Preprint
Article
Properties and Stability of Encapsulated Pomegranate Peels Extract Prepared by Co-crystallization
Altmetrics
Downloads
188
Views
18
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
10 July 2023
Posted:
11 July 2023
You are already at the latest version
Alerts
Abstract
Recently, there has been a lot of interest in the phenolics of pomegranate peels because of their health-promoting effects. The incorporation of encapsulated phenolic extracts in functional foods, beverages, and dietary supplements can enhance their nutritional and health benefits. This paper aims to provide an overview of the encapsulation of pomegranate peel phenolic extract by co-crystallization, focusing on the properties of the encapsulated extract. Pomegranate peel extract encapsulated in sucrose by co-crystallization at conditions determined in our previous work is characterized by evaluating its properties; moisture content, bulk density, solubility, hygroscopicity, color, degree of encapsulation by thermograms, crystallinity by X-ray scattering, microstructure by scanning electron microscope, and storage stability in terms of total phenolic content and antioxidant activity. The co-crystallized powder had a low moisture content (0.59%) and hygroscopicity (0.011%) and a high bulk density (0.803 g/cm3) and solubility (61 s). Its total phenolic content decreased by only 0.56% after storage at 60oC for 45 days, whereas its antioxidant activity was maintained at levels higher than 84%. The differential scanning calorimetry and the X-ray scattering techniques proved the successful encapsulation in the sucrose matrix and the fact that the extract remained liquid inside the porosity of the sucrose crystals.

Keywords:
Subject: Environmental and Earth Sciences - Waste Management and Disposal
1. Introduction
Pomegranate (Punica granatum L.) has gained considerable attention in recent years due to its diverse bioactive compounds, particularly phenolic compounds found in different parts of the fruit. Pomegranate peel, a byproduct of the pomegranate juice and fruit processing industry, contains a significant amount of phenolic compounds, including ellagitannins, anthocyanins, and flavonoids, which have demonstrated various health benefits, such as antioxidant, anti-inflammatory, and anticancer properties [1].
Multiple studies have reported in vitro biological activities of pomegranate peel extracts, including antioxidant, antitumor, anti-inflammatory, and antiproliferative properties. Kanatt et al. [2] investigated the antioxidant and antimicrobial potential of pomegranate peel extract (PPE) and concluded that PPE was very effective in scavenging hydroxyl and superoxide anion free radicals. In addition, the extract has good reducing power and iron chelating ability and exhibits good antibacterial activity against Staphylococcus aureus and Bacillus cereus, with a minimum inhibitory concentration of 0.01%. A higher concentration of 0.1% has an inhibitory effect on Pseudomonas but is ineffective against Escherichia coli and Salmonella typhimurium. Therefore, due to its versatility (e.g., as an ingredient in functional foods), PPE may be used in several industries.
Several studies have been carried out on the extraction of phenols from pomegranate peels using different methods such as pressurized water extraction, supercritical fluid enzyme-assisted extraction, ultrasonic-assisted extraction, and microwave-assisted extraction [3, 4, 5].
However, the practical application of phenolic compounds is often hindered by their inherent limitations, such as poor stability, low solubility, and susceptibility to degradation. To overcome these challenges and unlock the full potential of phenolic compounds, encapsulation techniques have emerged as an effective approach for enhancing their stability and bioavailability. Among various encapsulation methods, co-crystallization has recently gained recognition as a promising strategy for the encapsulation of phenolic extracts. Co-crystals are crystalline materials composed of two or more molecular entities held together by non-covalent interactions, resulting in unique physicochemical properties [6] By forming co-crystals with phenolic compounds, it becomes possible to modulate their properties, such as solubility, dissolution rate, and stability, thereby addressing their limitations and expanding their application potential [7].
The encapsulation of phenolic extracts through co-crystallization offers several advantages over traditional encapsulation techniques [8, 9]. Firstly, co-crystals provide a solid-state form that offers improved chemical stability, protecting phenolic compounds from degradation and oxidation. Secondly, the controlled formation of co-crystals allows for the enhancement of solubility and dissolution rate, leading to improved bioavailability. Moreover, the co-crystallization approach enables the incorporation of various excipients, such as natural polymers or surfactants, which can further enhance the stability and functional properties of phenolic encapsulates.
However, co-crystals are more commonly used in the pharmaceutical industry than in the food industry [10]. These uses fall into three broad categories based on the encapsulated active ingredient. The first category includes co-crystals of phenolic or carotenoid extracts, such as extracts of banana pulp and peel [11], green tea extracts [12], Butterfly pea flower extract [13], Brasella rubra extract [8], propolis extract [14], carrot extract [15], aronia extract [16], mint polyphenols [17] and pomegranate peel extract [18]. The second category includes pure compounds, such as glucose [6, 19], fructose and mixtures of glucose and fructose [19], magnesium sulfate and calcium lactate [20, 21], zinc sulfate [22], curcumin [23], Vitamin B12 [24], soluble fiber [25], and catechin hydrate and curcumin [26]. The third group includes oils and oleoresins, such as orange peel oil [27], cardamom oleoresin [9, 28], capsicum oleoresin [29], and ginger oleoresin [30]. It should be emphasized here that, according to the existing literature, the co-crystallization method has not been used for the encapsulation of pomegranate peel phenolic extracts.
