Preprint
Article
Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts
Altmetrics
Downloads
162
Views
63
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
09 June 2023
Posted:
09 June 2023
You are already at the latest version
Alerts
Abstract
Prunus dulcis is one of the most widely cultivated species in the world. Its fruit (almond) is rich in various nutritious and bioactive compounds that exert several beneficial effects. The aim of this study was to determine the chemical profile and evaluate the biological potential in vitro of almond shell extracts. The chemical analysis of shell extracts led to the identification of 15 com-pounds by HPLC-DAD of which 11 were first detected in the almond plant. Twenty-six volatile compounds were identified by the GC-MS technique, among them; seven were firstly detected in the studied plant. For the biological activities, the extracts demonstrated moderate inhibition potential against the antioxidant, antidiabetic, and cytotoxic activities. The methanol extract at 50 µg/mL showed the highest antioxidant (45%) and antidiabetic activities (45% against alpha-glucosidase and 31% against alpha-amylase extracts), while the cyclohexane and dichloromethane at 50 µg/mL showed the highest cytotoxic activity towards Hela (32.2% with cyclohexane), and RAW 264-7 (45% with dichloromethane). Overall, these findings demonstrate the potential of almond shell extracts as a source of bioactive compounds that could be applied in the pharmaceutical and medical fields.
Keywords:
Subject: Chemistry and Materials Science - Medicinal Chemistry
1. Introduction
Nowadays, fruits and their by-products are widely studied as a promising source of phytochemicals with various health benefits that have a high potential to be integrated into the pharmaceutical and cosmetic industries given their various pharmacological and pharmaceutical properties [1].
Nuts are a class of food rich with high lipids content. Its consumption provides us with a multitude of bioactive compounds that present several pharmacological properties. They are known for their distinguished flavor, their consumption provides us with wide array of bioactive molecules, and nutrients [2,3].
Prunus dulcis (P.dulcis) is one of the most cultivated nuts species in the world. It belongs to the Rosaceae family. The almond fruit is a stone fruit comprising a kernel, skin, shell, and hull.. It presents a high nutritional value given its richness with high quality fats (monounsaturated fatty acids (MUFA, 60%) such as palmitic, stearic and palmitoleic acid; polyunsaturated fatty acids (PUFA, 30%) such as linoleic acid), protein, carbohydrates fibers and vitamins [2,3] . Numerous bioactive compounds have been reported in the fruit of P. dulcis such as phenolic acids ( hydroxycinnamic acid, hydro benzoic acid sinapic acid, etc.), flavonoids (Catechin, epicatechin, cyanidin, etc.), lignans, stilbene, and terpenoids [1,3,4]. Various biological proprieties have been reported in the almond such as antimicrobial, antioxidant, anti-inflammatory, anticancer, laxative, hepatoprotective, cardiometabolic protective, neotropic, sedative, hypnotic, anxiolytic, and neuroprotective effects [4] .
Interestingly, the almond shell, which accounts for 33% of the total fruit mass and produces annually 0.8 to 1.7 million tons globally, has been traditionally utilized as animal feed. However, it remains less characterized among the other parts, traditionally used as animal food. It is rich in cellulose and lignin contents suggesting its potential for further exploration [5].
The present study aims to determine the chemical composition of P. dulcis shell extracts using HPLC-DAD and GC-MS methods as well as evaluate their biological potential in vitro, mainly the antioxidant (DPPH), antidiabetic (alpha-amylase, alpha-glucosidase), and cytotoxic (Hela, RAW 264.7 )
2. Results
2.1. Extraction yield:
In this study, maceration using an increasing polarity organic solvent (cyclohexane, dichloromethane, ethyl acetate, methanol, and H2O) was used in the extraction of the phytochemicals compounds. As presented in Table 1, the polar fractions (MeOH, H2O) presented higher yield values than the nonpolar fractions, with the highest value recorded with the H2O extract (2.22%), while the EtOAc extract presented the lowest yield value (0.03%). These findings suggest that the shell part is relatively richer in polar compounds such as polyphenols and polysaccharides. Conversely, the nonpolar fraction, which included dichloromethane and cyclohexane extracts, presented low yield values ranging from 0.09% to 0.14% respectively, suggesting the presence of nonpolar compounds, such as fatty acids, in the shell material. In this context, Sarwar et al. [6] studied the effect of different solvents in the yield extraction of two varieties of P. dulcis shell, using 80 or 100% MeOH and 80 or 100% EtOH. The results showed significant variation in the yield between the different solvents, with the highest recorded with the 80% MeOH (18.2% for the thick shell and 57.9% for the thin shell). In another study, Queirós et al. (2020) obtained an extraction yield value similar to our findings (3.4% with the ethanol-water extract).
