Preprint
Article
GDF15 promotes the Osteogenic Cell Fate of Periodontal Ligament Fibroblasts affecting their Mechanobiological Response
Altmetrics
Downloads
116
Views
38
Comments
0
A peer-reviewed article of this preprint also exists.
Submitted:
28 April 2023
Posted:
09 May 2023
You are already at the latest version
Alerts
Abstract
Periodontal ligament fibroblasts (PdLFs) exert important functions in oral tissue and bone remodeling following mechanical forces, which are specifically applied during orthodontic tooth movement (OTM). Located between the teeth and the alveolar bone, mechanical stress activates the mechanomodulatory functions of PdLFs including the regulation of local inflammation and activation of further bone-remodeling cells. Previous studies suggested the growth differentiation factor 15 (GDF15) as important pro-inflammatory regulator during the PdLFs mechanoresponse. However, the precise mechanism remains to be clarified, as GDF15 may act both intracrine and by receptor binding, potentially also in an autocrine manner. The extent to which PdLFs are susceptible to extracellular GDF15 has not yet been investigated. Thus, our study aims to examine the influence of GDF15 exposure on cellular properties of PdLFs and their mechanoresponse, which seems particularly relevant regarding disease- and aging-associated elevated GDF15 serum levels. Therefore, in addition to investigating potential GDF15 receptors, we analyzed its impact on proliferation, survival, senescence, and differentiation of human PdLFs, demonstrating a pro-osteogenic effect upon long-term stimulation. Furthermore, we detected an altered force-related inflammation and impaired osteoclast differentiation. Overall, our data suggest a major impact of extracellular GDF15 on PdLFs differentiation and their mechanoresponse.
Keywords:
Subject: Biology and Life Sciences - Life Sciences
1. Introduction
Orthodontic treatment of tooth malocclusions aims to improve and prevent medically relevant diseases of the teeth and supporting tissues such as the periodontium [1]. Not only highly prevalent in relation to the development of caries and periodontal inflammatory diseases, malocclusions and niches resulting from crowding of teeth may also lead to root recession and tooth loss in the long term [2,3]. As prophylactic therapy, orthodontic tooth movement (OTM) plays an important role in limiting the development and progression of these diseases. However, orthodontic therapy is associated with risks, including tooth root resorption, attachment or even tooth loss [4,5,6]. The underlying causes of increased occurrence of these risks appear to be multifactorial. In addition to genetic and epigenetic variations, changes in oral and systemic health as well as treatment-specific conditions are particularly reported [7,8]. In particular, extrinsic factors including hormones and growth factors also appear to influence the mechanofunctional remodeling processes of tissue and bone required for tooth movement [9].
The periodontal ligament (PdL) is a fibrous connective tissue located around the tooth root that serves to anchor the tooth in the alveolar bone and modulates the remodeling processes of tissue and bone induced by orthodontic forces leading to tooth movement [10]. The heterogeneous tissue includes diverse cell populations such as fibroblasts, osteoblasts, epithelial cell remnants of Malassez, macrophages and undifferentiated mesenchymal stem cells [11]. The predominant cell type are PdL fibroblasts (PdLFs), which, however, are characterized by many osteoblast-like properties as they express osteogenic markers like alkaline phosphatase and osteocalcin and are able to form mineral-like nodules in vitro [12,13,14].
During OTM, mechanical stimuli in form of tensile and compressive strains are generated, which trigger force-specific mechanobiological functions of PdLFs fostering a favorable microenvironment for tissue and bone remodeling [15,16,17]. This includes the modulation of aseptic transient inflammatory responses by specific pro- and anti-inflammatory cytokines and the activation of bone-remodeling cells. In this context, the RANKL/RANK/OPG signaling pathway is particularly crucial as it modulates osteoclast differentiation and activity [18,19,20,21]. In response to specific stimuli, receptor activator of NF-kB ligand (RANKL) can be secreted by cells of osteogenic origin, stimulating the differentiation of osteoclast precursors by binding to their transmembrane-receptor RANK. Osteoclastogenesis can be blocked by binding of RANKL to the decoy receptor osteoprotegrin (OPG), which can be also secreted by osteoblast-like cells. Unfavorable RANKL/OPG values are not only relevant in terms of bone remodeling defects, but also in root resorption as well as attachment and tooth loss [22].
Tensile forces particularly promote bone formation by increasing secretion of the anti-inflammatory interleukin IL-10 and stimulation of osteoblast differentiation [23,24]. Due to the intrinsic potential of PdL fibroblasts to differentiate into osteoblasts, increased expression of osteogenic markers such as alkaline phosphatase (ALP) and runt-related transcription factor 2 (RUNX2) as well as increased calcium deposits were detected in this context [25,26]. Contrary, compression promotes a bone-resorbing microenvironment by inducing hypoxia and the secretion of pro-inflammatory cytokines including IL-6, IL-8 and prostagladine E2 (PGE2), as well as RANKL by PdLFs [27,28,29,30].
As stress-dependently expressed and secreted multifunctional regulator [31,32], we recently identified growth differentiation factor 15 (GDF15) as important pro-inflammatory modulator in the mechanoresponse of PdLFs to compressive forces [33,34]. As member of the transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP) superfamily [31], GDF15 exerts important functions in the regulation of various cellular processes [32,35]. Synthesized as precursor protein, GDF15 undergoes disulfide-linked dimerization prior to secretion [36]. Extracellular GDF15 can bind to cell membrane receptors, with the α-like receptor of the GDNF family (GFRAL) being the best characterized receptor exclusively expressed in the central nervous system [37]. Additionally, it has been reported that members of the activin receptor-like kinase (ALK) family also bind GDF15 and mediate autocrine and paracrine functions [38,39,40]. Besides receptor-mediated signaling of GDF15, unprocessed GDF15 has also been reported to modulate gene expression after translocation into the nucleus [41,42].
GDF15 serum levels are highly elevated in association with various diseases as well as aging and have been implicated as potential biomarkers [43,44]. Considering its crucial role in the mechanoresponse of PdL fibroblasts [34,45], prolonged exposure to GDF15 could impair their functions and thus increase the risks for these patients in the context of orthodontic therapy. This seems particularly relevant considering that GDF15 has been shown to modulate osteoblast differentiation and function [46,47], thereby potentially also altering cell fate and mechanoresponse of PdLFs. Therefore, this study aims to investigate the effect of an extended GDF15 exposure on the cellular properties and mechanobiological functions of PdL fibroblasts.
2. Results
2.1. hPdLFs express potential receptors for GDF15
Considering the neuron-specific expression of the best-characterized GDF15 receptor GFRAL, members of the ALK protein family are discussed as possible receptors in other cell types [38,39,40]. We analyzed their expression in hPdLFs by quantitative PCR indicating gene expression of all family members (ALK1-7; Figure 1a). Western Blot analysis validated the expression of all members of the ALK family on protein levels (Figure 1b). Thus, extracellular GDF15 might potentially also stimulates PdL fibroblasts by ALK binding.
2.2. GDF15 exposure limits cell proliferation without affecting the survival of hPdLFs
To evaluate the impact of GDF15 on hPdLFs, we first analyzed metabolic activity after 12, 24, and 36 days of stimulation with 5 ng/ml and 20 ng/ml recombinant human GDF15 protein (rhGDF15; Figure 1c). A 12-day stimulation already resulted in reduced metabolic activity, which was also maintained with longer stimulation duration. Since changes in metabolic activity may be based on differences in cell number and thus proliferation level, we subsequently determined cell density (Figure 1d, e). After 12 days of stimulation, a significant decrease in cell density was observed, which became even more pronounced with increasing duration of exposure, but independent of the applied concentration of rhGDF15. To determine the proportion of proliferative cells, immunofluorescence staining of the proliferation marker Ki-67 was performed after 12, 24, and 36 days of rhGDF15 exposure (Figure 1d, f). Under all conditions, a decreasing proportion of Ki67-positive cells was observed over the culture period, resulting from either growth senescence due to limited space or induction of differentiation programs. However, this decrease was significantly stronger in rhGDF15-stimulated hPdLFs resulting in the lowest numbers of Ki-67-positive cells after 36 days of exposure compared to the control. A significant difference between both concentrations of the applied rhGDF15 could not be detected at any of the analyzed time points. Since GDF15 was reported to effect cell survival, we additionally analyzed the proportion of damaged cells using trypan blue staining (Figure 1g) and apoptosis using TUNEL assay (Figure 1h, i), respectively. However, we neither detected changes in cell survival in relation to culture duration nor caused by exposure to rhGDF15. Together, GDF15 limits the proliferative capacity of hPdLFs without affecting cell survival.
2.3. Long-term exposure to GDF15 promotes osteogenic cell fate of hPdLFs
The reduced proliferation of hPdLFs could be caused by increased cellular senescence or osteogenic differentiation, which are both potentially affected by GDF15, as previously shown in other studies [48,49]. Quantitative analysis of the immunofluorescent staining intensity of p21, an early senescence marker, revealed significantly higher expression levels after 12 and 24 days of rhGDF15 stimulation at both concentrations (Figure 2a, b). However, this increase could not be detected on the latest stimulation point, where p21 levels were not significantly different from the corresponding control. These findings were confirmed by analysis of β-galactosidase activity (Figure 2c, d). Thus, our data suggest enhanced activation of cellular senescence after 12 days of stimulation with rhGDF15, but not with prolonged exposure.