In our previous work [18], pomegranate peel phenolic extract was successfully encapsulated in the matrix of sucrose by co-crystallization utilizing three different experimental designs. The effect of various parameters, such as the temperature at which the extract is incorporated into the sucrose matrix, the solids concentration of the extract, and the dry extract to sucrose ratio, was examined and the optimum conditions were determined. Continuing our investigation of the utilization of pomegranate peels, the aim of this work is to characterize the co-crystallized pomegranate peel extract by evaluating its properties like moisture content, bulk density, solubility, hygroscopicity, color, degree of encapsulation in the sucrose matrix by thermograms, crystallinity by X-ray scattering, and microstructure by SEM. However, when designing a process for exploitation of a phenolic extract from food wastes, the storage stability of the extract is a significant consideration. Thus, the stability of the crude and the encapsulated phenolic, in terms of total phenolic content and antioxidant activity, was studied. This paper aims to shed light on the encapsulation of pomegranate peels phenolic extract by co-crystallization as a promising approach to overcome the limitations of phenolic compounds. By providing a comprehensive understanding of the co-crystallization process and its implications, this study seeks to contribute to the advancement of encapsulation technologies and their applications in the field of phenolic compound delivery systems.
2. Materials and Methods
2.1. Raw Materials
For the co-crystallization process, food quality crystal sucrose (Royal sugar) was purchased. Pomegranate peels (Wonderful variety) from the Parthenion area (Chalkidonos municipality, Thessaloniki, Greece), harvested in October 2021, were delivered and kept at –30 oC till usage. The peels, after cleaning and cutting into smaller, uniform pieces, were dried (Gallenkamp PCL, Model BR185HNI/NCC, Leister, England) for 48 h at a temperature of 40 oC, in the presence of air, until the moisture content reduced below 10% w/w wet basis. Following drying, the peels were ground in a laboratory mill (IKA Labortechnik, Germany). The average powder particle diameter was about 0.4 mm.
2.2. Preparation of Phenolic Extract
The extraction was performed using a Sonics and Materials 130 W, 20 kHz, VCX-130 (Danbury, CT, USA) ultrasound-assisted extraction system equipped with a 13 mm Ti-Al-V probe in pulsed mode. According to Kaderides et al. (2015), the optimum conditions to extract polyphenols from pomegranate peel are: type of solvent, water; solvent/solid ratio, 33/1 mL/g; amplitude level, 40% (50 W); pulse duration/pulse interval ratio, 7/6 s/s; extraction time, 10 min; extraction temperature, 35 °C. During the extraction process, the sample container was kept in a thermostatically controlled water bath. The resulting extract was subsequently filtered on Whatman filter paper No. 2 and evaporated at 40 ± 2 °C under 150 mbar vacuum using a rotary evaporator (Rotovapor R114, water bath B480, Büchi, Flawil, Switzerland) to a solids concentration of 60 °Brix The concentrated extract was stored in a dark bottle at –18 °C.
2.3. Co-Crystallization Procedure
Sucrose (75 g) was mixed with 12.5 mL of distilled water. The mixture was continuously stirred at 500 rpm using a vertical stirrer (IKA Eurostar 40 Digital, Germany) in a metal container placed on a heater and brought to 140 °C, a temperature that was determined as optimum [18]. Once the mixture reached the required temperature, heating was stopped, stirring was continued at 700 rpm, and pomegranate peel extract was quickly poured into the sucrose syrup in a dry extract to sucrose ratio of 0.591 g/g [18]. Immediately thereafter, the mixture was placed in a water bath (25 °C) and stirred until the temperature was below 60 °C (approximately 45 °C). The dry powder was then transferred to a glass container and stored in a desiccator for 24 h.
2.4. Characterization of the Product
- Moisture content
- The moisture content (% wet basis) of the co-crystallized product was calculated by drying at 105 °C until constant weight [31].
- Solubility
- The solubility was determined by dissolving 1 g of powder in 25 mL of distilled water at ambient temperature with continuous agitation at 890 rpm [32].
- Bulk density
- The bulk density was calculated by dividing the mass of a co-crystallized powder sample (about 2 g) by its volume in a 50 mL graduated cylinder [33].
- Hygroscopicity
- About 1 g of co-crystallized powder was placed in a desiccator with HNO3 solution at 23 °C and 76% relative humidity on dishes to create a high surface area between air and powder. The increase or decrease of powder weight per gram of its solids was measured [16].
- Color
- A Minolta colorimeter (CR-400, Konica-Minolta Sensing, Japan) was used for the evaluation of color using the CIE-L* a* b* uniform color space. The powder was placed in 5 cm-diameter dishes in a layer with a thickness of approximately 0.5 cm.
- Differential scanning calorimetry
- Samples (2-10 mg) of pure crystalline sucrose, co-crystallized sucrose without the active ingredient, and co-crystallized sucrose with the encapsulated ingredient are used for differential scanning calorimetry (DSC) analysis with a Perkin–Elmer Pyris 1 differential scanning calorimeter, as described by Kaderides and Goula [34]. The samples were heated from 25 to 250 oC with a rate of 10 oC/min in inert atmosphere. The reference was an empty pan, whereas liquid nitrogen was used for sample cooling before the runs.
- Degree of crystallinity
- The X-Ray diffraction pattern was determined by using a continuous scan mode (3003 TT, Rich. Seifert) at 40 kV with radiation of wavelength of 40 mÅ and 2θ data between 5 and 60o. All samples were dried at 60 oC before the assay.
- Morphology
- Particle morphology was evaluated using a Quanta-200 environmental scanning electron microscope system (FEI Company, USA). The samples were examined under high vacuum condition at an accelerating voltage of 10 kV.
- Storage stability
- The unencapsulated and encapsulated extracts were evaluated for total phenolic content and antiradical activity during storage at 60 °C. Extract samples in vials were stored in the dark in an air oven (Memmert, Schwabach, Germany) at controlled temperature for 45 days. Duplicate samples were collected every 2-3 days for analysis. The total phenolic content (TPC) was determined using the Folin-Ciocalteu method, whereas antiradical activity was measured using the DPPH method [32].
