Preprint
Article
Modulation of Apoptosis by Bovine Herpesvirus Type 4 Infection in Bovine Endometrial Cells and the Possible Role of LPS in this Process
Altmetrics
Downloads
89
Views
45
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
05 October 2023
Posted:
06 October 2023
You are already at the latest version
Alerts
Abstract
The prevalent pathogens associated with bovine uterine infections are bacteria that appear to increase the host's susceptibility to secondary infections with other bacteria or viruses, among which BoHV-4 is the most frequently found. In this work, the study of the pathways of apoptosis induction was carried out on an experimental model of primary culture of endometrial cells, in order to know the implication of BoHV-4 and the presence of bacterial LPS in the pathogenesis of the bovine reproductive tract. For this, different staining techniques and molecular analysis by RT-PCR were used. The results obtained allowed us to conclude that the level of cell death observed in the proposed primary culture is directly related to the time of viral infection and the presence of LPS in BoHV-4 infection. The apoptosis indices in cells infected with BoHV-4, BoHV-4 + LPS presented a maximum that correlates with the appearance of the cytopathic effect, and the maximum viral titers in the model studied. However, morphological, biochemical and molecular changes were evident during both early and late stages of apoptosis. These findings provide information on the factors that may influence the pathogenesis of BoHV-4 and help to better understand the mechanisms involved in virus infection.
Keywords:
Subject: Biology and Life Sciences - Virology
1. Introduction
Apoptosis is a mode of Programmed Cell Death (PCD) genetically regulated with the aim of eliminating surplus, damaged, or mutated cells; playing a key role in the innate response to viral infection [1]. Although it contributes to the prevention of pathogenesis, it often results in a potentially costly sacrifice for the cell [2,3].
Many viruses have developed apoptosis evasion strategies to efficiently establish a lytic or long-term latent infection [4,5,6,7]. Apparently, for herpesviruses, the induction of apoptosis would be a favorable event during the last stages of the viral replicative cycle, whereas the inhibition of apoptosis could be an important step in the transition to latency [8]. The Herpesviridae family is divided into three subfamilies (α, β, and γ) based on biological properties such as the host range and length of the infectious cycle. The members of each herpesvirus subfamily are known to encode anti-apoptotic genes [9]. Bovine gammaherpesvirus type 4 (BoHV-4) is associated with reproductive tract infections during the postpartum period [10and has been implicated in the pathogenesis of non-responsive postpartum metritis. However, its intrinsic pathogenic power seems low and the true incidence of viral metritis is unknown [11]. Symptoms develop only when BoHV-4 cooperates with gram-negative bacteria within the uterus, being currently considered as a cofactor for the development of an inflammatory reaction initiated by bacteria [10]. In vitro experiments demonstrated that tumor necrosis factor alpha (TNF-α), produced by macrophages stimulated by lipopolysaccharides (LPS), induces the expression of the immediate early gene IE2 of BoHV-4, stimulating its replication. This would generate the reactivation of the latent virus in persistently infected animals against a concomitant infection with bacteria [12]. BoHV-4 mainly infects blood lymphocytes (B and T-cells) and shows a particular tropism for the endometrium in which it causes the death of epithelial and stromal cells [13,14,15]. Despite the presence of anti-apoptotic genes in its genome (v-Bcl-2 and v-Flip), this virus seems to induce cell death by apoptosis, which is associated with the downregulation of Bcl-2, and allows BoHV-4 to complete its cycle of productive infection [16]. Epithelial cells are the first ones to be exposed to exogenous microbes and, for this reason; they play an essential role in the host defense against microbial invasion [17]. As the interaction between BoHV-4 infection and uterine response is still not well characterized, it is of great interest to analyze the mechanisms involved in controlling programmed cell death.
[8] Demonstrated variations in the sequence of the v-Flip and v-Bcl2 antiapoptotic genes in Argentine strains of BoHV-4, allowing us to infer that the different genetic characteristics of these genes could be associated with the viral genotype. Furthermore, it is interesting to highlight that the results of different studies carried out on the Madin-Darby bovine kidney (MDBK) cell line suggest that BoHV-4 can produce apoptosis through processes such as the induction of oxidative stress [18].
Our group previously showed that BoHV-4 has a replication preference for BEC cells (bovine endometrial cells) over the MDBK cell line. Moreover, in BEC primary cultures, this virus shows apoptotic cell death followed by a virus release at the last stage. At this moment, high viral titers are linked with apoptosis but the mitochondrial pathway would not be the leading mechanism [19]. It is clear that the apoptotic responses to BoHV-4 observed in the previously studied cell lines do not explain what occurs in vivo, being more suitable the study in primary culture cells.
Considering that postpartum infection of the bovine uterine tract with LPS-containing pathogens activates lytic replication of BoHV-4 in persistently infected macrophages [20] in this study we investigated the modulation of apoptosis by BoHV-4 infection in primary cultures of BEC cells and the role of LPS in this process.
2. Materials and methods
2.1. Isolation of BEC
The BEC cells obtained for primary culture and the fetal bovine serum (FBS) used for their growth were tested by virus isolation in MDBK cells and antigen detection by direct immunofluorescence (DIF) using FITC-labeled polyclonal antibodies (IBR/BHV-1, CJ-F-IBR-10ML; BVDV, CJ-F-BVD-10ML, VMRD, Pullman, WA), as previously described [21]. This test together with nucleic acid detection by PCR or nested RT-PCR [21,22,23] were conducted to rule out contamination in the starting material with bovine alphaherpesvirus 1 (BoHV-1), bovine gammaherpesvirus (BoHV-4), and bovine viral diarrhea virus (BVDV). Culture medium was replaced every 48 h until BEC cells reached confluence at 37 °C in a humidified incubator with a 5% CO2 atmosphere.
