Preprint
Brief Report
A Non-electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal
Altmetrics
Downloads
125
Views
39
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Alerts
Abstract
Bioelectric effect is known as combinatorial biofilm treatment with low dose of antibiotics with small electricity. When external electric field or current are applied, biofilms can be affected by the electrostatic force, non-uniform distribution of essential electrolytes, induction of low activity of enzyme, and increase of permeability of biofilms. In this work, we have focused on the reduction of applied electric power consumption that could avoid water electrolysis which was one of major challenges for biomedical applications. We have developed a new type of electrical signal that enhances the efficacy of biofilm removal. The results show combination of alternating and direct current remove biofilm effectively without causing electrolysis. We further developed an oral healthcare device as a bioelectric toothbrush and tested in dental clinic. The results demonstrated 75% reduction of gingivitis. In addition, we have tested a mimicked air conditioner biofilms that are root causes of aerobic hygiene infection. The result demonstrates 81.8% increased biofilm removal. In conclusion, a new bioelectric technology has been developed and demonstrated the biofilm removal efficacy without the electrolysis.
Keywords:
Subject: Biology and Life Sciences - Life Sciences
1. Introduction
1.1. Biofilms
Biofilms are major root causes of human infectious disease, including chronic inflammation [1,2]. Multispecies of bacteria comprised with extracellular matrix that prevents from antibiotic penetration as well as external biochemical stimulus [3,4]. Bacteria in biofilms exchange their gene information and mutate to resist antibiotic treatment [5]. Thus, traditional treatment of biofilm associated infections include invasive surgical procedures.
Developing a new technology for prevention of biofilm growth and non-invasive inhibition of biofilm infections are critical to provide good quality of patients’ lives, including gingivitis, orthopedic infections, chronic skin inflammation, chronic wound infections.
1.2. An alternative method for biofilm treatment
Since the biofilm associated infectious diseases require 500 – 5000 times of antibiotic concentration compared to the non-biofilm infection [4,5], reduction of antibiotic or an alternative therapy as a combinatorial method with electromagnetic waves has been investigated [6]. An integration of electricity with small dose of antibiotic, that is known as principles of the bioelectric effect, can be an appropriated candidate for future biofilm infection management [7]. The electricity induces electrical dipoles of the extracellular matrix, resulting in the molecular vibration and/or displacement, this can create increased permeability and decreased metabolic activity of essential enzyme [7]. This induced condition due to the external electricity can increase susceptibility of antibiotics that could reduce the required concentration of antibiotics. The details of mechanisms of actions are still under investigation [4,5,6,7].
Even if the bioelectric effect is one of promising technologies to overcome biofilm associated severe infections, the induced electrolysis of media due to the high electric voltage requirement is a major challenge to extend the applications toward biomedical area [8]. According to the literature upon 2015, the bioelectric effect work utilized the higher voltage than the threshold of water electrolysis (above 0.82V) [9]. The media electrolysis can cause severe side effects to human and microorganisms [8,10]. The electrolysis induces the separation of chemical elements and requires electrical energy (voltage) to decompose atoms. In water solution, the electrolysis creates hydrogen, chloride and oxygen ions and gases that can dramatically change normal metabolism including cell death [11]. Furthermore, generation of extra electrons due to the electrolysis can cause unexpected oxidation and reduction with host, could result in oxidative stress, including cell signaling malfunction and progression of several diseases (i.e. diabetes, cancer, and cardiovascular diseases) [12]. Thus, the electrolysis induced bioelectric effect cannot be applied for human clinical, ocean, and environmental biofilm associated fields.
Reduction of electricity with high efficacy of bioelectric effect has been investigated. The approach to achieve non-electrolysis induced bioelectric effect is focused on the mechanism of biofilm surface attachment “Van der Waals force”. Various of electric field have been applied under the threshold of electrolysis (0.82V) and demonstrated significant reduction of biofilms with combination of alternating and direct current (AC and DC) of electricity [13]. The efficacy of biofilm of superimposed AC and DC electricity was shown 71% total biomass reduction with a linear fit along the electricity as shown in Figure 1 [13].
In this review, we summarized two major applications of biofilm management based on the unique advantage of the non-electrolysis induced bioelectric effect. The oral biofilm infection and environmental contamination management has been demonstrated. For each application, a specific prototype device, such as a toothbrush for dental applications and a package for heat ventilation air conditioner (HVAC) has been designed, fabricated, and tested. Since the bioelectric effect is characterized free from any potential side effects of toxic molecule generation, the tested system has been successfully certified and tested for safety of international standard in USA, Japan, EU, China, and Korea.
1.3. Oral biofilms
Especially, maintaining of better oral health is considered as an indication of high quality of life. According to the WHO, however, approximately 3.5 billion people have oral diseases that is contributed by inefficient oral biofilm management [14]. Most of oral diseases are originated by biofilms, called plaque [15]. Oral biofilms are also associated with systematic disease. With oral infection condition, several systematic diseases are directly correlated, including stroke [16], diabetes [17], cardiovascular disease [18], Alzheimer [19]. Hence, appropriate oral biofilm cleaning is critical in both public and individual healthcare.
