Preprint
Article
Improving Flavor of Enzymatically Hydrolyzed Beef Liquid by Sonication
Altmetrics
Downloads
118
Views
37
Comments
0
This version is not peer-reviewed
Submitted:
24 July 2023
Posted:
24 July 2023
You are already at the latest version
Alerts
Abstract
Beef potentiator is an important flavor enhancer in food industry, while it is prone to generating insufficient taste compounds and off-odor compounds during enzymatic hydrolysis of beef, resulting in poor flavor of beef potentiator. It has been extensively reported that sonication is capable of improving food flavor. However, the effect of sonication on the flavor of enzymatically hydrolyzed beef liquid (EHBL) was scarcely reported. Herein, we investigated the effect of sonication on the flavor of EHBL using quantitative descriptive analysis (QDA), physicochemical analysis and SPME-GC-olfactometry/MS. QDA showed that sonication had a significant effect on taste improvement and off-odor removal of EHBL. Compared with the control, sonication (40 kHz, 80 W/L) increased the contents of total nitrogen, formaldehyde nitrogen, total sugar, reducing sugar, free amino acids (FAAs) and hydrolysis degree of EHBL by 19.25%, 19.80%, 11.83%, 9.52%, 14.37% and 20.45%. Notably, sonication markedly enhanced the contents of sweet FAAs, umami FAAs and bitter FAAs of EHBL by 19.66%, 14.04% and 9.18%, respectively, which contributed to the taste improvement of EHBL. SPME-GC-olfactometry/MS analysis showed that aldehydes and alcohols were the main contributors to aroma compounds of EHBL, sonication significantly increased the contents of key aroma compounds and 115.88% of alcohols content in EHBL. Notably, sonication decreased contents of the fishy odorants, hexanoic acid and nonanal, markedly by 35.29% and 26.03%, which was responsible for the aroma improvement of EHBL. Therefore, sonication could become a new potential tool to improve the flavor of EHBL.
Keywords:
Subject: Biology and Life Sciences - Food Science and Technology
1. Introduction
Thermal reaction flavour is a kind of food flavor enhancer and plays an important role in modern food industry, which is originated in European countries in the 1970s and is prepared via Maillard reaction using enzymatically hydrolyzed animal protein and reducing sugars as materials [1]. Nowadays, the annual output of thermal reaction flavour in European and American countries has exceeded 30,000 tons, accounting for more than 65% of the total flavour output, of which beef potentiator is the most popular thermal reaction flavour due to its popular flavor. Undoubtedly, beef potentiator will still have a huge demand in the future market, because flavor preference of consumers is difficult to change. Beef potentiator was prepared by mincing beef, grinding beef, boiling beef, enzymatic hydrolysis, adding reducing sugar and amino acids and Maillard reaction at 110–130 °C, then auxiliary materials (salt, monosodium glutamate, maltodextrin, preservative, etc.) were added at the end of Maillard reaction, followed by spray drying and packaging [2]. The main production process was shown in Figure 1. It is generally regarded that the enzymatic hydrolysis of beef is the most important process to obtain a beef potentiator with palateful flavor, because the free amino acids and peptides with low molecular weights generated in the process affect the flavor of beef potentiator significantly.
However, it is still difficult to obtain enzymatically hydrolyzed beef liquid (EHBL) with high contents of free amino acids and peptides with low molecular weight due to limitations of the activity and cost of enzymes and the processing conditions used. Furthermore, the off-odor (i.e., fishy odor) usually occurs during enzymatic hydrolysis of beef, which seriously lowers the quality of beef potentiator. Previous investigations demonstrated that beef aroma was associated with 38 aroma compounds [3], but the odor compounds relating to the off-odor of EHBL were still not clear till now. Thus, it is necessary to explore new method to improve the flavor of EHBL.
Ultrasound is an effective method to improve the enzymatic hydrolysis of protein and the physicochemical properties of enzymatically hydrolyzed products. Appropriate low intensity ultrasound can increase the hydrolysis degree and improve the flavor of hydrolyzed products [4]. At present, ultrasound has been applied in various foods manufacturing to enhance food properties and/or productivity. For example, Yang et al. (2020) treated grass carp protein during enzymatic hydrolysis using energy-divergent ultrasound with a power of 100 W/L for 20 min at 30 ± 2 °C. Results showed that content of free amino acids in the sonicated hydrolysate reached 242.83 mg/mL, which was 30.96% higher than that of the control (185.43 mg/mL), the taste of the sample was improved significantly and the bitterness and caramel color decreased markedly (p < 0.05), finally the overall acceptability of sample was remarkably enhanced [5]. In addition, previous investigation demonstrated that sonication (68 kHz, 60 W/L, 10 min, 8 circles) during soy sauce fermentation significantly enhanced the level of total FAAs in samples in comparison to the controls, suggesting that sonication promoted the amino acids release and accelerated flavor maturation of soy sauce [6]. The cavitation, mechanical and chemical effects of ultrasound may increase the degree of hydrolysis by altering the structure of enzymes and substrates, increasing specific surface area and molecular collision probability, thereby promoting the release of flavor substances such as free amino acids, small molecule peptides, nitrogen-containing substances, and reducing sugars [7,8,9]. But it is scarcely reported that whether sonication could improve the flavor of EHBL.
The objective of this study was to investigate (i) the effect of sonication on the flavor of EHBL and (ii) further explore the effect of sonication on the taste compounds and aroma compounds (especially off-aroma compounds) of EHBL.
2. Materials and Methods
2.1. Materials and Chemicals
Beef were commercially available from a local supermarket (Jimailong supermarket, Zhenjiang, China), Flavourzyme® 500 MG (EC3.4.11.1, complex of exoprotease and endoprotease from Aspergillus oryzae) and Neutrase 0.8L were purchased from Novozymes Biotechnology Co., Ltd (China), hexanoic acid, nonanal, 2-pentylfuran, (E)-2-octenal, (2E)-2-nonenal, (E,E)-2,4-decadien-1-al, 1-octen-3-ol and C6-C33 n-alkanes were provided by Aladdin Holdings Group (Shanghai, China, 2021). Other reagents with analytical purity were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China, 2021).
