Preprint
Article
Discovery of Antimicrobial Peptides That Can Accelerate the Diagnostics of Slow-Growing Mycobacteria Including Mycobacterium Tuberculosis by Culture
Altmetrics
Downloads
180
Views
68
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
21 July 2023
Posted:
26 July 2023
You are already at the latest version
Alerts
Abstract
Antimicrobial peptides (AMPs) can directly kill Gram-positive bacteria, Gram-negative bacteria, mycobacteria, fungi, enveloped viruses and parasites. At sublethal concentrations some AMPs and also conventional antibiotics can stimulate bacteria response to increase their resilience, also called the hormetic response. That can include stimulation of growth, mobility, and biofilm production. Here, we have discovered AMPs that stimulate the growth of certain mycobacteria. Peptide 14 for example showed growth stimulating effect on M. tuberculosis, M. bovis, M. avium subsp. paratuberculosis (MAP), M. marinum, M. avium-intracellulare, M. celatum and M. abscessus. The effect was more pronounced at low bacterial inocula. The peptides induce a faster transition from the lag phase to the log phase and keep the bacteria longer in the log phase before entering the stationary phase compared to non treated control. In some cases, an increase in the division rate was observed. An initial screen using MAP and a collection of 75 peptides revealed 13 peptides with a hormetic effect. For M. tuberculosis a collection of 25 artificial peptides were screened and 9 were found to reduce the time to positivity (TTP), improving growth. A screen of 43 naturally occurring peptides, 11 fragments of naturally occurring peptides and 5 designed peptides, all taken from the APD3, identified a further 44 peptides that also lowered TTP by at least 5%. Lasioglossin LL-III (Bee) and Ranacyclin E (Frog) were the most active natural peptides, the human cathelicidin LL37 fragment GF-17 and a porcine cathelicidin protegrin-1 fragment was the most active fragment of naturally occurring peptides. Peptide 14 showed growth-stimulating activity between 50 ng/ml and 10 µg/ml whereas the stability-optimised peptide 14D had a narrow activity range of 0.1 - 1 µg/ml. Peptides identified in this study are now part of novel products improving the diagnosis of mycobacteria in humans and animals.
Keywords:
Subject: Biology and Life Sciences - Immunology and Microbiology
1. Introduction
Mycobacterium tuberculosis (MTB) is the primary causal agent of tuberculosis in humans. MTB belongs to the M. tuberculosis complex (MTBC) which includes the closely related species M. bovis, M. africanum, M. microti, M. pinnipedii, M. caprae, and M. canetti. The slow-growing M. tuberculosis divides every 16-20 hours [1]. According to the Centres for Disease Control and Prevention (CDC), a fourth of the world’s population is infected with TB [2]. The World Health Organization (WHO) estimates 10.6 million TB cases in 2021 causing 1.6 million TB-related deaths worldwide (WHO, 2023). TB is a leading killer among HIV-infected people with weakened immune systems. In recent years the treatment of TB has become more challenging due to the emergence of drug-resistant strains (MDR- and XDR-TB).
Mycobacterium avium subsp. paratuberculosis (MAP) causes Johne’s disease, a chronic enteritis that can lead to severe economic losses in sheep, goat and dairy cattle farms. The disease has also been reported in horses, pigs, deer, alpacas, llamas, rabbits, stoats, foxes, and weasels [4]. The huge excretion of MAP from clinically diseased animals contaminates milk and meat supplies and is detected in pasteurised milk (including infant milk formula) and pastureland wash-offs which carry over through treatment plants to remain viable in potable water supplies.
Antimicrobial peptides (AMPs), also called host defence peptides, represent a ubiquitous response in nature to overcome microbial infections or to compete for an ecological niche [5]. The antimicrobial activities can include actions against Gram-negative and Gram-positive bacteria, including mycobacteria, fungi and enveloped viruses [6,7,8,9,10]. Of particular interest is their ability to kill multi-drug-resistant bacteria [11]. Within the last two decades, it has become increasingly clear that various antimicrobial peptides play a role in regulating the process of innate immunity. It has been reported that some AMPs can have direct and indirect chemotactic functions, regulate chemokine and cytokine production and promote wound healing [12,13,14,15,16,17]. With the multitude of cationic peptide sources, structures and spectra of activity come a number of complex and controversial structure-function theories attempting to describe and explain peptide modes of interaction that kill bacteria [18]. Most AMPs bind avidly to the lipopolysaccharides (LPS) of Gram-negative bacteria and lipoteichoic acids of Gram-positives, and subsequently associate with and depolarise and/or permeabilize cytoplasmic membranes [19]. In many cases, the peptide translocates into the microbial cell and attacks internal targets. AMPs can therefore affect very different processes and most likely have often not one target, but multiple. It has been reported that some AMPs bind to lipid II, DNA and RNA, chaperons and ribosome, proteases, ATP and ABC transporter [18,20,21,22,23,24,25,26,27,28]. At sublethal concentrations, some AMPs and antibiotics in general can stimulate growth, mobility, frequency of mutation and plasmid conjugative transfer [29]. For example, GL13K peptides have shown growth stimulation and an increase in metabolic activity for Pseudomonas aeruginosa [30].
WHO estimates about 60 million tests for human TB are done per year. The most common test is a microscopic evaluation of stained sputum smears which is simple, rapid and inexpensive. However, not only is the sensitivity of this method compromised when the bacterial load is low (less than 1x104 organisms/ml), but it also performs poorly in extra-pulmonary tuberculosis, paediatric tuberculosis and patients co-infected with HIV [31,32,33]. Other rapid tests like GeneXpert and IGRAs are now available but have their limitations. Limitations are a) DNA tests are susceptible to inhibitors in some samples, are also still prone to false positive reporting and also can only detect antibiograms through SNP’s that are known (many are not). B) IGRA cannot differentiate between active and latent infection and is not useful in some children, immunocompromised and also early acute infection. Culturing M. tuberculosis is the gold standard because it can identify and quantify bacterial load and allow antibiotic sensitivities or strain typing to be tested. However, worldwide only 3-4 million samples are sent for culture per year. This is due to the need for infrastructure and training, cost and the long-held perception that MTB grows too slowly to help initial diagnosis.
2. Methods
2.1. Peptides
From the 139 peptides used in the study, 2 peptides were purchased at Bachem (Germany), 35 at Umpep (Germany), and 40 at Kinexus Bioinformatics Corporation (Canada). 62 peptides were synthesized in our laboratory by automated solid-phase peptide synthesis (SPPS) on a MultiPep RSI peptide synthesizer (Intavis, Tuebingen; Germany) using the 9-fluorenyl-methoxycarbonyl-tert-butyl (Fmoc/tBu) strategy. Reactive side chains were protected by tBu (Tyr and Asp), trityl (Trt, for Asn, Cys, Gln and His), 2,2,4,6,7 pentamethyldihydrobenzofuran-5-sulfonyl (Pbf, for Arg) and tert-butoxycarbonyl (Boc, for Lys and Trp). For automated SPPS four equivalents of Fmoc amino acids (Bachem, Bubendorf, Switzerland) were coupled on TentaGel® HL RAM resin (25 μmol scale, loading 0.3- 0.4 mmol/g, Rapp Polymere, Tuebingen, Germany) after in situ activation with four equivalents of N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU; Carbosynth, Berkshire, United Kingdom) and eight equivalents of N-Methylmorpholine (NMM, Sigma, Dorset, United Kingdom). After a double coupling procedure (2x30 min) the Fmoc group was cleaved using 20% (v/v) piperidine (Thermofisher Acros Organics, Geel, Belgium) in dimethylformamide (DMF, Jencons-VWR, Leicestershire, United Kingdom). Peptide amides were cleaved from the resin with 95% (v/v) aqueous trifluoroacetic acid solution (TFA, Fisher Scientific, Loughborough, United Kingdom) containing 5% (v/v) triisopropylsilane (TIPS, Thermofisher Acros Organics, Geel, Belgium) / water (1:1) scavenger mixture within 3 h. Cleaved peptides were precipitated from ice-cold methyl tert-butyl ether (MTBE; Thermofisher Acros Organics, Geel, Belgium). After washing and collection by centrifugation crude peptides were dissolved in 20% (v/v) acetonitrile (ACN, Jencons-VWR, Leicestershire, United Kingdom) / 80% (v/v) water containing 1% (v/v) TFA to a concentration of 15 mg/ml and analysed by analytical reversed-phase (RP) HPLC on a Shim-pack VP-ODS (120 Å, 150x4.6 mm, Shimadzu, Milton Keynes, United Kingdom) using a Shimadzu LC2010AHT system. The binary solvent system contained 0.1% (v/v) TFA in H2O (solvent A) and 0.1% (v/v) TFA in acetonitrile (solvent B). The identity was verified by a liquid chromatography-electrospray ionisation mass spectrometry (LC-ESI-MS) Shimadzu LC2020 system equipped with a Jupiter 4μ Proteo C18 column (90 Å, 250x4.6 mm, Phenomenex, Cheshire, United Kingdom). The binary solvent system contained 0.01% (v/v) TFA in H2O (solvent A) and 0.01% (v/v) TFA in acetonitrile (solvent B).