2.2. Total Polyphenol Content (TPC):
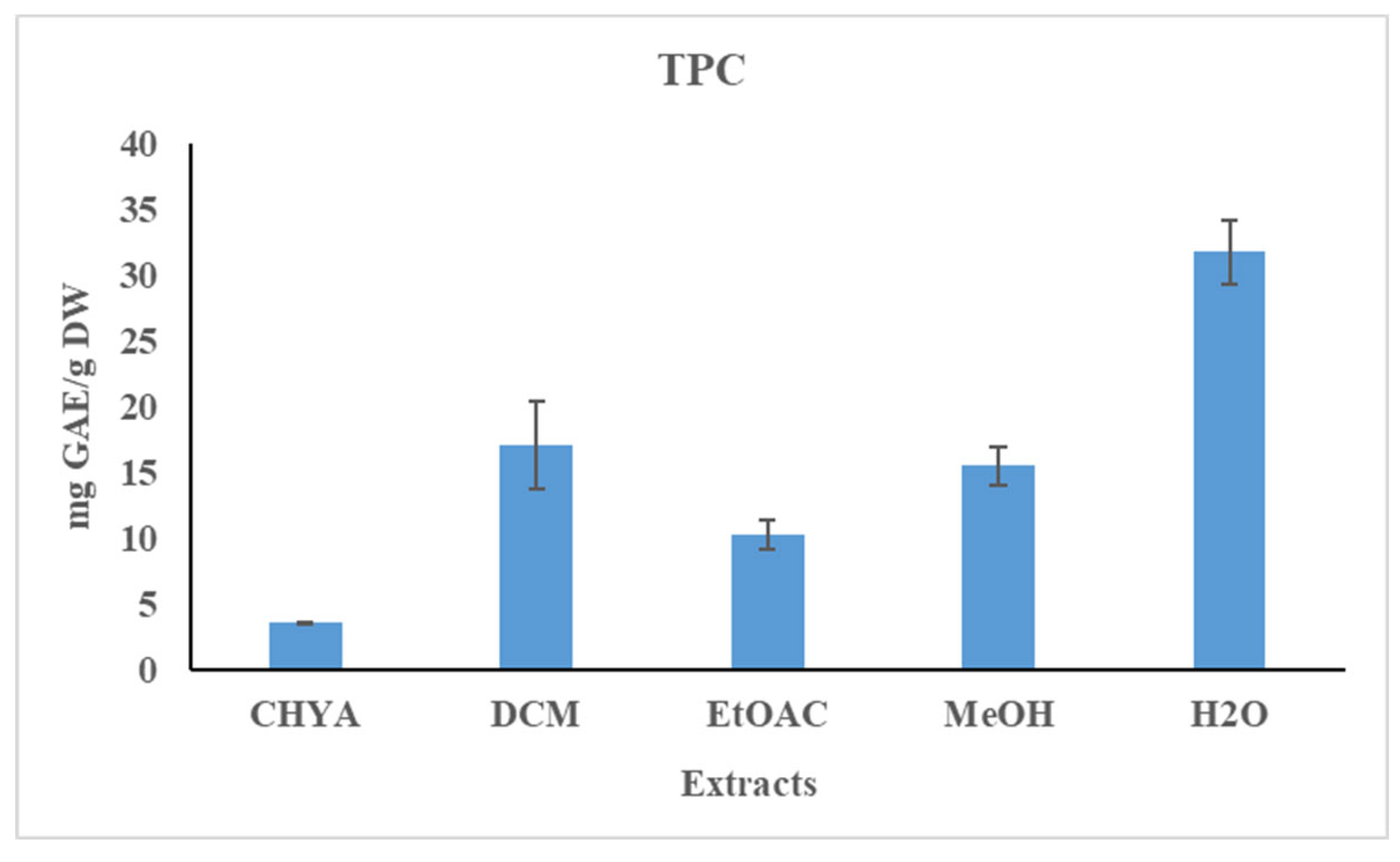
The total phenolic content of the almond shell extracts was determined using the Folin-Ciocalteu method. The results are shown in figure 1. Statistically, we note a significant difference between the extracts. As shown in the figure below, the polyphenol content of the obtained extracts is relatively low. The polar extracts have a higher TPC content than the apolar ones. The highest TPC content was recorded with the H2O extract (31.77 mg GAE / g of DW), while the lowest value was obtained with the CHYA extract (3.56 mg GAE / g of DW). Compared to the literature, the obtained TPC values are higher than that found by Sarwar et al. (2012) with the methanolic and the ethanolic shell extracts of the two almond varieties (values range from 1.4 to 2.3 mg GAE/g DW with the thick shell and from 2.3 to 7.21 mg GAE/g DW with the thin shell). In another study, Queirós et al. (2020) obtained a higher TPC content (188.6 mg GAE/g DW) with the almond shell ethanol-water extract.
Figure 1.
Total phenolic content (TPC) of P. dulcis shell extracts (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH). Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).
Figure 1.
Total phenolic content (TPC) of P. dulcis shell extracts (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH). Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).
2.3. Antioxidant Activity (DPPH):
The antioxidant activity of the almond shell extracts was assessed by the DPPH assay. The extracts concentration was adjusted to 50 µg/mL, the ascorbic acid was used as reference at 4 µg/mL. The results are presented in figure 2. Statistically, there is a significant difference between the different extraction solvents. As shown in Figure 2, only three extracts have shown antioxidant activity against the DPPH radical (DCM, MeOH, H2O). The highest inhibition values was recorded with the polar extracts: 37.6% with the MeOH extract and 16.6% with the H2O extract. This suggests that polar molecules seem to be responsible for the present effect. In fact, several polyphenol compounds identified in the almond, such as Catechin, epicatechin are known for their antioxidant properties. These compounds could interact synergistically with molecules of other classes, such as fatty acids, vitamins such as A, B (thiamine B1, riboflavin B2, niacin B3 and pyridoxine B6), and E present in almonds [8], and polysaccharides (glycosides) that also present as well according to recent studies [8]. Compared to the literature, a study conducted by Queirós et al. (2020), evaluating the antioxidant potential of almond shell extracts using the DPPH test, showed that the different extracts presented a moderate inhibition activity range of 12.1 to 18.22% for the thick shell and 15 to 57.9% for the thin shell. In another study, the DPPH assay was used to evaluate the antioxidant potential of almond shell extract. The results showed that the ethanol-water extract exerted a high inhibition activity with IC50 equal to 7.9 µg/mL [1].
2.4. Chromatographic analysis:
2.4.1. Identification of compounds in P. dulcis shell extracts by HPLC-DAD:
The identification of compounds in the almond shell extracts was performed using the HPLC-DAD technique. The retention time and the maximum wavelength (λmax) of each peak were compared to those of standards compounds, which were injected under identical conditions as the shell extracts. Following this identification, each recognized compound was quantified by referencing the calibration curve tailored to each corresponding standard. The analysis of the chemical composition of P. dulcis shell extracts led to the identification of 15 compounds as presented in Table 2. Of these, only four compounds were previously identified in the almond plant (Catechin, epicatechin, , rutin, and trans-cinnamic acid), while all the compounds were firstly identified in the shell part. The majority of the identified compounds were detected in the CHYA and the EtOAc extracts. The EtOAc extract was particularly rich in rutin, with a concentration of 13.06 mg per gram of extract. The Methanol (MeOH) extract had a high content of epicatechin, at 4.42 mg/g extract, while the water (H2O) extract was notable for its high Synephrine content, at 1.62 mg/g extract. According to the literature, few studies have been interested in the chemical composition of the almond shell, a study conducted by Moure et al. (2007), identified vanillic, syringic, and p-coumaric acids in the P. dulcis shell part. In another study, Kahlaoui et al. (2019) identified several polyphenolic compounds such as quercetin-3-glucoside, p-coumaric acid, caffeic acid, and protocatechuic acid in the hulls of several varieties of almonds.