In the following, we aimed to address the osteogenic differentiation and potential alterations by GDF15, which may be a considerable reason for the diminished proliferative capacity of stimulated hPdLFs apart from senescence. To this end, quantitative expression analysis of the osteogenic marker genes ALPL and RUNX2 were performed and showed increased levels after 12 and 24 days of rhGDF15 exposure (Figure 3a, b). However, compared to the corresponding control, no difference in osteogenic gene expression was detected at the last time point analyzed. Further analysis of alkaline phosphatase activity as a characteristic of osteogenic differentiation, clearly showed enhanced levels after 36 days of rhGDF15 exposure (Figure 3c, d), while no significant changes were detected at earlier time points (data not shown; p-values < 0.05). We used hPdLFs stimulated with dexamethasone and β-glycerol phosphate for 36 days as differentiation control [50].
Considering that ALP activity is critical for mineralization, we further analyzed calcium deposit formation as a marker of active osteoblasts at 36 days of rhGDF15 stimulation (Figure 3e, f). Likewise as for ALP activity, a slightly increased formation of mineralized deposits was observed upon long-term stimulation with rhGDF15, which also appeared to be concentration-independent. However, the increase was significantly higher for the osteogenic differentiation control.
Taken together, our data suggest that GDF15 promotes osteogenic differentiation of hPdLFs upon long-term exposure, whereas cellular senescence is more prominent with shorter stimulation periods.
2.4. Preceding long-term exposure of hPdLFs to rhGDF15 affect their mechanoreactivity
In the following, we aimed to determine the extent to which the shift towards an osteogenic cell fate by rhGDF15 exposure affects the functionality of hPdLFs responding to mechanical stimuli. To this end, we first applied biaxial tensile force for 24 hours on hPdLFs stimulated for 36 days with rhGDF15. Subsequent analysis of genes encoding the anti-inflammatory markers IL-10 (IL10; Figure 4a) and IL-1RA (IL1RN; Figure 4b), which are increased in hPdLFs following tensile forces [51], revealed no relevant changes due to rhGDF15 exposure. Even though the expression of IL1RN appears increased in GDF15-stimulated fibroblasts after tensile force, the baseline levels (red lines) are already enhanced, resulting in comparable fold changes of all conditions to the corresponding tensile-stressed cells (control: 2.42 ± 0.06; 5 ng/ml rhGDF15: 2.21 ± 0.07, p-value to control 0.99847; 20 ng/ml rhGDF15: 2.69 ± 0.09, p-value to control 0.98791). We next analyzed adherent THP1 cells used to visualize the inflammatory response by determining the activation of monocytic immune cells (Figure 4c, d). Whereas tensile force promoted an anti-inflammatory response with reduced THP1 activation, prior rhGDF15 exposure blocked this response.
Since bone formation and the activation of osteoblast activity are key features for the tensile site, we further analyzed ALP activity induction in hPdLFs after 24 hours of biaxial tensile stress (Figure 4e, f). Whereas the control treatment displayed increased ALP activity due to the application of tensile force, this was not detected for rhGDF15-stimulated hPdLFs pointing to a limited activation of further osteoblasts by mechanical stress. Of note, ALP activity in rhGDF15-exposed stretched hPdLFs was still significantly higher than in stressed control cells due to increased baseline levels (Figure 4f; (5 ng/ml rhGDF15 to control: p-value 0,0001 x 1027, ***; 20 ng/ml rhGDF15 to control: p-value 0.0002 x 105, ***). Furthermore, we examined the formation of calcium deposits after application of tensile stress (Figure 4g, h). While the previously detected GDF15-dependent increase in calcium deposit formation was also detected in force-stressed cells, neither the control cultures nor rhGDF15-stimulated hPdLFs showed tensile-related differences compared to unstressed hPdLFs, which might be expected due to the short duration of the stress application.
Based on its pivotal role in modulating the pro-inflammatory response to compressive stimuli [34], we next examined the effects of preceding long-term stimulation on the mechanoresponse of compressed hPdLFs. To this end, we performed quantitative expression analysis of important genes encoding the pro-inflammatory cytokines IL-6 (IL6) and COX2 (Figure 5a, b). Whereas no GDF15-dependent changes were detected in the expression of COX2, IL6 levels were significantly increased in compressed rhGDF15-stimulated hPdLFs. Of note baseline levels were not changed by rhGDF15 exposure. To investigate whether those changes in IL6 expression also cause an altered inflammatory response and thus activation of immune cells, we analyzed the adhesion of THP1 monocytic cells accordingly (Figure 5c, d). Thus, compared with controls, increased activation of monocytic cells by compressed hPdLFs was detected during long-term stimulation with rhGDF15.
We next focused on a possible impact on the activation of bone-resorbing osteoclasts by analyzing RANKL and OPG expression of force-stressed hPdLFs, which encode key players in the regulation of osteoclastogenesis (Figure 5e, f). Whereas compression promoted a pronounced increase in RANKL levels and a significant decrease in OPG levels in controls, these changes were not observed in rhGDF15-stimulated hPdLFs. To functionally address changes in osteoclast activation, macrophages resulting from pre-stimulated THP1 monocytic cells were cultured for six days with the medium supernatant of force-stressed hPdLFs to stimulate their differentiation into osteoclasts [52] (Figure 5f, g). Using TRAP assay, enhanced differentiation into mononuclear pre-osteoclasts and matured multinuclear osteoclasts was detected after stimulation with the medium supernatant of compressed control fibroblasts compared to the unstressed control. Consistent with the RANKL and OPG expression data, a decreased number of osteoclast activation was observed. However, it was still enhanced compared to the non-stressed control and the rhGDF15-stimulated fibroblasts.
Together, our data indicate a relevant impact of long-term stimulation with GDF15 on the cellular properties and the pro-inflammatory mechanobiological response of human PdL fibroblasts resulting in increased inflammation due to compressive forces but reduced osteoclast activation potentially via modulation of RANKL/OPG.
3. Discussion
The interdependent and interrelated responses of periodontal ligament cells to mechanical forces generated during orthodontic treatment are modulated by a variety of key factors [27,53]. A change in their balance or functionality due to extrinsic and intrinsic factors can affect tooth movement. Recently, we demonstrated a relevant pro-inflammatory role of GDF15 in the mechanosignaling of PdLFs [34]. However, elevated GDF15 serum levels have been detected in various diseases and within the orthodontic context more relevant in obesity and aging [44,54], which might affect tooth movement in those patient groups.
Thus to uncover potential effects of an increased GDF15 exposure on the mechanofunctionality of periodontal cells, we stimulated PdL fibroblasts up to 36 days with recombinant GDF15 protein prior subsequent analysis of the response to mechanical forces. Thereby, GDF15 stimulation resulted in significantly decreased proliferation correlating with an increased rate of cellular senescence at shorter exposures, while longer stimulation resulted in a more prominent level of osteogenic differentiation. Surprisingly, compressed hyperinflammatory PdLFs did not display correlated excessive osteoclast activation, in fact, it was significantly attenuated due to long-term GDF15 exposure.
GDF15 is known for its multiple functions in tissue proliferation and differentiation and has also been detected in fibroblasts. Kim et al. demonstrated an inhibiting effect of GDF15 on renal fibroblast growth in primary fibroblasts isolated from mouse kidney subjected to ureteral obstruction-induced fibrosis [55]. In addition, decreased growth and activation of lung fibroblasts due to GDF15-associated alterations in the TGF-Smad pathway have been described [56]. Contrarily, Guo et al. recently revealed that GDF15 can promote proliferation in newborn rat cardiac fibroblasts after irradiation, which was confirmed by the increased cell proliferation rate and increased expression of fibrosis markers (Col1α and αSma) after transfection with GDF15 in vitro [57]. However, our data support an anti-proliferative effect of GDF15, specifically on hPdLFs, in terms of decreased Ki67, whereas we could not detect an impact on apoptosis rate of hPdLFs.
We presumed, that increased cellular senescence and osteogenic differentiation could be reasons for the anti-proliferative effect of GDF15 on hPdLFs. GDF15 is highly upregulated with age and associated with many age-related diseases [58]. It is thus suggested as a biomarker for aging [44]. Besides, GDF15 levels correlate positively with cellular senescence induced by ionizing radiation in human aortic endothelial cells [48]. However, the pleiotropic regulator GDF15 is also associated with anti-senescence effects. Thus, Li et al. reported that GDF15 downregulates p21, a senescence marker, via activation of both PI3K/AKT and MAPK/ERK signaling pathways, resulting in cervical cancer cell proliferation [59]. We detected higher p21 levels and increased ß-galactosidase activity due to GDF15 stimulation in hPdLFs, rather supporting the function of GDF15 as a promoter of cellular senescence.
Apart from cellular senescence, we considered osteogenic differentiation as a potential reason for GDF15-related reduction of hPdLF proliferation. In this study, we provide supporting evidence regarding the osteogenic potential of GDF15 in PdL fibroblasts. Quantitative expression analysis of osteogenic marker genes ALPL and RUNX2 showed increased levels after 12 and 24 days of rhGDF15 exposure. ALP activity increased accordingly, but showed a temporal shift compared to expression pattern with its highest activity at 36 days of exposure. We further detected an increase in mineralized deposits of rhGDF15 exposed cells compared to control condition, which undermines the osteogenic effect of GDF15.
