Preprint
Article
Understanding the Impact of COVID-19 on Angioplasty Service and Outcome of Patients Treated for Critical Limb Ischaemia: A Single-Centre Retrospective Cohort Study
Altmetrics
Downloads
186
Views
87
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
02 June 2023
Posted:
05 June 2023
You are already at the latest version
Alerts
Abstract
We evaluated the impact of COVID-19 restriction on the angioplasty service and outcome of critical limb ischaemia (CLI) patients undergoing lower limb angioplasty in a UK secondary care setting. Consecutive patients were analysed retrospectively. Pre-COVID-19 (08/2018-02/2020), 106 CLI-patients (91% Fontaine 4; 60% diabetes mellitus) and during COVID-19 (03/2020-07/2021) 94 patients were treated (86% Fontaine 4; 66% diabetes mellitus). While the average monthly number of patients treated did not change, the proportion of day cases significantly increased (53% to 80%) and hospitalised patients decreased. Patients treated in <=14/5 days after referral significantly increased to 64/63%. Kaplan-Meier survival analysis (30-day/1-year) showed that neither wound healing nor mortality were significantly changed during COVID-19. In day cases, 1-year but not 30-day major amputations significantly increased, and clinically driven target lesion revascularisation decreased during COVID-19. 1-year mortality was significantly worse in hospitalised as compared to day cases (14% vs 43%) at similar wound healing rates (83% vs 84%). The most frequent known-causes-of-death were infectious disease (64%) and cardiovascular (21%) was less frequent. Despite COVID-19 restriction a safe and effective angioplasty service was maintained while shortening waiting times. Very high mortality rates in hospitalised patients may indicate that CLI should be treated more aggressively and earlier.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Peripheral artery disease (PAD) is a growing medical problem worldwide [1], affecting as many as 1 in 4 adults in developed countries [2]. The prevalence increases with age and in diabetes it may be present in up to 50% of patients [3]. In its most severe form, critical limb ischaemia (CLI) the level of blood flow reaching the limb is inadequate for baseline metabolism with patients suffering rest pain and restricting wound healing often leading to tissue loss and limb amputation. A rapid response to a diagnosis of CLI is crucial to prevent further tissue loss and save limbs [4].
Rates of 1-year all-cause mortality in patients hospitalized with CLI are alarmingly high (16-35%) [5]. This is associated with a 1-year risk of amputation of between 5 and 57% [5]. A recent meta-analysis with mean follow-up of 6.3 years showed that 21% of symptomatic PAD patients with claudication progress to CLI with 4-27% undergoing amputations [6]. In nearly half of patients with diabetic foot disease, PAD is present as diagnosed by low ankle brachial pressure index (ABPI) [7]. Supporting the class 1 indications for revascularization in CLI [8,9], a recent meta-analysis showed that even in patients with diabetes revascularization is effective to promote wound healing and prevent amputation in CLI [10].
In March 2020, the rapidly evolving situation regarding the COVID-19 virus and lockdown led to rapid and significant changes in hospital services. The guidance issued by the president of the Vascular Society (along with GIRFT and the Specialist Commissioners) stated: ‘Where possible, only urgent outpatients should be seen, and virtual clinics should be considered…Elective arterial surgery and venous surgery should be deferred… Those legs immediately threatened require urgent intervention. Others may be diverted to a hot foot clinic for further assessment. Interventional radiological approaches may allow more appropriate utilisation of scarce high dependency beds. There may be situations where primary amputation may be more appropriate than complex revascularisations, multiple debridements and potential prolonged hospital stay.’
Here, we evaluated the impact of COVID-19 service restrictions on our angioplasty service and outcome of CLI patients undergoing lower limb angioplasty at East Surrey Hospital.
2. Materials and Methods
2.1. Study Design, Setting and Patients
We performed a single-centre retrospective analysis of consecutive patients undergoing lower limb endovascular revascularisation from July 2018 to February 2020 (‘pre-COVID-19’) and March 2020 to July 2021 (‘COVID-19’) at East Surrey Hospital (Redhill, UK). The hospital is a secondary care district general hospital serving a local population of 744,000 people supported by the vascular surgery hubs at Royal Sussex County Hospital (Brighton and Sussex Medical School, Brighton, UK) and St. George’s University Hospital (London, UK). The team consists of vascular surgeons and interventional radiologists on joint appointments and angiology and diabetology clinical academics on joint appointments with the University of Surrey Medical School (Guildford, UK). We compared patient and procedural characteristics, technical success, peri-procedural complications, 30-day and 1-year wound healing, mortality, major amputations, and clinically driven target lesion revascularizations (TLR) of angioplasties performed in outpatients as day cases and hospitalised patients.
Our vascular spoke centre specializes in day case-based revascularisations in CLI patients as part of a multidisciplinary foot salvage service [11]. Independent of COVID-19, we aimed at following the PAD quality improvement framework (QIF) guidance for ‘non-admitted’ and ‘admitted’ pathways for CLI aiming at revascularization in less than 14 days or less than 5 days, respectively. Of note none of the hospitalised patients in the current study were primarily admitted through the vascular service. Patients presented to the vascular team with leading severe chronic ischaemia and deemed to require revascularization in less than 5 day were discussed with vascular hub and urgently transferred if faster revascularization could be achieved by doing this. Patients with acute limb ischaemia were also immediately transferred to a vascular hub.
