Preprint
Article
Quantification of hs-Troponin Level and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement
Altmetrics
Downloads
156
Views
50
Comments
0
A peer-reviewed article of this preprint also exists.












This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
20 October 2023
Posted:
24 October 2023
You are already at the latest version
Alerts
Abstract
Myocardial involvement among critical patients with coronavirus disease 2019 (COVID-19) has a worse outcome. The occurrence of an imbalance in oxygen supply results in the release of pro-inflammatory cytokines which leads to increased ventilation support requirement and increase risk of mortality. Our aim was to evaluate the association between hs-Troponin I level and global longitudinal strain as evidence of myocardial involvement among critical COVID-19 patients. Methods. We performed prospective cohort study from February 1st – July 31st, 2021 at Dr. Soetomo General Hospital, Surabaya as referral center for COVID-19 care. Of the 65 critical COVID-19 included, 41 (63.1%) were men, with a median (interquartile range) age of 51.0 years (20.0-75.0).Subjects were recruited according to WHO criteria for severe COVID-19 and CDC criteria for myocarditis. All subjects recruited were examined using echocardiography to measure global longitudinal strain (GLS) and blood samples were taken to measure hs-Troponin. Subjects were then followed to assess their needs for mechanical ventilation and in-hospital mortality. Severe COVID-19 subjects with cardiac injury were associated with an increased need for intubation (78.5%) and an increased incidence of myocarditis (50.8%). There was an association between the use of intubation and the risk of mortality in patients (66.7% vs. 33.3%, p-value <0.001). Both reduced GLS and increased hs-troponin are associated with increased myocarditis (p-value <0.001 and 0.004 respectively). Reduced GLS is associated with higher mechanical ventilation needs (12.17 + 4.79 vs.15.65 + 4.90, p-value 0.02) and a higher mortality rate (11.36 + 4.64 vs. 14.74 + 4.82; p-value 0.005). Increased hs-troponin is associated with higher mechanical ventilation needs (25.33% vs. 3.56%, p-value 0.002) and a higher mortality rate (34.57% vs. 5.76%, p-value 0.002). Compared with contemporary controls, critical COVID-19 patients with myocardial involvement and elevated cardiac troponin level are associated with higher mechanical ventilation needs and a higher mortality rate.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
COVID-19 is a worldwide pandemic with a mortality rate approaching ten percent in Indonesia [1]. Fever, cough, and dyspnea are common symptoms of COVID-19 which are exacerbated in the elderly and other comorbidities, such as chronic obstructive pulmonary disease or heart disease [2]. Cardiac injury as one of the cardiac manifestations that exacerbate the systolic and diastolic functions has potentially worsened the condition of COVID-19 patients and caused mortality [3].
Acute myocarditis in COVID-19 patients, which is associated with immunologic response and cytokine storm, destroyed the myocardium, which is characterized by an increased in a specific marker for cardiac enzymes, high-sensitive troponin I (hs c-TnI) [3,4,5].
Hue, et al. reported that acute myocarditis also causes heart failure accompanied by an increase in NT-proBNP associated with a fatal outcome, a high risk of death for COVID-19 patients [6]. Adaptive response appears due to cardiac burden in patients with COVID-19 infection. Echocardiography is an imaging modality used in patients with suspected cardiac injury [7,8].
Examination of several cardiac marker enzymes used to establish a diagnosis and predict the prognosis of patients with COVID-19 [9,10]. This study aims to determine the ability of hs cTnI and NT-pro BNP level as non-invasive prognostic tools and to predict the outcome of heart injury in COVID-19 patients without prior history of heart disease.
2. Materials and Methods
2.1. Data source
The primary data in this study are obtained by laboratory examinations to measure cardiac markers and echocardiography examinations by a certified cardiologist at the Dr. Soetomo General Hospital Surabaya, and were all adult COVID-19 patients.
2.2. Study population and design
Our observational analytic study used a prospective single-center cohort design. This study and patient treatment were conducted at Dr. Soetomo General Academic Hospital from July 1st to December 31st, 2020. The population in this study were all 18–90-year-old patients with clinical manifestations of COVID-19 and positive COVID-19 laboratory results according to WHO diagnostic criteria.
2.3. Data collection
Data was collected by total sampling, where data on all patients who met this study’s inclusion and exclusion criteria were collected. Primary data were obtained from interviews and examinations of patients, including physical, laboratory, and echocardiographic examinations. Data collection was carried out in a prospective cohort, where all patient histories were examined clinically outside the research team independently.
2.4. Statistical analysis
The independent and dependent variable data were analyzed by inferential statistical analysis. The data were tested for normality using a one-sample Kolmogorov–Smirnov and Saphiro-Wilk tests. The statistical difference test, which compared the value of variables to outcomes and the length of hospitalization, used the independent t–test, followed by the Linear Regression statistical test if the data were normally distributed, or the Wilcoxon Mann-Whitney test, followed by the Polynomial Regression statistical tests if the data were not normally distributed. Sex, blood type, ethnicity, and financing variables will be analyzed using the χ2 and Fisher-Exact test, followed by Logistic Regression statistical tests. The multivariate analysis statistical test will be conducted if there is a meaningful variable. All analysis results will be presented as narratives, tables, and graphs. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software 25.0.0 version for Windows, 2017 (Armonk, NY: IBM Corp. IBM Corp.).
3. Results
This study was conducted in the Intensive Care Unit (ICU) of Dr. Soetomo General Hospital, Surabaya from February 1st to July 31st 2021. Data was collected by total sampling.
3.1. Baseline Characteristics of Subjects
A total of 65 patients with severe COVID-19 with HFpEF who met the inclusion and exclusion criteria were included in the study. There were 33 subjects diagnosed with myocarditis according to clinical, echocardiographic, and laboratory criteria. Data normality analysis for numerical data was performed using the Kolmogorov-Smirnov test. Length of stay, GLS Average, and LAVI were found to be normally distributed.
Analysis of the basic characteristics of the subjects showed that the median age was 56 years old, and 63.1% of the subjects were male. Most research subjects had a history of hypertension (69.2%) and DM (60%). There was a significant rise in mortality in subjects who experienced increased of hs-Troponin level (p = 0.011), increased procalcitonin level (p = 0.001), increased serum creatinine (p < 0.001), decreased mean GLS (p = 0.005), an increased degree of diastolic dysfunction (p = 0.003), intubation (p < 0.001), and myocarditis (p = 0.002). Increased body mass index and higher NT-proBNP were also associated with increased mortality (p= 0.044 and p = 0.002, respectively). We present the overall data of baseline characteristics in Table 1.
3.2. Subanalysis of Myocardial injury and Severe COVID-19 Outcome
Bivariate analysis indicated relationships between increased NT-proBNP and hs-Troponin level on mortality (Figure 1).