2.2. Beef Homogenate Preparation and Treatment
Preparation of beef homogenate: Mixed beef and distilled water with 1:1 ratio (w/w), steamed the mixture at 100 °C for 1 h, then cut the beef into small pieces and ground them through colloid mill to make beef homogenate. Then, divided the beef homogenate into 6 aliquots, three aliquots were used for the control and the other 3 aliquots were used for the sample.
Control: Firstly, added Neutrase 0.8L into the beef homogenates (300 U/g beef homogenate), followed by incubating the homogenates at 60 °C in a water bath. After 1 h, Flavourzyme® 500 MG (300 U/g beef homogenate) was added into the aforementioned beef homogenates which were enzymatically hydrolyzed for 2 h at 60 °C. The pH of homogenates was controlled at about 6.5 ± 0.5 by adding 2 M NaOH solution.
Sample: the homogenates were treated as the above control except sonication during enyzymatic hydrolysis. Sonication (40 kHz, 80 W/L, 10 s on/10 s off) was performed immediately after adding Neutrase 0.8L.
2.3. Sensory Evaluation
QDA was performed to investigate the differences in taste and aroma between the sample and control. QDA was carried out with a panel of nine evaluators (5 males and 4 females, aged 24–46) in a professional flavour and fragrance company in Guangzhou, China (Guangzhou Ririxiang Food Co., Ltd). In odor evaluation, prior to training, the evaluators were asked to rank a series of 10-fold suprathreshold aqueous solutions (25 mL in Teflon vials) of ethanol (alcoholic), acetic acid (sour), nonanal (fatty), trimethylpyrazine (roast), bis (2-methyl-3-furyl) disulfide (meat-like) and trimethylamine (fishy). In taste evaluation, the following five reference solutions were prepared to recalibrate the five basic tastes: 4 mM monosodium glutamate (pH 5.6, umami), 40 mM saccharose (sweet), 12 mM NaCl (salty), 1.5 mM caffeine (bitter) and 10 mM lactic acid (sour). Then the evaluators were asked to score the tast and aroma intensities on a linear scale from 0 to 9. The experiments were carried out in a laboratory at 23 ± 2 °C, results of the sensory evaluation were discussed and reached agreement eventually by all the evaluators.
2.4. Analyses of Total nitrogen, Formaldehyde Nitrogen, Hydrolysis Degree, Total Sugar and Reducing Sugar
Prior to analysis, each sample and control was filtered with a qualitative filter paper. Total nitrogen content in sample was determined by Kjeldahl method, and formaldehyde nitrogen was determined by titration [10]. Hydrolysis degree of EHBL was measured based on the pH-stat method described by Rezvankhah et al. [11]. Total sugar was estimated by phenol-sulfuric acid method [12]. The reducing sugar content was assessed using 3,5-dinitrosalicylic acid titration [13].
2.5. Free Amino Acids Analysis
FAAs were estimated according to method described by Gao et al. with minor modifications [10]. The control and sample were diluted with 10% trichloroacetic acid solution, the mixtures were placed for 2 h, then were filtered through double filter paper. Then 1 mL of the filtrate was placed in a 1.5 mL centrifuge tube and centrifuged at 10000 g for 30 min. The supernatant was filtered through a 0.45 μm pore filter, and the filtrate was subjected to a high performance liquid chromatography (Waters Ltd, Milford, MA, USA) with a PICO.TAG amino acid analysis column (3.9 mm i.d. × 150 mm length). Ten microliters of the filtrate was injected to the column, then was eluted with at 1.0 mL/min and monitored at 254 nm. FAAs concentrations in sample and control were quantitated using amino acids standard solution (AAS18; Sigma, St Louis, MO).
2.6. Thresholds and Taste Intensity of FAAs
According to previously reported taste thresholds of FAAs, the taste intensity of each FAA was determined using the following equation:
2.7. Protease Activity Analysis during Sonication
During the enzymatic hydrolysis of beef homogenate, EHBL were taken (0 min, 30 min, 60 min, 90 min, 120 min, 150 min, 180 min) to determine the neutral protease activity of the control and sample, the activity of neutral protease in EHBL was determined using the method described by Gao et al. [6].
2.8. Volatile Compounds Extraction Using Solid-Phase Micro-Extraction (SPME)
To investigate the effect of sonication on EHBL odor, the sample (10 mL) and control (10 mL) were put into 20 mL headspace bottles, the volatile compounds were extracted from the control and sample using a SPME fiber coated with carboxypoly dimethylsiloxane (Supelco, Bellefonte, PA, USA). Before extraction, the sample were preheated for 30 min at 45 °C in a headspace bottle and stirred continuously with a magnetic stirrer. Then volatile compounds were adsorbed using SPME fiber at 45 °C for 30 min.
2.9. Analyses of GC-Olfactometry and Flavor Dilution Factor
As previously described by Gao et al. [14], the analyses of GC-Olfactometry (GC-O) and Flavor Dilution (FD) Factor were conducted using an Agilent 6890 gas chromatograph equipped with an Agilent 5973 N mass selection detector (Wilmington, DE, USA) and a sniffing port (ODP-2; Gerstel, Inc., Linthicum, MD, USA).
The FD factor of each volatile compound represents the maximum dilution that the odor can be perceived at the olfactory mouth by two-thirds of the evaluators [15,16,17]. In this study, the dilution of volatile compound was represented by the split ratios of GC injection, which were 1:1, 2:1, 4:1, 8:1, 16:1, 32:1, 64:1 and 128:1.
