Preprint
Article
A mixture of essential oils from three Cretan Aromatic Plants inhibits SARS-CoV-2 proliferation: A Proof-of-Concept intervention study in ambulatory patients
Altmetrics
Downloads
108
Views
55
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.pdf (240.08KB )
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Alerts
Abstract
Introduction: The need for therapeutic regimens for non-critically ill patients during the COVID-19 pandemic remains unmet. Previous work has shown that a combination of three aromatic plants’ essential oil (CAPeo) (Thymbra capitata (L.) Cav., Origanum dictamnus L., Salvia fruticosa Mill.) has remarkable in vitro antiviral activity. Given its properties, it was urgent to explore its potential in treating mild COVID-19 patients in primary care. Methods: 69 adult patients were included in a clinical, Proof-of-Concept (PoC) intervention study. Family physicians implemented the observational study in two arms (intervention and control group) during three study periods (IG2020, n=13, IG2021/22, n=25 and CG2021/22, n=31). The SARS-CoV-2 infection was confirmed by real-time PCR. The CAPeo mixture, was administered daily for 14 days, per os in the intervention group, while the control group received usual care. Results: The PoC study, found that the number and frequency of general symptoms, including general fatigue, weakness, fever and myalgia, decreased following CAPeo administration. The average presence (number) of symptoms decreased in IG (4.7 to 1.4) as well as in CG (4.0 to 3.1) by Day 7 compared to Day 1, representing a significant decrease in cumulative presence in IC (-3.3 vs. -0.9, p<0.001; η2=0.20) on Day 7 and on Day 14 (-4.2 vs. -2.9, p=0.027; η2=0.08). Discussion/Conclusion: Our findings suggest that CAPeo, possesses potent antiviral activity, in addition tο the Influenza A and B and the human rhinovirus HRV14 strains against SARS-CoV-2. The early and effective impact in alleviating key symptoms of COVID-19 may suggest this mixture can act as a complementary natural agent for mild COVID-19 patients
Keywords:
Subject: Medicine and Pharmacology - Clinical Medicine
1. Introduction
Since the COVID-19 pandemic outbreak in 2019, a previously unseen international effort has been undertaken to identify the underlying cause (the SARS-CoV-2 virus) and for the detailed analysis of its genome [1]. A global effort is directed toward efficient therapy [2,3,4] or the development of efficient vaccines [5,6]. Several established pharmaceutical molecules and patients’ plasma have been tested as drug candidates for treating COVID-19, with variable results (see [7,8,9] and references therein). Among the many products tested against COVID-19, several natural products, including herbal extracts, have also been assayed (critically reviewed in [10] and references therein), targeting mainly the viral proteases.
Much interest has been shown regarding the role of primary health care (PHC) in pandemic times, and evidence highlights distinct challenges to integrating and supporting PHC in response to infectious disease epidemics [11]. However, the contribution of PHC in research relevant to the identification and test of therapeutics against COVID-19 was limited in Europe.
Our group has conducted a large number of studies about the use of aromatic plants as an effective treatment of common health problems. A combination of three aromatic plants’ essential oil (CAPeo) (Thymbra capitata (L.) Cav., Origanum dictamnus L., Salvia fruticosa Mill. [12], and references therein) has been shown efficient against upper respiratory tract viral infections in humans [13,14]. Plant material has been identified by one of the authors (SP) and voucher specimens of the three species have been deposited at the Herbarium TAU of the Aristotle University of Thessaloniki (UOCSP101-1, UOCSP101-2, and UOCSP101-3). This sentence has been added in the revised manuscript. In vitro studies revealed the efficacy of CAPeo against Influenza A and B and the Human Rhinovirus HRV14. They reported an action by inhibiting the nuclear translocation of viral nucleoproteins [15], impairing viral protein transcription. In addition, the safety of CAPeo in humans (administered in soft gels, 1 ml/day of a 1.5% essential oil combination in extra virgin olive oil, [14] has been reported. Furthermore, we tested our preparation in vitro against SARS-CoV-2-infected cells. We found that it can promote the survival of cells following infection, reducing viral replication, both after pre- or co-incubation with CAPeo [16].
Based on the above-published experiences, it was considered essential to design and implement a Proof-of-of-Concept (PoC) intervention study (early-stage evidence that something works as it has been intended) with a control group of mild COVID-19 patients attending PHC services in Crete. Our primary objective was to explore to what extent the CAPeo can significantly alleviate the general and local symptoms of the disease. In parallel, the efficiency of CAPeo mixture was examined.
2. Materials and Methods
2.1. Setting
A private primary healthcare center with 3 GPs practices is located in a town near Heraklion, Crete’s capital. The study was conducted in August-October 2020 & August 2021-February 2022, in which different strains of SARS-CoV-2 were predominant. During the first period of data collection, the Alpha (B.1.1.7, VOC: 18-Dec-2020 with earliest documented samples in the United Kingdom, September 2020) and Beta (B.1.351, VOC: 18-Dec-2020 with earliest documented samples in the South Africa, May 2020) strains were predominant, while during the second period of data collection, the Delta (B.1.617.2, VOI: 4-Apr-2021, VOC: 11-May-202) and Omicron (B.1.1.529, VUM: 24-Nov-2021, VOC: 26-Nov-2021) strains were predominant [17,18].