Members of the TGF-ß-family are known to regulate osteoblast and osteoclast differentiation [60,61]. In addition to our own previous results in primary osteoblasts [33], a pro-osteogenic effect of GDF15 on various cell types has been observed in a number of studies. Thus, Uchiyama et al. demonstrated, that the number of bone marrow-derived mesenchymal stem cells (BM-MSCs) increased significantly after 7 days of stimulation with rhGDF15 [49]. Further analysis showed, that protein levels of representative markers of osteoblastic differentiation RUNX2 and OSX were increased in rhGDF15-treated human BM-MSCs compared to control cells. Moreover, transfection with GDF15 cDNA was shown to promote osteoblast differentiation in prostate cancer cells in vitro [62]. According to Siddiqui et al. [47], prostate cancer (PCa) secreted GDF15 promotes bone metastases and bone turnover. Further, rhGDF15 promotes osteogenic differentiation of mouse calvarial osteoblasts and GDF15 deletion inhibits PCa-mediated osteoblast differentiation and mineralization, suggesting an essential role of GDF15 in the stimulation of osteoblast differentiation by PCa. It should be noted that Westhrin et al. also reported an anti-osteogenic effect of GDF15 on human bone marrow-derived mesenchymal stem cells with reduced alkaline phosphatase activity, matrix mineralization and mRNA levels of RUNX2, Type I collagen and osteocalcin due to GDF15 stimulation for 17 days [46]. However, altered cell fate might influence cellular responses to mechanical forces.
We recently reported that GDF15-deficient hPdLF showed a significantly reduced secretion of the pro-inflammatory cytokines IL6, PGE2, and TNFα upon mechanical compression [34]. Combined with lower numbers of activated monocytic THP1 cells, our previous data suggest a proinflammatory role for GDF15 in hPdLFs. However, we left one relevant question unexplored. What is the role of intracellular GDF15 activity versus its effects by extracellular stimulation through receptor binding? Via different protein states, GDF15 can trigger intracrine signaling by translocation to the nucleus and auto- or paracrine signaling through membrane-bound receptors [39,41]. Artz et al. identified ALK-5 as a receptor expressed in mouse leucocytes that inhibits integrin activation triggered by GDF15 [40]. Moreover, GDF15 has been found to trigger analgesia in rat primary sensory neurons via ALK-2 receptor [39]. Here, we detected all members of the ALK family in human PdLFs.
Extending our previous study, our new data now suggest that the pro-inflammatory effect of GDF15 in compressed hPdLFs is mediated by auto- or paracrine action via protein-receptor binding. Thus, increased IL6 levels and activated monocytes were detected due to GDF15 stimulation. Interestingly, GDF15-stimulated mechano-controls showed comparable transcription levels of IL6, which is in contradiction to Li et al. reporting increased IL6 expression levels in unforced hPdLFs due to GDF15 exposure [45]. A plausible explanation might relate to the concentration of GDF15 used for cell exposure. While we stimulated with 5 to a maximum of 20 ng/ml GDF15 protein, which already corresponds to high concentrations secreted by various cells upon different stresses [63,64,65,66,67], Li et al. used an even higher concentration of 100 ng/ml. Nevertheless, both studies confirm a pro-inflammatory influence of GDF15 in hPdLFs.
Compressed fibroblasts also usually show an increased RANKL and a decreased OPG secretion leading to enhanced osteoclast activation [68,69,70]. Our data indicate, that long-term exposure to 5-20 ng/ml rhGDF15 significantly reduced those mechano-related effects as reduced RANKL/OPG expression ratios and osteoclast activation were detected. In contrast to this, short-term stimulation with significantly higher dose of 100 ng/ml GDF15 protein resulted in an increased RANKL/OPG expression ratio in human PdL cells mainly due to reduced OPG levels [45]. Since we stimulated cells with GDF15 for 36 days driving an osteogenic cell fate, the mechanoreactivity might differ due to cell differentiation. Comparable to our results, GDF15 stimulation of mouse calvarial osteoblasts (MCOs) also failed to induce changes in Opg expression levels [47]. In contrast to hPdLFs, GDF15-exposed MCOs showed increased Rankl levels when stimulated with GDF15 concentrations higher than 10 ng/ml. However, osteoclast activation was not functionally validated in both previous studies within this context [45,47], which limits comparison with our study in terms of possible differences between RNA and protein expression as well as secretion. Furthermore, considering the limited sequence homology of GDF15 between humans and rodents [71], possible effects of nonspecies-matched stimulation may account for differences to findings of Siddiqui, who used human recombinant GDF15 to stimulated mouse MCOs. Nevertheless, GDF15 stimulation of PdL fibroblasts and (PdL-derived) osteoblasts appears to affect the activation of osteoclasts by these cells, although this seems to depend on the specific conditions.
In summary, our results strongly suggest a relevant impact of GDF15 on the cell fate of PdL fibroblasts promoting osteogenic differentiation in the long-term. Moreover, GDF15 seems to impact the force-related inflammatory mechanoresponse of those cells and subsequently affected their activation of osteoclast. Since we used a simplified in vitro model to simulate orthodontic tooth movement with compressive and tensile strain, future studies should focus on these findings in vivo. Altogether, our study provides evidence that long-term elevated GDF15 levels, such as those associated with specific diseases as well as with aging [43,44], indeed impact the mechanoreactivity of PdL cells and thus may be relevant in orthodontic treatment of those patients.
4. Materials and Methods
4.1. Cell Culture
Human periodontal ligament fibroblasts (hPdLF, Lonza, Basel, Switzerland) were cultured in culture medium containing Dulbecco’s modified Eagle’s medium (DMEM, Capricorn Scientific, Ebsdorfergrund, Germany), 4.5 g/L Glucose, 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Carlsbad, CA, USA), 100 U/mL penicillin, 100 mg/L streptomycin and 50 mg/L L-ascorbic acid at 37°C, 5% CO2 and 95% humidity. Cells were passaged at 75% confluence with 0.05% Trypsin/EDTA (Thermo Fisher Scientific, Carlsbad, CA, USA). Cells of passages six to nine were used for the experiments.
THP1 monocytic cells (DMSZ, Braunschweig, Germany) were cultured at 37 °C, 5% CO2, and 95% humidity in RPMI 1640 medium (Thermo Fisher Scientific, Carlsbad, CA, USA) containing 10% FBS, 100 U/mL penicillin, and 100 g/mL streptomycin. Cells were passaged weekly and seeded at a density of 1 x 106 cells in a T175 culture flask (Thermo Fisher Scientific, Carlsbad, CA, USA).
4.2. Stimulation with recombinant human GDF15 protein
For evaluation of GDF15-induced effects, hPdLFs were stimulated for 12, 24, and 36 days with 5ng/ml or 20ng/ml recombinant human GDF15 protein (R&D Systems, Minneapolis, MN, USA) in culture flasks. As osteoblast differentiation control, hPdLFs were stimulated with 100nM dexamethasone and 10mM β-glycerol phosphate. Twelve days prior to the final experiments, 100.000 cells were seeded into 6-well plates. For immunofluorescent analysis and determination of THP1 activation, 5000 cells were seeded onto glass coverslips in 48-well plates and further cultured for 12 days in respective media.
4.3. Application of mechanical forces
At the final day of stimulation, a static compression of 2 g/cm² was achieved by applying sterilized glass plates for 24 hours according to the protocol of Kirschneck et al. [72] and as describe before [33,34,73]. For subsequent analysis of monocyte activation, force application was performed in 24-well plates by centrifugation via two rounds of twelve-hour centrifugations at 30 °C with a force of 7.13 g/cm2. Control cells were cultured at 30 °C for the duration of force application. A three-hour recovery break at 37 °C, under 5% CO2 and 95% humidity was applied between the centrifugation rounds.
For application of static isotrophic tensile force, hPdLFs were seeded on flexible bottomed 6-well plates (BioFlex® Culture Plates, FLEXCELL®, Asbach, Germany) coated with pronectin. Tensile forces of 15,9% were applied by spherical cap silicone stamps of a 25 mm radius and a high of 7,1 mm according to Nazet et al. [74]. The self-made stamps (S4 suhy dental a-silicone; Bisico, Bielefeld, Germany) were clamped into the bottom of the flexible membrane plates for 24 hours.
4.4. RNA expression analysis
For analyzing gene expression, cells were isolated with TRIzol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA). RNA isolation, cDNA synthesis, and quantitative PCR were performed as previously described [33,34,75]. Briefly, 1-bromo-3-chloro-propane (Sigma-Aldrich, St. Louis, MO, USA) and centrifugation was used for separation of RNA. Cleaning of RNA was performed with RNA Clean & Concentrator-5 kit (Zymo research, Freiburg, Germany). The quality and quantity of the RNA were measured with Nanodrop OneC (Thermo Fisher Scientific, Carlsbad, CA, USA). SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific, Carlsbad, CA, USA) and Oligo(dt)18 primers (Thermo Fisher Scientific, Carlsbad, CA, USA) were used for cDNA synthesis. Quantitative PCR was performed with Luminaris Color HiGreen qPCR Master Mix (Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacturer's protocol and analyzed with qTOWER3 (Analytik Jena, Jena, Germany). Primer design was performed as previously described [33,34][Schuldt, 2022 #803. Quality and specificity of the primers were analyzed using melting curves and agarose gel electrophoresis. Dilution series of cDNA were used to calculate primer efficiency. Table 1 contains all information for the used primers. RPL22 and TBP were used as reference genes for data analysis according to the ∆∆CT method.
