Preprint
Article
Development of Immersion and Oral Bivalent Nanovaccines for Streptococcosis and Columnaris Disease Prevention in Fry and Fingerling Asian Seabass (Lates calcarifer) Nursery Farms
Altmetrics
Downloads
92
Views
64
Comments
0
A peer-reviewed article of this preprint also exists.
Submitted:
08 November 2023
Posted:
08 November 2023
You are already at the latest version
Alerts
Abstract
In the present study, bivalent nanovaccines of S. iniae and F. covae were administered by immersion vaccination at 30 and 40 days after hatching (DAH), and the third vaccination was orally administered by feeding at 50 DAH. Five different groups were designed: group 1 was set as the control group, and groups 2-4 were vaccinated using the same protocols. ELISA revealed that the levels of total IgM and specific IgM to S. iniae, and F. covae were significantly elevated in groups 2-4 at 10, 20, and 30 days after vaccination (DAV). A qRT‒PCR analysis of immune-related genes revealed significantly higher IgT expression in the vaccinated groups compared with the control group, as revealed by 44-100-fold changes in the vaccinated groups compared with the control (p < 0.001) at every tested time point after vaccination. All vaccinated groups expressed IgM, MHCIIα and TCRα at significantly higher levels than the control group at 10 and/or 20 DAV (p < 0.05). In the S. iniae challenge tests, the survival of vaccinated groups 2-4 ranged from 62.15 ± 2.11 to 75.70 ± 3.36%, which significantly differed from that of the control group (44.44 ± 1.92%). Similarly, all vaccinated groups showed higher survival rates of 68.89 ± 3.85 to 77.78 ± 5.09% during F. covae challenge than the control groups (50.00 ± 3.33%) (p < 0.05).
Keywords:
Subject: Biology and Life Sciences - Animal Science, Veterinary Science and Zoology
1. Introduction
Asian seabass (Lates calcarifer) is a euryhaline fish indigenous to the Indo-Pacific region and is extensively farmed in Southeast Asia, notably in Thailand. Its capacity to survive in fresh and saltwater environments makes it an essential aquacultural commodity in Thailand, with significant economic value. The growth of the Asian seabass farming industry in the region has been propelled by this high demand, leading to a substantial expansion of its cultivation. The cultivation of Asian seabass has not only enhanced its domestic popularity in Thailand but has also resulted in exportation to countries such as China, Taiwan, Vietnam, Singapore, and Malaysia [1,2,3]. However, the Asian seabass farming industry is facing problems due to the outbreak of diseases such as bacterial and viral infections, which are causing significant damage to fish farms. Among those harmful diseases, streptococcosis and columnaris infections, which are caused by Streptococcus iniae and Flavobacterium covae, respectively, have been identified as major causative agents that significantly hamper Asian seabass aquaculture [4,5,6].
To overcome disease problems in fish aquaculture, vaccines are considered one of the most effective methods for disease prevention, acting as an alternative to chemical and drug treatment methods, which always have many adverse side effects both in the environment and for consumers [7]. Unlike in higher vertebrates, vaccination methods in fish can be conducted through injection, immersion, and oral administration. Of these, injection routes are classified as the most effective for immune responses and protection. However, these methods are time- and cost-consuming and always induce stress and mortality after vaccination [8]. Additionally, this method is too difficult to booster vaccinate in the nursery or juvenile to adults during culture in the grow-out stages. Therefore, immersion and oral administration methods are intensively considered because these routes are easy to perform in small fish, and a high number of fish can be simultaneously vaccinated in hatcheries or nurseries, and booster vaccination can also be more practical [9,10].
Compared with the injection route, immersion and oral vaccination have relatively low immune responses and protection efficacy [11]. To increase and meet the better efficacy of oral and immersion vaccines, nanotechnology is a novel technique developed to enhance vaccine efficacy in various fish species [11,12,13,14]. This technology involves reducing the size of vaccine particles, enabling them to adhere more efficiently to various mucosa-associated lymphoid tissues (MALTs), which effectively immune-function at the skin, nasal, gill, and gastrointestinal tract of fish, thus promoting better and longer-lasting immune responses and protection [14,15]. However, since various diseases occur at different times throughout the production cycle, administering bi- or polyvalent vaccines over the culture period can be targeted to effectively increase the quantity and quality of fish production and reduce production costs, which are preferable for fish farmers. Therefore, bivalent or multivalent vaccines would be a more attractive alternative.
Based on the current information, various bivalent nanovaccines have been developed and have shown excellent immune responses and protection in different fish species, including bivalent vaccines against columnaris and francisellosis in Nile tilapia [12].
At present, however, there are no reports on using nanovaccines in the aquaculture industry to prevent diseases, especially streptococcosis and columnaris, which are severe diseases that can cause significant damage to the Asian seabass aquaculture industry. Therefore, this study aims to investigate the efficacy of nanovaccines derived from the inactivated bacteria S. iniae and F. covae in enhancing the immune response, gene expression related to immunity, and disease resistance against these two target pathogens in Asian seabass at nursery farm scales. The information obtained from this study is expected to provide valuable information that can be applied to improve disease prevention in the Asian seabass aquaculture industry.
2. Materials and Methods
2.1. Fish and experimental designs
Healthy Asian seabass (Lates calcarifer) larvae [approximately 15 days after hatching (DAH)] with weights and lengths of 0.07 ± 0.02 g and 1.8 ± 0.17 cm, respectively, were raised at a nursery farm located in the Songklong subdistrict, Bangpakong district, Chachoengsao Province, Thailand. Fish larvae were raised and reared in 12 3.2 × 3.2 m3 cement ponds containing approximately 5,000 L (50 cm in depth) of 5 ppt estuarine water with 50,000 fish/pond (10 fish/L stocking density). Four groups (3 ponds/group) in 4 zones (1-4) for further vaccination experiments were constructed in nursery farm conditions. The water quality of the nursery pond was closely monitored and controlled daily to ensure its optimal levels for fish, with a temperature range of 28-30°C, pH 7-8, salinity 3-5 ppt, and alkalinity 90-100 mg/L as CaCO3. The feeding schedule consisted of commercial pelleted feed twice daily, 10% body weight, and the water was routinely changed every morning and evening, approximately 80%. Furthermore, routine fish sampling was conducted to test for potential diseases, including bacteria, viruses, and parasites, using standard microbiology methods [16].
All described experiments in the current study were conducted according to the Ethical Principles and Guidelines for the Use of Animals National Research Council of Thailand and approved by the Animal Ethics Committee, KU, Thailand (ACKU63-FIS-003).
2.2. Bacterial culture and formalin-killed vaccine preparations
Streptococcus iniae (AQAHMSi2) and Flavobacterium covae (AQAHMFc) were isolated from moribund Asian seabass [17]. The bacterium was cultured in tryptic soy broth (TSB) (Difco™, USA) for S. iniae and Shieh’s broth for F. covae. Incubate these bacteria at 30°C for 18–24 hours (h). After incubation, the bacterial cultures were harvested via centrifugation at 5,000 rpm for 10 minutes (min), and the resulting supernatant was discarded. The obtained pellets were washed twice with 0.85% NaCl. The bacterial cells were fixed with 1.0% formaldehyde solution for 24 h and washed twice with 0.85% NaCl. The bacterial cell density was measured using a spectrophotometer (Thermo Fisher Scientific, MA, USA) by monitoring the optical density at 600 nm. The purity of the cultured bacterium was confirmed through microscopic examination and Gram staining for further nanovaccine preparation.
2.3. Formulation of bivalent nanovaccines
The dry form of the nanovaccine of each S. iniae and F. covae was prepared by complexing antigens with cationic biopolymers to form nanoparticles following the protocol of Kitiyodom et al. (2019) [18] with some modifications. Both S. iniae and F. covae were separately prepared and combined when used as bivalent vaccinations. To formulate inactivated nanovaccine, both formalin-killed bacteria were adjusted based on an optical density until equivalent to 1 × 109 CFU/mL. The bacterial cells were sonicated at 40% amplitude for 10 minutes in an ice bath to breakdown them into nanosized particles. Chitosan solution (cationic biopolymer) (50–200 kDa, Sigma) at 0.25% w/v was prepared in 1% acetic acid.
