Get the free forming ions worksheet
Get, Create, Make and Sign ion formation worksheet

Editing formation of ions pdf online
How to fill out formation of ions worksheet with answers

Who needs formation of ions worksheet?
Understanding the formation of ions worksheet: A comprehensive exploration
The science behind ions: An overview
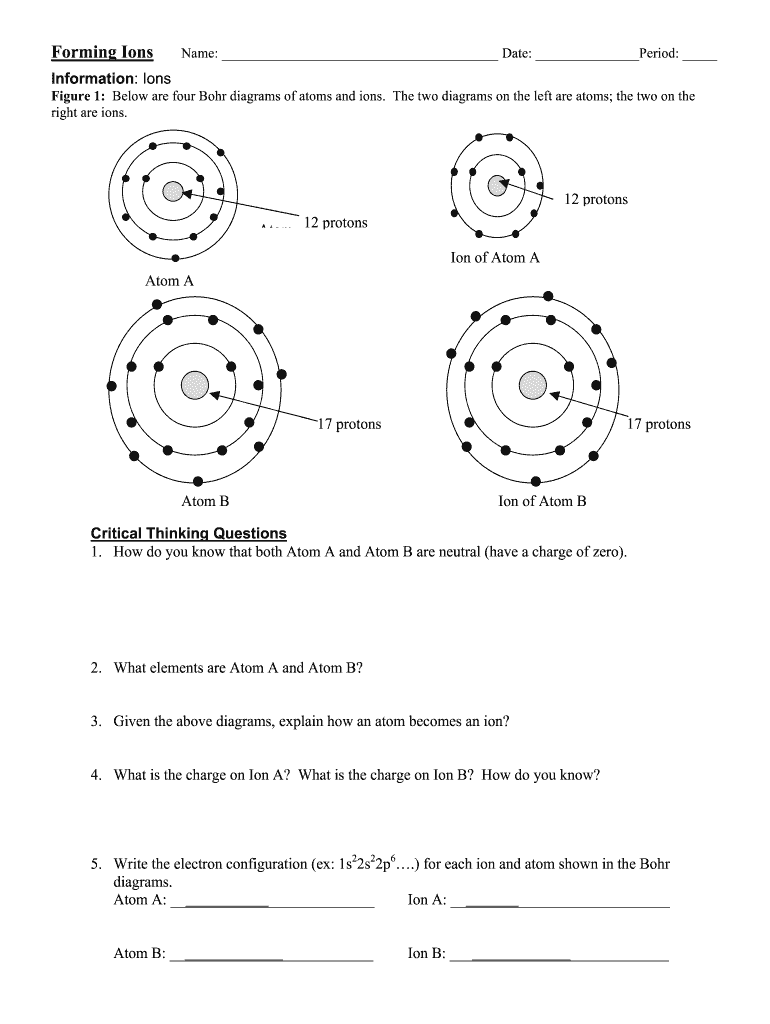
Ions play a crucial role in the fields of chemistry and biology, representing charged particles that arise when atoms gain or lose electrons. There are two main types of ions: cations and anions. Cations are positively charged ions formed when an atom loses one or more electrons, while anions are negatively charged ions that result from an atom gaining electrons. For instance, sodium (Na) typically forms a cation Na⁺, while chlorine (Cl) forms an anion Cl⁻. This fundamental understanding of ions is essential for exploring their behavior and interactions in various chemical processes.
The formation of ions involves several processes largely defined by the principles of atom structure and electron arrangement. When an atom loses an electron, it becomes positively charged due to having more protons than electrons. Conversely, when an atom gains an electron, it becomes negatively charged, resulting in an anion. Ionization processes can be found across various real-world applications, from the salt in our food (NaCl) to the minerals in our environment, making the study of ions profoundly relevant.
Structure and components of the worksheet
Creating an effective formation of ions worksheet involves meticulous attention to its structure and presentation. A well-designed worksheet should have a clear visual layout, often using mediaboxes to group relevant information. This organization helps in ensuring that students can easily navigate through the material without feeling overwhelmed. With clarity at the forefront, educators can effectively present complex ideas regarding ion formation and facilitate student engagement.
Incorporating interactive elements into the worksheet can significantly enhance the learning experience. For instance, using various layouts can cater to different learning styles, allowing students to explore ion formation in a way that resonates with them. Moreover, providing supplementary materials—like handouts with infographics and charts—can reinforce key concepts and aid in visual learning. Links to curated external resources can also offer students an avenue for deeper engagement, allowing for exploration beyond the classroom.
In-depth analysis of ion formation concepts
A vital aspect of understanding the formation of ions is grasping electron configurations and their influence on stability. The arrangement of electrons, particularly the valence electrons, determines how easily an atom can form an ion. These electrons exist in the outermost shell and are responsible for an atom's reactivity. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration equivalent to that of noble gases. This principle is central to why certain elements readily form ions while others do not.