2.5. Statistical Analysis
All characterizations were performed using independent triplicate samples and results are presented as mean ± standard deviation. Analysis of variance (ANOVA) was performed and a p value less than 0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Powder Properties
Powders encapsulated at the optimum conditions were analyzed for moisture content, bulk density, solubility, hygroscopicity, and color.
Moisture content of the co-crystallized product was about 0.59%, a value lower than those reported when spray drying was used [35]. Similar results were obtained by Bhandari and Hartel [19], who reported that the moisture content of co-crystallized powders was between 0.67% and 3.22% during encapsulation of glucose, fructose, and their mixtures in a sucrose matrix. Furthermore, Irigoiti et al. [14] found that the moisture content of co-crystallized powders of propolis extracts was between 0.03 and 2.09%. In general, according to the literature, the moisture content depends on several factors such as the type of active ingredient and the crystalline/amorphous structure ratio of the powder [9, 36]. Bhandari and Hartel [19], Deladino et al. [20] and Irigoiti et al. [14] studied the encapsulation of simple sugars (glucose and fructose), yerba mate extract, and mineral or propolis extract and found that as the concentration of the active ingredient in the sucrose matrix increased, the moisture content of the final product also increased. This observation may be due to the fact that as the ratio of extract solids to sucrose increases, so does the amount of extract added to the sucrose syrup, leading on the one hand to a reduction in the amount of water that can eventually be removed from the newly formed powder and on the other hand affecting hydrophilic sites, where water can be retained in the polysaccharide chain [37].
In general, the moisture content of a powder is a very important parameter for its bulk density. More specifically, with increasing water content, the tendency of particles to agglomerate increases and the bulk density decreases [16]. Bulk density of the co-crystallized product was about 0.803 g/cm3. Similar values were reported by Sarabandi et al. [36] and Tzatsi and Goula [16], who, when encapsulating marjoram and aronia extract, found that the bulk density of the final powder was between 0.7 and 0.73 g/cm3, and 0.63 and 0.97 g/cm3. Irigoiti et al. [14] also claimed that the bulk density of co-crystallized propolis extracts varied between 0.63 and 0.87 g/cm3. According to the literature, bulk density provides important information about economic fundamentals as it determines the amount of material required to fill a given volume of packaging [36]. Additionally, according to Kurozawa et al. [38], high bulk density generally means that there are fewer spaces between particles for air to occupy, which helps to prevent oxidation and improve powder stability.
The powder solubility was about 61 s. Similar results were obtained from Irigoiti et al. [14] and Kaur et al. [15], who observed that the solubility of co-crystallized powders varied between 41 and 47 s and 45 and 50 s, respectively. Furthermore, Rai et al. [30] mentioned that during the co-crystallization of ginger oleoresin into a sucrose matrix, the solubility of the final powder varied between 32 and 57 s. Solubility is important, on the one hand, to determine the extent of release of the active ingredient contained in the sucrose matrix, and on the other hand, to check the suitability of the product for the oral delivery of the active ingredient [36]. The dissolving power of powders depends on the size, shape, microstructure, and surface finish of the particles, the presence of additives and insoluble substances, and the type and composition of the matrix [9, 20, 36].
The color of the powder, specifically the brightness parameter L* (0 = black, 100 = white), is another characteristic that has been measured. According to Kaur et al. [15], the most crucial quality criterion for a product's aesthetic value is its color and the proportion of the active substance that is encapsulated inside the sucrose matrix directly affects the color of the powder [36, 39]. The lightness of the powder was found 83.24. With respect to the literature, the number of researchers who studied the color of a co-crystallized powder is very limited. Karangutkar and Ananthanarayan [8] mentioned that during the co-crystallization of Basella rubra extract into a sucrose matrix, the L* value of the powder ranged between 49.35 and 62.10. Furthermore, Irigoiti et al. [14] reported that the lightness of the powder with propolis extract varied from 62 to 82.26. These lower values may be associated with the higher temperature and extract solids concentration levels used in the specific studies. The possibility of caramelization increases with greater co-crystallization temperatures, increasing the tendency for a darker-colored powder with lower L*. Additionally, the evaporation of the extract may be the cause of the decrease in L* that occurs when the concentration of extract solids rises. The more the extract evaporates, the darker and more degraded the color of the extract becomes.
The moisture absorption rate for the co-crystallized product is about 0.011% and is lower than 2%, so the powder cannot be considered to be hygroscopic. At about 85% relative humidity, Deladino et al. [20] and Sarabandi et al. [36], similarly reported hygroscopicity values lower than 2%. These low values can be attributed to the crystalline structure's decreased water absorption when compared to the amorphous structure [19]. According to Sardar et al. [28], the amount of amorphous crystals in the glucose-fructose mixture determines the co-crystals' hygroscopicity. In generally, spray-dried phenolic extracts were found to be less fluid because they are more viscous than co-crystallized products. The problems of stickiness and hygroscopicity of spray-dried extracts are thereby eliminated by the co-crystallization method.
3.2. Differential Scanning Calorimetry
The differential scanning calorimetry technique was performed on three different samples, the pure crystalline sucrose, the co-crystallized sucrose without the active ingredient, and the co-crystallized sucrose with the encapsulated ingredient (Figure 1). Starting with the thermogram of the pure sucrose, there are three endothermic peaks, one very small around 155 °C, one near 192 °C, and one at a temperature higher than 200 °C and around 220 °C. According to the literature, the first very small peak may be mainly due to the residual moisture in amorphous regions of sucrose crystals and in other components, mainly metal salts [9, 10, 36, 39]. The second peak represents the melting of sucrose crystals and is in agreement with the majority of researchers, who also reached the same conclusion [9, 19, 21, 36, 39]. The third peak refers to the thermal degradation of sucrose [9, 36]. Finally, the small exothermic peak near 215 °C may be related either to possible interactions between impurities/salt impurities – sucrose or to caramelization products of sucrose [21].