To prepare the biopolymeric nanovaccine, sonicated bacterial cells were gently mixed with chitosan solution at a ratio of 1:0.5 (v/v). Chitosan was simultaneously complexed with antigen and formed cationic nanoparticles as mucoadhesive nanovaccines. The mixture was constantly stirred for 90 minutes at room temperature. Furthermore, the polymeric nanovaccine was transformed into dry form, treated with 2.5% sucrose, and then dried by a freeze-drying process. The vaccines were stored at -80°C. The physicochemical properties of the nanovaccine were measured by average diameter and zeta potential using a Malvern Instruments Zetasizer Nano ZX, employing the dynamic light scattering (DLS) technique described by Kitiyodom et al. (2019) [18].
Before use, a dry form of bivalent nanovaccine of each S. iniae and F. covae nanovaccine was diluted with 0.85% NaCl to reach 5 × 108 CFU/mL. Diluted nanovaccines of each bacterium were mixed at a ratio of 1:1, providing a 1 × 109 CFU/mL mixture in a 2 L sterile bottle and further used immediately for immersion vaccination after preparation. A preliminary analysis based on a mixture of S. iniae and F. covae nanovaccines showed that this ratio yielded higher ratios of specific IgM to S. iniae concentrations and specific IgM to F. covae concentrations compared with ratios of 1:2, 1:3, 2:1 and 3:1. This information was used for both oral and immersion vaccination.
For the oral vaccine, a nanovaccine of each S. iniae and F. covae nanovaccine with a concentration of approximately 8.5 × 106 CFU was thoroughly mixed to provide a bivalent vaccine of the two bacteria of 1.7 × 107 CFU. This concentration was used to scramble to fish feed at 1.7 × 107 CFU/fish in feed vaccination trials below.
2.4. Immersion vaccination
Three ponds in zone 1 were used as a control, and three ponds in zone areas 2-4, which were maintained by different workers, were used for vaccine treatments. When the fish reached 30 DAH, the water in each nursing pond in zones 1-4 was reduced and remained 10 cm deep (1.0 m3 in volume). Then, 2 L of the above-prepared vaccines was premixed with 2 L of culture water and further thoroughly splashed in the nursing pond to reach the final concentrations of both bacteria at 2 × 106 CFU/mL; fish larvae were exposed to vaccines for a full 20 min. Subsequently, similar spared water was added to a 50 cm depth to maintain a 4 × 105 CFU/mL vaccine concentration. Fish larvae were exposed to the last concentration for 24 h. Two liters of 0.85% NaCl were added to the three control ponds, which served as the control group. Afterward, the water was replaced under the same conditions for 10 days. After this, the second immersion vaccination was conducted. Fish larvae at 40 DAH were booster-vaccinated with the same conditions in the first immersion vaccination. During this period, aeration was fully supplied throughout the experiments.
2.5. Experimental feed preparation and oral vaccination
When the experimental fish reached 50 DAH, the total amount of a bivalent nanovaccine prepared in section 2.3 was calculated based on the target concentration of 1.7 × 106 CFU/fish and then mixed with 70 mL 0.85% NaCl/kg feed. The vaccine solution was thoroughly mixed with commercial feed (Thai Union Feedmill, Thailand) at a 5% feeding rate and air-dried until completely dry. Afterward, experimental fish groups in zones 2-4 were fed 2 times with the prepared feed to reach a total vaccine concentration of 3.4 × 107 CFU/fish/day. Fish were further fed experimental feed for 3 consecutive days to receive a net oral vaccine dose of approximately 1 × 108 CFU/fish. For the control group, fish were fed with feed mixed with 0.85% NaCl under the same conditions as vaccinated fish.
2.6. Effects of bivalent nanovaccine on IgM and immune responses of larvae to fingerling stages of Asian seabass
2.6.1. Fish sampling
During the vaccination experiments, the whole body of Asian seabass was collected 10 days after each vaccination (10, 20, and 30 DAV or 40, 50, and 60 DAH, respectively). At each DAV, 24 fish were randomly selected. The first 8 fish were used for total IgM detection and IgM specific to S. iniae and F. covae by ELISA, lysozyme, and bactericidal activity. The other 8 fish were used for immune-related gene expression. The last 8 fish were prepared for histopathological changes in mucosal-associated lymphoid tissues (MALTs) in the experimental fish’s skin, gills, and gut.
2.6.2. Extraction of whole-body protein
At 10, 20, and 30 DAV, the whole body of Asian seabass was collected using sterilized scissors to finely cut fish into 1.5 mL Eppendorf tubes. Subsequently, sample tissues were preserved in a protein extraction buffer containing a protease inhibitor cocktail (HiMedia, Mumbai, India) at a ratio of 100 mg of tissue per 1 mL of protein extraction buffer. The Asian seabass samples were homogenized using pellet pestles in a stable temperature condition at 4°C. After centrifugation at 2,000-2,500 rpm for 5 min at 4°C, the supernatant was transferred to a fresh 1.5 mL Eppendorf tube and stored at -80°C for ELISA and innate immune parameter analyses below.
2.6.3. Total RNA extraction and preparation of first strand cDNA
The whole fish samples were preserved in 1.0 mL of TriZOLTM reagent (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s protocol. Total RNA was subsequently isolated from the whole body, and the concentration was determined using a NanoDropTM spectrophotometer (Thermo Fisher Scientific, MA, USA). To synthesize first-strand cDNA, one microliter of 1,000 ng/μL total RNA was used as a template with the ReverTra Ace® qPCR RT Master Mix with gDNA Remover Kit (TOYOBO, Japan) according to the manufacturer’s protocol. The product of the first-strand cDNA synthesis was stored at -80°C for further experiments.
2.6.4. Histology analysis of mucosa-associated lymphoid tissues (MALTs)
Whole-body fish collected from all experimental groups after vaccination at each DAV were preserved and kept in Davidson’s fixative. The whole-body fish was then embedded in paraffin blocks after being dehydrated and processed by Fischer et al. (2008) [19]. Hematoxylin and eosin (H&E) were used to stain the target MALTs, including the skin, gills and intestine. Histopathological changes were observed under a light microscope (Olympus, MA, USA).
2.6.5. Total serum IgM
Total serum IgM protein from the whole body of experimental Asian seabass at 10, 20, and 30 DAV was used in the ELISA. The first step was to coat a flat-bottomed 96-well plate with bicarbonate/carbonate coating buffer, pH 9.6, with a volume of 50 mL, and incubate it at room temperature (RT) for 2 h and discard the solution. The microplate wells were then incubated overnight at 4°C with 50 mL of fish serum protein. After incubation, the wells were washed three times with wash buffer containing phosphate-buffered saline (PBS) and 0.05% Tween-20 (PBST; pH 7.4). Then, 50 mL of VisualProtein-BlockPRO™ Blocking Buffer (Energenesis Biomedical Co., Ltd., Taipei, Taiwan) was added to each well and incubated for 2 h at RT. After incubation, the wells were washed thrice with wash buffer and incubated for 5 mins at RT. After incubation, rabbit anti-Asian seabass IgM antibody (GenScript Biotech, USA) diluted at a ratio of 1:2,000 was added to each well at a volume of 50 mL and incubated for 2 h at RT. After incubation, the wells were washed thrice with wash buffer using an ImmunoWash machine and set for 5 mins for the final wash. Then, goat anti-rabbit IgM (Sigma, USA) diluted at a ratio of 1:5,000 was added to a final volume of 50 mL and incubated at RT for 2 hours. Afterward, wells were washed with wash buffer using the ImmunoWash machine 6 times and further incubated at RT for 5 mins for the 6th wash step. The TMB substrate One Component HRP Microwell Substrate (Surmodics IVD, Inc., USA) was added to a volume of 50 mL and incubated for approximately 1 min at RT, and then TMB Stop Solution (Surmodics IVD, Inc., USA) was added to stop the ELISA reaction. Finally, the IgM levels were measured in the absorbance of each reaction using the iMark™ Microplate Absorbance Reader at 450 nanometers (Bio-Rad Laboratories Ltd., USA).
2.6.6. Assessment of serum IgM specific to S. iniae and F. covae antigens
Detection of specific IgM against S. iniae and F. covae was performed using ELISA. Formalin killed S. iniae and F. covae bacterial solutions at a 1×108 CFU/mL concentration, which were prepared with the above protocol. Bacterial cells were sonicated with a VCX130 ultrasonic sonicator (Sonics & Materials, Inc., CT, USA). The first step was to coat a flat-bottomed 96-well plate with bicarbonate/carbonate coating buffer, pH 9.6, with a volume of 50 mL, and incubate it at RT for 2 h before removing the solution. Next, the bacterial solution prepared earlier was added to each well with a volume of 50 mL and incubated overnight at 4°C. Further steps for serum IgM specific to S. iniae and F. covae were conducted with the same conditions described in section 2.6.5.