Selected crude peptides were purified to the homogeneity of >92% by preparative RP HPLC on a Shimadzu LC2020 system equipped with a Jupiter 10μ Proteo C18 column (90 Å, 250x21.2 mm, Phenomenex) using a linear gradient system containing 0.01% (v/v) TFA in H2O (solvent A) and 0.01% (v/v) TFA in acetonitrile (solvent B). Pure products were finally characterized by analytical RP-HPLC and LCMS.
2.2. Mycobacterial culture
The following strains were used: Mycobacterium marinum NCTC 2275, Mycobacterium branderi (clinical human isolate), Mycobacterium intracellulare serotype 7 NCTC10682, Mycobacterium celatum (clinical isolate), Mycobacterium gastri ATCC25158, Mycobacterium abscessus (clinical human isolate), Mycobacterium porcinum strain L53 (buffalo milk isolate), Mycobacterium fortuitum ATCC6841, Mycobacterium vaccae NCTC10916, Mycobacterium smegmatis (laboratory strain MC2155), Mycobacterium avium subspecies paratuberculosis (MAP, cattle isolate), Mycobacterium tuberculosis (laboratory strain H37Rv). All mycobacterial species used in this study were grown in Middlebrook 7H9 media (BD, UK) with 0.2% glycerol (Sigma, UK), 0.1% casitone (Oxoid, UK) and 10% OADC (Becton Dickinson, UK) at 37°C (except M. marinum which was grown at 30°C). For MAP, Mycobactin J (IDVet, France) was added to the above growth media (final 2µg/ml). Initial screening assays with peptides were carried out in 1.6ml polystyrene semi-micro cuvettes (Fisherbrand, UK) with lids sealed with parafilm. Growth was measured by estimating optical density using a spectrophotometer every 2 days. Further confirmatory analysis was carried out in BACTEC MGIT 320 automated mycobacterial detection system using 7ml MGIT (Becton Dickinson, UK) mycobacterial culture tubes, supplemented with 0.8ml OADC/PANTA growth supplement prepared according to manufacturer recommendations.
2.3. Peptide screening
Initial assays to screen for peptide activity on mycobacterial growth used 1 ml culture of Middlebrooks 7H9 (Difco, UK) plus 10% OADC (Becton Dickinson, UK) and 2 ug/ml Mycobactin J (IDVet, France) inoculated to 0.1 OD600 from exponential growth starter cultures in semi-micro cuvettes (Fisherbrand, UK) with lids sealed with parafilm. Growth (37°C) was measured by estimating optical density (600nm) every 2 days. Further confirmatory analysis was carried out in BACTEC MGIT 320 automated mycobacterial detection system using 7ml MGIT (Becton Dickinson, UK) mycobacterial culture tubes, supplemented with 0.8ml OADC/PANTA growth supplement prepared according to manufacturer recommendations being inoculated as previously with exponential starter cultures and peptides up to various test final concentrations. Growth curves were derived by retrieving growth index values, based on the relative fluorescence of a proprietary oxygen depletion indicator, each day from an automated reader.
2.4. Quantitative PCR
qPCR was performed using (company name) machine with mycobacteria-specific Taqman primers cydA-F (5’ GACCACCAAGGCAAGCTGA 3’), cydA-R (5’ TCGCACAACGATTCGGC 3’) and labelled probe cydA-P (5’ FAM-CATCTTCATCGGCTGCTGCTGGAA-TAMRA 3’). Conditions used were 98°C for 2 min; 58°C for 30 sec; 72°C for 10 min. M. tuberculosis H37Rv was grown as detailed above and incubated in the presence (1µg/ml, 10µg/ml and 50µg/ml) or absence of peptides. All samples were done in duplicates, incubated in the MGIT system and removed only when the control (without peptide) went positive. Sterile water was used as a negative control. The culture tubes were centrifuged at 10,000 xg for 10 min and DNA was extracted using a Qiagen DNeasy blood and tissue kit. M. tuberculosis H37Rv standards were used to confirm copy number. M. tuberculosis standards were used to confirm the copy number for all experimental samples.
3. Results
3.1. Initial screen against Mycobacterium avium subsp. paratuberculosis (MAP)
The first screen was originally designed to identify peptides that could inhibit the growth of Mycobacterium avium subsp. paratuberculosis (MAP) as a starting point for the development of an antimicrobial drug candidate. A collection of 75 peptides from various projects was created, that had already shown antimicrobial activity against other microbes [10,34,35,36]. We expected to find two classes of peptides, 1) peptides that did not affect growth with a growth curve similar to the control and 2) peptides with antimicrobial activity with reduced growth resulting in a growth curve below the control. Of the 75 peptides screened at a concentration of 20 µg/ml, 41 peptides did not change the growth curve and 21 peptides showed growth inhibition. Unexpectedly, however, the results revealed a third class of peptides where the growth in presence of peptides increased compared to the control, indicating growth promotion. In total 13 peptides were identified with a growth promotion effect, peptides 14 and 69 among showing the strongest effects, see Table S1 A-C in the supplement. Peptide 102, a version of peptide 69 with a C-terminal modification showed a slightly reduced effect compared to peptide 69. The experiment was repeated with a smaller number of peptides to confirm this unexpected effect, see Figure 1.
As a consequence of this discovery, a series of experiments were performed to study what peptides can induce such growth stimulation and at what concentrations, see Figure 2.
This data showed that the inclusion of any cationic peptide was insufficient to cause an effect. There is a peptide sequence depended activity occurring. A comparison of peptide sequences from each different activity class (reduced growth, normal growth and accelerated growth) revealed that small changes in the primary structure can result in a strong effect on their biological activity. The primary structures of three 12mer peptides are presented in Figure 3. Each of these peptides belongs to one of the three activity classes.
3.2. Test of growth-enhancing activity in other mycobacterial species and inoculum effect
Peptide 14 (WKIVFWWRR- CONH2) was selected and tested for growth-enhancing activity in other mycobacterial species, including slow-growing mycobacterial species Mycobacterium marinum, Mycobacterium branderi, Mycobacterium avium-intracellulare and Mycobacterium celatum and fast-growing mycobacterial species Mycobacterium gastri, Mycobacterium abscessus, Mycobacterium porcinum, Mycobacterium fortuitum, Mycobacterium vaccae and Mycobacterium smegmatis. Peptide 14 was added to the cultures at 20 µg/ml and the OD600 was measured over time. The results in Figure 4 show that Peptide 14 was able to enhance the growth of most slow-growing mycobacteria (except Mycobacterium branderi) and one fast-growing mycobacterium (Mycobacterium abscessus).
Peptide 14 was then tested for growth-enhancing effects in relation to the starting inoculum and time point of treatment. An exponentially growing culture of MAP was sub-cultured and regrowth was monitored. Peptide 14 was added at 20 µg/ml directly after the dilution or after 3 days, see Figure 5. The degree of effect from the peptide was dependent on the size of the starting inoculum, with the lower inocula being more greatly affected. Improved growth stimulation through the addition of peptide during the growth phase was also demonstrated (Day 3).
3.3. Peptides stabilized against protease cleavage
During many days of mycobacterial culture growth enhancing peptides are exposed and possibly degraded by secreted and cell-surface-located proteases expressed by the bacteria themselves. We thus tested whether several modifications to increase the proteolytic stability of the peptides including a stereo-enantiomer of Peptide 14 showed any further growth effect. Peptide 14D (wkivfwwrr-CONH2), the retro-inverse sequence 14D-rev (rrwwfvikw-CONH2) and a version of Peptide 14 where proteolytic sensitive residues (arginine and lysine) were exchanged against ornithine (O) resulting in Peptide 14L-KR (WOIVFWWOO-CONH2) were synthesized [37]. In addition, the stereo-enantiomer of Peptide 69, Peptide 69D (rrwrivvirvrr-CONH2) and an ornithine exchange variant Peptide 69L-R (OOWOIVVIOVOO-CONH2) were synthesized and tested using MAP, see Figure 6. Except for peptide 14L-KR, the growth-enhancing activity remained, with peptide 14D and 14D-rev even showing some small improvement in activity, especially the longer the experiment lasted. This indicates that the L-peptide might be inactivated by proteases whereas the D-peptide continues to act, with a difference seen from day 15 onwards.