Regarding the second thermogram, which corresponds to co-crystallized sucrose without the encapsulated component, exactly the same pattern as the first is observed. This observation is in agreement with Deladino et al. [21], who also found no differences in the thermograms of pure and co-crystallized sucrose. However, there are research groups that emphasize that in the thermogram of co-crystallized sucrose there was a slight shift of the peaks to the left, which is due to the imperfect and irregular crystals of co-crystallized sucrose, some of which display an amorphous structure [9].
Finally, the third thermogram refers to co-crystallized sucrose with the addition of the active ingredient. As can be seen, there are no differences between the first and third thermograms. But what must be emphasized is that the endothermic peak of the active ingredient is absent, which, if this was considered separately, it would be found at temperatures lower than the melting temperature of sucrose crystals. This fact indicates both the improvement of the thermal stability of the extract by the co-crystallization process and its successful encapsulation in the sucrose matrix [8].
3.3. Degree of Crystallinity by X-ray Scattering
Another important feature examined is the retention of X-ray scattering (XRD) crystallinity in both pure crystalline sucrose and co-crystallized sucrose without or with the encapsulated component, as seen in Figure 2. It can be seen from the graphs that the degree of crystallinity of both co-crystallized sucrose without the active ingredient and in the presence of the active compound has been maintained at levels greater than 55%. According to Karangutkar and Ananthanarayan [8], products showing a degree of crystallinity greater than 50% are characterized as highly crystalline, between 20 and 50% as moderately crystalline, while below 20% as slightly crystalline.
Crystallinity was also maintained by other research groups, who investigated the encapsulation of phenolic extracts in a sucrose matrix. For example, both Deladino et al. [21] and Kaur et al. [15] observed that during the co-crystallization of Yerba mate phenolic extract and carotenoid extract, crystallinity was maintained. Also, they report that in the XRD graphs of the co-crystallized products, no new peak appears, indicating that the encapsulated extract retained its amorphous structure and did not crystallize. Similarly, in the present study, the addition of the active ingredient did not increase the degree of crystallinity, which proves that the phenolic extract of pomegranate peels did not crystallize but remained liquid inside the porosity of the sucrose crystals, with the result that when the co-crystallized powder is added to a product, it presents a better controlled release.
According to the literature, the preservation of crystallinity is due to the polar character of the extract, which was able to integrate unhindered into the sucrose matrix, and to the preservation of the covalent bonds of sucrose, resulting in the absence of changes in its structure and thus finally, the absence of reactions between the encapsulated components [8, 36, 40]. Research groups that detected a change in the degree of crystallinity of the co-crystallized products, and specifically a decrease compared to pure sucrose, encapsulated components of a hydrophobic nature. For example, Rai et al. [30] observed that during the co-crystallization of ginger oleoresins, the degree of crystallinity decreased by about 20%.
3.4. Morphology by Scanning Electron Microscope
Scanning electron microscopy was also used to characterize the morphology of pure crystalline sucrose, co-crystallized sucrose without the active ingredient, and co-crystallized sucrose with the encapsulated ingredient, as shown in Figure 3. Starting with pure sucrose, a continuous surface without voids and with small sharp corners is observed. Both Deladino et al. [21] and Tzatsi and Goula [16] reached similar results and concluded that the specific arrangement is due to the consecutive position of the atoms in the sucrose molecules. By observing the micrograph of the co-crystallized sucrose without the addition of the encapsulated component, it is clearly seen that there is a modification in the structure of the sucrose, as gaps appear between the crystals, while the corners remain sharp, thus verifying the preservation of crystallinity. A similar conclusion was reported by Kaur et al. [15], during the encapsulation of carotenoid extract.
Regarding the micrograph of the co-crystallized sucrose with the active ingredient, interstices with intense porosity are also shown. In addition, both sharp corners at interstices and amorphous regions appear at the same time, which indicate that the pomegranate peel extract is still in an amorphous state between gaps of the sucrose crystals. In some places, it is found that the extract is also on the surface of the sucrose crystals, which verifies the hygroscopicity value, as the low molecular weight sugars of the phenolic extract that remained on the surface tended to absorb moisture. Almost all research groups that used scanning electron microscopy concluded that their crystallized products showed gaps, with the active ingredient located between them and preserved in its original state. According to Tzatsi and Goula [16], who encapsulated extract from unused chokeberries by co-crystallization, after co-crystallization, the firm, dense, perfect crystal structure of sucrose turns into irregular, agglomerated micro-crystals with a spongy appearance and increased cavities and surface areas. Similar observations were made by Deladino et al. [20], Sardar and Singhal [9], and Karangutkar and Ananthanarayan [8], who studied the co-crystallization of yerba mate extract, cardamom oleoresin, and cranberry extract, respectively. On the contrary, during encapsulation of pomegranate peel extract by spray drying, the particles have a different morphology. Kaderides and Goula [35], who used common wall materials and orange juice by-products as encapsulating agents, reported that the encapsulated powders were spherical in shape with irregular surfaces that presented indentations, indicating that the walls had solidified before swelling began. Compared with capsules made from a mixture of maltodextrin and skimmed milk powder, the microcapsules with orange waste powder had a corrugated, more spherical surface, and the particles had few cracks or fissures. According to Tan et al. [41], cracks formed during encapsulation lead to greater exposure of bioactive compounds within particles to air and increased heat exposure during drying. Tzatsi and Goula [16], who encapsulated chokeberry extract by both spray drying and co-crystallization, reported similar differences. The spray-dried product has spherical particles and a matte surface with dents and wall voids, some of which may be due to air bubbles entrained during homogenization or atomization [32, 42]. On the contrary, the co-crystallized encapsulated extracts showed agglomerates and porous structure of co-crystals.