2.2. Participants
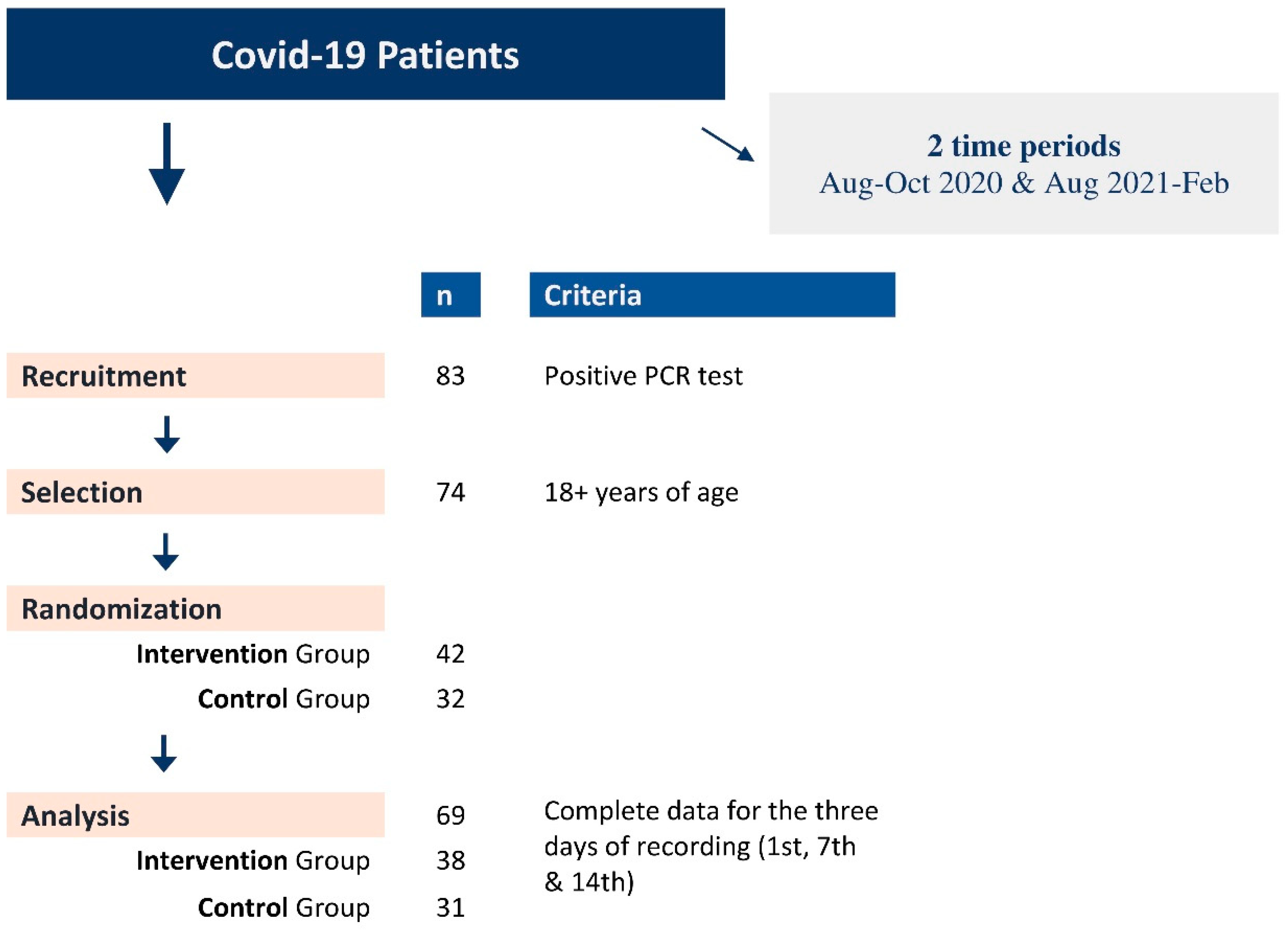
Adult patients reporting to a single primary care unit with symptoms related to an upper respiratory tract infection and suggestive of a SARS-CoV-2 infection were invited to participate in the study after the SARS-CoV-2 infection was confirmed by a real-time PCR test performed in the regional COVID-19 reference centre (Laboratory of Clinical Virology of the participating University). Eligible participants have been (randomly) allocated to the Intervention Group (IG) and the Control Group (CG). Thirteen patients from the first period of recruitment all participated in the IG (n=13; Aug-Oct 2020) and twenty-seven in the second (n=25; Aug 2021-Feb 2022) with the CG (n=31; Aug 2021-Feb 2022) (Figure 1, Table 1, Suppl. Table A). In addition, information regarding demographics, medical history, smoking habits, symptoms, and signs has been recorded in a pre-tested questionnaire for all study participants.
Table 1.
Descriptive characteristics of the 69 patients in Intervention & Control groups.
| Groups | ||||||||
|
Intervention (n=38) |
Control (n=31) |
|||||||
| n (%) | ||||||||
| Gender | males | 18 (47.4) | 14 (45.2) | |||||
| females | 20 (52.6) | 17 (54.8) | ||||||
| Age, years | mean age±stand.dev. | 38.4±12.8 | 42.1±16.1 | |||||
| Family members | yes | 11 (28.9) | 15 (48.4) | |||||
| Study phases | Aug-Oct 2020 | 13 (34.2) | - | |||||
| Aug 2021-Feb 2022 | 25 (65.8) | 31 (100.0) | ||||||
| Smokers | non | 22 (57.9) | 13 (42.0) | |||||
| former | 3 (7.9) | 9 (29.0) | ||||||
| current | 13 (34.2) | 9 (29.0) | ||||||
| Morbidity(at least one chronic disease) | yes a | 14 (36.8) | 9 (29.0) | |||||
| Recurrence of Covid-19(in last six months) | yes | -- | 1 (3.2) | |||||
| Vaccination for SARS-CoV-2(doses) | none | 19 (50.0) | 11 (35.5) | |||||
| one | 5 (13.2) | 3 (9.7) | ||||||
| two | 10 (26.3) | 14 (45.2) | ||||||
| three | 4 (10.5) | 3 (9.7) | ||||||
| Administration/intake of medicinal or other compound for the symptoms (before, at the point of or following inclusion to the study, additionally to CAPeo) | painkillers | 16 (42.1) | 19 (61.3) | |||||
| antibiotics | 4 (10.5) | -- | ||||||
a Includes nineteen different groups of diseases e.g. hypertension, diabetes mellitus, heart disease, cancer etc.
Figure 1.
Recruitment flowchart of Covid-19 patients and randomization in Intervention (IG) and Control (IG) groups.
Figure 1.
Recruitment flowchart of Covid-19 patients and randomization in Intervention (IG) and Control (IG) groups.