2.4.2. Identification of volatile compounds in P. dulcis shell extracts by GC-MS (before and after derivatization):
The volatile composition of the different almond shell extracts was determined by the GC-MS technique. The identified compounds were presented in tables 3 and 4. As shown in the tables below. The analysis by GC-MS method has led to the identification of 26 compounds before and after derivatization. Seven compounds were detected for the first time in the almond plant (2,3-dimethyldecane,undecane, 1,1'-bicyclohexyl, ben-zaldehyde, 3-hydroxy-4-methoxy , 2,4-Di-tert-butylphenol, dodecanoic acid, 1-methylethyl ester, and hydracrylic acid). Whereas only 11 compounds were previously reported in almond shell part: dodecane; tridecane, hexanoic acid; glycolic acid; glyceric acid; butanedioic acid; malic acid, Dodecanoic acid, Pentadecanoic acid; 9-octadecenoic acid,(E)-, and stearic acid. As shown in Table 3 and Table 4 most of the identified compounds have been detected in the apolar extracts. Various classes of compounds are present in the almond shell extract, such as alkanes (undecane; dodecane, tridecane…); organic acids (hexanoic acid, glycolic acid, butanedioic acid…); polyphenols (2,4-Di-tert-butylphenol). Some compounds were present in two or three extracts, such as 2,4-Di-tert-butylphenol, benzaldehyde, 3-hydroxy-4-methoxy- and palmitic acid. According to the literature, only few studies have investigated the volatile profile of the almond shell part. In this context, Moure et al. (2007) identified some compounds in the almond shell part by the GC-MS method: fatty acids (butanedioic, octadecanoic and trans-9 octadecenoic); phenolic acids (vanillic, syringic, and p-coumaric acids). Furthermore, in another study several volatile compounds were identified in the P. dulcis shell, such as syringol, 1-(4-hydroxy-3-methoxyphenyl)-propyne; hexadecanoic acid, and syrin-galdehyde [6].
2.5. Biological activities:
2.5.1. Antidiabetic activity:
- The evaluation of the antidiabetic potential of almond shell extracts was performed in vitro using alpha-glucosidase and alpha-amylase assays.
- Alpha-glucosidase activity:
To the best of our knowledge, no previous studies were conducted to assess the antidiabetic activity of the P. dulcis shell. The shell extracts were adjusted at 50 µg/mL. Acarbose was used as a reference at the same concentration. The results of the present activity are shown in Figure 3. As presented in the figure below, only three of the tested extracts have shown inhibition activity against the alpha-glucosidase enzyme (DCM, EtOAc, and MeOH). The highest inhibition value was recorded with the MeOH extract (45%), while the ethyl acetate extract presented the lowest one (7%). The variation in inhibition activity between the extracts could be correlated with their chemical composition. Indeed, several bioactive compounds, especially the polar compounds, could be responsible for this activity mainly the phenolic and polysaccharides. In the same context, a study conducted on the antidiabetic potential of almond kernel using the protein tyrosine phosphatase (1B PTP1B) inhibitory assay showed that seven alcoholic extracts exerted strong antidiabetic activity with an IC50 = 0.46 µg/mL with ETOH extract [1].
- Alpha-amylase activity:
Evaluation of the antidiabetic potential of almond shell extracts was performed using the alpha-amylase assay. The shell extracts and the reference concentration were adjusted at 50 µg/mL. The antidiabetic activity was illustrated in figure 4. As shown in the extracts figure below, all the tested had an inhibition activity against the alpha-amylase enzyme with some variation. The highest inhibition value was recorded with the CHYA extract (34.34%), while the lowest value was recorded with the H2O extract (20.71%). In comparison with the alpha-glucosidase inhibition activity, we deduce that the polar shell extracts are more effective with the alpha-glucosidase assay while the apolar ones are more active with the alpha-amylase assay. These results could be explained by the difference in the inhibition mechanism between the two enzymes as well as the compounds responsible for the inhibition activity. In the same context, Nowicka et al. (2016) have investigated the antidiabetic potential of several Prunus fruit smoothies and puree in vitro using the alpha glucosidase and the alpha amylase assays. The obtained results showed that the 100% apricot puree, 50% sour cherry juice 50% apricot puree, and 20% sour cherry juice 80% apricot puree exhibited the highest inhibition activity toward the alpha-amylase enzyme with an IC50 < 1mg/mL
2.5.2. Cytotoxic activity:
The determination of the cytotoxic potential of the obtained shell extracts was conducted using the MTT test. Two cancer cell lines (HELA and Raw 264-7). The extracts were adjusted at 50 µg/mL the tamoxifen was used as a reference at 100µM. The results of the inhibition activity are presented in Figure 5. As shown in the figure below, for the first cell line (HELA), we note that a significant difference was recorded between extracts with the highest inhibition activity with the apolar extracts (CHYA, DCM), while the MeOH extract has shown the lowest. For the second cell line (RAW 264-7), the apolar fractions have presented also the highest cytotoxic activity. These results suggest that apolar bioactive compounds could be responsible for this inhibition effect. Moreover, the shell extracts have demonstrated cytotoxic potential toward the two cell line. This could be explained by the presence of bioactive substances in the shell fractions capable of inhibiting the Hela and Raw 264-7 cells. In the same context, a study by Mericli et al. (2017) investigating the anticancer potential of P. dulcis seed oil against Colo-320 and Colo-741 cells showed that the almond seed oil from northern cyprus and turkey varieties was an effective antiproliferative agent against the two cancer cell lines. In another study, Amico et al. (2006) investigated the anticancer activity of terpenoids compounds obtained by bioguided fractionation assay of Sicilian almond hulls, the results showed that betulinic acid, a compound isolated from almond hulls in this study, exhibited anticancer potential against the MCF-7 cell line with an antiproliferative activity GI50 = 0.27 μM.