4.5. Protein preparation and expression analysis
For analyzing protein expression, cultured hPdLFS were isolated with ice-cold phosphate-buffered saline (PBS) and centrifugation. Further protein isolation and expression analysis by semi-dry western blot was performed as previously described [76]. Following primary antibodies from Santa Cruz Biotechnology (Dallas, Texas, USA) were used: rat-anti-ALK1 (sc-101556, 1:100), mouse-anti-ACTR-I (ALK2; sc-374523, 1:100), mouse-anti-BMPR-IA (ALK3; sc-134285, 1:100), mouse-anti-ACTR-IB (ALK4; sc-73677, 1:100), rat-anti-TGFβ-RI (ALK5; sc-101574, 1:100), mouse-anti-BMPR-IB (ALK6; sc-515886, 1:100) mouse-anti-ACTR-IC (ALK7; sc-374538; 1:100). Following secondary antibodies coupled to horseradish peroxidase (HRP) from Thermo Fisher Scientific (Carlsbad, CA, USA) were used: goat-anti-mouse IgG HRP (#31430, 1:5000) and goat-anti-rat IgG HRP (#31470, 1:5000).
4.6. Immunofluorescent Staining
To analyze cells characteristics, hPdLFs cultured on coverslips were fixed after specific treatment with 4% PFA for 10 minutes and washed three times with PBS containing 0.1% Triton™ X-100 (Merck Millipore, Burlington, Massachusetts, USA). Primary antibody incubation was performed for 3 hours in blocking solution consisting of PBS, 0.1% Triton™ X-100 and 4% bovine serum albumin, followed by three washing steps and subsequent secondary antibody incubation for 45 minutes in blocking solution. DAPI (Thermo Fisher Scientific, Carlsbad, CA, USA; 1:10,000 in PBS) was applied for 10 min to stain cell nuclei. The following antibodies were used: rabbit-anti-human p21 (18769S; Cell Signaling Technologies, Danvers, Massachusetts, USA; 1:100), rabbit-anti-human Ki67 (ab15580; Abcam, Cambridge, UK; 1:500), goat-anti-rabbit-Cy5.
4.7. MTT Assay
The cell vitality was assessed via MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay (Sigma Aldrich, Taufkirchen, Germany) according to the manufacturer’s instructions and measured the INFINITE M NANO microplate reader (Tecan Austria GmbH, Gröding, Austria).
4.8. Cell death assays
Trypan blue staining was performed on unfixed cells immediately after stimulation with rhGDF15. Cells were briefly washed with pre-warmed PBS, and 0.4% trypan blue solution was added to the PBS at a ratio of 1:2 for 5 minutes. After rinsing twice with PBS, microscopic images were captured immediately.
ApopTag® Fluorescein In Situ Apoptosis Detection Kit (Sigma Aldrich, St. Louis, Missouri, USA) was used according to the manufacturer’s protocol to detected apoptotic cells grown to 75% confluency on coverslips.
4.9. Senescence assay
To detect cellular senescence, 75% confluent hPdLFs cultured on coverslips were analyzed with the CellEvent™ Senescence Green Detection Kit according to the manufacturer’s protocol.
4.10. Alkaline phosphatase activity assay
To analysis the activity of alkaline phosphatase, cultured hPdLFs were fixed with 4% PFA for 10 min, washed with PBS and incubated with 1-Step™ NBT/BCIP Substrate Solution (Thermo Fisher Scinetific, Carlsbad, CA, USA) for 90 min. After washing with PBS, cells were directly imaged.
4.11. Alizarin red staining
Staining of calcium deposits to detect osteogenic differentiation was performed as previously described [77]. Briefly, cultured hPdLFs were fixed with 10 % PFA for 10 min prior staining with 40 mM Alizarin Red S (Merck Millipore, Burlington, Massachusetts, United States) for 20 min. Following a rinse with water, 10% acetic acid was used to dissolve the stained cells. Subsequent to heating at 85 °C for 10 min, cells were centrifuged at 20000 g for 15 min, and pH of the supernatant was neutralized with 10% ammonium hydroxide. OD405 was measured as duplicates using the Infinite M nano plate reader (Tecan Life Science, Männedorf, Switzerland).
4.12. THP1 activation assay
For visualization of the inflammatory response of stressed hPdLF, THP1 activation assay was performed as previously described [73]. To this end, 50 × 10³ non-adherent THP1 monocytic cells were stained with Celltracker CMFDA (Thermo Fisher Scientific, Carlsbad, CA, USA) and added to each well of a 24-well plate with cultured hPdLF. Non-adherent cells were removed by washing with prewarmed PBS and coverslips were fixed with 4% paraformaldehyde (PFA) for 10 min. After washing with PBS, cell nuclei were stained with DAPI (1:10.000 in PBS, Thermo Fisher Scientific, Carlsbad, CA, USA) and coverslips were mounted for microscopy on objects slides.
4.13. Osteoclast activation assay and TRAP staining
For the analysis of osteoclast differentiation induced by stressed hPdLFs, two-days phorbol 12-myristate 13-acetat (PMA, 100 ng/ml)-prestimulated macrophage-like THP1 cells were cultured in 96-well plates with the collected media supernatant of stressed hPdLFs. Subsequently, these cells were prefixed in 4% PFA for 10 min, then fixed for 1 min in 50:50 acetone/ethanol, air-dried and stained for tartrate-resistant acid phosphatase (TRAP). Staining solution consisting of 0.1 mg/ml Naphtol AS-MX phosphate, 0.5 mg /ml Fast Red Violet LB salt, 1 % N,N-dimethyl formamid in 50 mM sodium acetat trihydrate, 50 mM tartrate dehydrate and 0.1 % acetic acid (all Merck Millipore, Burlington, Massachusetts, United States) was applied for 60 min at 37 °C. Finally, cells were washed in PBS and directly imaged. Stimulation was performed with three independent collections of medium supernatant and analyzed on two coverslips per condition in each independent experiment.
4.14. Microscopy, Image Analysis and Statistics
Immunofluorescent stainings, TUNEL and senescence assays as well as THP1 activation assay were imaged with the inverted confocal laser scanning microscope TCS SP5 (Leica, Wetzlar, Germany). Fiji software (https://imagej.net/Fiji, accessed on 01.04.2017) was used for image analysis. Fluorescence intensity of p21 was assesed as previously reported [78]. Briefly, in 270 cells per condition, mean grey values (MGVs) of p21 stainings were measured and the background was subtracted for each measurement. MGV were normalized to control conditions and presented as percent changes. For visual presentation, MGV was displayed as intensity as thermal LUT. Each experimental condition was analyzed at least in biological triplicates with two technical replicates per condition in each independent experiment.
For statistical analysis and figure illustration, Graph Pad Prism (https://www.graphpad.com, accessed on 01.02.2021) and Adobe Photoshop CS5 (https://adobe.com, accessed on 01.02.2013) was used. One-way ANOVA and post hoc test (Tukey) were used as statistical tests. Significance levels: p value < 0.05 */#/§; p value < 0.01 **/##/§§; p value < 0.001 ***/###/§§§.
5. Conclusions
Orthodontic tooth movement is promoted by applied mechanical stimuli and relies on the crucial functions of PdL fibroblasts regarding the modulation of tissue and bone remodeling. Here we could demonstrate, that GDF15 alters the cell fate of PdLFs in an anti-proliferative and pro-osteogenic manner and further affects the mechanoresponse of PdLFs. These findings provide a link for future research to evaluate how orthodontic treatments are affected by elevated GDF15 blood levels, as seen in patients with advanced age and pathologic conditions such as inflammation, myocardial ischemia, and cancer [43].
Author Contributions
Conceptualization, Judit Symmank and Collin Jacobs; Funding acquisition, Christoph-Ludwig Hennig and Judit Symmank; Investigation, Lukas Lösch, Albert Stemmler, Adrian Fischer, Julia Steinmetz, Lisa Schuldt, Christoph-Ludwig Hennig and Judit Symmank; Methodology, Judit Symmank; Project administration, Judit Symmank and Collin Jacobs; Supervision, Judit Symmank and Collin Jacobs; Visualization, Judit Symmank; Writing – original draft, Lukas Lösch and Albert Stemmler; Writing – review & editing, Judit Symmank and Collin Jacobs.
Funding
This research was funded by the Deutsche Gesellschaft für Kieferorthopädie e.V. (DGKFO) and the Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde e.V. (DGZMK).
Data Availability Statement
The datasets of this study are available upon reasonable request from the corresponding author.
Acknowledgments
We would like to thank Katrin von Brandenstein for her excellent technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Navabi, N.; Farnudi, H.; Rafiei, H.; Arashlow, M.T., Orthodontic treatment and the oral health-related quality of life of patients. J Dent (Tehran) 2012, 9, 247-254 DOI.
