Preprint
Article
Evaluation of Artemisia absinthium. L, Essential Oil as a Potential Novel Prophylactic Against the Asian Citrus Psyllid Diaphorina citri Kuwayama
Altmetrics
Downloads
171
Views
96
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Alerts
Abstract
Interest in developing novel crop protectants has gained attention in the recent decade due to the hazardous effects of synthetic pesticides on humans and the environment. The citrus industry worldwide is threatened by the D. citri, the primary vector of phlo-em-limited bacterium (HLB) or citrus greening. However, there is no cure for citrus greening available. Diaphorina citri management largely depends on synthetic insecticides, but their massive application leads to resistance in pest populations. Therefore, alternative pest management strategies are needed. Our results indicated fewer D. citri adults settled on plants treated with ABEO than on control 48 h after re-lease. The psyllid fed on citrus leaves treated with ABEO significantly secreted less honeydew than the control. The essential oil showed potent ovicidal activity against the D. citri eggs with LC50 5.88 mg/mL. Furthermore, we also explore the fitness of D. citri on ABEO-treated and untreated Citrus × sinensis by using two-sex life table tools. The results indicated that the intrinsic rate of increase (r) was higher on untreated seedlings (0.10 d−1) than those treated with a sub-lethal dose of ABEO (0.07 d−1). Similarly, the net re-productive rate (R0) was higher for untreated seedlings (14.21 offspring) than those treated (6.405 offspring). Notably, the ABEO were safer against Aphis melifera, with LC50 ranging from 31.05 to 55.86 mg/mL, which is relatively higher than the LC50 24.40 mg/mL values against D. citri. Our results indicate that ABEO exhibits toxic and behavioral effects on D. citri that could be useful for managing this pest.
Keywords:
Subject: Biology and Life Sciences - Agricultural Science and Agronomy
1. Introduction
The Asian citrus psyllid D. citri Kuwayama (Hemiptera: Psyllidae), is a well-known significant citrus pest worldwide. Huanglongbing (HLB) is one of the most devastating diseases of Citrus spp worldwide that limits commercial production [1]. The HLB-infected trees show off-season bloom, twig dieback, stunted growth, early fruit dropping and ultimately, death of the whole plant may occur [2,3]. However, no cure for HLB has been reported [4], and controlling D. citri is the only effective way to manage HLB [5]. The primary control measures of D. citri largely depend on synthetic insecticides [6,7].
Similarly, Florida's citrus industry has been devastated by HLB and, since 2005, lost 74% of production [8]. Insecticidal suppression of D. citri has played a disproportionally significant role in HLB management. Eight to 12 insecticide applications are commonly used in one cropping season in China, Florida, Brazil, Mexico and other major citrus-growing countries worldwide [9]. Under such selection pressure, the resistance to organophosphate, pyrethroid, neonicotinoid and carbamate insecticides in D. citri has been reported [9,10]. Similarly, the massive use of these synthetic insecticides also negatively affects the environment, mammals, natural enemies, pest resurgence and development of resistance [11]. Therefore, further effort is needed to identify a greater diversity of more sustainable tools to improve HLB management.
Botanical insecticides have long been used as alternatives to synthetic chemical insecticides for pest management because botanicals cause no or minimal threat to the environment or human health [12]. Among the botanical pesticides, essential oils (EOs) are gaining the attention of scientists due to their ability to interfere with the primary metabolic, biochemical, physiological and behavioural functions of insects [13,14]. Artemisia absinthium L. (Asteraceae), known as wormwood, is an aromatic and medicinal herb of ethnopharmacological interest [15]. In addition, it has been reported to have toxicity against Callosobruchus maculatus and Bruchus rufimanus [16], repellent and larvicidal activity against mosquitos (Benelli, 2015; Govindarajan and Benelli, 2016), ingestion toxicity against Sitona lineatus [17] and Drosophila melanogaster [18], and fumigant toxicity against Tetranychus urticae and Bemisia tabasi [19].
This study aimed to investigate the toxicity and behavioral effect of Artemisia absinthium essential oil against D. citri. Furthermore, the effect of Essential oil on the fitness of D. citri was also assessed using two-sex life table tools.
2. Materials and Methods
2.1. Insects
The adults of the Diaphorina citri were collected from Citrus x aurantium L. grown at South China Agricultural University Guangzhou, China (23◦15 N, 113◦ 35 E). The adult psyllids were aspirated very carefully to avoid mechanical injury and were allowed to acclimatize to laboratory conditions 27± 2 ◦C, 65 % ± 5 % R.H., photoperiod 14:10 (Light: Dark) for 72 h before the bioassay. Males and females were separated based on their morphological characteristics. The yellow or orange color of the female abdomen indicates that it contains eggs [20,21].
2.2. Plant materials extraction procedure
The leaves and flowers of A. absinthium were collected from the Skardu Baltistan, Pakistan (N35◦16.775, E075◦38.402, Elevation 2396 meters) in August 2022. The plant species were identified by comparing the voucher specimen PH004 (ART004) Quaid-e-Azam University Islamabad, Pakistan [22]. The GC-MS analysis of A. absinthium essential oil was reported in our previous paper [23] (Table 1). It was stored in transparent glass vials (1.5mL) (CNW. Technologies (Shanghai) Inc.) and was kept at 4◦C for further uses.
2.3. Settling behavior of Diaphorina citri
The attractiveness and settling behaviour of D. citri adults towards sweet orange seedlings (10-15cm in length) sprayed with different concentrations 0.1, 0.5, 1, 2, 3% w/v of ABEO, diluted in 20% ethanol containing 0.01% Tween80, which corresponds to the dosage of 1, 5, 10, 15, 20 and 30 mg/mL respectively were observed in a choice experiment under controlled laboratory condition. One hundred adults (50 male and 50 female) were released into the centre of cages (60 cm x 30 cm x 60 cm), each cage with six C. sinensis seedlings and one from each treatment, including control. Seedlings were sprayed with 1 mL of desired concentrations of ABEO until the product runoff and allowed to dry for one hour under the hood. The flasks were randomly positioned inside the cage but equidistant from each other. There was a total of five replicate cages. The total numbers of D. citri adults settling on each seedling were recorded after an interval of 12 and 24 h after release. Within 2 hours after release, the cages were examined to check the mortality due to mechanical injury while aspirating was discarded. 30 mg/mL methanolic extract of Guava was used as a positive control as many reports show that Guava repels D. citri [24,25]. The numbers of D. citri adults settled on each seedling were compared among various treatments using one-way ANOVA, and means were separated with Tukey's HSD test (SPPSS 17.0).
2.4. Antifeedent activity of A. absinthium essential oil against D. citri
The feeding activity of D. citri was evaluated by the amount of honeydew excreted by the adults while they were kept to C. sinensis seedlings treated with ABEO. The feeding bioassay arenas consisted of agar-coated mini glass Petri dishes. Briefly, 3 mL of 1.5 % agar solution was added to 60-mm-diameter mini glass Petri dishes to form a solidified bed. Freshly excised leaves from C. sinensis grown in the laboratory were used for all bioassays. The leaf disk, average size 5.50 ± 0.3 cm in length, was dipped for 5 sec in the desired concentrations of ABEO and allowed to dry for one hour under the fume hood. Ten CO2 anesthetized D. citri adults were released in each petri dish, and the Petri dishes were capped with a lid lined with 60 mm filter paper. After one hour after release, the petri dish was examined to check the mortality due to mechanical injury while aspirating was discarded. Then the Petri dishes were closed with lab parafilm and turned upside down. The filter papers were removed and immersed in 1% w/v ninhydrin 48 h after release for three minutes and then dried at room temperature [26]. The amino acid present in the honeydew on filter paper reacts with ninhydrin forming dark purple spots. The feeding activities of D. citri were assessed by counting the number of purple spots under the stereomicroscope.
2.5. Ovicidal toxicity of ABEO
Ovicidal activities ABEO against D. citri were evaluated by confining the eggs containing sprayed C. sinensis seedlings with an aerial insect net (25 cm long, 20 cm wide). Sixteen mixed populations (8 male and eight female) of D. citri adults were aspirated. The aspirated psyllids were released into gauze nets for five days by covering them with an aerial insect net. After five days, the psyllids were removed from the gauze nets, and the number of eggs was counted under a stereomicroscope. The seedlings were sprayed using Shuaiyu SY-1-8 mini plastic trigger sprayer with the desired essential oil concentrations (approximately 1 mL per seedling). Each foliage shoot on a potted plant was sprayed twice. The plant was sprayed to runoff and allowed to dry for one hour under a fume hood. Then the seedlings were again confined with gauze nets and placed under laboratory condition, and the number of hatched nymphs were counted until all the eggs were hatched. The ovicidal activity was assessed regarding egg mortality rate (E.M.R.) using the formula below.
2.6. Effect of ABEO on fitness and development of Diaphorina citri
All the conditions were like the ovicidal toxicity bioassay. The C. sinensis seedlings were sprayed with LC20 concentration of ABEO. Seedlings were allowed to air dry under the hood for one hour and then exposed to five male and female virgin adult psyllids for mating and oviposition. Treated seedlings were covered with an aerial insect net. A hundred eggs were counted in treated and untreated tests on seedlings. The seedlings were observed daily until adult emergence was complete. The data of development time from eggs to adults formation, after adult formation as pre-oviposition, oviposition, and fecundity were calculated using age-stage, two-sex, life table software [27-29], the population growth rate (PGR.) was calculated using the equation [30,31].
Whereas
- Nf = Final number of D. citri
- N0>N0 = Initial number of D. citri
- Δt= Total number of days for the experiment
The result with positive values indicated an increasing population, PGR= 0 indicated a stable population, while negative values indicated a decline in population and led towards extinction.
2.7. Toxicity against non-targeted organisms
To evaluate the toxicity of ABEO against Apis mellifera, No-choice feeding bioassays were used [32] under laboratory conditions in a plastic container (0.5 L) with a thin net inserted in the cap following the procedure. Briefly, the following concentrations of ABEO (24, 36, 48, 60, and 72 mg/mL) were prepared in 50% sugar solution. Ten healthy foraging workers were introduced into the container. Each concentration was repeated thrice, and the control contained only 50% sugar solution. The mortality data were recorded within 48 h after exposure. The workers' bees were considered dead when they showed no movement upon probing with a camel brush. Each container was considered a single treatment and replicated five times. Imidacloprid (ug/mL) was used as a positive control.
2.8. Statistical analysis
Chi-square goodness of fit tests were used to evaluate the significance of choice between treated and untreated seedlings. The toxicity data was assessed by using Probit analysis to determine the 50% lethal concentration (LC50) and the 90% lethal concentration (LC90) (SPPSS 17.0). According to Levene's test, all data sets were homoscedastic, and the mean difference between treatments was separated by using Tukey's HSD test. The population parameters of D. citri were calculated using the TWO-SEX LIFE TABLE program. The values of population and age stage parameters of D. citri e.g., R0, r, k and sxj, fxj, lx, mx, exj, vxj, respectively, were calculated as described in the methodology of Chi (1988), Jaleel et al. (2018), and Saeed et al. (2022). The following equations explain the two-sex life table calculations.
3. Results
3.1. Effect of ABEO on settling behavior of Diaphorina citri
Concentration and time-dependent effects in settling D. citri adults were observed. The settling behavior of D. citri adults was not significantly different among the various ABEO concentrations tested compared with the control at 24 h (F= 18.98; df =4, 24; P= 0.243) after release. However, significant differences were observed after 48 h (F= 66; df= 4, 24; P= 0.005) and 72 h (F= 86; df= 4, 24; P= 0.001 after release (Figure 1). After 72 h, more D. citri adults were observed on control plants than on any of the ABEO treatments. However, no considerable repellant effect of 30 mg/mL methanolic extract of Psidium guajava was observed as a positive control during the settling behavior cage trails.
3.2. Effect of ABEO on Diaphorina citri feeding
The feeding activity of D. citri measured by the amount of honeydew extraction was presented in (Figure 2) The data indicated a concentration-dependent antifeedant effect of ABEO on the feeding activity of D. citri. Except for 1 mg/mL of ABEO assessed, all the treatments 5, 10, 20, and 30 mg/mL significantly reduced the amount of honeydew excretion compared to the control (F=84.47; df= 4, 24; P<0.0001). However, there was a reduction of 92 and 86% honeydew excretion by ABEO at 20 and 30 mg/mL. However, the effect of ABEO on D. citri feeding in term number of honeydew droplets recorded per filter paper disc is lower than cyantraniliprole, a synthetic anthranilic diamide insecticide, which caused an 80 % reduction in honeydew droplets secretion by D. citri at 0.1 µg/mL [33].
3.3. Effect of ABEO on eggs hatchability of D. citri
A concentration-dependent response of ABEO on eggs hatchability of D. citri was observed. The ABEO has shown potent ovicidal activity with LC50 5.88 mg/mL (Table 2). The number of eggs hatchability per plant was significantly lower than the control except for 1 mg/mL ABEO (F=63.82; df= 5, 29; P <0.0023). Whereas sweet orange potted plants treated with 30 mg/mL ABEO only 11.75% of eggs could hatch into adults, followed by 5, 10, and 20 mg/mL, only 30.44, 72.46 and 83.65% of eggs were able to hatch into adults. However, ABEO ovicidal activity is lower than cyantraniliprole, a synthetic anthranilic diamide insecticide, which caused complete inhibition of D. citri egg's hatchability at 0.025 µg/mL [33].
3.4. Population parameters
The intrinsic rate of increase (r) was higher on untreated sweet orange (0.10 d−1) than treated with LC20 concentration of ABEO (0.07 d−1). Similarly, the net reproductive rate (R0) was higher for untreated C. sinensis seedlings (14.21 offspring) than treated (6.40 offspring) with LC20 concentration of ABEO (Table 3).
Diaphorina citri, comprehensive age-stage survival rate (sxj) on treated and untreated C. sinensis seedlings, was determined. Our findings showed the possibility of a freshly hatched larva making it to age x and stage j (Figure 3). Because development rates varied across individuals on treated and untreated seedlings. The projected curves exhibited completely different layouts at each developmental stage. Individual survival rates rapidly dropped with age increased and showed an inverse relationship between treated and untreated seedlings (Figure 3). The developmental time of D. citri female was longer, and the survival rate was shorter on untreated than treated. While in the case of males, the development was shorter, and the survival rate was longer on untreated than on treated C. sinensis seedlings (Figure 3).
The highest value of age-stage specific fecundity (fxj) was higher on untreated sweet oranges as compared to treated (Figure 3). There is a direct relation in age-specific maternity (lx*mx) of D. citri. As the survival rate increases, fecundity increases in treated and untreated cases. However, the constant peak point of age-specific maternity (lx*mx) of D. citri was higher on untreated sweet oranges than on treated C. sinensis seedlings with ABEO (Figure 4).
The effects of treated and untreated sweet oranges on the population's expected average life expectancy (exj) at egg, nymph, and adults stages of D. citri were determined in (Figure 5). The longevity of the newly hatched D. citri eggs was longer on untreated sweet oranges as compared to treated. The maximum point of the graph on untreated sweet oranges eggs at zero age day their life expectancy (exj) was higher on untreated sweet oranges as zero age day (Figure 5). With heterogeneity in various developmental phases, an increasing tendency in female adult expectancy was identified on untreated sweet oranges in comparison to treated with ABEO. Overall, all stages of the highest life expectancy (exj) of D. citri were observed on untreated sweet oranges (Figure 5). Age-stage reproductive value (vxj) of D. citri in (Figure 6) explains an individual's contribution to the future population (i.e., the population forecasting scale) at age x and stage j.
3.5. Toxicity of ABEO against Aphis mellifera
4. Discussion
Botanicals insecticides are plant-derived materials and include pyrethrin, azadiractin and neem oil, garlic, capsaicin, and vegetable oil. Botanicals are generally short-lived in the environment, as they are broken down rapidly in the presence of light and air [34]. These include plant extracts and essential oils, which are eco-friendly, biodegradable, and nontoxic to mammals [15]. Essential oils from various plants are reported having antifeedant, repellent, and toxic against many insect pests [35]. These comprise various compounds but are dominated mainly by monoterpenes and sesquiterpenes [36]. These monoterpenes and sesquiterpenes can be lethal against insects in different ways. For example, limonene decreased oviposition in mite Oligonychus ununguis [37], carvacrol and thymol showed contact toxicity against Pochazia shantungensis [38], α-pinene, (-)-limonene and (-)-carvone showed fumigant and antifeedant activities against Solenopsis invicta and Meloidogyne incognita [39], thymol showed substantial contact toxicity against Blattella germanica (Yeom et al., 2012), and 1,8-cineole showed both contact and fumigant toxicities against Sitophilus oryzae and Ectomyelois ceratoniae [40]. Carvacrol showed contact toxicity against D. citri [15].
Plant volatile plays a crucial role in herbivores' host location and recognition [41]. Odors and plant colors mediate how the herbivores' insects find and recognize their potential host [42]. The citrus psyllid mainly relies on its olfactory and visual cues to locate and evaluates its host plants [43]. The volatile compounds emitted from non-host plants mask the host plant odor perceived by the phytophagous insects, which results in avoidance and non-preference of host plants[44]. Regarding the repellent activity of ABEO against D. citri, results indicated a concentration-dependent effect. The adults strongly preferred settling on the control C. sinensis seedlings to the treated seedlings. The psyllids settling was not significantly reduced 24 h after release compared to the control. However, after 48 and 72 h of release, only a few adults were observed on the treated plant compared to the control as D. citri were able to identify host plants volatiles from the mixture of non-host volatiles in the open atmosphere in a short period, approximately nine h [45]. A literature report indicated that the host finding and recognition ability of D. citri were reduced when non-host plant semiochemicals were used [44,46]. Many non-host plants have shown repellent activities against D. citri, including Guava [3,24,47], Allium spp [48,49]. HLB bacteria can only multiply in the body of the eukaryotic host [50]. The transmission of HLB bacterium from infected to uninfected tree primarily takes by nymphs and adults of D. citri [51]. Here we found that ABEO reduces the feeding activity of D. citri, measured as the number of purple spots on the treated leaf disc. ABEO at 20 and 20 and 30 mg/mL reduced 72.86 and 85.5% honeydew secretion compared to control. To get more accurate data on the antifeedant activity of ABEO against D. citri, further investigation should be conducted using EPG (Electrical penetration graph) technology.
Essential oils are effective against several insect species. They act as growth inhibitors, toxins, deterrents, repellents, and toxicants [52]. Essential oil of azadirachtin and Piper aduncum against nymph and adults of D. citri caused 90-100% mortality in nymph and below 80% in adults having the edge of nontoxic to ectoparasitoid Tamarixia radiata (Hymenoptera: Eulophidae) [53]. Similarly, the EOs from Syzygium aromaticum, Eucalyptus obliqua , Tithonia diversifolia and Citrus limoniaI showed considerable toxicity and repellent effects against D. citri [54,55]. Primarily D. citri management was prodigiously focused on controlling adult psyllids. For this, many classes of insecticides have been utilized [56]. Limited literature is available regarding the developmental and ovicidal products against D. citri. The current investigation showed that potted C. sinensis seedlings treated with ABEO give a concentration-dependent ovicidal activity. Only 11.75% and 30.44 eggs were able to hatch into adults when confined with the dry residue of ABEO at 20 and 30 mg/mL, while in control and 10 mg/mL concentration, the hatching percentages were 93.45 and 93.78%, respectively. The result showed that ABEO has ovicidal activity against D. citri. Neurotoxicity is the possible mode of action of EOs against insects [57,58]. The EOs are generally composed of complex mixtures of monoterpenes, biogenetically related phenols, and sesquiterpenes, which have a wide range of hydrophilic-hydrophobic properties which are able to easily penetrate insect cuticles and interfere with their physiological functions [59,60].
Despite the most promising properties of EOs as a natural insecticide, many technical issues are raised for their broader application due to their rapid volatility, and poor water solubility [13]. There are many challenges and constraints related to the commercialization of essential oils, including strict legislation [61], low persistence of effects, and lack of quality and insufficient quantities of raw materials for affordable prices [62]. Their rapid degradability and low persistence may significantly reduce the toxicity of EOs [62]. However, the efficacy and persistence of EOs can be enhanced by encapsulation, nanoparticles and nano gel formulation, and cyclodextrins (CDs) [63]. Overall, EOs have the potential to develop eco-friendly candidates for novel pest management, which should be a top priority to preserve ecosystems from contamination.
Essential oils and plant extracts are safer for the environment, humans, and non-targeted organisms than synthetic insecticides [64]. In our current investigation, the toxicity ABEO was evaluated against A. mellifera. The result indicated that EOshave lower toxicity, as the LC50 value was higher than 31.5 mg/mL compared to the LC50 value of 5.20 µg/insect against D. citri.
5. Conclusions
The current study concluded that A. absinthium essential oil showed repellent ovicidal activities against adult citrus psyllids, with minimal toxicity against honeybees tasted as non-targeted organisms under laboratory conditions. Further research should persuade regarding its broader application and impact on natural enemies. It is concluded that the A. absinthium essential oil might be developed as a novel prophylactic against D. citri with the edge of being environment friendly.
Author Contributions
“Conceptualization, S.A.H.R. and L.A.A.S methodology S.A.H.R. and L.A.A.S.; software, W.J; formal analysis,; investigation, M.S.G.; resources, S.A.H.R; data curation, F.M.A.A.G and W.J; writing—original draft preparation, S.A.H.R; writing—review and editing, S.A.H.R., F.A.A and M.W; visualization, M.S.G, F.A.A, F.A.A; supervision, F.A.A, F.M.A.A.G; project administration, ; funding acquisition F.M.A.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
Project number (PNURSP2023R336), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Project (RSPD2023R112), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R336), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. We also thank the Researchers Supporting Project (RSPD2023R112), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.”.
References
- Bové, J.M. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. Journal of plant pathology 2006, 7–37. [Google Scholar]
- Rizvi, S.A.H.; Ling, S.; Tian, F.; Liu, J.; Zeng, X. Interference mechanism of Sophora alopecuroides L. alkaloids extract on host finding and selection of the Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Environmental science and pollution research international 2019, 26, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Zaka, S.M.; Zeng, X.N.; Holford, P.; Beattie, G.A.C. Repellent effect of guava leaf volatiles on settlement of adults of citrus psylla, Diaphorina citri Kuwayama, on citrus. Insect Science 2010, 17, 39–45. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida entomologist 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Byrne, F.J.; Daugherty, M.P.; Grafton-Cardwell, E.E.; Bethke, J.A.; Morse, J.G. Evaluation of systemic neonicotinoid insecticides for the management of the Asian citrus psyllid Diaphorina citri on containerized citrus. Pest management science 2017, 73, 506–514. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hoy, M.A.; Singh, R.; Rogers, M.E. Laboratory and field evaluations of Silwet L-77 and kinetic alone and in combination with imidacloprid and abamectin for the management of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomologist 2008, 91, 87–100. [Google Scholar] [CrossRef]
- Witten, I.H.; Frank, E.; Hall, M.A.; Pal, C.J. Data Mining: Practical machine learning tools and techniques; Morgan Kaufmann: 2016.
- Singerman, A.; Rogers, M.E. The economic challenges of dealing with citrus greening: The case of Florida. Journal of Integrated Pest Management 2020, 11, 3. [Google Scholar] [CrossRef]
- Chen, X.-D.; Kaur, N.; Horton, D.R.; Cooper, W.R.; Qureshi, J.A.; Stelinski, L.L. Crude Extracts and Alkaloids Derived from Ipomoea-Periglandula Symbiotic Association Cause Mortality of Asian Citrus Psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Insects 2021, 12, 929. [Google Scholar] [CrossRef]
- Tian, F.; Rizvi, S.A.H.; Liu, J.; Zeng, X. Differences in susceptibility to insecticides among color morphs of the Asian citrus psyllid. Pesticide biochemistry and physiology 2020, 163, 193–199. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Chen, W.-J.; Wu, S.; Lei, Q.; Zhou, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and biodegradation of neonicotinoid insecticides. Environmental Research 2023, 218, 114953. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Zeng, X. Seriphidium brevifolium essential oil: a novel alternative to synthetic insecticides against the dengue vector Aedes albopictus. Environmental Science and Pollution Research 2020, 27, 31863–31871. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P. A review on prospects of essential oils as biopesticide in insect-pest management. Journal of Pharmacognosy and Phytotherapy 2009, 1, 052–063. [Google Scholar]
- Isman, M.B. Bridging the gap: moving botanical insecticides from the laboratory to the farm. Industrial Crops and Products 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Tian, F.; Xie, F.; Zeng, X. Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Industrial Crops and Products 2018, 121, 468–475. [Google Scholar] [CrossRef]
- Titouhi, F.; Amri, M.; Messaoud, C.; Haouel, S.; Youssfi, S.; Cherif, A.; Jemâa, J.M.B. Protective effects of three Artemisia essential oils against Callosobruchus maculatus and Bruchus rufimanus (Coleoptera: Chrysomelidae) and the extended side-effects on their natural enemies. Journal of Stored Products Research 2017, 72, 11–20. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; BINIAŚ, B. Effect of water extracts from Artemisia absinthium L. on feeding of selected pests and their response to the odor of this plant. Journal of Central European Agriculture 2016, 17. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Đorđević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta medica 2014, 80, 1698–1705. [Google Scholar] [CrossRef]
- Aslan, I.; Kordali, S.; Calmasur, O. Toxicity of the vapours of Artemisia absinthium essential oils to Tetranychus urticae Koch and Bemisia tabasi(Genn.). Fresenius Environmental Bulletin 2005, 14, 413–417. [Google Scholar]
- Husain, M.A.; Nath, D. The Citrus Psylla:(Diaphorina Citri, Kuw.) Psyllidae: Homoptera; Government of India Central Pubilication Branch: 1927.