2.2. BoHV-4 strain
The BoHV-4 field strain 07/435, originally isolated from the vaginal discharge of an aborted cow [22,24], was used for in vitro experiments. The virus stock was prepared by propagating the BoHV-4 strain for 48 h in confluent monolayers of MDBK cells in T-25 flasks (Greiner BioOne, Germany), which were seeded at 1 × 105 cells/ml. Supernatants were harvested and frozen at −80 °C. Virus titers were determined by the endpoint titration method on MDBK cells in 96-well microtiter plates (Greiner Bio-One, Germany) and expressed as log10 TCID50/ml [25].
2.3. Infection of cells cultures with BoHV-4 and LPS
BEC cell cultures (third passage) were grown in 6-well plates (Greiner Bio-One, Germany) by triplicate at a concentration of 7 × 105 cell/ml and incubated at 37 °C in a 5% CO2 atmosphere. Before performing the assays with the cells under study, confluent monolayers were infected with strain 07/435 at a multiplicity of infection (MOI) of 0.5. After 2 h of incubation, the supernatant was discarded and replaced with fresh medium (MEM-E with 10% FBS) followed by the LPS challenge (100 µg/ml) (LPS from Escherichia coli O55:B5; Sigma-Aldrich, L6529). Mock-infected and LPS-treated cells were used as negative controls.
2.4. Morphological analysis by staining with Rhodamine 123/Propidium Iodide
After 12, 24 and 48 hr post infection (hpi), the supernatant was removed by centrifugation at 200 g for 10 min at 4 °C. The pellet was then resuspended in 200 µl/tube of Rod-123 (1 g/ml, prepared in 0.1% PBS-BSA from a 1 mg/ml stock in absolute EtOH). After incubating for 10 min at 37 °C, the cells were washed with PBS and centrifuged again at 200 g for 10 min at 4 °C, and the supernatant was discarded. The pellet was then resuspended in 200 µl of PI (1/100 in PBS-BSA). Approximately 10 µl of the suspension was placed on slides for epifluorescence microscope observation (BX51, OLYMPUS, Japan) at a wavelength of 507 nm.
2.5. Detection of nuclear morphological changes by DAPI staining
BEC cell cultures were grown on 12 mm coverslips in 6-well plates (Greiner Bio-one, Germany), at a concentration of 3×105 cells/ml, and thereafter infected with BoHV-4 strain 07/435 at a MOI of 0.5. The virus was absorbed for 2 h. After 2 h of incubation, the supernatant was discarded and replaced with fresh medium (MEM-E with 10% FBS) followed by the LPS challenge (100 µg/ml) for 12, 24 and 48 hr. Mock-infected and LPS-treated cells were used as negative controls. The fusarium mycotoxin deoxynivalenol (DON) was used as positive control. At the end of each incubation period, the culture medium was discarded and cell monolayers were fixed in 4% paraformaldehyde. To evaluate changes in nuclear morphology, staining with 4 ‘6-diamino- 2-phenylindole (DAPI) was performed. Fixed cell monolayers were washed with PBS and incubated for 15 min at room temperature with 250 μl of DAPI (1 μg/ml)/well. After incubation, coverslips with cell monolayers were washed twice with distilled water and then stained for observation under epifluorescence microscope (BX51, Olympus, Japan) at a wavelength of 395 nm, as previously described [19].
2.6. Detection of DNA fragmentation by TUNEL assay
BEC cell cultures were grown on 12 mm coverslips in 6-well plates (Greiner Bio-one, Germany), at a concentration of 3 × 105 cells/ml, and thereafter infected with BoHV-4 strain 07/435 at a MOI of 0.5. After 2 h of incubation, the supernatant was discarded and replaced with fresh medium (MEM-E with 10% FBS) followed by the LPS challenge (100 µg/ml) for 12, 24 and 48 hr. Mock-infected and LPS-treated cells were used as negative controls. At the end of each incubation period, the culture medium was discarded and cells were fixed with 4% paraformaldehyde. To evaluate changes in nuclear morphology, staining with a commercial TUNEL kit (DeadEndTM Colorimetric TUNEL System, Promethean, Promethean) was used according to the manufacturer’s instructions.
2.7. Total RNA isolation, reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR), and mRNA expression analysis
The mRNA expression levels of BcL-2, Bax and Caspase 3 genes in cell cultures were determined by RT-qPCR. Infected and uninfected cells were harvested at 12, 24, 48 and 72 hpi and stored in BIO-ZOL Reagent (PB-L, Argentina) at 80 ◦C for subsequent RNA extraction, following the manufacturer’s instructions. On average, 1 μg of total RNA was used for first-strand cDNA synthesis using iScript™ (Bio-Rad Laboratories, Inc.) and according to the supplier’s protocol. cDNA was stored at -80 ∘C until qPCR was performed. The bovine glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) gene was selected as the endogenous control for RT-qPCR and its level of expression was quantified as described by Romeo et al., 2022 [19]. Specific primers for the BcL-2, Bax, Caspase 3 and GAPDH genes were used [26]. All samples were amplified in triplicate and the qPCR products were expressed as cycle threshold (Ct) values using the software StepOnePlus ® (Applied Biosystems, Foster City, CA, USA). The qPCR was performed using FastStart Universal SYBR Green Master Mix (Rox) (Roche Diagnostics Deutschland), following the manufacturer’s instructions, and a Real-Time PCR CFX96 Touch system (Bio-Rad Laboratories). The amplification was conducted under the following conditions: 10 min at 95°C, 40 cycles of 15 sec at 95°C, and 60 sec at 60°C.