2.7. Cellular and humoral immune response assays
2.7.1. Lysozyme activity
The activity of serum lysozyme was evaluated on the lysis of Micrococcus lysodeikticus (Sigma, MO, USA), as previously described [20]. Lysozyme activity levels were determined using the following formula: units/mL enzyme = [(A540 sample - A540 blank) dilution factor]/(0.001) × (0.1)
2.7.2. Bactericidal activity
The bactericidal activity (BA) of the whole-body protein samples prepared as described in section 2.6.2 was tested using S. iniae as the representative pathogenic bacterium in the bivalent nanovaccine formulation. The bacterium was cultured and prepared as described above. The BA of the experimental serum was determined by incubating 40 mL of protein serum with 10 μL of 1×105 CFU/mL bacterial suspension of S. iniae bacterium in a 1.5 mL tube (total final pathogen cells in the reaction being 1×103 CFU). The mixture was incubated for 2 h at RT. The surviving bacteria were counted on media specific to S. iniae [trypticase soy broth (Difco™ & BBL™, USA)]. Samples without serum and samples without bacteria were used as negative and positive controls (100% survival or 0% BA), respectively. The BA was calculated as the percentage of surviving bacteria after exposure to serum protein and present after plating on trypticase soy agar (Merck KGaA, Darmstadt, Germany) using the following formula: BA (%) = [(T0 – T24)/T0] × 100, where T0 is the total initial bacteria and T24 is the number of bacteria present after 24 h of exposure.
2.8. Expression of immune-related genes of larvae to fingerling stages of Asian seabass using quantitative real-time PCR (qRT‒PCR)
The immune-related genes IgM, IgT, IgD, CD4, MHCIIα, and TCRα were selected as target in the expression analysis. IgM and IgT primers were specifically designed based on their secreted Ig forms. All the primers used in the study were validated in Asian seabass by RT‒PCR amplification and nucleotide sequencing and are listed in Table 1.
The qRT‒PCR assays were performed using Brilliant III Ultra-Fast SYBR® Green (Agilent, CA, USA) in an Mx3005P QPCR Systems instrument (Agilent, CA, USA). The qPCRs were optimized in 15 μL reactions, including 10 μL of 2×SYBR Green QPCR Master Mix, 1 μL of 0.5 mM forward and reverse primers, 1 μL of cDNA template, and distilled water to adjust the reaction to a final volume of 15 μL. The qPCR cycling conditions consisted of an initial condition of one cycle of 95°C for 5 min, 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 90 s, and a final extension at 72°C for 10 min. Triplicate qRT-PCRs were conducted for each sample. The housekeeping gene β-actin was used to standardize the results by eliminating variation in mRNA and cDNA quantity and quality. The relative expression of immune-related genes in the whole body of Asian seabass at different time points was calculated using 2-ΔΔCT analysis following the protocol of Livak and Schmittgen (2001) [21].
2.9. Growth performance
The effects of bivalent nanovaccines on growth performance were assessed during a 30-day vaccination trial. The growth performances of all treatment groups were based on their body weight data. Thirty fish from each treatment group (10 fish/pond) were randomly monitored for growth performance parameters by weighing their bodies at 10, 20, and 30 DAV. Growth performances were reported as 1) absolute growth rate (AGR), including weight gain (WG) and average daily growth rate (ADG), and 2) specific growth rate (SGR). Moreover, the feed conversion ratio (FCR) was also measured in all experimental trials. All growth calculations were performed using the methods described by Bunnoy et al. (2019) [22].
where Wt is the final weight/length, Wi is the initial weight/length, and t is the trial duration (30 days).
WG (g or cm./30 days) = Wt -Wi
ADG (g or cm./day) = (Wt -Wi)/t
SGR (%/day) = log (Wt) – log (Wi)/t × 100
FCR = amount of total feed given/WG
2.10. Challenge with S. iniae and F. covae
At the end of the 30 DAV, one hundred eighty fish from each pond of zones 1-4 were randomly selected and transferred into 6 250-L fiberglass tanks (30 fish/tank) for challenge tests, three tanks for S. iniae and three tanks for F. covae. The preliminary pathogenicity of virulent S. iniae and F. covae on Asian seabass was determined to validate the optimum dose for the experimental challenge trials. The fourteen-day median lethal concentration (LC50) was assessed by direct immersion with the obtained final concentrations of 1 × 105 CFU/mL for F. covae and 1× 107 CFU/mL for S. iniae for 30 min, which was subsequently applied for the challenge test in this trial. The mortality and survival of the fish were recorded every 12 h for up to 14 days postchallenge. Reisolation of S. iniae and F. covae from moribund fish was performed to verify the cause of death in fish using a standard diagnostic protocol [16]. Cumulative survival analysis of Asian seabass challenged with S. iniae and F. covae was calculated using the Kaplan‒Meier method [15].
2.13. Statistical analysis
Protein serum IgM levels, innate immune response parameters, immune-related gene expression, and cumulative survival were obtained from three replicates, and the results are expressed as the mean ± standard deviation (SD). Data were statistically analyzed by one-way analysis of variance (ANOVA) and Duncan’s new multiple range test (DMRT) to determine differences among groups. All statistical analyses were performed using Statistical Package for Social Science (SPSS for Windows version 24.0, Chicago, IL, USA). The level of statistical significance between the control and experimental groups in the challenge test was indicated as * (p < 0.05), ** (p < 0.01), and ***(p < 0.001) using Student’s t test.
3. Results
3.1. Production and characterization of S. iniae and F. covae nanovaccines
In this part, S. iniae and F. covae nanovaccines were successfully developed for the first time. The physiological properties of these nanovaccines are indicated by average diameter and zeta potential values. As shown in Table 2, the zeta potential of both nanovaccines shifted from a negative charge for the sonicated antigen form to a positive charge for polymeric nanovaccines. It also confirmed the positive charge character of the cationic nanovaccines in both S. iniae and F. covae vaccines. Moreover, both vaccines still obtained this characteristic after transformation into the dry form. The results also showed that the average diameters of polymeric nanovaccines (dry form) were slightly greater than those of the solution form in both S. iniae and F. covae vaccines.
3.2. Effects of bivalent nanovaccine via immersion and oral vaccination on IgM and immune responses of larvae to fingerling stages of Asian seabass
3.1.1. Total serum IgM
At 10 DAV immersion, a significant increase in total IgM levels indicated by the obtained absorbance was observed in fish of groups 3 and 4 with 0.475 ± 0.04 and 0.725 ± 0.16, respectively, compared with the control group with 0.294 ± 0.05 (p < 0.05). However, group 2 did not show a significant difference in total IgM levels compared to the control group (p > 0.05). Following 20 DAV immersion, only group 4 exhibited a significantly higher whole IgM level (0.782 ± 0.20) than the control group (0.444 ± 0.28) (p < 0.05). There was no significant difference in total IgM levels for vaccinated fish in groups 2 and 3 compared with the control group (p > 0.05). At 30 DAV via oral administration, both groups 3 and 4 exhibited significantly increased total IgM levels of 0.794 ± 0.17 and 0.973 ± 0.28, respectively, compared to the control group (p < 0.05). However, group 2 showed no significant difference in total IgM levels compared to the control group (p > 0.05) (Figure 1A).
3.1.2. Serum IgM specific for S. iniae and F. covae
A significant increase in the protein serum IgM levels specific to S. iniae was observed in all groups immunized with bivalent nanovaccine compared with the control groups at every DAV (Figure 1B). In the same way, a significant increase in the protein serum IgM levels specific to F. covae was mainly observed in all groups immunized with bivalent nanovaccine compared to the control groups (Figure 1C).
At 10 DAV, the Asian seabass in groups 2, 3, and 4 exhibited significantly higher levels of specific IgM against S. iniae than the control group (p < 0.05). The specific IgM levels in groups 2, 3, and 4 were measured with absorbances of 0.918 ± 0.20, 0.827 ± 0.14, and 0.793 ± 0.38, respectively, while the control group had an absorbance of 0.440 ± 0.10. At 20 DAV, the fish in groups 2, 3, and 4 continued demonstrating significantly higher levels of specific IgM against S. iniae compared with the control group (p < 0.05), with absorbances of 1.074 ± 0.30, 1.094 ± 0.31, and 1.447 ± 0.32, respectively, while the control group had an absorbance of 0.514 ± 0.10. Similarly, at 30 DAV, the fish in groups 2, 3, and 4 maintained significantly higher levels of specific IgM against S. iniae (p < 0.05). The specific IgM levels in groups 2, 3, and 4 were measured with absorbances of 1.086 ± 0.08, 1.099 ± 0.18, and 1.449 ± 0.23, respectively, while the control group had an absorbance of 0.638 ± 0.09 (Figure 1B).