2.6. Principal component analysis (PCA):
Principal component analysis was performed to determine the correlation between the TPC and the different biological assays. The inertia axes was withheld from this analysis. As shown in figure 6, the total variation percentage recorded at 81.58%. The axes PC1 and PC2 expressed, respectively, 50.63% and 30.94% of variability. The “loading plot” (Figure 6) express the correlation between the tested activities, the TPC, and the correlation between the PC and the original variables. As presented in table 5, PC1 is positively correlated with DPPH, alpha-glucosidase, Raw276-4, and HELA with loading respectively 0.903, 0.547, 0.711, and 0.753. The PC2 is positively correlated with TPC and alpha-amylase with loading, respectively, 0.818, and 0.917. Based on the correlation matrix (Table 6) and Figure 8, we note the presence of moderate correlation between the TPC and the DPPH (0.388) which suggests the presence of other compounds classes such as fatty acids or polysaccharides contributing to the antioxidant activity detected in the present study. Moreover, the TPC present negative correlation with (alpha-glucosidase, alpha-amylase, and cytotoxic activity against RAW 276-4 and HELA) which means that phenolic compounds present in the extracts could be not responsible for the obtained results and then, we can suggest the presence of other classes of compounds responsible for these effects like the fatty acids, alkaloids, and polysaccharides. Furthermore, DPPH presents high correlation with alpha-glucosidase activity (0.680), suggesting that the substances exerting these effects could be the same. In addition, alpha-glucosidase present a low correlation with alpha-amylase, which could indicate that these activities were exerted by different bioactive compounds, and let us suggest the presence of a different class of bioactive molecules in the shell extracts. For the cytotoxic activity, the correlation between the two-cell line is high (0.679). As shown in figure 7, we can class the different extracts into three groups: C1 (MeOH), C2 (H2O), and C3 (CHYA, DCM, and EtOAc). The correlation between observations and variables presented in figure 8 shows that the MeOH extract is near to the DPPH and the alpha-glucosidase. This suggests that polar components such as polyphenols and polysaccharides may have a synergistic influence on the observed effects. In addition, the apolar fractions (CHYA, DCM, and EtOAc) are near to the alpha-amylase and the cytotoxic activities against RAW 276-4 and HELA, which suggest the presence of bioactive molecules in the mentioned fractions responsible for the present effects. In conclusion, based on the results of the biological assays and the statistical analysis mainly the PCA, we can deduct that the shell extracts are rich in several bioactive compounds of different classes and polarity such as Catechin, epicatechin, and Synephrine exerting moderate cytotoxic, antidiabetic and antioxidant effects.
3. Materials and Methods
3.1. Plant Material:
In this study, the shell material of P. dulcis (Achaak variety) was collected from the south-Est of Tunisia (Zarzis). After air-drying, the collected plant material was manually ground using mortar (LGC France) and stored at room temperature.
3.2. Extraction:
- Maceration:
Fifty grams of dried shell material was successively extracted, using organic solvents of increasing polarity cyclohexane (CHYA); dichloromethane (DCM); ethyl acetate (EtOAc); methanol (MeOH); and ultra-pure water (H2O) for 2 h with medium agitation at ambient temperature and pressure. The extraction mixture was subsequently filtered with Wattman paper .The solvents were then, evaporated under a vacuum at 35 ° C using a rotary evaporator (IKA; RV 10 auto V; Staufen Germany).
3.3. Total Phenolic Content (TPC):
The total polyphenolic content of P. dulcis was evaluated using the Folin-Ciocalteu method described by Ben khadher et al.
3.4. Determination of Radical Scavenging Activity:
The antioxidant scavenging activity was assessed using 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH); as reported by Rahmani et al [42] with some modifications. In a 96-well microplate (Micro Well; Thermo Fisher Scientific; Illkirch France); 20 μL of each diluted plant extract (0.5 mg/mL) was mixed with 180 μL of methanolic DPPH solution (0.2N). The reaction mixture was then incubated at 25 °C for 25 min. Subsequently, the absorbance was measured at 524 nm using a microplate reader (Multiskan Go F1-01620; Thermo Fisher Scientific; Vantaa, Finland). The ascorbic acid was used at 4 µg/mL as the reference for this activity.
DPPH inhibition was calculated as follows: % Inhibition = 100 × (Ablank −Asample)/Ablank.
3.5. Biological Activity:
3.5.1. Antidiabetic activity:
- Anti-α-Amylase Activity:
The anti-α-amylase activity of the shell extracts was performed as described by Ben khadher et al.[43].
- Anti-α-glucosidase Activity:
The anti-α-glucosidase activity of the different shell extracts was performed using the PNP-G method as described by Rahmani et al[42]. In the reaction mixture, 50 µL of phosphate buffer (0.1M, pH 6.9) was mixed with 100 µL of the alpha-glucosidase enzyme (1 U / ml) and 50µL of each extract (0.5 mg/mL), after 10 min of incubation at 25°C, 50µL of PNP-G (5 mM) was added to the mixture. After 5 min of incubation, the absorbance was measured at 405 nm. Inhibition of enzyme activity was calculated as follows:
% inhibition = 100 × (Ablank − Asample)/Ablank.
3.5.2. Cytotoxic Activity:
The Cytotoxic activity of the shell extracts was determined on the cell line RAW 264.7 (mouse macrophage cell line) and Hela (human cervical cancer cell line), as described by Ben Khadher et al. (2022)[43] with slight modification. In a 96-well microplate, cells have been distributed at 13 × 103 cells/well for Hela and 12 × 103 cells/well for RAW 264-7 in 100 µL. After 24 h of incubation at 37 °C, 100 µL of each extract diluted in the medium after being solubilised in DMSO was added to 100 µL of the corresponding culture medium; DMEM (Advanced DMEM, Thermo Fisher Scientific). The plate was then incubated for 48 h at 37°C. The cytotoxic potential of the samples was evaluated using the MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. After removing the supernatant, cells were treated with 50 µL of MTT solution then, the plate was incubated at 37°C for 40 min. then MTT was then eliminated and 80 µL of DMSO was added. The absorbance was determined at 605 nm using the microplate reader (Mullikan Go, F1-01620, Thermo Fisher Scientific, Vantaa, Fin-land). The Tamoxifen at 100 µM was used as a reference.
3.6. Chromatographic analysis:
3.6.1. High-Performance Liquid Chromatography Analysis (HPLC-DAD):
Analysis of the different shell extracts by HPLC-DAD was carried out in a Thermo Scientific Accela pump equipped with Accela PDA detector (280 nm wavelength was used for the detection of compounds). Separation has been performed using an RP-C18 column (Phenomenex; Le Pecq; France). The dimensions of the column are 25 cm × 4.6 mm and particle size 5 µm. Elution was carried out with flow rate of 0.5 mL/min.The mobile phase made up of acidified water (pH = 2.65) as solvent A and acidified water/ACN (20:80 v/v) as solvent B. The samples were eluted by the linear gradient as follows: from 12% B to 30% B for 15 min; from 30% B to 50% B for 2 min; from 50% B to 99.9% B for 3 min; and finally from 99.9% B to 12% B for 7 min. Extracts were prepared at 10 mg/mL using acidified water/ACN (80:20 v/v) and filtered using a filter (Sigma Aldrich, Millex-HA filter 0.45 µm, Saint-Quentin-Fallavier, France). The identification and the quantification of the molecules was determined by comparison them with the retention time and lambda max of known standards. Additionally, the quantity of each compound present in the shell extracts was determined using their specific calibration curves.
