Preprint
Review
Fermented Foods as a Potential Vehicle of Antimicrobial Resistant Bacteria and Genes
Altmetrics
Downloads
143
Views
48
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
10 July 2023
Posted:
12 July 2023
You are already at the latest version
Alerts
Abstract
Fermented food products are widely consumed for their nutritional and health-promoting properties earning them a central place in diets around the globe. However, these foods can present a paradox, as they have the potential to harbor not only beneficial probiotics but also antibiotic-resistant (AR) microbes and genes. The impact of AR in fermented foods has far-reached implications, such as its potential effects on human health, repercussions in the food industry, and environmental consequences. An in-depth analysis of AR in fermented foods, including dairy products, fermented fruits and vegetables, meat products, and beverages, would provide insights into the extent and ramifications of the issue with these foods. Therefore, this review systematically presents the status of AR in fermented foods, with a particular focus on AR bacteria and genes within this category of food products. The review also highlights the complexities of AR in fermented foods, emphasizing the role of bacterial adaptation during the fermentation process and the dynamics of bacterial gene transfer. Various contributing factors to AR are brought into focus, including intrinsic resistance among bacteria in fermented foods and the potential risk of contamination with pathogenic bacteria. Moreover, this review presents a range of mitigation strategies, from the development of novel antimicrobials to advances in fermentation technology and regulatory control. This comprehensive perspective on the intricate interplay between AR and fermented food will potentially pave the way for more targeted research and mitigation strategies in this critical area.
Keywords:
Subject: Biology and Life Sciences - Food Science and Technology
1. Introduction
Fermented foods have been an integral part of the global diet for centuries and are characterized by their production through controlled microbial growth and enzymatic action [1]. They encompass a wide range of food categories, including meat, dairy, vegetables, soybeans, legumes, cereals, and fruits. Fermentation serves multiple purposes, such as preservation and enhancement of organoleptic properties, offering not only unique flavors and textures but also potential health benefits. The fermentation process involves microorganisms, nutritional ingredients, and environmental conditions, resulting in numerous variations of fermented foods [2]. While fermented foods offer beneficial properties, the presence of antibiotic-resistant (AR) bacteria and genes within these food products raises concerns regarding their impact on human health. Understanding the relationship between fermented foods and AR is crucial for assessing the potential risks associated with their consumption.
Traditionally, fermentation served as a preservation method, utilizing antimicrobial metabolites to reduce the risk of contamination with pathogenic microorganisms. However, recent studies have revealed the presence of AR bacteria and genes in various types of fermented foods [3,4]. Spontaneously fermented foods pose a unique concern for AR as the presence of naturally occurring fermentative bacteria in the food matrix makes them less amenable to safety assurance measures [5]. Additionally, lactic acid bacteria (LAB), commonly found in fermented foods, have been associated with the dissemination of AR, especially when these products are consumed without heat treatment. The consumption of such foods may facilitate the exchange of functional AR genes (ARGs) between resident microorganisms and potential pathogens through horizontal gene transfer (HGT), as represented in Figure 1 [6].
Furthermore, while probiotics are generally considered safe, there have been reports of infections associated with probiotic Lactobacilli, particularly in immunocompromised individuals [7,8]. Alongside the possibility of probiotics being implicated in infectious processes, the concern for these bacteria acquiring resistance genes should also be addressed. In Europe, probiotics used in animal feed additives are deemed unsuitable if known exogenous resistance genes are present, especially when the acquired resistance can be further transferred [9]. By examining the presence and potential transferability of AR bacteria and genes in fermented foods, this review article aims to provide a comprehensive understanding of the implications of AR in these widely consumed products.
2. AR bacteria and genes in Fermented Foods
AR in fermented foods is a concerning phenomenon closely linked to the misuse of antibiotics in the food chain [10]. The fermentation process itself might contribute to the proliferation of bacteria, leading to an overall increase in the abundance of ARGs within fermented foods [3]. As reservoirs of AR bacteria and ARGs, fermented foods can potentially transfer these elements to other microorganisms, including pathogens. This transfer can have lasting effects on the microbial communities within the human body. Notably, fermented foods harbor a significant population of microorganisms, and the specific conditions within the fermentation environment, such as confined spaces and close cell-to-cell contact, provide an ideal setting for gene transfer. LAB and coagulase-negative Staphylococcus (CNS) species, commonly found in fermented foods, have been identified as carriers of ARGs, particularly against tetracyclines, penicillins, chloramphenicol, and macrolides [11]. Table 1 represents the major AR bacteria and ARGs isolated and identified from fermented foods.
In various fermented food products, metagenomic research has revealed the presence of ARGs belonging to several AR classes. Fermented dairy products exhibited an average count per million (CPM) of 3,686 ARGs per sample, while brine and sugar products had lower CPM values of 426 and 261, respectively [12]. Furthermore, using starter cultures in fermentations resulted in a five-fold higher abundance of ARGs than in spontaneous fermentations. Among 58 analyzed food samples, nine showed the presence of ARGs. These included kombucha, kefir, kimchi, pickled carrots, pickled vegetables, and apple cider vinegar samples. The presence of ARGs in fermented foods not only increases the risk of HGT but also amplifies the potential for ARG dissemination due to their mobility [12]. Therefore, understanding the prevalence and transferability of AR bacteria and genes in fermented foods is crucial for addressing the potential risks associated with their consumption.
2.1. ARGs and Associated Bacteria in Fermented Foods
2.1.1. Lactic Acid bacteria
ARGs have been consistently observed in LAB associated with various fermented foods. Specifically, resistance to tetracyclines, penicillins, chloramphenicol, and macrolides, such as erythromycin, is frequently detected among LAB strains, particularly in certain types of cheeses, fermented meats and spontaneously fermented vegetables [13,14]. In studies analyzing LAB isolates from Indonesian traditional fermented foods (dadih, tape ketan, bekasam, and tempoyak), a significant number of strains were found to be resistant to chloramphenicol and erythromycin [15]. Lactobacilli, commonly used as starter cultures in fermented milk products, exhibit widespread resistance to penicillins. Studies have identified acquired resistance of LAB to tetracycline, erythromycin, clindamycin, and chloramphenicol in Lactobacilli isolated from fermented foods. The resistance is mediated by specific genes such as tetM and tetS (associated with tetracycline resistance) and erm(B) (associated with erythromycin resistance). Furthermore, the cat gene, encoding chloramphenicol acetyltransferase and carried on plasmids, has been identified as a resistance determinant in those isolates [16,17].