2.10. Qualitative Analysis of Volatile Compounds
Qualitative analysis of volatile compounds was done with GC-MS system and a DB-Wax column (30 m × 0.25 mm × 0.25 μm; J&W Science, Folsom, CA, USA). High-purity helium (99.999%) was used as carrier gas with a flow rate of 1.0 mL/min (constant flow mode). The column was maintained for 3 min at 40 °C, heated from 40 °C to 200 °C with a speed of 5 °C /min, and heated from 200 °C to 230 °C with a speed of 10 °C /min. The mass spectrum conditions used were as follows: the ion source temperature was 230 °C, the electron energy was 70 eV, and the mass scanning range was 30–450 m/z. Volatile compounds were identified according to the Kovats retention index (RI) and by matching mass spectrometry in the NIST05 library (Gaithersburg, MD, USA.). In addition, RIs was determined under the same GC conditions using C6-C33 n-alkanes as the standards.
2.11. Quantitative Analysis of Key Odorants
External standard method was used to quantify the odor-active compounds in EHBL. The peak area of each odor-active compound was compared with peak area of its external standard compound to estimateits concentration. The response factor of each odor-active compound to its external standard compound was regarded as 1.
2.12. Odor Activity Value (OAV)
OAV was the ratio between the concentration of an odor compound and its odor threshold value detected in water. The contribution of an odor compound to EHBL was evaluated by its OAV, the contribution of an odorant with OAV beyond 1 was regarded as odor-active compound to EHBL.
2.13. Statistical Analysis
In this study, all tests except sensory tests were repeated thrice, and all data were expressed as mean ± standard deviation (SD). SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) was used for one-way ANOVA to determine that the differences were statistically significant within a 95% confidence interval, and the significance level was set to p < 0.05.
3. Results and Discussion
3.1. Sensory Evaluation
Sensory analysis is the most direct and effective method to evaluate food flavor. In this work, the effect of sonication on the flavor of EHBL was evaluated by QDA. As shown in Figure 2A, compared with the control, the sensory evaluation scores of umami, sweet and bitter tastes of the sample were 16.67%, 20.00%, 10.00% higher than those of the control, one-way ANOVA demonstrated that the sensory evaluation scores of umami and sweet tastes of the sample were significantly higher than those of the control (p < 0.05), indicating that sonication during the enzymatic hydrolysis improved the taste of EHBL markedly. As shown in Figure 2B, compared with the control, the sensory evaluation scores of fishy and fatty aromas of the sample were 40.00%, 33.33% lower than those of the control, one-way ANOVA demonstrated that the sensory evaluation scores of both aromas of the sample were significantly reduced than those of the control (p < 0.05), furthermore, sonication enhanced roast and alcoholic aromas of the sample to some extent. The above results indicated that sonication during the enzymatic hydrolysis improved the aroma of EHBL significantly. Previous investigations have extensively explored the effect of sonication on the flavor of fermented foods and enzymatically hydrolyzed products, results showed that moderate sonication could improve their taste and aroma [6,14,18], which was in line with the present sensory evaluation results. Overall, QDA proved that moderate sonication had a positive effect on the improvement of EHBL flavor, however, effect of sonication on the specific taste and aroma compounds of EHBL needed to further explore.
3.2. Analyses of Total Nitrogen, Formaldehyde Nitrogen, Hydrolysis Degree, Total Sugar and Reducing Sugar
As shown in Table 1, compared with the control, contents of total nitrogen, formaldehyde nitrogen, hydrolysis degree, total sugar and reducing sugar of the sample were 19.25%, 19.80%, 20.45%, 11.83% and 9.52% higher than those of the control, indicating that sonication (40 kHz, 80 W/L, 10 s on/10 s off) not only enhanced the hydrolysis degree of beef and the contents of sugars and formaldehyde nitrogen, but promoted solubility of N-containing compounds (i.e., proteins and peptides) and carbohydrates of beef (p < 0.05). Previous research indicated that appropriate sonication could increase 33–106% of the protein degradation rate by proteases and enhance the contents of hydrolyzed products of protein [19,20], which was consistent with our present results.
Previous research demonstrated that sonication changed the spatial structure of enzymes and substrates, which enlarged the specific area of substrate and increased the contact chance of protease and substrate, and then enhanced the protease activity and hydrolysis degree and contents of taste compounds [8,9], which could also explain the above phenomena observed in our experiment.
3.3. Analysis of Free Amino Acids
Free amino acids, especially glutamic acid, were regarded as key contributors to taste of food [6,18]. Compared with the control, the total FAAs level in the sample increased by approximately 14.37%, which was similar to the increase in formaldehyde nitrogen in the sample (19.80%). Specifically, sonication increased the levels of most sweet FAAs (19.66%), bitter FAAs (9.18%) and umami FAAs (14.04%), correspondingly, sonication increased the sweetness intensity, bitterness intensity and umami intensity of EHBL by 25.00%, 10.00% and 16.67% in the taste evaluation. In addition, it was found that sonication slightly reduced the glutamate level. Gao et al. found that low intensity sonication (60 W/L, 10 min × 8) during soy sauce fermentation enhanced the level of total free amino acids in soy sauce, but reduced its glutamate level [6], which was consistent with the present observation.
As shown in Table 2, the most abundant free amino acid was lysine, followed by leucine, alanine, phenylalanine and glutamic acid in the control and sample, indicating that sonication during the enzymatic hydrolysis process only increased the contents of FAAs in EHBL, but did not change their compositions of FAAs. As for taste intensity, although the variety of umami FAAs was the least, its taste intensity was relatively high due to the low thresholds of umami FAAs. Among the FAAs, the taste intensity values of glutamic acid, valine, phenylalanine and lysine in both EHBLs were greater than 2, indicating that they had significant contributions to EHBL. In addition, the taste intensity values of serine, glycine, proline, arginine, isoleucine and threonine were lower than their corresponding thresholds, suggesting that these six FAAs might have slight impacts on the taste of EHBL.
