenical, Alli), phentermine-topiramate (Qsymia), naltrexone-bupropion (Contrave), liraglutide (Saxenda), and semaglutide (Wegovy) for long-term use. However, these medications are associated with side effects such as diarrhea, stomach pain, dizziness, headache, nausea, vomiting, and increased heart rate. As a result, researchers have been exploring alternative anti-obesity agents derived from plants or other natural sources, including dietary fiber like cellulose [3], pectin [4], the fiber found in pear fruit pomace [5], and cereal [6]. These alternatives have shown potential benefits in controlling obesity, offering a safe, cost-effective, and holistic approach to long-term health promotion. These natural substances have demonstrated the ability to modify the absorption of nutrients and chemicals in the gastrointestinal tract [7], such as promoting satiation and improving satiety, and preventing obesity [8], as well as delaying digestion and inhibiting enzyme activity [9], especially nanomaterials like nanocellulose
Nanomaterials have been used in the human gastrointestinal tract (GIT) as additive and functional foods [10]. In particular, nanocellulose is a cellulose material with a broad spectrum of nanoscale range-based particles with different shapes, sizes, and surface properties [11]. There are many attractive properties of nanocellulose for food applications, such as low cost, biodegradability, renewable nanomaterials, high absorbance, and easy processing. However, nanocellulose is essential to understanding the behavior and toxicity and its effects on food digestion for using as a digestion modifier and limiting edible fat absorption. To generate the emulsions, nanocellulose has the ability to form stable oil-in-water (O/W) emulsions through the “Pickering mechanism” where it forms a protective steric barrier around the oil droplets [12]. Previous studies have suggested that the adsorption of nanocellulose onto lipid droplet surfaces can develop a physical barrier that inhibits the adsorption of lipase and bile salts and retarding lipid digestion in GIT. Furthermore, the presence of nanocellulose or dietary fiber in the aqueous phase of emulsions can lead to interactions with bile salts, phospholipids, or calcium and potentially increase viscosity. These effects can potentially alter lipid digestion [13,14]. Hence, the utilization of nanocellulose, an insoluble fiber found in nature, holds promise in addressing the potential benefits of controlling lipid digestibility and assimilation.
Based on a previous report, nanocellulose has been shown with non-toxic for humans and compatible with biological tissue [15], and can not be digested during human GIT. Li et al. [16] investigated three types of nanocellulose, such as cellulose nanocrystals and cellulose nanofibers, on lipid in vitro gastrointestinal digestion using corn oil-in-water emulsions and showed the different performances during each digestion stage to control the reduction of lipid digestion or release of free fatty acid (FFA). According to the FFA determination, the degree of lipid digestion was influenced by both the crystalline structure and form of nanocellulose, especially the morphology. Deloid et al. [17] reported the ability of cellulose nanofibril (CNF) and cellulose nanocrystal (CNC) to reduce the hydrolysis of fatty foods consisting of heavy cream, coconut oil, mayonnaise, and corn oil. Moreover, CNF and CNC at 0.75 % (w/w) had no significant in vitro toxicity [15]. Some previous research used oil-in-water emulsion with various nanocellulose to reduce triglyceride hydrolysis in fatty food and FFA release during simulated in vitro digestion [13,18,19]. By the way, the research on the applications of nanocellulose with a granular or spherical shape in lipid digestibility to release free fatty acids has not been reported. Some previous studies have suggested that granular nanocellulose (GNC) exhibits promising applications as functional material due to its exceptional thermal stability [20] and highly polydisperse nanoparticles [21]. In addition, Ram et al. [22] reported that GNC had been extensively evaluated in various fields, including synthesizing adsorbents for metal ions in wastewater, supercapacitors, carriers for drug delivery, and cellular uptake.
This research aimed to evaluate the feasibility of the granular nanocellulose particles (GNC) from sugarcane bagasse obtained from enzymatic hydrolysis under an optimum condition to reduce lipid digestion. The role of GNC in terms of concentration and simulation between GNC and olive oil formation was carried out. Moreover, the characteristics of GNC during the in vitro gastrointestinal simulation (particle size distribution, zeta potential, and interfacial between GNC and oil) were investigated. Furthermore, the cell cytotoxicity on HIEC-6 was assayed using the MTT method. The release of FFA on lipid digestibility and permeability through the HIEC-6 intestinal epithelium was evaluated to indicate the feasibility of reducing fat assimilation for application as a potential and alternative nano-biomaterial for food additive or supplement in fatty food for weight control, weight loss, and the management of obesity.
2. Materials and Methods
2.1. Materials
Potassium chloride (KCl), sodium hydrogen carbonate (NaHCO3), and calcium chloride (CaCl2) were purchased from LOBA Chemie (Mumbai, India). Potassium dihydrogen phosphate (KH2PO4) and ammonium carbonate (NH4)2CO3 were provided from QRëC® (New Zealand). Magnesium chloride (MgCl2), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were obtained from RCI Labscan (Bangkok, Thailand). Sodium chloride (NaCl) was purchased from CARLO ERBA Reagents (Val-de-Reuil, France). α-amylase (A3176, ≥5 units/mg solid, pepsin (P7000, ≥250 units/mg solid), bile salts (B8756, for microbiology), pancreatin (P7545, 8USP), Calcofluor White Stain and Nile red were from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulphoxide (DMSO) was obtained from Bio Basic Inc. (Markham, Canada). Water was purified with a Milli-Q system (Millipore Milli-Q purification system). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was provided from Bio Basic Inc. (Markham, Canada). OptiMEM 1 Reduced Serum Medium, HEPES, GlutaMAX, Epidermal Growth Factor (EGF), and fetal bovine serum (FBS) were from Thermo Fisher Scientific, Gibco (MA, USA).