The standard device for oral hygiene is a toothbrush. However, although people brush their teeth every day, half population of the planet have oral diseases [14]. This means that current oral biofilm management system & device should be improved. The oral healthcare system is based on dental clinic visit with home maintenance. Depending on the medical infrastructure, the dental service is varied significantly, including insurance, cost, and quality [20]. Practical oral hygiene maintenance in everyday life is more important than discrete visits of dental clinic for prevention of severe progression on oral diseases [21]. For instance, it is required to develop more efficient oral biofilm cleaning home device.
1.4. Hygiene biofilms
Furthermore, an environmental hygiene control of home appliance is being critical as increased demand of life quality with well-being. Especially, a heat and ventilation with air conditioning system (HVAC) becomes critical to good condition of aerobic hygiene. The root cause of HVAC contamination is considered a biofilm formation on the evaporator part which provide in high moisture due to the compressions and relaxation of refrigerant. The biofilm in HVAC is also a high-risk cause of respiratory infections [22]. Since biofilms can be established with 10 - 24 hours [23], an effective and real-time biofilm removal in the HVAC is required.
Application of small electricity to biofilm can disrupt their structure and metabolism due to the electrostatic force (Van der Waals) that is known as bioelectric effect (BE) [24,25]. The alternating current (AC) can induce enhanced permeability of extracellular matrix of biofilm which can enhance on antibiotic penetration [25]. Direct current (DC) applies for static force on biofilms, resulting in surface detachment with non-uniform distribution of electrolytes [9,26]. This electric power utilized technology has a unique advantage that can be compatible for integration on current home appliance electronics. The major challenges of previous work are induction of media electrolysis that produces toxic biocides to human and microorganisms [10,13].
We have developed a new bioelectric effect technology utilized below the electrolysis threshold voltage. The AC and DC signals are applied simultaneously to create synergistic effect of both mechanisms that enable to decrease the required electric power under the level of the electrolysis [13]. We have summarized on our development and validation of new bioelectric effect oral healthcare device as well as HVAC biofilm cleaning system without causing electrolysis. Clinical trials have been conducted with the BE toothbrush. The results demonstrated significant reduction inflammation under the BE toothbrush condition.
2. Materials and Methods
2.1. Principles of bioelectric effect (BE)
Biofilms are defined in 1970 as slime films with multispecies of bacterial composition [27,28,29,30]. Application of electricity on biofilm is reported by Dr. Costerton for the first time in 1990’s [25]. Since then, several researchers have demonstrated reduction of biofilms, but electrolysis is usually included as mechanism of actions [9,26,31]. In 2015, Dr. Kim reported a non-electrolysis induced bioelectric effect (BE) and quantified the efficacy of biofilm reduction along the energy [13]. Simultaneously applied AC and DC electric field has been demonstrated significant inhibition of biofilms in various bacterial strains [32] with antimicrobial agent combination [33]. The non-electrolysis bioelectric effect has been shown as micro & nanosystem in a microfluidic drug discovery platform [23,33] and biomedical device [34,35]. In this work, we applied both the AC and DC bioelectric effect for oral healthcare with HVAC hygiene devices. The system tested in focused on electrolysis, oral gingival inflammation, and total biofilm reduction of the HVAC evaporator.
2.2. Description of bioelectric effect (BE)
The electrical signal comprised of 0.7V sinusoidal signal at the 10 MHz frequency with 0.7V of DC offset. The frequency has been selected based on the previous work [13,33,34,35] and DC offset defined to avoid electrolysis threshold (below 0.82V) [13]. The schematic of electrical signal is shown in Figure 2(a). The electronic circuit that is embedded on the toothbrush has been developed (Figure 2(b)).
2.3. Quantification of electrolysis experiments
The key contribution of the BE in this work was characterized the method with a biocompatible electricity that is below the threshold potential of media electrolysis (0.82 V at 25 °C in pH 7) [36]. Quantitative studies of the electrolysis effect of the electrical signal were conducted by measuring pH changes using a pH indicator (#36828, Fluka Analytical) which actively reacts at pH 4 – 10. A 1 mL of bacterial growth media (LB media, Life Technologies Inc. Carlsbad, Cam USA) was placed in sterilized cuvettes and applied the 0.7 V amplitude sinusoidal signal at 10 MHz (AC) with 0.7 V DC for the media by a function generator (Agilent Technology, USA) for 24 hours. Then, the pH indicator was added to the solution and measured a specific wavelength optical density (OD616). Each experiment was repeated three times and presented an average value with their standard deviations. Figure 3 shows the schematic and photo of experimental set up.
2.4. Developing a BE toothbrush
Based on the advantage of low electricity of the technology, we have developed a BE embedded toothbrush for the first time. The device is characterized by integration of two metallic electrodes on the brush. The material (stainless steel) has been chosen to be anticorrosive and conductive for electric field supply. The BE generating circuit (figure 1(b)) included on the main electronics of the toothbrush. Details of toothbrush design rule has been reported previously [37].