In a study examining 16 fermented Chinese foods and eight sausages and pickles, LAB isolates displayed varying levels of AR. LABs in pickle samples showed susceptibility to tetracycline, ampicillin, and clindamycin but showed resistance to other antibiotics. LABs in sausage samples from different provinces also exhibited differing levels of resistance, with some strains showing resistance to multiple antibiotics [18]. PCR analysis of antibiotic-resistant LAB strains revealed the presence of frequently detected ARGs, such as ermB, and tetM, found on both plasmid and chromosomal DNA. tetM gene was consistently identified in all tetracycline-resistant LAB strains, with some strains carrying the gene on both plasmids and chromosomes, indicating multiple mechanisms and locations for ARG occurrence [18]. Additionally, certain strains of Lactobacillus delbrueckii subsp. bulgaricus, commonly used in yogurt cultures, demonstrated intrinsic resistance towards mycostatin, nalidixic acid, neomycin, polymyxin B, trimethoprim, colimycin, sulfamethoxazole, and sulphonamides [19].
2.1.2. Staphylococcus spp.
Coagulase-negative Staphylococcus (CNS) species, frequently found in fermented animal products such as certain meats, cheeses, and fermented fish products, have been associated with the presence of ARGs. The common ARGs include resistance to tetracyclines, penicillins, chloramphenicol, and macrolides [20].
In a Swiss study, tetracycline resistance was detected in Staphylococcus isolates used as starter cultures in meat, as well as in Bifidobacterium lactis and Lactobacillus reuteri [21]. Another study conducted in Nigeria examined 255 CNS isolates obtained from six different Nigerian fermented foods (including kindirmo, nono, iru, wara, ogi, and kunu) and found that a substantial proportion of the isolates exhibited resistance to different antibiotics. Specifically, a high percentage of isolates demonstrated resistance to ampicillin, trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, and oxacillin. Staphylococcus caprae showed particularly high resistance levels to multiple antibiotics, including ciprofloxacin, ofloxacin, gentamicin, erythromycin, and cefotaxime [22]. In yet another study, the genetic diversity and AR profiles of Staphylococcus saprophyticus isolates from fermented foods and clinical samples were assessed, revealing notable resistance rates to lincomycin, erythromycin, and tetracycline [23].
2.1.3. Enterococcus spp.
Enterococcus species, commonly found in Turkish white cheese, have exhibited resistance to streptomycin, erythromycin, oxacillin, and vancomycin. In fact, a high prevalence of vancomycin resistance was observed in Enterococcus faecalis (96.8%) and Enterococcus faecium (76%) isolates [24]. The presence of specific resistance genes in Enterococcus faecium strain SZ109 was also investigated, with the ermB gene associated with erythromycin resistance, detected on both the plasmid and chromosome. Additionally, the aphA3 gene, conferring resistance to antibiotics like kanamycin and neomycin, was exclusively found on the plasmid, while the mefA gene, responsible for macrolide resistance, was exclusively located on the chromosome [18]. A comprehensive study assessed the resistance patterns of 55 Enterococci strains, including 41 Enterococcus faecium and 14 Enterococcus faecalis strains, isolated from various traditional fermented foods of animal and vegetable origins, as well as water samples. The analysis revealed that certain strains exhibited resistance to vancomycin, while a high incidence of AR was detected among Enterococcus faecalis isolates to rifampicin, ciprofloxacin, and quinupristin/dalfopristin. Intermediate resistance to erythromycin and tetracycline was also observed. Among Enterococcus faecium isolates, resistance was found against rifampicin, ciprofloxacin, erythromycin, levofloxacin, and nitrofurantoin [25]. These findings underscore the presence of AR in Enterococcus species isolated from fermented foods and emphasize the importance of understanding and monitoring their resistance profiles for food safety and public health considerations.
Table 1.
Antibiotic-resistant bacteria and antibiotic-resistant genes in fermented foods.
| Food type | Bacteria present | ARGs | Reference |
|---|---|---|---|
| Cheese | LAB, Staphylococcus (CNS) | Tetracycline, Chloramphenicol, erythromycin | [13,14] |
| Fermented meat/sausage | LAB, Staphylococcus (CNS) Salmonella Listeria monocytogenes |
Tetracycline, Chloramphenicol, erythromycin, Penicillins Amoxicillin, Gentamycin, Streptomycin, tetracycline Amoxicillin, benzyl penicillin, tetracycline, ciprofloxacin |
[11] [14] [14] |
| Fermented vegetables | LAB | Tetracycline, Chloramphenicol, erythromycin, Clindamycin | [11,13,14] |
| Yogurt |
Lactobacillus Streptococcus |
Mycostatin, nalidixic acid, neomycin, polymyxin B, trimethoprim, colimycin, sulfamethoxazole, and sulphonamides Colimycin, gentamicin, kanamycin, mycostatin, nalidixic acid, neomycin, polymyxin b, trimethoprim/sulfamethoxazole, streptomycin, and sulfonamides |
[19] [19] |
| Vinegar | Acetic acid bacteria | Trimethoprim, ciprofloxacin, erythromycin | [26] |
| Turkish cheese | Enterococcus | streptomycin, erythromycin, oxacillin, and vancomycin | [24] |
| Fermented fish | Staphylococcus (CNS) | tetracyclines, penicillins, chloramphenicol, and macrolides | [20] |
2.1.4. AR genes in Other Bacteria
Research has focused on AR in Acetobacter and Komagataeibacter species, predominantly derived from vinegar, revealing resistance to trimethoprim, erythromycin, ciprofloxacin, and chloramphenicols [26]. Another study investigating AR microorganisms in meat and minced meat used for fermented sausage production found Salmonella strains exhibiting resistance to amoxicillin (44.5%), gentamycin (38%), streptomycin (44.5%), and tetracycline (55.5%). Similarly, Listeria monocytogenes displayed resistance to amoxicillin (38.5%), benzylpenicillin (30.8%), tetracycline (53.8%), and ciprofloxacin (38.5%). Escherichia coli strains showed resistance to amoxicillin (33.3%), neomycin (30%), streptomycin (40%), and tetracycline (40%) [14]. Moreover, fifteen strains of Streptococcus thermophilus isolated from yogurt cultures exhibited varying levels of resistance to colimycin, gentamicin, kanamycin, mycostatin, nalidixic acid, neomycin, polymyxin B, trimethoprim/sulfamethoxazole, streptomycin, and sulfonamides [19]. Furthermore, high tetracycline and erythromycin resistance occurrences have been observed in Lactococcus and Streptococcus thermophilus strains isolated from dairy products [21]. These findings highlight the prevalence of AR in diverse bacterial species associated with fermented foods and accentuate the need for continued surveillance and management strategies to mitigate the risks posed by AR strains.