2.2. Granular Nanocellulose Particle (GNC) Preparation
The granular nanocellulose particle was produced from the alkaline pretreated and bleached sugarcane bagasse cellulose by enzymatic hydrolysis based on Jirathampinyo et al. [23]. Briefly, sugarcane bagasse was treated with 10% (w/v) sodium hydroxide at a 1:20 (w/v) ratio under an autoclave condition for 15 min. The solid residue was washed and dried. Then, the dried solid was bleached by sodium chlorite (2%, w/v) at 75°C for 120 min and washed with distilled water to pH 7.0. The extracted cellulose of sugarcane bagasse was obtained and hydrolyzed by enzymatic hydrolysis under an optimum condition. The mixture of extracted cellulose (0.13%, w/v) and commercial cellulase (containing endoglucanase 174 U/mL) in sodium acetate buffer (0.05 M, pH 5.0) was incubated at 29.5°C in a shaking incubator with a shaking rate of 120 rpm for 1 h. The mixture was centrifuged for 15 min. The solid residue was dispersed in deionized (DI) water in an ultrasonic bath (The Branson 2510, 40 Hz) for 10 min and centrifuged at 3,500 rpm for 10 min. The supernatant was collected and referred to as the granular nanocellulose particle (GNC-E) suspension
2.3. Simulated Gastrointestinal Tract (GIT) Fluids and Enzyme Solutions Preparation
The simulated GIT system, saliva, gastric and intestinal fluids were prepared. Simulated saliva fluid was prepared to obtain final electrolyte concentrations: 18.875 mM KCl, 17.0 mM NaHCO3, 4.625 mM KH2PO4, 0.06 mM (NH4)2CO3, and 0.05625 mM MgCl2. In the simulated gastric fluid, 59 mM NaCl, 31.25 mM NaHCO3, 8.625 mM KCl, 1.125 mM KH2PO4, 0.625 mM (NH4)2CO3, and 0.15 mM MgCl2 were prepared. Then, the pH value at 3 was adjusted with 1 M HCl. Likewise, simulated small intestinal fluid was prepared to a final concentration of electrolytes of 106.25 mM NaHCO3, 48 mM NaCl, 8.5 mM KCl, 1 mM KH2PO4, and 0.4125 mM MgCl2. pH value was adjusted to 7 with 1 M HCl. Enzyme solutions were prepared daily in each simulated fluid to receive the concentration of α-amylase (1,000 mg/L), pepsin (31,660.61 mg/L), and pancreatin (8,000 mg/L). Before use, the individual enzyme solution was pre-incubated at 37°C. Bile salt was prepared to obtain a concentration of 25,000 mg/L in simulated small intestinal fluid
2.4. In Vitro Simulated GIT Digestion System
All simulated GIT fluids and enzyme solutions were prepared and pre-incubated at 37°C before being used in the in vitro simulated GIT digestion. The procedure was based on Jakobek et al. [24]. The oral, stomach and small intestinal stages were consistently in the system throughout the simulated GIT model. The ingested GNC suspension and GNC-olive oil mixture were added at the beginning of the digestion system. The initial solution was added to the oral phase containing 3.5 ml of simulated salivary fluid, 975 μl of H2O, 25 μl of CaCl2 (0.3 M), and 500 μl of α-amylase and mixed by vortex for 30s. The oral phase solution was mixed with the simulated gastric fluid (7.5 ml), 295 µl of H2O, 5 µl of CaCl2 (0.3 M), 200 µl of HCl (1 M), and 2 ml of pepsin. The mixture was vortexed and incubated in a water bath with shaking for 2 h at 37 °C. The simulated intestinal phase contained 11 ml of intestinal fluid, 3.61 ml of H2O, 40 µl of CaCl2 (0.3 M), 150 µl of NaOH (1 M), 5 ml of pancreatin, and 0.2 ml of bile salt was prepared. After that, the simulated oral and gastric digest solution was added and mixed with 150 μl of NaOH (1 M), 5 ml of pancreatin, and 0.2 ml of bile salt. The intestinal phase mixture was incubated in the shaking water bath for 2 h at 37 °C. All the solution from each stage was collected to analyze the characteristic of GNC during the GI tract system.
2.5. The Role of GNC in Releasing FFA Content in the Simulated GIT System
2.5.1. GNC Concentrations
The granular nanocellulose particle concentrations (0.01-0.08% w/v of the final concentration) were investigated for effect on releasing free fatty acid in GIT simulation. The suspension was mixed with 3 ml olive oil by vortex for 30 s, followed by simulated gastrointestinal tract model digestion. The final digesta after simulation were titrated with 50 mM sodium hydroxide using thymolphthalein as an indicator. The releasing FFA concentration was determined.
2.5.2. The Simulation Mixture of the GNC and Olive Oil
The GNC and olive oil simulation mixtures were prepared by vortex mixing for 30 s to obtain the GNC-mixing form, and the GNC emulsion form was generated by sonication for 21 s. Both GNC and olive oil simulation mixtures were added to the GIT system. The final digestion products were titrated with 50 mM sodium hydroxide using thymolphthalein as an indicator. The measurement of released-free fatty acid content was conducted.
2.6. The Characteristics of GNC During In Vitro GIT Simulation
2.6.1. The Particle Size Distribution and Zeta Potential Determination
A Zetasizer (Malvern Nano particle analyzer series, Nano ZS) was performed to determine the particle size distribution and zeta potential value of the GNC during in vitro GIT simulation. The reflective index at 1.47 and a temperature of 25°C with 0.01% of each sample were measured.
2.6.2. The GNC-Olive Oil Droplets Characterization
Simulating between GNC and olive oil formation (GNC-olive oil mixture and emulsion) in the initial stage prior to ingestion into the GIT simulation were observed by bright-field microscopy (CX43, Olympus corporation Japan) using 20× objective lenses. The oil droplet was stained with Nile red.
2.6.3. The Interfacial of GNC and Olive Oil Emulsion Observation
The GNC-olive oil emulsion of the initial and final digesta stages of GIT simulation was detected by confocal laser scanning microscope (ZEISS LSM 900) using 60× objective lenses. The oil droplet and GNC particles were stained with Nile red and calcofluor white, respectively.
2.7. Cytotoxicity of GNC
HIEC-6 (ATCC® CRL-3266TM) cells were cultured in the completed medium using OptiMEM 1 reduced serum medium containing 20 mM HEPES, 10 mM GlutaMAX, 10 ng/mL epidermal growth factor (EGF), and 4% fetal bovine serum (FBS) and incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The HIEC-6 cultures were seeded and maintained in a 96-well plate at 20,000 cells/well density until 90% confluence. After the 24 h preconditioned, cell cultures were treated with various concentrations of GNC (0.01-0.50% w/v) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 h using the medium as the control. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [25] was used for determining cell viability. The MTT solution (concentration 5 mg/mL in phosphate buffer saline; PBS) was added and then continued incubated for 4 h. After removing the MTT solution, DMSO was added to dissolve the formazan crystals for determining the absorbance at 550 and 620 nm using a microplate reader (VarioskanTM Flash Multimode Reader, Thermo scientific®, USA). Compared to the control, the percentage of cell viability was estimated.
2.8. The Lipid Digestibility and Permeability of HIEC-6 Cell Monolayer
The final digesta after simulation was treated in the intestinal phase for 2 h digestion times. The FFA concentration from lipid digestibility was determined using a colorimetric assay kit. Figure 1 demonstrates a schematic of a transwell support system for permeability assay of HIEC-6 cell monolayer. HIEC-6 cells were seeded at 1×105 cells/ insert on polyethylene terephthalate (PET) micropore (0.4 µm of diameter) membranes into transwell inserts placed in a multiwell 24 plates and then incubated at 37°C in a humidified atmosphere of 5% CO2. The medium was changed every two days until day 8, and the transepithelial electrical resistance (TEER) was measured using a volt-ohmmeter to assess the integrity of the HIEC-6 monolayer. The final digest solutions of the GIT digestion were mixed with the medium in a 1:3 (v/v) ratio and treated on the apical side. After adding the digest solution for 2 h, the free fatty acid and total triglyceride contents of the basolateral sides were determined using a colorimetric assay kit.
2.9. Statistic Analysis
The experimental results were carried out in triplicate, and their statistical significance was expressed in terms of average mean and standard deviations (SD). The IBM SPSS Statistics of Windows version 25.0 (IBM Corp, Armonk, NY, USA) was used to evaluate the statistical differences using One-way ANOVA at a p-value of less than 0.05 (P<0.05).
3. Results and Discussion
3.1. The Characteristics of Granular Nanocellulose Particles (GNC) from Sugarcane Bagasse
The morphology of produced GNC obtained from sugarcane bagasse by enzymatic hydrolysis is demonstrated in the uniform and aggregated particles, as shown in Figure 2. The GNC revealed a slight low in the crystallinity with a Crl value of about 30.6 compared to the original cellulose. The particle size distributions of GNC had in the range of 220-458 nm diameter with an average of 342 nm-sized and a zeta potential of -49.0 mV, leading to suitable for good suspension stability with the negative charge. Moreover, the polydispersity index (PDI) of the produced GNC indicated considerable homogeneity of the GNCs size.
3.2. The Role of GNC in Releasing FFA Content after In Vitro Gastrointestinal Digestion
The influence of GNC on triacylglycerol hydrolysis during the gastrointestinal tract was investigated. Alkali titration determined the released-FFA content using olive oil as the triacylglycerol source. GNC concentrations and GNC-olive oil simulation mixtures were conducted to evaluate the effect of releasing free fatty acid after in vitro gastrointestinal digestion.