For randomized double-blinded clinical trials, non-BE toothbrush is the same device to the BE toothbrush, but the BE circuit is not working.
2.5. Clinical trials with the toothbrush
We have conducted clinical trials to validate the performance of the BE toothbrush. The decrease of inflammation of gingival area was investigated by measuring the gingival index (GI) that is conducted in Department of Dentistry, College of Medicine, University of Ulsan, Korea. All studies were approved by the Institutional Review Board (IRB) of Ulsan University Hospital, Korea.
Biofilm accumulation of gingival area is root cause of inflammation. Thus, if the plaque has been significantly reduced, it is also expected to decrease of gingival inflammation that is quantitatively measurable via gingival index [38]. The study designed as randomized double blindness with four visits before and after different toothbrush uses for two weeks. The washout period was designed two weeks that the inflammation of gingival expected. The process flow of the GI clinical trial is shown in Figure 4.
The data analysis was focused on the relative changes of GI before and after measurement. The statistical analysis was performed (ANOVA, P-value).
2.6. Developing a HVAC biofilm cleaner
The electronics for BE application was the same circuit described in Figure 2. For mimicked evaporator assembly, seven electrodes with 1.5 mm width are placed at 6 mm pitch on the side of electronics package as shown in Figure 5. During biofilm growth experiments, the package has been placed into a growth media (LB media, Life Technologies Inc. Carlsbad, Cam USA) and grow biofilms for 48 hours. The BE was applied for 1 hour and followed quantitative analysis using the standard fluorescence staining method.
2.7. Testing of the HVAC system and data analysis
We have chosen bacterial strain as Escherichia coli W3110 that is a standard biofilm [13,32,33,34,35]. The biofilm growth was followed by the standard procedures based on the previous work [32,33,34,35]. Initially all samples were grown biofilms with favorable conditions, including supply of nutrients, electrolytes, pH, enzyme via the standard growth media and temperature with incubator set 37℃. After 48 hours of biofilm growth, a randomly chosen one electrode exposed area was stained by fluorescent dye (Alexa Fluor 647 Ester, thermos Fisher Scientific Inc. USA) as the initial condition. The remaining samples were turned on the BE applications for 1 hours. One randomly chosen plate was stained the green fluorescent dye as the BE-treated samples. The testing was repeated 6 times (N=6) and the result summarized in Table 3.
The total biofilm has been quantified through image analysis; 1) fluorescent microscopy images (IX83, Olympus fluorescence microscopy), 2) the image converted to the binary image (black and white, Image J 1.53, NIH, Bethesda, MD, USA), 3) calculated the percentage of surface coverage as shown in Figure 8 and Table 3.
3. Results
3.1. Quantification of electrolysis
Since electrolysis of the media involves hydrogen gas generation resulting in decreased concentration of hydrogen ions, the pH of the medium was expected to become slightly basic due to the electrolysis. Using a pH 8 buffer solution, a strong absorbance peak was shown at a wavelength of 616 nm (OD616). Thus, the electrolysis effect was quantified by measuring the OD616 after applying electric fields.
The results show the BE does not induce significant electrolysis due to the electrical energy supply. Compared to the threshold of the electrolysis (0.82V DC), the BE was shown a minimal pH change (less than 0.05 changes of pH). Therefore, this concludes that the BE does not induce massive electrochemical condition changes in biofilm growth media.
Table 1.
Measurement of optical density at 616nm for electrolysis quantification (N=6).
| OD616 | Control | BE | 0.82V |
|---|---|---|---|
| Average | 0.007 | 0.006 | 0.360 |
| Stdev | 0.045 | 0.012 | 0.250 |
| P-value | NA | > 0.05 | < 0.05 |
3.2. Reduction of GI
The oral biofilm is a root cause of gingival inflammation. Thus, quantification of gingival index (GI) can be corresponded to the plaque [38]. Participants visited dental clinic and results of GI has been shown in Figure 7 and Table 2. The GI has been significantly reduced in the condition of BE toothbrush (ANOVA, P<0.05). As the bioelectric effect can remove biofilms from the surface based on the electrical force, we think that biofilm reduction can be increased and results in the reduction of GI. This result suggests that the BE toothbrush can be an effective new method for oral healthcare management based on the significant oral biofilm inflammation decrease.
3.3. Reduction of HVAC biofilms
When the BE applied for 1 hour after biofilm growth for 48 hours, the total biomass has been significantly reduced as shown in Figure 7. The initial biofilms were in average 23.575% of surface coverage with 7.794% standard deviations. However, 1 hour application of the BE, the average surface overage of biofilms significantly decreased 4.294% in average with 3.473% standard deviation. The statistical significance has been confirmed one-way ANOVA analysis with P < 0.05 (P=0.00025) as shown details in Table 3. Compared to the initial biofilms, the BE technology demonstrates approximately 81.8% increased biofilm treatment efficacy in Figure 9.
Figure 8.
Representative fluorescence and binary images (Image J 1.53., NIH, USA) of initial and BE 1 hour applied biofilms. (a) initial biofilms after 48 hours growth (green color represents biofilms) (b) binary image converted version (black color represents biofilms), (b) after 1 hour application of the BE, significant reduced biofilms are presented [33].
Figure 8.
Representative fluorescence and binary images (Image J 1.53., NIH, USA) of initial and BE 1 hour applied biofilms. (a) initial biofilms after 48 hours growth (green color represents biofilms) (b) binary image converted version (black color represents biofilms), (b) after 1 hour application of the BE, significant reduced biofilms are presented [33].

Table 3.
Quantitative data of biofilm surface coverage (N=6) [39].
Table 3.
Quantitative data of biofilm surface coverage (N=6) [39].
| # of Sample | Initial (%) Surface Coverage |
After 1 hour BE (%) Surface Coverage |
Reduction (%) (After-Initial)/(Initial) |
|---|---|---|---|
| 1 | 20.275 | 1.708 | -91.6% |
| 2 | 34.489 | 1.721 | -95.0% |
| 3 | 19.136 | 2.337 | -87.8% |
| 4 | 31.733 | 2.822 | -91.1% |
| 5 | 14.499 | 10.026 | -30.9% |
| 6 | 21.320 | 7.148 | -66.5% |
| Average | 23.575 | 4.294 | -81.8% |
| Stdev (P-value) | 7.794 | 3.473 | P<0.05 (P=0.00025) |
Figure 9.
Percentage of the surface biofilms between control (initial) and BE 1 hour treated conditions [39].
Figure 9.
Percentage of the surface biofilms between control (initial) and BE 1 hour treated conditions [39].

4. Discussion
Biofilms are 80% of severe human infectious diseases and cost for management over $1,283 bn USD in the world [40]. There is a great interest of developing an effective biofilm management technology. One of promising technology is the bioelectric effect, but previous work limited the applications due to the electrolysis of media which can increase oxidative stress to human health, including significant progress of major diseases including cancer. Thus, the demonstration of non-electrolysis induced bioelectric effect is critical to effective biofilm related biomedical and environmental grand challenges.
The BE does not cause electrolysis as shown in results. This is critical for developing an alternative eco-friendly biofilm management technology which does not create biocides. The electrolysis of water generates diverse chemical toxic molecules, including peroxides (H2O2), hydrogen oxide (OH-), hydronium ion (H3O+), and hypochlorite (ClO-) from anode and cathode [41]. These molecules can modify local pH from 3.5 to 10.5, resulting in inhibition of cell metabolism through reduction of essential enzyme activities. The biocides can even eradicate normal microorganisms [36]. Hence, non-electrolysis technology based on the BE is critical to open the applications of biomedical field as a biocompatible biofilm inhibition method. Long term stability of the electrolysis under the BE would be investigated further for medical device development.
The bioelectric effect (BE) can propagate under the water conditions which characterize low electrical impedance for electric current. Propagation of bioelectricity (0.7V electricity) is approximately within 2cm range in water conditions [37] which is effective area for oral biofilm applications. Especially, the area in between the gum line and teeth, known as the deep pocket region, where biofilm can accumulate significantly and hardly can be cleaned by current bristle due to the tight area, is considered as the root cause of oral disease, including bleeding, inflammation, tartar, and loss of teeth. Since the BE can reach to the deep pocket area via salivary water condition, the BE toothbrush demonstrates reduction of gingival index (GI). The test is going to be extended over 3 months for the details of histological investigation of the inflammation cells. Furthermore, quantitative analysis of total oral biofilm for orthodontic patients who has significant problems with massive biofilms on the brace is under investigation using the BE toothbrush. The histological inflammation and plaque analysis would provide substantial discovery of bioelectric effect not only the efficacy of biofilm removal, but also cell level effect of the BE.
During the HVAC testing, one sample has been shown the decreased biofilm growth (sample#5 in Table 3). However, we included it for data analysis. Even if we try to provide for consistent biofilm growth conditions with appropriate media, temperature, and humidity, biofilm growth can be varied in nature, such as DNA expression, transcription, and amino acid formation [42]. Sophisticated testing setup including microsystem can be utilized for reduced biofilm variance would be investigated further research. In addition, the research would be extended to the various home appliance, including washer and humidifier which often causes biofilms hygiene issues.
The result from the HVAC system can be applied in various biofilm associated contamination field. Biofilms cause healthcare hygiene issues in environments and home applications. For instance, evaporator of the air conditioner induces high moisture conditions which is favorable to the biofilm growth. This can result in respiratory diseases with bad smell from the system [22]. The BE technology has been applied to remove the air conditioning system biofilms based on the advantages of non-electrolysis with ultra-low electric power consumption [39]. The vessel biofouling is also initiated by biofilms formation on the surface [43]. Thus, the technology can be utilized to prevent biocidal effect from the marine microorganisms with the biofouling inhibition efficacy. This ocean applications are currently on-going for expansion of the BE technology.
We are going to further investigate to the oral biofilm management. Dental implant patients require life through diligent work for plaque removal. The BE technology can be an appropriate method for dental implant patients both toothbrush and mouth guard type device. Currently, the system is on developing for medical applications. Details of mechanism of action by the BE is also under investigation through the in-vitro tests, focused on the quantitative study of biofilm detachment in various skin depth.