Ionization can occur through various techniques, including chemical and thermal ionization processes. Chemical ionization involves reactions with other elements, whereby an atom interacts with species that can donate or accept electrons. On the other hand, thermal ionization refers to the changes that occur when an atom absorbs significant energy, leading to alterations in atomic structure. Factors such as electronegativity and environmental conditions like temperature and pressure also play crucial roles in influencing these ion formation processes.
Engaging activities on the worksheet
Engaging students through interactive exercises is essential for reinforcing the concepts of ion formation. A fill-in-the-blanks activity that focuses on key definitions and examples can help solidify understanding. Additionally, a matching columns exercise allows learners to pair different ions with their respective charges and electron configurations. These activities not only promote active participation but also facilitate retention of information, making lessons more effective.
Moreover, incorporating problem-solving scenarios invites students to apply theoretical concepts to practical chemistry problems involving ion formation. These real-world applications can ignite interest and demonstrate the relevance of ions in daily life. Guided questions such as reflecting on the implications of ion formation in everyday scenarios encourage critical thinking. Predictive scenarios also provide a platform for exploring how changes in external conditions can affect ion stability, fostering a deeper understanding of the topic.
Utilizing pdfFiller for effective document management
As educators prepare formation of ions worksheets, using tools like pdfFiller can streamline their document management process. The platform allows users to securely share these educational materials, making it easy for students to access and submit completed worksheets without hassle. The collaborative tools offered by pdfFiller enhance interactivity, enabling teachers to leave annotations and comments directly on the worksheets, fostering an environment of real-time feedback and support.
Additionally, pdfFiller provides online editing tools that allow educators to make modifications as needed, ensuring that the materials remain current and relevant. Customization options are particularly beneficial as they enable the creation of worksheets tailored to address specific learning needs. This adaptability promotes inclusivity and ensures every student can grasp the complexities of ion formation. Moreover, pdfFiller integrates seamlessly with other educational resources, creating a holistic learning experience.
The role of technology in learning about ions
Incorporating technology into education has transformed the way students learn about complex topics such as the formation of ions. Digital worksheets offer numerous advantages over traditional materials, making it easier for students to engage with the content. They provide interactive features that can help visualize intricate concepts, enhancing understanding. Moreover, technology can foster an immersive learning environment that caters to varied learning preferences.
Innovations in chemistry education have paved the way for advanced formats that articulate ideas formerly confined to textbooks. Incorporating visual aids within digital resources can clarify ion structures and behaviors, leading to a more comprehensive grasp of the subject matter. Utilizing multimedia elements can also create opportunities for students to engage with ions through simulations or videos, enhancing their educational experience. Adopting technology in chemistry fosters a learning atmosphere that is both engaging and effective.
Future of chemistry worksheets and education
Looking ahead, the future of chemistry education, especially topics like the formation of ions, is bound to evolve. Current trends in STEM education emphasize hands-on, experiential learning and real-world applications, encouraging students to grasp the concept's relevance. This shift represents a growing recognition of the significance of ion formation in various scientific fields, demonstrating its applicability in biology, medicine, and environmental science.
The importance of feedback in developing worksheets cannot be understated. Continuous updates and revisions based on student performance data are vital for ensuring that educational materials remain effective and relevant. The global perspective on chemistry education presents an opportunity to analyze and compare different educational systems, revealing diverse teaching methods and approaches. Such an analysis can contribute to improving how ion formation is introduced and taught across various learning contexts, engaging students on a broader scale.
Assessing learning outcomes: Measuring effectiveness
Effectively measuring students' understanding of ion formation requires robust evaluation techniques. Completing worksheets is a significant indicator of mastery over the topics covered. Educators should establish clear criteria for assessing comprehension and providing constructive feedback that encourages growth. This systematic approach helps identify areas where additional support may be needed, ensuring that all students can effectively engage with the material.
Feedback mechanisms play a crucial role in continuous improvement within the educational context. Collecting student insights regarding their experiences with worksheets can direct future refinements to the materials provided. Adaptive learning paths also enhance this process by allowing educators to customize follow-up material based on assessment outcomes. This approach ensures that students receive the necessary support tailored to their learning pace and style.
The importance of ion formation in broader scientific contexts
Ion formation holds significance beyond chemistry and is intertwined with various broader scientific fields. In biology, for instance, ions are vital in processes such as nerve impulse transmission and muscle contraction. The role of ions in physiological functions makes it essential to explore their formation and interactions further. Similarly, in medicine, understanding ion balance is crucial for treatments involving electrolyte solutions and intravenous therapies.
Environmental science also relies heavily on the understanding of ions, especially in contexts such as water quality assessments and climate change impacts. Case studies exploring ion formation can reveal valuable insights into research applications, demonstrating how ions influence ecological interactions and chemical reactions in the environment. Therefore, promoting an understanding of ion formation in diverse scientific contexts fosters a more holistic educational experience for students.
Fill formation of ions worksheet answer key : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Fill out your forming ions worksheet online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.