3.2.1. The Role of GNC Concentration
The effect of GNC concentration on releasing FFA from olive oil hydrolysis in the simulated gastrointestinal tract was evaluated and shown in Figure 3. The results indicated that the GNC concentration of 0.02-0.08% (w/v) has significantly affected the liberation of FFA, resulting in a decrease in FFA concentration compared to without GNC. At 0.08% of GNC concentration, showed the highest decreasing FFA content and found the FFA concentration about 138.3±3.4 µM with a 20% reduction of the control (180.0±6.5 µM). However, there were no significant differences in releasing FFA when using 0.02-0.08% (w/v) of GNC concentration on olive oil digestion under simulated intestinal conditions. This finding was consistent with the work of Liu and Kong [26], who reported that nano-fibrillated cellulose of 0.22 and 1.1% (w/w) have no significant differences in decreasing the amount of FFA released at the end of intestinal digestion. In addition, at high concentrations of 1% cellulose nanofiber (CNF), 0.25-0.36% TEMPO-CNF and 2-3% cellulose nanocrystals (CNC) [27] revealed in the delayed initial release of free fatty acids during the digestion of Tween 80 stabilized lipid emulsions. However, the differences in fiber source, purity, dosage, experimental conditions, and test methodologies have contributed to the different results reported by other studies [26]. Previous research suggested that the increment in nanocellulose content can increase viscosity, which may impact lipase activity [28]. Viscous fibers have been found to reduce triacylglycerol hydrolysis, thereby decreasing the area available for lipase access [29] and lowering the release of FFA content. Additionally, the polar or ionic fiber site may interact with lipase, leading to a reduction or inhibition of pancreatic lipase activity, according to studies conducted by Chen et al. [30], Skjold-Jørgensen et al. [31], and Yu et al. [32].
3.2.2. The Role of Simulation Mixture of the GNC and Olive Oil
The different simulation mixtures between GNC and olive oil were prepared as shown in Figures 4a and 4b for investigating the influence on releasing free fatty acid during in vitro gastrointestinal tract system. GNC-olive oil emulsion (Figure 4b) displayed the homogeneity of the GNC-olive oil phase, indicating the Pickering emulsion formation of nanocellulose-stabilized [33]. In the GNC-olive oil mixture presented inversely, the coalescence of oil droplets occurred, resulting in the separation of the oil and GNC suspension phase (Figure 4a). The results explained that the stability of the GNC-olive oil mixture was not good enough. To understand the interfacial phenomenon, the oil droplet size and distribution in the GNC-olive oil mixture and the Pickering emulsion were estimated, and the optical microscopy photographs are illustrated in Figure 4c and Figure 4d, respectively. The oil droplets in the GNC-olive oil mixture prepared by vortex mixing revealed large droplet sizes and a heterogeneous distribution (Figure 4c). The microscopy photography observed droplets aggregated into clusters in the oil-to-GNC suspension mixture. For the Pickering emulsion of GNC-olive oil, the small oil droplets were suspended within the emulsion, as shown in Figure 4d. The results demonstrated that the GNC colloidal particles enhanced the emulsion stability and protected their aggregation by absorbing the oil droplet surfaces. The results corresponded to Wen et al. [34] reported that larger emulsion droplets were much less stable under low shear by mixing than smaller droplets formed at higher nanoparticle concentrations. Furthermore, the behavior of GNC-olive oil emulsion during simulated gastrointestinal digestion significantly reduced the olive oil hydrolysis with approximately 22% hydrolysis inhibition (Figure 4e). Nanocellulose satisfies the increasing demands for a sustainable and environmentally friendly stabilizer. Nanocelluloses are likely to form o/w emulsions, which is an emulsion stabilized by solid particles (Pickering emulsions) [35] due to their amphiphilic surface nature, which originates from the hydrophobic face and hydrophilic edge of cellulose chains [36]. These formation offers a wide range of potential applications such as drug delivery, food, and composite materials because it generally provides a more stable system than surfactant stabilized emulsion.
3.3. Characteristics of GNC During In Vitro Simulated Gastrointestinal System
3.3.1. The Particle Size Distribution and Zeta Potential Value
The particle size distributions of ingested GNC suspension compared to GNC-olive oil emulsion during the simulated GI tract are shown in Figure 5. In addition, Table 1 summarizes the average particle size, the polydispersity index (PDI), and the zeta potential value of each suspension. In the initial stage, the particle size distributions of the ingested GNC suspension (Figure 5a) and GNC-olive oil emulsion (Figure 5b) were similar in diameter between 190-458 nm, with an average size distribution of 295.3 nm. The polydispersity indexes (PDI) of both the GNC suspension and GNC emulsion was 0.551, presenting the monodispersity. Moreover, the zeta potential remained within -38.2 mV, indicating good stability of the GNC suspension and GNC emulsion. According to the report, a zeta potential value of around 30 mV with a negative charge is suitable for maintaining the stability of the suspension and preventing the nanocellulose from aggregating [37].
In the oral phase, ingested GNC suspension and GNC-olive oil emulsion showed different size distributions, as shown in Figure 5a and Figure 5b, respectively. The results revealed that of the ingested GNC particles, approximately 90% were distributed in the 342-615 nm range, slightly larger than in the initial stage due to GNC particle swelling and fluctuation in the oral phase. Therefore, the PDI value was 0.835, indicating a broad particle size distribution but still a good dispersity. While as the GNC emulsion in the oral phase demonstrated that the particle size of the GNC (72.5% of the total particle) was significantly increased and varied between 1484-2669 nm containing about 20% intensity of particle size at 1990 nm. However, the particle size distribution presented the homogenous with the PDI value 0.597 due to GNC formed emulsion with olive oil led to the GNC-oil droplet particles increasing in particle size. The results revealed that the zeta valve was -7.56 mV resulting in a low dispersion of GNC emulsion, causing the aggregation of GNC might have occurred in the oral phase. The results accorded to Capron et al. [38], the results found that various nanocellulose types, including cellulose nanocrystals and cellulose nanofibers with different sizes and lengths, maintained their particle size stability during the oral stage. However, some studies have suggested that the smaller cellulose nanocrystals led to flocculation during the oral phase [39] and swelling after entering the oral stage. This effect may result from anionic mucin molecules in the simulated saliva fluid adsorbing to the surfaces of particles [40].
The particle size distributions of ingested GNC suspension significantly increased along the stomach phase. Figure 5a and 5b illustrates the GNC particle size around 825 nm with 55% intensity, respectively. In contrast, the size of GNC in the emulsion decreased into a range of 1106-1484 nm with an average size of 1281 nm, compared to the oral phase. The results presented that both ingested GNC and GNC emulsions have a high homogenous particle size during the gastric phase, with a PDI value of about 0.111 and 0.352, respectively. Moreover, the ingested GNC suspension and GNC emulsion were not stable in the gastric phase due to the zeta potential values being lower than -2.00 mV because of the low pH value and high ionic strength. These factors compressed the electric double layer, leading to a decrease in the zeta potential value [16,41,42]. The increasing size of GNC indicates the occurrence of flocculation of nanocellulose particles, which is consistent with prior research [41,43,44]. Moreover, the gel-like structure resulting from the aggregation of oil droplets in the gastric environment was caused by the negative charge of nanocellulose, which is highly susceptible to alterations in ionic strength [39,45].