3.6.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis:
The volatile composition of almond shell extracts was determined using the method mentioned by Rahmani et al. (2020)[42] with some modifications. Shell extracts was solubilized in their extraction solvents (except for water extract, where methanol was used) at 3 mg/mL. the analysis was carried out on Saturn 2000 gas chromatography (Les Ulis, France), equipped with a fused silica capillary DB-5MS column (5% phenylmethylpolysyloxane, 30 × 0.25 mm, film thickness 0.25 μm). H2 was used as a carrier gas in this analysis. Chromatographic conditions were: 60 ° C for 1 min, then up to 150°C, at a gradient of 10°C/min, then hold for one minute. A second gradient was applied to reach 260 at 12°C/min, and then was held for 10 min. The trap temperature was 220 °C and that of the transfer line was 240 °C. Mass scanning was performed from 70 to 650 m/z. 5 µL of each extract was injected.
- Derivatization method:
The derivatization method was conducted as described by ben khadher et al (2022).
- Compounds identification:
Identification of volatile compounds by GC-MS of P. dulcis shell extracts has been performed using the Xcalibur software version 3.0.63 by comparison of their mass spectra and linear retention index (LRI) with those recorded in the NIST MS library version 2.4 built on 25 March 2020.
3.7. Statistical Analysis:
All data were expressed as means ± standard deviations from triplicate measurements. Confidence limits were set at p < 0.05 and calculated according to the ANOVA test using the Statistical Package for the Social Sciences (SPSS) 22 (version IBM. 22.0. 2013, San Francisco, CA, USA). The difference between the extraction solvents was determined using the Tukey’s test. Principal component analysis (PCA) was performed using XLSTAT (version 2021.3.1, Addinsoft, Pearson edition, Waltham, MA, USA)
4. Conclusions
The present study asses the chemical profile as well as the biological potential of P. dulcis shell extracts. The chemical analysis led to the identification of 15 compounds by HPLC-DAD of which 11 were firstly identified in the almond plant, and 26 volatile compounds by GC-MS among them seven were identified for the first time in the P. dulcis plant. For the biological activities, shell extracts displayed moderate antioxidant, antidiabetic, and cytotoxic potential. Further studies are required to confirm the obtained results and to isolate the active compounds responsible for those activities.
Author Contributions
Talel ben Khadher carried out the practical part and drafting of the manuscript. Jalloul Bouajila, Mohamed Mars., Samir Aydi, and Sameh Sassi-Aydi. were involved in the correction of the manuscript, the orientation of work, and the coordination of the project.
Funding
This work has been supported financially by the ERASMUS PLUS program and the Tunisian Minister of Higher Education and Scientific Research, which provided a PhD scholarship for the student Talel ben khadher.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- Qureshi, M.N.; Numonov, S.; Abudurexiti, A.; Aisa, H.A. Phytochemical Investigations and Evaluation of Antidiabetic Potential of Prunus Dulcis Nuts. LWT-Food Science and Technology 2016, 66, 311–317. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chemistry 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus Amygdalus L.) and Its by-Products. Food chemistry 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef] [PubMed]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from Almond (Prunus Dulcis) Shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of Walnut, Almond, and Pine Nut Shells Regarding Chemical Composition and Extract Composition. Biomass Conv. Bioref. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Sarwar, S.; Anwar, F.; Raziq, S.; Nadeem, M.; Zreen, Z.; Cecil, F. Antioxidant Characteristics of Different Solvent Extracts from Almond (Prunus Dulcis L.) Shell. JMPR 2012, 6, 3311–3316. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Medicine and Cellular Longevity 2015, 2016, 5692852. [Google Scholar] [CrossRef]
- Moure, A.; Pazos, M.; Medina, I.; Domínguez, H.; Parajó, J.C. Antioxidant Activity of Extracts Produced by Solvent Extraction of Almond Shells Acid Hydrolysates. Food Chemistry 2007, 101, 193–201. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus Dulcis L.). Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Vangdal, E.; Brede, C. Analysis of Phenolic Compounds in Six Norwegian Plum Cultivars (Prunus Domestica L.). J. Agric. Food Chem. 2009, 57, 11370–11375. [Google Scholar] [CrossRef]
- He, D.; Shan, Y.; Wu, Y.; Liu, G.; Chen, B.; Yao, S. Simultaneous Determination of Flavanones, Hydroxycinnamic Acids and Alkaloids in Citrus Fruits by HPLC-DAD–ESI/MS. Food Chemistry 2011, 127, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, B.; Simčič, M.; Trebše, P. Anthocyanins and Hydroxycinnamic Acids of Lambert Compact Cherries (Prunus Avium L.) after Cold Storage and 1-Methylcyclopropene Treatment. Food Chemistry 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Davarcı, F.; Paslı, A.A.; Demir, N.; Özçelik, B. Determination of Phenolic Compounds by Ultra High Liquid Chromatography-Tandem Mass Spectrometry: Applications in Nuts. LWT - Food Science and Technology 2015, 64, 42–49. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Bouajila, J.; Cazaux, S.; Abderrabba, M. The Impact of Regional Locality on Chemical Composition, Anti-Oxidant and Biological Activities of Thymelaea Hirsuta L. Extracts. Phytomedicine 2018, 41, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Ao, W.-L.-J.; Wang, X.-L.; Bao, X.-H.; Wang, J.-H. Two New Flavonoid Glycosides from Artemisia Frigida Willd. Journal of Asian Natural Products Research 2010, 12, 950–954. [Google Scholar] [CrossRef]
- Sancho, M.I.; Almandoz, M.C.; Blanco, S.E.; Castro, E.A. Spectroscopic Study of Solvent Effects on the Electronic Absorption Spectra of Flavone and 7-Hydroxyflavone in Neat and Binary Solvent Mixtures. International journal of molecular sciences 2011, 12, 8895–8912. [Google Scholar] [CrossRef]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of Shikonin and Its Ester Derivatives from the Roots of Echium Italicum L. Journal of Chromatography A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Ashrafian, S.; Farimani, M.M.; Sonboli, A.; Ashrafian, H.; Kabiri, M.; Rezadoost, H. Simultaneous Characterization of Nine Isolated Flavonoids in Iranian Dracocephalum Species and in Silico Study of Their Inhibitory Properties against MTH1 Enzyme. South African Journal of Botany 2022, 146, 254–261. [Google Scholar] [CrossRef]