Peptide 14D was then used to test if growth enhancement was retained after the peptide was removed. MAP was grown with and without Peptide 14D at 20 µg/ml and the growth was monitored by absorbance measurement at 600 nm, see Figure 7. After 20 days the Peptide 14D treated sample was intensively washed, diluted and split into 2 samples. One was again exposed to Peptide 14D at 20 µg/ml, whereas the other was given new peptide-free media. Washed samples reverted to growth curves similar to the control showing that growth stimulation by Peptide 14D was reversible.
3.4. Peptides tested on Mycobacterium tuberculosis
With an estimated 10.6 million TB cases in 2021 causing 1.6 million TB-related deaths worldwide (WHO, 2023) it is undoubtfully clear that new diagnostics and treatments are of utmost importance. Therefore, the next question to answer was if those peptides can also affect the growth of Mycobacterium tuberculosis (MTB) and if yes, can they improve the diagnostics of MTB? Because of the slow growth of this organism the time to positivity (TTP) in a clinical sample is very high compared to other bacteria. For example, one study reported an average TPP of 14 days using the Mycobacterium Growth Indicator Tube (MGIT) system (Becton Dickinson, Allschwil, Switzerland), with all positive samples detected within 28 days [38]. We aimed to identify peptides that could reduce the TTP in a commercial MTB test. In consequence, the first screen was performed by using the MGIT system.
A subset of peptides that were tested against MAP was also tested for their effect on Mycobacterium tuberculosis (MTB). In this assay, MGIT was used in a BD BACTEC MGIT320 System. Oxygen depletion by bacterial growth activates the fluorescence of a special dye in the MGIT which can be monitored over time as Growth Units, see Figure 8. A similar activity pattern was observed with MTB as had been with MAP. Out of 27 peptides screened at 10 µg/ml, two peptides showed slight improvements (Peptide 69 and Peptide 14), 12 showed similar activity as the control and 13 peptides showed less growth than the control.
In further experiments, dose dependency was measured for a selected set of peptides, see Figure 9. Peptide 14D and Peptide 14Drev were found to be more toxic towards M. tuberculosis compared to Peptide 14, but at lower concentrations (1µg/ml for Peptide 14D and 0.5 µg/ml for Peptide 14Drev) a strong sustained growth stimulation was observed. Peptide 14 showed a wide concentration range with growth stimulation between 50 ng/ml to 10 µg/ml whilst Peptide 69D also showed toxicity at higher concentrations and growth-stimulating activity between 10 ng/ml and 1 µg/ml.
To further confirm the effect of 14D on M. tuberculosis, a quantitative analysis of copy number was performed. As DNA was extracted when M. tuberculosis control went positive, it gave further insight into the effect of the peptide on M. tuberculosis growth in comparison to the control. This showed that M. tuberculosis spiked with 14D at 1 µg/ml not only had gone positive on the MGIT system before the control but also had a higher copy number. This correlates with our hypothesis that 14D at this concentration accelerates growth.
Where culturing of MTB is a very slow method it is currently the only one to allow the determination of any drug resistance. Drug resistance is becoming a problem in the effective treatment of TB and therefore culturing TB is still critical. It was important to check if the peptide also affects multi-drug resistant MTB. The effect of peptide 14D at 1 µg/ml was then tested on a multi-drug resistant strain of M. tuberculosis, see Figure 11. Indeed, peptide 14D could affect the growth of multi-drug resistant MTB, especially by reducing the lag time before growth starts.
After those encouraging results, the next set of experiments was designed to show whether peptides from our library (most of them designed) and naturally occurring peptides can reduce the Time To Positivity (TTP) using standard automated MGIT using a BD BACTECMGIT 320 system used for clinical testing, see Table 1. That would open up the possibility to develop them into enhancers of MTB diagnostics by culture. Since the effect of the peptides depends on the inoculum, two inocula sizes were used and experiments were performed in duplicate. Nine out of 25 tested peptides showed consistently lower TTPs, 36%. As observed before, small changes in the sequence led to strong effects, for example, peptides 59-61 showed strong effects, whereas very similar peptides (62 and 63) delayed TTP in low inocula. Similarly, peptides 64-72 share a single motif, but only Peptide 69 lowered TTP consistently. The human cathelicidin LL37 showed lower TTP as well.
3.5. Test of natural peptides against M. tuberculosis
A screen was performed with naturally occurring peptides (n=43, crude peptides), synthetic fragments of naturally occurring peptides (n=11, crude) and short-designed peptides (n=5, crude) taken from the ADP3 (http://aps.unmc.edu/AP/) and confirmed for their antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) (unpublished results). In two independent experiments, their effect on TTP of M. tuberculosis was determined, inoculated from an exponentially grown stock culture and emulsified before inoculation by passing through a G25 needle, see Table 2. Taking into consideration experiment 1 and experiment 2, see Table 2, 44 peptides showed an average faster TTP larger than 5%. Nine peptides showed a faster TTP of 10% or more. All of these peptides showed a consistent effect between the two screens, however, the protegrin-1 fragment, where the last two amino acids are omitted, showed a very strong effect only in the second screen. Full-length Protegrin-1 shows antibacterial as well as anticancer activity [39,40]. Lasioglossin LL-III, isolated from the eusocial bee Lasioglossum laticeps can kill Gram-positive, and Gram-negative bacteria and various cancer cells, however, it is only mildly toxic against human cells. An internal target was suggested and it was shown that the peptide does not disrupt the membrane (non-lytic) [41,42]. The full-length peptide Ranacyclin E from the frog skin of Rana esculenta [43] is active against Gram-positive bacteria, some Gram-negative like Yersinia pseudotuberculosis and some fungi. GF-17 is a fragment of the human antimicrobial peptide LL37 a cathelicidin, called GF-17. This fragment was described with strong antibacterial and antiviral activity [44,45]. The full-length peptide LL37 showed a similar but less pronounced effect, see Table 1, Peptide 78.
Peptide 14D was selected to test the concentration effect on the TTP in MGIT using a BD BACTECMGIT 320 system, see Table 3. The peptides showed faster TTP values within a concentration range between 0.1 and 1 µg/ml, whereas 1 µg/ml had the strongest effects. At 50 µg/ml, a strong delay of growth is observed. The data correlate well with the growth observed over 20 days, see Figure 9.
Peptide 14D was used to check if other machines that are routinely used in medical microbiology for the detection of mycobacteria show shorter TTP as well. BacT/Alert (Biomerieux, France) and VersaTrek (ThermoFisher, US) were tested and shorter TTP was shown for both systems, see Figure S1 in the supplement.
4. Discussion
Antimicrobial peptides are naturally occurring molecules in microbes, plants and animals that perform a variety of different biological functions including antimicrobial and immunomodulatory activities. By screening short artificial peptides for their antibacterial activity against the very slow fastidious pathogen Mycobacterium avium subspecies paratuberculosis (MAP) we discovered 13 peptides that had growth-stimulating activity with Peptide 14 and Peptide 69 among the peptides showing the strongest effects, see Table S1 and Figure 1. A comparison of peptide sequences revealed that small changes in the sequence could induce dramatically different effects on growth. For example, the peptides in Figure 3 show three 12mer peptides with the conserved core motif RIVVIR found in the bovine AMP bactenecin. Peptide 70 (RLARIVVIRVRR-CONH2) has no activity on the growth of MAP. Changes of only two amino acids at the N-terminal region increased positive charge (R) and increased hydrophobicity as well as the ability to integrate into membranes (W) consequently showing an acceleration of growth of MAP, Peptide 69 (RRWRIVVIRVRR-CONH2). Reducing the positive charge (A) and increasing hydrophobicity and ability to interact with membranes (A, W) at the C-terminal region showed a reduction of growth in MAP, Peptide 72 (RLARIVVIRWAR-CONH2). Further studies revealed that Peptide 14 displayed growth stimulation in both slow and some selected fast-growing mycobacterial species. Growth stimulation was strongest in low-load inocula and was reversible (Figure 5 and Figure 7). Modifications of peptides 14 and 69 showed that the stereoisomer peptide 14D, peptide 14Drev and peptide 69D (all containing only D-amino acids) further increased growth stimulation slightly, indicating interactions were not stereo-selective (Figure 6, 9 and Table 3). Further studies using Mycobacterium tuberculosis identified the same two peptides (14 and 69) that improved growth. Dose dependency of Peptide 14 showed a large range of activity (50 ng/ml and 10 µg/ml) of the growth stimulatory effect. All tested D-peptides showed a smaller activity range for growth stimulatory effect and in addition revealed a stronger antimicrobial effect at higher concentrations, see Figure 8 and Table 3. Peptide 14D stimulated the growth of both antibiotic-sensitive and multi-drug resistant Mycobacterium tuberculosis similarly.