During small intestinal digestion, the GNC particles, approximately 77%, returned to the initial size in the 342-458 nm range of the ingested GNC suspension with a broad distribution and less stability. In contrast, all GNC particle sizes in the emulsion were inverse to 220 nm and had excellent homogeneity and stability. The zeta potential values experienced a significant increase during the small intestinal stage due to the neutral pH condition. The increase in pH values could help eliminate the charge shielding of nanocellulose. Additionally, the absorption of many anions, such as bile salts, free fatty acids, and phospholipids, onto the lipid surface can enhance the negative surface charge [17]. This increase in particle size can be attributed to the highly negative zeta potential increased electrostatic repulsive force among lipid droplets [41], and various relatively small colloidal particles, including micelles, vesicles, and insoluble calcium soaps, may form during the lipid digestion process [46].
These results noticed that the presence of food, such as olive oil, and various parameters in the gastrointestinal tract, including residence time, pH, ion strength, mechanical force, and GIT secretions, such as enzymes, bile salts, acids, and hormones have the effects on the behavior [47]. Likewise, the presence of a food matrix can also affect the behavior of nanocellulose in the gastrointestinal tract [19,48]. In GIT simulation, the lipids emulsion stabilized become unstable, and the surfactants that adsorb more strongly to the droplet surfaces, resulting in the large surface area of lipids exposed to lipase and gastrointestinal fluids, thereby presenting a low efficiency at inhibiting lipid digestion [49]. Mun et al. [50] reported that the stability of lipid droplets against disruption and coalescence during lipid digestion is primarily determined by the hydrophilic and hydrophobic properties of the emulsifiers coating the interfacial layer of the droplets. Additionally, the properties of this layer can impact the hydrolysis of lipid droplets by lipases in the small intestine. By the way, nanocellulose, which has the ability to stabilize emulsions, has numerous potential uses due to its amphiphilic surface characteristics. This property makes it a more reliable stabilizer than surfactants, as it arises from the hydrophilic edge and hydrophobic face of cellulose chains [35].
3.3.2. The Interfacial Properties of GNC-Olive Oil Emulsion During the Simulated GIT System
Visual observations via the confocal laser scanning microscope imaging revealed that the GNC interfaced with olive oil to generate the emulsion droplet along the digestion stage of the GIT, as shown in Figure 6. The GNC particle represented the blue color of the Calcoflour-white staining, while oil droplets showing in red color were stained with Nile red. The GNC-oil droplets dispersed in the oil-to-water (Pickering) emulsion of the initial phase. Figure 6a demonstrated that the GNC particles distributed surrounding the olive oil droplets and obviously interacted with the oil molecule, as shown in the zoomed image in Figure 6b. After intestinal phase digestion of the GIT simulation, the oil droplets were revealed in small size and were less than in the initial stage, primarily due to lipid digestion resulting in the free oil droplet distribution (Figure 6c). The interfacial GNC-olive oil droplets in the emulsion remained in the digesta of the intestinal phase after the simulation of GIT digestion, as zoomed in Figure 6d. Hence, it can be inferred that GNC potentially alters the colloidal interactions among the oil droplets, affecting their aggregation state and the surface area of lipids accessible to enzyme activity. This hypothesis is consistent with previous research findings [17,39,51].
3.4. Cytotoxicity of GNC along the GIT Simulation
The cytotoxicity of GNC to HIEC-6 (ATCC® CRL-3266™) cell was investigated, and the cell viability was evaluated using the MTT assay. The results revealed that GNC had low cytotoxicity at tested concentrations (0.01-0.10% w/v) and demonstrated high cell viability with more than 80% cell viability, ranging between 80.0±4.1 to 99.0±3.3% compared to the without GNC (Figure 7). However, a higher GNC concentration at 0.50% (w/v) found a cell toxicity effect with 68.3±7.2% cell viability. The results were consistent with the previous studies investigating the impact of nanocellulose materials, including cellulose nanocrystals and cellulose nanofibrils, on in vitro toxicity in gastrointestinal (GIT) cells. Vital et al. [52] showed that the ingested nanocellulose is not toxic to the gastrointestinal tract. Furthermore, the non-toxicity of nanocellulose, focusing on natural sources, was observed at concentrations ranging from ∼0.2% to 1.0% (w/w) [53]. Deloid et al. [15] reported that the ingested nanocellulose was slightly acute toxicity and was not at risk when consumed in small amounts. The results found that cellulose nanofibrils (50 nm width) and cellulose nanocrystals (25 nm width) at 0.75% (w/w) did not exhibit any toxicity in the in vitro studies.
3.5. The Releasing FFA in Simulated GIT System
The influence of GNC on lipid digestion during a simulated GIT system was investigated by estimating the released FFA content at 0 and 2 h of digestion times. The results compared the lipid digestibility in digesta with and without ingested GNC suspension and GNC-olive oil emulsion. The concentration of released FFA increased when digestion time increased. As shown in Figure 8, the increase in released FFA occurred in those without GNC digesta more than in those containing GNC after a digestion time of up to 2 h. The FFA concentration increased by approximately 90% without GNC, while about 61.2% and 69.9% increased in the digesta containing ingested GNC suspension (Figure 8a) and GNC-olive oil emulsion (Figure 8b). Moreover, the FFA contents in the digesta containing ingested GNC suspension and GNC emulsion were lower than those without GNC, with about a reduction of 30% in lipid digestion. The results indicated that GNC affected the reduction of lipid digestibility, leading to a lower FFA released along the gastrointestinal simulation. These results corresponded to Rungraung et al. [40] indicated that nanofibrillated cellulose (NFC) or nanocrystalline cellulose (NCC) at a concentration range between various 0.05-0.20% w/w on oil-filled beads decreased the rate of lipid digestion, 0.20% (w/w) of NFC and NCC showed FFA content of final digesta around 60% and 49%, respectively. Ni et al. [39] showed that cellulose nanoparticles on the gastrointestinal fate and emulsion digestion (10% oil v/v) reduced the FFA released by 71.4±1.5% and 49.6±2.1% using cellulose nanoparticle sized 310±15.9 nm and 955±20.6 nm, respectively. In addition, Liu and coworkers [27] studied the three types of nanocellulose, including cellulose nanocrystal (CNC), cellulose nanofibril (CNF), and TEMPO-oxidized cellulose nanofibrils (TEMPO-CNF) on lipolysis rate with the released FFA content. The results demonstrated that 1% CNF, 0.25–0.36% TEMPO-CNF or 2–3% CNC) delayed initial in vitro digestion of emulsions, though the final lipolysis extent was nearly the same amongst all (47–55%).
3.6. The FFA and TG Permeability in HIEC-6 Cell Monolayer
The integrity of the permeability was evaluated by the transepithelial electrical resistance (TEER) value of the HIEC-6 cell monolayer growing on the transwell insert membranes. The TEER value of the functional HIEC-6 cell monolayer was 108±12 ohm×cm2 on day 8, indicating the monolayers remained intact. The morphology feature of the HIEC-6 cell monolayer was similar to the human small intestine [54], whereas the most frequently applied cellular model was the human colon carcinoma Caco-2 cell line [52]. According to Takenaka [55], HIECs showed permeable values of paracellularly absorbed hydrophilic molecules, and their TEER value on day 5 was 98.9±17.5 ohm×cm2, which remained constant on day 8. This value was similar to the TEER values reported for the human small intestine (duodenum, jejunum, and ileum).