At 10 DAV, a significant increase in specific IgM levels against F. covae was observed in fish groups 2 and 3 compared with the control group (p < 0.05). The specific IgM levels in groups 2 and 3 were measured with absorbances of 0.462 ± 0.12 and 0.448 ± 0.10, respectively, while the control group had an absorbance of 0.282 ± 0.07. However, no significant difference was observed in the specific IgM levels against F. covae in group 4 compared to the control group (p > 0.05), with an absorbance of 0.336 ± 0.09. At 20 DAV, the fish in groups 2, 3, and 4 continued to exhibit significantly higher levels of specific IgM against F. covae than the control group (p < 0.05). The specific IgM levels in groups 2, 3, and 4 showed absorbances of 0.636 ± 0.15, 0.744 ± 0.18, and 0.840 ± 0.15, respectively, while the control group had an absorbance of 0.402 ± 0.09. Similarly, at 30 DAV, the fish in groups 2, 3, and 4 exhibited significantly higher levels of specific IgM against F. covae than the control group (p < 0.05). The specific IgM levels in groups 2, 3, and 4 indicated absorbances of 0.860 ± 0.15, 0.845 ± 0.18, and 1.102 ± 0.25, respectively, while that in the control group was 0.477 ± 0.10 (Figure 1C).
3.1.3. Nonspecific humoral immune responses
3.1.3.1. Lysozyme activity
The lysozyme activity in the protein serum of all vaccinated groups increased rapidly at 10 DAV after the first vaccination, reaching 88.71 ± 33.67, 157.09 ± 29.45, and 168.96 ± 27.00 unit/mL for groups 2, 3, and 4, respectively, and still significantly differed at 20 DAV and 30 DAV, which exceeded the levels of the control group by a substantial margin (p < 0.05). In comparison, the protein serum lysozyme activity of the control group fluctuated slightly from 10 DAV to 30 DAV, which was not a significant change (p > 0.05) (Figure 2A).
3.1.3.2. Bactericidal activity (BA)
At 10 and 20 DAV, a significant increase in bactericidal activity against S. iniae was observed only in Asian seabass that were immunized with bivalent nanovaccine group 4 (p < 0.05), with 57.21 ± 8.86% and 64.22 ± 6.21%, respectively. Interestingly, at 30 DAV, a significant increase in the BA against S. iniae was observed in all groups immunized with bivalent nanovaccines, with 61.03 ± 9.21%, 66.166 ± 6.96%, and 79.04 ± 6.66% for groups 2, 3 and 4 (p < 0.05), respectively (Figure 2B).
3.2. Effects of bivalent nanovaccines on the expression of immune-related genes
The relative expression levels of immune-related genes, including IgM, IgT, IgD, MHCIIα, TCRα, and CD4, in the whole body are presented in Figure 3A-4F. At 10 DAV, the expression of the IgM gene was significantly upregulated in all vaccinated fish groups by 2.63 ± 0.81-, 1.572 ± 0.48-, and 2.03 ± 0.58-fold, respectively, compared to the control group (p < 0.05) (Figure 3A). Similar results were observed at 20 DAV, when all vaccinated fish showed significant upregulation of IgM expression levels (p < 0.05) compared with the control (Figure 3A). However, at 30 DAV, a significant difference in IgM compared with the control was observed only in group 4 (Figure 3A). The expression patterns of the IgT gene were found to be significantly similarly upregulated at 10 and 20 DAV. At 10 DAV, vaccinated groups 3 and 4 demonstrated highly significant differences in IgT greater than the control group by 74.68 ± 14.08- and 56.92 ± 16.64-fold, respectively (p < 0.001) (Figure 3B). At 20 DAV, these two groups still showed 83.33 ± 19.25- and 60.13 ± 13.97-fold upregulation, respectively, compared to the control group (p < 0.001) (Figure 3B). At 30 DAV, the IgT gene expression in all vaccinated fish groups was also upregulated significantly by 7.43 ± 4.44-, 72.66 ± 10.17-, and 61.89 ± 4.45-fold for groups 2, 3, and 4, respectively (p < 0.05 and 0.001) (Figure 3B). The expression of MHCIIα was observed at 20 DAV in all vaccinated fish groups 2, 3, and 4 by 2.22 ± 0.73-, 2.73 ± 0.69-, and 2.74 ± 0.79-fold changes, respectively, compared with the control group (p < 0.05) (Figure 3D). The expression of TCRα was also observed at 10 DAV in fish vaccinated groups 2 and 3 by 1.14 ± 0.32- and 1.29 ± 0.34-fold changes, respectively. On day 20, TCRα expression was upregulated in all vaccinated fish groups (groups 2, 3, and 4) by 1.41 ± 0.19-, 2.07 ± 0.42-, and 2.17 ± 0.49-fold, respectively, compared to the control group (p < 0.05) (Figure 3E). Regarding the IgD and CD4 genes, no significant differences were observed in most vaccinated fish groups at 10, 20, and 30 DAV when compared to the control group (p > 0.05) (Figure 3C and 3F).
3.3. Effects of bivalent nanovaccine on histopathological changes in MALTs
At each tested period after vaccination, 8 fish of each group were used to analyze the histological alteration in MALTs. Interestingly, at every tested time point after vaccination, the fish in all the vaccinated groups exhibited similar histology, which different from that of the control fish.
3.3.1. SALT
Histological analysis of SALT in Asian seabass following bivalent nanovaccination showed widened lateral line pores after the first, second, and third vaccinations at 10, 20, and 30 DAV, respectively. Fish in all vaccinated groups had significantly wider lateral line tubules compared to the control group (Figure 4). Additionally, after the first and second vaccinations, a notable presence of blue-stained cells was observed around the lateral line tubules in the vaccinated group compared to the control group. Moreover, on the dermis and epidermal layers of the vaccinated fish after the first, second, and third vaccinations, a substantial number of immune-involved-like cells were found in the respective areas compared to the control group (Figure 5).
3.3.2. GIALT
At 10, 20, and 30 DAV, the histological changes in the fish gills revealed no significant differences in the number of goblet cells (Figure 6). However, it was observed that the epithelial cells of the gill lamellae in vaccinated fish appeared enlarged when compared to the control group (Figure 6). Moreover, in the interbranchial lymphoid tissue (ILT), which is part of the GIALT located on the gills, there were no significant changes after the first vaccination (10 DAV). Interestingly, however, after the second and third vaccinations, the ILT showed noticeable expansion and infiltration of white blood cells (Figure 7).
3.3.3. GALT
The results revealed no abnormal changes in the overall structure of the intestine or epithelial cells at 10 and 20 DAV, indicating the absence of significant histopathological alterations in the cells and tissues. No clinical signs or histopathological lesions were observed in the fish that received the nanovaccines compared with the control group. However, a slight increase in the number of goblet cells responsible for mucus production was noted in both the vaccinated and unvaccinated fish. Nevertheless, no significant differences were found compared to the control group (Figure 8A-L).
At 30 DAV, the fish intestine’s overall structure, epithelial cells, and goblet cells displayed no significant histopathological changes. However, a notable difference was observed in the number of goblet cells in all vaccinated groups, which exhibited a significant increase in these cells compared with the control group. Additionally, the intestine of vaccinated fish showed a thicker laminar propria layer than that of the control group (Figure 8P-8R).
3.4. Growth performance
The effect of the bivalent nanovaccines on the growth performance of vaccinated Asian seabass is shown in Table 3. After 30 DAV, no significant differences in growth parameters were measured, i.e., WG, ADG, SGR, and FCR, between the control and the treatment groups (p > 0.05).
3.5. Challenge tests with S. iniae and F. covae
The survival rates of Asian seabass immunized with bivalent nanovaccines in all groups, including groups 2, 3, and 4, were 62.15 ± 2.11, 60.00 ± 3.00, and 75.70 ± 3.36%, respectively, after challenge with S. iniae. These values were significantly higher than those of the control group (p < 0.05), with a cumulative survival rate of 44.44 ± 1.92% (Figure 9A). Similarly, the Asian seabass immunized with bivalent nanovaccines in all groups, including groups 2, 3 and 4, showed cumulative survival rates of 68.89 ± 3.85, 64.44 ± 5.09, and 77.78 ± 5.09%, respectively, after challenging the fish with F. covae, which were significantly higher than that of the control group (p < 0.05), with a cumulative survival of 50.00 ± 3.33% (Figure 9B).