Nine artificial peptides were identified that consistently lowered TTP for M. tuberculosis using culture techniques and cut-offs in line with routine diagnostic protocols. In addition, we identified 44 peptides from the ADP3 that lowered TTP for MTB growth by 5-15.3% and 9 peptides which lowered TTP by 10% or more (Table 1 and Table 2).
We observed that peptides are active as low as 10 ng/ml, we thus hypothesise that these types of peptides are sensed by mycobacteria and in slow-growing mycobacteria in particular induce a hormetic response. It is well described that Gram-positive and Gram-negative bacteria can sense antimicrobial peptides, for example, PhoP/PhoQ a two-component sensor/regulator system in Salmonella typhimurium and Aps in Staphylococcus epidermidis [46]. We have observed three changes in the growth of the mycobacteria tested. The first change is the faster transition from the lag phase into the log phase compared to untreated cells. Lower inoculation sizes tend to have a longer lag phase and therefore the effects of the peptide are most pronounced at lower inoculum sizes. The second change observed is a faster decision rate, which was more pronounced with MAP and not consistently seen with Mycobacterium tuberculosis. Further research is needed to investigate this in more detail. These two changes give hope that those peptides can indeed be used to improve mycobacterial diagnostics by culture. The third change observed was a longer continuation of the log phase compared to the untreated control, indicating higher cell numbers at the end of the growth phase. These could be interesting for research purposes. In Figure 12 all three changes are presented in two modelled growth curves. Further results for the mode of action will be published in a separate paper.
The WHO estimates that about 3-4 million culture tests are performed worldwide to grow Mycobacteria, especially M. tuberculosis. Phenotypic antibiograms require culture and provide the most accurate way to determine resistance and inform on management therapy. Our discovery that the strongest effect of these peptides occurs at very low bacterial loads suggests that the sensitivity of culturing Mycobacterium tuberculosis could be improved. In consequence, this discovery was the platform for the product development of a growth enhancer for different mycobacteria. These products have been introduced to the market. A clinical study evaluating growth-enhancing peptides in combination with a new sample preparation procedure has been performed and showed higher sensitivity (publication will be submitted separately). Further work is needed to deduce the full mechanism of action of these agents and their possible role in the recovery of latent, viable unculturable forms of pathogenic mycobacteria.
We conclude that some natural and artificial cationic antimicrobial peptides demonstrate a hormetic effect on mycobacteria, mainly on slow-growing mycobacteria. Whereas a range of different sequences shows activity, small changes in the sequence can destroy the activity. Inverse and retro-inverse peptides show activity as well, but at a smaller concentration range and displaying stronger toxicity. The hormetic effect is dose-dependent and occurs already at a concentration of 10ng/ml. In addition, the effect is dependent on the starting inocula, the lower the inocula the greater the effect. Different equipment that is routinely used in medical microbiology for the detection of mycobacteria were tested with selected peptides and all showed improvement in the time to positivity. Different peptides were developed further into novel products for improved diagnostics of mycobacteria that have already reached the market.
Author Contributions
KH: intellectual design, data analysis, funding support, writing and editing the manuscript. TM: data collection and data analysis, editing the manuscript, PL: peptide synthesis and characterisation, editing the manuscript, JS: data collection and analysis, SH: peptide synthesis and characterisation, editing the manuscript, TB: intellectual design, data analysis, funding support, editing the manuscript.
Funding
This study was funded by a grant from SBRI, UK, Phase I & II Healthcare: Better Health Outcomes Award and InnovateUK, development grant TS/M009068/1.
Acknowledgments
KH thanks life for the opportunity to get a chance despite the odds to be able to continue researching and consequently writing this manuscript.
Conflicts of Interest
Several peptides described in this manuscript are patented. Tim Bull and Kai Hilpert are founders and directors of TiKa diagnostics.
References
- Cox, R.A. Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: an integrative theoretical approach. Microbiology 2004, 150, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- CDC Data and Statistics on TB. Available online: https://www.cdc.gov/tb/statistics/default.htm (accessed on Nov 1, 2018).
- WHO Tuberculosis - Key facts. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on Nov 1, 2018).
- Stevenson, K.; Alvarez, J.; Bakker, D.; Biet, F.; de Juan, L.; Denham, S.; Dimareli, Z.; Dohmann, K.; Gerlach, G.F.; Heron, I.; et al. Occurrence of Mycobacterium avium subspecies paratuberculosis across host species and European countries with evidence for transmission between wildlife and domestic ruminants. BMC Microbiol. 2009, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Patrzykat, A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets. Infect. Disord. 2002, 2, 79–83. [Google Scholar] [CrossRef]
- Hancock, R.E. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Mania, D.; Hilpert, K.; Ruden, S.; Fischer, R.; Takeshita, N. Screening for antifungal peptides and their modes of action in Aspergillus nidulans. Appl. Environ. Microbiol. 2010, 76. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M. Minidefensins and other antimicrobial peptides: candidate anti-HIV microbicides. Expert Opin. Ther. Targets 2003, 7, 329–341. [Google Scholar] [CrossRef]
- Silva, J.P.; Appelberg, R.; Gama, F.M. Antimicrobial peptides as novel anti-tuberculosis therapeutics. Biotechnol. Adv. 2016, 34, 924–940. [Google Scholar] [CrossRef]
- Ramón-García, S.; Mikut, R.; Ng, C.; Ruden, S.; Volkmer, R.; Reischl, M.; Hilpert, K.; Thompson, C.J. Targeting mycobacterium tuberculosis and other microbial pathogens using improved synthetic antibacterial peptides. Antimicrob. Agents Chemother. 2013, 57. [Google Scholar] [CrossRef]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Curr. Med. Chem. 2017, 24, 4303–4314. [Google Scholar] [CrossRef]
- Territo, M.C.; Ganz, T.; Selsted, M.E.; Lehrer, R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 1989, 84, 2017–20. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Iwabuchi, K.; Matsuda, H.; Ogawa, H.; Nagaoka, I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 2002, 14, 421–6. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004, 172, 4987–94. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007, 178, 1829–34. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Sørensen, O.; Ståhle-Bäckdahl, M.; Kratz, G.; Weber, G.; Borregaard, N. The Cathelicidin Anti-Microbial Peptide LL-37 is Involved in Re-Epithelialization of Human Skin Wounds and is Lacking in Chronic Ulcer Epithelium. J. Invest. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Escámez, M.J.; García, M.; Duarte, B.; Holguín, A.; Retamosa, L.; Jorcano, J.L.; Río, M. del; Larcher, F. In vitro and In vivo Wound Healing-Promoting Activities of Human Cathelicidin LL-37. J. Invest. Dermatol. 2008, 128, 223–236. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–23. [Google Scholar] [CrossRef]
- Hilpert, K.; McLeod, B.; Yu, J.; Elliott, M.R.; Rautenbach, M.; Ruden, S.; Bürck, J.; Muhle-Goll, C.; Ulrich, A.S.; Keller, S.; et al. Short cationic antimicrobial peptides interact with ATP. Antimicrob. Agents Chemother. 2010, 54. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta - Biomembr. 2009, 1788, 1564–1569. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takeshima, K.; Park, C.B.; Kim, S.C.; Matsuzaki, K. Interactions of the novel anfimicrobial peptide buforin 2 with lipid bilayers: Proline as a translocation promoting factor. Biochemistry 2000, 39, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Krajewski, K.; Lee, H.-F.; Antony, S.; Johnson, A.A.; Amin, R.; Roller, P.; Kvaratskhelia, M.; Pommier, Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006, 34, 5157–5165. [Google Scholar] [CrossRef]
- Carlsson, A.; Engström, P.; Palva, E.T.; Bennich, H. Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect. Immun. 1991, 59, 3040–5. [Google Scholar] [CrossRef]
- 26. Mardirossian, M.; Sola, R.; Beckert, B.; Collis, D.W.P.; Di Stasi, A.; Armas, F.; Hilpert, K.; Wilson, D.N.; Scocchi, M. Proline-rich peptides with improved antimicrobial activity against E. coli, K. pneumoniae and A. baumannii. ChemMedChem 2019.
- Mardirossian, M.; Sola, R.; Beckert, B.; Valencic, E.; Collis, D.W.P.; Borišek, J.; Armas, F.; Di Stasi, A.; Buchmann, J.; Syroegin, E.A.; et al. Peptide Inhibitors of Bacterial Protein Synthesis with Broad Spectrum and SbmA-Independent Bactericidal Activity against Clinical Pathogens. J. Med. Chem. 2020, 63, 9590–9602. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Pérébaskine, N.; Benincasa, M.; Gambato, S.; Hofmann, S.; Huter, P.; Müller, C.; Hilpert, K.; Innis, C.A.; Tossi, A.; et al. The Dolphin Proline-Rich Antimicrobial Peptide Tur1A Inhibits Protein Synthesis by Targeting the Bacterial Ribosome. Cell Chem. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Agathokleous, E.; Santocono, C.; Russo, F.; Vetrani, I.; Fedele, M.; Calabrese, E.J. Hormetic dose responses induced by antibiotics in bacteria: A phantom menace to be thoroughly evaluated to address the environmental risk and tackle the antibiotic resistance phenomenon. Sci. Total Environ. 2021, 798, 149255. [Google Scholar] [CrossRef]
- Gorr, S.U.; Brigman, H. V.; Anderson, J.C.; Hirsch, E.B. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS One 2022, 17, e0273504. [Google Scholar] [CrossRef]
- Nema, V. Tuberculosis diagnostics: Challenges and opportunities. Lung India 2012, 29, 259. [Google Scholar] [CrossRef]
- Luelmo, F. What is the role of sputum microscopy in patients attending health facilities. In Toman’s tuberculosis: case detection, treatment, and monitoring - questions and answers.; Frieden, T., Ed.; World Health Organization: Geneva, 2004; pp. 7–10. [Google Scholar]
- Suleiman, K. AN ACTIVIST’S GUIDE TO Tuberculosis Diagnostic Tools; Lessem, E., Ed.; Treatment Action Group (TAG): New York, 2017. [Google Scholar]
- Hilpert, K.; Volkmer-Engert, R.; Walter, T.; Hancock, R.E.W. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 2005, 23. [Google Scholar] [CrossRef]
- Fjell, C.D.; Jenssen, H.; Hilpert, K.; Cheung, W.A.; Panté, N.; Hancock, R.E.W.; Cherkasov, A. Identification of novel antibacterial peptides by chemoinformatics and machine learning. J. Med. Chem. 2009, 52. [Google Scholar] [CrossRef]
- Knappe, D.; Ruden, S.; Langanke, S.; Tikkoo, T.; Ritzer, J.; Mikut, R.; Martin, L.L.L.; Hoffmann, R.; Hilpert, K. Optimization of oncocin for antibacterial activity using a SPOT synthesis approach: Extending the pathogen spectrum to Staphylococcus aureus. Amino Acids 2016, 48, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Knappe, D.; Kabankov, N.; Hoffmann, R. Bactericidal oncocin derivatives with superior serum stabilities. Int. J. Antimicrob. Agents 2011, 37, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, F.C.; Budnick, G.E.; Elliott, T.; Gillim-Ross, L.; Hildred, M. V.; Mahlmeister, P.; Parrish, N.; Pentella, M.; Vanneste, J.; Wang, Y.F.; et al. Probability of negative Mycobacterium tuberculosis complex cultures based on time to detection of positive cultures: A multicenter evaluation of commercial-broth-based culture systems. J. Clin. Microbiol. 2012, 50, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Findlay, E.G.; Currie, S.M.; Davidson, D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014, 9, 55–73. [Google Scholar] [CrossRef]
- Kokryakov, V.N.; Harwig, S.S.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O. V; Korneva, H.A.; Lehrer, R.I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993, 327, 231–6. [Google Scholar] [CrossRef]
- Čeřovský, V.; Buděšínský, M.; Hovorka, O.; Cvačka, J.; Voburka, Z.; Slaninová, J.; Borovičková, L.; Fučík, V.; Bednárová, L.; Votruba, I.; et al. Lasioglossins: Three Novel Antimicrobial Peptides from the Venom of the Eusocial Bee Lasioglossum laticeps (Hymenoptera: Halictidae). ChemBioChem 2009, 10, 2089–2099. [Google Scholar] [CrossRef]
- Battista, F.; Oliva, R.; Vecchio, P. Del; Winter, R.; Petraccone, L. Insights into the Action Mechanism of the Antimicrobial Peptide Lasioglossin III. Int. J. Mol. Sci. 2021, Vol. 22, Page 2857 2021, 22, 2857. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Mignogna, G.; Andreu, D.; Shai, Y.; Barra, D.; Simmaco, M. Ranacyclins, a New Family of Short Cyclic Antimicrobial Peptides: Biological Function, Mode of Action, and Parameters Involved in Target Specificity. 2003. [CrossRef]
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Han, H.; Donald W. Miller, A.; Wang, G. Solution Structures of Human LL-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. 2006. [CrossRef]
- Otto, M. Bacterial sensing of antimicrobial peptides. Contrib. Microbiol. 2009, 16, 136–49. [Google Scholar] [CrossRef]
Figure 1.
Growth curve of Mycobacterium avium subsp. paratuberculosis (MAP) with and without peptide treatment at 20 µg/ml. Growth was followed by measuring the optical density at 600 nm.
Figure 1.
Growth curve of Mycobacterium avium subsp. paratuberculosis (MAP) with and without peptide treatment at 20 µg/ml. Growth was followed by measuring the optical density at 600 nm.

Figure 2.
Schematic representation of experiments performed in this study. Growth means studies that follow the growth from start to end, whereas TTP (Time To Positivity) just looks at the early stages of growth until the mycobacteria can be detected by an MGIT in a BD BACTECMGIT 320 System. MAP stands for Mycobacterium avium subsp. Paratuberculosis, MTB for Mycobacterium. Tuberculosis and MDR for multi-drug resistance. Stable peptides refer to peptides that are not easily degraded by proteases.
Figure 2.
Schematic representation of experiments performed in this study. Growth means studies that follow the growth from start to end, whereas TTP (Time To Positivity) just looks at the early stages of growth until the mycobacteria can be detected by an MGIT in a BD BACTECMGIT 320 System. MAP stands for Mycobacterium avium subsp. Paratuberculosis, MTB for Mycobacterium. Tuberculosis and MDR for multi-drug resistance. Stable peptides refer to peptides that are not easily degraded by proteases.

Figure 3.
Primary structure of three 12mer antimicrobial peptides, A) Peptide 69 (RRWRIVVIRVRR-CONH2) that improves growth, B) Peptide 72 (RLARIVVIRWAR- CONH2) that reduced growth and C) Peptide 70 (RLARIVVIRVRR-CONH2) that showed no effect on growth. PEPDRAW was used to generate these structures (http://pepdraw.com/).
Figure 3.
Primary structure of three 12mer antimicrobial peptides, A) Peptide 69 (RRWRIVVIRVRR-CONH2) that improves growth, B) Peptide 72 (RLARIVVIRWAR- CONH2) that reduced growth and C) Peptide 70 (RLARIVVIRVRR-CONH2) that showed no effect on growth. PEPDRAW was used to generate these structures (http://pepdraw.com/).