3.5. Change in Total Phenolic Content
It can be seen from Figure 4 that the phenolic extract encapsulated in a sucrose matrix was better preserved than the liquid extract. The improved stability of the co-crystallized sample is probably due to the incorporation of unstable phenolic components into the porosity of the sucrose aggregates, which in turn ensure them to reduce the interaction of the active ingredient with environmental factors, thus offering protection against oxidation and degradation reactions [8].
Regarding the change in the phenolic concentration of the encapsulated extract, it can be seen that during the 40 days there was an ups and down of the total phenolic content. The results show that the total phenolic content decreased by only 0.56% compared to the initial value. A consequence of this is that the co-crystallization technique was able to effectively protect and preserve the phenolic components in the sucrose matrix. Sarabandi et al. [36] reported that co-crystallization ensures high stability of the active ingredient and this trend can be attributed to the ability of the sucrose crystal structure to effectively protect the encapsulated compounds. It is worth emphasizing that from the course of change of the total phenolic content of the co-crystallized powder, an increasing trend of the curve is noted after about 12 days, indicating an increase in the concentration of phenolic components. There are many literature references that report an increase in the concentration of total phenolic content in encapsulated samples during the study of their stability. For example, Kaderides et al. [43] reported an increase in phenolics in the encapsulated phenolic extract from pomegranate peels, by the spray drying technique, mainly in the first ten days. This increase was attributed to both the hydrolysis of conjugated polyphenols and structural changes in molecules of the phenolic compounds as well as in the formation of compounds that react with the Folin-Ciocalteu reagent. A similar trend was found by Flores et al. [44], who encapsulated raspberry peel extract with whey protein and found that the phenolic content increased by the duration of storage. In addition, the retention of phenolic content at the end of the 40-day time period has also been observed by other research groups. Irigoiti et al. [14], when encapsulating propolis extract in a sucrose matrix, also observed good retention of phenolic content and they even emphasized that the higher the concentration of the extract in the sucrose matrix, the more stable the final product is, presuming that this trend is due to the fact that greater amounts of active ingredient can be retained in the porosity of the sucrose aggregates.
The spray-dried powder from pomegranate peels extract produced by Kaderides et al. [43] demonstrated higher stability compared to the product of the present work, since it had a flat surface with dents suggesting that the wall had solidified before expansion and shrinkage began. Tzatsi and Goula [16], who encapsulated chokeberry extract by both spray drying and co-crystallization, also reported that the spray-dried powder showed better stability than the co-crystallized product, because it had a smooth surface indicating that the wall had solidified before shrinkage. On the contrary, according to Sarabandi et al. [36], the co-crystallized treatments exhibit greater stability when compared to powders made by spray drying. This trend was attributed to sucrose's superior ability to protect the encapsulated compounds due to its crystalline structure as opposed to the amorphous powder made by spray drying. Additionally, certain spray-dried capsules' pores lead to less entrapment.
Regarding the stability of the liquid, non-encapsulated extract, over time a decrease of 9.93% was observed compared to the initial value. It was found that in the second measurement, there was a sharp increase in the total phenolic content, which may be due either to the thermal degradation of phenolics and the formation of derivative products which are recognized as phenolic components [18] or to the formation of compounds that react with the Folin-Ciocalteu reagent and affect the absorbance measurement [13]. Then, however, a continuous decrease is observed, which can be attributed to oxidation and hydrolysis of the polyphenols, as direct contact with environmental factors led to the destruction of a part of the phenolic content. Similar results were reached by Kaderides et al. [43], who reported that the concentration of total phenolic components in the liquid extract of pomegranate peels decreased over storage time. This outcome was attributable to the polyphenols' hydrolysis and oxidation. Laine et al. [45] proposed a number of hypotheses regarding the cause of ellagitannin loss during storage, including polymerization reactions during oxidation [46], interactions with carbohydrates by forming complexes with them [47], and hydrolysis that liberates ellagic acid from the ellagitannin structure [46]. According to Xu et al. [48], the exposure to air and light caused the total phenolic content of mulberry powder to decrease by 34.21% after 20 days of storage, whereas Cam et al. [1], who assessed the phenolic compounds of pomegranate peels during storage, found a significant decrease in the phenolic content in the first 15 days of storage.