2.2. Patient Assessment and Procedure Planning
All patients were presented and discussed at our multi-disciplinary team (vascular surgery, angiology, interventional radiology in attendance) or multi-disciplinary diabetic foot team (vascular surgery, angiology, diabetology, microbiology, podiatry, tissue viability in attendance) meetings. The diagnosis of PAD was based on ABPI < 0.9 with concordant Doppler waveforms [12] and the classification into clinical stages made according to the Fontaine Classification (Fontaine II for claudication, Fontaine III for rest pain or Fontaine IV when rest pain or ischaemic ulcers/gangrene were present and plausibly related to ischaemia. CLI was defined as Fontaine III and IV. In case of incompressible ABPI (>1.2), discordance with Doppler waveform, or borderline values that appeared implausible compared to the clinical picture, toe-brachial index (TBI) or intra-arterial angiography were performed [8,13,14]. Indications for revascularisations were based on current PAD treatment guidelines [8,13,14] including short distance lifestyle-limiting claudication not responding to or amenable to exercise therapy, rest pain, non-healing ulcer or gangrene. Individualised optimal revascularization strategy (angioplasty vs open surgery), surgical risk, technical feasibility, and procedure planning were assessed by the team’s interventionalists and vascular surgeons based on clinical characteristics, symptoms and imaging including Duplex ultrasound and CT angiograms [14]. Finally, the multi-disciplinary treatment plan, risks, and expected benefits were discussed with the patient, informed consent signed and procedure scheduled.
2.3. Day Case Criteria and Procedure Preparation
Outpatients were accepted for ‘day case’-based endovascular intervention if all the following criteria were met: body mass index <35 kg/m2, American Society of Anesthesiology score <IV, eGFR >29 ml/min, sheath ≥7F needed for procedure, not socially isolated with <1 h drive to hospital, telephone available with responsible adult present overnight, and no anti-coagulation requiring bridging. In individual cases, patients with lower eGFR or higher ASA were accepted or procedures with 7F performed with special arrangements. When patients fulfilling all medical criteria but were not able to arrange for a responsible adult to stay with them over night or requested to stay in, we admitted the patients ‘overnight’. If the pre- or postprocedural risk was deemed high by the interventionalists or open revascularization required, then patients were referred to be treated at one of the partner vascular surgery hubs.
2.4. Peri- and Post-Procedural Management
Metformin was paused 24 h prior to procedure. Warfarin was paused to reach a target INR <1.5. Direct-acting oral anticoagulants were paused for 48 h prior to the procedure and restarted on the next day. Aspirin and clopidogrel were not paused. Strict fasting status was not required and patients were allowed a small breakfast up to 3 h before the procedure.
All procedures were performed by an experienced consultant angiologist or interventional radiologists according to current treatment guidelines aiming at establishing at least single vessel straight line flow to the foot. In cases of crural artery disease, preference was given to the artery supplying the angiosome in which the ulcer or gangrene was present. All patients received a Duplex ultrasound scan of the access sites on the table by the interventionalist. Access site punctures were mostly performed under ultrasound guidance. Technical success was defined as the visually successful treatment of the target lesion(s) with less than 30% remaining stenosis. If possible, vascular occlusion devices were used (Angioseal, St Jude Medical, Minnesota). A target procedure finishing time was set at 14:00 to allow sufficient observation time in the day case unit prior to discharge from hospital or to the ward.
Post procedures, patients lay flat for 1 h followed by 1 h of bed rest with elevated head, followed by a light snack and drink if desired and discharge after groin check at 4 h. If access was difficult or there was superficial bleeding visible through the transparent dressing, a Duplex ultrasound was performed to exclude a haematoma or pseudo-aneurysm. Unless there was a contra-indication, patients were started on dual antiplatelet therapy consisting of Aspirin 75 mg and Clopidogrel 75 mg for 1-3 months (3 months only when drug eluting stents were implanted) with a clopidogrel loading dose of 300 mg. In case of oral anti-coagulation, we added only clopidogrel temporarily. In addition, medications were reviewed and adapted towards optimal medical management if required.
All patients were scheduled within a month of discharge for a Duplex ultrasound examination of the access site and intervened vessel segment together with ABPI and vascular clinic review. Wound care continued throughout within our network or local podiatry.
2.5. Clinical Outcome Assessment
We recorded wound closure, mortality, major amputation or clinically driven target lesion revascularization (TLR) within 30 days and 1 year following angioplasty based on electronic patient records at East Surrey Hospital. When patients died during the timeperiod the electronic patient records were searched for the Medical Examiners note and the cause of death recorded.
2.6. Statistical Analyses
Data are presented as mean and standard deviation. Mean values were compared using one-way ANOVA and if p<0.05 consecutive Bonferroni post-hoc between group comparisons were considered. Data for which normality could not reasonable assumed and data and categorical parameters were compared using the Mann Whitney U-Test. Wound closure, mortality, major amputation or TLR within 30 days and 1 year following angioplasty were assessed with Kaplan-Meier survival analysis and Log Rank (Mantel-Cox) test. Analyses were performed with SPSS 28 (IBM).
3. Results
3.1. Baseline and Procedural Characteristics
One hundred and six patients with CLI were treated pre-COVID-19 (91% Fontaine 4; 60% diabetes mellitus) and 94 (86% Fontaine 4; 66% diabetes mellitus) were treated during COVID-19. See Figure 1 for time-course of patient numbers. Overall, the clinical characteristics did not differ except for statistically significant higher percentage of patients using statins during COVID-19 (Table 1). With regards to procedural characteristics (Table 2) during COVID-19 the mean treated lesion length was larger (253±158 mm vs 141±97 mm, p<0.001), and stented segments shorter (135±59 mm vs 87±82 mm, p=0.005). The post-procedural ABPI was significantly higher (0.87±0.20 vs 0.81±19, p=0.045).
3.2. COVID-19 Related Changes in Angioplasty Service
While the average monthly number of patients treated did not change (pre-COVID-19: 5.3±1.7, COVID-19: 5.0±2.1, p=0.612), the day cases-based outpatient procedures increased (53% to 80%, p<0.001). Within these the number of same day discharges (39% to 72%, p=0.009) increased. Conversely, the overall treatment of hospitalized patients more than halved (47% to 20%, (p<0.001). The numbers of both planned urgent hospitalisations (13% to 0%, p=0.010) and emergency admissions (34% to 20%, p=0.010) were lower during COVID-19 as compared to pre-COVID-19. Most hospitalized patients were admitted via the emergency department with foot pain, infected foot or leg wounds. Planned urgent hospitalisations were initiated in outpatients by the vascular team for combined urgent wound debridements or minor amputations of necrotic toes and combined with revascularisation.
The mean interval between multi-disciplinary treatment decision and angioplasty procedure significantly decreased during COVID-19. The percentage of outpatients treated within 14 days after referral, as suggested by the PAD QIF, increased from 39% (average waiting time: 25±20 days) to 65% (15±13 days, p=0.009) and hospitalized patients treated within 5 days from 44% (11±13 days) to 63% (5±6 days, p=0.008). The average length of stay also significantly decreased by a third during COVID-19 (22±20 days vs 7±19 days, p<0.001).
3.3. Effect of COVID-19 on 30 Day and 1-Year Outcomes
As summarized in Table 3, there were no significant differences in outcomes pre-COVID-19 and during COVID-19 at 30-days in terms of wound healing, mortality, major amputation, or TLR. In the pre-COVID-19 period, the 30-day wound healing was significantly greater in day cases, compared to hospitalised patients, but 30-day mortality, major amputation and TLR rate were similar between groups.
There were no statistically significant differences between 1-year wound healing or mortality between pre-COVID-19 and COVID-19 periods. However, mortality was strikingly higher in in hospitalized patients compared to outpatient day cases independent of COVID-19 (43% vs 14%; Table 4, Figure 2). A total of 48 patients died (pre-COVID-19: n=28, during COVID-19: n=20). Figure 3 shows the distribution according to the documented cause of death. Surprisingly, the majority of known causes of death were infectious disease, namely sepsis, pneumonia and COVID pneumonia, accounting for 75% of all deaths pre-COVID-19 (n=21) and 50% during COVID-19 (n=10). These were more frequent than cardiac or stroke which together only accounted for 14% (n=4) and 30% of deaths (n=6, p=0.831, pre vs during COVID-19).
During COVID-19, major amputations were significantly more frequent and TLR significantly less frequent, compared to pre-COVID-19. This was only statistically significant in day case patients (Table 4, Figure 4).
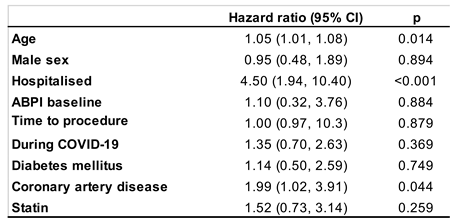
We also performed a Cox regression analysis to determine major contributors for mortality (Table 5). This analysis showed that increasing age, being hospitalised and a diagnosis of coronary artery disease contributed to mortality. However, none of male sex, being treated during COVID-19, baseline ABPI, time to procedure, diabetes mellitus or statin use were associated with mortality.
4. Discussion
The current data show how COVID-19 restrictions affected endovascular treatments and outcomes of CLI patients in an NHS district general hospital. Care pathways were changed to protect patients from COVID-19 infection and to open bed capacity for COVID-19 patients. This negatively impacted resources available for non-COVID-19 patients with staff redeployment outside usual specialist roles. However, the Vascular Society recognized that ‘CLI/diabetic foot…Those legs immediately threatened require urgent intervention’ and that ‘Interventional radiological approaches may allow more appropriate utilisation of scarce high-dependency beds’. Following this guidance and contrary to frequent reports in news media, in our service the number of CLI patients treated remained stable during COVID-19 and waiting times significantly improved towards nationally recommended targets that were set before COVID-19 but were upheld during COVID-19 (revascularisation ≤14 days via non-admitted pathway and ≤5 days via admitted pathway for CLI).
We show that the number and proportion of day cases increased, while angioplasties in hospitalised patients decreased. This occurred in part because patients who would usually have been treated as inpatients, particularly through urgently planned hospitalisation, were instead managed as day cases. Day case procedures remained possible as they followed a green pathway separated from the rest of the hospital and because some staff performing MDTs and interventions were not re-deployed and were able to continue their vascular duties. As depicted in Figure 1, even during COVID-19 lockdowns, individual day case patients were admitted overnight when there was no other adult living with them, and lockdown prohibited anyone staying with them. In all cases, the clinical severity of CLI was so high that the patients accepted the increased risk of contracting COVID-19. Fortunately, our data now indicate that day case patients (same day discharge and overnight) were not at increased peri-procedure risk when treated during COVID-19 as indicated by the similar 30-day outcomes pre-COVID-19 as compared to during COVID-19 (Table 3). There were two and three COVID pneumonia deaths pre- and during COVID-19, respectively. However, only one patient died during first lockdown with COVID-19 pneumonia at 14 days after the day case procedure which would be compatible with having contracted COVID-19 infection it in the hospital. The remaining deaths occurred in >84 days post-procedure and are unlikely linked to the hospital stay. Our similar 1-year mortality rates pre- and during COVID-19 provide further reassurance.
Day case-based angioplasty is becoming increasingly common, and we have previously reported our experience and 30-day outcomes [11]. Potential risk factors for outpatient procedures in CLI were recently discussed [15]. Our current data support the safety and efficacy of day case angioplasties [11] even in patients with CLI [15], as the complication rates were low and technical and clinical success high, with increased ABPI in most patients and high rates of wound healing independent of COVID-19. The complications, 30-day mortality, major amputation and TLR did not differ between day cases and hospitalized patients. In addition, day cases had higher wound healing rates already at 30 days as compared to those who were hospitalized and despite longer waiting times. Of note, all patients were followed up including review and optimization of medical therapy which likely contributed to the outcomes [14] highlighting the need for day case procedure being accompanied by a follow-up program including optimal medical management.
The most striking finding of the current analysis was the extremely large 1-year mortality (43%) in patients who were hospitalised, independent of the COVID-19 period. This aligns with published data in German hospitals [5]. Our data suggest that a requirement for hospitalization is a major risk factor for mortality in CLI. We believe that this indicates that CLI patients need to be identified and treated early to avoid hospitalization. It is noteworthy that cardiovascular and cerebrovascular causes of death were less common in our cohort than inflammatory disease outside the leg, presenting as sepsis and/or pneumonia. A recent meta-analysis has also shown lower than expected cardiovascular-related mortality [6] reporting 5-year cumulative incidence of mortality of 27% in symptomatic PAD patients, but only 13% cardiovascular mortality supporting our findings. While in some of the cases the infected foot wound might have been the focus of sepsis, our finding leads to the question of whether, and how, CLI could be linked with increased systemic susceptibility to inflammatory disease. Possible explanations might include a link between CLI and frailty, and/or increased nosocomial exposure to respiratory pathogens, and/or frequent antibiotic treatment of infected wounds altering bacterial flora and selecting for pathogenic and/or resistant strains.
4.1. Limitations
This is a single-centre, retrospective, observational study, the data for which reflect unselected consecutive cases of CLI requiring angioplasty. Our findings may thus not be representative of other institutions, as our multidisciplinary team unusually for the UK includes an academic interventional angiologist who was not re-deployed during COVID-19. Another limitation relates to the fact that, notwithstanding the similarities of baseline characteristics, our data cannot show if the increase in proportion of day-case procedures was fully explained by altered clinical decision-making during COVID-19 pandemic restrictions, or by subtly altered case-mix. This might include a smaller proportion of presentations being to the Accident & Emergency department, with more people presenting instead via vascular/foot clinic. A further possibility is that there was an increase in frequency of patients being referred directly to regional vascular hubs for emergency amputations. However, our observation of lower numbers of hospitalised emergency admissions for acute feet aligns with international trends of lower admissions for foot ulcers during COVID-19 [16-18]. A French nationwide study reported lower hospitalisation rates in diabetic foot ulcer patients along with the lower revascularisation rates [17], which aligns with our current data and may point towards lower access to care during COVID-19 lockdown. Different to the French study, we observed higher major amputation rates, particularly in day cases. We speculate that, during lockdown, patients might have presented too late for foot/leg salvage attempts by revascularisation. An alternative explanation is that, to reduce cumulative risk of SARS-CoV-2 exposure during frequent hospital visits for dressings, investigations, and repeat angioplasties with relatively lower chances of success, clinicians might have had a lower threshold for recommending amputation during the COVID-19 lockdowns as also recommended by the Vascular Society. Our data suggest that patients were not at increased procedure-related risk when treated during COVID-19, as indicated by the similar 30-day outcomes (Table 3). Our similar 1-year mortality rates before and during COVID-19 provide further reassurance. Finally, the time from referral to revascularization in hospitalized patients in the current study does not necessarily reflect a typical population of ‘admitted’ pathway CLI patients. As our hospital is a vascular spoke, patients with acute and rapidly progressing ischaemia and therefore requiring urgent revascularization for this reason within 5 days, are typically discussed with and transferred to the vascular hub hospital for emergency treatment. One could argue that the required time to revascularisation of our patients should be rather evaluated with the less than 14 days target like the ‘day cases’.
5. Conclusions
In conclusion, our data show that, while adapting to COVID-19 restrictions, we maintained a safe and effective angioplasty service and shortened our waiting times. Very high mortality rates in patients after hospitalisation, unrelated to COVID-19 period, indicate that CLI needs to be treated much more aggressively and earlier when CLI is still stable to avoid disease progression requiring hospitalisation. Further development of day casebased angioplasties as part of integrated foot salvage services may be the way forward to meet this growing medical need.
Author Contributions
Conceptualization, Alexander Rodway and Christian Heiss; Data curation, Christian Heiss; Formal analysis, Jenny Harris, Simon Skene and Christian Heiss; Investigation, Charlotte Allan, Felipe Pazos-Casal, Ciara Giltinan, Ali Dehghan-Nayeri, Andre Santos, Nikolaos Ntagiantas, Ivan Walton, Richard Brown, Ajay Pankhania and Christian Heiss; Methodology, Jenny Harris, Lydia Hanna, Martin Whyte, Simon Skene and Christian Heiss; Project administration, Christian Heiss; Resources, Christian Heiss; Software, Christian Heiss; Supervision, Alexander Rodway, Gary Maytham and Christian Heiss; Validation, Alexander Rodway, Jenny Harris, Lydia Hanna, Charlotte Allan, Felipe Pazos-Casal, Ciara Giltinan, Andre Santos, Martin Whyte, Richard Brown, Simon Skene, Benjamin Field, Gary Maytham and Christian Heiss; Visualization, Lydia Hanna and Christian Heiss; Writing – original draft, Christian Heiss; Writing – review & editing, Alexander Rodway, Jenny Harris, Lydia Hanna, Charlotte Allan, Felipe Pazos-Casal, Ciara Giltinan, Ali Dehghan-Nayeri, Andre Santos, Martin Whyte, Nikolaos Ntagiantas, Ivan Walton, Richard Brown, Simon Skene, Ajay Pankhania, Benjamin Field, Gary Maytham and Christian Heiss.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, all measurements were conducted as part of routine clinical care within two National Health Service acute hospitals in the UK. The confidentiality of patient data was strictly observed and only anonymised data were analysed and, as a service evaluation, there was no requirement for research ethics committee approval.
Informed Consent Statement
Patient consent was waived as this was part of a service evaluation, without requirement for research ethics committee approval.