4. Discussion
Intensive Asian seabass cultures are constantly faced with an increase in the risk of disease outbreaks, leading to a reduction in fish production and mass mortalities [23]. Streptococcosis and columnaris disease are common causes of disease outbreaks in many freshwater fish species worldwide, mainly in the Asian seabass culture in Thailand [5,24]. The efficacy of antibiotics and chemicals in managing these diseases is variable. Vaccination offers a pragmatic and trustworthy means of averting diseases in aquaculture, diminishing occurrences of mass mortality, and lessening the reliance on harmful antibiotics and chemicals [25,26]. These strategies have been commercially applied to many economic fish species [27]. In comparison to the vaccination routes, injection administration is the most effective regarding immune responses and protection levels [8]. However, this method is relatively unacceptable for commercial scales with high labor, high stress, mortality induction, booster vaccination difficulty during fish growth in grow-out ponds, cost requirements, etc. Therefore, immersion and oral vaccination have been developed to increase their efficacy in the fish aquaculture industry. These methods were previously found to exhibit low efficacy in protection but have been lately developed to meet an acceptable level important for industry. With the progress and development of nanotechnology, nanovaccines with mono- and bivalent characteristics have been effectively developed [12,28].
Therefore, the development of bivalent nanovaccines against S. iniae and F. covae was conducted in the present study to generate high efficacy in resistance to these two harmful pathogenic bacteria in Asian seabass larvae.
Based on the physical characteristics of S. iniae and F. covae nanovaccines, it was demonstrated that the average particle size and zeta potential were in line with the suitable and influential characteristics of nanovaccines that are smaller than 500 nm and have positive charges. The cationic biopolymer generated positively charged nanovaccines, enhancing adhesion on the mucosal surface and improving protection against diseases. These properties have been suggested as the optimal characteristics for effective and favorable vaccines to be efficiently absorbed by fish immune-associated organs or tissues [29].
Recently, mono- and bivalent nanovaccines have been developed to provide better immune responses and protection in various fish species, especially tilapia, from several fish pathogens, such as Aeromonas veronii [30], Flavobacterium oreochromis [12], Francisella orientalis [14], Streptococcus agalactiae [31,32,33] and tilapia lake virus [34,35,36]. These nanovaccines were formulated from various materials, including chitosan or its derivatives, alginate, nanoclay, halloysite nanotubes, and poly[(methyl methacrylate)-co-(methyl acrylate)-co-(methacrylic acid)]-poly(d,l-lactide-co-glycolide) (PMMMA-PLGA). With oral, immersion, and intramuscular injections, these nanovaccines effectively elevated protection from the pathogens mentioned above [37]. Of these, chitosan and its derivatives are low-cost and effective adjuvants, enhancing better immune responses such as immunostimulants [38,39].
To date, various monovalent nanovaccines have been reported in some fish species; in the present study, chitosan-based bivalent nanovaccines created from S. iniae and F. covae bacteria were further applied to fish larvae in the nursery farms of farmers via the first immersion and the other two immersions during routine water exchanges of larval stages and oral booster vaccination during fingerling periods, respectively. These combination methods have been reported to prevent stress conditions that may severely rise during or after excessive handling or overcrowding conditions in vaccination periods [40,41]. Our research intends to explore cost-effective, safe, practical, and high-efficacy vaccines for dual-preventing harmful pathogenic bacteria causing streptococcosis and columnaris diseases at Asian seabass farm scales.
Principally, total serum IgM is expected to be the first indicator used to demonstrate immune responses after vaccination, and nanovaccines were effectively surpassed to increase this target parameter [13]. The application of the current nanovaccines significantly increased serum IgM levels in the 3rd and 4th vaccinated groups at 10 and 30 DAVs, similar to bivalent mucoadhesive nanovaccines of Flavobacterium oreochromis (For) and Francisella orientalis (Fo) and monovalent nanovaccines of For and Fo, which showed significant IgM levels compared with the control group. However, when specific IgM to S. iniae or F. covae bacteria was measured, all nanovaccine-immunized groups showed significantly higher specific IgM than the unvaccinated control group at every DAV. In contrast to the previous study, total IgM levels of Nile tilapia in bivalent mucoadhesive nanovaccine For- and Fo-vaccinated groups were significantly suppressed compared with the For or Fo monovalent vaccines [12], suggesting that the bivalent S. iniae or F. covae nanovaccine is strong enough to enhance specific IgM levels against both S. iniae or F. covae.
Additionally, our findings showed that fish immunized with bivalent nanovaccines showed an increase in innate immune parameters, including lysozyme activity and bactericidal activity. This information agreed with the results from previous studies conducted by Bunnoy et al. [12], which showed that the application of bivalent mucoadhesive nanovaccines to prevent francisellosis and columnaris diseases in Nile tilapia also significantly elevated these innate immune-parameters against Francisella orientalis and Flavobacterium oreochromis, suggesting that the innate immune responses of fish are simultaneously induced by bivalent nanovaccine application. Furthermore, this vaccine effectively upregulates several immune-related genes, such as IgM, IgD, IgT, MHCIIα, TCRα, and CD4, which are key components of specific immune systems. This information was firmly in line with previous studies [12,14,28,42,43,44], suggesting that the bivalent nanovaccines in the current study are potent immunogens that vigorously drive the two arms of both innate and adaptive immune systems [12]. Particularly for IgM and IgT, almost all vaccinated treatments showed very high upregulation, indicating that two immersion and single booster vaccinations of the bivalent nanovaccines effectively enhance both systemic and local-specific immune responses. Similar responses were also observed in several monovalent nanovaccine experiments [14,18,30] and a bivalent nanovaccine [12].
Histopathology intensively supported the surpassing local immune responses found in the above information. Our study demonstrates that bivalent nanovaccines could permeate the skin and gills, the main organs targeted by the immersion vaccination method [45]. Interestingly, the interbranchial lymphoid tissue (ILT) in the gills of all vaccinated fish became elevated, with numerous immune-like cells related to the immune responses. However, this structure, the ILT, does not seem to have any equivalent among lymphoid tissues, and its function is still unknown, although it shares some properties with secondary lymphoid structures [46]. Additionally, an elevation of the epithelium layer was observed in the gills of the vaccinated fish. These changes have never been reported previously in immersion vaccination trials, but similar characteristics can be observed in previous experiments, where fish were exposed to various factors [47,48,49]. This mysterious observation requires further investigation.
Furthermore, histopathological changes in mucus cells on epithelial layers were also observed in the gut of all vaccinated fish after the second booster vaccination via oral administration at 30 DAV. This phenomenon strongly indicates that an effective oral vaccination route impacts immune responses in GALT, which is crucial for the local gastrointestinal tract resistant components of fish [50,51,52]. Unfortunately, no available literature dealing with vaccination in fish using bivalent nanovaccines can be used for comparative analysis.
In the current study, research on combined nanovaccines using Gram-positive and Gram-negative bacteria for Asian seabass or other fish species is still scarce. Therefore, there is insufficient data to clearly analyze and compare the mechanism of combined nanovaccine responses in Asian seabass in the current study. Further research is needed to address these gaps in the future. However, previous studies in other fish species, especially tilapia, have demonstrated positive results, indicating the high impacts of nanovaccine application with various practical administrations.
The high efficacy of chitosan-based nanovaccines indicated by the RPS or survival rate was found in various experiments. For example, chitosan-based monovalent nanovaccines against Aeromonas veronii [30], Flavobacterium columnare [13,18], Streptococcus agalactiae [31,32], and tilapia lake virus [35] immunized with oral or immersion vaccinations showed high RPS or survival ranging from 52.2-100%. In our results, chitosan-based bivalent nanovaccines showed survival rates of 60.00-75.70% for S. iniae challenge and 64.44-77.78% for F. covae testing, similar to those of previous reports. This result suggested that the chitosan-based bivalent nanovaccines, which were immunized via the combination of immersion and oral administration in Asian seabass larvae to fingerlings, led to an additive action, dually protecting Asian seabass from columnaris and streptococcosis effectively.
Considerably, some immune parameters of the vaccinated groups 2-4 differed at some periods, which may be effectively due to different management methods of workers and should be optimized and considered when establishing a vaccination strategy for nursery farms.