2.8. Data analysis
With the objective of measuring the loss of mitochondrial permeability indirectly expressed as the percentage of Rod-123+ cells (% Rod+), an experiment was carried out under a randomized complete block design with three repetitions and with a factorial arrangement. One of the factors was time with three levels (6, 12 and 24 hpi) and the other factor was cell lines with four levels (Negative Control, LPS, BoHV-4 and BoHV-4+LPS).
A second experiment was conducted to determine the presence of nuclear alterations (DAPI) and detection of in situ DNA fragmentation (TUNEL) were expressed as RAI (Relative Apoptosis Index), under a randomized complete block design with three repetitions and a factorial arrangement. One of the factors was time with three levels (12, 24 and 48 hpi) and the other factor was cell lines with four levels (Negative Control, LPS, BoHV-4 and BoHV-4+LPS).
A third experiment was conducted to obtain the relative expression ratios, under a design in randomized complete blocks with a factorial arrangement and with three repetitions. One of the factors was time with three levels (12, 24 and 48 hpi) and the other factor was the Bax, Bcl2 genes and the relationship between them. For qPCR results, the Relative Expression Software Tool (REST®, Qiagen Inc., Germantown, MD, USA) was used.
The data were analyzed by analysis of variance, followed by mean comparison using the least significant difference test. For all hypothesis tests performed, a significance level of 5% (α = 0.05) was considered. The analysis was carried out through the R program (2023). The values shown in graphs are presented as the mean ± standard deviation (SD) of three independent experiments each done in triplicate. GraphPad Prism 8.0 was used for data plotting for all experiments.
3. Results
3.1. Evaluation of early apoptosis using Rhodamine 123/Propidium Iodide
The assessment of mitochondrial membrane integrity as an early apoptosis marker through staining with Rod-123 demonstrated a significant interaction (p < 0.05) between time and treatment for the last two evaluated time points. As shown in Figure 1, after 12 hpi, the percentage of Rod-123+ cells significantly decreased (p < 0.05) in the culture infected with BoHV-4 compared to the uninfected and LPS-treated BEC controls. Additionally, in the culture with BoHV-4+LPS, the decrease in membrane integrity was significant (p < 0.05) compared to the culture only infected with the virus. Lastly, at 24 hpi, significant differences (p < 0.05) were observed among the different treatments, with a decrease in the percentage of Rod-123+ cells in LPS-treated cells compared to the control, in virus-infected cells compared to LPS-treated cells, and in cells doubly exposed compared to those only infected with BoHV-4.
3.2. Nuclear morphology changes in BEC cells
DAPI staining of BEC cells infected with the 07/435 strain of BoHV-4 revealed changes in nuclear morphology consistent with those reported in the literature, characteristic of late apoptosis, such as chromatin condensation and fragmentation (Figure 2). The results of the RAI analysis, obtained from the count of cells with nuclear alterations, demonstrated a significant interaction between time and different treatments (Figure 3). The cell cultures exhibited cell rounding and reduction in cellular volume along with significant nuclear contraction as early as 12 hours post-infection (hpi). This trend continued over time, reaching a significantly higher RAI (p < 0.05) at 48 hpi (Figure 3) in both BoHV-4 and BoHV-4+LPS-exposed cells. For the remaining evaluated time points, no significant differences (p < 0.05) were observed among the different treatments.
3.3. TUNEL
The cell count with alterations compatible with apoptosis, following TUNEL staining (Figure 4), allowed for the calculation of the RAI in cells exposed to different treatments relative to the negative control. The results demonstrated significant changes (p < 0.05) at 24 and 48 hpi in cells only infected with BoHV-4 and BoHV-4+LPS, compared to cells only treated with LPS. Furthermore, the RAI values obtained at 48 hpi were 20 times higher than those obtained at 24 hpi, both in cells infected with BoHV-4 and in cells with BoHV-4+LPS (Figure 5).
3.4. Pro-anti-apoptotic potential of BoHV-4: Relative expression of Bax, Bcl-2, and Caspase 3
The anti-/pro-apoptotic capacity of the virus was evaluated through the mRNA expression of the mitochondrial proteins Bax and Bcl-2, in BEC cells treated only with BoHV-4. The results of RT-qPCR revealed that the expression levels of the pro-apoptotic gene Bax were similar at 12 hpi and 24 hpi, significantly increasing (p < 0.05) at 48 hpi (Figure 6). There was a reduction in the expression levels of the anti-apoptotic gene Bcl-2 at 12 hpi and 24 hpi. However, its expression level increased significantly (p < 0.05), reaching its peak at 48 hpi compared to 12 hpi. The analysis of the Bax/Bcl-2 ratio showed no significant differences at the evaluated post-infection times (p > 0.05). However, at 12 hpi, a value of the Bax/Bcl-2 ratio < 1 indicates the presence of an anti-apoptotic stimulus, while at 24 hpi and 48 hpi, a value of the ratio close to 1 indicates a balance between pro- and anti-apoptotic genes.
When the final stage of apoptosis was evaluated, significant differences (p < 0.05) were observed at all evaluated time points for the expression of Caspase 3 mRNA in CEB cells after infection with BoHV-4 and/or treatment with LPS (Figure 7). A significant decrease in Caspase 3 expression was observed at 12 hpi (p < 0.05) in all treatments compared to the control. After 24 hpi, Caspase 3 expression increased significantly (p < 0.05) in cells infected with BoHV-4 (134 times higher), while in the presence of both, the virus and LPS (BoHV-4+LPS), the effect was contrary, showing a significant decrease (p < 0.05). After 48 hpi, there were only significant changes in cells exposed to LPS, with an increase in the expression of the studied gene, while at 72 hpi, significant increases (p < 0.05) were observed in all treatments compared to the control, where Caspase 3 expression was 131 times higher in cells infected with BoHV-4 (Figure 7).
4. Discussion
Apoptosis is a complex process that involves a wide variety of biochemical and morphological changes that lead to the complete disappearance of the cell without inducing an inflammatory response [27]. Many viruses modulate this PCD as a strategy to safeguard their replication and survival in infected cells [28].
In this work, diverse experiments were carried out in order to relate the pathogenicity of the Argentine strain 07/435 of BoHV-4 with the apoptosis evidenced in vitro in primary culture of BEC cells and the role of LPS in this process. For the analysis of the pro- and anti-apoptotic potential of the virus, different techniques were used to detect early and late apoptotic events at different times post infection.
The change in the mitochondrial membrane permeability is a phenomenon that often precedes all the other modifications inherent to apoptosis. The results showed an alteration of the membrane potential in the presence of BoHV-4 and BoHV-4+LPS starting at 12 hpi, which decreases considerably at 24 hpi, indicating that mitochondrial damage already exists at this time post-infection. It is notable, in this last time evaluated, that the effect of BoHV-4 + LPS is cumulative, where the loss of mitochondrial membrane permeability is greater compared to treatment with BoHV-4 alone or LPS alone. This finding suggests that the presence of bacterial LPS predisposes to a greater mitochondrial alteration response against a subsequent BoHV-4 infection.
Subsequently, changes in nuclear morphology, which could suggest early apoptosis, were assessed through DAPI staining. Significant changes were recorded after 48 hpi in cells infected with the virus and in cells infected and treated with LPS. These results would indicate that the cells are in an apoptotic process. However, some authors mention that morphological changes are not always specific indicators of cell death by apoptosis and may vary between different cell lines [29]. For that reason, they recommend complementing the analysis of this parameter with other methodology, such as the study of DNA fragmentation. Consequently, we proceeded to determine the DNA fragmentation comparing the RAIs of BEC cells after different treatments using TUNEL. Changes in nuclear morphology can be initiated as early as 30 min to 4 h after apoptotic stimulation, and precede changes in the cytoskeleton. In contrast, DNA fragmentation occurs later (24 hpi), hence TUNEL staining is a good indicator of late apoptosis process [30,31,32]. Therefore, DAPI and TUNEL techniques were used in a complementary way as indicators of early and late cell damage processes, respectively. TUNEL staining indicated significant apoptosis starting at 24 hpi in cells infected with BoHV-4 and in the presence of BoHV-4 + LPS, while at 48 hpi the apoptosis indices in the aforementioned treatments were higher. These results correlate with the observed cytopathic effect which became evident at 24 hpi and was marked after 48 hpi, indicating a large percentage of dead cells. In turn, it is consistent with the viral titers obtained in previous works [22], which means that at 48 hpi cell death by apoptosis would allow the release of progeny, thus increasing the extracellular viral load.
The analysis of the mRNA expression of mitochondrial Bcl2, Bax, and the Bax/Bcl-2 ratio in BoHV-4-infected BEC cells demonstrated a “balance.” These results can be interpreted as a strategy of the virus to favor the latency state in the early stages of the infection, avoiding the apoptosis of the infected cells. These results are consistent with those reported in the literature [13], where BoHV-4 efficiently infected purified populations of bovine endometrial epithelial and stromal cells, leading to non-apoptotic cell death and de novo viral production. Finally, the expression of Caspase 3 was evaluated, since it is a central enzyme in the apoptosis process that plays a fundamental role in the activation and execution of the apoptotic cascade. The results obtained from the expression of Caspase 3 were variable according to the different times evaluated. However, it can be noted that LPS by itself is not a potent inducer of Caspase 3 expression, in the BEC cells evaluated. On the other hand, it is evident that BoHV-4 can induce a significant increase in Caspase 3 mRNA levels after 24 hpi and 72 hpi and this effect seems to be inhibited in the presence of bacterial LPS. It is important to highlight that these results were obtained in the context of a specific study using BEC cells in particular. Results may vary depending on cell type and experimental conditions used. For example, [33], who studied the effect of LPS on neuronal cell apoptosis, demonstrated a significant increase in the expression of caspase 3 together with the pro-apoptotic protein Bax.
Although only Caspase 3 gene expression was evaluated, the results confirm that changes occur at the level of mRNA expression in this cell type. These increases could suggest that BoHV-4 stimulates apoptosis through the caspase pathway, but not in the presence of LPS, where it would use another pathway or would not be the main one.