5. Conclusions
We have demonstrated a non-electrolysis induced BE technology for effective oral and hygiene associated biofilm management. The technology characterizes the electric voltage under the threshold of electrolysis which can be a biosafe and eco-friendly method for biofilm infection treatment. The BE integrated toothbrush has been developed and tested in clinical trials focused on the quantification of total oral biofilms inflammation (gingival index). Integration of the BE on the toothbrush has been shown 75% more inflammation inhibition compared to the non-BE toothbrush users. Also, the HVAC system biofilm inhibition system using the BE shows 81.8% reduction of total biofilm. Since the BE does not induce biocidal chemical generation, we believe that this technology can be an appropriate alternative method to effective biofilm associated infection and contamination management.
Author Contributions
Y.W. Kim, J. Lee, S.K. Han and T. Park designed this project and write the manuscript. B. Koo, S.K. Han, Y.W. Kim, H.M. Park, B.D. Lee developed the system and tested. J. Lee conducted the clinical trials. All authors have been contributed to the review and writing the manuscript.
Funding
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank for College of Medicine, University of Ulsan for technical supports. This research was supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (2021050012).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance - development, composition and regulation - therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.-I.; Nagahori, R.; Yamada, S.; Sugimoto, S.; Sato, C.; Sato, M.; Iwase, T.; Hashimoto, K.; Mizunoe, Y. The Composition and Structure of Biofilms Developed by Propionibacterium acnes Isolated from Cardiac Pacemaker Devices. Front. Microbiol. 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Ciarolla, A.A.; Lapin, N.; Williams, D.; Chopra, R.; Greenberg, D.E. Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics 2023, 12, 54. [Google Scholar] [CrossRef]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The Two Weapons against Bacterial Biofilms: Detection and Treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef]
- Cogan, S.F.; A Ludwig, K.; Welle, C.G.; Takmakov, P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 2016, 13, 021001–021001. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric Effect and Bacterial Biofilms. a Systematic Review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 432, 580–585. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Y.; Rubinsky, B. Molecular and histological study on the effects of electrolytic electroporation on the liver. Bioelectrochemistry 2019, 124, 79–89. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Kim, Y.W.; Subramanian, S.; Gerasopoulos, K.; Ben-Yoav, H.; Wu, H.-C.; Quan, D.; Carter, K.; Meyer, M.T.; E Bentley, W.; Ghodssi, R. Effect of electrical energy on the efficacy of biofilm treatment using the bioelectric effect. npj Biofilms Microbiomes 2015, 1, 15016. [Google Scholar] [CrossRef]
- Petersen, P.E.; Baez, R.J.; Ogawa, H. Global application of oral disease prevention and health promotion as measured 10 years after the 2007 World Health Assembly statesment on oral health, Community Dent Oral Epidemiol. 2020, 48:338–348.
- Abebe, G.M. Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. Int J Oral Dent Health 2021,7:127.
- Alhadainy, H.A.; Keefe, T.; Abdel-Karim, A.H.; Abdulrab, S.; Halboub, E. Association between dental diseases and history of stroke in the United States. Clin. Exp. Dent. Res. 2021, 7, 845–851. [Google Scholar] [CrossRef]
- Albert, D.A.; Ward, A.; Allweiss, P.; Graves, D.T.; Knowler, W.C.; Kunzel, C.; Leibel, R.L.; Novak, K.F.; Oates, T.W.; Papapanou, P.N.; et al. Diabetes and oral disease: implications for health professionals. Ann. New York Acad. Sci. 2012, 1255, 1–15. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Ming, Y.; Hsu, S.-W.; Yen, Y.-Y.; Lan, S.-J. Association of oral health–related quality of life and Alzheimer disease: A systematic review. J. Prosthet. Dent. 2020, 124, 168–175. [Google Scholar] [CrossRef]
- Ghanbarzadegan, A.; Balasubramanian, M. Inequality in dental services: a scoping review on the role of access toward achieving universal health coverage in oral health. BMC Oral Health 2021, 21, 404. [Google Scholar] [CrossRef]
- Wårdh, I.; Jonsson, M.; Wikström, M. Attitudes to and knowledge about oral health care among nursing home personnel - an area in need of improvement. Gerodontology 2012, 29, e787–e792. [Google Scholar] [CrossRef]
- Nitschke, M.; Appleton, S.L.; Li, Q.; Tucker, G.R.; Shah, P.; Bi, P.; Pisaniello, D.L.; Adams, R.J. Lung function reductions associated with motor vehicle density in chronic obstructive pulmonary disease: a cross-sectional study. Respir. Res. 2016, 17, 138. [Google Scholar] [CrossRef]