3.4. Analysis of Neutral Protease Activity
As shown in Figure 3, protease activities of both EHBLs gradually decreases over time except for those of EHBLs sampled at 60 min (supplementing Flavourzyme® 500 MG), but protease activities of sonicated EHBL are significantly higher than those of EHBL during the whole enzymatic hydrolysis (p < 0.05), suggesting that appropriate sonication was capable of enhancing the protease activities during enzymatic hydrolysis. Previous investigations indicated that low intensity ultrasound (20 kHz, < 110.6 W/cm2) increased the activities of neutrase and papain and energy-gathered ultrasound (63.2 W/cm2, 4 min) enhanced the alcalase activity [9]. Huang et al. concluded that ultrasound with appropriate frequency and low intensity could improve conformations of enzyme and substrate via the magnetostrictive, mechanical oscillation and cavitation actions of ultrasound, resulting in the enhancement of enzyme activity and substrate degradation rate [8]. The present result conformed to the results of previous reports.
3.5. Characterization of Volatile Compounds
As shown in Table 3, a total of 33 volatile compounds including alcohols, acids, ketones, aldehydes, furans, esters, alkanes and other compounds are identified in the sample and control. Among them, aldehydes and alcohols account for 72.72% of the identified volatile compounds in both EHBLs.
In this work, only hexanoic acid, a degraded product of fatty acid, was detected, which was consistent with the weak sweaty aroma in our sensory evaluation. Unsaturated fat acids can be degraded into short chain aldehydes and unsaturated aldehydes [21,22]. Aldehydes are believed to not only contribute to the aroma of food including beef, but also generate other aroma compounds through carbonyl-amine reaction with amino acids [23]. Aldehydes dominate the aroma of enzymatically hydrolyzed products due to their low odor thresholds, and they are mainly derived from the oxidative decomposition of fats and the thermally degraded products of reducing sugars and amino acids [18,24]. In this study, a total of 14 aldehydes including octanal, nonanal, decanal, etc. were identified, which were important components of EHBL aroma. Of them, nonanal had an obvious rose/citrus-like aroma, which was mainly produced by oleic acid oxidation [25]. Nonanal was reported to be a key aroma compound in roasted beef and contribute to the overall flavor of beef [26].
A total of 10 volatile alcohols, the oxidative decomposition products of fats or the reduction products of carbonyl compounds, were identified in both EHBLs [27]. 1-Pentanol, 3-ethyl-2-pentanol and 4-methyl-5-decanol were only detected in the sample, indicating that sonication promoted the oxidative decomposition of fats in EHBL. Generally, unsaturated alcohols contribute more to the aroma of food due to their lower thresholds compared to saturated alcohols. As an unsaturated alcohol, 1-octen-3-ol is a well-known compound with strong mushroom-like aroma [28]. This compound also has fishy and fatty aromas and is produced by enzymatic or nonenzymatic degradation of linoleic acid [29]. However, it might contribute slightly to the aroma of EHBL due to its low concentration. In addition, hexanol, octanol and nonanol were reported to have grassy and fruity aromas, which were helpful for improving the aroma of food [30]. It was worth noting that some volatile substances including (E)-2-hexadecenal, 2,4-dimethylhexane, 3-hydroxy-2-butanone, 3-ethyl-2-methyl-1,3-hexadiene and 1-nonene-4-ol were only detected in the control. It was speculated that these substances contained branched and unsaturated bonds which were easily damaged by the cavitation effect of ultrasound, and then degraded into other substances.
Most esters are volatile compounds with fruity aroma, which are mainly produced by esterification reaction between acids and alcohols. In this work, only diethyl phthalate with an imperceptible aroma was detected in both EHLBs, indicating its contribution the aroma of EHLB could be neglected. Furans are produced during caramelization and sugar degradation in the Maillard reaction, which were reported to be important aroma compounds in meat products due to their nonnegligible effect on the overall aroma [31]. Thus, 2-pentylfuran was an important odorant detected in both EHBLs.
3.6. Key Odor-active Compounds Identified by OAVs
OVA is an important quantitative index to evaluate the contribution of volatile substance to food aroma [32]. In order to further clarify the influence of sonication on aroma of EHBL, OAVs of aroma-active compounds in the control and sample were measured in this study. As shown in Table 4, 13 volatile odorants including 9 aldehydes, 2 alcohols, 1 acid and 1 furan with OAV ≥ 1 in both EHBLs were identified, of them, aldehydes accounted for 69.23%, which contributed the most to EHBL aroma. As aforementioned, sonication significantly reduced the fishy odor of sample, as shown in Table 4, the concentrations OAVs of compounds with fishy odor, i.e., hexanoic acid and nonanal, were decreased significantly, which decreased by 35.29% and 26.03%, respectively. The possible reason for this phenomenon was that the fishy odor substances were unstable. The aldehydes and the structure of double bonds were destroyed by sonication Furthermore, some aldehydes identified, i.e., octanal, nonanal, (E)-2-octenal, (E,E)-2,4-decadien-1-al, exhibited nutty, butter, burning and fruity aromas would also contributed to the overall aroma of EHBL. Based on the OAVs and FD factors in Table 4, it can concluded that aldehydes were the largest contributors to EHBL aroma, while sonication effectively promoted the production of most aldehydes and reduced the contents of fishy aroma compounds, making EHBL aroma more popular.
For alcohols, only 1-hexanol and 1-octene-3-ol were identified as aroma-active compounds. After sonication, the concentration of alcohols in the sample was increased by 115.88% compared with that in the control. The concentrations and OAVs of 1-hexanol and 1-octanol in the sample increased to some extent, but the concentrations of 1-octen-3-ol in the sample and control were basically the same. Notably, the increase of 1-hexanol in the sample was more significantly, the concentration of 1-hexanol in the sample was 13.4 times of that in the control. As a high molecular weight fatty alcohol, 1-hexanol was described as having fruity and grassy fragrance. 1-octen-3-ol was mainly formed by arachidonic acid through oxidation of lipoxygenase to form mushrooms-like odor [30]. They were important for the richness of EHBL aroma.