The results obtained allow us to affirm that BoHV-4 infection generates morphological, biochemical, and molecular changes during early and late stages of programmed cell death. Furthermore the magnitude of cell death observed in the proposed primary culture is directly related to the time of viral infection, and to the presence of LPS in BoHV-4 infection. Similar results were observed by [18] in MDBK cells, where they demonstrated an inoculum- and infection time- dependent induction of apoptosis, where antioxidants prevented it but did not affect viral replication, confirming that apoptosis is not essential in productive BoHV-4 infection. On the other hand, [8] demonstrated the variability in the ability to induce changes compatible with apoptosis of phylogenetically divergent BoHV-4 strains, and the variations present according to the type of infected cell. This is similar to what was found by other authors regarding the time of presentation of apoptosis indicator characteristics, such as morphological changes or nuclear condensation, who point out that there are variations according to different cell types [29,34]. Furthermore, it is important to consider that some stimuli can cause cell death by apoptosis or necrosis depending on the intensity and duration, as well as the cell type on which they act [32].
5. Conclusions
The data presented in this study showed that the degree of apoptosis in the proposed primary culture is directly related to the time of viral infection, and to the presence of LPS in BoHV-4 infection. This finding provides insight into the factors that may influence the pathogenesis of BoHV-4 and helps to better understand the mechanisms involved in viral infection. Understanding the antiapoptotic mechanisms of bovine herpesvirus will greatly enhance the ability to develop new antiviral compounds and vaccines for treatment and prevention. These new antiviral compounds could inhibit virus release, adjust latency to reduce viral infection, or promote death of infected cells in early infection.
Author Contributions
Conceptualization, Florencia Romeo. Andrea Verna.; Data curation, Santiago Delgado.; Formal analysis, Santiago Delgado.; Funding acquisition, Andrea Verna. Sandra Pérez.; Investigation, Florencia Romeo. Marisol Yavorsky. Susana Pereyra. Andrea Verna.; Methodology, Florencia Romeo.; Project administration, Andrea Verna.; Resources, Andrea Verna.; Supervision, Enrique Louge, Lucia Martínez Cuesta, Sandra Pérez, Andrea Verna.; Validation, Santiago Delgado.; Visualization, Florencia Romeo. Marisol Yavorsky. Writing - original draft, Florencia Romeo.; Writing - review & editing, Erika González Altamiranda, Lucia Martínez Cuesta.
Funding
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica through PICT 2015–2263 and PICT 2020-01012, being part of a thesis by Florencia Romeo in partial fulfillment of the requirements for the Doctor’s degree (Doctorado en Ciencias Agrarias, Facultad de Ciencias Agrarias, Universidad Nacional de Mar del Plata, Argentina).
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dra Andrea Verna reports financial support was provided by Agencia Nacional de Promoción Científica y Tecnológica PICT 2015-2263- PICT 2020-01012.
References
- Everett, H.; McFadden, G. Apoptosis: an innate immune response to virus infection. Trends Microbiol. 1999, 7, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E. Viruses, apoptosis, and neuroinflammation—a double-edged sword. J. NeuroVirology 2015, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.A.; Norris, P.S.; Ware, C.F. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 2002, 3, 1013–1018. [Google Scholar] [CrossRef]
- Sarid, R.; Sato, T.; Bohenzky, R.A.; Russo, J.J.; Chang, Y. Kaposi's sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat. Med. 1997, 3, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total. Environ. 2019, 710, 136397. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.; Schneider, P.; Hofmann, K.; Fickenscher, H.; Meinl, E.; Neipel, F.; Mattmann, C.; Burns, K.; Bodmer, J.-L.; Schröter, M.; et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 1997, 386, 517–521. [Google Scholar] [CrossRef]
- Kofod-Olsen, E.; Ross-Hansen, K.; Schleimann, M.H.; Jensen, D.K.; Møller, J.M.L.; Bundgaard, B.; Mikkelsen, J.G.; Höllsberg, P. U20 Is Responsible for Human Herpesvirus 6B Inhibition of Tumor Necrosis Factor Receptor-Dependent Signaling and Apoptosis. J. Virol. 2012, 86, 11483–11492. [Google Scholar] [CrossRef] [PubMed]
- Morán, P.; Manrique, J.; Pérez, S.; Romeo, F.; Odeón, A.; Jones, L.; Verna, A. Analysis of the anti-apoptotic v-Bcl2 and v-Flip genes and effect on in vitro programmed cell death of Argentinean isolates of bovine gammaherpesvirus 4 (BoHV-4). Microb. Pathog. 2020, 144, 104170. [Google Scholar] [CrossRef] [PubMed]
- Nguyen ML, Blaho JA. Apoptosis during herpes simplex virus infection. Adv Virus Res. 2007;69:67-97. [CrossRef] [PubMed]
- Chastant-Maillard, S. Impact of Bovine Herpesvirus 4 (BoHV-4) on Reproduction. Transbound. Emerg. Dis. 2013, 62, 245–251. [Google Scholar] [CrossRef]
- Frazier, K.; Pence, M.; Mauel, M.J.; Liggett, A.; Hines, M.E.; Sangster, L.; Lehmkuhl, H.D.; Miller, D.; Styer, E.; West, J.; et al. Endometritis in Postparturient Cattle Associated with Bovine Herpesvirus-4 Infection: 15 Cases. J. Veter- Diagn. Investig. 2001, 13, 502–508. [Google Scholar] [CrossRef]