- Erdenetsogt, U.; Nadmid, S.; Paulus, C.; Chanagsuren, G.; Dolgor, E.; Gotov, C.; Dahse, H.-M.; Luzhetskyy, A.; Dagvadorj, E. Bioactive Flavonoids from Plant Extract of Pyrethrum Pulchrum and Its Acute Toxicity. Natural product research 2021, 35, 5960–5963. [Google Scholar] [CrossRef]
- Sei-Ichi, K.; Toda, K.; Matsumoto, K.; Ishihara, C.; Nonobe, S.; Matsunaga, C.; Gomi, Y.K.; Senga, S.; Kawaguchi, K.; Yamamoto, A. Isolation and Characterization of a Novel Oligomeric Proanthocyanidin with Significant Anti-Cancer Activities from Grape Stems (Vitis Vinifera). Scientific reports 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Bukvicki, D.R.; Tyagi, A.K.; Gottardi, D.G.; Veljic, M.M.; Jankovic, S.M.; Guerzoni, M.E.; Marin, P.D. Assessment of the Chemical Composition and In Vitro Antimicrobial Potential of Extracts of the Liverwort Scapania Aspera. Natural Product Communications 2013, 8, 1934578X1300800932. [Google Scholar] [CrossRef]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical Composition, Cytotoxicity and Molecular Profiling of Cordia Africana Lam. on Human Breast Cancer Cell Line. Natural Product Research 2021, 35, 4133–4138. [Google Scholar] [CrossRef]
- Rath, D.; Panigrahi, S.K.; Kar, D.M.; Maharana, L. Identification of Bioactive Constituents from Different Fractions of Stems of Cuscuta Reflexa Roxb. Using GC-MS. Natural Product Research 2018, 32, 1977–1981. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical Composition and Antifungal Properties of Essential Oils of Three Pistacia Species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Goyal, M. Qualitative and Quantitative Evaluation of Phytochemicals in Leaf Extract of Alstonia Scholaris (L.) R. Br.By GC-MS Technique. Qualitative and quantitative evaluation of phytochemicals in leaf extract of Alstonia scholaris (L.) R. Br.By GC-MS technique 2019, 9. [Google Scholar]
- Guedes De Pinho, P.; Gonçalves, R.F.; Valentão, P.; Pereira, D.M.; Seabra, R.M.; Andrade, P.B.; Sottomayor, M. Volatile Composition of Catharanthus Roseus (L.) G. Don Using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry. Journal of Pharmaceutical and Biomedical Analysis 2009, 49, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus Gerardiana and Pinus Roxburghii Seed Extracts. BioMed Research International 2022, 2022, e5938610. [Google Scholar] [CrossRef] [PubMed]
- Yetayih, M. Extraction and GC-MS Analysis of the Essential Oil from the Peel of Solanum Incanum and Its Antibacterial Activity Studies. Extraction and GC-MS analysis of the essential oil from the peel of Solanum incanum and its antibacterial activity studies 2020, 32. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Nedelcheva, D.V.; Parushev, S.P.; Atanassov, A.I.; Hvarleva, T.D.; Djakova, G.J.; Bankova, V.S.; Popov, S.S. Preliminary Study on Biomarkers for the Fungal Resistance in Vitis Vinifera Leaves. Journal of Plant Physiology 2008, 165, 791–795. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis Vinifera Cv. Pinot Noir). Plant Physiology and Biochemistry 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Kumar, A. GC-MS Analysis of Phytochemical Constituents in Ethanolic Extract of Punica Granatum Peel and Vitis Vinifera Seeds. GC-MS analysis of phytochemical constituents in ethanolic extract of Punica granatum peel and Vitis vinifera seeds 2011, 2. [Google Scholar]
- Orsavova, J.-M. , LadislavaAU-Ambrozova, Jarmila V. AU-Vicha, RobertAU-Mlcek, JiriTI-Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids International Journal of Molecular Sciences 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Sermakkani GC-MS Analysis of Cassia Italica Leaf Methanol Extract. GC-MS analysis of Cassia italica leaf methanol extract 2012, 5.
- Fiehn, O.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Identification of Uncommon Plant Metabolites Based on Calculation of Elemental Compositions Using Gas Chromatography and Quadrupole Mass Spectrometry. Anal. Chem. 2000, 72, 3573–3580. [Google Scholar] [CrossRef]
- Eldjoudi, D.A.; Ruiz-Fernandez, C.; González-Rodriguez, M.; Atmane, S.A.; Cordero-Barreal, A.; Farrag, Y.; Pino, J.; Sineiro, J.; Lago, F.; Conde-Aranda, J.; et al. Analgesic and Antiinflammatory Effects of Nigella Orientalis L. Seeds Fixed Oil: Pharmacological Potentials and Molecular Mechanisms. Phytotherapy Research 2022, 36, 1372–1385. [Google Scholar] [CrossRef]
- Komakech, R.; Shim, K.-S.; Yim, N.-H.; Song, J.H.; Yang, S.; Choi, G.; Lee, J.; Kim, Y.; Omujal, F.; Okello, D.; et al. GC–MS and LC-TOF–MS Profiles, Toxicity, and Macrophage-Dependent in Vitro Anti-Osteoporosis Activity of Prunus Africana (Hook f.) Kalkman Bark. Sci Rep 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. Journal of Functional Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayı, H.; Hanoglu, A.; Yigit Hanoglu, D.; Ozkum Yavuz, D.; Ozek, T.; Vatansever, S. Fatty Acid Composition and Anticancer Activity in Colon Carcinoma Cell Lines of Prunus Dulcis Seed Oil. Pharmaceutical Biology 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative Terpenoids from Almond Hulls (Prunus Dulcis): Identification and Structure−Activity Relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Rahmani, R.; Bouajila, J.; Jouaidi, M.; Debouba, M. African Mustard (Brassica Tournefortii) as Source of Nutrients and Nutraceuticals Properties. Journal of Food Science 2020. [Google Scholar] [CrossRef]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis Vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]
Figure 2.
Antioxidant Activity of P.dulcis shell extracts (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; ascorbic acid: VIT C: used as a reference at 4 µg/mL). Extracts was tested at 50 µg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).
Figure 2.
Antioxidant Activity of P.dulcis shell extracts (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; ascorbic acid: VIT C: used as a reference at 4 µg/mL). Extracts was tested at 50 µg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).

Figure 3.
Antidiabetic Activity (alpha-glucosidase) of different extracts of P. dulcis shell Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Acarbose: was used as reference at 50 µg/mL). Extracts was tested at 50 µg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).
Figure 3.
Antidiabetic Activity (alpha-glucosidase) of different extracts of P. dulcis shell Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Acarbose: was used as reference at 50 µg/mL). Extracts was tested at 50 µg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).

Figure 4.