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Behavioral evidence for a female-produced sex attractant in Diaphorina citri. Entomologia Experimentalis et Applicata 2008, 128, 450–459. [Google Scholar] [CrossRef]
- Bano, A.; Ahmad, M.; Hadda, T.B.; Saboor, A.; Sultana, S.; Zafar, M.; Khan, M.P.Z.; Arshad, M.; Ashraf, M.A. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. Journal of ethnobiology and ethnomedicine 2014, 10, 43. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Tao, L.; Zeng, X. Chemical composition of essential oil obtained from Artemisia absinthium L. Grown under the climatic condition of Skardu Baltistan of Pakistan. Pak. J. Bot 2018, 50, 599–604. [Google Scholar]
- Silva, J.A.; Hall, D.G.; Gottwald, T.R.; Andrade, M.S.; Maldonado, W.; Alessandro, R.T.; Lapointe, S.L.; Andrade, E.C.; Machado, M.A. Repellency of selected Psidium guajava cultivars to the Asian citrus psyllid, Diaphorina citri. Crop Protection 2016, 84, 14–20. [Google Scholar] [CrossRef]
- Barman, J.C.; Campbell, S.A.; Zeng, X. Exposure to Guava affects citrus olfactory cues and attractiveness to Diaphorina citri (Hemiptera: Psyllidae). Environmental entomology 2016, 45, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Stelinski, L.L.; Rogers, M.E. Biochemical basis of organophosphate and carbamate resistance in Asian citrus psyllid. Journal of economic entomology 2012, 105, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environmental Entomology 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Jaleel, W.; Yin, J.; Wang, D.; He, Y.; Lu, L.; Shi, H. Using two-sex life tables to determine fitness parameters of four Bactrocera species (Diptera: Tephritidae) reared on a semi-artificial diet. Bulletin of entomological research 2018, 108, 707–714. [Google Scholar] [CrossRef]
- Saeed, R.; Mahmood, Z.; Shah, S.I.A.; Jaleel, W.; Ahmad, J.N.; Malik, T.H.; Jan, M.T.; Ghramh, H.A.; Ahmad, Z.; Khan, K.A. Using two-sex life table tools to compare the population parameters of Oxycarenus hyalinipennis costa (Lygaeidae: Hemiptera) when fed on Bt and non-Bt cotton seeds. Journal of King Saud University-Science 2022, 34, 102073. [Google Scholar] [CrossRef]
- Walthall, W.K.; Stark, J.D. Comparison of two population-level ecotoxicological endpoints: The intrinsic (rm) and instantaneous (ri) rates of increase. Environmental Toxicology and Chemistry: An International Journal 1997, 16, 1068–1073. [Google Scholar]
- Bowles, S.; Gintis, H. The evolution of strong reciprocity: cooperation in heterogeneous populations. Theoretical population biology 2004, 65, 17–28. [Google Scholar] [CrossRef]
- Anwar, M.I.; Sadiq, N.; Aljedani, D.M.; Iqbal, N.; Saeed, S.; Khan, H.A.A.; Naeem-Ullah, U.; Aslam, H.M.F.; Ghramh, H.A.; Khan, K.A. Toxicity of different insecticides against the dwarf honey bee, Apis florea Fabricius (Hymenoptera: Apidae). Journal of King Saud University-Science 2022, 34, 101712. [Google Scholar] [CrossRef]
- Tiwari, S.; Stelinski, L.L. Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions. Pest management science 2013, 69, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Suladze, T.; Kintsurashvili, L.; Mshvildadze, V.; Todua, N.; Chincharadze, D.; Legault, J.; Vachnadze, N. Study Of The Cytotoxic Activity Of Alkaloid-Containing Fractions Isolated From Certain Plant Species Growing And Introduced In Georgia. EXPERIMENTAL AND CLINICAL MEDICINE GEORGIA 2023. [Google Scholar] [CrossRef]
- da Silva Sa, G.C.; Bezerra, P.V.V.; da Silva, M.F.A.; da Silva, L.B.; Barra, P.B.; de Fátima Freire de Melo Ximenes, M.; Uchoa, A.F. Arbovirus vectors insects: are botanical insecticides an alternative for its management? Journal of Pest Science 2023, 96, 1–20. [Google Scholar] [CrossRef]
- Yang, Y.; Aghbashlo, M.; Gupta, V.K.; Amiri, H.; Pan, J.; Tabatabaei, M.; Rajaei, A. Chitosan nanocarriers containing essential oils as a green strategy to improve the functional properties of chitosan: A review. International journal of biological macromolecules 2023, 123954. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: with special reference to limonene and its suitability for control of insect pests. Agricultural and Food Science 2008, 10, 243–259. [Google Scholar] [CrossRef]
- Park, J.-H.; Jeon, Y.-J.; Lee, C.-H.; Chung, N.; Lee, H.-S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Scientific reports 2017, 7, 40902. [Google Scholar] [PubMed]
- Karabörklü, S.; Ayvaz, A. A comprehensive review of effective essential oil components in stored-product pest management. Journal of Plant Diseases and Protection 2023, 1–33. [Google Scholar] [CrossRef]
- Ben Abada, M.; Soltani, A.; Tahri, M.; Haoual Hamdi, S.; Boushih, E.; Fourmentin, S.; Greige-Gerges, H.; Mediouni Ben Jemâa, J. Encapsulation of Rosmarinus officinalis essential oil and of its main components in cyclodextrin: application to the control of the date moth Ectomyelois ceratoniae (Pyralidae). Pest Management Science 2023. [Google Scholar] [CrossRef]
- Karalija, E.; Šamec, D.; Dahija, S.; Ibragić, S. Plants strike back: Plant volatiles and their role in indirect defence against aphids. Physiologia Plantarum 2023, e13850. [Google Scholar] [CrossRef]
- Silva, M.S.; Patt, J.M.; de Jesus Barbosa, C.; Fancelli, M.; Mesquita, P.R.R.; de Medeiros Rodrigues, F.; Schnadelbach, A.S. Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae) responses to plant-associated volatile organic compounds: A mini-review. Crop Protection 2023, 106242. [Google Scholar] [CrossRef]
- Ling, S.; Rizvi, S.A.H.; Xiong, T.; Liu, J.; Gu, Y.; Wang, S.; Zeng, X. Volatile signals from guava plants prime defense signaling and increase jasmonate-dependent herbivore resistance in neighboring citrus plants. Frontiers in plant science 2022, 13. [Google Scholar] [CrossRef]
- Gallinger, J.; Rid-Moneta, M.; Becker, C.; Reineke, A.; Gross, J. Altered volatile emission of pear trees under elevated atmospheric CO2 levels has no relevance to pear psyllid host choice. Environmental Science and Pollution Research 2023, 1–12. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Hall, D.G.; Liu, B.; Duan, Y.-P.; Li, T.; Hu, H.-Q.; Fan, G.-C. Host-choice behavior of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) under laboratory conditions. Journal of insect behavior 2015, 28, 138–146. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, X.; Srivastava, A.K.; Tan, Q.; Low, W.; Yan, X.; Wu, S.; Sun, X.; Hu, C. Boron deficiency mediates plant-insect (Diaphorima citri) interaction by disturbing leaf volatile organic compounds and cell wall functions. Tree Physiology 2023. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Gottwald, T.; Nguyen, N.; Ichinose, K.; Le, Q.; Beattie, G.; Stover, E. REFEREED MANUSCRIPT. Proceedings of Proc. Fla. State Hort. Soc; pp. 104–109.
- Mann, R.; Rouseff, R.; Smoot, J.; Castle, W.; Stelinski, L. Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bulletin of entomological research 2011, 101, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Rouseff, R.L.; Smoot, J.; Rao, N.; Meyer, W.L.; Lapointe, S.L.; Robbins, P.S.; Cha, D.; Linn, C.E.; Webster, F.X. Chemical and behavioral analysis of the cuticular hydrocarbons from Asian citrus psyllid, Diaphorina citri. Insect science 2013, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, J.; Li, Y.; Liu, Y.; Liang, J.; Wang, C.; Fang, F.; Deng, X.; Zheng, Z. Pathogenicity and Transcriptomic Analyses of Two “Candidatus Liberibacter asiaticus” Strains Harboring Different Types of Phages. Microbiology Spectrum 2023, e00754–00723. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Heck, M. Variations on a theme: Factors regulating interaction between Diaphorina citri and" Candidatus Liberibacter asiaticus" vector and pathogen of citrus huanglongbing. Current Opinion in Insect Science 2023, 101025. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of essential oils as natural biopesticides; recent advances. Critical Reviews in Food Science and Nutrition 2023, 1–21. [Google Scholar] [CrossRef]
- Hu, W.; Zheng, R.; Feng, X.; Kuang, F.; Chun, J.; Xu, H.; Chen, T.; Lu, J.; Li, W.; Zhang, N. Emergence inhibition, repellent activity and antifeedant responds of mineral oils against Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). International Journal of Pest Management 2023, 69, 27–34. [Google Scholar] [CrossRef]
- Wuryantini, S.; Yudistira, R. The toxicity of the extract of tobacco leaf Nicotiana tabacum L, marigold leaf Tithonia diversifolia (HAMSLEY) and citrus japansche citroen peel Citrus limonia against citrus psyllid (Diaphorina citri Kuwayama), the vector of citrus HLB disease. Proceedings of IOP Conference Series: Earth and Environmental Science; p. 012039.
- Hall, D.G.; Borovsky, D.; Chauhan, K.R.; Shatters, R.G. An evaluation of mosquito repellents and essential plant oils as deterrents of Asian citrus psyllid. Crop Protection 2018, 108, 87–94. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.; Wang, Z.; Liu, J.; Zeng, X. Identification of detoxification genes in imidacloprid-resistant Asian citrus psyllid (Hemiptera: Lividae) and their expression patterns under stress of eight insecticides. Pest Manag Sci 2019, 75, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-arami, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. Journal of chemical ecology 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bullangpoti, V.; Wajnberg, E.; Audant, P.; Feyereisen, R. Antifeedant activity of Jatropha gossypifolia and Melia azedarach senescent leaf extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and their potential use as synergists. Pest management science 2012, 68, 1255–1264. [Google Scholar] [CrossRef]
- Lee, S.; Peterson, C.J.; Coats, J. Fumigation toxicity of monoterpenoids to several stored product insects. Journal of Stored Products Research 2003, 39, 77–85. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Scientific reports 2015, 5, 12690. [Google Scholar] [CrossRef]
- Pavela, R. Limitation of plant biopesticides. In Advances in Plant Biopesticides, Springer: 2014; pp. 347-359.
- Bandi, S.M.; Mishra, P.; Venkatesha, K.; Aidbhavi, R.; Singh, B. Insecticidal, residual and sub-lethal effects of some plant essential oils on Callosobruchus analis (F.) infesting stored legumes. International Journal of Tropical Insect Science 2023, 1–13. [Google Scholar] [CrossRef]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food research international 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. Journal of Environmental Science and Health, Part B 2019, 54, 366–375. [Google Scholar] [CrossRef]
Figure 1.
Settling preference of D. citri adults on sweet orange seedlings treated with various concentrations of ABEO 24, 48, and 72 h after release of adults. Bars within a panel not labeled by the same letter are significantly different according to Tukey’s test (P < 0.05). A 3 % methanolic extract of Guava was used as a positive control.
Figure 1.
Settling preference of D. citri adults on sweet orange seedlings treated with various concentrations of ABEO 24, 48, and 72 h after release of adults. Bars within a panel not labeled by the same letter are significantly different according to Tukey’s test (P < 0.05). A 3 % methanolic extract of Guava was used as a positive control.

Figure 2.
Effect of ABEO on D. citri adult feeding as measured by the number of honeydew droplets produced. Citrus leaf discs treated with various concentrations of ABEO in 20% ethanol+ 0.05 % or 20% ethanol+ 0.05 % Tween 80 (as control) were exposed to 10 D. citri adults. Bars not labeled by the same letter are significantly different from one another according to Tukey’s test (P < 0.05). .
Figure 2.
Effect of ABEO on D. citri adult feeding as measured by the number of honeydew droplets produced. Citrus leaf discs treated with various concentrations of ABEO in 20% ethanol+ 0.05 % or 20% ethanol+ 0.05 % Tween 80 (as control) were exposed to 10 D. citri adults. Bars not labeled by the same letter are significantly different from one another according to Tukey’s test (P < 0.05). .

Figure 3.
Effect of ABEO on the age-stage-specific survival rate (sxj) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.
Figure 3.
Effect of ABEO on the age-stage-specific survival rate (sxj) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.

Figure 4.
Effect of ABEO on the age-specific survival rate (lx), female age-specific fecundity (fx), age-specific fecundity (mx), and age-specific maternity (lx*mx) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.
Figure 4.
Effect of ABEO on the age-specific survival rate (lx), female age-specific fecundity (fx), age-specific fecundity (mx), and age-specific maternity (lx*mx) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.
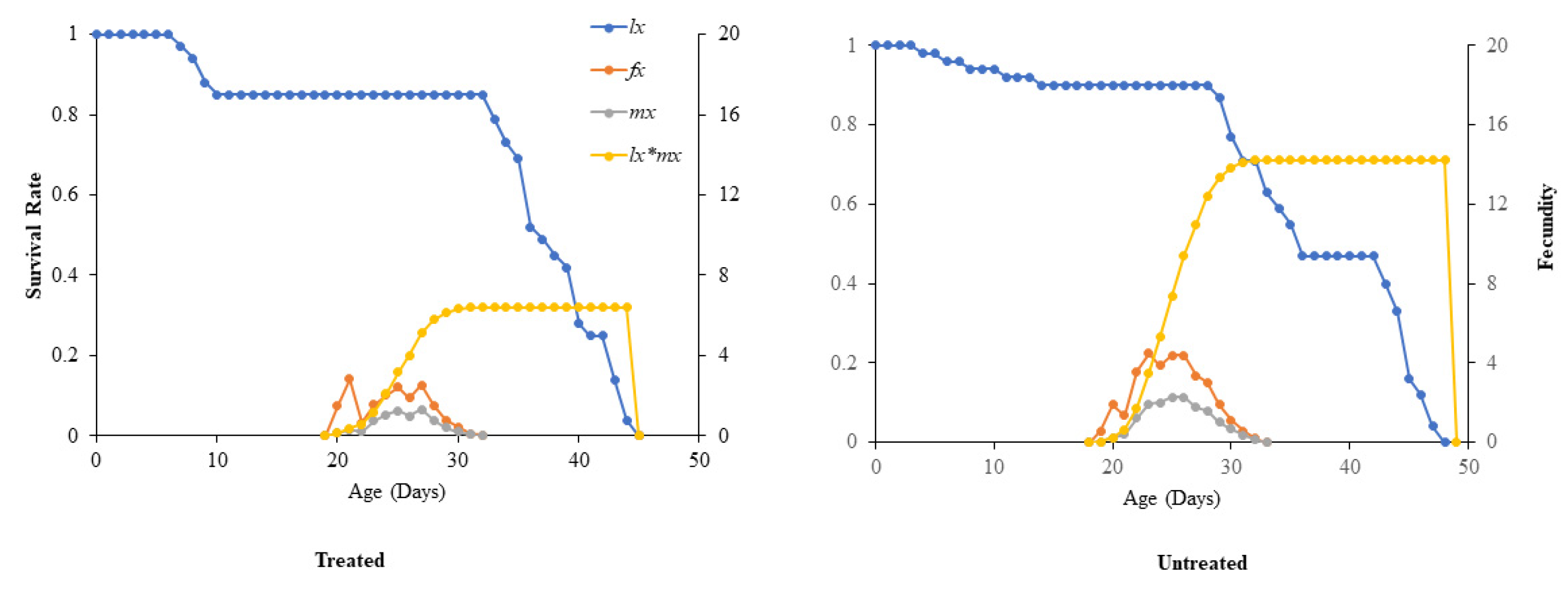
Figure 5.
Effect of ABEO on the life expectancy (exj) of the D. citri on sweet orange seedlings compared to untreated sweet orange seedlings.
Figure 5.
Effect of ABEO on the life expectancy (exj) of the D. citri on sweet orange seedlings compared to untreated sweet orange seedlings.

Figure 6.
Effect of ABEO on the reproductive value (vxj) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.
Figure 6.
Effect of ABEO on the reproductive value (vxj) of the D. citri on sweet orange seedlings in comparison to untreated sweet orange seedlings.

Table 1.
A. absinthium essential oil dominant constituents identified through GC/MS (Source Rizvi et al., 2018c).
Table 1.
A. absinthium essential oil dominant constituents identified through GC/MS (Source Rizvi et al., 2018c).
| Peak # | RTa | Compounds Nameb | Relative % | KI(Exp)c |
|---|---|---|---|---|
| 1 | 12.351 | β-myrcene | 0.86 | 1147 |
| 2 | 13.291 | Pinocarvone | 0.62 | 1172 |
| 3 | 21.838 | α -Gurjunene | 1.68 | 1416 |
| 4 | 22.976 | α -Humulene | 0.94 | 1452 |
| 5 | 23.811 | α-Copaene | 3.51 | 1478 |
| 6 | 24.054 | g-Curcumene | 0.45 | 1486 |
| 7 | 24.754 | epi-Cubenol | 2.67 | 1508 |
| 8 | 26.599 | β -Calacorene | 2.10 | 1570 |
| 9 | 26.737 | (-)-Spathulenol | 1.94 | 1575 |
| 10 | 26.861 | Germacrene-D-4-ol | 3.48 | 1579 |
| 11 | 27.559 | Guaiol | 19.34 | 1602 |
| 12 | 27.837 | Thujol | 2.69 | 1620 |
| 13 | 28.356 | 4-epi-Cubedol | 1.68 | 1631 |
| 14 | 28.64 | Cubenol | 1.89 | 1641 |
| 15 | 29.011 | γ-Eudesmol | 1.19 | 1654 |
| 16 | 29.113 | 8-epi-γ-Eudesmol | 1.14 | 1657 |
| 17 | 29.269 | a-Cadinol | 2.76 | 1663 |
| 18 | 29.844 | Geranial | 8.83 | 1686 |
| 19 | 31.067 | Chamazulene | 5.94 | 1728 |
| 20 | 31.455 | 1,3-Dicyclopentylcyclopentane | 0.93 | 1746 |
| 21 | 32.355 | Fraganol | 0.95 | 1769 |
| 22 | 32.92 | Tetrakis(1-methyl)-Pyrazine | 2.26 | 1797 |
| 24 | 36.568 | Cubedol | 1.16 | 1941 |
| 25 | 36.748 | Geranyl-p-Cymene | 1.63 | 1948 |
| 26 | 37.999 | Nerolidol-epoxyacetate | 1.12 | 1999 |
| 27 | 38.176 | Geranyl-α-terpinene | 5.64 | 2007 |
| 28 | 39.549 | Spathulenol | 0.83 | 2066 |
| 29 | 40.341 | Heneicosane | 1.60 | 2100 |
| 30 | 40.507 | Eugenol | 1.21 | 2102 |
| 31 | 41.557 | Carvacrol | 5.47 | 2147 |
| 32 | 41.721 | α-Bisabolol | 6.17 | 2166 |
| 33 | 43.784 | 1-ethyl-4-methoxy-benzene | 0.53 | 2256 |
| 34 | 44.735 | Tricosane | 1.48 | 2300 |
| 35 | 44.931 | 1-Heptatriacotanol | 1.03 | 2309 |
| 36 | 48.786 | Pentacosane | 2.20 | 2500 |
| 37 | 52.539 | Heptacosane | 1.28 | 2700 |
| 38 | 56.106 | Nonacosane | 0.80 | 2899 |
| Total identified | 99.9 | |||
| Oil yield (%) | 0.46 | |||
| Monoterpenes | 20.42 | |||
| Sesquiterpenes | 52.69 | |||
| Others | 26.89 |
a Retention time. b Compounds are listed in order of their retention time. c Retention index relative to C7-C40 n-alkanes on a DB-1 (30 m x 0.22 mm i.d., 0.25 µm film thickness). e Identification methods: RI, based on comparison of calculated RI with those reported in Adams or NIST 08 and previous literature.
Table 2.
Ovicidal activity of ABEO on D. citri eggs hatchability measured by number D. citri adult emergence.
Table 2.
Ovicidal activity of ABEO on D. citri eggs hatchability measured by number D. citri adult emergence.
| Treatment | % of egg mortality | LC50 (95% CL) mg/mL | Slope ± SE | X² (d.f.) | P-value |
|---|---|---|---|---|---|
| Control | 7.76 ± 1.37 | 5.88 (2.27-12.09) | 1.40 ± 0.134 | 16.83 (3) | 0.326 |
| 1 mg/mL | 14.78 ± 1.87 | ||||
| 5 mg/mL | 29.00 ± 1.67 | ||||
| 10 mg/mL | 55.67 ± 0.89 | ||||
| 15 mg/mL | 80.42 ± 0.65 | ||||
| 30 mg/mL | 92.44 ± 0.28 |
aEggs containing sweet orange potted plant were sprayed with 1 mL of different concentrations of ABEO. b 50% lethal dose. c 90% lethal dose. d 0.025 µg/mL of cyantraniliprole caused complete inhibition of eggs' hatchability.
Table 3.
Effect of ABEO on reproductive and population parameters of the D. citri on treated sweet orange seedlings compared to untreated sweet orange seedlings.
Table 3.
Effect of ABEO on reproductive and population parameters of the D. citri on treated sweet orange seedlings compared to untreated sweet orange seedlings.
| Traits | Treated | Untreated |
|---|---|---|
| r (per day) | 0.07 | 0.10 |
| ʎ (per day) | 1.07 | 1.11 |
| GRR (offspring) | 7.53 | 16.02 |
| R0(offspring/individual) | 6.40 | 14.21 |
r; The intrinsic rate of increase (per days); ʎ; The finite rate of increase (per days); GRR; Gross reproductive rate (offspring); R0; The net reproductive rate (offspring/individual).
Table 4.
Toxicity of A. absinthium essential oil against Aphis mellifera.
| Concentration mg/mL | Exposeda | % Mortality±SD | LD50(LCL-UCL)b | LD90(LCL-UCL)c | X² (d/f)d | P-value |
|---|---|---|---|---|---|---|
| 24 | 64 | 21.65±0.87 | 31.05(25.58-34.69) | 55.86(46.01-67.81) | 0.94(2) | 0.23 |
| 36 | 60 | 35,32±0.59 | ||||
| 48 | 67 | 52.01±0.87 | ||||
| 60 | 67 | 65.34±0.60 | ||||
| 72 | 63 | 84.32±0.51 | ||||
| 0 | 65 | 4.11±0.24 | ||||
| Imidacloprid (ug/mL) | 60 | 98±0.11 | (17.21)11.45-24.34 | 71.32 (86.32-92.32) | 4.11 (3) | 0.51 |
a Total number of bees treated. b 50 % lethal dose. d 90% lethal dose. e χ2 chi square, d.f. degrees of freedom.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Evaluation of Artemisia absinthium. L, Essential Oil as a Potential Novel Prophylactic Against the Asian Citrus Psyllid Diaphorina citri Kuwayama
Syed Arif Hussain Rizvi
et al.
,
2023
Citrus Essential Oils as an Alternative Method of Control of the Fungus Alternaria alternata (Fr.: Fr.) Keissler
Fernando Alves de Azevedo
et al.
,
2023
Bio-Insecticidal Potential of Tangerine Peel Oil Against Greenhouse Whitefly: A Green Biopesticide Candidate
Nancy Anabel Flores-Mediavilla
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