The results revealed the permeability of the final digesta products from GI tract simulation containing the ingested GNC suspension and GNC-olive oil emulsion compared without GNC. The FFA and triglyceride (TG) concentrations were detected on the basolateral side after 2 h of permeable time. The results showed that the FFA contents significantly decreased with the ingested GNC suspension (Figure 9a) and GNC-olive oil emulsion (Figure 9b) than without GNC. These results indicated that GNC reduced fatty acid permeability across the HIEC-6 monolayer. In contrast, the results presented no significant difference in TG contents on the basolateral side between with and without GNC, resulting in GNC not affecting to generate TG after permeable through the HIEC-6 intestinal epithelium (Figure 9c and 9d). These results suggested that the ingested GNC and GNC-olive oil emulsion affected the reduction of FFA permeability of the HIEC-6 monolayer. These results corresponded to DeLoid et al. [17], who discovered that the presence of cellulose nanofibrils (CNF) resulted in a decrease of approximately 52% and 32% in FFA and TG concentration, respectively, on the basolateral side after a 2 h absorption period. Furthermore, Liu et al. [27] demonstrated that 1% CNF or 2-3% CNC delayed the digestion of lipid emulsion by approximately 47-55%. Bai et al. [13] and Winuprasith et al. [56] reported that CNF and CNC decreased lipid digestion and FFA content. The results suggested that the slower rate of lipid digestion was possibly due to three mechanisms. Firstly, the coalescence of oil on nanocellulose reduced the surface area for lipase binding. Secondly, the sequestration of bile salts by nanocellulose impaired interfacial displacement, and solubilization of lipid digestion products by bile salts occurred [17,39]. Thirdly, nanocellulose may alter the colloidal interactions between the lipid droplets, which changes their aggregation state and the surface area of lipids exposed to lipase [51].
4. Conclusions
This research indicated the critical role of the granular nanocellulose particle (GNC) from sugarcane bagasse in reducing lipid digestibility and absorption during in vitro simulated gastrointestinal tract (GIT) system. GNC concentrations (0.02-0.08%, w/v) had significantly affected free fatty acid (FFA) release. The simulation formulation model between GNC and olive oil as the Pickering emulsion revealed more oil droplet size distribution and stability in the initial stage before adding it into the GIT system. The particle size distribution and zeta potential value of the ingested GNC suspension and GNC-olive oil emulsion were to be different in size distribution and dispersity during the in vitro GIT simulation. The interfacial of GNC and oil droplets were investigated by confocal laser scanning microscopy. The cell cytotoxicity on HIEC-6 demonstrated low toxicity at the tested concentration (0.01-0.10%, w/v) with 80% cell viability. The release of FFA on lipid digestibility and permeability through the HIEC-6 intestinal epithelium monolayer was decreased in the digesta containing the ingested GNC suspension and GNC-olive oil emulsion. This work indicated the role of GNC in reducing lipid digestion and absorption along in vitro GIT simulation. The GNC will be further used as an alternative nanomaterial for food additives or supplements in fatty food for weight control due to their inhibiting lipid digestibility and reducing lipid absorption.
Author Contributions
W.C.: Experimental, Methodology, Investigation, Data curation, Writing - original draft preparation; N.C.: Suggestion, Review & editing; C.S.: Review & editing, Funding acquisition; J.T.: Conceptualization, Validation, and formal analysis, Funding acquisition, Review & editing, Supervision, Correspondence, Project administration.
Funding
This research project was supported by Chiang Mai University
Data Availability Statement
The research created experimental data that can be found in the tables and figures presented in this manuscript.
Acknowledgments
The authors thank the Department of Chemistry of the Faculty of Science, Faculty of Pharmacy, and Faculty of Medicine at Chiang Mai University for providing the analytical tools in this research study. The authors would like to thank the Teaching Assistant and Research Assistant (TA-RA) Scholarships from the Graduate School at Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, T.-Y.; Wang, M.M.C.; Hsieh, S.-K. ; Hsieh, M-H.; Chen, W.-Y, Tzen J.T.C. Pancreatic lipase inhibition of strictinin isolated from Pu’er tea (Cammelia sinensis) and its anti-obesity effects in C57BL6 mice. J. Funct. Foods. [CrossRef]
- Calcaterra, V.; Rossi, V. ; Mari. A.; Casini, F. Bergamaschi F, Zuccotti G.V, Fabiano V. Medical treatment of weight loss in children and adolescents with obesity. Pharmacol. Res, 1064. [Google Scholar] [CrossRef]
- Iqbal, S.; Zhang, P.; Wu, P.; Yin, Q.; Hidayat, K.; Chen, X.D. Modulation of viscosity, microstructure and lipolysis of W/O emulsions by cellulose ethers during in vitro digestion in the dynamic and semi-dynamic gastrointestinal models. Food. Hydrocoll. 2022, 128, 107584. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, J.; Lyu, J.; Chen, J.; Wang, R.; Liu, X.; Richel, A. Structural conversion of pectin fractions during heat processing in relation to the ability of inhibiting lipid digestion: A case study of hawthorn pectin. Food. Hydrocoll. 2021, 117, 106721. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Niu, K.; Yu, Z.; Yang, Y. Insoluble dietary fiber of pear fruit pomace (Pyrus ussuriensis Maxim) consumption ameliorates alterations of the obesity-related features and gut microbiota caused by high-fat diet. J. Funct. Foods. 2022, 99, 105354. [Google Scholar] [CrossRef]
- Low, D.Y.; Pluschke, A.M.; Gerrits, W.J.J.; Zhang, D.; Shelat, K.J.; Gidley, M.J.; Williams, B.A. Cereal dietary fibres influence retention time of digesta solid and liquid phases along the gastrointestinal tract. Food. Hydrocoll. 2020, 104, 105739. [Google Scholar] [CrossRef]
- Khorasaniha, R.; Olof, H.; Voisin, A.; Armstrong, K.; Wine, E.; Vasanthan, T.; Armstrong, H. Diversity of fibers in common foods: Key to advancing dietary research. Food. Hydrocoll. 2023, 139, 108495. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food. Hydrocoll. 2010, 24(1), 42–48. [Google Scholar] [CrossRef]
- Fabek, H.; Goff, H.D. Simulated intestinal hydrolysis of native tapioca starch: Understanding the effect of soluble fibre. Bioact. Carbohydr. Diet. Fibre. 2015, 6(2), 83–98. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, X.; Yagoub, A.E.; Wahia, H.; Zhou, C. Application and challenge of nanocellulose in the food industry. Food. Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; Fox, D.M.; Hamad, W.Y.; Heux, L.; Jean, B.; Korey, M.; Nieh, W.; Ong, K.J.; Reid, M.S.; Renneckar, S.; Roberts, R.; Shatkin, J.A.; Simonsen, J.; Stinson-Bagby, K.; Wanasekara, N.; Youngblood, J. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47(8), 2609–2679. [Google Scholar] [CrossRef]
- Fitri, I.; Mitbumrung, W.; Akanitkul, P.; Rungraung, N.; Kemsawasd, V.; Jain, S.; Winuprasith, T. Encapsulation of β-Carotene in oil-in-water emulsions containing nanocellulose: Impact on emulsion properties, In vitro digestion and bioaccessibility. Polym. 2022, 14(7), 1414. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food. Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Tangsrianugul, N.; Winuprasith, T.; Suphantharika, M.; Wongkongkatep, J. Effect of hydrocolloids on physicochemical properties, stability, and digestibility of Pickering emulsions stabilized by nanofibrillated cellulose. Food. Funct. 2022, 13(2), 990–999. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano. 2019, 6(7), 2105–2115. [Google Scholar] [CrossRef]