3. Role of Bacterial Adaptation and Gene Transfer in Fermented Foods
Fermented foods comprise a complex micro-environment characterized by diverse microbial communities, creating an ideal environment for horizontal gene transfer [27]. HGT in fermented foods plays a crucial role in the adaptation of bacteria to evolving nutritional needs and environments [28]. For example, during the fermentation process of foods such as cheese, wine, yogurt, and vinegar, the acquisition of the horizontal transfer region occurs, facilitating the transfer of genetic material among microbial populations [29]. This can potentially improve the quality and shelf life of fermented foods. Research shows that the occurrence of HGT in a specific environment is beneficial for starter culture domestication; a process that involves selectively cultivating and adapting specific microorganisms to exhibit desired traits and behaviors during the fermentation process, leading to improved fermentation efficiency, digestibility, palatability, and longevity [29,30]. Microbial fermentation leading to HGT in fermented beverages, such as wine and beer, has also been reported, potentially impacting their shelf life and sensory attributes [31]. However, it is important to note that while HGT contributes to the adaptation and quality of fermented food, it can also facilitate the transfer of ARGs among microbial populations [3]. Within the fermentation environment, microbial interactions, such as conjugation or transduction, may also occur during manufacturing and storage [32].
3.1. Horizontal Gene Transfer in Fermented Food
The transfer of ARGs through HGT in fermented foods has been extensively studied, with sausages, kefir, yogurt, and cheese being key examples. These studies also highlight the potential of ARG transmission to both animal and human microbiota. Leroy et al, observed the transfer of plasmids carrying ARG during sausage fermentation involving Staphylococcus xylosus, isolated from fermented sausage [33]. Furthermore, the analysis of mobile genetic elements (MGE) domains in kefir revealed the presence of ARG lmrD (provides resistance to lincosamides). The contig containing this gene was found in close proximity to a transposase open reading frame (ORF) within ten neighboring ORFs, suggesting potential gene mobility in the fermentation-associated microbial community [3]. Metagenomic analysis of fermented milk products such as yogurt, nunu, kefir, and Koumiss demonstrated the presence of plasmid-borne ARGs within the fermented milk microbiome [34]. Additionally, plasmid-mediated transfer of AR has been demonstrated between strains of L. curvatus during sausage fermentation [35]. The ripening of fermented sausages also facilitates a high frequency of plasmid transfer among Enterococcus faecalis strains, highlighting the close contact and genetic transfer facilitated during the ripening of sausages [36]. Cheese, known for its favorable conditions for microbial survival and growth, can harbor AR pathogens such as Listeria due to its moderately high-water activity and low salt content and serve as a potential reservoir for the acquisition of resistance genes by Enterococci. The horizontal transfer of the tetM gene from L. monocytogenes to E. faecalis was investigated in Minas Frescal cheese. The results revealed that both donor and recipient strains remained viable throughout the 21-day shelf life, with transconjugant bacteria detected from the second day onwards [37]. Commercial dairy-derived Lactobacilllus strains from cheese and yogurts carried plasmid-mediated streptomycin and gentamicin resistance genes in L. rhamnosus and L. plantarum, indicating the potential for HGT among probiotic strains [38]. During soybean fermentation, a plasmid carrying a lincosamide resistance gene (lnuA) was transferred between coagulase-negative Staphylococci in the presence of lincomycin. Interestingly, the plasmid was efficiently transferred between these bacteria during passage through murine intestines, even in the absence of lincomycin, highlighting the enhanced transferability under in vivo conditions [39]. A similar study conducted by Li et al.,[40] reported the transfer of vanA gene associated with vancomycin resistance during the early stages of fermented soybean meal (FSBM) production, with transconjugants steadily increasing throughout the fermentation process. Notably, these transconjugants were able to pass through the digestive tract of growing pigs and detected after three days of feeding. These findings underscore the potential dissemination of ARGs carried by commercial probiotics used in animal feed and feed additives within the digestive tracts of animals. Such dissemination poses a risk of further infections under extreme conditions, highlighting fermented food as a potential source of ARGs to human gut microbiota via HGT.
3.2. Stress Response Contributing to Gene Transfer
During fermentation, bacteria encounter various stressors such as low pH, high salt concentration, and the presence of antimicrobial compounds produced during the fermentation process. These stressors may exert selective pressure on bacterial populations, leading to the survival and proliferation of adapted strains. Some bacteria possess intrinsic mechanisms to counteract these stresses, while others may acquire adaptive traits through HGT. Stressful conditions, such as low temperatures, can favor the transfer of genetic elements, including ARGs [41,42]. LAB, predominantly found in cold dairy environments, has been frequently linked with AR. In the case of cheese, temperature, and pH influenced antibiotic gene transfer. At a processing temperature of 30oC, the highest levels of donor and recipient bacteria were observed after 48 hours, while transconjugant bacteria peaked at 48 hours. At 10oC, growth rate and acidification were reduced, resulting in delayed detection of vancomycin-resistant transconjugants that reached their highest number during ripening [36]. Furthermore, Tarrah et al [43] discovered a genomic island closely associated with S. thermophilus isolated from dairy environments, containing the tetS gene, along with genes related to plasmids, beta-lactamase class C-like, and penicillin-binding proteins superfamily. However, no HGT was detected from S. thermophilus to L. rhamnosus and L. delbrueckii during skim milk fermentation, refrigerated storage, and simulated gastrointestinal transit. On the other hand, the transfer frequencies of tetracycline, ampicillin, and chloramphenicol resistance genes significantly increased following high-pressure processing exposure, commonly used in the preservation of fermented food, both in vitro and in the food matrix, when compared to the non-pressurized control strains [44]
3.3. Transfer of ARGs from Fermented Food to Human Microbiota
Transfer of ARGs within fermented foods presents a dual concern, as they impede microbial evolution within the food matrix while also posing a risk to human health. Bacteria that enter our gut through food intake have the potential to exchange functional ARGs with both saprophytic bacteria and nearby pathogens through HGT, leading to the emergence of resistant or multi-resistant pathogenic strains limiting treatment options for infections [3]. The consumption of livestock products facilitates the establishment of transfer connections between bacteria, as it brings bacterial populations harboring diverse sets of ARGs [3]. Lactobacilli, due to their wide environmental distribution, can act as potential vectors for the dissemination of AR, raising concerns about their use as probiotics due to the acquisition and transfer of transferable AR determinants [45]. Previous research has demonstrated the transfer of ARGs between Lactobacillus species and even to other pathogens. Transfer of vancomycin resistance from Enterococci (both of human and animal origin) to a probiotic Lactobacillus acidophilus was observed in vitro and in vivo in mice, highlighting the potential long-term consequences of accumulating ARGs in the gut [45]. Given the high frequency of transfer of resistant determinants in mouse models, it is likely that similar events occur in the human gut, which serves as a reservoir for ARGs [46]. Consumption of fermented products in conjunction with appropriate triggers further increases the potential for HGT within the human gut [3]. Enterococci, known for causing human diseases, possess acquired antibiotic resistance along with intrinsic resistance to multiple antibiotics, low pH, high salt concentrations, and high temperatures, enabling their survival in harsh environments. AR Enterococci are commonly found in animal-derived foods and thrive during sausage fermentation. Transfer of tetracycline-resistant determinant tet(M) from E. faecium isolated from dry sausage to clinical isolates of E. faecalis and E. faecium was associated with the type 1 Integron [4], highlighting the significance of fermented food as reservoirs of ARGs and their potential transfer to human strains upon consumption.
4. Other Contributing Factors to AR in Fermented Food
Lactobacillus species, commonly used in the fermentation process, exhibit both acquired and intrinsic resistance to antibiotics, raising concerns about their safety in food products. Intrinsic resistance, unique to this bacterial group, varies among species [19]. LAB demonstrates natural tolerance to a range of antibiotics, including bacitracin, cefoxitin, ciprofloxacin, fusidic acid, gentamicin, metronidazole, nitrofurantoin, norfloxacin, sulphadiazine, teicoplanin, trimethoprim/sulfamethoxazole, kanamycin, streptomycin, and vancomycin [17,19]. Specifically, L. rhamnosus strains isolated from Parmigiano Reggiano cheese showed tolerance to aminoglycosides, glycopeptides, and quinolones [5,47]. Some Lactobacillus species used as probiotics are intrinsically resistant to vancomycin and aminoglycosides, where the mechanism of vancomycin resistance is well characterized [48] Vancomycin interacts with peptidoglycan precursors that are found on the cell wall side of the membrane of the cytoplasm and bind onto the D-alanine/D-alanine terminal of the pentapeptide, blocking peptidoglycan precursor polymerization. In certain LAB species, the terminal D-alanine residue in the muramyl pentapeptide is changed by D-lactate or D-serine, blocking vancomycin binding and thus developing resistance to the antibiotic [49]. Similarly, Leuconostoc and Pediococci isolated from alcoholic beverages and food products exhibited vancomycin resistance [19,50].
Moreover, numerous Lactobacillus species were intrinsically resistant to aminoglycosides, including gentamicin, kanamycin, streptomycin, neomycin, ciprofloxacin, and trimethoprim [51,52]. Lactobacillus rhamnosus, L. acidophilus, L. delbrueckii subsp. bulgaricus and L. helveticus lack cytochrome-mediated drug transport, rendering them intrinsically resistant to aminoglycosides [6,47]. Folate auxotrophic Lactobacilli, such as L. acidophilus, L. salivarius, L. johnsonii, L. brevis, L. casei, L. rhamnosus, L. delbrueckii, L. gasseri, L. fermentum, L. plantarum, L. reuteri, L. sakei, L. helveticus, and L. crispatus, were found to be intrinsically resistant to trimethoprim [51,53].
Similarly, Bifidobacterium obtained from probiotic cultures exhibited resistance to vancomycin, gentamicin, kanamycin, streptomycin, fusidic acid, trimethoprim, norfloxacin, nalidixic acid, metronidazole, polymyxin B, and colistin, although the mechanisms of resistance remain unknown [54]. In naturally fermented Alorena table olives, Lactobacillus pentosus and Leuconostoc pseudomesenteroides strains showed intrinsic resistance to vancomycin, teicoplanin, streptomycin, kanamycin, and trimethoprim/sulfamethoxazole. This multi-resistance may be attributed to chromosomally encoded efflux pumps coded by norA, mepA, and mdeA genes [55].
Among LAB, Enterococci are intrinsically resistant to cephalosporins, low levels of clindamycin, and aminoglycosides in milk and meat samples [56]. High-level gentamicin resistance was observed in many dairy isolates, despite their intrinsic resistance to low levels of gentamicin (17). Enterococcus faecium strain 68, used as a probiotic for humans and animals and as a silage inoculant, was found to be intrinsically resistant to kanamycin, streptomycin, and oxacillin [19]. Lactococcus lactis was recorded to be naturally resistant to colistin, fosfomycin, pipemidic acid, and rifamycin [57]. From different strains obtained from yoghurt culture, 31 strains of Lactobacillus delbrueckii subsp. bulgaricus seemed to be intrinsically tolerant to mycostatin, nalidixic acid, neomycin, polymyxin B, trimethoprim, colimycin, sufamethoxazole, and sulphonamides. Susceptibilities to cloxacillin, dihydrostreptomycin, doxycycline, furadantin, novobiocin, oleandomycin, oxacillin, and streptomycin [58].
5. Mitigation strategies
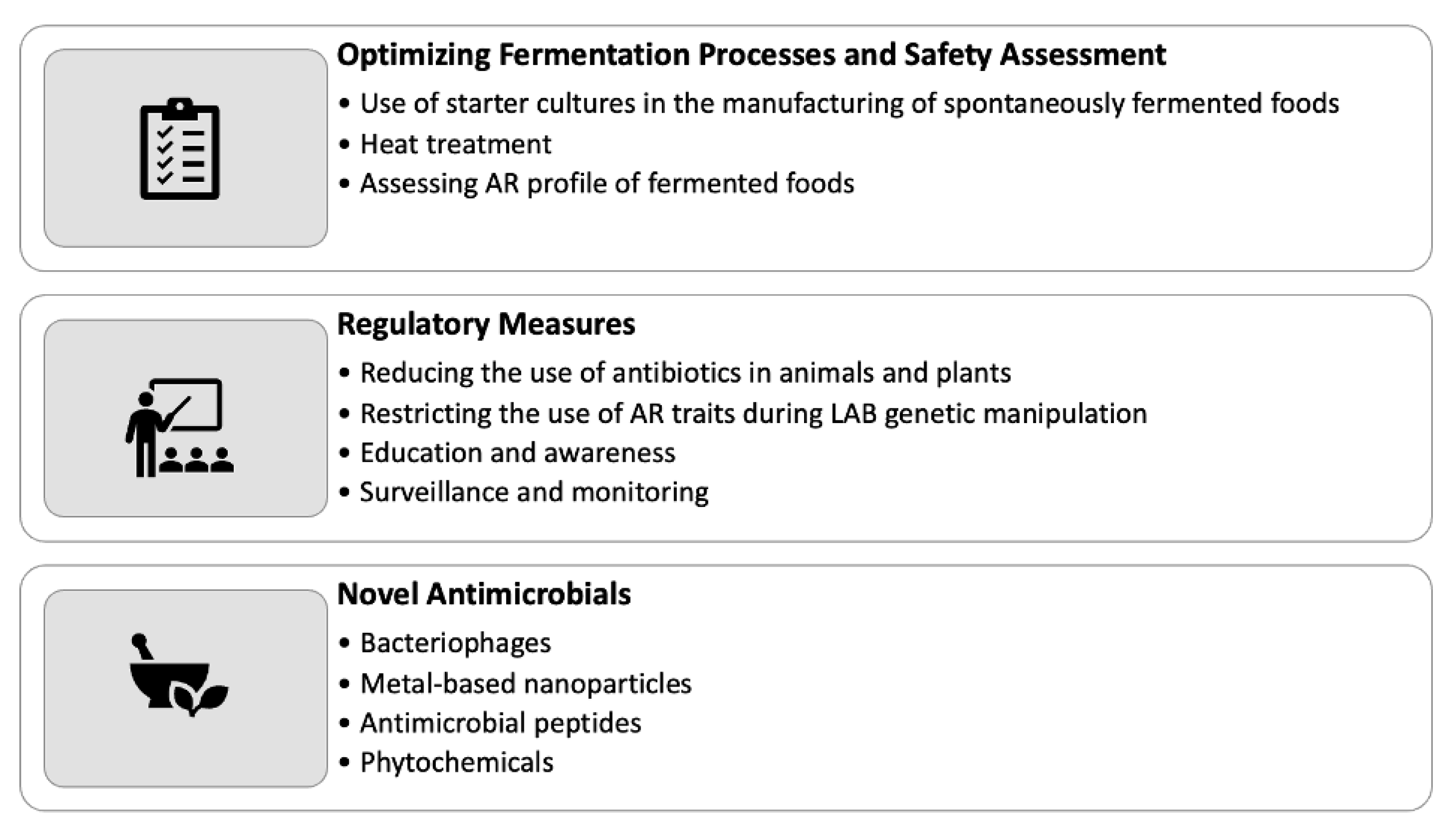
The presence of AR bacteria and genes in fermented foods raises concerns regarding their impact on human health. To mitigate these risks, several strategies can be implemented in the production and consumption of fermented foods (Figure 2), which are described below:
5.1. Optimizing Fermentation Processes and Safety Assessment
Implementing starter cultures during the manufacturing process of spontaneously fermented plant-based foods can effectively manage AR [59]. Recent advancements enable the use of multi-strain starters comprising microorganisms unique to these foods, allowing for better control over fermentation outcomes [60]. However, it is important to note that the use of starter cultures may result in the loss of some organoleptic properties associated with natural fermentation. Another approach to managing AR in fermented foods is through the application of heat treatment or pasteurization techniques, which can diminish or eradicate microorganisms, including AR strains [5,61]. Nevertheless, it is essential to consider that these methods may impact the organoleptic qualities, live microbial content, and potential health benefits associated with live microbes in the final product. Assessing antibiotic susceptibility within the diverse bacterial community present in fermented foods is crucial