- Sa-Pinto, A.C.; Rego, T.M.; Marques, L.S.; Martins, C.C.; Ramos-Jorge, M.L.; Ramos-Jorge, J., Association between malocclusion and dental caries in adolescents: A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2018, 19, 73-82. https://doi.org/10.1007/s40368-018-0333-0. [CrossRef]
- Alsulaiman, A.A.; Kaye, E.; Jones, J.; Cabral, H.; Leone, C.; Will, L.; Garcia, R., Incisor malalignment and the risk of periodontal disease progression. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2018, 153, 512-522. https://doi.org/10.1016/j.ajodo.2017.08.015. [CrossRef]
- Yamaguchi, M.; Fukasawa, S., Is inflammation a friend or foe for orthodontic treatment?: Inflammation in orthodontically induced inflammatory root resorption and accelerating tooth movement. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms22052388. [CrossRef]
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E., Root resorption associated with orthodontic tooth movement: A systematic review. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2010, 137, 462-476; discussion 412A. https://doi.org/10.1016/j.ajodo.2009.06.021. [CrossRef]
- Wishney, M., Potential risks of orthodontic therapy: A critical review and conceptual framework. Australian dental journal 2017, 62 Suppl 1, 86-96. https://doi.org/10.1111/adj.12486. [CrossRef]
- Theodorou, C.I.; Kuijpers-Jagtman, A.M.; Bronkhorst, E.M.; Wagener, F., Optimal force magnitude for bodily orthodontic tooth movement with fixed appliances: A systematic review. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2019, 156, 582-592. https://doi.org/10.1016/j.ajodo.2019.05.011. [CrossRef]
- Talic, N.F., Adverse effects of orthodontic treatment: A clinical perspective. The Saudi dental journal 2011, 23, 55-59. https://doi.org/10.1016/j.sdentj.2011.01.003. [CrossRef]
- Parcianello, R.G.; Amerio, E.; Giner Tarrida, L.; Nart, J.; Flores Mir, C.; Puigdollers Perez, A., Local hormones and growth factors to enhance orthodontic tooth movement: A systematic review of animal studies. Orthodontics & craniofacial research 2022, 25, 281-303. https://doi.org/10.1111/ocr.12544. [CrossRef]
- Andrade Jr, I.; Taddei, S.R.A.; Souza, P.E.A., Inflammation and tooth movement: The role of cytokines, chemokines, and growth factors. Semin. Orthod. 2012, 18, 257-269. https://doi.org/10.1053/j.sodo.2012.06.004. [CrossRef]
- Nanci, A.; Bosshardt, D.D., Structure of periodontal tissues in health and disease. Periodontol 2000 2006, 40, 11-28. https://doi.org/10.1111/j.1600-0757.2005.00141.x. [CrossRef]
- Basdra, E.K.; Komposch, G., Osteoblast-like properties of human periodontal ligament cells: An in vitro analysis. Eur J Orthod 1997, 19, 615-621. https://doi.org/10.1093/ejo/19.6.615. [CrossRef]
- Somerman, M.J.; Young, M.F.; Foster, R.A.; Moehring, J.M.; Imm, G.; Sauk, J.J., Characteristics of human periodontal ligament cells in vitro. Archives of oral biology 1990, 35, 241-247. https://doi.org/10.1016/0003-9969(90)90062-f. [CrossRef]
- Arceo, N.; Sauk, J.J.; Moehring, J.; Foster, R.A.; Somerman, M.J., Human periodontal cells initiate mineral-like nodules in vitro. Journal of periodontology 1991, 62, 499-503. https://doi.org/10.1902/jop.1991.62.8.499. [CrossRef]
- Li, M.; Zhang, C.; Yang, Y., Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone & joint research 2019, 8, 19-31. https://doi.org/10.1302/2046-3758.81.BJR-2018-0060.R1. [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P., Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. European journal of oral sciences 2007, 115, 355-362. https://doi.org/10.1111/j.1600-0722.2007.00469.x. [CrossRef]
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M., Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res 1998, 33, 500-508. https://doi.org/10.1111/j.1600-0765.1998.tb02350.x. [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H., Osteoimmunology: The conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 2017, 97, 1295-1349. https://doi.org/10.1152/physrev.00036.2016. [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S., Rankl/rank/opg pathway: A mechanism involved in exercise-induced bone remodeling. BioMed research international 2020, 2020, 6910312. https://doi.org/10.1155/2020/6910312. [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T., Rankl biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. https://doi.org/10.1186/s41232-019-0111-3. [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L., Osteoclast differentiation and activation. Nature 2003, 423, 337-342. https://doi.org/10.1038/nature01658. [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D., Root resorption and the opg/rankl/rank system: A mini review. Journal of oral science 2008, 50, 367-376. https://doi.org/10.2334/josnusd.50.367. [CrossRef]
- Zhang, L.; Ding, Y.; Rao, G.Z.; Miao, D., Effects of il-10 and glucose on expression of opg and rankl in human periodontal ligament fibroblasts. Braz. J. Med. Biol. Res. 2016, 49, e4324. https://doi.org/10.1590/1414-431X20154324. [CrossRef]
- Yan, T.; Xie, Y.; He, H.; Fan, W.; Huang, F., Role of nitric oxide in orthodontic tooth movement (review). International journal of molecular medicine 2021, 48. https://doi.org/10.3892/ijmm.2021.5001. [CrossRef]
- Tang, N.; Zhao, Z.; Zhang, L.; Yu, Q.; Li, J.; Xu, Z.; Li, X., Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch. Med. Sci. 2012, 8, 422-430. https://doi.org/10.5114/aoms.2012.28810. [CrossRef]
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S., Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7872-7880 DOI.
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.C., Orthodontic tooth movement: The biology and clinical implications. The Kaohsiung journal of medical sciences 2018, 34, 207-214. https://doi.org/10.1016/j.kjms.2018.01.007. [CrossRef]
- Ullrich, N.; Schroder, A.; Jantsch, J.; Spanier, G.; Proff, P.; Kirschneck, C., The role of mechanotransduction versus hypoxia during simulated orthodontic compressive strain-an in vitro study of human periodontal ligament fibroblasts. International journal of oral science 2019, 11, 33. https://doi.org/10.1038/s41368-019-0066-x. [CrossRef]
- Vansant, L.; Cadenas De Llano-Perula, M.; Verdonck, A.; Willems, G., Expression of biological mediators during orthodontic tooth movement: A systematic review. Archives of oral biology 2018, 95, 170-186. https://doi.org/10.1016/j.archoralbio.2018.08.003. [CrossRef]
- Brooks, P.J.; Nilforoushan, D.; Manolson, M.F.; Simmons, C.A.; Gong, S.G., Molecular markers of early orthodontic tooth movement. The Angle orthodontist 2009, 79, 1108-1113. https://doi.org/10.2319/121508-638R.1. [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K., et al., Mic-1, a novel macrophage inhibitory cytokine, is a divergent member of the tgf-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America 1997, 94, 11514-11519. https://doi.org/10.1073/pnas.94.21.11514. [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A., Gdf15, an update of the physiological and pathological roles it plays: A review. Pflugers Arch. 2020, 472, 1535-1546. https://doi.org/10.1007/s00424-020-02459-1. [CrossRef]
- Symmank, J.; Zimmermann, S.; Goldschmitt, J.; Schiegnitz, E.; Wolf, M.; Wehrbein, H.; Jacobs, C., Mechanically-induced gdf15 secretion by periodontal ligament fibroblasts regulates osteogenic transcription. Scientific reports 2019, 9, 11516. https://doi.org/10.1038/s41598-019-47639-x. [CrossRef]
- Stemmler, A.; Symmank, J.; Steinmetz, J.; von Brandenstein, K.; Hennig, C.L.; Jacobs, C., Gdf15 supports the inflammatory response of pdl fibroblasts stimulated by p. Gingivalis lps and concurrent compression. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms222413608. [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jorgensen, S.B.; Steinberg, G.R., Gdf15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nature reviews. Endocrinology 2021, 17, 592-607. https://doi.org/10.1038/s41574-021-00529-7. [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C., Insights into mechanisms of gdf15 and receptor gfral: Therapeutic targets. Trends in endocrinology and metabolism: TEM 2020, 31, 939-951. https://doi.org/10.1016/j.tem.2020.10.004. [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D., et al., Gfral is the receptor for gdf15 and the ligand promotes weight loss in mice and nonhuman primates. Nature medicine 2017, 23, 1150-1157. https://doi.org/10.1038/nm.4392. [CrossRef]
- Wu, Q.; Jiang, D.; Matsuda, J.L.; Ternyak, K.; Zhang, B.; Chu, H.W., Cigarette smoke induces human airway epithelial senescence via growth differentiation factor 15 production. Am. J. Respir. Cell Mol. Biol. 2016, 55, 429-438. https://doi.org/10.1165/rcmb.2015-0143OC. [CrossRef]
- Lin, W.; Zhang, W.W.; Lyu, N.; Cao, H.; Xu, W.D.; Zhang, Y.Q., Growth differentiation factor-15 produces analgesia by inhibiting tetrodotoxin-resistant nav1.8 sodium channel activity in rat primary sensory neurons. Neurosci. Bull. 2021, 37, 1289-1302. https://doi.org/10.1007/s12264-021-00709-5. [CrossRef]
- Artz, A.; Butz, S.; Vestweber, D., Gdf-15 inhibits integrin activation and mouse neutrophil recruitment through the alk-5/tgf-betarii heterodimer. Blood 2016, 128, 529-541. https://doi.org/10.1182/blood-2016-01-696617. [CrossRef]
- Min, K.W.; Liggett, J.L.; Silva, G.; Wu, W.W.; Wang, R.; Shen, R.F.; Eling, T.E.; Baek, S.J., Nag-1/gdf15 accumulates in the nucleus and modulates transcriptional regulation of the smad pathway. Oncogene 2016, 35, 377-388. https://doi.org/10.1038/onc.2015.95. [CrossRef]