5. Conclusions
In the current study, developing a novel and innovative bivalent nanovaccine can potentially become one of the most effective methods for controlling pathogenic S. iniae and F. covae in Asian seabass. This nanovaccine enhances the efficacy of vaccination against streptococcosis and columnaris disease by boosting both innate and adaptive immunity, as indicated by innate immune parameters, elevated protein serum antibody levels of both total and specific IgM, upregulated gene expression related to immunity, and improved survival rates and disease resistance. The combined suitable and practical routes of immersion and oral vaccinations are less labor intensive, cost-effective, safe, practical, and high-efficacy vaccines and provide dual protection against streptococcosis and columnaris diseases at nursery Asian seabass farm scales, which are further crucial key roles and stepping stones for developing and sustaining the Asian seabass aquaculture industry worldwide.
Author Contributions
Conceptualization, P.S.; methodology, P.X.; validation, P.M., W.K., P.D., A.B. and K.N.; formal analysis, P.M.; investigation, P.S.; resources, P.M.; data curation, X.X. and A.B.; writing—original draft preparation, P.M.; writing—review and editing, P.S.; visualization, P.M.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Thai Union Feedmill Public Company Limited (Thailand) and the National Science and Technology Development Agency (NSTDA) for the partial financial support provided under the project code P-19-51469.
Acknowledgments
The authors would like to thank Thai Union Feedmill Public Company Limited (Thailand) and the National Science and Technology Development Agency (NSTDA) for the financial support provided and the scholarships awarded to the first author of the manuscript.
Conflicts of Interest
The authors declare that this research was conducted without any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Lawley, D. Repositioning Australian Farmed Barramundi: Online Consumer Survey Findings; University of Sunshine Coast, Sunshine Coast, QLd, 2010.
- Robinson, N.A.; Schipp, G.; Bosmans, J.; Jerry, D.R. Modelling selective breeding in protandrous, batch-reared Asian sea bass (Lates calcarifer, Bloch) using walkback selection. Aquac. Res. 2010, 41, e643–e655. [Google Scholar] [CrossRef]
- Vandeputte, M.; Labbé, L. Cultured aquatic species information programme. Salmo trutta. In Cultured Aquatic Species Fact Sheets; FAO Fisheries and Aquaculture Department: Rome, Italy, 2012. [Google Scholar]
- Declercq, A.M.; Haesebrouck, F.; Van den Broeck, W.; Bossier, P.; Decostere, A. Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Vet. Res. 2013, 44, 1–17. [Google Scholar] [CrossRef]
- Suanyuk, N.; Sukkasame, N.; Tanmark, N.; Yoshida, T.; Itami, T.; Thune, R.; Tantikitti, C.; Supamattaya, K. Streptococcus iniae infection in cultured Asian sea bass (Lates calcarifer) and red tilapia (Oreochromis sp.) in southern Thailand. Songklanakarin J. Sci. Technol. 2010, 32, 341–348. [Google Scholar]
- Tran Vi, H.; Dang Ha, Q.V.; Nguyen Huu, D.; Wergeland, H.I. Experimental Streptococcus iniae infection in barramundi (Lates calcarifer) cultured in Vietnam. Int. J. Aquatic Sci. 2013, 4, 3–12. [Google Scholar]
- Quesada, S.P.; Paschoal, J.A.R.; Reyes, F.G.R. Considerations on the aquaculture development and on the use of veterinary drugs: special issue for fluoroquinolones da review. J. Food Sci. 2013, 78, R1321–R33. [Google Scholar] [CrossRef]
- Vinitnantharat, S.; Gravningen, K.; Greger, E. Fish vaccines. Adv. Vet. Med. 1999, 41, 539–550. [Google Scholar]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- H. Mondal, J. Thomas, A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thompson, K.D.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Pirarat, N.; Kitiyodom, S.; Thangsunan, P.; Sukkarun, P.; Prukbenjakul, P.; Panthukumphol, N.; Morishita, M.; Srisapoome, P.; Rodkhum, C. Development of a bivalent mucoadhesive nanovaccine to prevent francisellosis and columnaris diseases in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 138, 108813. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Thompson, K.D.; Costa, J.; Elumalai, P.; Katagiri, T.; Temisak, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Immersion vaccination by a biomimetic-mucoadhesive nanovaccine induces humoral immune response of red tilapia (Oreochromis sp.) against Flavobacterium columnare challenge. Vaccines 2021, 9, 1253. [Google Scholar] [CrossRef]
- Thangsunan, P.; Kitiyodom, S.; Srisapoome, P.; Pirarat, N.; Yata, T.; Thangsunan, P.; Boonrungsiman, S.; Bunnoy, A.; Rodkhum, C. Novel development of cationic surfactant-based mucoadhesive nanovaccine for direct immersion vaccination against Francisella noatunensis subsp. orientalis in red tilapia (Oreochromis sp.). Fish Shellfish Immunol. 2022, 127, 1051–1060. [Google Scholar] [CrossRef]
- Angulo, C.; Tello-Olea, M.; Reyes-Becerril, M.; Monreal-Escalante, E.; Hernández-Adame, L.; Angulo, M.; Mazon-Suastegui, J.M. Developing oral nanovaccines for fish: A modern trend to fight infectious diseases. Rev. Aquac. 2021, 13, 1172–1192. [Google Scholar] [CrossRef]
- Kent, M.L.; Feist, S.W.; Harper, C.; Hoogstraten-Miller, S.; Mac Law, J.; Sanchez-Morgado, J.M.; Tanguay, R.L.; Sanders, G.E.; Spitsbergen, J.M.; Whipps, C.M. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp. Biochem. Physiol. 2009, 149, 240–248. [Google Scholar] [CrossRef]
- Tumree P.; Bunnoy A.; Tang X.; Srisapoome P. Efficacy of the whole-cell based monovalent and bivalent vaccines of Streptococcus iniae and Flavobacterium covae in fingering Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, (Under review).
- Kitiyodom, S.; Yata, T.; Yostawornkul, J.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Enhanced efficacy of immersion vaccination in tilapia against columnaris disease by chitosan-coated “pathogen-like” mucoadhesive nanovaccines. Fish Shellfish Immunol. 2019, 95, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 49, 86. [Google Scholar] [CrossRef]
- Ito, Y.; Yamada, H.; Imoto, T. Colorimetric assay for lysozyme using Micrococcus luteus labeled with a blue dye, remazol brilliant blue R, as a substrate. Chem. Pharm. Bull. 1992, 40, 1523–1526. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Probiotic effects of a novel strain, Acinetobacter KU011TH, on the growth performance, immune responses, and resistance against Aeromonas hydrophila of bighead catfish (Clarias macrocephalus Günther, 1864). Microorganisms 2019, 7, 613. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef]
- Chokmangmeepisarn, P.; Thangsunan, P.; Kayansamruaj, P.; Rodkhum, C. Resistome characterization of Flavobacterium columnare isolated from freshwater cultured Asian sea bass (Lates calcarifer) revealed diversity of quinolone resistance-associated genes. Aquaculture 2021, 544, 737149. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Leal, C.A.G.; Carvalho-Castro, G.A.; Sacchetin, P.S.C.; Lopes, C.O.; Moraes, A.M.; Figueiredo, H.C.P. Oral and parenteral vaccines against Flavobacterium columnare: evaluation of humoral immune response by ELISA and in vivo efficiency in Nile tilapia (Oreochromis niloticus). Aquac. Int. 2010, 18, 657–666. [Google Scholar] [CrossRef]
- Priya, T.J.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture-prospects and challenges. Vaccine 2022, 40, 5873–81. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Kitiyodom, S.; Srisapoome, P.; Rodkhum, C. Mucoadhesive cationic lipid-based Flavobacterium oreochromis nanoencapsulation enhanced the efficacy of mucoadhesive immersion vaccination against columnaris disease and strengthened immunity in Asian sea bass (Lates calcarifer). Fish Shellfish Immunol. 2022, 127, 633–646. [Google Scholar] [CrossRef]