- Jacca, S.; Franceschi, V.; Colagiorgi, A.; Sheldon, M.; Donofrio, G. Bovine Endometrial Stromal Cells Support Tumor Necrosis Factor Alpha-Induced Bovine Herpesvirus Type 4 Enhanced Replication1. Biol. Reprod. 2013, 88, 135. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, G.; Herath, S.; Sartori, C.; Cavirani, S.; Flammini, C.F.; Sheldon, I.M. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 2007, 134, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, G.; Ravanetti, L.; Cavirani, S.; Herath, S.; Capocefalo, A.; Sheldon, I.M. Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: a new insight into bacterial and viral interaction for uterine disease. Reproduction 2008, 136, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Deim, Z.; Szeredi, L.; Egyed, L. Detection of bovine herpesvirus 4 DNA in aborted bovine fetuses. . 2007, 71, 226–9. [Google Scholar] [PubMed]
- Sciortino, M.T.; Perri, D.; Medici, M.A.; Foti, M.; Orlandella, B.M.; Mastino, A. The Gamma-2-Herpesvirus Bovine Herpesvirus 4 Causes Apoptotic Infection in Permissive Cell Lines. Virology 2000, 277, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- U. , U.P.; Pagnini, U.; Montagnaro, S.; Pacelli, F.; De Martino, L.; Florio, S.; Rocco, D.; Iovane, G.; Pacilio, M.; Gabellini, C.; et al. The involvement of oxidative stress in bovine herpesvirus type 4-mediated apoptosis. Front. Biosci. 2004, 9, 2106–2114. [Google Scholar] [CrossRef]
- Romeo, F.; Delgado, S.; Uriarte, E.L.; Storani, L.; Cuesta, L.M.; Morán, P.; Altamiranda, E.G.; Odeón, A.; Pérez, S.; Verna, A. Study of the dynamics of in vitro infection with bovine gammaherpesvirus type 4 and apoptosis markers in susceptible cells. Microb. Pathog. 2022, 169, 105645. [Google Scholar] [CrossRef]
- Tebaldi, G.; Jacca, S.; Montanini, B.; Capra, E.; Rosamilia, A.; Sala, A.; Stella, A.; Castiglioni, B.; Ottonello, S.; Donofrio, G. Virus-Mediated Metalloproteinase 1 Induction Revealed by Transcriptome Profiling of Bovine Herpesvirus 4-Infected Bovine Endometrial Stromal Cells. Biol. Reprod. 2016, 95, 12–12. [Google Scholar] [CrossRef]
- Romeo, F.; Louge-Uriarte, E.; Gonzalez-Altamiranda, E.; Delgado, S.; Pereyra, S.; Morán, P.; Odeón, A.; Pérez, S.; Verna, A.E. Gene expression and in vitro replication of bovine gammaherpesvirus type 4. Arch. Virol. 2021, 166, 535–544. [Google Scholar] [CrossRef]
- Romeo, F.; Spetter, M.J.; Moran, P.; Pereyra, S.; Odeon, A.; Perez, S.E.; Verna, A.E. Analysis of the transcripts encoding for antigenic proteins of bovine gammaherpesvirus 4. J. Veter- Sci. 2020, 21, e5. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.J.; Uriarte, E.L.L.; Armendano, J.I.; Morrell, E.L.; Cantón, G.J.; Verna, A.E.; Dorsch, M.A.; Pereyra, S.B.; Odeón, A.C.; Saliki, J.T.; et al. Detection methods and characterization of bovine viral diarrhea virus in aborted fetuses and neonatal calves over a 22-year period. Braz. J. Microbiol. 2020, 51, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Verna, A.; Manrique, J.; Pérez, S.; Leunda, M.; Pereyra, S.; Jones, L.; Odeón, A. Genomic analysis of bovine herpesvirus type 4 (BoHV-4) from Argentina: High genetic variability and novel phylogenetic groups. Veter- Microbiol. 2012, 160, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reed, LJ, Müench, H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg.
- Suzuki, T.; Sakumoto, R.; Hayashi, K.-G.; Ogiso, T.; Kunii, H.; Shirozu, T.; Kim, S.-W.; Bai, H.; Kawahara, M.; Kimura, K.; et al. Involvement of interferon-tau in the induction of apoptotic, pyroptotic, and autophagic cell death-related signaling pathways in the bovine uterine endometrium during early pregnancy. J. Reprod. Dev. 2018, 64, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.J. Viruses and apoptosis. . 2001, 82, 65–76. [Google Scholar] [CrossRef]
- McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 1995;46:153-85. [CrossRef] [PubMed]
- Mesner, P.; Epting, C.; Hegarty, J.; Green, S. A timetable of events during programmed cell death induced by trophic factor withdrawal from neuronal PC12 cells. J. Neurosci. 1995, 15, 7357–7366. [Google Scholar] [CrossRef]
- Maruyama, W.; Irie, S.; Sato, T.-A. Morphological changes in the nucleus and actin cytoskeleton in the process of Fas-induced apoptosis in Jurkat T cells. Histochem. J. 2000, 32, 495–503. [Google Scholar] [CrossRef]
- Daniel, B.; A DeCoster, M. Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res. Protoc. 2004, 13, 144–150. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Hoda, F.E.; Noor, A.M. Studying the effect of LPS on cytotoxicity and apoptosis in PC12 neuronal cells: Role of Bax, Bcl-2, and Caspase-3 protein expression. Toxicol. Mech. Methods 2010, 20, 316–320. [Google Scholar] [CrossRef]
- Gillet, L.; Dewals, B.; Farnir, F.; de Leval, L.; Vanderplasschen, A. Bovine Herpesvirus 4 Induces Apoptosis of Human Carcinoma Cell Lines In vitro and In vivo. Cancer Res 2005, 65, 9463–9472. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Percentage of Rod-123+ BEC cells after different times post infection with BoHV-4 and/or treatment with LPS. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times.
Figure 1.