- Lee, J.; Kim, I.; Yoo, E.; Baek, S.J., Competitive inhibition by nag-1/gdf-15 nls peptide enhances its anti-cancer activity. Biochemical and biophysical research communications 2019, 519, 29-34. https://doi.org/10.1016/j.bbrc.2019.08.090. [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H., Growth/differentiation factor-15 (gdf-15): From biomarker to novel targetable immune checkpoint. Frontiers in immunology 2020, 11, 951. https://doi.org/10.3389/fimmu.2020.00951. [CrossRef]
- Liu, H.; Huang, Y.; Lyu, Y.; Dai, W.; Tong, Y.; Li, Y., Gdf15 as a biomarker of ageing. Experimental gerontology 2021, 146, 111228. https://doi.org/10.1016/j.exger.2021.111228. [CrossRef]
- Li, S.; Li, Q.; Zhu, Y.; Hu, W., Gdf15 induced by compressive force contributes to osteoclast differentiation in human periodontal ligament cells. Experimental cell research 2020, 387, 111745. https://doi.org/10.1016/j.yexcr.2019.111745. [CrossRef]
- Westhrin, M.; Moen, S.H.; Holien, T.; Mylin, A.K.; Heickendorff, L.; Olsen, O.E.; Sundan, A.; Turesson, I.; Gimsing, P.; Waage, A., et al., Growth differentiation factor 15 (gdf15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum gdf15 levels are associated with multiple myeloma bone disease. Haematologica 2015, 100, e511-514. https://doi.org/10.3324/haematol.2015.124511. [CrossRef]
- Siddiqui, J.A.; Seshacharyulu, P.; Muniyan, S.; Pothuraju, R.; Khan, P.; Vengoji, R.; Chaudhary, S.; Maurya, S.K.; Lele, S.M.; Jain, M., et al., Gdf15 promotes prostate cancer bone metastasis and colonization through osteoblastic ccl2 and rankl activation. Bone research 2022, 10, 6. https://doi.org/10.1038/s41413-021-00178-6. [CrossRef]
- Park, H.; Kim, C.H.; Jeong, J.H.; Park, M.; Kim, K.S., Gdf15 contributes to radiation-induced senescence through the ros-mediated p16 pathway in human endothelial cells. Oncotarget 2016, 7, 9634-9644. https://doi.org/10.18632/oncotarget.7457. [CrossRef]
- Uchiyama, T.; Kawabata, H.; Miura, Y.; Yoshioka, S.; Iwasa, M.; Yao, H.; Sakamoto, S.; Fujimoto, M.; Haga, H.; Kadowaki, N., et al., The role of growth differentiation factor 15 in the pathogenesis of primary myelofibrosis. Cancer medicine 2015, 4, 1558-1572. https://doi.org/10.1002/cam4.502. [CrossRef]
- Langenbach, F.; Handschel, J., Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell. Res. Ther. 2013, 4, 117. https://doi.org/10.1186/scrt328. [CrossRef]
- Long, P.; Hu, J.; Piesco, N.; Buckley, M.; Agarwal, S., Low magnitude of tensile strain inhibits il-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of il-10 in human periodontal ligament cells in vitro. Journal of dental research 2001, 80, 1416-1420. https://doi.org/10.1177/00220345010800050601. [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G., High-dose pma with rankl and mcsf induces thp1 cell differentiation into human functional osteoclasts in vitro. Molecular medicine reports 2017, 16, 8380-8384. https://doi.org/10.3892/mmr.2017.7625. [CrossRef]
- Asiry, M.A., Biological aspects of orthodontic tooth movement: A review of literature. Saudi J. Biol. Sci. 2018, 25, 1027-1032. https://doi.org/10.1016/j.sjbs.2018.03.008. [CrossRef]
- Sarkar, S.; Legere, S.; Haidl, I.; Marshall, J.; MacLeod, J.B.; Aguiar, C.; Lutchmedial, S.; Hassan, A.; Brunt, K.R.; Kienesberger, P., et al., Serum gdf15, a promising biomarker in obese patients undergoing heart surgery. Frontiers in cardiovascular medicine 2020, 7, 103. https://doi.org/10.3389/fcvm.2020.00103. [CrossRef]
- Kim, Y.I.; Shin, H.W.; Chun, Y.S.; Park, J.W., Cst3 and gdf15 ameliorate renal fibrosis by inhibiting fibroblast growth and activation. Biochemical and biophysical research communications 2018, 500, 288-295. https://doi.org/10.1016/j.bbrc.2018.04.061. [CrossRef]
- Kim, Y.I.; Shin, H.W.; Chun, Y.S.; Cho, C.H.; Koh, J.; Chung, D.H.; Park, J.W., Epithelial cell-derived cytokines cst3 and gdf15 as potential therapeutics for pulmonary fibrosis. Cell death & disease 2018, 9, 506. https://doi.org/10.1038/s41419-018-0530-0. [CrossRef]
- Guo, H.; Zhao, X.; Li, H.; Liu, K.; Jiang, H.; Zeng, X.; Chang, J.; Ma, C.; Fu, Z.; Lv, X., et al., Gdf15 promotes cardiac fibrosis and proliferation of cardiac fibroblasts via the mapk/erk1/2 pathway after irradiation in rats. Radiat. Res. 2021, 196, 183-191. https://doi.org/10.1667/RADE-20-00206.1. [CrossRef]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S., Gdf15, an emerging key player in human aging. Ageing research reviews 2022, 75, 101569. https://doi.org/10.1016/j.arr.2022.101569. [CrossRef]
- Li, S.; Ma, Y.M.; Zheng, P.S.; Zhang, P., Gdf15 promotes the proliferation of cervical cancer cells by phosphorylating akt1 and erk1/2 through the receptor erbb2. J. Exp. Clin. Cancer Res. 2018, 37, 80. https://doi.org/10.1186/s13046-018-0744-0. [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P., Tgf-beta and bmp signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone research 2016, 4, 16009. https://doi.org/10.1038/boneres.2016.9. [CrossRef]
- Quinn, J.M.; Itoh, K.; Udagawa, N.; Hausler, K.; Yasuda, H.; Shima, N.; Mizuno, A.; Higashio, K.; Takahashi, N.; Suda, T., et al., Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2001, 16, 1787-1794. https://doi.org/10.1359/jbmr.2001.16.10.1787. [CrossRef]
- Wakchoure, S.; Swain, T.M.; Hentunen, T.A.; Bauskin, A.R.; Brown, D.A.; Breit, S.N.; Vuopala, K.S.; Harris, K.W.; Selander, K.S., Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate 2009, 69, 652-661. https://doi.org/10.1002/pros.20913. [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N., The mic-1/gdf15-gfral pathway in energy homeostasis: Implications for obesity, cachexia, and other associated diseases. Cell metabolism 2018, 28, 353-368. https://doi.org/10.1016/j.cmet.2018.07.018. [CrossRef]
- Conte, M.; Ostan, R.; Fabbri, C.; Santoro, A.; Guidarelli, G.; Vitale, G.; Mari, D.; Sevini, F.; Capri, M.; Sandri, M., et al., Human aging and longevity are characterized by high levels of mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 600-607. https://doi.org/10.1093/gerona/gly153. [CrossRef]
- Andersson-Hall, U.; Svedin, P.; Mallard, C.; Blennow, K.; Zetterberg, H.; Holmang, A., Growth differentiation factor 15 increases in both cerebrospinal fluid and serum during pregnancy. PloS one 2021, 16, e0248980. https://doi.org/10.1371/journal.pone.0248980. [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ortenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T., et al., Pharmacological but not physiological gdf15 suppresses feeding and the motivation to exercise. Nature communications 2021, 12, 1041. https://doi.org/10.1038/s41467-021-21309-x. [CrossRef]
- Kim, Y.; Noren Hooten, N.; Evans, M.K., Crp stimulates gdf15 expression in endothelial cells through p53. Mediators of inflammation 2018, 2018, 8278039. https://doi.org/10.1155/2018/8278039. [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K., Levels of rankl and opg in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthodontics & craniofacial research 2006, 9, 63-70. https://doi.org/10.1111/j.1601-6343.2006.00340.x. [CrossRef]
- Yamaguchi, M.; Aihara, N.; Kojima, T.; Kasai, K., Rankl increase in compressed periodontal ligament cells from root resorption. Journal of dental research 2006, 85, 751-756. https://doi.org/10.1177/154405910608500812. [CrossRef]
- Oshiro, T.; Shiotani, A.; Shibasaki, Y.; Sasaki, T., Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat. Rec. 2002, 266, 218-225. https://doi.org/10.1002/ar.10061. [CrossRef]
- Bottner, M.; Laaff, M.; Schechinger, B.; Rappold, G.; Unsicker, K.; Suter-Crazzolara, C., Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (gdf-15/mic-1). Gene 1999, 237, 105-111. https://doi.org/10.1016/s0378-1119(99)00309-1. [CrossRef]
- Kirschneck, C.; Batschkus, S.; Proff, P.; Kostler, J.; Spanier, G.; Schroder, A., Valid gene expression normalization by rt-qpcr in studies on hpdl fibroblasts with focus on orthodontic tooth movement and periodontitis. Scientific reports 2017, 7, 14751. https://doi.org/10.1038/s41598-017-15281-0. [CrossRef]
- Symmank, J.; Appel, S.; Bastian, J.A.; Knaup, I.; Marciniak, J.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Wolf, M., Hyperlipidemic conditions impact force-induced inflammatory response of human periodontal ligament fibroblasts concomitantly challenged with p. Gingivalis-lps. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms22116069. [CrossRef]
- Nazet, U.; Schroder, A.; Spanier, G.; Wolf, M.; Proff, P.; Kirschneck, C., Simplified method for applying static isotropic tensile strain in cell culture experiments with identification of valid rt-qpcr reference genes for pdl fibroblasts. Eur J Orthod 2020, 42, 359-370. https://doi.org/10.1093/ejo/cjz052. [CrossRef]
- Schuldt, L.; Reimann, M.; von Brandenstein, K.; Steinmetz, J.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Symmank, J., Palmitate-triggered cox2/pge2-related hyperinflammation in dual-stressed pdl fibroblasts is mediated by repressive h3k27 trimethylation. Cells 2022, 11. https://doi.org/10.3390/cells11060955. [CrossRef]
- Symmank, J.; Bayer, C.; Schmidt, C.; Hahn, A.; Pensold, D.; Zimmer-Bensch, G., Dnmt1 modulates interneuron morphology by regulating pak6 expression through crosstalk with histone modifications. Epigenetics 2018, 13, 536-556. https://doi.org/10.1080/15592294.2018.1475980. [CrossRef]
- Symmank, J.; Chorus, M.; Appel, S.; Marciniak, J.; Knaup, I.; Bastian, A.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C., et al., Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts. Scientific reports 2020, 10, 15706. https://doi.org/10.1038/s41598-020-72736-7. [CrossRef]
- Schuldt, L.; von Brandenstein, K.; Jacobs, C.; Symmank, J., Oleic acid-related anti-inflammatory effects in force-stressed pdl fibroblasts are mediated by h3 lysine acetylation associated with altered il10 expression. Epigenetics 2022, 1-13. https://doi.org/10.1080/15592294.2022.2090654. [CrossRef]
Figure 1.