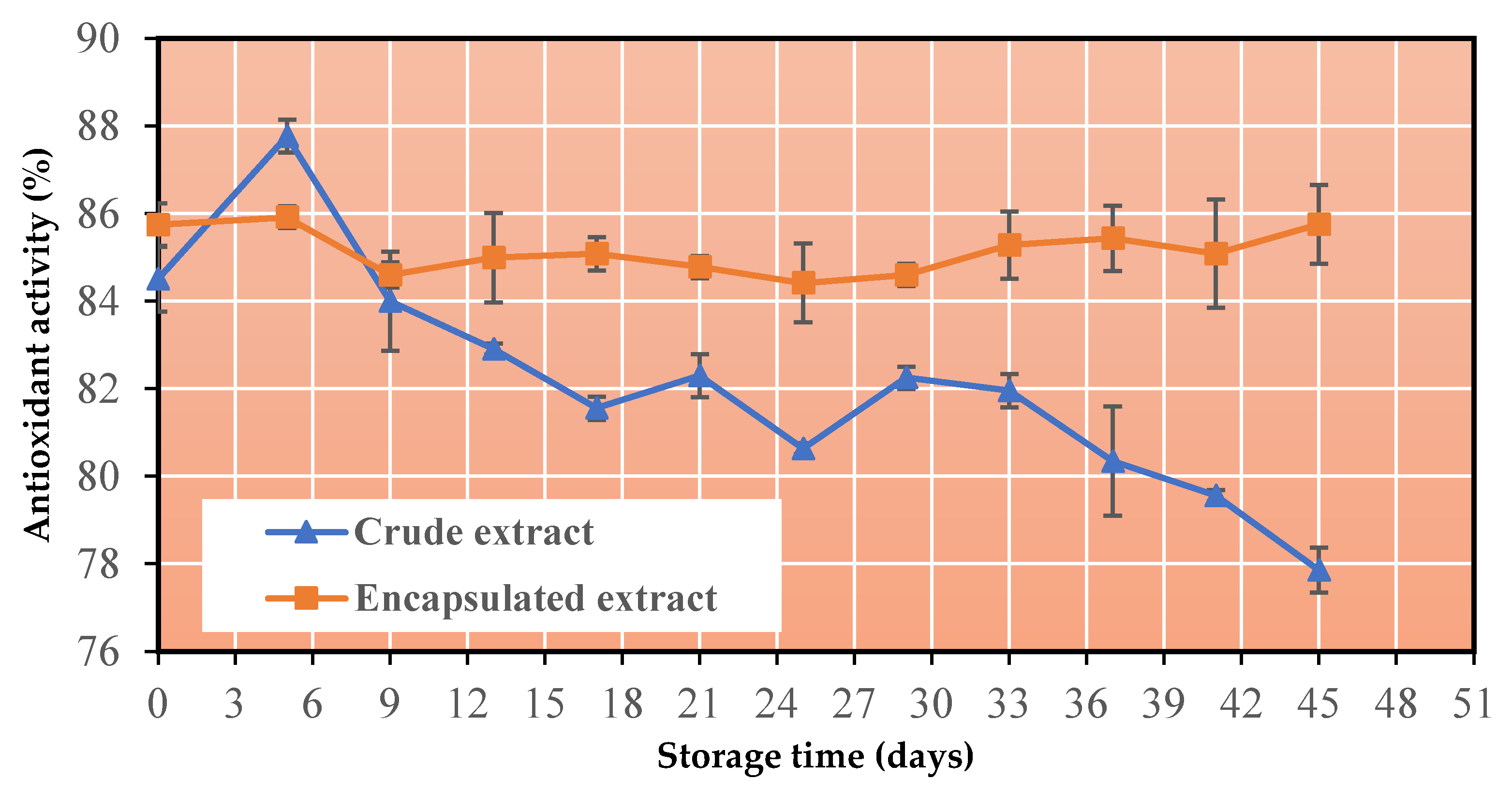
3.6. Change in antioxidant capacity
Another important characteristic identified in the co-crystallized powder is the preservation of antioxidant activity during storage at 60°C. As can be seen in Figure 5, the antioxidant activity of the encapsulated extract was maintained at higher levels than in the liquid extract and in fact greater than 84%.
Regarding the antioxidant activity, the encapsulated extract is found to follow a similar pattern to that of the total phenolic content curve. More specifically, there are some fluctuations during the 40 days, which match those of Figure 4. A result of this is that in this particular research study, there is a direct correlation between the total phenolic content and the antioxidant activity of the samples. Many researchers correlate the antioxidant activity with the phenolic content of a sample. For example, Negrao-Murakami et al. [49] correlated the high antioxidant activity of encapsulated concentrated mate tea with the good stability of its phenolics during storage. Fang and Bhandari [50] and Flores et al. [44] also showed that the change of antioxidant activity during storage was similar to that of total phenolic content for laurel powder and encapsulated extract of bilberry peels, respectively. The modification in the phenolic profiles may be responsible for the slight variations in antioxidant activity. According to Laine et al. [51], phenolic compounds are "team players", which means they cooperate with one another to promote each other's antioxidant functions. The formation of phenolics with equivalent or better antioxidant activity may make up for the loss of the original phenolics. For instance, according to Bors and Michel [52], the hydrolysis of ellagitannins may increase antioxidant activity by increasing the number of free hydroxyl groups, whereas the oxidation of hydrolyzable tannins can result in oligomerization through phenolic coupling, which subsequently increases the number of reactive sites and enhances antioxidant activity. Kaderides et al. [5], who studied the stability of pomegranate peel extract encapsulated by spray drying, concluded that the antioxidant activity remained at high levels (> 90%), over the entire storage period (60 oC, 45 days). They also reported that newly formed phenolics with equal or greater antioxidant capabilities may make up for the loss of original phenolics. Punicalagin, for instance, is hydrolyzed to create chemicals like punicalin, gallagic acid, gallic acid, and ellagic acid, all of which have the potential to be antioxidants [53].
Regarding the liquid extract, a sharp increase is first observed, which is followed by a fluctuation and at the end, a further decrease. The total decrease in antioxidant activity at the end of the specific time period of 40 days was 7.86%. Interest of this is that once again the correlation between total phenolic content and antioxidant activity is verified, and the reasons for the increase or decrease respectively in the antioxidant activity are similar to the reasons for the increase or decrease in the total phenolic content.
4. Conclusions
The growing concern about the safety of synthetic antioxidants has led to an increased interest in the study of effective and economical natural antioxidants. Pomegranate peels may be a good commercial source of phenolic compounds, which can be isolated and stabilized by various methods. The subject of the present work is the characterization of pomegranate peel extract encapsulated in sucrose by co-crystallization at conditions determined in our previous work. It was proven that:
- The moisture content of the co-crystallized product is about 0.59%, a value lower than those reported when spray drying was used.
- The moisture absorption rate for the co-crystallized product is about 0.011%, so the powder cannot be considered to be hygroscopic.
- The co-crystallized powder has a high bulk density (0.803 g/cm3) and solubility (61 s).
- The phenolic extract does not crystallize but is still in an amorphous state between gaps of the sucrose crystals.