Percentage of Rod-123+ BEC cells after different times post infection with BoHV-4 and/or treatment with LPS. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times.
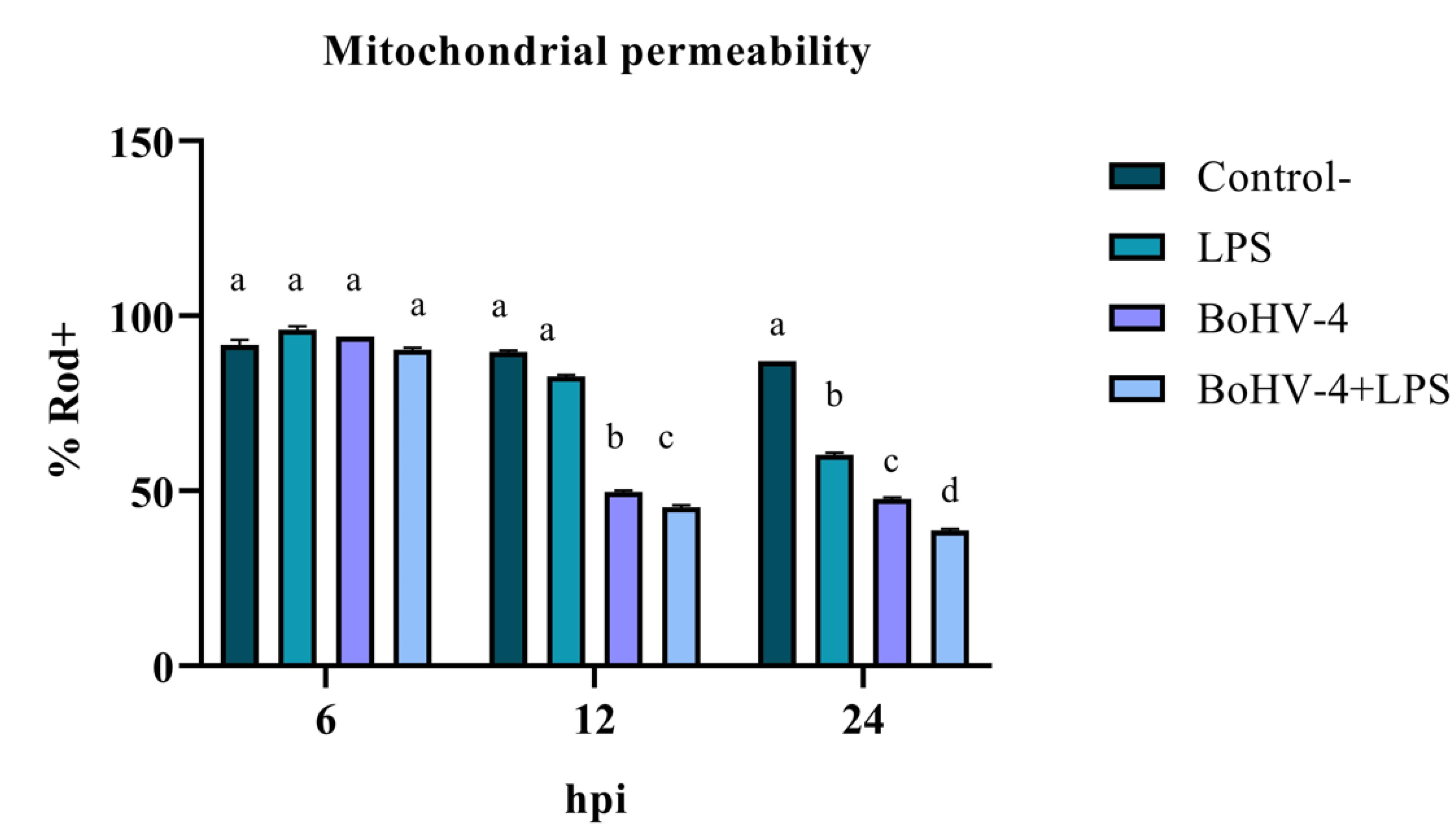
Figure 2.
Conventional (left) and confocal (right) fluorescence microscopy of DAPI stained BEC cells infected with BoHV-4. Nuclear morphological changes characteristic of apoptosis (asterisks) were observed with a magnification of 40X .
Figure 2.
Conventional (left) and confocal (right) fluorescence microscopy of DAPI stained BEC cells infected with BoHV-4. Nuclear morphological changes characteristic of apoptosis (asterisks) were observed with a magnification of 40X .

Figure 3.
Means and standard deviations of Relative Apoptosis Index (RAI), determined after infection with BoHV-4 and/or LPS treatment at 12, 24 and 48 hpi in BEC cells (DAPI staining). Means accompanied by the same letter indicate non-significant differences (α= 0.05) between each treatment at reading time.
Figure 3.
Means and standard deviations of Relative Apoptosis Index (RAI), determined after infection with BoHV-4 and/or LPS treatment at 12, 24 and 48 hpi in BEC cells (DAPI staining). Means accompanied by the same letter indicate non-significant differences (α= 0.05) between each treatment at reading time.

Figure 4.
Representative images of apoptotic cells detected by TUNEL (arrows) in BoHV-4-infected BEC cells after 24 (A) and 48 (B) hpi. 20X magnification.
Figure 4.
Representative images of apoptotic cells detected by TUNEL (arrows) in BoHV-4-infected BEC cells after 24 (A) and 48 (B) hpi. 20X magnification.