for ensuring food safety. While culture-dependent methods have limitations in detecting low-frequency resistance genes due to the vast number of uncultured microorganisms, high-throughput technologies such as metagenomics offer a culture-independent approach [52,62]. Metagenomics enables the identification of uncultured bacteria, the detection of novel AR genes and gene transfer methods, and the comprehensive safety assessment of traditional fermented foods [52]. It is important to note that AR evaluation should not be confined to PCR detection of known AR genes but should also involve genome sequencing to discover and describe novel AR genes and transfer mechanisms [63]. In addition to the strategies mentioned above, integrating other measures can further contribute to controlling AR in fermented foods. These may include implementing stringent hygiene practices, optimizing fermentation conditions, and adopting good manufacturing practices, which indirectly affect the microbiota associated with fermented foods.
5.2. Regulatory Measures for Combating AR in Fermented Foods
A key mitigation strategy for addressing AR in fermented foods involves implementing regulatory changes at various levels. One crucial step is to reduce AR in food animals and plants through the cautious use of antibiotics. Additionally, it is essential to restrict the use of antibiotics that can potentially lead to cross-resistance to compounds used in human medicine, such as tylosin and virginiamycin, to control the transfer of ARGs [19]. In the field of genetic manipulation of LAB, AR traits are being replaced with selectable indicators for various purposes, including the development of food-grade vectors. This shift aims to avoid undesirable resistance transfer or conferment to endogenous bacteria. While it is possible to generate AR probiotics by introducing diverse resistance to bacteria, it is crucial to ensure that probiotics do not carry more resistance than necessary for their intended purpose [19]. To guarantee the safety of probiotic lactic acid bacteria (e.g., Lactobacilli, Lactococci, Enterococci, and Bifidobacteria) for human consumption, strict adherence to safety and quality standards, guidelines, and regulations is essential throughout the pre-and post-marketing stages. Robust measures should be in place to assess and monitor the biosafety of probiotic strains, ensuring their suitability for use in food products. This includes a comprehensive evaluation of their antibiotic resistance profiles to prevent the dissemination of resistant strains [19]. Regulatory initiatives should also focus on promoting responsible antibiotic use across the entire food production chain. This involves educating and raising awareness among stakeholders about the potential consequences of antibiotic misuse and overuse. Encouraging the adoption of alternative disease prevention and treatment methods, such as vaccination, probiotics, and phage therapy, can help reduce reliance on antibiotics and mitigate the emergence and spread of AR. Implementing robust surveillance and monitoring systems is crucial for tracking the prevalence and trends of AR in fermented foods. This includes regular monitoring of antibiotic residues, microbial quality, and resistance profiles in raw materials, production processes, and finished products. Prompt detection and response to emerging AR threats can enable timely intervention and the development of targeted control strategies.
5.3. Novel Antimicrobials
As AR continues to pose a significant health concern, the urgent need for novel therapeutic alternatives and alternative antimicrobial therapies that are less prone to developing resistance becomes paramount. One promising approach involves the use of bacteriophages, metal-based nanoparticles, antimicrobial peptides, phytochemicals, antibodies, and gene-based CRISPR methods [64]. These innovative strategies offer diverse mechanisms of action and can target specific pathogens or resistance mechanisms, providing potential solutions to mitigate antibiotic resistance in fermented foods. Utilizing natural plant-derived compounds is a particularly promising avenue for controlling microorganisms and combating AR. Compounds such as trans-cinnamaldehyde, eugenol, thymol, carvacrol, resorcylic acid, and caprylic acid exhibit strong antibacterial action [65]. For instance, extracts from cloves and cinnamon have been reported to possess anti-listerial effects in cheese, highlighting the potential of these natural compounds as alternatives to traditional antibiotics [65]. In addition to the mentioned antimicrobial strategies, other novel approaches are being investigated to combat antibiotic resistance in fermented foods. These include the development of engineered probiotics capable of producing antimicrobial compounds, the application of CRISPR-Cas technology to selectively target and eliminate ARGs, and the use of advanced delivery systems to enhance the efficacy of antimicrobial agents [66,67]. By exploring and harnessing these cutting-edge techniques, the food industry can find effective means to counteract antibiotic resistance and ensure the safety and quality of fermented food products.
Given the complex nature of AR, a comprehensive approach that integrates multiple mitigation strategies is crucial. This includes combining traditional control measures, regulatory changes, novel antimicrobials, and innovative technologies to create a multifaceted approach that addresses the diverse challenges posed by antibiotic resistance in fermented foods. Collaboration between researchers, food producers, regulatory bodies, and healthcare professionals is vital to drive innovation, foster knowledge exchange, and develop effective strategies that can successfully mitigate antibiotic resistance in the food industry. By incorporating novel antimicrobials, exploring natural compounds, and adopting innovative approaches, the food industry can make significant strides in combating antibiotic resistance in fermented foods. These strategies not only contribute to safeguarding public health but also promote the sustainability and long-term viability of the fermented food sector.
5. Conclusions and Future Directions
Given the complex nature of AR, a comprehensive approach that integrates multiple mitigation strategies is crucial. This includes combining traditional control measures, regulatory changes, novel antimicrobials, and innovative technologies to create a multi-faceted approach that addresses the diverse challenges posed by antibiotic resistance in fermented foods. Collaboration between researchers, food producers, regulatory bodies, and healthcare professionals is vital to drive innovation, foster knowledge exchange, and develop effective strategies that can successfully mitigate antibiotic resistance in the food industry. By incorporating novel antimicrobials, exploring natural compounds, and adopting innovative approaches, the food industry can make significant strides in combating antibiotic resistance in fermented foods. These strategies not only contribute to safeguarding public health but also promote the sustainability and long-term viability of the fermented food sector.
Author Contributions
Writing—original draft preparation, P.G.V., L.S.V., D.J. and K.V.; writing—review and editing, P.G.V., L.S.V., D.J. and K.V.; visualization, P.G.V and K.V.; supervision, K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr Opin Biotechnol 2017, 44, 94–102. [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11. [CrossRef]
- Tóth, A.G.; Csabai, I.; Maróti, G.; Jerzsele, Á.; Dubecz, A.; Patai, Á. V; Judge, M.F.; Nagy, S.Á.; Makrai, L.; Bányai, K.; et al. A Glimpse of Antimicrobial Resistance Gene Diversity in Kefir and Yoghurt. 2020. [CrossRef]
- Jahan, M.; Zhanel, G.G.; Sparling, R.; Holley, R.A. Horizontal Transfer of Antibiotic Resistance from Enterococcus Faecium of Fermented Meat Origin to Clinical Isolates of E. Faecium and Enterococcus Faecalis. Int J Food Microbiol 2015, 199, 78–85. [CrossRef]
- Jasiak, K.; Amund, D. Are Spontaneously Fermented Plant-Based Foods Potential Sources of Transferable Antibiotic Resistance Genes? Food Front 2022, 3, 46–55. [CrossRef]
- Hummel, A.S.; Hertel, C.; Holzapfel, W.H.; Franz, C.M.A.P. Antibiotic Resistances of Starter and Probiotic Strains of Lactic Acid Bacteria. Appl Environ Microbiol 2007, 73, 730–739. [CrossRef]
- Cannon, J.P.; Lee, T.A.; Bolanos, J.T.; Danziger, L.H. Pathogenic Relevance of Lactobacillus: A Retrospective Review of over 200 Cases. Eur J Clin Microbiol Infect Dis 2005, 24, 31–40. [CrossRef]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus Sepsis Associated with Probiotic Therapy. Pediatrics 2005, 115, 178–181. [CrossRef]
- Anadón, A.; Rosa Martínez-Larrañaga, M.; Aranzazu Martínez, M. Probiotics for Animal Nutrition in the European Union. Regulation and Safety Assessment. Regul Toxicol Pharmacol 2006, 45, 91–95. [CrossRef]
- The WHO Regional Office for Europe.
- Wolfe, B.E. Are Fermented Foods an Overlooked Reservoir of Antimicrobial Resistance? Curr Opin Food Sci 2023, 51, 101018. [CrossRef]
- Leech, J.; Cabrera-Rubio, R.; Walsh, A.M.; Macori, G.; Walsh, C.J.; Barton, W.; Finnegan, L.; Crispie, F.; O’Sullivan, O.; Claesson, M.J.; et al. Fermented-Food Metagenomics Reveals Substrate-Associated Differences in Taxonomy and Health-Associated and Antibiotic Resistance Determinants. mSystems 2020, 5. [CrossRef]
- Flórez, A.B.; Delgado, S.; Mayo, B. Antimicrobial Susceptibility of Lactic Acid Bacteria Isolated from a Cheese Environment. Can J Microbiol 2005, 51, 51–58. [CrossRef]
- Fraqueza, M.J. Antibiotic Resistance of Lactic Acid Bacteria Isolated from Dry-Fermented Sausages. Int J Food Microbiol 2015, 212, 76–88. [CrossRef]
- SUKMARINI, L.; MUSTOPA, A.Z.; NORMAWATI, M.; MUZDALIFAH, I. Identification of Antibiotic-Resistance Genes from Lactic Acid Bacteria in Indonesian Fermented Foods. Hayati 2014, 21, 144–150. [CrossRef]
- Çataloluk, O.; Gogebakan, B. Presence of Drug Resistance in Intestinal Lactobacilli of Dairy and Human Origin in Turkey. FEMS Microbiol Lett 2004, 236, 7–12. [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus Spp. to Antimicrobial Agents. Int J Food Microbiol 2003, 82, 1–11. [CrossRef]
- Pan, L.; Hu, X.; Wang, X. Assessment of Antibiotic Resistance of Lactic Acid Bacteria in Chinese Fermented Foods. Food Control 2011, 22, 1316–1321. [CrossRef]
- Mathur, S.; Singh, R. Antibiotic Resistance in Food Lactic Acid Bacteria--a Review. Int J Food Microbiol 2005, 105, 281–295. [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-Derived Coagulase-Negative Staphylococcus as Starter Cultures for Fermented Foods. Food Sci Biotechnol 2020, 29, 1023–1035. [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int J Environ Res Public Health 2013, 10, 2643–2669. [CrossRef]
- Fowoyo, P.T.; Ogunbanwo, S.T. Antimicrobial Resistance in Coagulase-Negative Staphylococci from Nigerian Traditional Fermented Foods. Ann Clin Microbiol Antimicrob 2017, 16. [CrossRef]
- Lee, B.; Jeong, D.W.; Lee, J.H. Genetic Diversity and Antibiotic Resistance of Staphylococcus Saprophyticus Isolates from Fermented Foods and Clinical Samples. J Korean Soc Appl Biol Chem 2015, 58, 659–668. [CrossRef]
- Çitak, S.; Yucel, N.; Orhan, S. Antibiotic Resistance and Incidence of Enterococcus Species in Turkish White Cheese. Int J Dairy Technol 2004, 57, 27–31. [CrossRef]
- Sánchez Valenzuela, A.; Lavilla Lerma, L.; Benomar, N.; Gálvez, A.; Pérez Pulido, R.; Abriouel, H. Phenotypic and Molecular Antibiotic Resistance Profile of Enterococcus Faecalis and Enterococcus Faecium Isolated from Different Traditional Fermented Foods. Foodborne Pathog Dis 2013, 10, 143–149. [CrossRef]
- Cepec, E.; Trček, J. Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. Int J Environ Res Public Health 2022, 19. [CrossRef]
- Giraffa, G.; Carminati, D. Molecular Techniques in Food Fermentation: Principles and Applications. Molecular Techniques in the Microbial Ecology of Fermented Foods 2008, 1–30. [CrossRef]
- Gibbons, J.G.; Rinker, D.C. The Genomics of Microbial Domestication in the Fermented Food Environment. Curr Opin Genet Dev 2015, 35, 1–8. [CrossRef]
- Wang, R.; Wu, J.; Jiang, N.; Lin, H.; An, F.; Wu, C.; Yue, X.; Shi, H.; Wu, R. Recent Developments in Horizontal Gene Transfer with the Adaptive Innovation of Fermented Foods. Crit Rev Food Sci Nutr 2023, 63, 569–584. [CrossRef]
- Steensels, J.; Gallone, B.; Voordeckers, K.; Verstrepen, K.J. Domestication of Industrial Microbes. Current Biology 2019, 29, R381–R393. [CrossRef]
- Roach, M.J.; Borneman, A.R. New Genome Assemblies Reveal Patterns of Domestication and Adaptation across Brettanomyces (Dekkera) Species. BMC Genomics 2020, 21, 1–14. [CrossRef]
- Irlinger, F.; Mounier, J. Microbial Interactions in Cheese: Implications for Cheese Quality and Safety. Curr Opin Biotechnol 2009, 20, 142–148. [CrossRef]
- Leroy, S.; Christieans, S.; Talon, R. Tetracycline Gene Transfer in Staphylococcus Xylosus in Situ During Sausage Fermentation. Front Microbiol 2019, 10, 392. [CrossRef]
- You, L.; Yang, C.; Jin, H.; Kwok, L.Y.; Sun, Z.; Zhang, H. Metagenomic Features of Traditional Fermented Milk Products. LWT 2022, 155, 112945. [CrossRef]
- Vogel, R.F.; Becke-Schmid, M.; Entgens, P.; Gaier, W.; Hammes, W.P. Plasmid Transfer and Segregation in Lactobacillus Curvatus LTH1432 in Vitro and during Sausage Fermentations. Syst Appl Microbiol 1992, 15, 129–136. [CrossRef]
- Cocconcelli, P.S.; Cattivelli, D.; Gazzola, S. Gene Transfer of Vancomycin and Tetracycline Resistances among Enterococcus Faecalis during Cheese and Sausage Fermentations. Int J Food Microbiol 2003, 88, 315–323. [CrossRef]
- Haubert, L.; Cruxen, C.E. dos S.; Fiorentini, Â.M.; Silva, W.P. da Tetracycline Resistance Transfer from Foodborne Listeria Monocytogenes to Enterococcus Faecalis in Minas Frescal Cheese. Int Dairy J 2018, 87, 11–15. [CrossRef]
- Seifabadi, F.S.; Baserisalehi, M. Plasmid-Mediated Antibiotic-Resistant Pattern of Lactobacillus Spp. Isolated From Dairy Products. Avicenna Journal of Clinical Microbiology and Infection 2021, 8, 1–4. [CrossRef]
- Heo, S.; Bae, T.; Lee, J.H.; Jeong, D.W. Transfer of a Lincomycin-Resistant Plasmid between Coagulase-Negative Staphylococci during Soybean Fermentation and Mouse Intestine Passage. FEMS Microbiol Lett 2019, 366, 113. [CrossRef]
- Li, N.; Yu, H.; Liu, H.; Wang, Y.; Zhou, J.; Ma, X.; Wang, Z.; Sun, C.; Qiao, S. Horizontal Transfer of VanA between Probiotic Enterococcus Faecium and Enterococcus Faecalis in Fermented Soybean Meal and in Digestive Tract of Growing Pigs. J Anim Sci Biotechnol 2019, 10, 1–11. [CrossRef]
- Miller, J.H.; Novak, J.T.; Knocke, W.R.; Pruden, A. Elevation of Antibiotic Resistance Genes at Cold Temperatures: Implications for Winter Storage of Sludge and Biosolids. Lett Appl Microbiol 2014, 59, 587–593. [CrossRef]
- Schjørring, S.; Krogfelt, K.A. Assessment of Bacterial Antibiotic Resistance Transfer in the Gut. Int J Microbiol 2011. [CrossRef]
- Tarrah, A.; Pakroo, S.; Corich, V.; Giacomini, A. Identification and Transferability of Tetracycline Resistance in Streptococcus Thermophilus during Milk Fermentation, Storage, and Gastrointestinal Transit. Fermentation 2021, Vol. 7, Page 65 2021, 7, 65. [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W.; Adamski, P. High-Pressure Processing Effect on Conjugal Antibiotic Resistance Genes Transfer in Vitro and in the Food Matrix among Strains from Starter Cultures. Int J Food Microbiol 2023, 388, 110104. [CrossRef]
- Mater, D.D.G.; Langella, P.; Corthier, G.; Flores, M.-J. Fax +41 61 306 12 34 E-Mail [email protected] A Probiotic Lactobacillus Strain Can Acquire Vancomycin Resistance during Digestive Transit in Mice. J Mol Microbiol Biotechnol 2008, 14, 123–127. [CrossRef]
- Broaders, E.; Gahan, C.G.; Marchesi, J.R. Gut Microbes Mobile Genetic Elements of the Human Gastrointestinal Tract Potential for Spread of Antibiotic Resistance Genes. www.landesbioscience.com Gut Microbes 271 Gut Microbes 4, 271–280. [CrossRef]
- Coppola, R.; Succi, M.; Tremonte, P.; Reale, A.; Salzano, G.; Sorrentino, E. Antibiotic Susceptibility of Lactobacillus Rhamnosus Strains Isolated from Parmigiano Reggiano Cheese. Lait 2005, 85, 193–204. [CrossRef]
- Goldstein, E.J.C.; Tyrrell, K.L.; Citron, D.M. Lactobacillus Species: Taxonomic Complexity and Controversial Susceptibilities. Clin Infect Dis 2015, 60 Suppl 2, S98–S107. [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic Resistance in Probiotic Bacteria. Front Microbiol 2013, 4, 51661. [CrossRef]
- Simpson, W.J.; Hammond, J.R.M.; Miller, R.B. Avoparcin and Vancomycin: Useful Antibiotics for the Isolation of Brewery Lactic Acid Bacteria. Journal of Applied Bacteriology 1988, 64, 299–309. [CrossRef]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus Spp. Appl Environ Microbiol 2018, 85. [CrossRef]
- Abriouel, H.; Casado Muñoz, M. del C.; Lavilla Lerma, L.; Pérez Montoro, B.; Bockelmann, W.; Pichner, R.; Kabisch, J.; Cho, G.S.; Franz, C.M.A.P.; Gálvez, A.; et al. New Insights in Antibiotic Resistance of Lactobacillus Species from Fermented Foods. Food Res Int 2015, 78, 465–481. [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118. [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic Susceptibility of Potentially Probiotic Lactobacillus Species. J Food Prot 1998, 61, 1636–1643. [CrossRef]
- Casado Muñoz, M. del C.; Benomar, N.; Lerma, L.L.; Gálvez, A.; Abriouel, H. Antibiotic Resistance of Lactobacillus Pentosus and Leuconostoc Pseudomesenteroides Isolated from Naturally-Fermented Aloreña Table Olives throughout Fermentation Process. Int J Food Microbiol 2014, 172, 110–118. [CrossRef]
- Knudtson, L.M.; Hartman, P.A. Antibiotic Resistance Among Enterococcal Isolates from Environmental and Clinical Sources. J Food Prot 1993, 56, 489–492. [CrossRef]
- Van Veen, H.W.; Konings, W.N. The ABC Family of Multidrug Transporters in Microorganisms. Biochim Biophys Acta 1998, 1365, 31–36. [CrossRef]
- Sozzi, T.; Smiley, M.B. Antibiotic Resistances of Yogurt Starter Cultures Streptococcus Thermophilus and Lactobacillus Bulgaricus. Appl Environ Microbiol 1980, 40, 862–865. [CrossRef]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, Vol. 3, Page 49 2017, 3, 49. [CrossRef]
- Maqueda, M.; Pérez-Nevado, F.; Regodón, J.A.; Zamora, E.; Álvarez, M.L.; Rebollo, J.E.; Ramírez, M. A Low-Cost Procedure for Production of Fresh Autochthonous Wine Yeast. J Ind Microbiol Biotechnol 2011, 38, 459–469. [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr Rev Food Sci Food Saf 2020, 19, 184–217. [CrossRef]
- Devirgiliis, C.; Zinno, P.; Stirpe, M.; Barile, S.; Perozzi, G. Functional Screening of Antibiotic Resistance Genes from a Representative Metagenomic Library of Food Fermenting Microbiota. Biomed Res Int 2014, 2014. [CrossRef]
- Teuber, M.; Meile, L.; Schwarz, F. Acquired Antibiotic Resistance in Lactic Acid Bacteria from Food. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology 1999, 76, 115–137. [CrossRef]
- Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.J.; Hudson, A.O.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics (Basel) 2019, 8. [CrossRef]
- Hambrick, E.C. Listeria Monocytogenes : Food Sources, Prevalence & Management Strategies.
- Neil, K.; Allard, N.; Roy, P.; Grenier, F.; Menendez, A.; Burrus, V.; Rodrigue, S. High-efficiency Delivery of CRISPR-Cas9 by Engineered Probiotics Enables Precise Microbiome Editing. Mol Syst Biol 2021, 17, 10335. [CrossRef]
- Romero-Luna, H.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Peredo-Lovillo, A. Bioactive Peptides Produced by Engineered Probiotics and Other Food-Grade Bacteria: A Review. Food Chem X 2022, 13, 100196. [CrossRef]
Figure 1.
Potential events in fermented food production leading to the transfer of AR bacteria and genes to human gut microbiota.
Figure 1.
Potential events in fermented food production leading to the transfer of AR bacteria and genes to human gut microbiota.
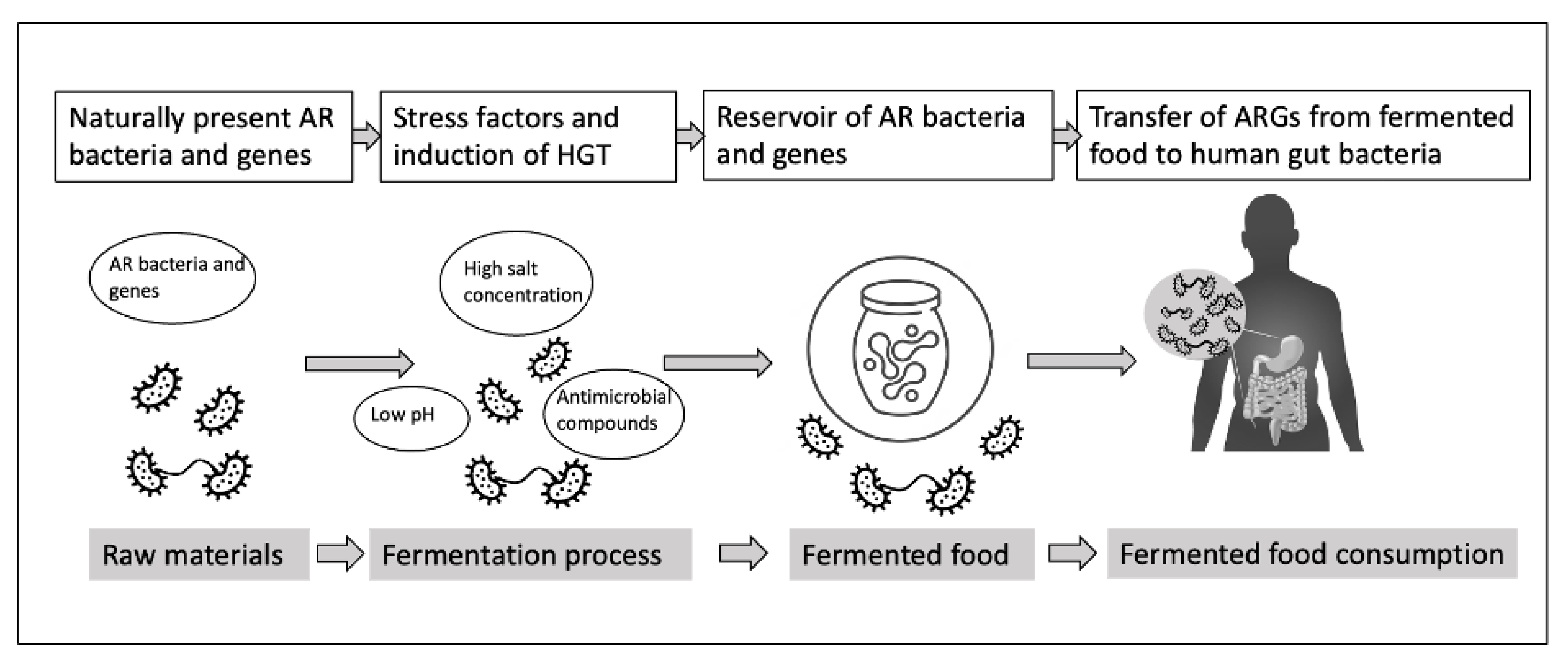
Figure 2.
Strategies for reducing AR in fermented food production.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