- The obtained thermographs indicate both the improvement of the thermal stability of the extract by the co-crystallization and its successful encapsulation in the sucrose matrix.
- The co-crystallized powder has an excellent storage stability, as it preserves its phenolic content and antioxidant activity at high levels after storage at 60°C for 45 days.
Overall, the encapsulation of pomegranate peel phenolic extract by co-crystallization presents a promising avenue for utilizing and enhancing the bioactivity of these valuable compounds. This research aims to contribute to the understanding of co-crystallization technique for pomegranate peel phenolic extract and inspire further exploration of its applications, promoting sustainable utilization of pomegranate byproducts and fostering the development of functional products with potential health benefits.
Author Contributions
Conceptualization, A.M.G.; methodology, A.M.G.; software, E.C.; validation, E.C.; formal analysis, E.C.; investigation, E.C. and A.M.G.; resources, A.M.G.; data curation, E.C.; writing—original draft preparation, E.C. and A.M.G.; writing—review and editing, A.M.G.; visualization, E.C.; supervision, A.M.G.; project administration, A.M.G.; funding acquisition, A.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Çam, M.; İçyer, N. C., Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [CrossRef]
- Kanatt, S. R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Techn. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurized water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A. M.; Adamopoulos, K. G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. IFSET 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A. M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process 2019, 137, 1–11. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Navarro, A. Physicochemical properties and stability of sucrose/glucose agglomerates obtained by co-crystallization. J. Food Process Eng. 2018, 41, e12901. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–94. [Google Scholar] [CrossRef]
- Karangutkar, A. V.; Ananthanarayan, L. Co-crystallization of Basella rubra extract with sucrose: Characterization of co-crystals and evaluating the storage stability of betacyanin pigments. J. Food Eng. 2020, 271, 109776. [Google Scholar] [CrossRef]
- Sardar, B. R.; Singhal, R. S. Characterization of co-crystallized sucrose entrapped with cardamom oleoresin. J Food Eng. 2013, 117, 521–529. [Google Scholar] [CrossRef]
- Maulny, A. P. E.; Beckett, S. T.; Mackenzie, G. Physical properties of co-crystalline sugar and honey. J. Food Sci. 2005, 70, E567–E572. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S. C. Encapsulation of Natural Antioxidant Compounds from Culinary Banana by Cocrystallization. J. Food Process Preserv. 2017, 41, e13033. [Google Scholar] [CrossRef]
- Akbari, M.; Sadeghi Mahoonak, A.; Sarabandi, K.; Ghorbani, A. Physiochemical characterization and antioxidant activities of green tea extract microencapsulated by co-crystallization technique. Food Sci. Technol. 2019, 15, 179–193. [Google Scholar]
- Marpaung, A. M.; Lee, M.; Kartawiria, I. S. The Development of Butterfly pea (Clitoria ternatea) Flower Powder Drink by Co-crystallization. IFSTJ 2020, 3, 34–37. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Yamul, D. K.; Navarro, A. S. Co-crystallized sucrose with propolis extract as a food ingredient: Powder characterization and antioxidant stability. Food Sci. Technol. 2021, 143, 111164. [Google Scholar] [CrossRef]
- Kaur, P.; Elsayed, A.; Subramanian, J.; Singh, A. Encapsulation of carotenoids with sucrose by co-crystallization: Physicochemical properties, characterization and thermal stability of pigments. Food Sci. Technol. 2021, 140, 110810. [Google Scholar] [CrossRef]
- Tzatsi, P.; Goula, A. M. Encapsulation of Extract from Unused Chokeberries by Spray Drying, Co-crystallization, and Ionic Gelation. Waste Biomass Valorization 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mohammadi, A. Stabilization of peppermint polyphenols within crystalline sucrose matrix: fortification of gummy candy as a food model system. J. Food Process. Preserv. 2022, 46, e16720. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Kenanidou, N.; Spyropoulos, C.; Xenitopoulou, D.; Zlati, E.; Goula, A. M. Encapsulation of pomegranate peel extract in sucrose matrix by co-crystallization. Sustain. Chem. Pharm. 2023, 31, 100949. [Google Scholar] [CrossRef]
- Bhandari, B. R.; Hartel, R. W. Co-crystallization of sucrose at high concentration in the presence of glucose and fructose. J. Food Sci. 2002, 67, 1797–1802. [Google Scholar] [CrossRef]
- Deladino, L.; Anbinder, P. S.; Navarro, A. S.; Martino, M. N. Co-crystallization of yerba mate extract (Ilex paraguariensis) and mineral salts within a sucrose matrix. J Food Eng. 2007, 80, 573–580. [Google Scholar] [CrossRef]
- Deladino, L.; Navarro, A. S.; Martino, M. N. Microstructure of minerals and yerba mate extract co-crystallized with sucrose. J. Food Eng. 2010, 96, 410–415. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Gallo, L.; Bucalá, V.; Martino, M.; Navarro, A. Co-crystallization of zinc sulfate with sucrose: A promissory strategy to render zinc solid dosage forms more palatable. J. Food Eng. 2016, 170, 100–107. [Google Scholar] [CrossRef]
- Nugroho, D.; Sugih, A. K. Determination of process parameters for curcumin–dextrose cocrystallization. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Malang, East Java, Indonesia, 4-5 November 2017. [Google Scholar]
- Bajaj, S. R.; Singhal, R. S. Enhancement of stability of vitamin B12 by co-crystallization: A convenient and palatable form of fortification. J. Food Eng. 2021, 291, 110231. [Google Scholar] [CrossRef]
- Queiroz, M. B.; Sousa, F. R.; da Silva, L. B.; Alves, R. M. V.; Alvim, I. D. Co-crystallized sucrose-soluble fiber matrix: Physicochemical and structural characterization. Food Sci Technol. 2022, 154, 112685. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; Zhang, D.; Wang, Y.; Zhang, Y.; Jiang, S.; Meng, X. Encapsulation of catechin or curcumin in co-crystallized sucrose: Fabrication, characterization and application in beef meatballs. Food Sci. Technol. 2022, 168, 113911. [Google Scholar] [CrossRef]
- Beristain, C. I.; Vazquez, A.; Garcia, H. S.; Vernon-Carter, E. J. Encapsulation of orange peel oil by co-crystallization. Food Sci. Technol. 1996, 29, 645–647. [Google Scholar] [CrossRef]
- Sardar, B. R.; Tarade, K. M.; Singhal, R. S. Stability of active components of cardamom oleoresin in co-crystallized sugar cube during storage. J. Food Eng. 2013, 117, 530–537. [Google Scholar] [CrossRef]
- Federzoni, V.; Alvim, I. D.; Fadini, A. L.; Silva, L. B. D.; Queiroz, M. B. Co-crystallization of paprika oleoresin and storage stability study. Food Sci. Technol. 2019, 39, 182–189. [Google Scholar] [CrossRef]
- Rai, K.; Chhanwal, N.; Shah, N. N.; Singhal, R. S. Encapsulation of ginger oleoresin in co-crystallized sucrose: development, characterization and storage stability. Food Funct. 2021, 12, 7964–7974. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, Vol. II. 2005, Association of Official Analytical Chemists, Arlington, VA.