Myocardial injury is indicated by increased cardiac markers particularly, NT-proBNP and hs-Troponin. Bivariate analysis was carried out to evaluate the relationship between the two laboratory parameters on the outcomes of patients with severe degrees of COVID-19. There was a significant relationship between the increase in NT-proBNP and the degree of diastolic dysfunction in patients (p < 0.001). An increase in hs-Troponin was associated with a higher incidence of intubation and a higher degree of diastolic dysfunction (p = 0.044 and p = 0.001, respectively). The analysis is as listed in Table 2 and can be observed in Figure 2 and Figure 3.
Table 2.
Analysis of laboratory parameters in myocardial injury to the outcome of patients with severe degree of COVID-19.
Table 2.
Analysis of laboratory parameters in myocardial injury to the outcome of patients with severe degree of COVID-19.
| Variable | Event | Non-event | p-value |
|---|---|---|---|
| Intubation | Intubation | Non-intubation | |
| NT-proBNP | 742 (32, 10000) | 246.30 (37, 10000) | 0.068 |
| Hs-Troponin | 25.33 (1, 1407) | 3.56 (2, 120) | 0.002 |
| Procalcitonine | 1.85 (0.05, 100) | 0.59 (0.05, 100) | 0.044 |
| Serum Creatinine | 1.40 (0.10, 10) | 0.75 (0.50, 1.10) | 0.001 |
| ECMO | ECMO | Non-ECMO | |
| NT-proBNP | 742 (32, 10000) | 419.20 (37, 10000) | 0.640 |
| Hs-Troponin | 32.79 (2, 1357) | 16.44 (1, 1407) | 0.210 |
| Procalcitonine | 2.01 (0.38, 100) | 1.38 (0.01, 100) | 0.623 |
| Serum Creatinine | 0.80 (0.10, 3.30) | 1.10 (0.40, 10.00) | 0.370 |
| Diastolic Dysfunction | Grade 1 | Grade 2 | |
| NT-proBNP | 302.30 (32, 10000) | 2290.05 (178, 10000) | <0.001 |
| Hs-Troponin | 7.19 (1, 1356) | 91.47 (4, 1407) | 0.001 |
| Procalcitonine | 1.20 (0.09, 100) | 1.97 (0.01, 100) | 0.655 |
| Serum Creatinine | 1.00 (0.40, 10.00) | 1.30 (0.10, 6.80) | 0.454 |
Figure 2.
Box Plot Analysis of Increase in (A) NT-proBNP and (B) hs-Troponin to Intubation Events.

Figure 3.
Boxplot Analysis of the Relationship between Increased (A) NT-proBNP and (B) hs-Troponin to the Degree of Diastolic Dysfunction.
Figure 3.
Boxplot Analysis of the Relationship between Increased (A) NT-proBNP and (B) hs-Troponin to the Degree of Diastolic Dysfunction.

Analysis of laboratory parameters through Receiver Operating Characteristic (ROC) showed that NT-pro-BNP, hs-Troponin, and Procalcitonin were satisfactory at predicting mortality outcomes in patients with severe COVID-19 [(NT pro-BNP: AUC 0.720; p = 0.002; 95% CI 0.595–0.844), (hs-Troponin: AUC 0.722; p = 0.002; 95% CI 0.599–0.846), (Procalcitonin: AUC 0.731; p = 0.001; 95% CI 0.607–0.856)] (Figure 3.4).
Figure 4.
Analysis of Receiver Operating Characteristic (ROC) of laboratory parameters on the outcome (mortality) of research subjects.
Figure 4.
Analysis of Receiver Operating Characteristic (ROC) of laboratory parameters on the outcome (mortality) of research subjects.

Correlative analysis of laboratory parameters using the Spearman showed a moderate correlation between NT-proBNP and hs-Troponin (Table 3.3). It was found that the correlation between NT-proBNP and hs-Troponin had a moderate relationship (r = 0.412; p-value < 0.001). There was a weak relationship between hs-troponin increase and lower length of stay (r 0.287, p = 0.02). There was no relationship between the increase in NT-proBNP and the length of stay (r 0.230, p = 0.065).
Table 3.
Analysis of echocardiographic parameters on the outcome of patients with severe COVID-19.
| Variable | Event | Non-event | p-value |
|---|---|---|---|
| Intubation | Intubated | Not intubated | |
| Length of stay | 19.71 ± 9.93 | 18.07 ± 7.05 | 0.567 |
| GLS Average | 12.17 ± 4.79 | 15.65 ± 4.90 | 0.02 |
| LAVI | 20.36 ± 7.74 | 22.02 ± 5.51 | 0.455 |
| ECMO | ECMO | Non-ECMO | |
| Length of stay | 23.15 ± 9.88 | 18.40 ± 9.08 | 0.102 |
| GLS Average | 11.94 ± 4.71 | 13.17 ± 5.07 | 0.432 |
| LAVI | 22.21 ± 6.54 | 20.34 ± 7.50 | 0.415 |
| Diastolic Dysfunction | Grade 1 | Grade 2 | |
| Length of stay | 20.49 ± 9.67 | 16.39 ± 7.99 | 0.115 |
| GLS Average | 13.35 ± 5.14 | 11.79 ± 4.49 | 0.261 |
| LAVI | 19.26 ± 6.77 | 24.52 ± 7.47 | 0.008 |
3.3. Subanalysis of Echocardiography Parameters and Severe COVID-19 Outcome
Echocardiography is a non-invasive examination that can be done quickly and easily to analyze the anatomy and function of the heart. Echocardiographic assessment shows that the mean parameters of GLS and the degree of dysfunction are associated with mortality in COVID-19 patients. Sub-analysis of echocardiographic parameters on the outcomes of COVID-19 subjects showed that a decrease in the mean GLS was associated with an increase in the need for intubation. Sub-analysis of echocardiographic features on the outcome of patients with severe degree of COVID-19 as shown in Table 3 and Figure 5.
The analysis of laboratory parameters using Receiver Operating Characteristic (ROC) analysis showed that GLS could predict mortality outcomes in patients with severe COVID-19 (AUC 0.700; p-value 0.006; 95% CI 0.568–0.833). ROC analysis can be observed as presented in Figure 6.
3.4. Subanalysis of Clinical Characteristics and Myocarditis in Severe COVID-19
The diagnosis of myocarditis was made based on clinical, echocardiographic, and laboratory criteria. Bivariate analysis was carried out on the relationship between clinical characteristics and the incidence of myocarditis. Subjects with myocarditis were associated with male, older age, a history of hypertension, and incidence of AKI during hospitalization. The incidence of myocarditis can be associated with an increase in serum creatinine (p < 0.001), an increase in NT-proBNP (p <0.001), an increase in hs-troponin (p < 0.001), a decrease in the average GLS (p 0.004), and use of intubation (p < 0.001). We present data on the relationship of clinical characteristics to the incidence of myocarditis in Table 4.