2.3. CAPeo production and phytochemical analysis
Spanish oregano (Coridothymus capitatus (L) Rchb. F. synonym of Thymbra capitata (L) Cav.), dictamnus or Cretan dittany (Origanum dictamnus L) and Greek sage (Salvia fruticosa Mill.) were cultivated under total Good Agricultural Practice and High Precision Agriculture, based on an Ecological Niche Modelling tool, we have recently developed [19], to maximize their essential oil composition and content. A barcoding monitor of each batch was always performed to ensure the constant genotype of plants. The essential oils of the three plants are produced from collected leaves of the plant material, air dried in the dark at room temperature (25°C) for ten days. Essential oils were extracted by steam distillation of the dried plant leaves under GMP conditions. The final extract contained four parts Corydothymus capitatus (L) extract and two parts Salvia fruticosa Mill. extract, and one-part Origanum dictamnus L extract.
For analysis, after steam distillation, 1 ml of volatile oils were diluted with 2 ml of ether, filtered through anhydrous sodium sulfate to remove water traces, and stored at 4°C. Analysis was performed, as described previously [14], by Gas Chromatography-Mass Spectroscopy (GC-MS, Shimadzu, QP 5050 A), with a MDN-5 column and a Quadrupole Mass Spectrometer as the detector, after injection of 2 μL. The carrier gas was helium, a flow rate 0.9 ml/min. The sample was measured in a split mode procedure (1:35). For GS-MS detection, an electron ionization system was used with ionization energy at 70 eV.
2.4. CAPeo administration
The CAPeo mixture, in the form of two 0.5 ml soft capsules, in a concentration of 15 ml/L, was administered daily for two weeks (14 days) per os in the subjects that have allocated in the IG, while the subjects in the CG received usual care, including instructions for monitoring the progress of the illness and analgesics if needed.
2.5. Collected data
Data were collected on Day 1, Day 7, and Day 14; following the initial face-to-face consultation, data collection and consultations were performed remotely (by phone) or via home visits by trained medical personnel. The severity of symptoms was assessed through the utilisation of a five-point Likert scale, starting from zero (no severity), 1 (very mild), 2 (mild), 3 (moderate), and 4 (severe). Data were recorded on Day 1 and taken as the baseline. The primary outcome defined to assess the clinical effectiveness of the CAPeo was symptom reduction, in terms of severity and frequency, defined as the total number of symptoms over the 14 days, and with measurement on Day 7, and Day 14. For reporting and given the small interval before the confirmed diagnosis and study inclusion, the date of the confirmed diagnosis, i.e., Day 1 of the study, was considered Day 1 for symptoms and severity and frequency assessment.
2.6. Statistical analysis
Data were analyzed using the SPSS software (IBM SPSS Statistics for Windows, 2021, ver28.0 Armonk, NY: IBM Corp). Frequencies of descriptive characteristics in two groups (IG & CG) were assessed, as well as the presence and severity of the symptoms. The severity was estimated as a cumulative score (summing up all symptoms’ scoring severity). Repeated-measures analysis of variance was used to assess the Δ-changes between the three days (Day 1 or baseline, Day 7, and Day 14), while the analysis of covariance was used to test the differences between the groups. As covariates were used, age, gender, smoking habit, morbidity, vaccination for SARS-CoV-2, phases of the study, and use of painkillers or/and antibiotics. A critical value of 0.05 was taken as the threshold of statistical significance.
2.7. Ethics
In a separate file (since authors’ details)
3. Results
Phytochemical constituents of CAPeo
The mixture of essential oils contains carvacrol (53%), eucalyptol (13%), and β-Caryophyllene (3%). Concentrations of the compounds p-Cymene, γ-Terpinene, Borneol, and α-Terpineol were 1.32, 1.17, 1.68, and 1.06%, respectively, while the concentrations of the remaining 15 compounds were less than 1%. For the complete analysis of compounds, please refer to previous reports [14,15], while their chemical structure is presented in Figure 2. These concentrations refer to the stock essential oil mixture, while a concentration of 1.5% in extra-virgin olive oil (for human studies) or DMSO (Sigma-Aldrich, for cell studies) was used. This refers to the dilution of 1:1, mimicking the suggested daily dose of the CAPeo extract in humans (1 ml of 1.5% of CAPeo in olive oil) for managing upper respiratory tract infections [13,14]. As the pharmacokinetics and bioavailability of CAPeo are under investigation, we have used bibliography data, suggesting a variable absorption of phenolic compounds ranging from 27 to 0.0006% and blood recovery ≤1% for the majority of compounds [20,21]. Therefore, to mimic available concentrations in humans, different dilutions (1:10, 1:100, and 1:1000 of the clinically administered concentration -15 mL extract/L, 1 mL/day-) in DMSO) were used for cell studies. The same concentrations were used in a previous study to determine the protective and therapeutic effects of CAPeo in cells infected with other upper respiratory viruses [15].
Figure 2.
Hierarchical frequency of the presence (%) of the 12 local symptoms, in Intervention (IG) and Control (IG) groups.
Figure 2.
Hierarchical frequency of the presence (%) of the 12 local symptoms, in Intervention (IG) and Control (IG) groups.

Demographic and health habits data of the participants
The study involved 69 patients meeting the selection criteria (39 in the IG and 31 in the CG) and completing the study’s data (Table 1, Figure 1, Suppl. Table A). Fifty-two percent (52%) and fifty-five percent (55%) from IG and CG were women, respectively, with a mean age of 38.4 and 42.1 years. Smoking was reported in 34.2% and 29.0% of the participants in the IG and CG, respectively, with 36.8% and 29.0% reporting having at least one chronic disease. Recurrence of COVID-19 was reported for the second time in one CG patient, while 50.0% of IG (all including patients in the first phase) and 35.5% of CG reported being unvaccinated. Finally, during the illness, painkillers were used by 42.1% of IG and by 61.3% of CG. No statistically significant differences were present among the three study groups (IG2020, IG2021/22, and CG2021/22 with only one exception regarding the use of painkillers (Suppl. Table A).