- Li, X.; Kuang, Y.; Jiang, Y.; Dong, H.; Han, W.; Ding, Q.; Lou, J.; Wang, Y.; Cao, T.; Li, J.; Jiao, W. In vitro gastrointestinal digestibility of corn oil-in-water Pickering emulsions stabilized by three types of nanocellulose. Carbohydr. Polym. 2022, 277, 118835. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; Ng, K.W.; Loo, S.C.J.; Bell, D.C.; Brain, J.; Demokritou, P. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS. Nano. 2018, 12(7), 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food. Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Murray, B.; Russell, J.A.; Boxal, S. Modulating in vitro gastric digestion of emulsions using composite whey protein-cellulose nanocrystal interfaces. Colloids. Surf. B. Biointerfaces. 2017, 158, 137–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.; Wu, M.; Rahmaninia, M.; Xu, C.; Li, B. Tailoring functionality of nanocellulose: Current status and critical challenges. Nanomater. 2023, 13(9), 1489. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Preparation and liquid crystalline properties of spherical cellulose nanocrystals. Langmuir. 2008, 24(1), 5–8. [Google Scholar] [CrossRef]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. J. Chem. Eng. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Jirathampinyo, S.; Chumchoochart, W.; Tinoi, J. Integrated biobased processes for nanocellulose preparation from rice straw cellulose. Processes. 2023, 11(4), 1006. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food. Chem. 2021, 363, 130283. [Google Scholar] [CrossRef] [PubMed]
- van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 1994, 174(1), 311–320. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerr, W.L.; Kong, F. Characterization of lipid emulsions during in vitro digestion in the presence of three types of nanocellulose. J. Colloid. Interface. Sci. 2019, 545, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, R.; Yokoyama, W.; Zhong, F. Investigation of the effect of nanocellulose on delaying the in vitro digestion of protein, lipid, and starch. Food. Hydrocoll. For. Health. 2022, 2, 100098. [Google Scholar] [CrossRef]
- Zhai, H.; Gunness, P.; Gidley, M.J. Effects of cereal soluble dietary fibres on hydrolysis of p -nitrophenyl laurate by pancreatin. Food. Funct. 2016, 7(8), 3382–3389. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yao, R.; Yan, S.; Wang, Q. Mechanism of lipid metabolism regulation by soluble dietary fibre from micronized and non-micronized powders of lotus root nodes as revealed by their adsorption and activity inhibition of pancreatic lipase. Food. Chem. 2020, 305, 125435. [Google Scholar] [CrossRef]
- Skjold-Jørgensen, J.; Bhatia, V.K.; Vind, J.; Svendsen, A.; Bjerrum, M.J.; Farrens, D. The enzymatic activity of lipases correlates with polarity-induced conformational changes: A Trp-induced quenching fluorescence study. Biochem. 2015, 54(27), 4186–4196. [Google Scholar] [CrossRef]
- Yu, B.; Tang, Q.; Fu, C.; Regenstein, J.; Huang, J.; Wang, L. Effects of different particle-sized insoluble dietary fibre from citrus peel on adsorption and activity inhibition of pancreatic lipase. Food. Chem. 2023, 398, 133834. [Google Scholar] [CrossRef]
- Souza, A.G.d.; Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.d.S. Cinnamon essential oil nanocellulose-based pickering emulsions: Processing parameters effect on their formation, stabilization, and antimicrobial activity. Polysaccharides. 2021, 2(3), 608–625. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d-limonene Pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mate. 2017, 18(1), 959–971. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules. 2012, 13(1), 267–275. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS. Sustain. Chem. Eng. 2020, 8(5), 2287–2299. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid. Interface. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Ni, Y.; Gu, Q.; Li, J.; Fan, L. Modulating in vitro gastrointestinal digestion of nanocellulose-stabilized pickering emulsions by altering cellulose lengths. Food. Hydrocoll. 2021, 118, 106738. [Google Scholar] [CrossRef]
- Rungraung, N.; Jain, S.; Mitbumrung, W.; Khomein, P.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Controlling the in vitro gastrointestinal digestion of emulsified lipids by encapsulation within nanocellulose-fortified alginate beads. Food. Struct. 2022, 32, 100266. [Google Scholar] [CrossRef]
- Le, H.D.; Loveday, S.M.; Singh, H.; Sarkar, A. Gastrointestinal digestion of pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Release of short-chain fatty acids. Food. Chem. 2020, 320, 126650. [Google Scholar] [CrossRef]
- Li, K.; Xie, L.; Wang, B.; Yan, J.; Tang, H.; Zhou, D. Mechanistic investigation of surfactant-free emulsion polymerization using magnetite nanoparticles modified by citric acid as stabilizers. Langmuir. 2020, 36(28), 8290–8300. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Minařík, A.; Kašpárková, V. Pickering oil-in-water emulsions stabilized by carboxylated cellulose nanocrystals – Effect of the pH. Food. Hydrocoll. 2018, 80, 60–67. [Google Scholar] [CrossRef]
- Du Le, H.; Loveday, S.M.; Singh, H.; Sarkar, A. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food. Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Scheuble, N.; Schaffner, J.; Schumacher, M.; Windhab, E.J.; Liu, D.; Parker, H.; Steingoetter, A.; Fischer, P. Tailoring emulsions for controlled lipid release: establishing in vitro–in vivo correlation for digestion of lipids. ACS. Appl. Mater. Interfaces. 2018, 10(21), 17571–17581. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of vitamin D(3) in pickering emulsion stabilized by nanofibrillated mangosteen cellulose: Effect of environmental stresses. J. Food. Sci. 2019, 84(11), 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid. Interface. Sci. 2017, 246, 165–180. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid. Interface. Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Mun, S.; Choi, Y.; Kim, Y.-R. Lipase digestibility of the oil phase in a water-in-oil-in-water emulsion. Food. Sci. Biotechnol. 2015, 24(2), 513–520. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food. Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Vital, N.; Ventura, C.; Kranendonk, M.; Silva, M.J.; Louro, H. Toxicological assessment of cellulose nanomaterials: Oral exposure. Nanomater. 2022, 12(19), 3375. [Google Scholar] [CrossRef]
- Silbir, S.; Yekta Göksungur, M. Nanocellulose production and its food applications. 2nd Congress on Food Structure. Antalya, Turkey, 2016.
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Human small intestinal epithelial cells differentiated from adult intestinal stem cells as a novel system for predicting oral drug absorption in humans. Drug. Metab. Dispos. 2014, 42(11), 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, Å.; Lutz, M.; Tannergren, C.; Wingolf, C.; Borde, A.; Ungell, A.L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur. J. Pharm. Sci. [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 2. In vitro lipid digestion. Food. Hydrocoll. 2019, 96, 709–716. [Google Scholar] [CrossRef]
Figure 1.
Schematic of a transwell support system for permeability assay of HIEC-6 cell monolayer and morphology of HIEC-6 cell line (day 8), 20×.
Figure 1.
Schematic of a transwell support system for permeability assay of HIEC-6 cell monolayer and morphology of HIEC-6 cell line (day 8), 20×.

Figure 2.
The SEM image of the produced GNC from sugarcane basse by enzymatic hydrolysis (30,000×).

Figure 3.
The releasing FFA from olive oil digestion in the gastrointestinal tract (GIT) simulation in different GNC concentrations.
Figure 3.
The releasing FFA from olive oil digestion in the gastrointestinal tract (GIT) simulation in different GNC concentrations.