GDF15 limits cell proliferation of hPdLFs without affecting cell survival in the long-term. (a, b) RNA (a) and protein (b) expression of activin receptor-like kinases (ALK1-7) in hPdLFs. (c) Metabolic activity of hPdLFs after stimulation with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 12, 24 and 36 days (d) displayed in relation to the 12-days control. (d-f) Ki-67-positive hPdLFs (magenta) after stimulation with rhGDF15 with cell nuclei (blue; d). The number of cells per mm² is displayed in (e) and (f) shows the proportion of Ki-67-positive hPdLFs. (g) The number auf trypan blue-positive hPdLFs after rhGDF15 stimulation indicating the proportion of dead cells. (h, i) TUNEL-positive hPdLFs (green, h) after rhGDF15 stimulation with cell nuclei (blue) and displayed as proportion to the cell number (i). * p < 0.05; **/## p < 0.01; ***/### p < 0.001; */**/*** control in relation to 5 ng/ml rhGDF15; ##/### control in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 20 µm in (d) and 25 µm in (h). dRN, difference between baseline and measured fluorescence; n.s., not significant.
Figure 1.
GDF15 limits cell proliferation of hPdLFs without affecting cell survival in the long-term. (a, b) RNA (a) and protein (b) expression of activin receptor-like kinases (ALK1-7) in hPdLFs. (c) Metabolic activity of hPdLFs after stimulation with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 12, 24 and 36 days (d) displayed in relation to the 12-days control. (d-f) Ki-67-positive hPdLFs (magenta) after stimulation with rhGDF15 with cell nuclei (blue; d). The number of cells per mm² is displayed in (e) and (f) shows the proportion of Ki-67-positive hPdLFs. (g) The number auf trypan blue-positive hPdLFs after rhGDF15 stimulation indicating the proportion of dead cells. (h, i) TUNEL-positive hPdLFs (green, h) after rhGDF15 stimulation with cell nuclei (blue) and displayed as proportion to the cell number (i). * p < 0.05; **/## p < 0.01; ***/### p < 0.001; */**/*** control in relation to 5 ng/ml rhGDF15; ##/### control in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 20 µm in (d) and 25 µm in (h). dRN, difference between baseline and measured fluorescence; n.s., not significant.

Figure 2.
GDF15 promote cellular senescence of hPdLFs. (a, b) p21 intensity in hPdLFs stimulated with 5 ng/ml and 20 ng/ml rhGDF15 for 12, 24 and 36 days (d) shown as thermal LUT (a). Grey scattered lines surround the cell nuclei. Mean p21 intensity in shown in relation to the 12-days control in (b). (c, d) β-galactosidase (β-Gal)-positive hPdLFs (magenta) after rhGDF15 stimulation with cell nuclei (blue, c). The proportion of β-Gal-positive hPdLFs is displayed in (d). **/## p < 0.01; ***/### p < 0.001; */**/*** control in relation to 5 ng/ml rhGDF15; ##/### control in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 10 µm in (a) and 25 µm in (c).
Figure 2.
GDF15 promote cellular senescence of hPdLFs. (a, b) p21 intensity in hPdLFs stimulated with 5 ng/ml and 20 ng/ml rhGDF15 for 12, 24 and 36 days (d) shown as thermal LUT (a). Grey scattered lines surround the cell nuclei. Mean p21 intensity in shown in relation to the 12-days control in (b). (c, d) β-galactosidase (β-Gal)-positive hPdLFs (magenta) after rhGDF15 stimulation with cell nuclei (blue, c). The proportion of β-Gal-positive hPdLFs is displayed in (d). **/## p < 0.01; ***/### p < 0.001; */**/*** control in relation to 5 ng/ml rhGDF15; ##/### control in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 10 µm in (a) and 25 µm in (c).

Figure 3.
Long-term exposure to GDF15 foster the osteogenic differentiation of hPdLFs. (a, b) ALPL and RUNX2 expression encoding osteoblast-related markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 12, 24 and 36 days (d) displayed in relation to the control at each time point. (c, d) ALP activity (dark blue; c) of 36-days rhGDF15-stimulated hPdLFs displayed in relation to the control in (d). (e, f) Alizarin red intensity of hPdLFs in wells stimulated 36 d with rhGDF15 (e) displayed in relation to the control in (f). Stimulation with DMS and βGP was used as positive control. * p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; ##/### in relation to 5 ng/ml rhGDF15; §§/§§§ in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 25 µm in (c).
Figure 3.
Long-term exposure to GDF15 foster the osteogenic differentiation of hPdLFs. (a, b) ALPL and RUNX2 expression encoding osteoblast-related markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 12, 24 and 36 days (d) displayed in relation to the control at each time point. (c, d) ALP activity (dark blue; c) of 36-days rhGDF15-stimulated hPdLFs displayed in relation to the control in (d). (e, f) Alizarin red intensity of hPdLFs in wells stimulated 36 d with rhGDF15 (e) displayed in relation to the control in (f). Stimulation with DMS and βGP was used as positive control. * p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; ##/### in relation to 5 ng/ml rhGDF15; §§/§§§ in relation to 20 ng/ml rhGDF15; one-way ANOVA and Tukey post hoc test. Scale bar: 25 µm in (c).
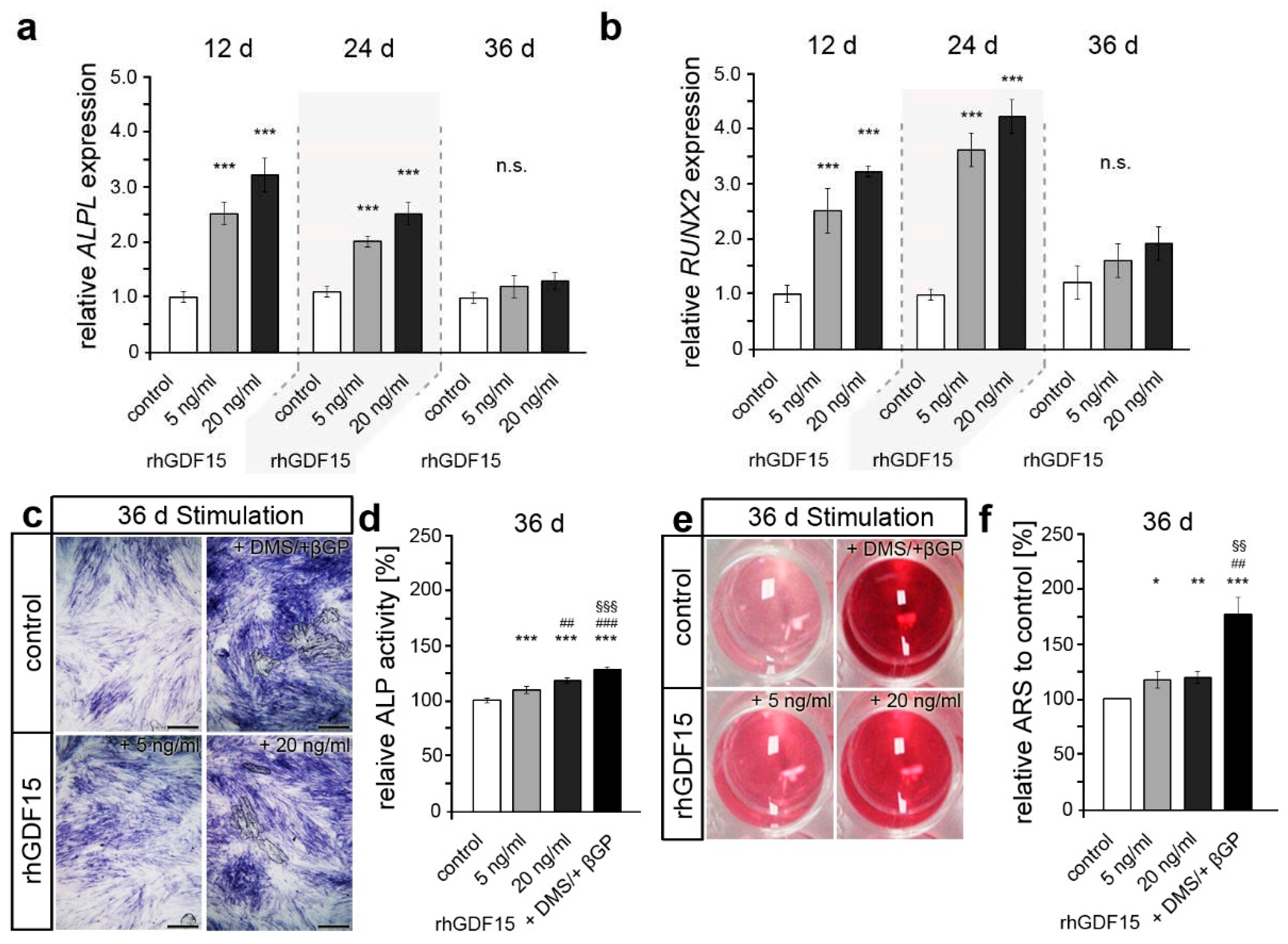
Figure 4.