It was also found that patients with an increased degree of diastolic dysfunction were associated with a higher mortality rate (p = 0.032). Echocardiographic features that support an increase in diastolic dysfunction were also associated with an increase in incidence of myocarditis, including an increase in mitral velocity (MV level, p = 0.047) and the occurrence of tricuspid regurgitation which describes an increase in left atrial pressure (TR Vmax, p = 0.029 and TR maxPG, p = 0.026).
3.5. Subanalysis of Myocarditis Clinical Characteristics and Mortality
A sub-analysis was performed on the relationship between clinical characteristics of myocarditis subjects and mortality. Myocarditis patients who experience diastolic dysfunction were associated with increased mortality (p = 0.005).
Table 5.
Relationship between Clinical Characteristics of Myocarditis Subjects and Mortality in Severe COVID-19 Patients.
Table 5.
Relationship between Clinical Characteristics of Myocarditis Subjects and Mortality in Severe COVID-19 Patients.
| Myocarditis Variables | Survived (%) | Mortality (%) | p-value |
| Total | 9 (27.3%) | 24 (72.7%) | - |
| Length of Stay | 23 ± 11 | 16 ± 9 | 0.095 |
| Intubation | 8 (25%) | 24 (75%) | 0.273 |
| ECMO | 4 (57.1%) | 3 (42.9%) | 0.068 |
| Demographic Parameters | |||
| Age | 44.0 (29, 63) | 56.6 (25, 73) | 0.392 |
| Gender | |||
| Male | 7 (35%) | 13 (65%) | 0.216 |
| Female | 2 (15.4%) | 11 (84.6%) | |
| Anthropometry | |||
| Body Mass Index | 28 (22, 36) | 26.5 (17, 45) | 0.766 |
| Past Medical History | |||
| Hypertension | 5 (20%) | 20 (80%) | 0.117 |
| Diabetes Mellitus | 4 (22.2%) | 14 (77.8%) | 0.475 |
| Pregnancy | 1 (25%) | 3 (75%) | 0.705 |
| Acute Kidney Injury | 2 (13.3%) | 13 (86.7%) | 0.101 |
| Laboratory Parameters | |||
| Increased NT | 8 (26.7%) | 22 (73.3%) | 0.629 |
| Increased HS | 8 (25.8%) | 23 (74.2%) | 0.477 |
| Serum Creatinine | 1.00 (0.70, 3.70) | 0.10 (0.10, 8.30) | 0.036 |
| NT-pro BNP | 895.70 (72, 10000) | 3180.10 (120, 10000) | 0.179 |
| Hs-Troponin | 46.55 (2, 162) | 135.99 (5, 1407) | 0.079 |
| Procalcitonin | 0.70 (0.33, 100) | 2.80 (0.05, 100) | 0.437 |
| CRP | 14.30 (4.60, 23.30) | 17.85 (0.50, 90.80) | 0.538 |
| NLR | 13.22 (4.64, 28.76) | 13.24 (2.10, 44.80) | 0.890 |
| D-dimer | 5720 (940, 22300) | 5580 (700, 12870) | 0.984 |
| Echocardiography Parameters | |||
| Biplane EF | 57 (53, 77) | 65 (51, 85) | 0.592 |
| Average GLS | 10.90 (4, 16) | 10.40 (5, 22) | 0.981 |
| LAVI | 19.36 ± 7.00 | 22.19 ± 9.18 | 0.409 |
| MV E Vel | 0.62 (0.42, 0.85) | 0.63 (0.43, 0.92) | 0.238 |
| Average E/E’ | 5.87 (4.74, 10.53) | 7.09 (3.68, 14.00) | 0.619 |
| TR Vmax | 0.00 (0.00, 2.84) | 3.10 (0.00, 4.56) | 0.018 |
| TR MaxPG | 0.00 (00.00, 32.00) | 38.37 (00.00, 83.18) | 0.018 |
| Diastolic Dysfunction | |||
| Grade 1 | 9 (45%) | 11 (55%) | 0.005 |
| Grade 2 | 0 (0%) | 13 (100%) | |
DM, Diabetes Mellitus; AKI, Acute Kidney Injury; CRP, C-Reactive Protein; EF, Ejection Fraction; LAVI, Left Atrial Volume Index; GLS, Global Longitudinal Strain; TR, Tricuspid Regurgitation; ECMO, Extracorporeal Membrane Oxygenator.
4. Discussion
4.1. Clinical Characteristic of the Research Subjects
COVID-19 patients are differentiated into 5 degrees of severity by WHO based on complaints and clinical signs. There are two host responses to COVID infection, the immune defense-based protective phase, and the inflammation-driven damaging phase. In the first phase, the focus is on improving the immune response. In contrast, in the second phase, efforts are made to suppress the excess immune response [11]. Increased inflammatory markers are obtained to moderate, severe, and critical degrees. The increase in such inflammatory markers occurs as a host immune response to the virus, which may be accompanied by acute myocardial injury [11,12,13].
Our research data shows that patients with a higher body mass index (BMI) have better survival abilities. This condition is called the “obesity survival paradox (OSP).” This tendency to bad outcomes is since obesity is associated with various cardiovascular comorbidities. However, further studies are needed on the relationship between obesity and mortality in severe COVID-19 patients. OSP in pneumonia generally has been elaborated by a meta-analysis study conducted by Nie, et al. in 2014 [14]. There are three explanations for the inverse correlation of obesity with mortality in pneumonia. First, obese patients tend to be aware of cardiovascular risks and perform adequate therapy. Second, tumor necrosis factor-alpha (TNF-a) is a pro-inflammatory cytokine important in inflammatory and immune responses. Fatty tissue produces TNF-a receptors, indicated by a decrease in pneumonia severity in the obese group. Third, pneumonia patients with normal weight may not have good metabolic ability to cope with increased catabolic stress [12,14].
The coronavirus binds to target cells through the ACE2 receptor, a homolog of angiotensin-converting enzymes, and converts angiotensin II into angiotensin I also reduce vasoconstriction mediated by RAAS and has a pro-inflammatory role of angiotensin II. The binding of SARS-CoV-2 to the ACE1 receptor results in a series of post-receptor signal changes that result in vasoconstriction, pro-inflammatory response, and endothelial dysfunction and affects myocardial injury and pro-thrombotic processes. The mechanisms mediated by the ACE2 receptor are related to the effects of hypertension, diabetes mellitus, and history of CHD on more severe manifestations of COVID-19 [14,15].