- Tsali, A.; Goula, A. M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Goula, A. M.; Adamopoulos, K. G.; Kazakis, N. A. Influence of spray drying conditions on tomato powder properties. Dry. Technol. 2004, 22, 1129–1151. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A. M. Development and characterization of a new encapsulating agent from orange juice by-products. Food Res. Int. 2017, 100, 612–622. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A. M. Encapsulation of pomegranate peel extract with a new carrier material from orange juice by-products. J. Food Eng. 2019, 253, 1–13. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mahoonak, A. S.; Akbari, M. Physicochemical properties and antioxidant stability of microencapsulated marjoram extract prepared by co-crystallization method. J. Food Process Eng. 2019, 42, e12949. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of olive leaves: spray drying of olive leaf extract. Waste Biomass Valorization 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Kurozawa, L. E.; Park, K. J.; Hubinger, M. D. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J. Food Eng. 2009, 94, 326–333. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Agudelo-Mesa, L.; Martino, M. Yerba mate antioxidant powders obtained by co-crystallization: Stability during storage. J. Food Eng. 2014, 124, 158–165. [Google Scholar] [CrossRef]
- Nik, A. B.; Vazifedoost, M.; Didar, Z.; HajirostamLoo, B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019, 3, 243–250. [Google Scholar] [CrossRef]
- Tan, S. P.; Tuyen, C. K.; Parks, S. E.; Stathopoulos, C. E.; Roach, P. D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Jones, J. R.; Prime, D.; Leaper, M. C.; Richardson, D. J.; Rielly, C. D.; Stapley, A. G. Effect of processing variables and bulk composition on the surface composition of spray dried powders of a model food system. J. Food Eng. 2013, 118, 19–30. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A. M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef] [PubMed]
- Flores, F. P.; Singh, R. K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Does Microencapsulation Improve Storage Stability of Cloudberry (Rubus chamaemorus) Ellagitannins? In Water Properties in Food, Health, Pharmaceutical and Biological Systems, 1st ed.; Reid, D.S., Sajjaanantakul, Τ., Lillford, P.J., Charoenrein, S., Eds.; Blackwell publishing: Iowa, USA, 2010; pp. 563–569. [Google Scholar]
- Lei, Z. Monomeric ellagitannins in oaks and sweetgum. Doctoral dissertation, Virginia Polytechnic Institute and State University, Virginia, 25 April 2022.
- Haslam, E. Plant polyphenols: vegetable tannins revisited, 1st ed.; Cambridge University Press: Cambridge, USA, 1998; pp. 90–139. [Google Scholar]
- Xu, L.; Cheng, J. R.; Liu, X. M.; Zhu, M. J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. Lwt 2019, 100, 62–68. [Google Scholar] [CrossRef]
- Negrão-Murakami, A. N.; Nunes, G. L.; Pinto, S. S.; Murakami, F. S.; Amante, E. R.; Petrus, J. C. C.; Prudêncio, E. S.; Amboni, R. D. M. C. Influence of DE-value of maltodextrin on the physicochemical properties, antioxidant activity, and storage stability of spray dried concentrated mate (Ilex paraguariensis A. St. Hil.). Food Sci. Technol. 2017, 79, 561-567.
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A. B.; Bharali, D. J.; Nallanthighal, S.; Coon, J. K.; Mousa, S. A.; Reliene, R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int. J. Nanomedicine 2015, 10, 475. [Google Scholar]
Figure 1.
DSC thermographs of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.
Figure 1.
DSC thermographs of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.


Figure 2.
X-ray diffraction characterization of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.
Figure 2.
X-ray diffraction characterization of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.


Figure 3.
SEM microphotographs of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.
Figure 3.
SEM microphotographs of (a) pure crystalline sucrose; (b) co-crystallized sucrose without the active ingredient; (c) co-crystallized sucrose with the encapsulated ingredient.

Figure 4.
Total phenolics content of crude and encapsulated extract during storage at 60°C.

Figure 5.
Antioxidant activity of crude and encapsulated extract during storage at 60°C.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Properties and Stability of Encapsulated Pomegranate Peels Extract Prepared by Co-crystallization
Evangelos Chezanoglou
et al.
,
2023
Microencapsulation and Characterization of Natural Polyphenols from PHF Extract
Syed Ammar Hussain
et al.
,
2018
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