- Kim, Y.W.; Mosteller, M.P.; Subramanian, S.; Meyer, M.T.; E Bentley, W.; Ghodssi, R. An optical microfluidic platform for spatiotemporal biofilm treatment monitoring. J. Micromechanics Microengineering 2015, 26, 015013. [Google Scholar] [CrossRef]
- Zuki, F.M.; Edyvean, R.G.J.; Pourzolfaghar, H.; Kasim, N. Modeling of the Van Der Waals Forces during the Adhesion of Capsule-Shaped Bacteria to Flat Surfaces. Biomimetics 2021, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Wellman, N.; Fortun, S.M.; McLeod, B.R. Bacterial biofilms and the bioelectric effect. Antimicrob. Agents Chemother. 1996, 40, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzadeh, E.; Wei, C.; Farzadkia, M.; Rezaee, A. Effects of Low Frequency-Low Voltage Alternating Electric Current on Apoptosis Progression in Bioelectrical Reactor Biofilm. Front. Bioeng. Biotechnol. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, G.D.; Stoodley, P.; Kathju, S.; Zhao, Y.; McLeod, B.R.; Balaban, N.; Hu, F.Z.; Sotereanos, N.G.; Costerton, J.W.; Stewart, P.; et al. Engineering Approaches for the Detection and Control of Orthopaedic Biofilm Infections. Clin. Orthop. Relat. Res. 2005, 59–66. [Google Scholar] [CrossRef]
- Bajpai, P. The Control of Microbiological Problems. Pulp and Paper Industry. 2015, 103–195. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: from the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Lappin-Scott, H.; Burton, S.; Stoodley, P. Revealing a world of biofilms — the pioneering research of Bill Costerton. Nat. Rev. Microbiol. 2014, 12, 781–787. [Google Scholar] [CrossRef]
- Freebairn, D.; Linton, D.; Harkin-Jones, E.; Jones, D.S.; Gilmore, B.F.; Gorman, S.P. Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert Rev. Med Devices 2013, 10, 85–103. [Google Scholar] [CrossRef]
- Kim, Y.W.; Meyer, M.T.; Berkovich, A.; Subramanian, S.; Iliadis, A.A.; Bentley, W.E.; Ghodssi, R. A surface acoustic wave biofilm sensor integrated with a treatment method based on the bioelectric effect. Sens. Actuator A Phys. 2016, 238, 140–149. [Google Scholar] [CrossRef]
- Subramanian, S.; Gerasopoulos, K.; Guo, M.; Sintim, H.O.; Bentley, W.E.; Ghodssi, R. Autoinducer-2 analogs and electric fields - an antibiotic-free bacterial biofilm combination treatment. Biomed. Microdevices 2016, 18, 95. [Google Scholar] [CrossRef]
- Subramanian, S.; Huiszoon, R.C.; Chu, S.; Bentley, W.E.; Ghodssi, R. Microsystems for biofilm characterization and sensing – A review. Biofilm 2020, 2, 100015. [Google Scholar] [CrossRef]
- Huiszoon, R.C.; Subramanian, S.; Rajasekaran, P.R.; Beardslee, L.A.; Bentley, W.E.; Ghodssi, R. Flexible Platform forIn SituImpedimetric Detection and Bioelectric Effect Treatment ofEscherichia ColiBiofilms. IEEE Trans. Biomed. Eng. 2019, 66, 1337–1345. [Google Scholar] [CrossRef]
- Chen, B.-K.; Wang, C.-K. Electrolyzed Water and Its Pharmacological Activities: A Mini-Review. Molecules 2022, 27, 1222. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, J.; Lee, T.-H.; Lim, S. Bioelectric effect utilized a healthcare device for effective management of dental biofilms and gingivitis. Med Eng. Phys. 2022, 104, 103804. [Google Scholar] [CrossRef]
- Schwartz, S.B.; Christensen, J.R.; Fields, H. 31 - Examination, Diagnosis, and Treatment Planning, Editor(s): Arthur J. Nowak, John R. Christensen, Tad R. Mabry, Janice A. Townsend, Martha H. Wells, Pediatric Dentistry (Sixth Edition), Elsevier, 2019, Pages 419-454.
- Han, S.K.; Kim, Y.W.; Koo, B.-S.; Choi, H.W.; Lee, S. Developing an Electrical System toward the Prevention of Heat Ventilation Air Conditioning Contamination. Appl. Sci. 2022, 12, 11842. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. npj Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Yuxi, X.; Jin, Z.; R, Qiu. In situ glass antifouling using Pt nanoparticle coating for periodic electrolysis of seawater, Appl Surf Sci, 2015, Volume 357, Part A, Pages 60-68.
- Gordon, V.D.; Davis-Fields, M.; Kovach, K.; A Rodesney, C. Biofilms and mechanics: a review of experimental techniques and findings. J. Phys. D: Appl. Phys. 2017, 50, 223002. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
Figure 1.
(a) total biomass reduction (optical density measurement) along with various type of electricity (AC: alternating current only, DC: direct current only, SP: both AC and DC electricity), (b) total biomass along with external electricity (below the threshold of electrolysis) [13].
Figure 1.
(a) total biomass reduction (optical density measurement) along with various type of electricity (AC: alternating current only, DC: direct current only, SP: both AC and DC electricity), (b) total biomass along with external electricity (below the threshold of electrolysis) [13].

Figure 2.