Hypertension and DM are the most common comorbidities found in COVID-19 patients [16]. This is aligned with the clinical demographic of our research subjects. The presence of hypertension in the population of our study subjects was higher compared to multicenter studies conducted in China and the United States of America, which were 24% and 35% [16,17,18]. The percentage of DM patients was also higher in our study than in a study conducted in China (9.7%, 95% CI: 7.2–12.2%) [18]. The difference in the percentage of comorbidities in our study can be due to inclusion criteria that include patients with severe COVID-19. Wang, et al. stated that COVID-19 patients admitted to the ICU had a higher comorbidity rate than those who were not admitted to the ICU (72.2% vs.37.3%) [19]. Patients needing intubation support were associated with an increase in mortality (p = <0.001) [19]. Like another study that demonstrated that severe pneumonia is independently associated with ICU care, the use of mechanical ventilation, and mortality [12,20].
4.2. Subanalysis of Myocardial Injury to the Outcome of the Patients
Increased concentrations of cardiac biomarkers (NT-proBNP and hs-Troponin) correlated with the severity of COVID-19 infection [9,10]. Improved other inflammatory responses, such as procalcitonin and CRP, were also associated with increased mortality [12,20]. Based on the description of the basic data of laboratory parameters, higher levels of NT-proBNP and hs-Troponin were obtained in subjects with mortality output (NT-proBNP 1380.50 vs. 238.10, p = 0.002; hs-Troponin 34.57 vs. 5.76, p-value 0.001). Nevertheless, only an increase in hs-Troponin exceeding normal values is associated with increased mortality. Several clinical factors strongly influence some of the underlying things, including the adjustment of the normal value of NT-proBNP. In addition, there was also an association of increased procalcitonin with mortality (p = 0.001).
The relationship of hs-troponin to other clinical outcomes showed an association of hs-troponin increase with the need for intubation and diastolic dysfunction (p = 0.002 and 0.001, respectively). Hs-Troponin levels in circulation indicate a systemic inflammatory impact caused by COVID-19 infection, so it can be used in predicting major adverse cardiovascular events (MACE) [12,20,21]. The mechanism of myocardial injury can also be seen from an imbalance in oxygen demand, increased ventricular strains, direct myocyte trauma, and the response to increased catecholamines [12,20,21].
In sepsis conditions, increased hs-troponin is associated with LV dysfunction, as seen from transesophageal echocardiography examination. Ventricular dilatation and stress to the LV wall are other causes of increased hs-Troponin in sepsis conditions [22]. Studies conducted by Mehta, et al. in 2004 showed that patients with increased hs-Troponin were associated with regional wall motion abnormalities (RWMA; 56% vs. 6%; p = 0.002), decrease in EF (46% vs. 62%; p = 0.04), and increased mortality (56% vs. 24%; p = 0.04). It also indicates the linkage of the inflammatory process underlying the increase in hs-troponin, which is also associated with alterations in the picture of diastolic dysfunction in patients [23].
The hs-Troponin level can predict 30-day death, namely AUC 0.81 (95%CI 0.73–0.88). Other laboratory parameters also support this capability in predicting mortality [(NT-proBNP AUC 0.80; 95%CI 0.74–0.86; Procalcitonin AUC 0.77; 95%CI 0.70–0.84)]. The results of the AUC analysis on our study samples also showed a fairly good ability of hs-Troponin, NT-proBNP, and procalcitonin in predicting mortality. In addition, it supports its usefulness in predicting the need for mechanical ventilation.
Through the AUC test in our study, we obtained almost similar mortality prediction capabilities between hs-Troponin, NT-proBNP, and procalcitonin. Our research shows AUC NT pro-BNP 0.720 (p = 0.002; 95% CI 0.595–0.844) and hs-Troponin 0.722 (p = 0.002; 95% CI 0.599- 0.846). This value is similar to the meta-analysis study conducted by Wibowo, et al. in 2021, namely the ability of hs-Troponin to predict mortality with AUC 0.73 (0.69–0.77) [23,24].
Through Spearman correlation analysis, a correlation of hs-troponin increase with NT-proBNP increase was obtained in our study subjects with a moderate correlation. This supports that serial examination in conjunction with NT-proBNP examination can help analyze the prognosis of existing biomarkers.
NT-proBNP is secreted in response to increased myocardial wall stress. The MMR process regulates this condition through pro-inflammatory molecules such as lipopolysaccharides, interleukin 1, CRP, and cardiac troponin I, independent of ventricular function. Elevated circulating natriuretic levels are associated with myocardial injury, inflammatory processes, and interaction with ACE2. In addition, its increase can also result from acute heart failure conditions [25,26].
Increased mortality was also observed to be significantly associated with an increase in procalcitonin (p-value 0.001). An increase in procalcitonin is observed particularly in severe COVID-19 patients [26,27]. This condition can be observed in COVID-19 infection and sepsis. This is demonstrated by a study conducted by Firani and Priscilla (2022), which evaluated the significance of procalcitonin in predicting mortality (OR 17.78, p = 0.001). The relationship between the increase in procalcitonin and the increase in hs-troponin can be related to the condition of increasing troponin, which can also be observed in conditions of sepsis [28].
Study conducted by Lippi and Plebani in 2020 showed an increase in procalcitonin occurring in 50% of severe COVID-19 infections and 80% of critical patients. Meanwhile, bacterial co-infection is only obtained in 20% of severe COVID-19 patients and 50% of critical degrees [29].
Our research data also shows levels of CRP (15.60 vs. 14.60), NLR (11.78 vs.9.86), and D-dimer (5240 vs. 4620) which is higher in the event group. Increased inflammatory markers in severe COVID-19 patients may be associated with cytokine storms in the second week of infection. In this condition, leukocytes become over-activated, which results in the release of cytokines. The relationship between increased neutrophils and decreased lymphocytes in COVID-19 infection is unclear. However, this is often associated with the involvement of neutrophil extracellular traps (NETs). This causes neutrophil infiltration into the pulmonary capillaries and results in organ damage and acute respiratory distress syndrome (ARDS). In comparison, the decrease in lymphocytes is caused by the expression of ACE2 receptors by lymphocytes which results in apoptosis [27].
4.3. A Subanalysis of the Echocardiographic Profile of Severe COVID-19 Patient Outcomes
GLS examination shows cardiac abnormalities in the early stages, with normal values of −17% to −18% [8,23,24]. Systemic inflammation induced by COVID-19 can cause LV and RV disorders and result in heart failure [8]. The cardiovascular comorbidities result in a series of cellular changes, including changes in endocardial fibers that result in decreased GLS [8,30]. This also explains that while a decrease in GLS can describe myo-pericardial injury due to an acute process in COVID-19, other chronic conditions can also affect the decrease in GLS [8,30]. The study by Lairez, et al. in 2021 showed no correlation between the decrease in GLS and the increase in hs-troponin level [31].
The correlation analysis of GLS with hs-Troponin using the Spearman method we performed also showed no relationship between GLS decrease and hs-Troponin increase (r 0.245; p = 0.05). However, the decrease in GLS was associated with an increase in mortality (11.36 + 4.64 vs. 14.74 ± 4.82; p-value 0.005). The GLS examination also showed a fairly good ability to predict mortality (AUC 0.700; p-value 0.006; 95% CI 0.568–0.833).