Effect on the frequency of symptoms
Figure 2 and Figure 3 show in hierarchical frequency the presence (%) of the 12 local and nine general symptoms, respectively, in the two groups of the study. At the onset (Day 1 or baseline), the headache was reported at a high prevalence in both groups [IG 24 (63.2%) and CG 619 (1.3%)] decreased in both groups on Day 7 A[IG 2(5.3%) and CG 9(29.0%)] (Figure 3). The impact of CaPeo was less evident in the local symptoms such as dry cough, anosmia, or lack of taste on Day 14 in both groups; however, their frequency was lower in the IG (Figure 4).
Figure 3.
Hierarchical frequency of the presence (%) of the 9 general symptoms, in Intervention (IG) and Control (IG) groups.
Figure 3.
Hierarchical frequency of the presence (%) of the 9 general symptoms, in Intervention (IG) and Control (IG) groups.

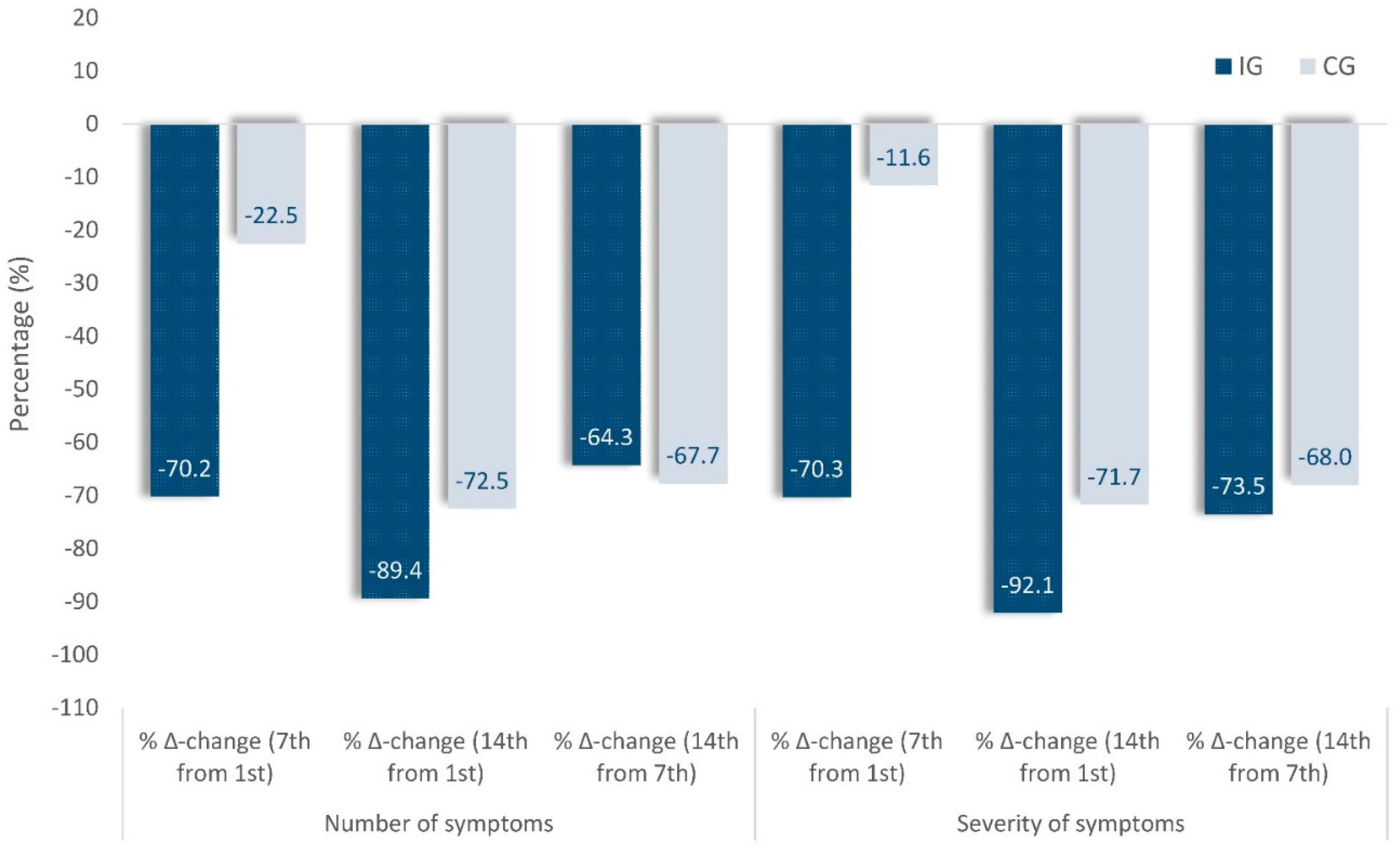
Figure 4.
Percentage changes in number and severity of symptoms on the 7th day compared to the 1st(baseline), in Intervention (IG) and Control (IG) groups.
Figure 4.
Percentage changes in number and severity of symptoms on the 7th day compared to the 1st(baseline), in Intervention (IG) and Control (IG) groups.
Effect on the severity of symptoms
Table 2 shows the Δ-changes of the cumulative presence (number) and severity of the symptoms. A decrease in the average presence (number) of symptoms in IG (4.7 to 1.4 symptoms) as well as in CG (4.0 to 3.1) was observed on Day 7 compared to Day 1 (baseline). It represents a significantly more significant decrease in the cumulative presence (number) in IC (-3.3 vs. -0.9, p < 0.001; η2 = 0.20) on Day 7 as on Day 14 as well (-4.2 vs. -2.9, p = 0.027; η2 = 0.08). Concerning the intensity (severity) of the symptoms, there is a difference in the baseline between the intervention and the control group, as shown in Table 2, with 11.58 (0.92) marginal mean in the IG in comparison to 9.16 (1.03) in the CG. Furthermore, a decrease was found in the average intensity (severity) of symptoms in both IG (11.58 to 3.44) and in CG (9.16 to 8.10), but with a significantly more significant reduction in intensity in the IC (-8.14 vs. -1.06, p < 0.001; η 2= 0.20) as on Day 14 too (-10.67 vs. -6.57, p=0.007; η2=0.12). The observed differences remain among the three study groups (IG2020, IG2021/22, and CG2021/22; Suppl. Table Β) due to the finding that the two IGs recorded a similar reduction from Day 1 to Day 7 (-8.0 and -7.8 vs. -1.4, p = 0.006; η2 = 0.12) and Day 14 (-9.3 and -10.9 vs. -6.9, p=0.033; η2 = 0.07) regarding the severity in the symptoms in comparison with the CG. To this effect and in terms of their percentage changes (Figure 4), on Day 7 compared to Day 1(baseline), there is a decrease in the average presence (number) of symptoms in IG by -70.2% compared to -22.5% in CG or -70.3% and 11.6% respectively in the severity of the symptoms.
