January 17, 2025 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Genetically modified mice hint at tau hyperphosphorylation's early role in neurodegenerative diseases
Tau is a microtubule-associated protein that helps to stabilize the structure of neurons, specifically by supporting microtubules, cylindrical structures that contribute to cell motility, intracellular transport and the maintenance of a cell's shape over time. While tau has an important neurophysiological function, when it undergoes pathological changes and accumulates in the brain, this protein has been found to contribute to some neurodegenerative diseases, broadly defined as tauopathies.
Specifically, past research linked the levels of tau in the brains of patients diagnosed with Alzheimer's disease and frontotemporal dementia to the extent of their neuronal dysfunction and neurodegeneration. While the association between abnormal tau accumulation and neurodegeneration is well-established, the precise processes leading to tau-mediated toxicity in the brains of patients diagnosed with tauopathies are not yet fully understood.
One reason why uncovering these processes has proved difficult is existing methods do not allow neuroscientists to safely examine them in living humans. In addition, animal models that closely mirror the initiation and progression of human tauopathies have so far been lacking.
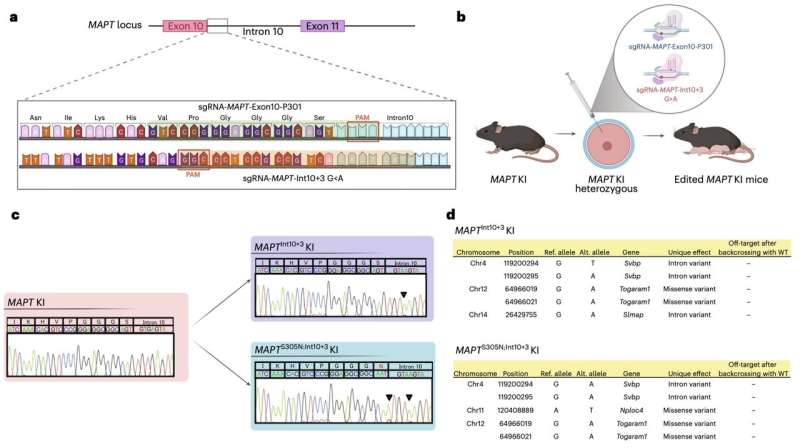
Researchers at the RIKEN Center for Brain Science, the UK Dementia Research Institute at University College London and other institutes recently introduced new genetically modified mouse models that express humanized versions of MAPT, a gene known to encode the tau protein. Using these models, outlined in a paper published in Nature Neuroscience, the researchers gathered new insight suggesting that an alteration known as tau hyperphosphorylation plays a key role in the early stages of tau pathology.
"Tau pathology is a hallmark of several neurodegenerative diseases, including frontotemporal dementia and Alzheimer's disease," Naoto Watamura, Martha S. Foiani and their colleagues wrote in their paper.
"However, the sequence of events and the form of tau that confers toxicity are still unclear, due in large part to the lack of physiological models of tauopathy initiation and progression in which to test hypotheses. We have developed a series of targeted mice expressing frontotemporal-dementia-causing mutations in the humanized MAPT gene to investigate the earliest stages of tauopathy."
Tau hyperphosphorylation occurs when phosphate groups attach to tau molecules, altering and disrupting their normal functioning. Watamura, Foiani and their colleagues genetically altered mice brains to produce pathological changes that resemble those observed in humans affected by tauopathies.
They mutated mice genes associated with frontotemporal dementia, specifically two lines of the MAPT gene. Subsequently, they looked at how tau hyperphosphorylation influenced pathological changes in the mice.
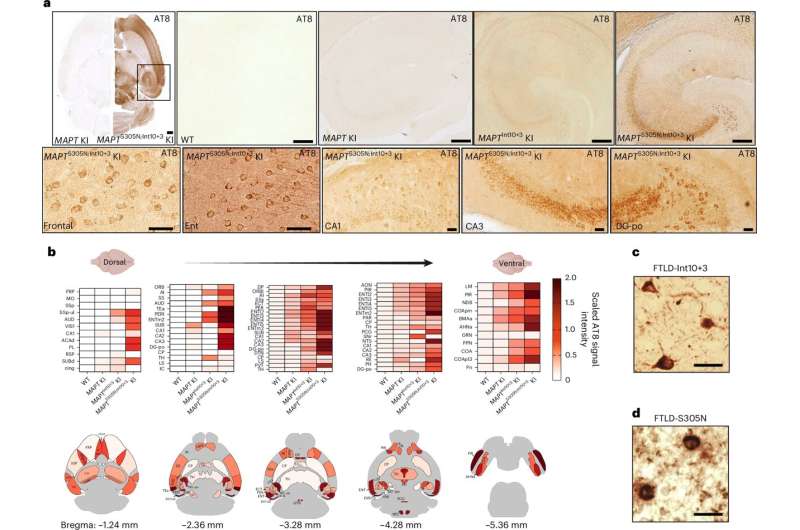
"MAPTInt10+3G>A and MAPTS305N;Int10+3G>A lines show abundant hyperphosphorylated tau in the hippocampus and entorhinal cortex, but they do not develop seed-competent fibrillar structures," wrote Watamura, Foiani and their colleagues. "Accumulation of hyperphosphorylated tau was accompanied by neurite degeneration, loss of viable synapses and indicators of behavioral abnormalities."
The researchers observed that hyperphosphorylated tau accumulated in key regions of the mice's brains, prompting a loss of synapses and neurodegeneration. Overall, these findings suggest that tau hyperphosphorylation could play a key role in the early stages of tauopathies.
"Our results demonstrate that neuronal toxicity can occur in the absence of fibrillar, higher-order structures and that tau hyperphosphorylation is probably involved in the earliest etiological events in tauopathies showing isoform ratio imbalance," wrote the researchers.
The results of this recent study and the mouse model it introduced could soon pave the way for further research examining the link between tau hyperphosphorylation and early tau pathology. By shedding new light on the early mechanisms of Alzheimer's disease, frontotemporal dementia and other tauopathies, these efforts could help to identify promising therapeutic targets for preventing or slowing down neurodegeneration.
More information: Naoto Watamura et al, In vivo hyperphosphorylation of tau is associated with synaptic loss and behavioral abnormalities in the absence of tau seeds, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01829-7.
© 2025 Science X Network


