In addition, studies conducted by Bevilacqua, et al. in 2021 state that a decrease in LV GLS (cutoff −16.1%) can predict a decrease in the PaO2/FiO2 (P/F) ratio of < 100 mmHg during maintenance, which marks the need for mechanical ventilation use (HR 4.0, 95% CI 1.4–11.1, p = 0.008) [32].
Our study shows a correlation of GLS decrease with increased intubation needs (12.17 + 4.79 vs. 15.65 ± 4.90, p = 0.02). AUC analysis also showed GLS decreasing ability to predict a good increase in intubation needs (AUC 0.707, p = 0.018, 95% CI 0.514–0.873).
4.4. Analysis of the Relationship of Clinical Characteristics to the Incidence of Myocarditis
COVID-19 infection is associated with the incidence of myocardial injury, one of which is caused by myocarditis [5]. Myocarditis is an inflammatory process involving the myocardium [7]. The US Centers for Disease Control and Prevention (CDC) categorizes myocarditis into probable and confirmed cases based on clinical manifestations and diagnostic features. Establishing the diagnosis of myocarditis using MRI and/or biopsy, however, due to limitations, the use of additional diagnoses using CMR and biopsy could not be performed in our study. The classification of myocarditis in our study is based on the presence of 1 or more new clinical presentations or worsening (chest pain/suppression/discomfort, tightness, palpitations, or the occurrence of syncope) with ≥ 1 new findings from increased hs-troponin, abnormal ECG picture, abnormal heart function indicated by Regional Wall Motion Abnormalities (RWMA) disorders through echocardiography, or if CMR findings that support myocarditis are obtained without being accompanied by other causes of complaints according to Advisory Committee on Immunization Practices (ACIP).
A study by Maino, et. al. in 2021 showed that a cardiovascular magnetic resonance (CMR) examination was carried out for symptomatic COVID-19 patients. The examination showed cardiac involvement, edema and myocardial scars, in 58% of patients through late gadolinium enhancement (LGE) [21]. Knight, et al. in 2020 also conducted a study using CMR examination during early recovery to assess the presence, type, and extent of myocardial injury in troponin-positive COVID-19 patients (during treatment) [33]. Their study showed that abnormalities in CMR are common even though heart function is generally normal. CMR indicate ischemic heart disease (17%), suspicion of myocarditis picture through LGE (38%), and sometimes indicate multiple pathologies (ischemic and non-ischemic, 14%). In addition, the lack of edema in this group indicates that scars such as myocarditis may be permanent [33].
An increase in hs-Troponin alone cannot describe the incidence of myocarditis, therefore its increase can also result from microangiopathy and myocardial infarction [34]. Biopsy examinations show diffuse monocular infiltration or high viral load by the SARS-CoV-2 virus [35,36]. However, limited infrastructure does not allow biopsy or CMR examinations to be carried out. In our study, myocardial injury assessment is performed based on CDC criteria. This statement is supported by various literature that showed hs-Troponin as biomarker that is very useful in assessing myocardial injury as well as studies by researchers that showed hs-Troponin as a gold standard for early diagnosis of cardiac complications with good clinical relevance [35,36]. Hs-Troponin is even able to assess subclinical myocardial inflammation which is helpful in determining early treatment and assessing the prognostics of cardiac complications in patient with COVID-19 [35].
Myocardial injury, as shown by an increase in hs-troponin level, was obtained in 36% of patients who were hospitalized due to COVID-19 infection. However, in the case of fulminant myocarditis, normal hs-troponin is often obtained. Hence, there are restrictions on the use of hs-troponin as a single examination in diagnosing myocarditis. Besides that, subclinical myocardial dysfunction was reported in 79% of COVID-19 patients who underwent strain imaging examination using speckle-tracking echocardiography (GLS). Therefore, the use of these two parameters simultaneously needs to be analyzed in diagnosing myocarditis [36].
We conducted a follow-up analysis on the myocarditis group and found that there was an association in an increase in hs-troponin level, a decrease in GLS, and an increase in the degree of diastolic dysfunction, to the incidence of myocarditis (p < 0.001, 0.004, and 0.032 respectively). The increased risk of death in myocarditis group is supported by the impact of the myocardial injury on the cardiovascular system. Myocardial injury and decreased GLS can recognize both subclinical and acute myocardial dysfunction, which is associated with mortality events. Meanwhile, the evaluation of the two simultaneously supports the diagnosis of myocarditis. The myocardial injury underlying myocarditis also increases the filling pressure of LV and may lead to diastolic dysfunction. Diastolic dysfunction contributes to an increase in the degree of aggravation and mortality in COVID-19 patients. Analysis of the myocarditis group showed that increased diastolic dysfunction was associated with increased mortality (p < 0.005).
The degree of diastolic dysfunction is an evaluation to assess the therapeutic response and predictors of HF-related hospitalization and mortality outcomes in patients with HFpEF according to the American Society of Echocardiography (ASE). However, no study has analyzed the degree of diastolic dysfunction in COVID-19 patients. Research conducted by AlJaroudi, et al. 2012 in the general population explains that diastolic dysfunction is an independent predictor of mortality. Improvements in diastolic dysfunction ≥ 2 showed better mortality outcomes. The degree of diastolic dysfunction illustrates the impact of the correlation between cardiovascular comorbidities and the cardiac response to COVID-19 infection. Stretching of the myocardium will occur due to a persistent increase in LV filling pressure and result in LA dilatation which at an advanced stage increases LAVI. This explains that higher degrees of diastolic dysfunction are associated with the anatomical and functional impact of the myocardium in compensating for changes in LV charging pressure that occur because of myocardial injury in COVID-19 patients [37].
4.5. Study Limitation
This study is a single-center study, so further research is needed with a larger number of subjects with a multi-center study approach to provide an overview of the clinical characteristics of cardiac injury in patients with COVID-19 infection. The diagnosis of myocarditis using CMR, and biopsy could not be performed in our study, however the CMR and biopsy examination may help to determine the definitive cause of myocardial injury that occurs in severe COVID-19 patients.
5. Conclusions
Our study reveals a positive correlation between high hs-troponin level and the need of intubation and the diastolic dysfunction in patients with severe COVID-19. A higher NT-proBNP level was found with an increased degree of diastolic dysfunction in patients with severe COVID-19. Patients with severe degree of COVID-19 with cardiac injury are associated with the need of intubation and incidence of myocarditis. There was a correlation between the incidence of myocarditis and the risk of mortality in patients. High hs-troponin and NT-proBNP level were found with increased mortality in patients with severe degree of COVID-19. This study also showed that hs-troponin and NT-proBNP have a good ability to predict the mortality of patients with severe COVID-19. However, this study showed no correlation between the increase of NT-proBNP level and the duration of hospitalization. In addition, there was a weak correlation between the increase in hs-troponin level and short duration of hospitalization.