- Mody, N.; Sharma, R.; Agrawal, U.; Vyas, S.P. Nanocarriers: A versatile approach for mucosal vaccine delivery. Ther. Deliv. 2015, 6, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Sukkarun, P.; Kitiyodom, S.; Yostawornkul, J.; Chaiin, P.; Yata, T.; Rodkhum, C.; Boonrungsiman, S.; Pirarat, N. Chitosan-polymer based nanovaccine as promising immersion vaccine against Aeromonas veronii challenge in red tilapia (Oreochromis sp.). Fish Shellfish Immunol. 2022, 129, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pumchan, A.; Sae-Ueng, U.; Prasittichai, C.; Sirisuay, S.; Areechon, N.; Unajak, S. A novel efficient piscine oral nano-vaccine delivery system: Modified halloysite nanotubes (HNTs) preventing streptococcosis disease in tilapia (Oreochromis sp.). Vaccines 2022, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Suwanbumrung, D.; Wongkhieo, S.; Keaswejjareansuk, W.; Dechbumroong, P.; Kamble, M.T.; Yata, T.; Kitiyodom, S.; Rodkhum, C.; Thompson, K.D.; Namdee, K.; Pirarat, N. Oral delivery of a Streptococcus agalactiae vaccine to Nile tilapia (Oreochromis niloticus) using a novel cationic-based nanoemulsion containing bile salts. Fish Shellfish Immunol. 2023, 139, 108913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Z.; Hu, C.; Bellis, S.L.; Yang, W.; Su, Y.; Zhang, X.; Wu, Y. Controlled and targeted release of antigens by the intelligent shell for improving the applicability of oral vaccines. Biomaterials 2016, 77, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-M.; Wei, X.-F.; Zhou, G.-Q.; Liu, M.-Z.; Li, P.-F.; Zhu, B. Mannose functionalized biomimetic nanovaccine enhances immune responses against tilapia lake virus. Aquaculture 2022, 560, 738535. [Google Scholar] [CrossRef]
- Ramos, E.A.; Relucio, J.L.V.; Torres-Villanueva, C.A.T. Gene expression in tilapia following oral delivery of chitosan-encapsulated plasmid DNA incorporated into fish feeds. Mar. Biotechnol. 2005, 7, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-F.; Gong, Y.-M.; Xia, J.-Y.; Liu, M.-Z.; Li, P.-F.; Wang, G.-X.; Zhu, B. Biomimetic nanovaccine based on erythrocyte membrane enhances immune response and protection against tilapia lake virus. Virology 2023, 580, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.D.; Rodkhum, C.; Bunnoy, A.; Thangsunan, P.; Kitiyodom, S.; Sukkarun, P.; Yostawornkul, J.; Yata, T. Addressing nanovaccine strategies for tilapia. Vaccines 2023, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Gupta, M.; Gupta, V.; Gogoi, H.; Bhatnagar, R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int. J. Nanomedicine 2018, 13, 7959–7970. [Google Scholar] [CrossRef]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-based nanomaterial as immune adjuvant and delivery carrier for vaccines. Vaccines (Basel) 2022, 10, 1906. [Google Scholar] [CrossRef]
- Plumb, J.A.; Vinitnantharat, S. Vaccination of channel catfish, Ictalurus punctatus (Rafinesque), by immersion and oral booster against Edwardsiella ictalurid. J. Fish Dis. 1993, 16, 65–71. [Google Scholar] [CrossRef]
- Thinh, N.; Kuo, T.; Hung, L.; Loc, T.; Chen, S.; Evensen, Ø.; Schuurman, H.J. Combined immersion and oral vaccination of Vietnamese catfish (Pangasianodon hypophthalmus) confers protection against mortality caused by Edwardsiella ictalurid. Fish Shellfish Immunol. 2009, 27, 773–776. [Google Scholar] [CrossRef]
- Dan, X.-M.; Zhang, T.-W.; Li, Y.-W.; Li, A.-X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Antigen uptake and expression of antigen presentation-related immune genes in flounder (Paralichthys olivaceus) after vaccination with an inactivated Edwardsiella tarda immersion vaccine, following hyperosmotic treatment. Fish Shellfish Immunol. 2016, 55, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Q.; Ni, C.; Li, S.; Wu, H.; Wang, Q.; Xiao, J.; Zhang, Y. Gene expression profiling in live attenuated Edwardsiella tarda vaccine immunized and challenged zebrafish: insights into the basic mechanisms of protection seen in immunized fish. Dev. Comp. Immunol. 2013, 40, 132–141. [Google Scholar] [CrossRef]
- Soto E.; Griffin M.J.; Tobar J.A. Mucosal vaccines, In Mucosal Health in Aquaculture, Beck B.H.; Peatman E., Eds.; Academic Press, San Diego, 2015, pp. 297-323.
- Aas, I.B.; Austbø, L.; König, M.; Syed, M.; Falk, K.; Hordvik, I.; Koppang, E.O. Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J. Immunol. 2014, 193, 3463–3469. [Google Scholar] [CrossRef]
- Haaparanta, A.; Valtonen, E.; Hoffmann, R.W. Gill anomalies of perch and roach from four lakes differing in water quality. J. Fish Biol. 1997, 50, 575–591. [Google Scholar] [CrossRef]
- Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Monteiro, S.; Cortes, R.; Pacheco, F.; Fernandes, L.S. Seasonal effect of land use management on gill histopathology of Barbel and Douro Nase in a Portuguese watershed. Sci. Total Environ. 2021, 764, 142869. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, M.; Köllner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.-J.; Fichtner, D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Korbut, R.; Mehrdana, F.; Kania, P.W.; Larsen, M.H.; Frees, D.; Dalsgaard, I.; Jørgensen, L.V.G. Antigen uptake during different life stages of zebrafish (Danio rerio) using a GFP-tagged Yersinia ruckeri. PLoS One 2016, 11, e0158968. [Google Scholar] [CrossRef]
- Ohtani, M.; Villumsen, K.R.; Koppang, E.O.; Raida, M.K. Global 3D imaging of Yersinia ruckeri bacterin uptake in rainbow trout fry. PLoS One 2015, 10, e0117263. [Google Scholar] [CrossRef]
Figure 1.
Total IgM (A) and IgM specific to S. iniae (B) and F. covae (C) in Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 1.
Total IgM (A) and IgM specific to S. iniae (B) and F. covae (C) in Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).

Figure 2.
Lysozyme activity (A) and bactericidal activity (B) of Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 2.
Lysozyme activity (A) and bactericidal activity (B) of Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).

Figure 3.
Relative gene expression levels of specific immune-related genes, including IgM (A), IgT (B), IgD (C), MHCIIα (D), TCRα (E), and CD4 (F), in the whole body of Asian seabass vaccinated with bivalent nanovaccines at 10, 20 and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05) or *** (p < 0.001).
Figure 3.
Relative gene expression levels of specific immune-related genes, including IgM (A), IgT (B), IgD (C), MHCIIα (D), TCRα (E), and CD4 (F), in the whole body of Asian seabass vaccinated with bivalent nanovaccines at 10, 20 and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05) or *** (p < 0.001).

Figure 4.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Llp: lateral line tubules, Mu: muscle (H&E staining 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 4.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Llp: lateral line tubules, Mu: muscle (H&E staining 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).

Figure 5.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Sc: scale, Hy: hypodermis layer, De: dermis, Mu: muscle (H&E staining, 100× magnification).
Figure 5.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Sc: scale, Hy: hypodermis layer, De: dermis, Mu: muscle (H&E staining, 100× magnification).

Figure 6.
Histopathological changes in the gills of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; PL: Primary lamella, SL: Secondary lamella, Ep: Epithelium and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 6.
Histopathological changes in the gills of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; PL: Primary lamella, SL: Secondary lamella, Ep: Epithelium and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).

Figure 7.
Histopathological changes in the gills, focusing on interbranchial lymphoid tissues (ILTs), of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Black arrow: enlarged and cumulative immune-like cells of the ILTs (H&E staining, 100× magnification).
Figure 7.
Histopathological changes in the gills, focusing on interbranchial lymphoid tissues (ILTs), of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Black arrow: enlarged and cumulative immune-like cells of the ILTs (H&E staining, 100× magnification).