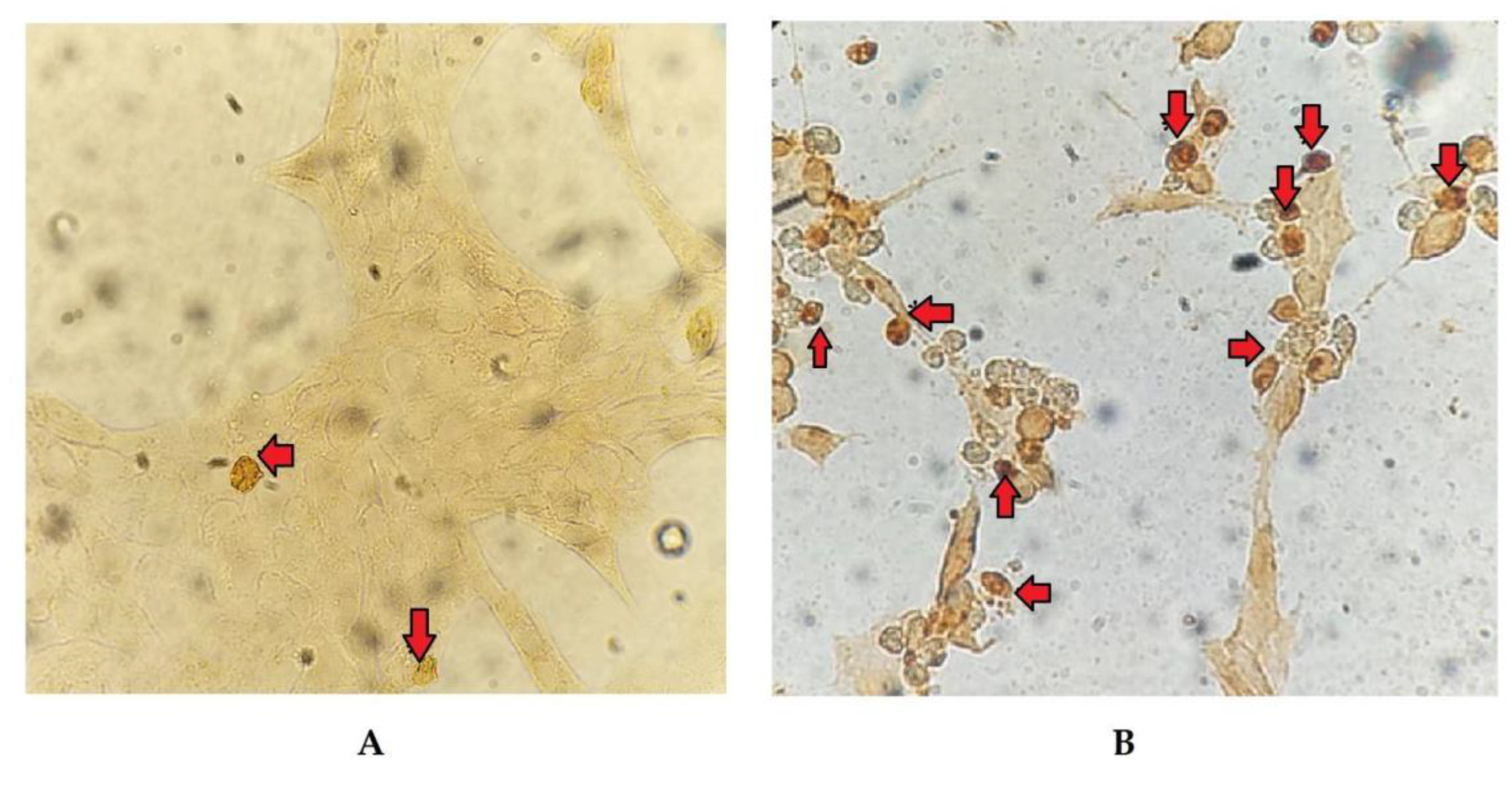
Figure 5.
Relative Apoptosis Index (RAI) in BEC cells infected with BoHV-4 and/or treated with LPS. Detection through TUNEL. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times.
Figure 5.
Relative Apoptosis Index (RAI) in BEC cells infected with BoHV-4 and/or treated with LPS. Detection through TUNEL. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times.

Figure 6.
Means and standard deviations of the relative expression of Bax, Bcl-2 and Bax/Bcl-2 in BEC cells at different times post infection with BoHV-4. The data were normalized to the expression of the reference gene GAPDH and are presented in arbitrary units. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between levels of times for each gene.
Figure 6.
Means and standard deviations of the relative expression of Bax, Bcl-2 and Bax/Bcl-2 in BEC cells at different times post infection with BoHV-4. The data were normalized to the expression of the reference gene GAPDH and are presented in arbitrary units. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between levels of times for each gene.

Figure 7.
Relative expression of Caspase 3 in BEC cells after different times post-infection with BoHV-4 and/or treatment with LPS. The data were normalized to the expression of the reference gene GAPDH and are presented in arbitrary units. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times. .
Figure 7.
Relative expression of Caspase 3 in BEC cells after different times post-infection with BoHV-4 and/or treatment with LPS. The data were normalized to the expression of the reference gene GAPDH and are presented in arbitrary units. Means accompanied by the same letter indicate non-significant differences (α= 0.05) between the treatments at the evaluated times. .

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Modulation of Apoptosis by Bovine Herpesvirus Type 4 Infection in Bovine Endometrial Cells and the Possible Role of LPS in this Process
Florencia Romeo
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