Author Contributions
Conceptualization, M.Y.A., L.F.K.W., R.A.N. and T.S.P.; methodology, L.F.K.W., R.A.N. and R.I.G.; software, M.J.A.F.; validation, M.Y.A., F.F.A. and B.S.P.; formal analysis, T.S.P.; investigation, L.F.K.W. and R.A.N.; resources, N.L.; data curation, T.S.P.; writing—original draft preparation, Y.A. and L.F.K.W.; writing—review and editing, R.A.N. and N.L.; visualization, C.P.B. and R.A.F.; supervision, A.S., Y.H.O., A.L., B.B.D. and B.S.P.; project administration, L.F.K.W.; funding acquisition, Y.H.O. and F.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Dr. Soetomo Academic General Hospital Research Grant, grant number 420/UN3.1.1/IPJ/Sp1/2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Dr. Soetomo Academic General Hospital (protocol number: 0133/KEPK/I/2021, issued on January 29th, 2021 under the name of Mochamad Yusuf Alsagaff as Principal Investigator).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
The author would like to thank all staff and residents from Center for Integrated Cardiac Services at the Dr. Soetomo General Academic Hospital, Surabaya, Indonesia. Special thanks for Prof. Djoni Wahyuhadi as Directore of Dr. Soetomo General Academic Hospital and Boedi Santoso as Dean of Faculty of Medicine Universitas Airlangga for their financial and moral support to finish our research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kemenkes, R.I. Situasi Terkini COVID19. 2020. Internet. Available online: https://covid19.kemkes.go.id/situasi-infeksi-emerging/situasi-terkini-perkembangan-coronavirus-disease-covid-19-5-mei-2020.
- Hageman, J.R. The Coronavirus Disease 2019 (COVID-19). Situation Report-59 World Health Organization 2020, 49, e99–e100. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Yan, J.T.; Zhou, N.; Zhao, J.P.; Wang, D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 567–571. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; Schwartz, A.; Uriel, N. COVID-19 and Cardiovascular Disease. AHA Journals 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Sagarad, S.V.; Thakur, B.S.; Reddy, S.S.; Balasubramanya, K.; Joshi, R.M.; Kerure, S.B. NT-proBNP in Myocarditis after a Scorpion Sting Envenomation. J Clin Diagn Res 2013, 7, 118–121. [Google Scholar] [CrossRef]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J 2020, 42, 206. [Google Scholar] [CrossRef]
- Hékimian, G.; Combes, A. Myocardites. La Revue de Médecine Interne 2017, 38, 531–538. [Google Scholar] [CrossRef]
- Hong, G.H.; Hays, A.G.; Gilotra, N.A. The Evolving Role of Echocardiography During the Coronavirus Disease 2019 Pandemic. Heart Int 2022, 16, 28–36. [Google Scholar] [CrossRef]
- Han, H.; Xie, L.; Liu, R.; Yang, J.; Liu, F.; Wu, K.; Chen, L.; Hou, W.; Feng, Y. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. JMV 2020, 92, 819–823. [Google Scholar] [CrossRef]
- Hu, L.; Chen, S.; Fu, Y.; Gao, Z.; Long, H.; Ren, H.W.; Zuo, Y.; Wang, J.; Li, H.; Xu, Q.B.; Yu, W.X.; Liu, J.; Shao, C.; Hao, J.J.; Wang, C.Z.; Ma, Y.; Wang, Z.; Yanagihara, R.; Deng, Y. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019. Clin Infect Dis 2020, 71, 2089–2098. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis 2020, 63, 682–689. [CrossRef]
- Nie, W.; Zhang, Y.; Jee, S.H.; Jung, K.J.; Li, B.; Xiu, Q. Obesity survival paradox in pneumonia: A meta-analysis. BMC Med 2014, 12, 61. [Google Scholar] [CrossRef]
- de Almeida-Pititto, B.; Dualib, P.M.; Zajdenverg, L.; Dantas, J.R.; de Souza, F.D.; Rodacki, M.; et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Cleland, J.G.F.; Katus, H.A. Myocardial injury in severe COVID-19 infection. Eur Heart J 2020, 41, 2080–2082. [Google Scholar] [CrossRef]
- Maino, A.; Di Stasio, E.; Grimaldi, M.C.; Cappannoli, L.; Rocco, E.; Vergallo, R.; et al. Prevalence and characteristics of myocardial injury during COVID-19 pandemic: A new role for high-sensitive troponin. Int J Cardiol 2021, 338, 278–285. [Google Scholar] [CrossRef]
- Ostermann, M.; Ayis, S.; Tuddenham, E.; Lo, J.; Lei, K.; Smith, J.; et al. Cardiac Troponin Release is Associated with Biomarkers of Inflammation and Ventricular Dilatation During Critical Illness. Shock 2017, 47, 702. [Google Scholar] [CrossRef]
- Mehta, N.J.; Khan, I.A.; Gupta, V.; Jani, K.; Gowda, R.M.; Smith, P.R. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004, 95, 13–17. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Astuti, A.; Tiksnadi, B.B.; Martanto, E.; Martha, J.W.; et al. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care Med 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, D.; Wen, X.S.; Cheng, X.C.; Sun, M.; He, B.; et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 2020, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.C.; Ahmed, A.; Burger, A.L.; Muthspiel, M.; Jäger, B.; Wojta, J.; et al. Biomarkers Associated with Cardiovascular Disease in COVID-19. Cells 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Mudatsir, M.; Fajar, J.K.; Wulandari, L.; Soegiarto, G.; Ilmawan, M.; Purnamasari, Y.; et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res 2020, 9, 1107. [Google Scholar] [CrossRef]
- Firani, N.K.; Prisilla, J. Procalcitonin and Troponin-I as Predictor of Mortality in Acute Myocardial Infarction Patients. Indonesian Journal of Clinical Pathology and Medical Laboratory 2022, 28, 170–174. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta 2020, 505, 190–191. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Henkens, M.T.H.; Wang, P.; Schummers, G.; Raafs, A.G.; Krapels, I.P.C.; et al. A global longitudinal strain cut-off value to predict adverse outcomes in individuals with a normal ejection fraction. ESC Heart Failure 2021, 8, 4343. [Google Scholar] [CrossRef]
- Lairez, O.; Blanchard, V.; Houard, V.; Vardon-Bounes, F.; Lemasle, M.; Cariou, E.; et al. Cardiac imaging phenotype in patients with coronavirus disease 2019 (COVID-19): results of the cocarde study. Int J Cardiovasc Imaging 2021, 37, 449. [Google Scholar] [CrossRef]
- Bevilacqua, M.; De Togni, P.; Cattazzo, F.; Dell’Atti, D.; Dalbeni, A.; Mazzaferri, F.; et al. Global Longitudinal Strain to Predict Respiratory Failure and Death in Patients Admitted for COVID-19–Related Disease. Am J Cardiol 2022, 165, 109–115. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Akbar, M.R.; Purnomowati, A.; Martha, J.W. Prognostic performance of troponin in COVID-19: A diagnostic meta-analysis and meta-regression. International Journal of Infectious Diseases 2021, 105, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Pranata, R.; Astuti, A.; Tiksnadi, B.B.; Martanto, E.; Martha, J.W.; Purnomowati, A.; Akbar, M.R. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care 2021. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Aronow, W.S. COVID-19, cardiovascular diseases and cardiac troponins. Future Cardiol 2022, 18, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.P.; Čiháková, D.; Gilotra, N.A. COVID-19 and Myocarditis: Review of Clinical Presentations, Pathogenesis and Management. Heart Int 2022, 16, 20–27. [Google Scholar] [CrossRef]
- Aljaroudi, W.; Alraies, M.C.; Halley, C.; Rodriguez, L.; Grimm, R.A.; Thomas, J.D.; et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012, 125, 782–788. [Google Scholar] [CrossRef]
Figure 1.