(a) Schematic of bioelectric effect signal. (Combination of AC and DC signal), (b) Schematic of electronic system to generate the bioelectric effect (integrated to the toothbrush, HVAC cleaner).
Figure 2.
(a) Schematic of bioelectric effect signal. (Combination of AC and DC signal), (b) Schematic of electronic system to generate the bioelectric effect (integrated to the toothbrush, HVAC cleaner).

Figure 3.
(a) Schematic of cuvette for electrical signal applications, (b) 6 parallel experimental setup for electrolysis testing.
Figure 3.
(a) Schematic of cuvette for electrical signal applications, (b) 6 parallel experimental setup for electrolysis testing.
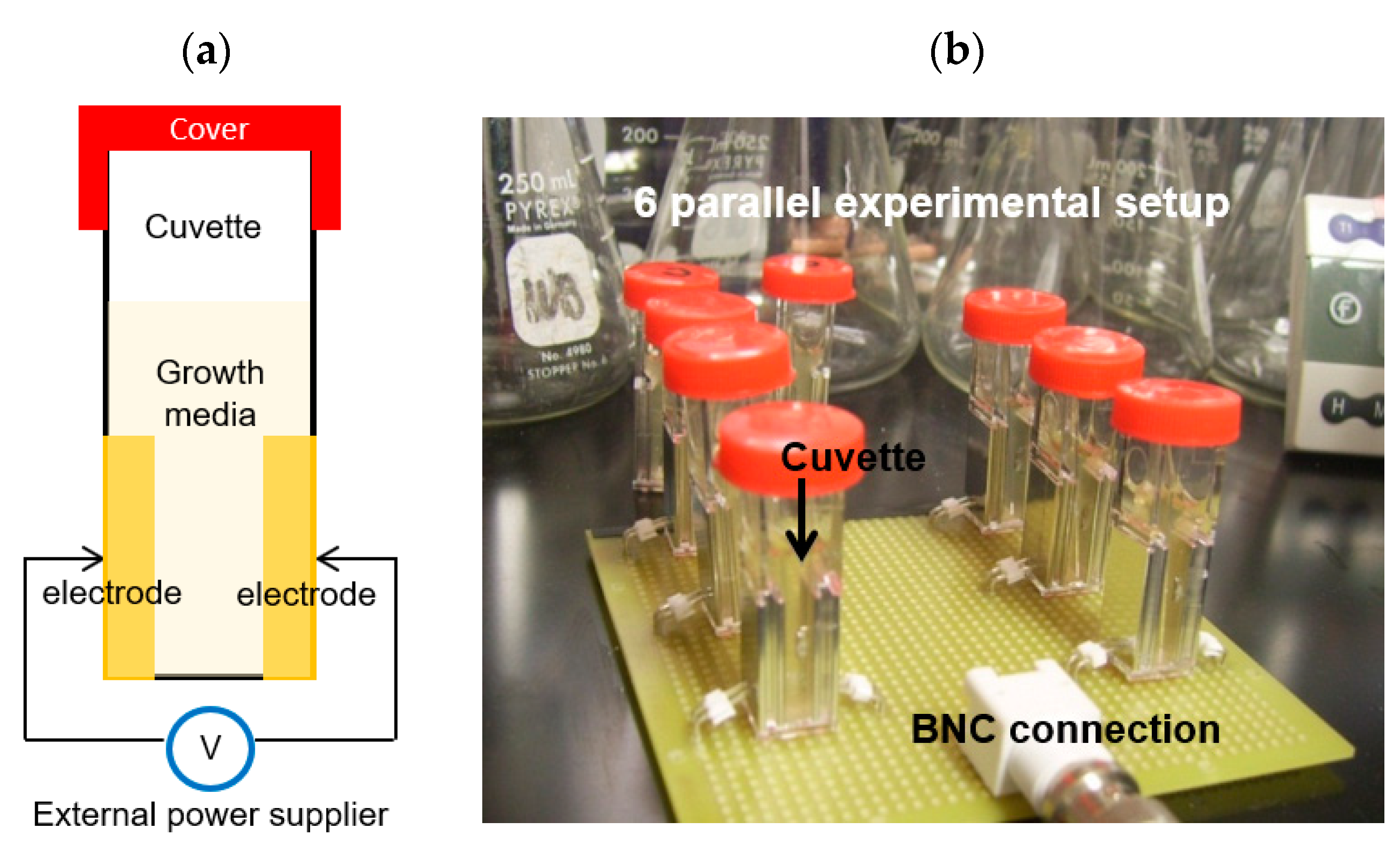
Figure 4.
Schematic of GI clinical trial process [37].
Figure 4.
Schematic of GI clinical trial process [37].

Figure 5.
(a) Schematic of BE assembly evaporator plates, (b) photo of testing set up in water, (c) photo of testing setup in biofilm growth media for 48 hours. [39].
Figure 5.
(a) Schematic of BE assembly evaporator plates, (b) photo of testing set up in water, (c) photo of testing setup in biofilm growth media for 48 hours. [39].

Figure 6.