Antidiabetic activity (alpha-amylase) of different extracts of P. dulcis shell cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Acarbose: was used as reference at 50 µg/mL). Extracts was tested at 50 μg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05)..
Figure 4.
Antidiabetic activity (alpha-amylase) of different extracts of P. dulcis shell cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Acarbose: was used as reference at 50 µg/mL). Extracts was tested at 50 μg/mL. Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05)..

Figure 5.
Cytotoxic activity of P. dulcis shell extracts against two cell types (HELA; RAW 264-7 ). Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Tamoxifen: was used as a reference at 100 µM). Extracts was tested at 50 µg/mL Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).
Figure 5.
Cytotoxic activity of P. dulcis shell extracts against two cell types (HELA; RAW 264-7 ). Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH; Tamoxifen: was used as a reference at 100 µM). Extracts was tested at 50 µg/mL Mean values ± SD (n = 3); Different letters on the histograms mean a significant difference (p <0.05).

Figure 6.
Principal Components analysis (PCA) “loading plot” of (TPC: total phenolic content), (DPPH: Antioxidant activity) and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.
Figure 6.
Principal Components analysis (PCA) “loading plot” of (TPC: total phenolic content), (DPPH: Antioxidant activity) and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.
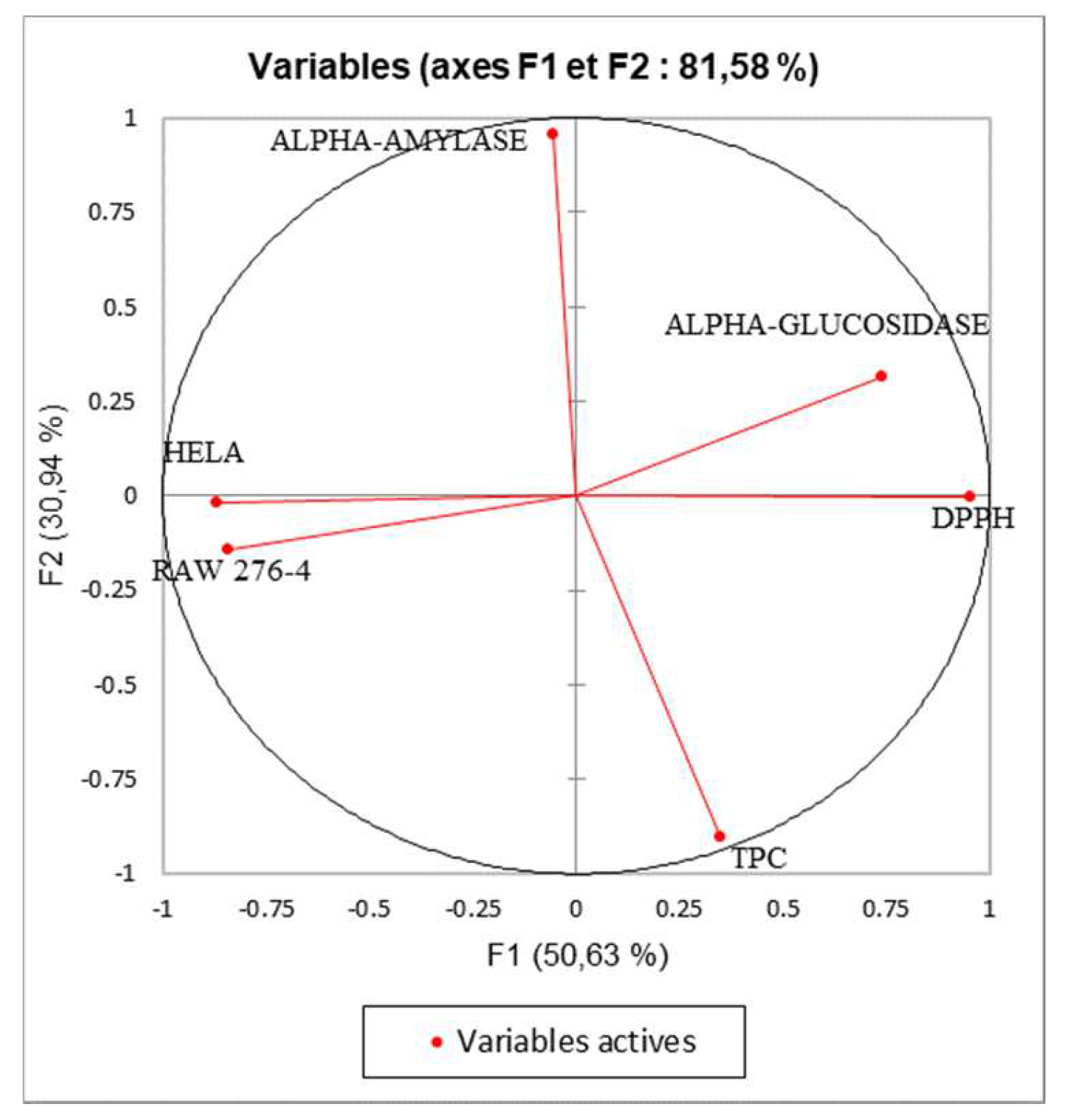
Figure 7.
Principal component analysis (PCA) “score plot” of TPC: total phenolic content), (DPPH: antioxidant activity), and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.
Figure 7.
Principal component analysis (PCA) “score plot” of TPC: total phenolic content), (DPPH: antioxidant activity), and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.

Figure 8.
Principal Components Analysis (PCA) “Biplot” of TPC: total phenolic content), (DPPH: antioxidant activity), and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.
Figure 8.
Principal Components Analysis (PCA) “Biplot” of TPC: total phenolic content), (DPPH: antioxidant activity), and biological activities (Alpha-amylase, Alpha-glucosidase: Antidiabetic activity); (HELA and RAW 276-4: cytotoxic activity) of shell extracts of P. dulcis.

Table 1.
Extraction yields of shell extracts of P. dulcis (DW %) (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
Table 1.
Extraction yields of shell extracts of P. dulcis (DW %) (Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
| Fractional extraction | |||||
| CHYA | DCM | EtOAc | MeOH | H2O | |
| Maceration | 0.14 | 0.09 | 0.03 | 0.63 | 2.22 |
Table 2.
Identification of compounds by HPLC-DAD of almond shell extracts Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
Table 2.