Data Availability Statement
As this was part of a NHS service evaluation, the data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Behrendt, C.A.; Thomalla, G.; Rimmele, D.L.; Petersen, E.L.; Twerenbold, R.; Debus, E.S.; Kolbel, T.; Blankenberg, S.; Schmidt-Lauber, C.; Peters, F.; et al. Editor's Choice - Prevalence of Peripheral Arterial Disease, Abdominal Aortic Aneurysm, and Risk Factors in the Hamburg City Health Study: A Cross Sectional Analysis. Eur J Vasc Endovasc Surg 2023, 65, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Stoberock, K.; Kaschwich, M.; Nicolay, S.S.; Mahmoud, N.; Heidemann, F.; Rieß, H.C.; Debus, E.S.; Behrendt, C.-A. The interrelationship between diabetes mellitus and peripheral arterial disease – a systematic review. Vasa 2021, 50, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ireland, V.S.o.G.B.a. Peripheral Arterial Disease - Quality Improvement Framework. 2019.
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Luders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015, 36, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Lundin, F.; Wahlberg, E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur J Vasc Endovasc Surg 2016, 51, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D.; et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007, 50, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsater, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Brownrigg, J.R.; Andros, G.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev 2016, 32 Suppl 1, 136–144. [Google Scholar] [CrossRef]
- Rodway, A.; Stafford, M.; Wilding, S.; Ntagiantas, N.; Patsiogiannis, V.; Allan, C.; Field, B.; Clark, J.; Casal, F.P.; Pankhania, A.; et al. Day case angioplasty in a secondary care setting - initial experience. Vasa 2021, 50, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Rodway, A.D.; Cheal, D.; C, A.; Pazos-Casal, F.; Hanna, L.; Field, B.C.T.; Pankhania, A.; Aston, P.J.; Skene, S.S.; Maytham, G.D.; et al. Ankle Doppler for cuffless ankle brachial index estimation and peripheral artery disease diagnosis independent of diabetes. J. Clin. Med. 2023, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg 2019, 58, S1–S109. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Olinic, D.M.; Belch, J.J.F.; Brodmann, M.; Mazzolai, L.; Stanek, A.; Madaric, J.; Krentz, A.; Schlager, O.; Lichtenberg, M.; et al. Management of chronic peripheral artery disease patients with indication for endovascular revascularization. Vasa 2022, 51, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Thieme, M.; Krankenberg, H.; Schilling, T.; Betge, S.; Korosoglou, G.; Rammos, C.; Vosseler, M.; Espinola-Klein, C.; Heilmeier, B.; Muller, O.J.; et al. Endovascular interventions in outpatient care. Vasa 2023. [Google Scholar] [CrossRef] [PubMed]
- Kendirci, M.; Sahiner, I.T.; Sezikli, I.; Akin, M.; Yasti, A.C. Effects of the COVID-19 pandemic on the management of diabetic foot ulcers: experiences from a dedicated diabetic foot care center. Wounds 2022, 34, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Mariet, A.S.; Benzenine, E.; Bouillet, B.; Verges, B.; Quantin, C.; Petit, J.M. Impact of the COVID-19 Epidemic on hospitalization for diabetic foot ulcers during lockdown: A French nationwide population-based study. Diabet Med 2021, 38, e14577. [Google Scholar] [CrossRef] [PubMed]
- de Mestral, C.; Gomez, D.; Wilton, A.S.; Lee, D.S.; Albalawi, Z.; Austin, P.C.; Jacob-Brassard, J.; Urbach, D.R.; Al-Omran, M.; Baxter, N.N. A Population-Based Analysis of Diabetes-Related Care Measures, Foot Complications, and Amputation During the COVID-19 Pandemic in Ontario, Canada. JAMA Netw Open 2022, 5, e2142354. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Evolution of numbers of patient who underwent endovascular revascularisations at Surrey and Sussex Healthcare NHS Trust 07/2018-07/2021.
Figure 1.
Evolution of numbers of patient who underwent endovascular revascularisations at Surrey and Sussex Healthcare NHS Trust 07/2018-07/2021.

Figure 2.
Kaplan-Maier survival analysis for 1-year wound healing (A, B) and survival (C, D) pre-COVID-19 (A, C) and during COVID-19 (B, D).
Figure 2.
Kaplan-Maier survival analysis for 1-year wound healing (A, B) and survival (C, D) pre-COVID-19 (A, C) and during COVID-19 (B, D).