Hexanoic acid and heptaldehyde were associated with sourness. In addition, the OAVs of hexadecanal, (E)-2-octenal were relatively low, but they endowed fruity, butter, fatty and nutty aroma to EHBL, making its overall aroma more harmony. Among these key odor-active substances, octanal, (2E)-2-nonenal, trans-2-decenal and (E,E)-2,4-decadien-1-al had higher OAVs, meanwhile, they had high FD factors, suggesting they contributed greatly to the aroma of both EHBLs. Overall, the observed changes in OAVs of the major aroma-active compounds were consistent with the sensory evaluation results in Figure 2.
4. Conclusions
In summary, appropriate sonication (40 kHz, 80 W/L) is a feasible method to improve the flavor of EHBL via enhancing the contents of taste compounds of EHBL and removing the off-odor compounds of EHBL. Therefore, ultrasonic technology has a great application potential in the production of EHBL with high-quality flavor. Currently, research on the biochemical mechanism of sonication improving the flavor of EHBL is ongoing.
Author Contributions
Chao Ye: investigation, data curation, formal analysis, visualization and writing-original draft. Zhankai Zhang: investigation, data curation, formal analysis and writing-original draft. Zhi-Hong Zhang: data curation and formal analysis. Ronghai He: conceptualization, resources, supervision and validation. Xue Zhao: investigation, data curation and formal analysis. Xianli Gao: conceptualization, project administration, methodology, resources, supervision and Writing-review & editing.
Funding
This study was supported by the Primary Research & Development Plan of Jiangsu Province (BE2020329), Key R & D Plan of Shandong Province (2022CXGC010603), Guangdong Provincial Key Laboratory of Advanced Biofermentation Technology Enterprise in Flavoring & Food (2017B030302002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, X.; Yan, S.; Yang, B.; Lu, J.; Jin, Z. Comparison of physicochemical properties of beef potentiators prepared by synergistic fermentation and traditional method. Int. J. Food Sci. Technol. 2013, 48, 1932–1939. [Google Scholar] [CrossRef]
- Gao, X.; Yan, S.; Yang, B.; Lu, J.; Jin, Z. A novel method for beef potentiator preparation and identification of its characteristic aroma compounds. J. Sci. Food Agric. 2013, 94, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Chambers, E., IV; Miller, R.; Vázquez-Araújo, L.; Bhumiratana, N.; Philip, C. Development of A Lexicon for Beef Flavor in Intact Muscle. J. Sens. Stud. 2011, 26, 413–420. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, M.; Fan, H.; Liu, Y. Effect of microwave combined with ultrasonic pretreatment on flavor and antioxidant activity of hydrolysates based on enzymatic hydrolysis of bovine bone. Food Biosci. 2021, 44, 101399. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Ren, X.; Oladejo, A.O.; Lu, F.; Ma, H. Effects and mechanism of ultrasound pretreatment of protein on the Maillard reaction of protein-hydrolysate from grass carp (Ctenopharyngodon idella). Ultrason. Sonochemistry 2020, 64, 104964. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Liu, E.; Yang, M.; Chen, S.; Hu, F.; Ma, H.; Liu, Z.; Yu, X. Enhancing the taste of raw soy sauce using low intensity ultrasound treatment during moromi fermentation. Food Chem. 2019, 298, 124928. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.M.; Lai, O.M.; Abas, F.; Tan, C.P. Effects of sonication on the extraction of free-amino acids from moromi and application to the laboratory scale rapid fermentation of soy sauce. Food Chem. 2017, 215, 200–208. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochemistry 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Ma, H.; Huang, L.; Jia, J.; He, R.; Luo, L.; Zhu, W. Effect of energy-gathered ultrasound on Alcalase. Ultrason. Sonochemistry 2011, 18, 419–424. [Google Scholar] [CrossRef]