Boxplot Analysis on the Relationship of (a) Increased NT-proBNP and (b) hs-Troponin to Mortality.
Figure 1.
Boxplot Analysis on the Relationship of (a) Increased NT-proBNP and (b) hs-Troponin to Mortality.
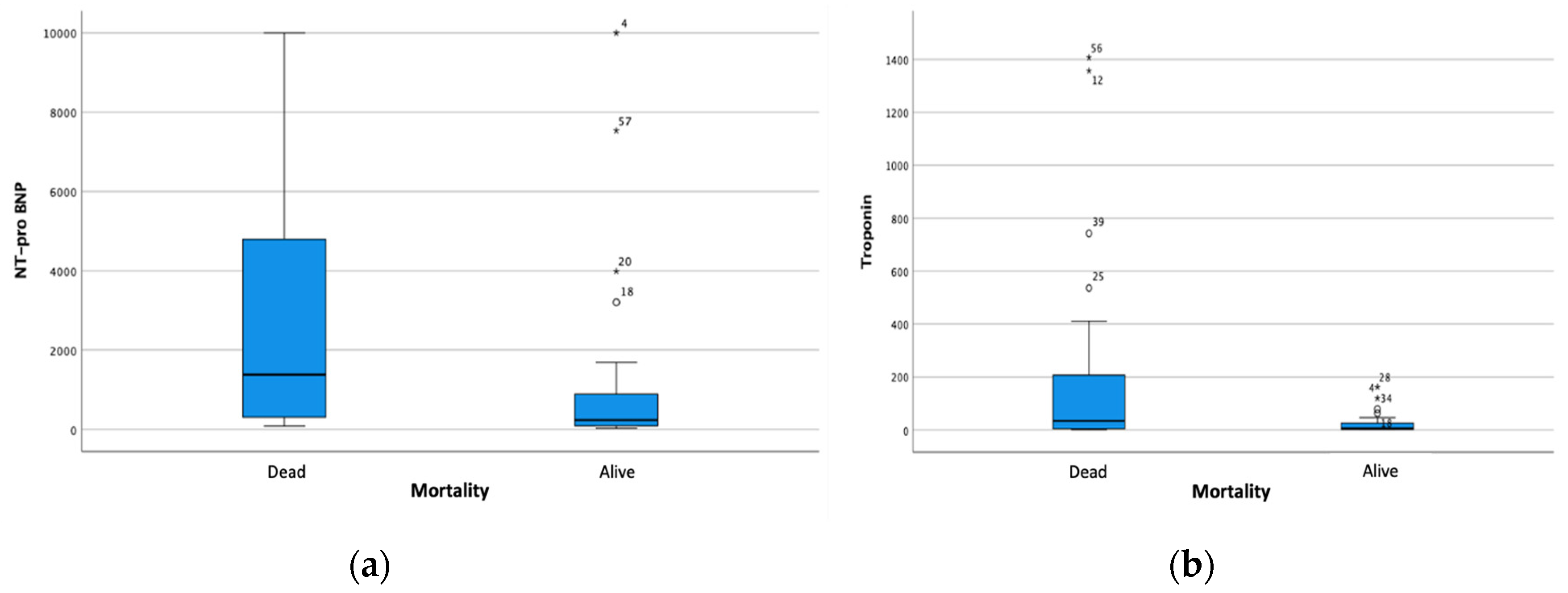
Figure 5.
Boxplot analysis represents (A) an increase in LAVI is associated with an increase in the degree of diastolic dysfunction, (B) a decrease in the mean GLS is associated with an increase in the degree of diastolic dysfunction, and (C) a decrease in the mean GLS is associated with an increased of intubation.
Figure 5.
Boxplot analysis represents (A) an increase in LAVI is associated with an increase in the degree of diastolic dysfunction, (B) a decrease in the mean GLS is associated with an increase in the degree of diastolic dysfunction, and (C) a decrease in the mean GLS is associated with an increased of intubation.

Figure 6.
Receiver Operating Characteristic (ROC) analysis of GLS parameters on mortality.

Table 1.
Baseline Characteristics of Subjects.