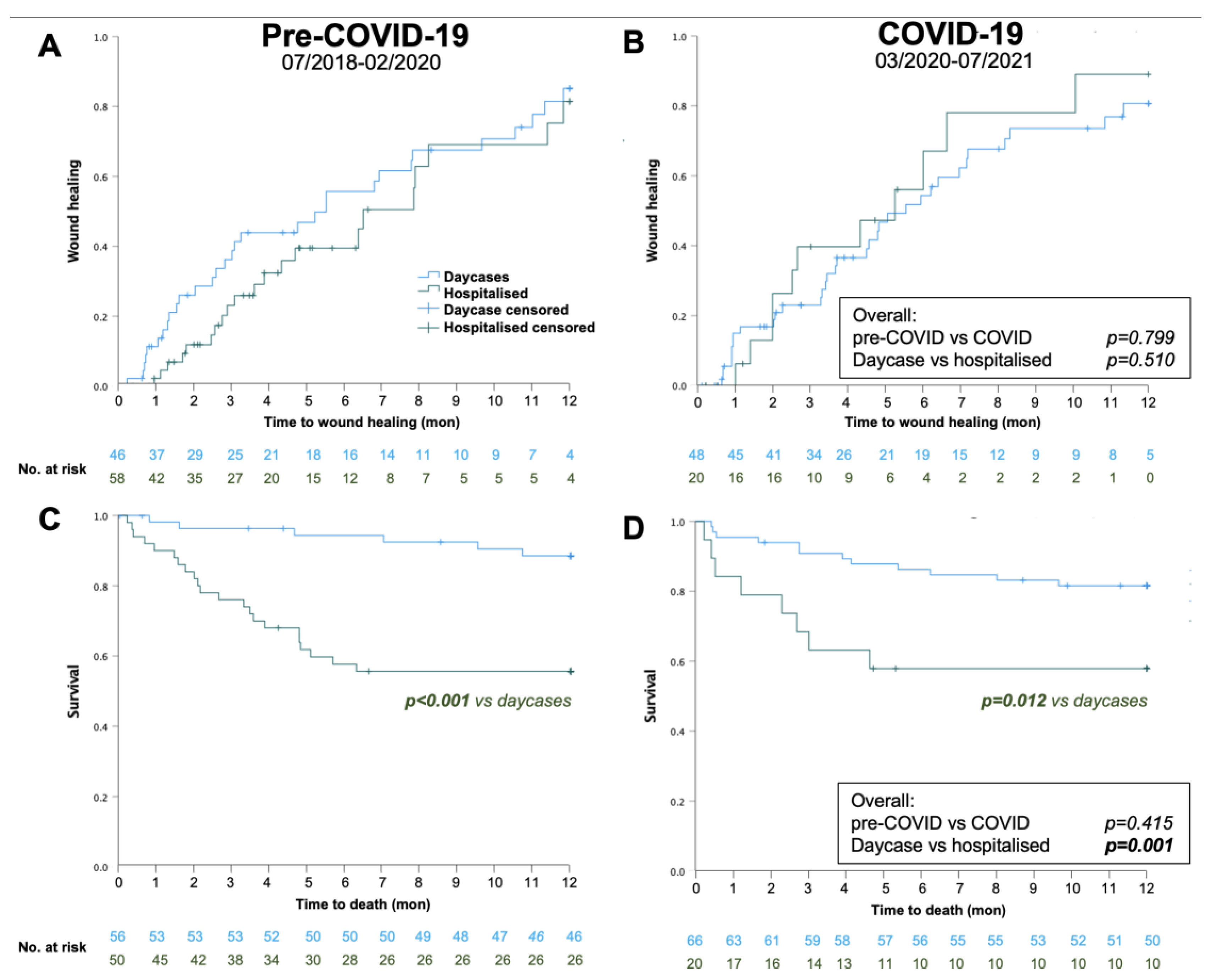
Figure 3.
Cause of death pre- and during COVID-19 (n=28 and n=20) as noted on medical examiner’s report.
Figure 3.
Cause of death pre- and during COVID-19 (n=28 and n=20) as noted on medical examiner’s report.
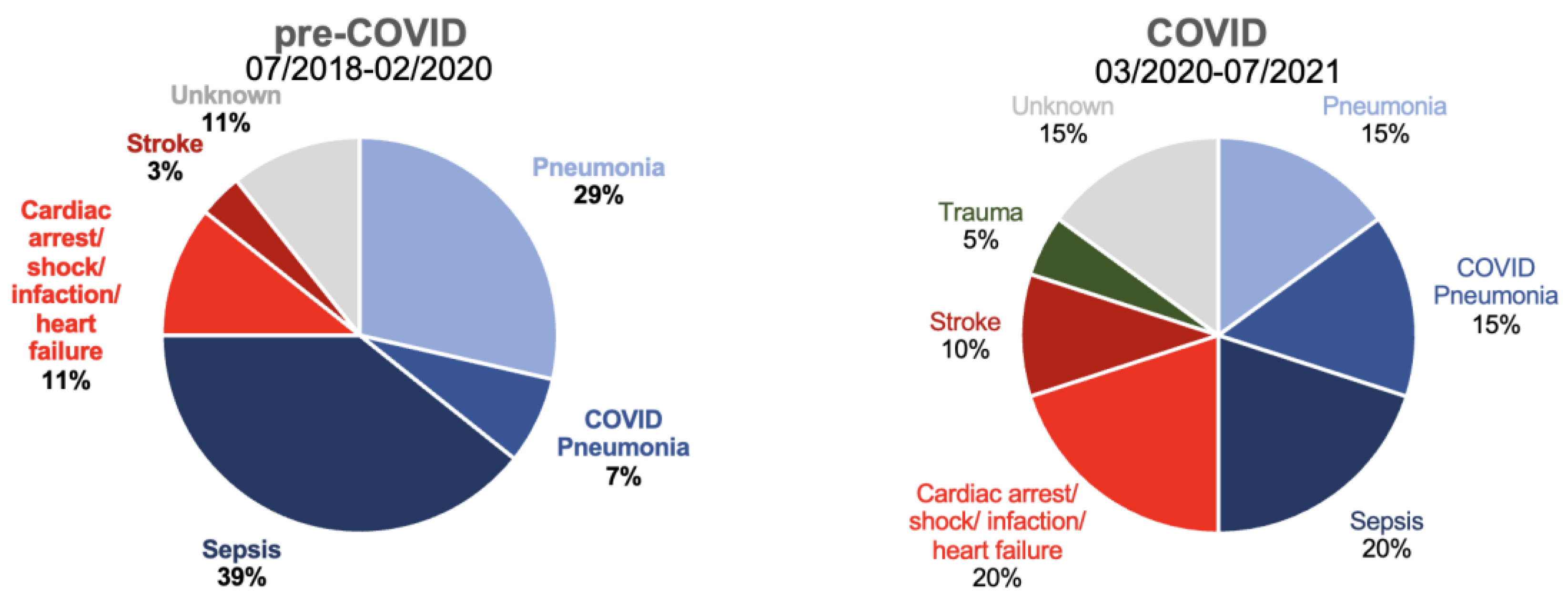
Figure 4.