Identification of compounds by HPLC-DAD of almond shell extracts Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
| mg/g extract | |||||||
| compounds | Rt (min) | Ref | |||||
| Extracts | |||||||
| CHYO | DCM | EtOAc | MeOH | H2O | |||
| Catechin | 0.87 | 0.39 | [10] | ||||
| Rutin | 0.93 | 13.06 | [11] | ||||
| Synephrine | 1.09 | 1.62 | [12] | ||||
| Epicatechin | 1.9 | 4.42 | [13] | ||||
| trans-3-hydroxycinnamic acid | 3.5 | 0.08 | [14] | ||||
| trans-cinnamic acid | 10.8 | 0.02 | [15] | ||||
| 7,8-dihydroxy-2,2-dimethylchromane-6-carboxylic acid | 10.93 | 0.31 | [16] | ||||
| 5,7-dihydroxy-3',4',5'-trimethoxyflavone | 19.51 | 0.31 | [17] | ||||
| 3-tert-butyl-4-hydroxybenzoic acid | 19.55 | 0.02 | [16] | ||||
| 7-hydroxyflavone | 19.71 | 0.59 | [18] | ||||
| Shikonin | 20.72 | 0.19 | [19] | ||||
| 3,3′-dimethoxyflavone | 21.59 | 0.23 | [20] | ||||
| 3,6,3′-trimethoxyflavone | 21.74 | 0.33 | 0.11 | [21] | |||
| Isobutyl 4-hydroxybenzoate | 21.02. | 0.25 | [16] | ||||
| 5-hydroxy-3'-methoxyflavone | 21.95 | 0.33 | [22] | ||||
Table 3.
Identification of volatile compounds by GC-MS of almond shell extracts (before derivatization) Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
Table 3.
Identification of volatile compounds by GC-MS of almond shell extracts (before derivatization) Cyclohexane: CHYA; Dichloromethane: DCM; Ethyl acetate: EtOAc; Methanol: MeOH).
| N | RI | Compounds | Area (E+07) | Ref | ||||
| Extracts | ||||||||
| CHYA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1075 | 2,3-Dimethyldecane | 12.6 | [23] | ||||
| 2 | 1100 | Undecane | 0.0922 | [24] | ||||
| 3 | 1205 | Dodecane | 6.76 | [25] | ||||
| 4 | 1306 | Tridecane | 1.63 | [26] | ||||
| 5 | 1377 | 1,1'-Bicyclohexyl | 25300 | 4.06 | [26] | |||
| 6 | 1535 | Benzaldehyde, 3-hydroxy-4-methoxy- | 370 | 4.99 | [25] | |||
| 7 | 1560 | 2,4-Di-tert-butylphenol | 6.24 | 3.55 | 7.868 | [27] | ||
| 8 | 1566 | Dodecanoic acid, 1-methylethyl ester | 3.69 | [28] | ||||
Table 4.
Identification of volatile compounds by GC-MS of almond shell extracts (after derivatization) (CHYA: Cyclohexane; DCM: Dichloromethane; EtOAc: Ethyl acetate; MeOH: Methanol).
Table 4.
Identification of volatile compounds by GC-MS of almond shell extracts (after derivatization) (CHYA: Cyclohexane; DCM: Dichloromethane; EtOAc: Ethyl acetate; MeOH: Methanol).
| N | RI | Compounds | Area(E+07) | Ref | ||||
| Extracts | ||||||||
| CHYA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1025 | Cyclohexanol | 15.4 | 4.02 | [29] | |||
| 2 | 1038 | Furfuryl alcohol | 3.13 | 14.3 | 39.1 | 23.4 | [29] | |
| 3 | 1075 | Lactic Acid | 45.9 | 58.2 | 86.5 | [30] | ||
| 4 | 1094 | Hexanoic acid | 15.1 | [30] | ||||
| 5 | 1094 | Glycolic acid | 121 | [30] | ||||
| 6 | 1162 | Hydracrylic acid | 13.8 | 32.9 | [31] | |||
| 7 | 1266 | Glycerol | 88.9 | 1130 | 621 | [26] | ||
| 8 | 1338 | Glyceric acid | 41.7 | 109 | [28] | |||
| 9 | 1351 | Butanedioic acid | 97.9 | 0.691 | [32] | |||
| 10 | 1384 | Nonanoic acid | 51.6 | [26] | ||||
| 11 | 1500 | Malic acid | 1760 | 1310 | [33] | |||
| 12 | 1591 | 2,4-Di-tert-butylphenol | 22.2 | [27] | ||||
| 13 | 1672 | Dodecanoic acid | 23.8 | [34] | ||||
| 14 | 1865 | Myristic acid | 12.8 | [34] | ||||
| 15 | 1959 | Pentadecanoic acid | 10.6 | [33] | ||||
| 16 | 2054 | Palmitic acid | 511 | 4.60 | 2.84 | [35] | ||
| 17 | 2074 | Myo-Inositol | 428 | 1150 | [36] | |||
| 18 | 2241 | 9-octadecenoic acid, (E)- | 278 | [37] | ||||
| 19 | 2248 | Stearic acid | 165 | 121 | 392 | [38] | ||
Table 5.
Correlations between variables and factors.
| F1 | F2 | |
| TPC | 0.121 | 0.818 |
| DPPH | 0.903 | 0.000 |
| ALPHA-GLUCOSIDASE | 0.547 | 0.100 |
| ALPHA-AMYLASE | 0.003 | 0.917 |
| RAW 276-4 | 0.711 | 0.021 |
| HELA | 0.753 | 0.000 |
Table 6.
Correlation matrix (Pearson (n)).
| Variables | TPC | DPPH | ALPHA-GLUCOSIDASE | ALPHA-AMYLASE | RAW 276-4 | HELA | |
| TPC | 1 | 0.388 | -0.018 | -0.828 | -0.150 | -0.209 | |
| DPPH | 0.388 | 1 | 0.680 | 0.011 | -0.757 | -0.762 | |
| ALPHA-GLUCOSIDASE | -0.018 | 0.680 | 1 | 0.185 | -0.490 | -0.535 | |
| ALPHA-AMYLASE | -0.828 | 0.011 | 0.185 | 1 | -0.099 | 0.063 | |
| RAW 276-4 | -0.150 | -0.757 | -0.490 | -0.099 | 1 | 0.679 | |
| HELA | -0.209 | -0.762 | -0.535 | 0.063 | 0.679 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts
Talel Ben khadher
et al.
,
2023
Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-apoptotic Effects
Nunzio Antonio Cacciola
et al.
,
2019
Antioxidant and Anticancer Activity of Phenolic Extracts from Psidium guajava Linn Leaves by Novel Assisted Extraction Techniques
Md. Anisur Rahman Mazumder
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated