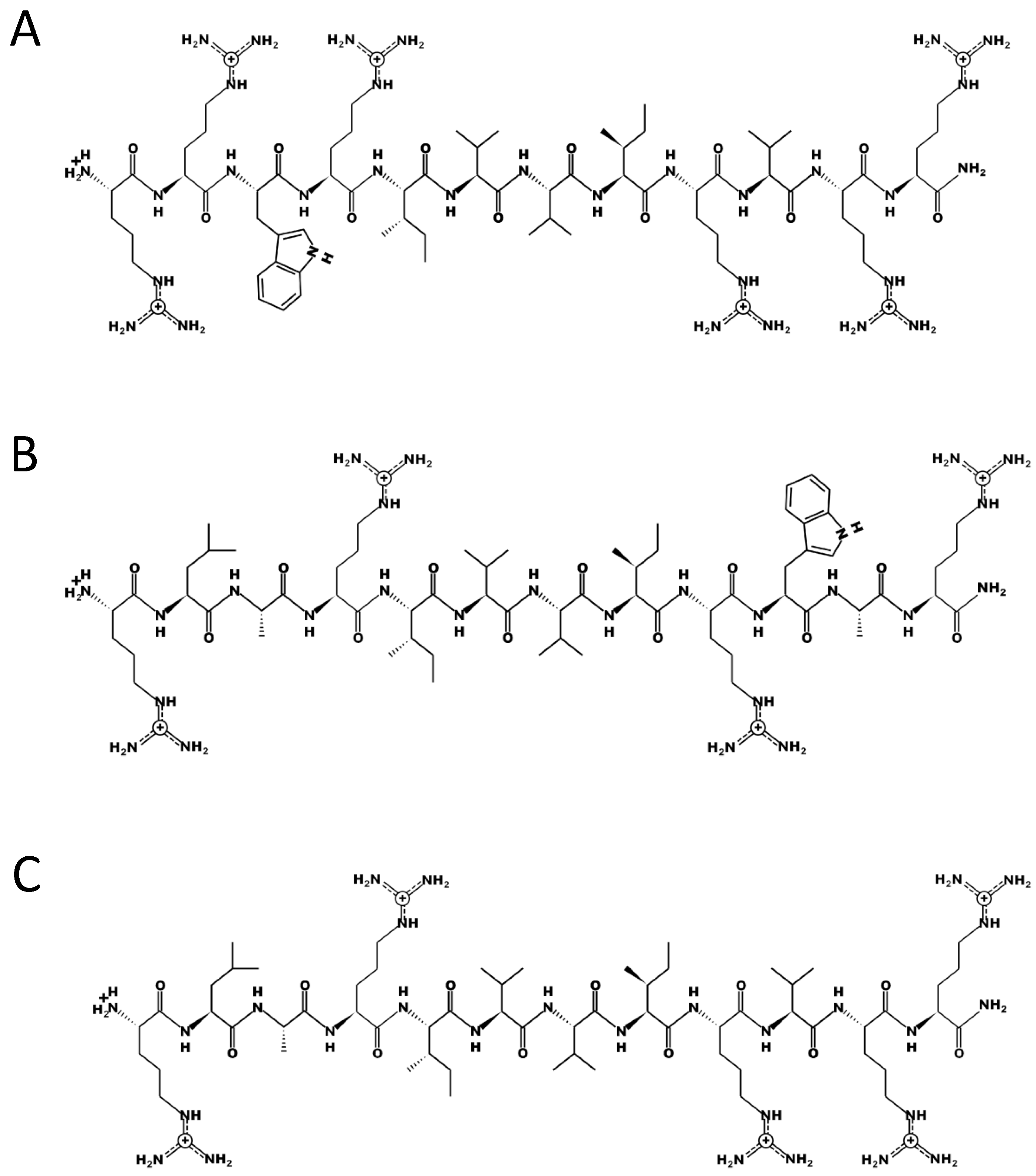
Figure 4.
Monitoring the growth curve of slow- and fast-growing mycobacteria with and without peptide 14 at 20 µg/ml. T in the legend indicates treatment with peptide 14. The slow-growing mycobacteria included A) Mycobacterium marinum, B) Mycobacterium branderi, C) Mycobacterium avium-intracellulare and D) Mycobacteria celatum. The fast-growing mycobacteria included E) Mycobacterium gastri, F) Mycobacterium abscessus, G) Mycobacterium porcinum, H) Mycobacterium fortuitum, I) Mycobacterium vaccae and J) Mycobacterium smegmatis.
Figure 4.
Monitoring the growth curve of slow- and fast-growing mycobacteria with and without peptide 14 at 20 µg/ml. T in the legend indicates treatment with peptide 14. The slow-growing mycobacteria included A) Mycobacterium marinum, B) Mycobacterium branderi, C) Mycobacterium avium-intracellulare and D) Mycobacteria celatum. The fast-growing mycobacteria included E) Mycobacterium gastri, F) Mycobacterium abscessus, G) Mycobacterium porcinum, H) Mycobacterium fortuitum, I) Mycobacterium vaccae and J) Mycobacterium smegmatis.

Figure 5.
Growth of MAP with Peptide 14 (red squares) and without peptide treatment (blue diamonds) monitored using the absorbance at 600 nm. Peptide 14 was added either directly to the diluted culture or on day 3 (green triangles) of the experiment. Different starting inoculum sizes were used, measured by absorbance at 600 nm A) 0.1, B) 0.05, C) 0.01 and D) 0.001.
Figure 5.
Growth of MAP with Peptide 14 (red squares) and without peptide treatment (blue diamonds) monitored using the absorbance at 600 nm. Peptide 14 was added either directly to the diluted culture or on day 3 (green triangles) of the experiment. Different starting inoculum sizes were used, measured by absorbance at 600 nm A) 0.1, B) 0.05, C) 0.01 and D) 0.001.

Figure 6.
Growth of MAP with and without several peptides at 10 µg/ml measured by absorbance at 600 nm. 14L stands for Peptide 14 (WKIVFWWRR- CONH2), 14D for Peptide 14D (wkivfwwrr- CONH2), 14 rev for the retro-inverse sequence (rrwwfvikw- CONH2), 14 L-KR for an ornithine exchange peptide (WOIVFWWOO- CONH2), 69D for Peptide 69D (rrwrivvirvrr-CONH2) and 69L-R for an ornithine exchange variant (OOWOIVVIOVOO-CONH2).
Figure 6.
Growth of MAP with and without several peptides at 10 µg/ml measured by absorbance at 600 nm. 14L stands for Peptide 14 (WKIVFWWRR- CONH2), 14D for Peptide 14D (wkivfwwrr- CONH2), 14 rev for the retro-inverse sequence (rrwwfvikw- CONH2), 14 L-KR for an ornithine exchange peptide (WOIVFWWOO- CONH2), 69D for Peptide 69D (rrwrivvirvrr-CONH2) and 69L-R for an ornithine exchange variant (OOWOIVVIOVOO-CONH2).

Figure 7.
Growth curve of MAP monitored via the absorbance at 600 nm over 20 days. The first experiment compared MAP without treatment (MAP only) and treatment with 20 µg/ml of Peptide 14D (MAP+Peptide 14D (1st)). After 20 days the Peptide 14D treated sample was intensively washed, diluted in new media, divided and growth monitored. One part was treated again with Peptide 14 at 20 µg/ml (MAP+Peptide 14D after washed out 1st treatment (2nd)) whereas the other was without treatment (MAP only washed after Peptide14D treatment).
Figure 7.
Growth curve of MAP monitored via the absorbance at 600 nm over 20 days. The first experiment compared MAP without treatment (MAP only) and treatment with 20 µg/ml of Peptide 14D (MAP+Peptide 14D (1st)). After 20 days the Peptide 14D treated sample was intensively washed, diluted in new media, divided and growth monitored. One part was treated again with Peptide 14 at 20 µg/ml (MAP+Peptide 14D after washed out 1st treatment (2nd)) whereas the other was without treatment (MAP only washed after Peptide14D treatment).

Figure 8.
Growth curves of M. tuberculosis in presence of different peptides determined using MGIT in a BD BACTECMGIT 320 System. The numbers are the peptide identification used in Table S1.
Figure 8.
Growth curves of M. tuberculosis in presence of different peptides determined using MGIT in a BD BACTECMGIT 320 System. The numbers are the peptide identification used in Table S1.

Figure 9.
Growth curves of M. tuberculosis in presence of different peptides, A) Peptide 14, B) Peptide 14D, C) Peptide 14Drev and D) Peptide 69D at a range of concentrations, determined using MGIT in a BD BACTECMGIT 320 System.
Figure 9.
Growth curves of M. tuberculosis in presence of different peptides, A) Peptide 14, B) Peptide 14D, C) Peptide 14Drev and D) Peptide 69D at a range of concentrations, determined using MGIT in a BD BACTECMGIT 320 System.