Long-term GDF15-exposed hPdLFs show a reduced anti-inflammatory and pro-osteogenic mechanoresponse to tensile forces. (a, b) Quantitative expression levels of IL10 (a) and IL1RN (b) encoding anti-inflammatory markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 36 days (d) and stressed with tensile forces for 24 hours (+TF) displayed in relation to the untreated control. (c, d) Adherent (activated) THP1 cells (green) on stimulated and stressed hPdLFs (blue, cell nuclei) displayed as number of THP1 cells per hPdLFs and in relation to the untreated control in (d). (e, f) ALP activity (dark blue) of 36-days rhGDF15-stimulated hPdLFs stressed by TF (c), displayed in relation to the control in (d). (g, h) Alizarin red staining intensity of hPdLFs in wells stimulated 36 d with rhGDF15 and stressed by TF (g), displayed in relation to the control in (h). Stimulation with DMS and βGP was used as positive control. Red lines showing baseline levels of the respective condition. */#/§ p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; #/##/### in relation to control +TF; §§/§§§ baseline (red) in relation to 5 ng/ml or 20 ng/ml rhGDF15 +TF; one-way ANOVA and post hoc test (Tukey). Scale bars: 25 µm in (e).
Figure 4.
Long-term GDF15-exposed hPdLFs show a reduced anti-inflammatory and pro-osteogenic mechanoresponse to tensile forces. (a, b) Quantitative expression levels of IL10 (a) and IL1RN (b) encoding anti-inflammatory markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 36 days (d) and stressed with tensile forces for 24 hours (+TF) displayed in relation to the untreated control. (c, d) Adherent (activated) THP1 cells (green) on stimulated and stressed hPdLFs (blue, cell nuclei) displayed as number of THP1 cells per hPdLFs and in relation to the untreated control in (d). (e, f) ALP activity (dark blue) of 36-days rhGDF15-stimulated hPdLFs stressed by TF (c), displayed in relation to the control in (d). (g, h) Alizarin red staining intensity of hPdLFs in wells stimulated 36 d with rhGDF15 and stressed by TF (g), displayed in relation to the control in (h). Stimulation with DMS and βGP was used as positive control. Red lines showing baseline levels of the respective condition. */#/§ p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; #/##/### in relation to control +TF; §§/§§§ baseline (red) in relation to 5 ng/ml or 20 ng/ml rhGDF15 +TF; one-way ANOVA and post hoc test (Tukey). Scale bars: 25 µm in (e).

Figure 5.
Long-term GDF15-exposed hPdLFs show an increased pro-inflammatory response to compressive stimuli with limited activation of osteoclasts. (a, b) Quantitative expression levels of IL6 (a) and COX2 (b) encoding pro-inflammatory markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 36 days (d) and stressed with compressive forces for 24 hours (+CF), displayed in relation to the untreated control. (c, d) Adherent (activated) THP1 cells (green) on stimulated and stressed hPdLFs (blue, cell nuclei) displayed as number of THP1 cells per hPdLFs and in relation to the untreated control in (d). (e, f) Quantitative expression levels of OPG (e) and RANKL (f) in stimulated and stressed hPdLFs displayed in relation to the untreated control. (g, h) TRAP-positive THP1 cells (magenta; g) indicating the differentiation into osteoclasts, when stimulated with the medium supernatant of 36-days rhGDF15-stimulated hPdLFs additionally stressed by CF. The proportion of TRAP-positive osteoclasts is displayed in (h). Red lines showing baseline levels of the respective conditions. */#/§ p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; #/##/### in relation to control +TF; §§/§§§ baseline (red) in relation to 5 ng/ml or 20 ng/ml rhGDF15 +TF; one-way ANOVA and Tukey post hoc test. Scale bars: 25 µm in (c) and (g).
Figure 5.
Long-term GDF15-exposed hPdLFs show an increased pro-inflammatory response to compressive stimuli with limited activation of osteoclasts. (a, b) Quantitative expression levels of IL6 (a) and COX2 (b) encoding pro-inflammatory markers in hPdLFs stimulated with 5ng/ml and 20 ng/ml recombinant human GDF15 (rhGDF15) for 36 days (d) and stressed with compressive forces for 24 hours (+CF), displayed in relation to the untreated control. (c, d) Adherent (activated) THP1 cells (green) on stimulated and stressed hPdLFs (blue, cell nuclei) displayed as number of THP1 cells per hPdLFs and in relation to the untreated control in (d). (e, f) Quantitative expression levels of OPG (e) and RANKL (f) in stimulated and stressed hPdLFs displayed in relation to the untreated control. (g, h) TRAP-positive THP1 cells (magenta; g) indicating the differentiation into osteoclasts, when stimulated with the medium supernatant of 36-days rhGDF15-stimulated hPdLFs additionally stressed by CF. The proportion of TRAP-positive osteoclasts is displayed in (h). Red lines showing baseline levels of the respective conditions. */#/§ p < 0.05; **/##/§§ p < 0.01; ***/###/§§§ p < 0.001; */**/*** in relation to control; #/##/### in relation to control +TF; §§/§§§ baseline (red) in relation to 5 ng/ml or 20 ng/ml rhGDF15 +TF; one-way ANOVA and Tukey post hoc test. Scale bars: 25 µm in (c) and (g).

Table 1.
qPCR primer sequences of human genes indicated in 5`-3` direction. bp, base pairs. Length, amplicon length.
Table 1.
qPCR primer sequences of human genes indicated in 5`-3` direction. bp, base pairs. Length, amplicon length.
| Gene | Gene Symbol | NCBI Gene ID | Primer Sequence | Length |
|---|---|---|---|---|
| Activin A receptor like type 1 |
ACVRL1 (alias ALK1) |
94 | fw: GTGGAGTGTGTGGGAAAAGG rev: CATGTCTGAGGCGATGAAGC |
180 bp |
| Activin A receptor type 1 |
ACVR1 (alias ALK2) |
90 | fw: GCATTCCCAGAGCACCAATC rev: GGCCACTTCCCACAAAACAA |
166 bp |
| Activin A receptor type 1B |
ACVR1B (alias ALK4) |
91 | fw: TTAGTGCCCTCTGACCCTTC rev: ATCATCTTCCCCATCACCCG |
122 bp |
| Activin A receptor type 1C |
ACVR1C (alias ALK7) |
130399 | fw: TGTTGGTCTGGTTTACTGGGA rev: TGCTTCACAACTTTGCCACT |
181 bp |
| Alkaline Phosphatase | ALPL | 249 | fw: ACTGCAGACATTCTCAAA rev: GAGTGAGTGAGTGAGCA |
190 bp |
| Bone morphogenetic protein receptor type 1A | BMPR1A | 657 | fw: ACCACTTCCAGCCCTACATC rev: TTGACACACACAACCTCACG |
172 bp |
| Bone morphogenetic protein receptor type 1B |
BMPR1B (alias ALK6) |
658 | fw: GCATCAAGAAGTTACGCCCC rev: TGGGACTCTGACATTTTGGC |
160 bp |
| Interleukin 6 | IL6 | 3569 | fw: CATCCTCGACGGCATCTCAG rev: TCACCAGGCAAGTCTCCTCA |
164 bp |
| Interleukin 10 | IL10 | 3586 | fw: AGCCATGAGTGAGTTTGACA rev: AGAGCCCCAGATCCGATTTT |
141 bp |
| Interleukin 1 receptor antagonist | IL1RN | 3557 | fw: GATGTGCCTGTCCTGTGTCA rev: ACTCAAAACTGGTGGTGGGG |
146 bp |
| Mitochondrially encoded cytochrome c oxidase II |
MT-COX2 (alias COX2) |
4513 | fw: GATGATTGCCCGACTCCCTT rev: GGCCCTCGCTTATGATCTGT |
185 bp |
| Ribosomal protein L22 | RPL22 | 6146 | fw: TGATTGCACCCACCCTGTAG rev: GGTTCCCAGCTTTTCCGT TC |
98 bp |
| RUNX family transcription factor 2 | RUNX2 | 6146 | fw: CCCACGAATGCACTATCC rev: GGACATACCGAGGGACA |
120 bp |
| TATA-box binding protein |
TBP | 6908 | fw: CGGCTGTTTAACTTCGCTTCC rev: TGGGTTATCTTCACACGCCAAG |
86 bp |
| TNF receptor superfamily member 11b |
TNFRSF11B (alias OPG) |
4982 | fw: GAAGGGCGCTACCTTGA | 142 bp |
| rev: GCAAACTGTATTTCGCTC | ||||
| TNF Superfamily Member 11 |
TNFSF11 (alias RANKL) |
8600 | fw: ATCACAGCACATCAGAGCAGA rev: TCACTTTATGGGAACCAGATGGG |
160 bp |
| Transforming growth factor beta receptor 1 |
TGFBR1 (alias ALK5) |
7046 | fw: AAAACTTGCTCTGTCCACGG rev: TGCCAGTCCTAAGTCTGCAA |
157 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