Table 2.
Mean changes in number and severity of symptoms between Intervention-Control groups at 7th and 14th days in relation to baseline (1st record day).
Table 2.
Mean changes in number and severity of symptoms between Intervention-Control groups at 7th and 14th days in relation to baseline (1st record day).
| Groups | ||||||
| Intervention | Control | |||||
| Symptoms | Days of follow-up | marginal means (stand. errors) | p-value | η2 | ||
| Number | 1st (baseline) | 4.7 (0.3) | 4.0 (0.4) | |||
| 7th | 1.4 (0.4) | 3.1 (0.4) | ||||
| Δ-change (7th from 1st) | -3.3 (0.4) | -0.9 (0.5) | <0.001 | 0.20 | ||
| 14th | 0.5 (0.2) | 1.1 (0.3) | ||||
| Δ-change (14th from 7th) | -0.9 (0.3) | -2.1 (0.3) | 0.008 | 0.16 | ||
| Δ-change (14th from 1st) | -4.2 (0.3) | -2.9 (0.4) | 0.027 | 0.08 | ||
| Severity | 1st (baseline) | 11.58 (0.92) | 9.16 (1.03) | |||
| 7th | 3.44 (1.07) | 8.10 (1.20) | ||||
| Δ-change (7th from 1st) | -8.14 (1.14) | -1.06 (1.28) | <0.001 | 0.20 | ||
| 14th | 0.91 (0.55) | 2.59 (0.62) | ||||
| Δ-change (14th from 7th) | -2.53 (0.73) | -5.51 (0.82) | 0.014 | 0.10 | ||
| Δ-change (14th from 1st) | -10.67 (0.91) | -6.57 (1.02) | 0.007 | 0.12 | ||
Based on 12 topics and 9 general symptoms. The severity based on 5-points Likert scale as: 0=none, 1=very mild, 2=mild, 3=moderate and 4=severe. Scores of mean changes were extracted as summing-up all symptoms intensity. Comparisons of Δ-changes between groups were performed by using repeated measures analysis of covariance and as covariates were used: age, gender, smoking habit, morbidity, vaccination for SARS-CoV-2, phases of the study, and using of painkillers or and antibiotics.
4. Discussion
Main findings
This observational study revealed that general symptoms, including general fatigue, fever, and myalgia, were among the most frequent (almost fifty percent and more) reported symptoms. Regarding the local symptoms, the headache was the predominant reported symptom. It was evident that the CAPeo effectively and significantly decreased the most frequently reported general symptoms (general fatigue, weakness, fever, and myalgia) and local (headache). The changes also in the cumulative presence (number) and severity of symptoms supported the positive effect of CAPeo in the patients with SARS-CoV-2.
Our findings in light of the emerging evidence
It is well known that the symptoms of the mild infection of SARS-CoV-2 gradually decrease with time, while there are cases with very mild symptoms, especially with the omicron-variant. Thus, the key research question that this study needs to address is to what extent this natural product could change the evaluation of this illness and reduce the duration and severity of the symptoms.
Unfortunately, there are few studies with comprehensive reporting regarding the evolution of COVID-19 symptoms for comparison [22,23,24]. Fourteen days after COVID-19 testing, several symptoms persist; they include headache, fatigue, myalgias, anosmia, ageusia, and respiratory distress.
Our study is contributing to a recently published review article that states that several medicinal plants with antiviral activity may be utilized to treat viral infections or as supportive therapy [25]. Even though this review sets a limitation regarding the lack of information on the safety profile and dosage of herbal treatment for various diseases, the used in this study, a combination of three aromatic plants’ essential oil, shares no such limitation as it is evidenced has been in previous studies in humans [13,14].
In our group, treated with CAPeo, we reported an almost complete resolution of headache, fatigue, fever, and myalgia symptoms in COVID-19. However, the critical impact of the CAPeo on the number and severity of symptoms is visible within seven days of initiating CAPeo administration. We have therefore concluded that CAPeo alleviates general symptoms very quickly, with markedly better outcomes than those observed in the untreated population.
We decided to include family members (father, mother, and sister of one patient), and individuals working together or living in the same house. We consider this a vital reporting aspect in establishing guidelines for sequential testing, managing oligo- or asymptomatic patients in outpatient settings, and informing future clinical study design across settings, as highlighted by a recent report [26]. The median incubation period of five days, and even of approximately four and three days for the Delta and Omicron variants, respectively, creates a false sense of safety but also presents a challenge in terms of study inclusion and proper trial conduct and reporting [27].
No side effects have been reported, and the CAPeo in the form of soft capsules was easily recommended by the family physicians and well accepted by the patients and families.
CAPeo contains 25 different microconstituents (please refer to Supplemental Table 4 of Reference [14] for a detailed presentation of concentrations of specific constituents). The main compounds are carvacrol (53%), eucalyptol (13%), β-Caryophyllene (3%), p-Cymene (1.32%), γ-Terpinene (1.17%), Borneol (1.68%), and α-Terpineol (1.06%). Recent reviews [12] indicate that none of these compounds have been previously reported as having antiviral properties. However, recent work by our group has identified specific viral targets for some of these constituents, with a direct impact on viral replication [28].