- Gao, X.; Cui, C.; Ren, J.; Zhao, H.; Zhao, Q.; Zhao, M. Changes in the chemical composition of traditional Chinese-type soy sauce at different stages of manufacture and its relation to taste. Int. J. Food Sci. Technol. 2011, 46, 243–249. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Yarmand, M.S.; Ghanbarzadeh, B.; Mirzaee, H. Generation of bioactive peptides from lentil protein: degree of hydrolysis, antioxidant activity, phenol content, ACE-inhibitory activity, molecular weight, sensory, and functional properties. J. Food Meas. Charact. 2021, 15, 5021–5035. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, X. L.; Liu, E. M.; Zhang, J. K.; Yang, L. X.; Huang, Q. R.; Chen, S.; Ma, H. L.; Ho, C. T.; Liao, L. Accelerating aroma maturation of raw soy sauce using low intensity sonication. Food Chem. 2020, 329, 127118. [Google Scholar] [CrossRef]
- Ozkara, K.T.; Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. GC-MS-Olfactometric Differentiation of Aroma-Active Compounds in Turkish Heat-Treated Sausages by Application of Aroma Extract Dilution Analysis. Food Anal. Methods 2018, 12, 729–741. [Google Scholar] [CrossRef]
- Takakura, Y.; Sakamoto, T.; Hirai, S.; Masuzawa, T.; Wakabayashi, H.; Nishimura, T. Characterization of the key aroma compounds in beef extract using aroma extract dilution analysis. Meat Sci. 2014, 97, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.-H.; He, R.; Xu, R.; Zhang, L.; Gao, X. Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism. Foods 2022, 11, 2190. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Wang, J.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, T.; Li, J. Combined ultrasound and heat pretreatment improve the enzymatic hydrolysis of clam (Aloididae aloidi) and the flavor of hydrolysates. Innov. Food Sci. Emerg. Technol. 2020, 67, 102596. [Google Scholar] [CrossRef]
- Chen, X.; Luo, Y.; Qi, B.; Luo, J.; Wan, Y. Improving the hydrolysis efficiency of soy sauce residue using ultrasonic probe-assisted enzymolysis technology. Ultrason. Sonochemistry 2017, 35, 351–358. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Tang, S.; Mi, S.; Lu, L.; Zeng, Q.; Xia, M.; Cai, Z. Improved effect of ultrasound-assisted enzymolysis on egg yolk powder: Structural properties, hydration properties and stability characteristics. Food Chem. 2022, 382, 132549. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kerth, C.R.; Miller, R.K. Beef flavor: a review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography–mass spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kitabayashi, K.; Fukusima, C.; Sugiyama, S.; Hashimoto, T. Effect of storage period before reheating on the volatile compound composition and lipid oxidation of steamed meat of yellowtail Seriola quinqueradiata. Fish. Sci. 2015, 81, 1145–1155. [Google Scholar] [CrossRef]
- Wang, H. L.; Yang, P.; Liu, C.; Song, H. L.; Pan, W. Q.; Gong, L. Characterization of key odor-active compounds in thermal reaction beef flavoring by SGC×GC-O-MS, AEDA, DHDA, OAV and quantitative measurements. J. Food Compos. Anal. 2022, 114, 104805. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC–MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef]
- Zhuang, J.; Xiao, Q.; Feng, T.; Huang, Q.; Ho, C.-T.; Song, S. Comparative flavor profile analysis of four different varieties of Boletus mushrooms by instrumental and sensory techniques. Food Res. Int. 2020, 136, 109485. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Sun, Y.; Dong, Y.; Gao, Q. Response of Atlantic salmon (Salmo salar) flavor to environmental salinity while culturing between freshwater and seawater. Aquaculture 2020, 530, 735953. [Google Scholar] [CrossRef]
- Ye, Y.; Ye, S.; Wanyan, Z.; Ping, H.; Xu, Z.; He, S.; Cao, X.; Chen, X.; Hu, W.; Wei, Z. Producing beef flavors in hydrolyzed soybean meal-based Maillard reaction products participated with beef tallow hydrolysates. Food Chem. 2022, 378, 132119. [Google Scholar] [CrossRef]
- Zhang, Z.; Blank, I.; Wang, B.; Cao, Y. Changes in odorants and flavor profile of heat-processed beef flavor during storage. J. Food Sci. 2022, 87, 5208–5224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Y.; Pu, D. D.; Sun, B. G.; Ren, F. Z.; Zhang, Y. Y.; Chen, H. T. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flow chart of beef potentiator preparation.

Figure 2.
Sensory evaluation of the control and sample.

Figure 3.
Effect of sonication on neutral protease activity of EHBL. Annotation: C-EHBL represents the control and S-EHBL represents the sample.
Figure 3.
Effect of sonication on neutral protease activity of EHBL. Annotation: C-EHBL represents the control and S-EHBL represents the sample.
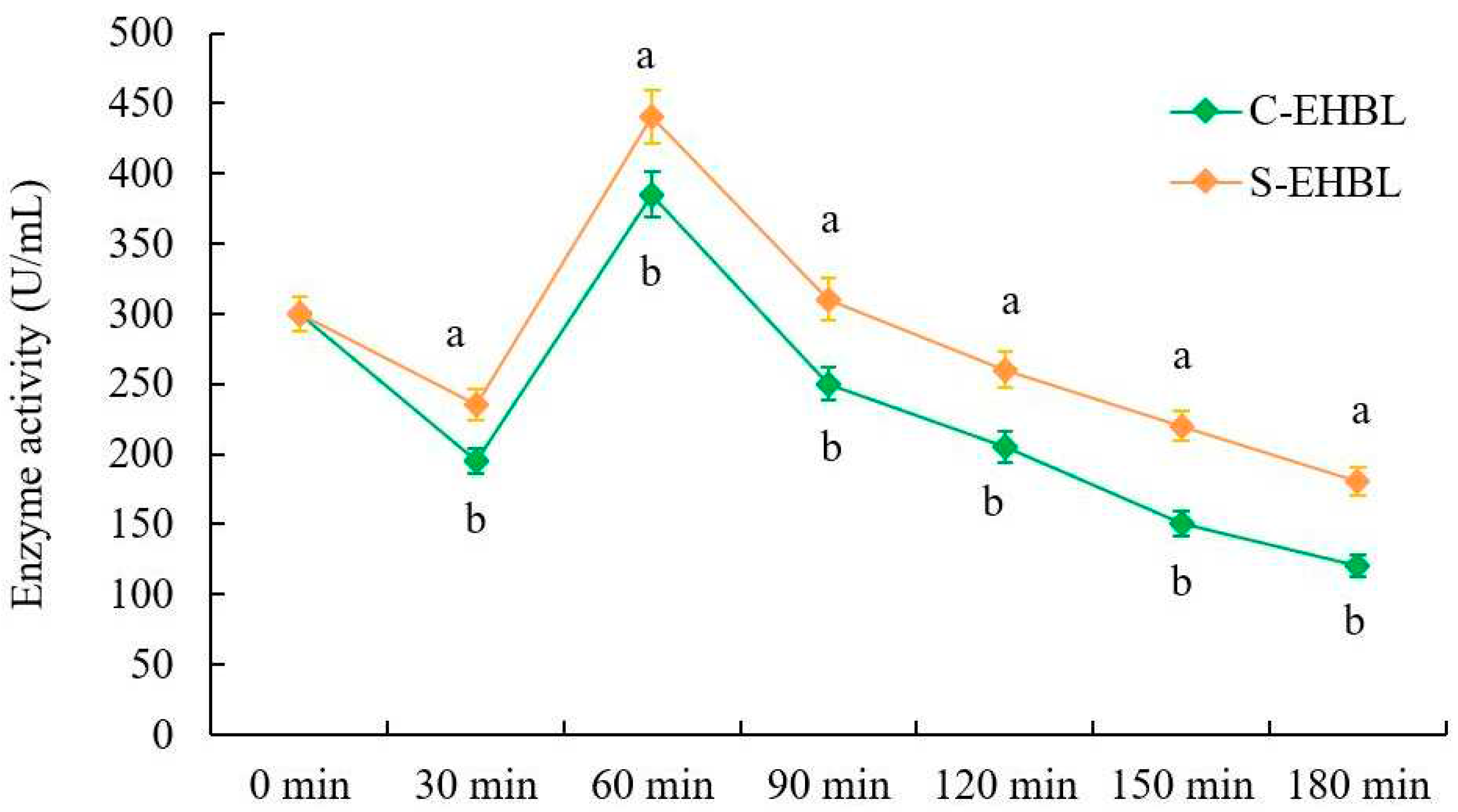
Table 1.