Kaplan-Maier survival analysis for 1-year major amputation (A, B) and clinically driven target lesion revascularization (TLR; C, D) pre-COVID-19 (A, C) and during COVID-19 (B, D).
Figure 4.
Kaplan-Maier survival analysis for 1-year major amputation (A, B) and clinically driven target lesion revascularization (TLR; C, D) pre-COVID-19 (A, C) and during COVID-19 (B, D).

Table 1.
Clinical and demographic characteristics of patient population pre and during COVID. Values are mean and standard deviation (or percent of total if indicated); p values are from t-test or Mann-Whitney-U test.
Table 1.
Clinical and demographic characteristics of patient population pre and during COVID. Values are mean and standard deviation (or percent of total if indicated); p values are from t-test or Mann-Whitney-U test.
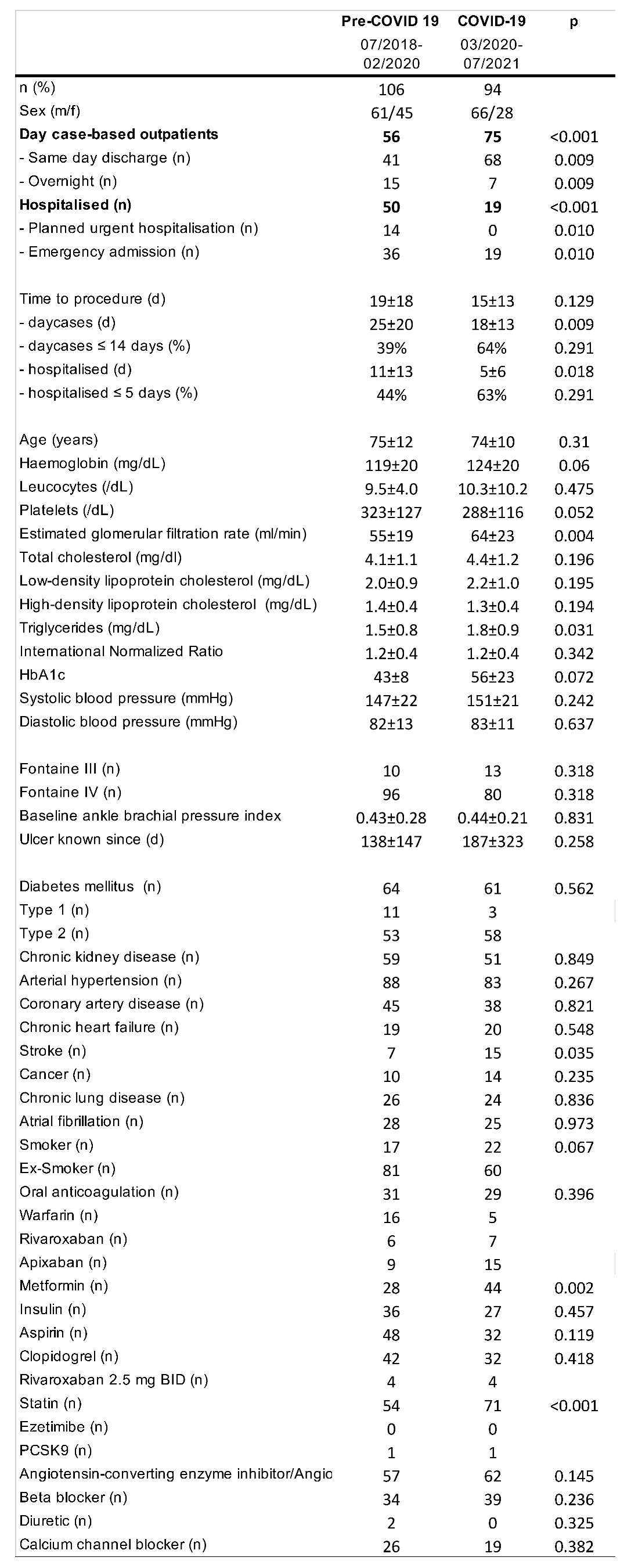 |
Table 2.
Procedural characteristics pre and during COVID. Values are mean and standard deviation (or percent of total if indicated); p values are from t-test or Mann-Whitney-U test. Length of stay reported as median and interquartile range (IQR).
Table 2.
Procedural characteristics pre and during COVID. Values are mean and standard deviation (or percent of total if indicated); p values are from t-test or Mann-Whitney-U test. Length of stay reported as median and interquartile range (IQR).