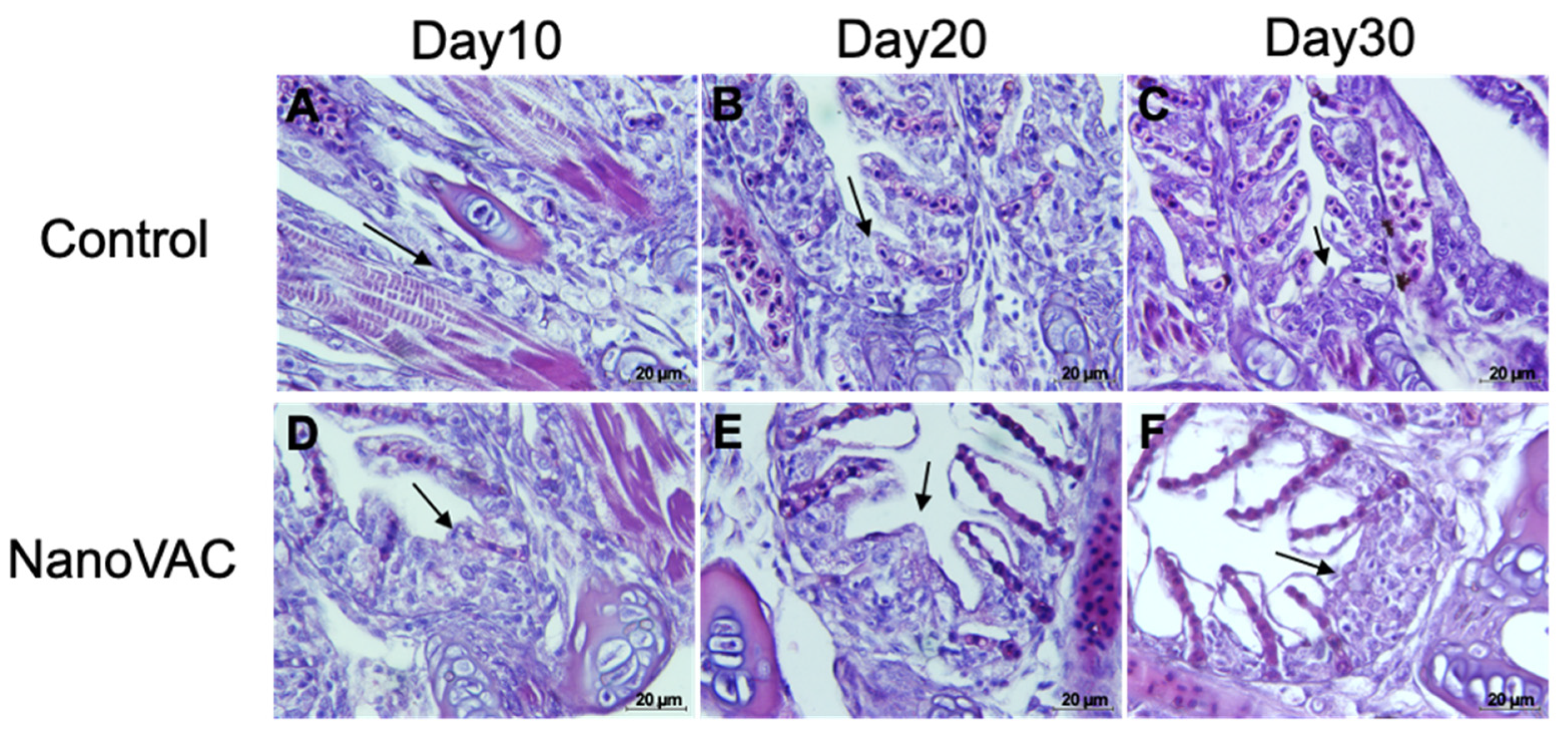
Figure 8.
Histopathological changes in the gut of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; LU: Lumen, Ep: Epithelium, Lp: Laminar propria and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 8.
Histopathological changes in the gut of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; LU: Lumen, Ep: Epithelium, Lp: Laminar propria and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).

Figure 9.
Survival analysis of Asian seabass vaccinated with bivalent nanovaccines after challenge with the single pathogens S. iniae (A) and F. covae (B). Data and survival plots were generated using the Kaplan‒Meier method. The levels of statistical significance between the control and treatment groups are indicated by * (p < 0.05) (n = 30).
Figure 9.
Survival analysis of Asian seabass vaccinated with bivalent nanovaccines after challenge with the single pathogens S. iniae (A) and F. covae (B). Data and survival plots were generated using the Kaplan‒Meier method. The levels of statistical significance between the control and treatment groups are indicated by * (p < 0.05) (n = 30).

Table 1.
Primers used in this study for determining immune-related gene expression.
| Primer names | Genes | Nucleotide sequences (5’→3′) | Annealing temperature (oC) | Product size (bp) | References |
|---|---|---|---|---|---|
| Lc_β-actin | β-actin | F-5′-TACCCCATTGAGCACGGTATTG-3′ R-5′-TCTGGGTCATCTTCTCCCTGTT-3′ |
60 | 160 | XM_018667666.1 |
| Lc_IgM | Immunoglobulin M (IgM) (secreted form) |
F-5′-TGTCAAGGTAAACGAGGGAGC-3′ R-5′-TCCCCTGGATCCATTCGTCA-3′ |
60 | 152 | ASM164080v1 |
| Lc_IgT | Immunoglobulin T (IgT) (secreted form) |
F-5′-GAGGCAACTTACAGAGGAACCATA-3′ R-5′-CTGGTCACTTCTCCATCAATTTCC-3′ |
60 | 194 | ASM164080v1 |
| Lc_IgD | Immunoglobulin D (IgD) (membrane-bound form) |
F-5′-GAGTGTGAATGTTGCTGGGC -3′ R-5′-TTGGCCTGAAAGGTGACGTA -3′ |
60 | 150 | ASM164080v1 |
| Lc_CD4 | CD4 receptor (CD4) | F-5′-AGTGCAATGGATTGGGGTAGATAA-3′ R-5′-GTTGCAGGCTCTGTAACTTTGATT-3′ |
60 | 156 |
XM_018672258 |
| Lc_TCRα | T-cell receptor alpha (TCRα) | F-5′-GGCCGTTCGGATAGAAGGAG-3′ R-5′-AGAGCCATTGTGTTCACCGT-3′ |
60 | 153 | ASM164080v1 |
| Lc_MHCIIα | Major histocompatibility complex class IIα (MHCIIα) | F-5′-TTCCTACCTCCCTGATCTACCC-3′ R-5′-CTGAAGTCGCTGTTGGAGTAGT-3′ |
60 | 178 | ASM164080v1 |
Table 2.
Characterization of S. iniae and F. covae nanovaccines.
| Bacterial culture | Formulation | Average diameter (nm) | Zeta potential (mV) |
|---|---|---|---|
| Streptococcus iniae | Sonicated antigen (bacterial cells) | 203 ± 10 | -36.87 ± 0.93 |
| Polymeric nanovaccine (solution form) | 246 ± 16 | 45.39 ± 1.31 | |
| Polymeric nanovaccine (dry form) | 304 ± 25 | 47.60 ± 0.96 | |
| Flavobacterium covae | Sonicated antigen (bacterial cells) | 324 ± 6 | -21.86 ± 0.89 |
| Polymeric nanovaccine (solution form) | 394 ± 14 | 38.25 ± 1.06 | |
| Polymeric nanovaccine (dry form) | 426 ± 18 | 36.48 ± 1.02 |
Table 3.
Growth performance of Asian seabass 30 days after vaccination with bivalent nanovaccines (DAV).
Table 3.
Growth performance of Asian seabass 30 days after vaccination with bivalent nanovaccines (DAV).
| Growth parameters | Treatments | |||
|---|---|---|---|---|
| Control | NanoVAC-G2 | NanoVAC-G3 | NanoVAC-G4 | |
| Weight gain (WG, g) | 0.542 ± 0.16 | 0.545 ± 0.17 | 0.552 ± 0.12 | 0.544 ± 0.15 |
| Specific growth rate (SGR, %/day) | 7.18 ± 0.75 | 7.18 ± 0.72 | 7.24 ± 0.79 | 7.19 ± 0.74 |
| Average daily gain (ADG, g/fish/day) | 0.0181 ± 0.0016 | 0.0182 ± 0.0018 | 0.0184 ± 0.0014 | 0.0181 ± 0.0011 |
| Feed conversion ratio (FCR) | 1.85 ± 0.44 | 1.83 ± 0.37 | 1.81 ± 0.39 | 1.84 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Development of Immersion and Oral Bivalent Nanovaccines for Streptococcosis and Columnaris Disease Prevention in Fry and Fingerling Asian Seabass (Lates calcarifer) Nursery Farms
Pakapon Meachasompop
et al.
,
2023
First Investigation of the Optimal Vaccination Timing for Nile Tilapia (Oreochromis niloticus) Larvae against Streptococcus agalactiae
Benchawan Kumwan
et al.
,
2023
RBD-Adjuvanted Preparation: Evaluation of IgG and IgM Antibodies for One Year and Cross-Reactivity with Omicron Variant in Mice
Gabrielle Gimenes Lima
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated