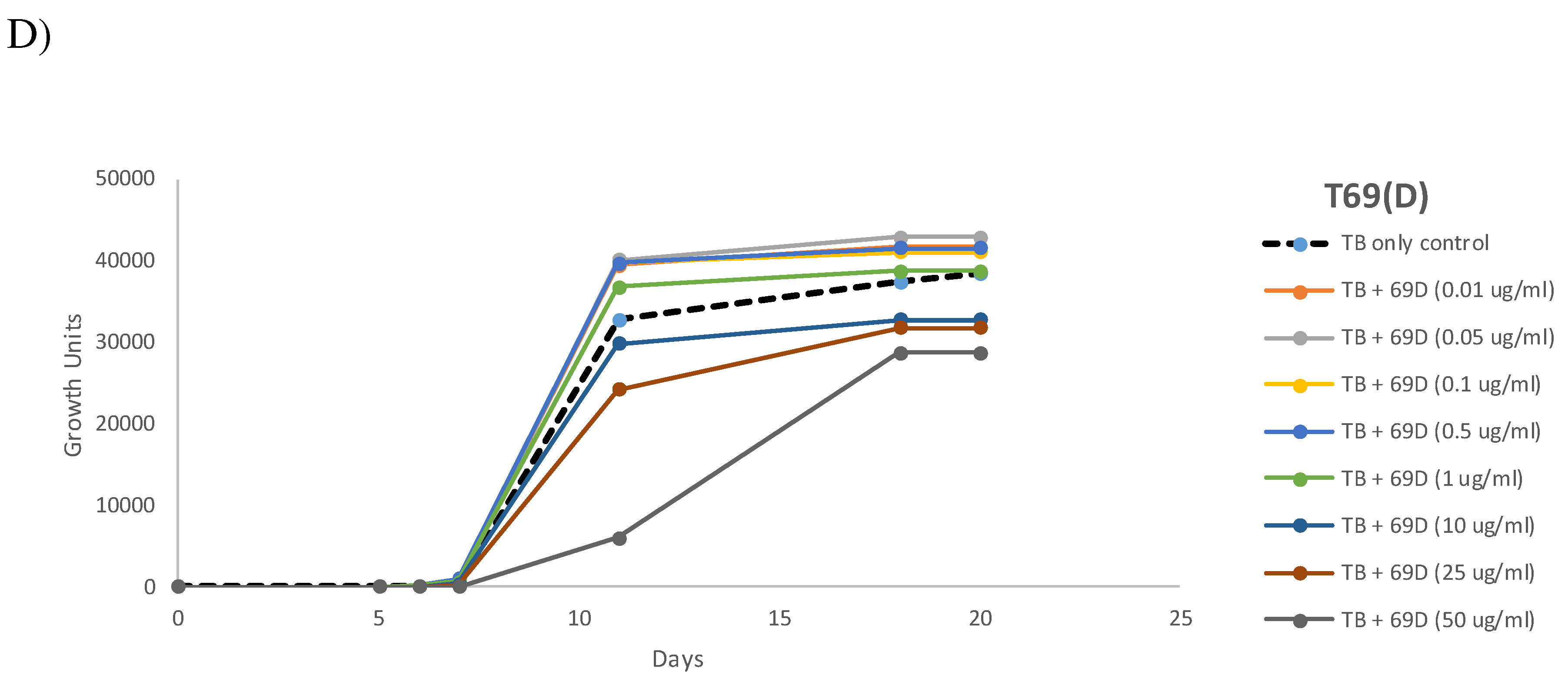
Figure 10.
qPCR analysis of M. tuberculosis in the absence (MTB control) and presence of 14D at different concentrations. .
Figure 10.
qPCR analysis of M. tuberculosis in the absence (MTB control) and presence of 14D at different concentrations. .

Figure 11.
Growth Curve of low inoculum MDR-TB treated with Peptide 14D at 1 µg/ml showing increased growth (red diamonds) compared with untreated control (black circles).
Figure 11.
Growth Curve of low inoculum MDR-TB treated with Peptide 14D at 1 µg/ml showing increased growth (red diamonds) compared with untreated control (black circles).

Figure 12.
Modelled growth curves of Mycobacterium tuberculosis, untreated in grey and treated with a peptide with a hormetic effect in black.
Figure 12.
Modelled growth curves of Mycobacterium tuberculosis, untreated in grey and treated with a peptide with a hormetic effect in black.

Table 1.
Effect of selected peptides at 10 µg/ml on the time to positivity (TTP) of M. mycobacterium measured in MGIT in a BD BACTECMGIT 320 System. Experiments 1a and 1b are technical repeats, independent of experiments 2a and 2b (technical repeats).
Table 1.
Effect of selected peptides at 10 µg/ml on the time to positivity (TTP) of M. mycobacterium measured in MGIT in a BD BACTECMGIT 320 System. Experiments 1a and 1b are technical repeats, independent of experiments 2a and 2b (technical repeats).
| Peptide code | Sequence | Time to positivity (days.hours) Experiment 1a | Time to positivity (days.hours) Experiment 1b | Time to positivity (days.hours) Experiment 2a | Time to positivity (days.hours) Experiment 2b |
|---|---|---|---|---|---|
| TB control (no peptide) | 7.02 | 7.01 | 15.01 | 15.20 | |
| 3 | WKWRVRVTI | 6.08 | 6.17 | 14.22 | 15.02 |
| 5 | FFIYVWRRR | 5.21 | 6.08 | 14.06 | 14.16 |
| 9 | KRRWRIWLV | 6.07 | 6.04 | 15.08 | 14.21 |
| 12 | RRRIKIRWY | 6.14 | 5.20 | 14.08 | 14.22 |
| 14 | WKIVFWWRR | 6.01 | 6.12 | 13.23 | 14.01 |
| 16 | RLKRWWKFL | 6.01 | 5.22 | 14.17 | 14.20 |
| 20 | KWKWWWRKI | 6.11 | 6.00 | 14.01 | 14.16 |
| 22 | IRMRIRVLL | 6.00 | 6.07 | 15.19 | 15.10 |
| 29 | RWRWWWRVY | 6.13 | 6.00 | 15.12 | 15.01 |
| 49 | VRLRIRVRVIRK | 5.22 | 6.06 | 15.12 | 15.02 |
| 59 | ILKWKRKWWKWRR | 5.17 | 5.22 | 13.19 | 12.22 |
| 60 | ILKWKIFKWKWFR | 6.03 | 6.02 | 14.19 | 14.16 |
| 61 | ILKWKTKWWKWFR | 6.20 | 5.23 | 13.11 | 14.21 |
| 62 | ILKWKMFKWKWFR | 6.07 | 7.02 | 16.20 | 17.16 |
| 63 | ILPWKWPWWPWRR | 6.22 | 6.22 | 17.01 | 17.10 |
| 64 | RLARIVVIRVAR | 6.19 | 6.16 | 15.21 | 16.13 |
| 65 | RGARIVVIRVAR | 6.16 | 6.03 | 16.11 | 16.01 |
| 66 | RRARIVVIRVAR | 6.05 | 6.16 | 17.06 | 17.16 |
| 67 | RLWRIVVIRVAR | 6.19 | 6.11 | 16.00 | 16.05 |
| 68 | RLRRIVVIRVAR | 6.09 | 6.17 | 15.00 | 15.23 |
| 69 | RRWRIVVIRVRR | 6.02 | 6.02 | 13.12 | 13.02 |
| 70 | RLARIVVIRVRR | 6.03 | 6.19 | 14.22 | 15.06 |
| 71 | RLWRIVVIRVKR | 6.04 | 6.06 | 14.01 | 14.02 |
| 72 | RLARIVVIRWAR | 6.22 | 7.01 | 15.01 | 15.13 |
| 76 | VRWRIRVAVIRA | 6.13 | 6.16 | 16.08 | 16.14 |
| 77 | VRLRIRVAVIRA | 6.14 | 6.04 | 14.22 | 15.03 |
| 78 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 6.08 | 6.09 | 14.15 | 15.00 |
Table 2.
A screen of 43 naturally occurring peptides, 11 fragments of naturally occurring peptides and 5 designed peptides, all taken from the APD3 was performed at 10 µg/ml in two independent experiments on the TTP of M. tuberculosis measured in MGIT in a BD BACTECMGIT 320 System. Fragments are labelled with the * symbol.
Table 2.
A screen of 43 naturally occurring peptides, 11 fragments of naturally occurring peptides and 5 designed peptides, all taken from the APD3 was performed at 10 µg/ml in two independent experiments on the TTP of M. tuberculosis measured in MGIT in a BD BACTECMGIT 320 System. Fragments are labelled with the * symbol.
| Name/Source | Sequence |
Time to positivity (day.hours) Experiment 1 |
Time to positivity (day.hours) Experiment 2 |
Average % faster than control |
|---|---|---|---|---|
| Control (TB only) | 11.10 | 9.10 | ||
| Protegrin 1/Pig* | RGGRLCYCRRRFCVCV | 10.23 | 6.22 | 15.3 |
| Lasioglossin LL-III /Bee | VNWKKILGKIIKVVK | 9.18 | 8.08 | 13.1 |
| Ranacyclin E/Frog | SAPRGCWTKSYPPKPCK | 10.07 | 8.01 | 12.2 |
| GF-17/Human* | GFKRIVQRIKDFLRNLV | 10.02 | 8.07 | 11.8 |
| Arenicin 1/Annelid worm* | RWCVYAYVRVRGVLVR | 10.15 | 8.02 | 10.5 |
| PP13/Wasp* | IRLHRLYTWKATIYTR | 10.17 | 8.02 | 10.2 |
| Peptide B9 / Frog | FLPLIAGLIGKLF | 10.19 | 8.01 | 10.0 |
| CM15/Bee venom | KWKLFKKIGAVLKVL | 10.19 | 8.01 | 10.0 |
| Macropin 1 /Wasp | GFGMALKLLKKVL | 10.12 | 8.07 | 10.0 |
| Salusin-beta/Scorpion* | FIFIRWLLKLGHHGRA | 10.18 | 8.03 | 9.8 |
| GLK-19/Designed* | KKLLGKLLKKLGKLLL | 10.17 | 8.04 | 9.7 |
| Odorranain-H1/Frog* | FGKILGVGKKVLCGLS | 10.19 | 8.03 | 9.6 |
| Temporin-HN1 /Frog | AILTTLANWARKFL | 10.23 | 8.01 | 9.3 |
| CPF-AM4/Frog | GLGSLVGNALRIGAKLL | 10.17 | 8.06 | 9.3 |
| Tachyplasin II/Crab | RWCFRVCYRGICYRKCR | 10.18 | 8.06 | 9.1 |
| Mastoparan-VT6 /Wasp | INLKAIAALVKKLL | 10.21 | 8.04 | 9.0 |
| Temporin-1TSa /Frog | FLGALAKIISGIF | 11.00 | 8.02 | 8.9 |
| Temporin-1DRa /Frog | HFLGTLVNLAKKIL | 10.18 | 8.09 | 8.5 |
| Temporin-HN2 /Frog | NILNTIINLAKKIL | 11.03 | 8.02 | 8.4 |
| NRC-15/Fish* | WGKLFKLGLHGIGLLH | 10.21 | 8.07 | 8.3 |
| CM-3/Designed | ALKAALLAILKIVRVI | 11.04 | 8.03 | 8.0 |
| Piscidin 1/Fish* | FFHHIFRGIVHVGKTI | 10.18 | 8.12 | 7.8 |
| Eumenitin/Wasp | LNLKGIFKKVASLLT | 11.00 | 8.08 | 7.6 |
| OdVP2/Wasp | ILGIITSLLKSLGKK | 10.22 | 8.11 | 7.3 |
| Clavanin C/Sea squirt | VFHLLGKIIHHVGNFV | 10.21 | 8.12 | 7.2 |
| P-04/Tunicate | FFPYAALKWLRKLLKK | 11.02 | 8.08 | 7.2 |
| Temporin L /Frog | FVQWFSKFLGRIL | 11.06 | 8.05 | 7.1 |
| Protegrin 4/Pig* | GRLCYCRGWICFCVGR | 11.11 | 8.01 | 7.1 |
| D28/Designed* | FLGVVFKLASKVFPAV | 11.02 | 8.09 | 7.0 |
| TsAP-1/Scorpion | FLSLIPSLVGGSISAFK | 11.07 | 8.05 | 7.0 |
| Brevinin-1Ta/Frog | FITLLLRKFICSITKKC | 11.07 | 8.05 | 7.0 |
| Bombolitin V/Bee | INVLGILGLLGKALSHL | 10.22 | 8.13 | 6.8 |
| Temporin-LTb /Frog | FIITGLVRGLTKLF | 11.07 | 8.06 | 6.7 |
| Temporin-1Cb /Frog | FLPLFASLIGKLL | 11.08 | 8.06 | 6.6 |
| Hylin a1/Frog | GAILPLALGALKNLIK | 11.14 | 8.02 | 6.3 |
| Arenicin-2/Lugworm | YAYVRIRGVLVRYRRC | 11.13 | 8.03 | 6.3 |
| Temporin-PTa /Frog | FFGSVLKLIPKIL | 11.07 | 8.08 | 6.3 |
| Decoralin/Wasp | SLLSLIRKLIT | 11.12 | 8.04 | 6.3 |
| Pd_mastoparan PDD-B /Wasp | INWLKLGKKILGAL | 11.06 | 8.09 | 6.3 |
| NRC-15/Flounder | GFWGKLFKLGLHGIGL | 11.02 | 8.14 | 5.9 |
| Clavanin E/Sea squirt | LFKLLGKIIHHVGNFV | 11.05 | 8.12 | 5.8 |
| Temporin 1Vb /Frog | FLSIIAKVLGSLF | 11.10 | 8.08 | 5.8 |
| Alyteserin-2b/Toad | ILGAILPLVSGLLSNKL | 11.18 | 8.02 | 5.6 |
| Polyphemusin/Crab* | WCFRVCYKGFCYRKCR | 11.05 | 8.13 | 5.6 |
| IsCT2 /Scorpion | IFGAIWNGIKSLF | 10.23 | 8.21 | 4.9 |
| Indolicidin /Cow | ILPWKWPWWPWRR | 11.03 | 8.18 | 4.8 |
| Temporin-SN1/Frog | FFPFLLGALGSLLPKIF | 10.22 | 8.23 | 4.6 |
| Pelophylaxin-4 /Frog | ILPFLAGLFSKIL | 11.05 | 8.18 | 4.5 |
| P9/Designed | KRRWRIWLV | 11.10 | 8.14 | 4.4 |
| Brevinin 1Tb/Frog | LVPLFLSKLICFITKKC | 11.10 | 8.15 | 4.2 |
| Casecidin 17/Cow | YQEPVLGPVRGPFPIIV | 11.12 | 8.14 | 4.1 |
| Brevinin-1DYc/Frog | LLAGLPKLLCLFFKKC | 11.10 | 8.16 | 4.0 |
| Temporin-1Oc /Frog | FLPLLASLFSRLF | 11.10 | 8.17 | 3.8 |
| Temporin A /Frog | FLPLIGRVLSGIL | 11.02 | 9.00 | 3.7 |
| Fallaxidin/Frog* | LSFLPKVIGVIGHLIH | 11.18 | 8.13 | 3.2 |
| Pantinin-3 /Scorpion | FLSTIWNGIKSLL | 11.08 | 8.23 | 2.8 |
| Temporin-1Vc /Frog | FLPLVTMLLGKLF | 11.12 | 8.21 | 2.5 |
| P8/Designed* | FFIYVWRRR | 11.16 | 8.22 | 1.6 |
| Tritrpticin /Pig | VRRFPWWWPFLRR | 11.08 | 9.13 | -0.3 |
Table 3.
Effect of Peptide 14D at different concentrations on the time to positivity (TTP) of M. tuberculosis in two independent experiments measured in MGIT in a BD BACTECMGIT 320 System. The value between the TTP of the peptide-treated sample minus the TTP of the control is shown in the next column, given in hours.
Table 3.
Effect of Peptide 14D at different concentrations on the time to positivity (TTP) of M. tuberculosis in two independent experiments measured in MGIT in a BD BACTECMGIT 320 System. The value between the TTP of the peptide-treated sample minus the TTP of the control is shown in the next column, given in hours.
| Peptide 14D concentration (µg/ml) |
Time to positivity (days.hours) Experiment 1 |
Difference to untreated control (hours) |
Time to positivity (days.hours) Experiment 2 |
Difference to untreated control(hours) |
|---|---|---|---|---|
| 0 | 6.19 | 6.12 | ||
| 0.1 | 5.12 | -31 | 5.19 | -17 |
| 0.5 | 5.17 | -26 | 5.17 | -19 |
| 1 | 5.05 | -38 | 5.08 | -28 |
| 10 | 7.03 | 8 | 7.08 | 20 |
| 25 | 9.21 | 74 | 9.17 | 77 |
| 50 | 19.15 | 308 | 21.5 | 353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Discovery of Antimicrobial Peptides That Can Accelerate the Diagnostics of Slow-Growing Mycobacteria Including Mycobacterium Tuberculosis by Culture
Kai Hilpert
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