| Variable | Total (n = 65) |
Survived (n = 32) |
In hospital death (n = 33) |
p-value |
| Age | 51 (20, 75) | 46 (20, 75) | 56 (25, 75) | 0.085 |
| Gender | ||||
| Male | 41 (63.1%) | 20 (48.8%) | 21 (51.2%) | 0.579 |
| Female | 24 (36.9%) | 10 (41.7%) | 14 (58.3%) | |
| Body Mass Index (kg/m2) | 27 (17, 45) | 28 (22, 37) | 26 (17, 45) | 0.044 |
| Past Medical History | ||||
| Hypertension | 45 (69.2%) | 18 (40%) | 27 (60%) | 0.135 |
| Diabetes Mellitus | 39 (60%) | 15 (38.5%) | 24 (61.5%) | 0.128 |
| Pregnancy | 8 (12.3%) | 5 (62.5%) | 3 (37.5%) | 0.270 |
| Acute Kidney Injury | 23 (35.4%) | 3 (13%) | 20 (87%) | <0.001 |
| Laboratory Parameters | ||||
| Serum Creatinine (mg/dL) | 1.10 (0.10, 10.00) | 0.80 (0.40, 3.70) | 1.70 (0.10, 10.00) | <0.001 |
| Increased NT-proBNP | 54 (83.1%) | 23 (42.6%) | 31 (57.4%) | 0.173 |
| NT-proBNP (pg/mL) | 581.90 (31, 10000) | 238.10 (32, 10000) | 1380.50 (82, 10000) | 0.002 |
| Increased Hs-Troponin | 37 (56.9%) | 12 (32.4%) | 25 (67.6%) | 0.011 |
| Hs-Troponin (ng/L) | 17.56 (1, 1407) | 5.76 (2, 162) | 34.57 (1, 1407) | 0.002 |
| Procalcitonin (ng/mL) | 1.41 (0.01, 100) | 0.65 (0.01, 100) | 2.32 (0.05, 100) | 0.001 |
| CRP (mg/dL) | 14.90 (0.36, 90.80) | 14.6 (0.36, 28.90) | 15.6 (0.50, 90.80) | 0.571 |
| NLR | 10.85 (2.10, 44.80) | 9.86 (3.87, 28.76) | 11.78 (2.10, 44.80) | 0.519 |
| D-dimer | 5030.00 (700, 25230) | 4620 (940, 22300) | 5240 (700, 25230) | 0.146 |
| Echocardiography Parameters | ||||
| Biplane EF | 66.00 (51, 85) | 67.50 (53, 79) | 65.00 (51, 85) | 0.391 |
| Average GLS | 12.92 ± 4.99 | 14.74 ± 4.82 | 11.36 ± 4.64 | 0.005 |
| LAVI | 20.72 ± 7.31 | 20.05 ± 6.11 | 21.28 ± 8.24 | 0.503 |
| Velocity MV E | 0.59 (0.36, 0.92) | 0.59 (0.41, 0.85) | 0.58 (0.36, 0.92) | 0.818 |
| Average E/e’ | 6.99 (3.68, 14.00) | 6.39 (4.62, 10.65) | 6.47 (3,68, 14.00) | 0.927 |
| TR MaxPG | 00.00 (0.00, 83.18) | 0.00 (0.00, 56.66) | 0.00 (00.00, 83.18) | 0.018 |
| TR Vmax | 00.00 (00.00, 4.56) | 0.00 (0.00, 3.76) | 0.00 (0.00, 4.56) | 0.019 |
| Degree of diastolic dysfunction | ||||
| Grade 1 | 47 (72.3%) | 27 (57.4%) | 20 (42.6%) | 0.003 |
| Grade 2 | 18 (27.7%) | 3 (16.7%) | 15 (83.3%) | |
| Other clinical parameters | ||||
| Length of stay | 19 ± 9 | 21 ± 9 | 18 ± 10 | 0.115 |
| Intubation | 51 (78.5%) | 17 (33.3%) | 34 (66.7%) | <0.001 |
| ECMO | 13 (20%) | 7 (53.8%) | 6 (46.2%) | 0.534 |
| Myocarditis | 33 (50.8%) | 9 (27.3%) | 24 (72.7%) | 0.002 |
1 DM, Diabetes Mellitus; AKI, Acute Kidney Injury; CRP, C-Reactive Protein; NLR, Neutrophil to Lymphocyte Ratio; EF, Ejection Fraction; LAVI, Left Atrial Volume Index; GLS, Global Longitudinal Strain; TR, Tricuspid Regurgitation; ECMO, Extracorporeal Membrane Oxygenator.
Table 4.
Correlation between Clinical Characteristics and The Event of Myocarditis in Severe COVID-19 Patients.
Table 4.
Correlation between Clinical Characteristics and The Event of Myocarditis in Severe COVID-19 Patients.
| Variable | Non-Myocarditis (n = 32) |
Myocarditis (n = 33) |
p-value |
| Age | 49 (20, 75) | 55 (25, 73) | 0.753 |
| Gender | |||
| Male | 21 (51.2%) | 20 (48.8%) | 0.675 |
| Female | 11 (45.8%) | 13 (54.2%) | |
| Body Mass Index | 27 (22.37) | 27 (17, 45) | 0.693 |
| Past Medical History | |||
| Hypertension | 20 (44.4%) | 25 (55.6%) | 0.247 |
| Diabetes Mellitus | 21 (53.8%) | 18 (46.2%) | 0.362 |
| Pregnancy | 4 (50%) | 4 (50%) | 0.628 |
| Acute Kidney Injury | 8 (34.8%) | 15 (65.2%) | 0.085 |
| Laboratory Parameters | |||
| Serum Creatinine | 0.80 (0.40, 10.00) | 1.60 (0.10, 8.30) | <0.001 |
| NT-pro BNP | 238.10 (32, 7536) | 2524.40 (72, 10000) | <0.001 |
| Increased NT-proBNP | 24 (44.4%) | 30 (55.6%) | 0.087 |
| Hs-Troponin | 3.56 (1, 36) | 78.32 (2, 1407) | <0.001 |
| Increased Hs-Troponin | 6 (16.2%) | 31 (83.8%) | <0.001 |
| Procalcitonin | 1.29 (0.01, 97.65) | 1.92 (0.05, 100) | 0.158 |
| CRP | 11.75 (0.36, 30.10) | 15.70 (0.50, 90.80) | 0.462 |
| NLR | 9.47 (3.21, 25.21) | 13.22 (2.10, 44.80) | 0.181 |
| D-dimer | 4440.00 (960, 25230) | 5720 (700, 22300) | 0.300 |
| Echocardiography Parameters | |||
| Biplane EF | 67 (60, 79) | 66 (51, 85) | 0.669 |
| MV Evel | 0.58 (0.36, 0.81) | 0.62 (0.42, 0.92) | 0.047 |
| average E/E’ | 6.38 (3.77, 10.65) | 6.76 (3.68, 14.00) | 0.679 |
| TR maxPG | 0.00 (0.00, 56.66) | 7.01 (0.00, 83.18) | 0.026 |
| TR Vmax | 0.00 (0.00, 3.76) | 1.32 (0.00, 4.56) | 0.029 |
| LAVI | 19.99 ± 5.68 | 21.42 ± 8.63 | 0.436 |
| Average GLS | 14.70 ± 5.20 | 11.19 ± 4.16 | 0.004 |
| Degree of diastolic dysfunction | |||
| Grade 1 | 27 (57.4%) | 20 (42.6%) | 0.032 |
| Grade 2 | 5 (27.8%) | 13 (72.2%) | |
| Other Clinical Parameters | |||
| Intubation | 19 (37.3%) | 32 (62.7%) | <0.001 |
| ECMO | 6 (46.2%) | 7 (53.8%) | 0.804 |
| Length of stay | 21 ± 9 | 18 ± 10 | 0.309 |
| Mortality | 11 (31.4%) | 24 (68.6%) | 0.002 |
DM, Diabetes Mellitus; AKI, Acute Kidney Injury; CRP, C-Reactive Protein; EF, Ejection Fraction; LAVI, Left Atrial Volume Index; GLS, Global Longitudinal Strain; TR, Tricuspid Regurgitation; ECMO, Extracorporeal Membrane Oxygenator.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Quantification of hs-Troponin Level and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement
Mochamad Yusuf Alsagaff
et al.
,
2023
Cardiac MRI as a Risk Stratification Tool in COVID -19 Myocarditis
Olga Nedeljković-Arsenović
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