Physicochemical properties of the control and sample.
| Total nitrogen (g/L) |
Formaldehyde nitrogen (g/L) | Hydrolysis degree (%) | Total sugar (g/L) |
Reducing sugar (g/L) |
|
|---|---|---|---|---|---|
| Control | 8.83 ± 0.21b | 1.01 ± 0.01b | 16.33 ± 0.48b | 0.93 ± 0.02b | 0.84 ± 0.02b |
| Sample | 10.53 ± 0.15a | 1.21 ± 0.02a | 19.67 ± 0.51a | 1.04 ± 0.01a | 0.92 ± 0.03a |
Annotation: results are expressed as mean ± standard deviation (n=3); values followed by different letters in the same column indicated significant differences between the data (p < 0.05).
Table 2.
FAAs compositions of the control and sample.
| FAAs | Control (g/L) | Sample (g/L) |
Taste attributes | Thresholds (g/L) | Taste intensities | |
|---|---|---|---|---|---|---|
| Control | Sample | |||||
| Aspartic acid | 0.14 ± 0.01b | 0.25 ± 0.01a | Umami | 0.17 | 0.82 | 1.47 |
| Glutamic acid | 0.43 ± 0.01a | 0.40 ± 0.01a | Umami | 0.075 | 5.73 | 5.33 |
| Umami FAAs | 0.57 | 0.65 | 6.55 | 6.80 | ||
| Serine | 0.35 ± 0.02a | 0.33 ± 0.01a | Sweet | 1.50 | 0.23 | 0.22 |
| Glycine | 0.48 ± 0.03a | 0.52 ± 0.01a | Sweet | 1.30 | 0.37 | 0.40 |
| Threonine | 0.12 ± 0.01b | 0.27 ± 0.02a | Sweet | 5.20 | 0.02 | 0.05 |
| Alanine | 0.75 ± 0.03a | 0.73 ± 0.01a | Sweet | 0.60 | 1.25 | 1.21 |
| Proline | 0.09 ± 0.01b | 0.30 ± 0.01a | Sweet | 3.00 | 0.03 | 0.10 |
| Lysine | 1.16 ± 0.04b | 1.28 ± 0.01a | Sweet | 0.50 | 2.32 | 2.56 |
| Sweet FAAs | 2.95 | 3.53 | 4.22 | 4.54 | ||
| Histidine | 0.16 ± 0.01a | 0.16 ± 0.01a | Bitter | 0.20 | 0.80 | 0.80 |
| Arginine | 0.03 ± 0.01b | 0.12 ± 0.01a | Bitter | 0.50 | 0.06 | 0.24 |
| Valine | 0.37 ± 0.02a | 0.34 ± 0.02a | Bitter | 0.10 | 3.70 | 3.40 |
| Tyrosine | 0.29 ± 0.01b | 0.34 ± 0.01a | Bitter | 0.46 | 0.63 | 0.74 |
| Methionine | 0.37 ± 0.02a | 0.39 ± 0.02a | Bitter | 0.30 | 1.23 | 1.30 |
| Leucine | 1.04 ± 0.05b | 1.15 ± 0.06a | Bitter | 0.95 | 1.09 | 1.21 |
| Isoleucine | 0.32 ± 0.02a | 0.25 ± 0.02b | Bitter | 0.90 | 0.36 | 0.28 |
| Phenylalanine | 0.47 ± 0.04b | 0.59 ± 0.04a | Bitter | 0.23 | 2.04 | 2.57 |
| Bitter FAAs | 3.05 | 3.33 | 9.91 | 10.54 | ||
| Cysteine | 0.39 ± 0.03b | 0.45 ± 0.01a | Tasteless | |||
| Total | 6.96 | 7.96 | 20.68 | 21.88 | ||
Annotation: results are expressed as mean ± standard deviation (n=3); values followed by different letters in the same row indicated significant differences between the data (p < 0.05).
Table 3.
Volatile compounds identified in the control and sample.