Limitations
We conducted an observational study in primary health care where only patients with mild symptoms were studied, and our findings lack the robustness of the evidence generated by a randomized control study (RCT). In addition, our study included patients from the last three waves of the COVID-19 pandemic in the IG. In contrast, patients from the last two waves (Delta and Omicron variants) were included in the CG. Generally, mixing patients from various waves without an equal allocation of them into two groups may introduce a selection bias that impacts the results’ interpretation. However, the secondary analysis supported the main findings of this study. Moreover, real-world observational data do not replace RCTs; such evidence may offer valuable evidence in real-world settings while a pandemic is still unfolding.
The underlying mechanism of action for this beneficial effect of CAPeo in the evolution of COVID-19 requires further in-depth study; however, at least one component of our preparation (p-cymene) has been recently found to possess an extreme antiviral action against SARS-CoV-2 [28]. In addition to the possible direct antiviral effect of CAPeo, reported in cells, its beneficial effect may be, at least partially, due to its anti-inflammatory effect in humans [14]. Anosmia and ageusia, however, persisted at 13.2% in the IG at the end of the two weeks of provision of CAPeo. Whether this is due to a late recovery of nasal and buccal mucosa or the persistence of the virus in a small percentage of patients [29] is unclear. It is to note, however, that this symptom was persistent (~50%) in ambulatory patients with two negative tests in a previous study [30].
5. Conclusions
In conclusion, our findings from the performed clinical study, jointly with the reported in vitro studies, suggest that CAPeo, a mixture of essential oils of three Cretan aromatic plants, possesses a potent antiviral activity, in addition to Influenza and the Human Rhinovirus HRV14 [15], against SARS-CoV-2. It has an early and influential impact in alleviating the acute symptoms of COVID-19 patients. It suggests this mixture of essential oils as a complementary natural agent for mild COVID-19 patients in primary healthcare settings. If these results are confirmed in an already planned prospective clinical study, CAPeo might be a novel, inexpensive therapeutic agent in cases of ambulatory mild COVID-19 patients.
6. Patents
SAP, CL, and EC are inventors in patents CN102762218, EP2482831 and WO2011045557, with priority numbers WO2010GB01836 20100929 and GB20090017086 20090929, related to the antiviral activity of the CAPeo.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
CL conceived the Proof-of-Concept clinical study. CL, EP, ES, and ML equally participated in the design of the Proof-of-Concept clinical study and the analysis thereof. EP, AD, and CL were responsible for trial conduct and all clinical operation aspects, including the preliminary trial report, whereas ML performed the statistical analysis. MNK coordinated and conducted data collection. SAP was responsible for the quality assurance and quality control of CAPeo mixture, and GS performed the COVID-19 real-time PCR in clinical samples. CL and EC wrote the first draft of the paper, while all authors contributed to the reduction of the manuscript. All authors approved the submission.
Ethics: The study received approval by the University of Crete Bioethics Committee (No 78/01.04.2020). This study was registered in ClinicalTrials.gov, with the number NCT04705753.
Funding
The clinical study, which received approval by the Bioethics Committee of the University of Crete), was exclusively supported by the capacity of the Clinic of Social and Family Medicine at the University of Crete. CAPeo, in the form of soft capsules, has been provided by Olvos Science SA.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
CIntellectual Royalties (that are given to any inventor) are provided to CL, EC and SAP). No commercial or other association exists with this Greek company.
References
- O’Leary, Valerie Bríd, and Saak Victor Ovsepian. "Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2)." Trends in Genetics 36, no. 11 (2020): 892-93.
- Baum, A., D. Ajithdoss, R. Copin, A. Zhou, K. Lanza, N. Negron, M. Ni, Y. Wei, K. Mohammadi, B. Musser, G. S. Atwal, A. Oyejide, Y. Goez-Gazi, J. Dutton, E. Clemmons, H. M. Staples, C. Bartley, B. Klaffke, K. Alfson, M. Gazi, O. Gonzalez, E. Dick, Jr., R. Carrion, Jr., L. Pessaint, M. Porto, A. Cook, R. Brown, V. Ali, J. Greenhouse, T. Taylor, H. Andersen, M. G. Lewis, N. Stahl, A. J. Murphy, G. D. Yancopoulos, and C. A. Kyratsous. "Regn-Cov2 Antibodies Prevent and Treat Sars-Cov-2 Infection in Rhesus Macaques and Hamsters." Science 370, no. 6520 (2020): 1110-15.
- Hansen, J., A. Baum, K. E. Pascal, V. Russo, S. Giordano, E. Wloga, B. O. Fulton, Y. Yan, K. Koon, K. Patel, K. M. Chung, A. Hermann, E. Ullman, J. Cruz, A. Rafique, T. Huang, J. Fairhurst, C. Libertiny, M. Malbec, W. Y. Lee, R. Welsh, G. Farr, S. Pennington, D. Deshpande, J. Cheng, A. Watty, P. Bouffard, R. Babb, N. Levenkova, C. Chen, B. Zhang, A. Romero Hernandez, K. Saotome, Y. Zhou, M. Franklin, S. Sivapalasingam, D. C. Lye, S. Weston, J. Logue, R. Haupt, M. Frieman, G. Chen, W. Olson, A. J. Murphy, N. Stahl, G. D. Yancopoulos, and C. A. Kyratsous. "Studies in Humanized Mice and Convalescent Humans Yield a Sars-Cov-2 Antibody Cocktail." Science 369, no. 6506 (2020): 1010-14.
- Weinreich, D. M., S. Sivapalasingam, T. Norton, S. Ali, H. Gao, R. Bhore, B. J. Musser, Y. Soo, D. Rofail, J. Im, C. Perry, C. Pan, R. Hosain, A. Mahmood, J. D. Davis, K. C. Turner, A. T. Hooper, J. D. Hamilton, A. Baum, C. A. Kyratsous, Y. Kim, A. Cook, W. Kampman, A. Kohli, Y. Sachdeva, X. Graber, B. Kowal, T. DiCioccio, N. Stahl, L. Lipsich, N. Braunstein, G. Herman, and G. D. Yancopoulos. "Regn-Cov2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19." N Engl J Med 384, no. 3 (2021): 238-51.