(a) Quantification of optical density due to the electrolysis, (b) photo of the color changes under the control (no electric field), BE (bioelectric effect electric field), and 0.82V (threshold electric potential of media electrolysis).
Figure 6.
(a) Quantification of optical density due to the electrolysis, (b) photo of the color changes under the control (no electric field), BE (bioelectric effect electric field), and 0.82V (threshold electric potential of media electrolysis).

Figure 7.
Reduction of the GI in Non-BE and BE toothbrush. Statistically significant difference has been shown in BE condition (P<0.05).
Figure 7.
Reduction of the GI in Non-BE and BE toothbrush. Statistically significant difference has been shown in BE condition (P<0.05).
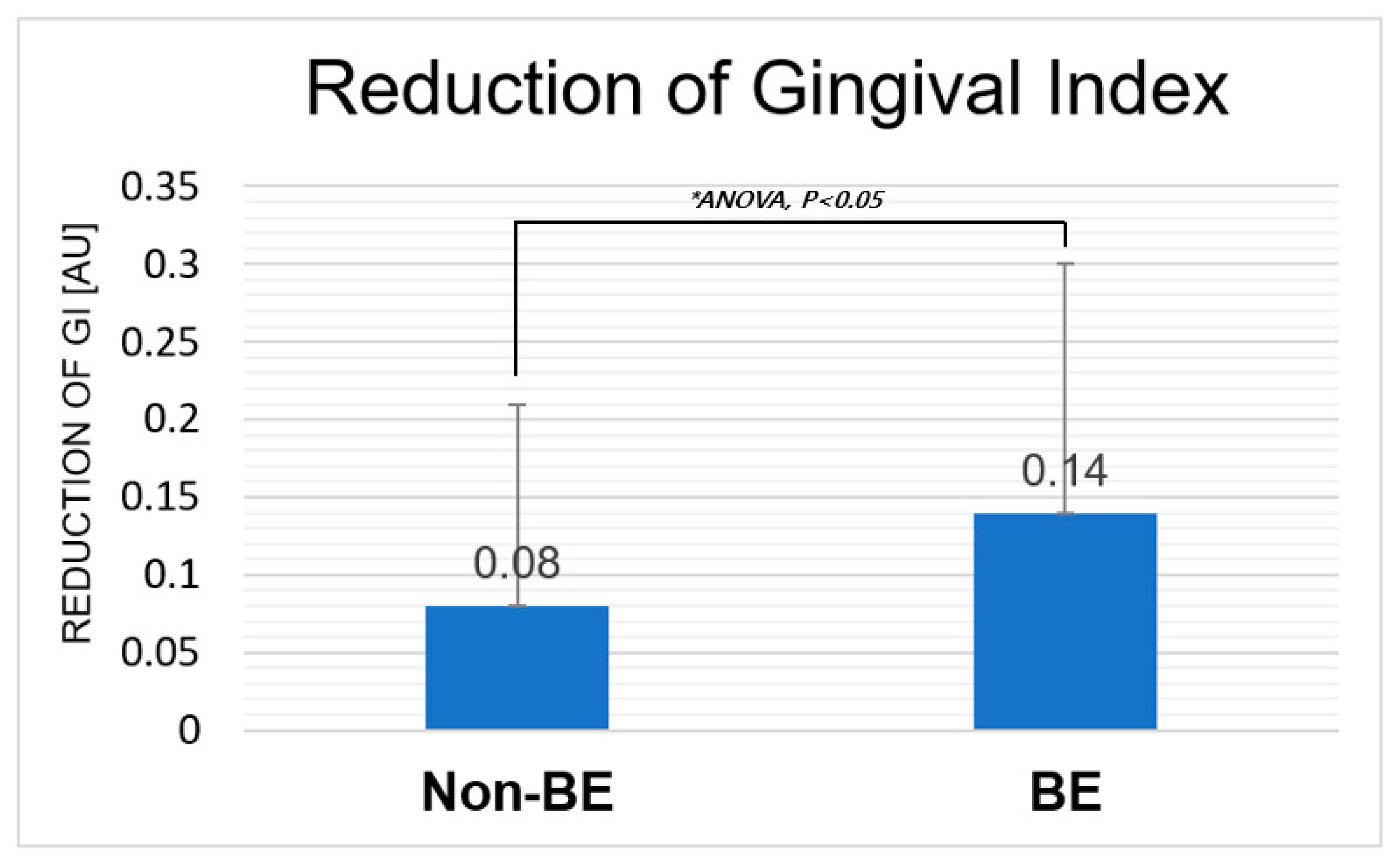
Table 2.
Quantitative data of gingival index (N=40) [37].
Table 2.
Quantitative data of gingival index (N=40) [37].
| Reduction of Gingival index | Non-BE | BE |
|---|---|---|
| Average (Stdev) | 0.08 (0.13) | 0.14 (0.16) |
| P-value (Between non-bioelectric and bioelectric effect) |
NA | 0.034 (P<0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
A Study on Biofilm Removal Efficacy of Bioelectric Toothbrush
Hyun Mok Park
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