| Compounds | CAS | RI (DB-Wax) |
Aroma Description | Identification | |
|---|---|---|---|---|---|
| Control | Sample | ||||
| 1-Pentanol | 71-41-0 | 1257 | Balsamic | nd | MS/RI/O |
| 3-Ethyl-2-pentanol | 609-27-8 | 1452 | un | nd | MS/RI |
| 1-Hexanol | 111-27-3 | 1365 | Resin, flower, green | MS/RI/O | MS/RI/O |
| Heptaldehyde | 111-71-7 | 1178 | Fat, citrus, rancid | MS/RI/O | MS/RI/O |
| (E)-Hept-2-enal | 18829-55-5 | 1245 | Soap, fat, almond | MS/RI/O | MS/RI/O |
| Hexanoic acid | 142-62-1 | 1832 | Sweat | MS/RI/O | MS/RI/O |
| Heptanol | 111-70-6 | 1470 | Fruity, green | MS/RI/O | MS/RI/O |
| 1-Octen-3-ol | 3391-86-4 | 1397 | Mushroom, fishy, grass, fatty | MS/RI/O | MS/RI/O |
| 2-Pentylfuran | 3777-69-3 | 1241 | Green bean, butter, pungent | MS/RI/O | MS/RI/O |
| Dodecane | 112-40-3 | 1210 | Alkane | MS/RI/O | MS/RI/O |
| Octanal | 124-13-0 | 1282 | Fat, soap, lemon, green | nd | MS/RI/O |
| (E)-2-Octenal | 2548-87-0 | 1350 | Meaty, fatty, green, nut, fat | MS/RI/O | MS/RI/O |
| 1-Octanol | 111-87-5 | 1555 | Fruity | MS/RI/O | MS/RI/O |
| 4-Methyl-5-decanol | 213547-15-0 | 1955 | un | nd | MS/RI |
| 1-Nonanal | 124-19-6 | 1387 | Fat, citrus | MS/RI/O | MS/RI/O |
| (2E)-2-Nonenal | 18829-56-6 | 1530 | Cucumber, fat, green | MS/RI/O | MS/RI/O |
| 1-Nonano | 143-08-8 | 1504 | Fat, green | MS/RI/O | MS/RI/O |
| Decanal | 112-31-2 | 1485 | Soap, orange peel, tallow | MS/RI/O | MS/RI/O |
| Trans-2-Decenal | 3913-81-3 | 1592 | Tallow, mushroom | MS/RI/O | MS/RI/O |
| Nonadecane | 629-92-5 | 1911 | Alkane | MS/RI/O | MS/RI/O |
| Undecanal | 112-44-7 | 1649 | Oil, pungent, sweet | MS/RI/O | MS/RI/O |
| (E,E)-2,4-Decadien-1-al | 25152-84-5 | 1715 | Fried, wax, fat | MS/RI/O | MS/RI/O |
| (E)-2-Hexadecenal | 22644-96-8 | 2655 | un | MS/RI | nd |
| Pentadecanal | 2765-11-9 | 2060 | Fresh | nd | MS/RI/O |
| Diethyl phthalate | 84-66-2 | 1711 | un | MS/RI/O | MS/RI/O |
| Docosanal | 57402-36-5 | 2754 | un | nd | MS/RI |
| Hexadecanal | 629-80-1 |
2156 | Strawberry and bayberry like aroma | nd | MS/RI/O |
| 2,4-Dimethylhexane | 589-43-5 | 820 | un | MS/RI | nd |
| 3-Hydroxy-2-butanone | 513-86-0 | 1290 | Butter, cream, milky | MS/RI/O | nd |
| Undecane | 1120-21-4 | 1110 | Alkane | MS/RI/O | nd |
| 3-Ethyl-2-methyl-1,3-hexadiene | 61142-36-7 |
1030 | un | MS/RI | nd |
| 1-Nonen-4-ol | 35192-73-5 | 1657 | un | MS/RI | nd |
| N-heneicosanal | 51227-32-8 | 2846 | un | MS/RI | nd |
Annotation: nd not detected in GC-MS or GC-O; un unavailable; MS, mass spectrometry; RI, retention index; O, olfactometry.
Table 4.
Odor-active compounds identified in the control and sample.
| No. | Compounds | Threshold (μg/kg) | Concentration (μg/kg) | OAV | FD | |||
|---|---|---|---|---|---|---|---|---|
| Control | Sample | Control | Sample | Control | Sample | |||
| 1 | Octanal | 0.70 | 50.25 ± 2.65a | 48.55 ± 1.35b | 71.79 | 69.36 | 32 | 32 |
| 2 | Hexanoic acid | 0.08 | 1.87 ± 0.11a | 1.21 ± 0.10b | 23.38 | 15.13 | 32 | 16 |
| 3 | 1-Hexanol | 5.60 | 3.63 ± 0.1b | 41.32 ± 2.25a | 0.65 | 7.38 | — | 4 |
| 4 | Heptaldehyde | 2.80 | 25.02 ± 1.32b | 32.90 ± 2.51a | 8.94 | 11.75 | 4 | 4 |
| 5 | Nonanal | 1.00 | 78.65 ± 2.3a | 58.18 ± 2.1b | 78.65 | 58.18 | 32 | 16 |
| 6 | Decanal | 0.10 | 2.59 ± 0.04a | 2.78 ± 0.05a | 25.9 | 27.8 | 16 | 16 |
| 7 | Hexadecanal | 0.91 | nd | 2.13 ± 0.11 | nd | 2.34 | nd | 1 |
| 8 | 2-Pentylfuran | 5.80 | 12.56 ± 1.51a | 10.60 ± 1.41a | 2.17 | 1.83 | 1 | 1 |
| 9 | 1-Octen-3-ol | 1.50 | 28.55 ± 1.08a | 28.15 ± 0.95a | 19.03 | 18.77 | 8 | 8 |
| 10 | (E)-2-Octenal | 3.00 | 14.96 ± 0.21a | 14.36 ± 0.18a | 4.99 | 4.79 | 2 | 2 |
| 11 | (2E)-2-Nonenal | 0.08 | 18.54 ± 1.4a | 20.02 ± 1.5a | 231.75 | 250.25 | 128 | 128 |
| 12 | (E,E)-2,4-Decadien-1-al | 0.07 | 7.56 ± 0.2a | 8.11 ± 0.3a | 108.00 | 115.86 | 64 | 64 |
| 13 | trans-2-Decenal | 0.30 | 27.54 ± 1.8b | 31.25 ± 2.2a | 91.80 | 104.17 | 32 | 64 |
Annotation: nd not detected in GC-MS or GC-O; — the odor was not perceived using GC-O on DB-Wax column; for the concentrations of Odor-active compounds, results are expressed as mean ± standard deviation (n=3); values followed by different letters in the same row indicated significant differences between the data (p < 0.05).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