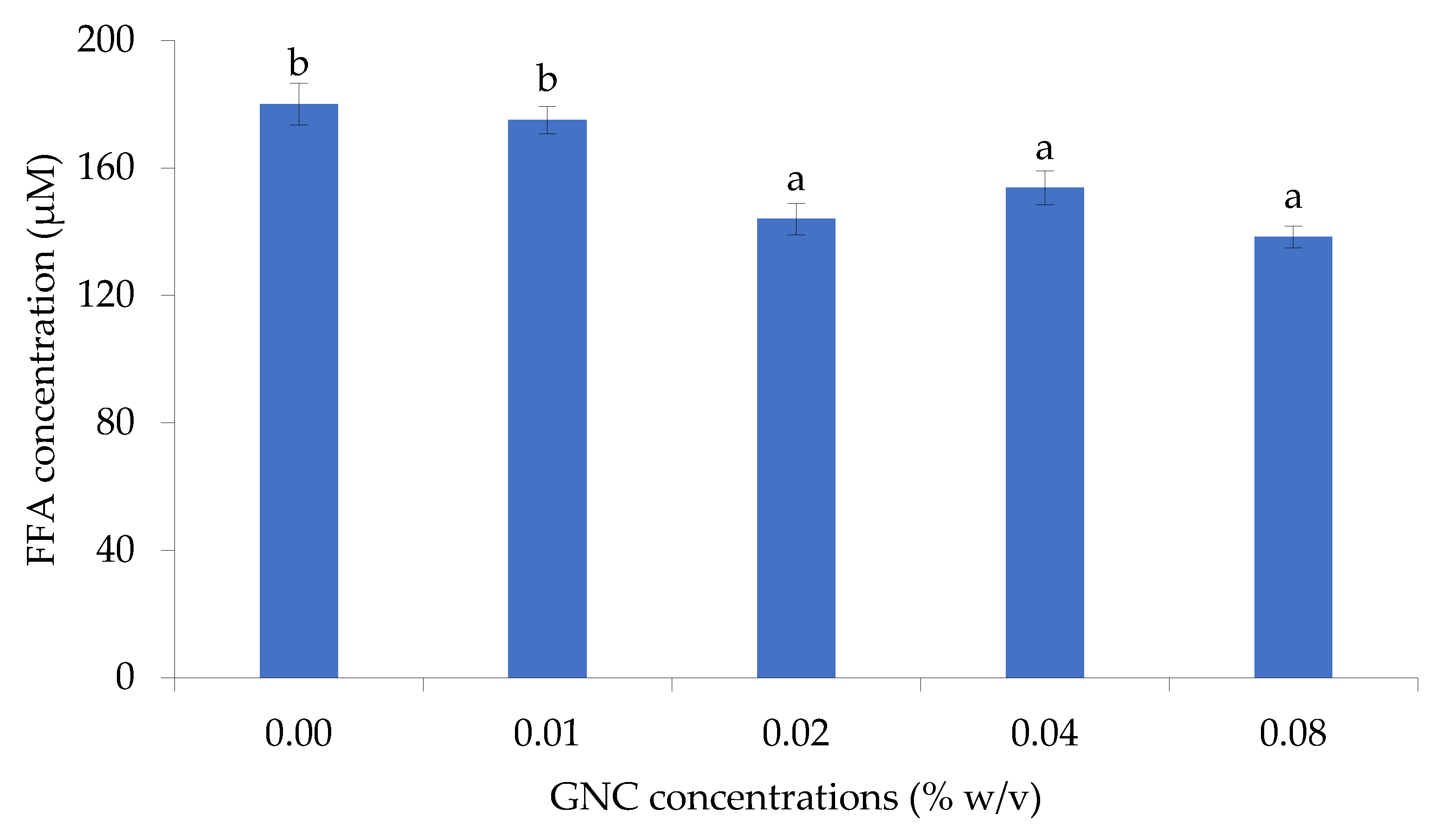
Figure 4.
The digital images of simulation mixtures between GNC and olive oil (a) GNC-olive oil mixture and (b) GNC-olive oil emulsion; The optical microscopy images of (c) GNC-olive oil mixture and (d) GNC-olive oil emulsion and (E) the free fatty acid concentration after olive oil hydrolysis in the small intestinal stage of GIT system.
Figure 4.
The digital images of simulation mixtures between GNC and olive oil (a) GNC-olive oil mixture and (b) GNC-olive oil emulsion; The optical microscopy images of (c) GNC-olive oil mixture and (d) GNC-olive oil emulsion and (E) the free fatty acid concentration after olive oil hydrolysis in the small intestinal stage of GIT system.

Figure 5.
The particle size distributions of (a) ingested GNC suspension and (b) GNC-olive oil emulsion during in vitro gastrointestinal tract at different GI stages.
Figure 5.
The particle size distributions of (a) ingested GNC suspension and (b) GNC-olive oil emulsion during in vitro gastrointestinal tract at different GI stages.

Figure 6.
Confocal laser scanning microscopic images of GNC-olive oil interaction (a) in the initial stage, 60× (b) initial stage, zoomed 300× and (c) in the digesta after GIT simulation, 60×; (d) in the digesta after GIT simulation, zoomed 300×. Noted; GNC particle (stained in blue color) and olive oil (stained in red color).
Figure 6.
Confocal laser scanning microscopic images of GNC-olive oil interaction (a) in the initial stage, 60× (b) initial stage, zoomed 300× and (c) in the digesta after GIT simulation, 60×; (d) in the digesta after GIT simulation, zoomed 300×. Noted; GNC particle (stained in blue color) and olive oil (stained in red color).
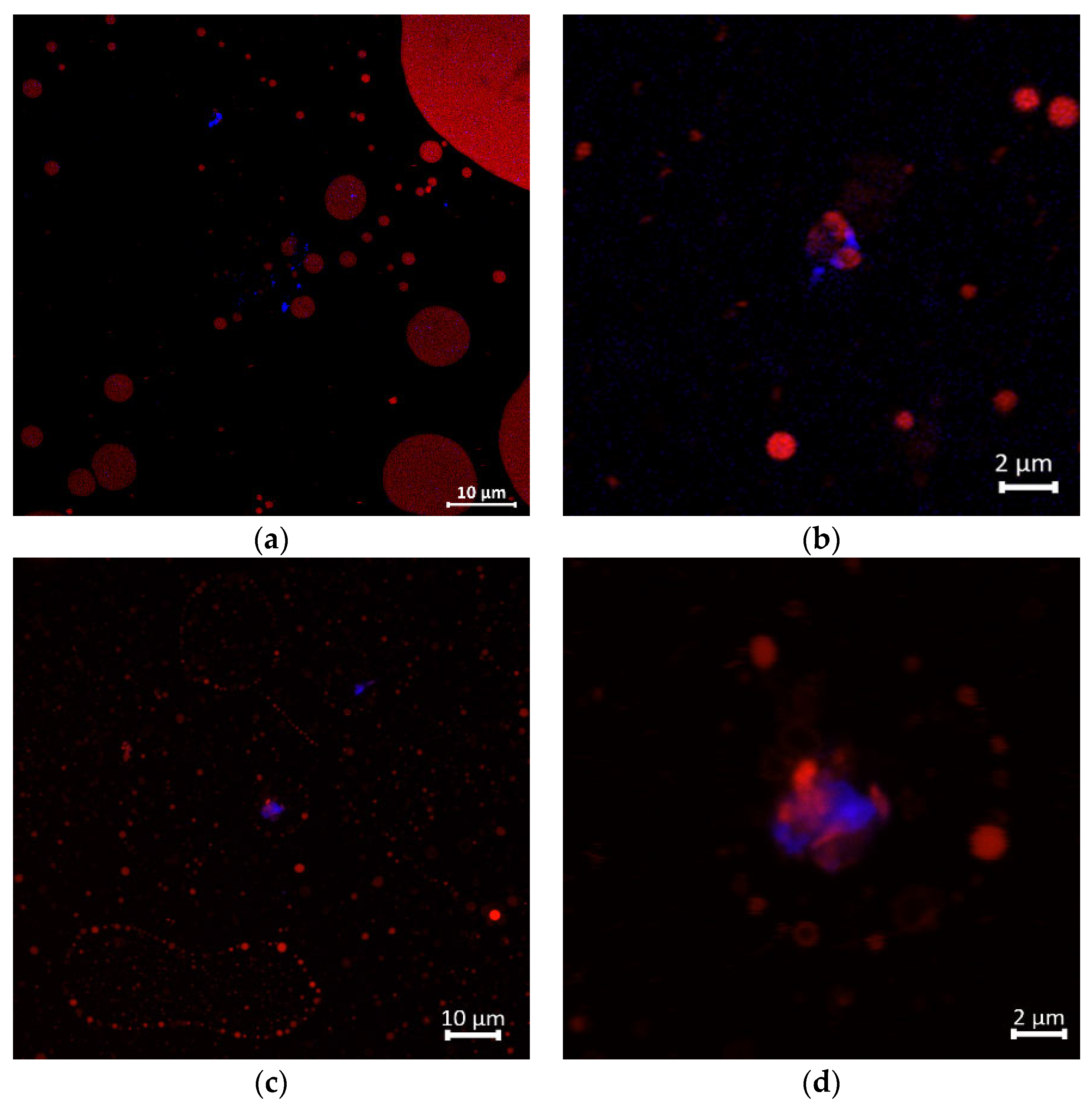
Figure 7.
The toxicity on HIEC-6 cell viability of GNC at various concentrations after 24 h.

Figure 8.
The releasing of free fatty acid during the intestinal digest solution at 0 and 2 h of digestion time contained (a) the ingested GNC suspension and (b) the GNC-olive oil emulsion.
Figure 8.
The releasing of free fatty acid during the intestinal digest solution at 0 and 2 h of digestion time contained (a) the ingested GNC suspension and (b) the GNC-olive oil emulsion.

Figure 9.
The fatty acid concentration in the basolateral phase of (a) the ingested GNC; (b) GNC-olive oil emulsion, and the triacylglycerol permeability (absorption) of (c) the ingested GNC; (d) GNC-olive oil emulsion through HIEC-6 monolayer.
Figure 9.
The fatty acid concentration in the basolateral phase of (a) the ingested GNC; (b) GNC-olive oil emulsion, and the triacylglycerol permeability (absorption) of (c) the ingested GNC; (d) GNC-olive oil emulsion through HIEC-6 monolayer.
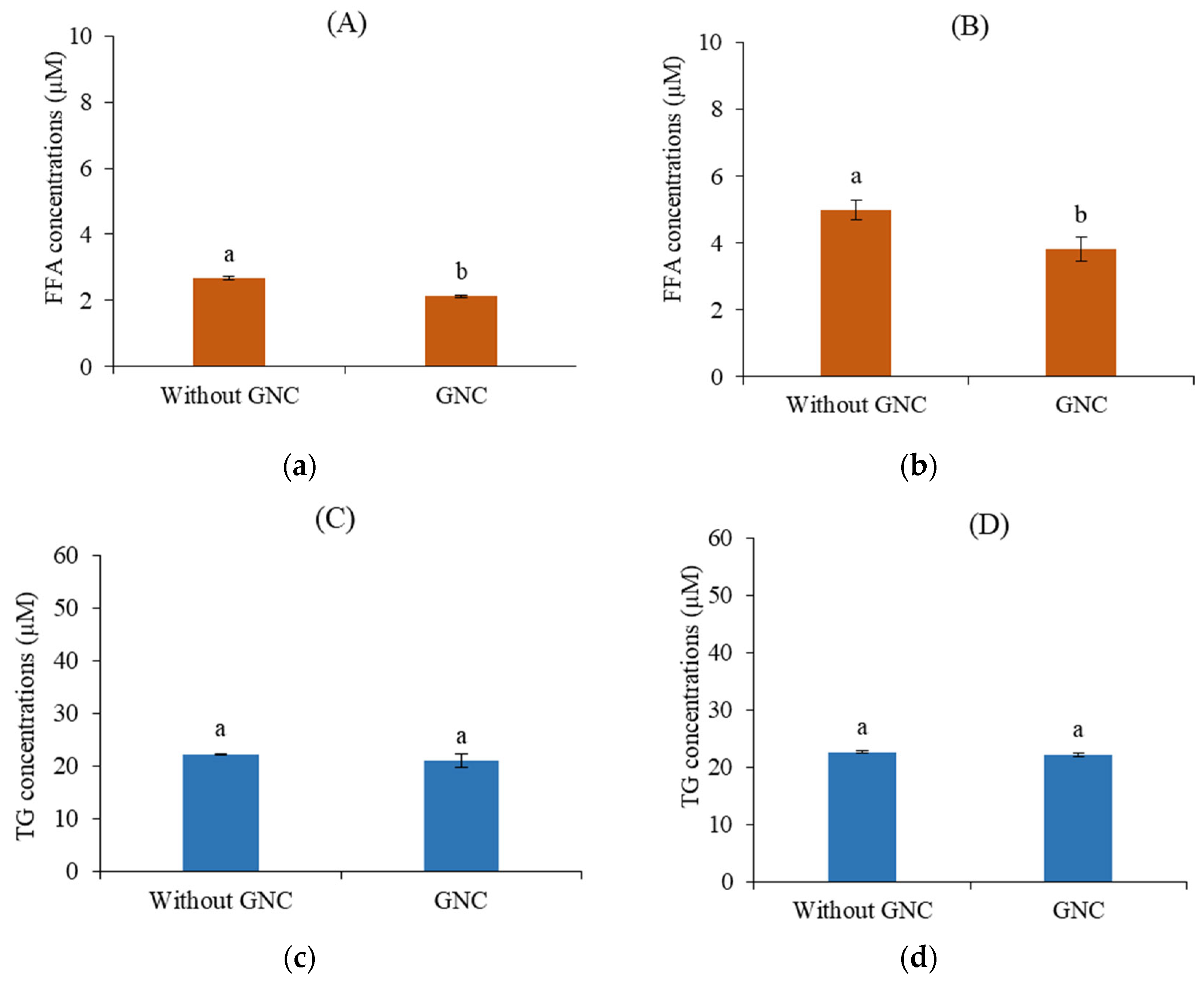
Table 1.
The particle size distributions at the highest intensity, polydispersity indexes (PDI), and zeta potential value of ingest GNC suspension and GNC-olive oil emulsion during in vitro GI tract.
Table 1.
The particle size distributions at the highest intensity, polydispersity indexes (PDI), and zeta potential value of ingest GNC suspension and GNC-olive oil emulsion during in vitro GI tract.
| Sample | Characteristics | Initial phase | Oral phase | Stomach phase | Small intestine phase |
|---|---|---|---|---|---|
|
Ingested GNC suspension |
Particle size (nm) | 295.3 | 458.7 | 825.0 | 458.7 |
| PDI | 0.551 | 0.835 | 0.211 | 0.826 | |
| Zeta potential (mV) | -38.2 | -33.8 | -10.5 | -31.4 | |
| GNC-olive oil emulsion | Particle size (nm) | 295.3 | 1990.0 | 1281.0 | 220 |
| PDI | 0.551 | 0.597 | 0.452 | 0.111 | |
| Zeta potential (mV) | -38.2 | -7.56 | -0.5 | -33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Important Role and Properties of Granular Nanocellulose Particle in the In Vitro Simulated Gastrointestinal System and Lipid Digestibility and Permeability
Warathorn Chumchoochart
et al.
,
2023
Development and Evaluation of a Dry Emulsion of Ostrich Oil as a Dietary Supplement
Juthaporn Ponphaiboon
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