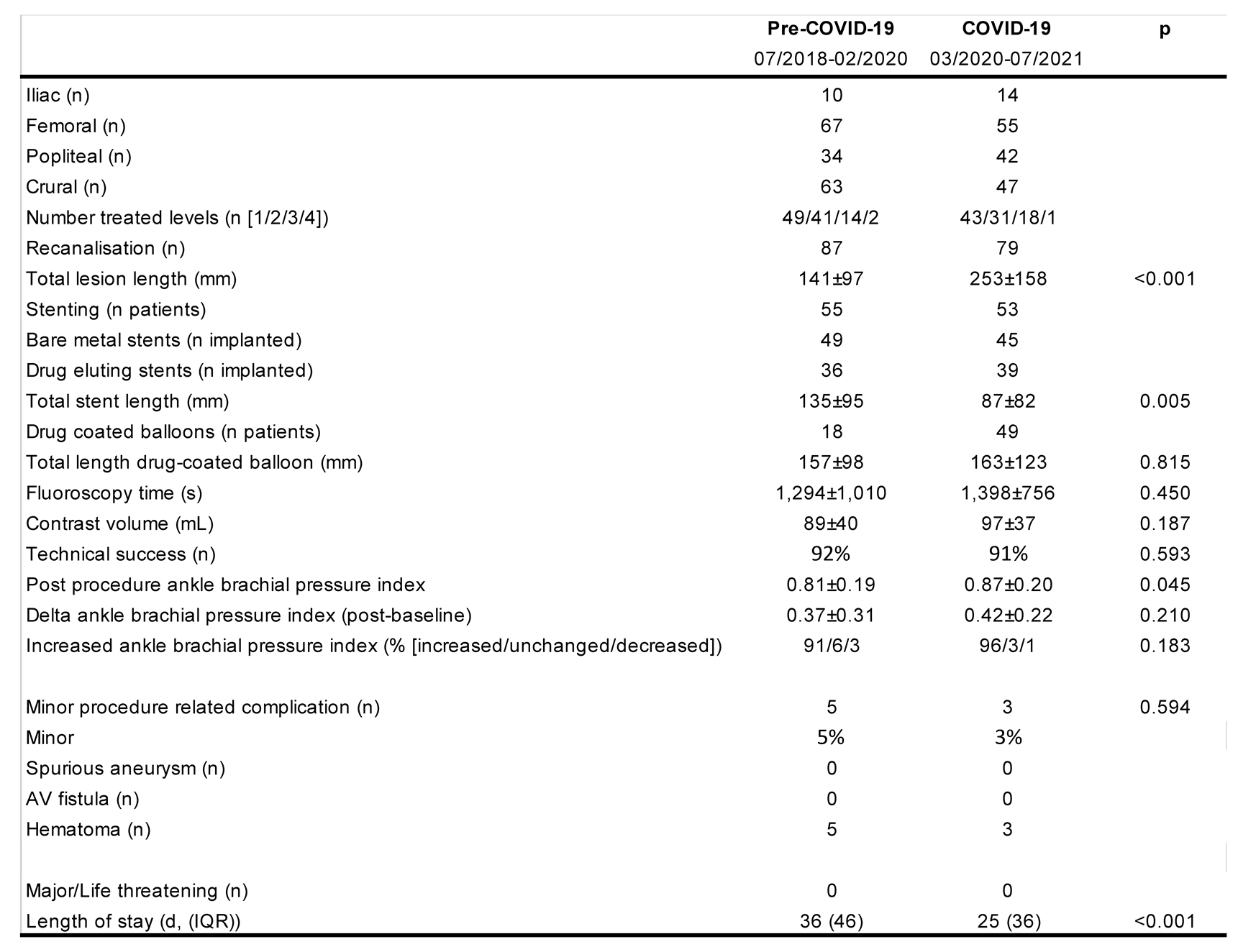 |
Table 3.
30-day outcomes of patients receiving angioplasties for CLI pre and during COVID. Values number of cases with respective endpoint (for all patients and stratified by day case/hospitalized, percentage next to event refers to cumulative survival at time of last event); p value is from log-Rank (Mantel-Cox) test.
Table 3.
30-day outcomes of patients receiving angioplasties for CLI pre and during COVID. Values number of cases with respective endpoint (for all patients and stratified by day case/hospitalized, percentage next to event refers to cumulative survival at time of last event); p value is from log-Rank (Mantel-Cox) test.
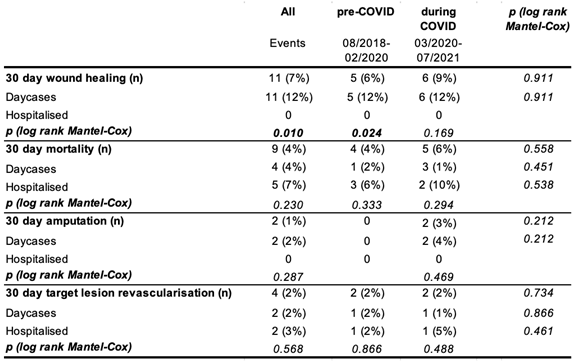 |
Table 4.
One year outcome of patients receiving angioplasties for CLI pre and during COVID. Values number of cases with respective endpoint (for all patients and stratified by day case/hospitalized, percentage next to event refers to cumulative survival at time of last event); p value is from log-Rank (Mantel-Cox) test.
Table 4.
One year outcome of patients receiving angioplasties for CLI pre and during COVID. Values number of cases with respective endpoint (for all patients and stratified by day case/hospitalized, percentage next to event refers to cumulative survival at time of last event); p value is from log-Rank (Mantel-Cox) test.
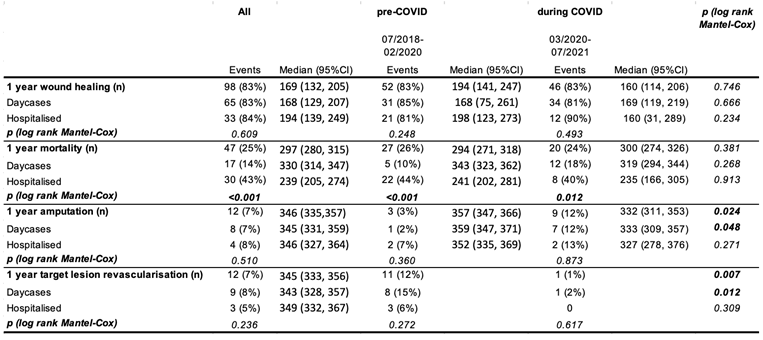 |
Table 5.
Cox regression analysis to determine significant contributors of mortality (ABPI=ankle brachial pressure index).
Table 5.
Cox regression analysis to determine significant contributors of mortality (ABPI=ankle brachial pressure index).
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Understanding the Impact of COVID-19 on Angioplasty Service and Outcome of Patients Treated for Critical Limb Ischaemia: A Single-Centre Retrospective Cohort Study
Alexander D. Rodway
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