- Polack, F. P., S. J. Thomas, N. Kitchin, J. Absalon, A. Gurtman, S. Lockhart, J. L. Perez, G. Pérez Marc, E. D. Moreira, C. Zerbini, R. Bailey, K. A. Swanson, S. Roychoudhury, K. Koury, P. Li, W. V. Kalina, D. Cooper, R. W. Frenck, Jr., L. L. Hammitt, Ö Türeci, H. Nell, A. Schaefer, S. Ünal, D. B. Tresnan, S. Mather, P. R. Dormitzer, U. Şahin, K. U. Jansen, and W. C. Gruber. "Safety and Efficacy of the Bnt162b2 Mrna Covid-19 Vaccine." N Engl J Med 383, no. 27 (2020): 2603-15. [CrossRef]
- Voysey, M., S. A. C. Clemens, S. A. Madhi, L. Y. Weckx, P. M. Folegatti, P. K. Aley, B. Angus, V. L. Baillie, S. L. Barnabas, Q. E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A. M. Collins, R. Colin-Jones, C. L. Cutland, T. C. Darton, K. Dheda, C. J. A. Duncan, K. R. W. Emary, K. J. Ewer, L. Fairlie, S. N. Faust, S. Feng, D. M. Ferreira, A. Finn, A. L. Goodman, C. M. Green, C. A. Green, P. T. Heath, C. Hill, H. Hill, I. Hirsch, S. H. C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C. C. D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A. M. Lawrie, A. Lelliott, V. Libri, P. J. Lillie, R. Mallory, A. V. A. Mendes, E. P. Milan, A. M. Minassian, A. McGregor, H. Morrison, Y. F. Mujadidi, A. Nana, P. J. O’Reilly, S. D. Padayachee, A. Pittella, E. Plested, K. M. Pollock, M. N. Ramasamy, S. Rhead, A. V. Schwarzbold, N. Singh, A. Smith, R. Song, M. D. Snape, E. Sprinz, R. K. Sutherland, R. Tarrant, E. C. Thomson, M. E. Török, M. Toshner, D. P. J. Turner, J. Vekemans, T. L. Villafana, M. E. E. Watson, C. J. Williams, A. D. Douglas, A. V. S. Hill, T. Lambe, S. C. Gilbert, and A. J. Pollard. "Safety and Efficacy of the Chadox1 Ncov-19 Vaccine (Azd1222) against Sars-Cov-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the Uk." Lancet 397, no. 10269 (2021): 99-111.
- Bolarin, J. A., M. A. Oluwatoyosi, J. I. Orege, E. A. Ayeni, Y. A. Ibrahim, S. B. Adeyemi, B. B. Tiamiyu, L. A. Gbadegesin, T. O. Akinyemi, C. K. Odoh, H. I. Umeobi, and A. B. Adeoye. "Therapeutic Drugs for Sars-Cov-2 Treatment: Current State and Perspective." Int Immunopharmacol 90 (2021): 107228.
- Wang, M. Y., R. Zhao, L. J. Gao, X. F. Gao, D. P. Wang, and J. M. Cao. "Sars-Cov-2: Structure, Biology, and Structure-Based Therapeutics Development." Front Cell Infect Microbiol 10 (2020): 587269.
- Wang, Y., P. Huo, R. Dai, X. Lv, S. Yuan, Y. Zhang, Y. Guo, R. Li, Q. Yu, and K. Zhu. "Convalescent Plasma May Be a Possible Treatment for Covid-19: A Systematic Review." Int Immunopharmacol 91 (2021): 107262. [CrossRef]
- Benarba, B., and A. Pandiella. "Medicinal Plants as Sources of Active Molecules against Covid-19." Front Pharmacol 11 (2020): 1189. [CrossRef]
- Desborough, J., S. H. Dykgraaf, C. Phillips, M. Wright, R. Maddox, S. Davis, and M. Kidd. "Lessons for the Global Primary Care Response to Covid-19: A Rapid Review of Evidence from Past Epidemics." Fam Pract 38, no. 6 (2021): 811-25. [CrossRef]
- Pirintsos, S. A., M. Bariotakis, M. Kampa, G. Sourvinos, C. Lionis, and E. Castanas. "The Therapeutic Potential of the Essential Oil of Thymbra Capitata (L.) Cav., Origanum Dictamnus L. And Salvia Fruticosa Mill. And a Case of Plant-Based Pharmaceutical Development." Front Pharmacol 11 (2020): 522213.
- Anastasaki, M., A. Bertsias, S. A. Pirintsos, E. Castanas, and C. Lionis. "Post-Market Outcome of an Extract of Traditional Cretan Herbs on Upper Respiratory Tract Infections: A Pragmatic, Prospective Observational Study." BMC Complement Altern Med 17, no. 1 (2017): 466.
- Duijker, G., A. Bertsias, E. K. Symvoulakis, J. Moschandreas, N. Malliaraki, S. P. Derdas, G. K. Tsikalas, H. E. Katerinopoulos, S. A. Pirintsos, G. Sourvinos, E. Castanas, and C. Lionis. "Reporting Effectiveness of an Extract of Three Traditional Cretan Herbs on Upper Respiratory Tract Infection: Results from a Double-Blind Randomized Controlled Trial." J Ethnopharmacol 163 (2015): 157-66. [CrossRef]
- Tseliou, Melpomeni, Stergios A. Pirintsos, Christos Lionis, Elias Castanas, and George Sourvinos. "Antiviral Effect of an Essential Oil Combination Derived from Three Aromatic Plants (Coridothymus Capitatus (L.) Rchb. F., Origanum Dictamnus L. And Salvia Fruticosa Mill.) against Viruses Causing Infections of the Upper Respiratory Tract." Journal of Herbal Medicine 17-18 (2019): 100288.
- Pirintsos, S., A. Panagiotopoulos, M. Bariotakis, V. Daskalakis, C. Lionis, G. Sourvinos, I. Karakasiliotis, M. Kampa, and E. Castanas. "From Traditional Ethnopharmacology to Modern Natural Drug Discovery: A Methodology Discussion and Specific Examples." Molecules 27, no. 13 (2022). [CrossRef]
- Organization, National Public Health. "Covid-19 Report." 2022.
- WHO. "Tracking Sars-Cov-2 Variants." (2022), https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- Bariotakis, Michael, Luciana Georgescu, Danae Laina, Ioanna Oikonomou, George Ntagounakis, Margarita-Ioanna Koufaki, Maria Souma, Michalis Choreftakis, Ourania Grigoriadou Zormpa, Petr Smykal, George Sourvinos, Christos Lionis, Elias Castanas, Regina Karousou, and Stergios A. Pirintsos. "From Wild Harvest Towards Precision Agriculture: Use of Ecological Niche Modelling to Direct Potential Cultivation of Wild Medicinal Plants in Crete." Science of The Total Environment 694 (2019): 133681. [CrossRef]
- Manach, C., G. Williamson, C. Morand, A. Scalbert, and C. Rémésy. "Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies." Am J Clin Nutr 81, no. 1 Suppl (2005): 230s-42s.
- Scalbert, Augustin, Christine Morand, Claudine Manach, and Christian Rémésy. "Absorption and Metabolism of Polyphenols in the Gut and Impact on Health." Biomedicine & Pharmacotherapy 56, no. 6 (2002): 276-82. [CrossRef]
- Allen, W. E., H. Altae-Tran, J. Briggs, X. Jin, G. McGee, A. Shi, R. Raghavan, M. Kamariza, N. Nova, A. Pereta, C. Danford, A. Kamel, P. Gothe, E. Milam, J. Aurambault, T. Primke, W. Li, J. Inkenbrandt, T. Huynh, E. Chen, C. Lee, M. Croatto, H. Bentley, W. Lu, R. Murray, M. Travassos, B. A. Coull, J. Openshaw, C. S. Greene, O. Shalem, G. King, R. Probasco, D. R. Cheng, B. Silbermann, F. Zhang, and X. Lin. "Population-Scale Longitudinal Mapping of Covid-19 Symptoms, Behaviour and Testing." Nat Hum Behav 4, no. 9 (2020): 972-82.
- Lechien, J. R., C. M. Chiesa-Estomba, S. Place, Y. Van Laethem, P. Cabaraux, Q. Mat, K. Huet, J. Plzak, M. Horoi, S. Hans, M. Rosaria Barillari, G. Cammaroto, N. Fakhry, D. Martiny, T. Ayad, L. Jouffe, C. Hopkins, and S. Saussez. "Clinical and Epidemiological Characteristics of 1420 European Patients with Mild-to-Moderate Coronavirus Disease 2019." J Intern Med 288, no. 3 (2020): 335-44. [CrossRef]
- Tenforde, M. W., S. S. Kim, C. J. Lindsell, E. Billig Rose, N. I. Shapiro, D. C. Files, K. W. Gibbs, H. L. Erickson, J. S. Steingrub, H. A. Smithline, M. N. Gong, M. S. Aboodi, M. C. Exline, D. J. Henning, J. G. Wilson, A. Khan, N. Qadir, S. M. Brown, I. D. Peltan, T. W. Rice, D. N. Hager, A. A. Ginde, W. B. Stubblefield, M. M. Patel, W. H. Self, and L. R. Feldstein. "Symptom Duration and Risk Factors for Delayed Return to Usual Health among Outpatients with Covid-19 in a Multistate Health Care Systems Network - United States, March-June 2020." MMWR Morb Mortal Wkly Rep 69, no. 30 (2020): 993-98. [CrossRef]
- Demeke, Chilot Abiyu, Alem Endashaw Woldeyohanins, and Zemene Demelash Kifle. "Herbal Medicine Use for the Management of Covid-19: A Review Article." Metabolism Open 12 (2021): 100141. [CrossRef]
- Wernhart, S., T. H. Förster, and E. Weihe. "Outpatient Management of Oligosymptomatic Patients with Respiratory Infection in the Era of Sars-Cov-2: Experience from Rural German General Practitioners." BMC Infect Dis 20, no. 1 (2020): 811.
- Lauer, S. A., K. H. Grantz, Q. Bi, F. K. Jones, Q. Zheng, H. R. Meredith, A. S. Azman, N. G. Reich, and J. Lessler. "The Incubation Period of Coronavirus Disease 2019 (Covid-19) from Publicly Reported Confirmed Cases: Estimation and Application." Ann Intern Med 172, no. 9 (2020): 577-82.
- Panagiotopoulos, A., M. Tseliou, I. Karakasiliotis, D. M. Kotzampasi, V. Daskalakis, N. Kesesidis, G. Notas, C. Lionis, M. Kampa, S. Pirintsos, G. Sourvinos, and E. Castanas. "P-Cymene Impairs Sars-Cov-2 and Influenza a (H1n1) Viral Replication: In Silico Predicted Interaction with Sars-Cov-2 Nucleocapsid Protein and H1n1 Nucleoprotein." Pharmacol Res Perspect 9, no. 4 (2021): e00798.
- Singanayagam, A., M. Patel, A. Charlett, J. Lopez Bernal, V. Saliba, J. Ellis, S. Ladhani, M. Zambon, and R. Gopal. "Duration of Infectiousness and Correlation with Rt-Pcr Cycle Threshold Values in Cases of Covid-19, England, January to May 2020." Euro Surveill 25, no. 32 (2020).
- Weinbergerova, B., J. Mayer, S. Hrabovsky, Z. Novakova, Z. Pospisil, L. Martykanova, K. Hortova, L. Mandelova, K. Hejduk, R. Chloupková, M. Pospisil, M. Doubkova, V. Marek, R. Novotna, M. Dolecek, H. M. Kubesova, K. Brat, R. Parizkova, P. Husa, M. Mechl, Z. Kral, and M. Lengerova. "Covid-19’s Natural Course among Ambulatory Monitored Outpatients." Sci Rep 11, no. 1 (2021): 10124. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
A mixture of essential oils from three Cretan Aromatic Plants inhibits SARS-CoV-2 proliferation: A Proof-of-Concept intervention study in ambulatory patients
Christos Lionis
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated












