Preprint
Article
Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum
Altmetrics
Downloads
106
Views
42
Comments
0
A peer-reviewed article of this preprint also exists.
Submitted:
25 July 2023
Posted:
26 July 2023
You are already at the latest version
Alerts
Abstract
Japanese pepper (sansho, Zanthoxylum piperitum) contains several types of sanshools belonging to N-alkylamides. Because of long-chain unsaturated fatty acids present in their structure, sanshools are prone to oxidative deterioration, which poses problems in processing. Here, we evaluated the effects of antioxidants from the genus Zanthoxylum in preventing sanshool degradation using accelerated tests. An ethanolic extract of segment membranes of the sansho fruit pericarp was incubated at 70 °C for 7 days with different antioxidants to determine the residual amount of hydroxy-α-sanshool (HαS) in the extract. α-Tocopherol (α-Toc) showed excellent HαS-stabilizing activity at low concentrations. Among phenolic acids, we noted that the HαS-stabilizing activity increased with the number of hydroxy groups per molecule. For example, gallic acid and its derivatives exhibited excellent sanshool-stabilizing activity. Quercetin was found to be a superior HαS stabilizer compared with hesperetin and naringenin. However, the effective concentration was much higher for phenolic compounds than for α-Toc. These substances are believed to play a role in preventing the decomposition of sanshools in the pericarp of sansho. These sanshool stabilizers should be useful in the development of new beverages, foods, cosmetics, and pharmaceuticals that take advantage of the taste and flavor of sansho.
Keywords:
Subject: Chemistry and Materials Science - Food Chemistry
1. Introduction
The Japanese pepper (sansho, Zanthoxylum piperitum) has been used as a spice and traditional medicine in Japan since ancient times. Wakayama in central Japan cultivates the variety Budo Sansho, which accounts for 60–70% of Japan's sansho production. The harvest of ripened sansho fruits is mainly conducted from the second half of July to the first half of August. After harvesting, the fruits are dried, and the seeds are removed. The resulting dried pericarps are preserved for later use. Half of the dried pericarps is utilized as spices, and the other half as herbal medicine. For manufacturing herbal medicines, boiling water extract of the pericarps is dried and mixed with maltose; this powder is mainly used in medicines like Daikenchuto (TU-100) [1,2].
Sansho has a long history as a spice, and in recent years, its pungent taste accompanied with numbness has led to a boom in its production. The pungent taste of Japanese peppers arises from sanshools. To date, 13 types of sanshools have been identified in the genus Zanthoxylum [3]. Among them, hydroxy-α-sanshool (HαS) is the main compound in Japanese and Chinese pepper (Huajiao, Sichuan peppercorn, Zanthoxylum bungeanum) [3,4,5,6]. Terpenes, such as citronellal, geraniol, and geranial, are the major fragrance components [7].
For using sansho as a spice or for preparing its extract, it is necessary to grind the pericarp. The pungency, aroma, and color tone of the dried pericarps of sansho are relatively stable at room temperature. However, when the pericarp is ground into a powder, the taste and aroma quickly disappear. Moreover, the intensity of the aroma and the tingling taste of huajiao are not stable [8]. The tendency of sanshool to decompose when powdering sansho pericarp suggests that the pericarp contains substances or conditions that stabilize sansho. HαS has been reported to easily hydrolyze and oxidize under normal storage conditions [8,9]. Sanshools are long-chain polyunsaturated amides. Therefore, it is conceivable that the instability of sanshools may be due to the oxidation of the polyunsaturated chain or amide hydrolysis under acidic conditions. We selected antioxidants reported to be present in the genus Zanthoxylum, and measured their stabilizing activity against sanshools using a screening system.
2. Materials and Methods
2.1. Sansho and Chemicals
Zanthoxylum piperitum fruits (variety name: Budo Sansho, product of Wakayama Prefecture, Japan) were harvested from Kainan, Wakayama, Japan. Ripened fruits were dried in a drier at 40 and 50 °C for 6 h each, and then at 60 °C for 2 h. Upon drying, the fruit got split and the pericarp was separated by sieving off the seeds. HαS and hydroxyl-β-sanshool (HβS) were obtained from MedChemExpress (Monmouth Junction, NJ, USA). Hydroxyl-ε-sanshool (HεS) was purchased from MuseChem (Fairfield, NJ, USA). Hydroxyl-γ-sanshool (HγS) was obtained from Chengdu Biopurify Phytochemicals Ltd. (Sichuan, China). HPLC-grade acetonitrile, α-tocopherol (α-Toc), 2,2,5,7,8-pentamethyl-6-chromanol, gallic acid monohydrate, phytol ((2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-ol), and ascorbic palmitate were purchased from Fujifilm-Wako Chemicals (Tokyo, Japan). 2-Hydroxybenzoic acid (salicylic acid), 4-hydroxy benzoic acid (PHBA), ethyl gallate, propyl gallate, 4-hydroxy-3-methoxybenzoic acid (vanillic acid), hydroxyquinone, and 3,4-dihydroxybenzoic acid (protocatechuic acid) were purchased from Tokyo Chemical Industry (Tokyo, Japan). Quercetin, naringenin, hesperetin, caffeic acid, p-coumaric acid, and ferulic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.
2.2. Preparation of Segment Membrane
The pericarp of sansho consists mainly of flavedo and segment membranes (Figure 1). The segment membranes are located between the pericarp and seeds, and are attached to the inside of the pericarp. After separating the flavedo and segment membranes by placing the pericarp in a mortar and holding it down with a pestle, the membranes were peeled off from the pericarp using tweezers. The isolated segment membranes were ground in a laboratory mill (LM-PLUS, Osaka Chemical Co., Ltd.) and passed through a sieve with 250 μm pores to obtain powdered segment membranes.
2.3. Accelerated Test of HαS-Stabilizing Activity
Segment membrane powder (0.4 g) was suspended in 8 mL of ethanol, and the suspension was mixed by vortexing. The suspension was centrifuged at 2,000 ×g for 2 min and the supernatant was recovered. The content in 1 mL of the supernatant was approximately 2.5 μmol. Two milliliter of the supernatant and 2 mL of an ethanolic solution of candidate antioxidant were mixed in a 10 ml Spitz tube and incubated in an oil bath at 70 °C. The amount of HαS in the sample on day 7 of incubation was measured using HPLC. All accelerated tests were performed in triplicate. The ratio of the amount of HαS on day 7 of incubation to the amount on day 0 was calculated and expressed as the survival rate (%).
2.4. HPLC and LC-MS
HPLC was performed on a Shimadzu LC-2010 instrument (SHIMADZU, Kyoto, Japan). All samples were injected into an InertSustainSwift C18 4.6 × 250 mm column (GL Sciences, Tokyo, Japan). The analytes were eluted with mixed solvent A (30% acetonitrile): B (80% acetonitrile) at a flow rate of 1.0 mL/min. The conditions were as follows: 0 min: 0% B; 35 min: 45% B; 50 min: 100% B; 51 min: 0% B; 55 min: 0% B. All analyses were performed at 40 °C and the absorbance of eluate was monitored at 270 nm. Liquid chromatography–mass spectrometry (LC-MS) was performed by electrospray ionization (ESI)-MS in a positive mode using a Bruker micrOTOFTM ESI-TOF mass spectrometer (Bruker Corporation, Billerica, MA, USA) interfaced with an Agilent 1200 HPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA). A portion of the sample was loaded onto an Inertsil ODS-3 (2.1 × 150 mm) column. Separation was achieved by elution with a mixture of solvents A (30% acetonitrile) and B (80% acetonitrile) at a flow rate of 1.0 mL/min. The conditions were as follows: 0 min: 0% B; 35 min: 45% B; 50 min: 100% B; 51 min: 0% B; 55 min: 0% B. All analyses were performed at 40 °C and the absorbance of eluate was monitored at 270 nm.
2.5. Quantitation of HαS and HβS
HαS and HβS were quantitated using HPLC. Calibration curves were prepared using different concentrations of HαS and HβS standards to derive a relationship between the peak area and concentration.
2.6. Quantitation of Tocopherol
Analysis of α-, β-, γ-, and δ-tocopherols was performed using HPLC by Japan Food Research Laboratories (Suita, Osaka, Japan).
3. Results
3.1. Sansho Harvest Season
Sansho fruits are harvested in three seasons in the central prefecture of Wakayama, Japan (Table 1). The unripe fruits are harvested in May. The seeds of these fruits have not yet hardened, and the whole fruit is used for Tsukudani (a preservable food, boiled in soy sauce). Ripe fruits are harvested from the second half of July to the first half of August. After drying, they are used as raw materials for herbal medicines and spices. In September, the sansho fruit turns red and is harvested as fully ripened sansho pepper, which is also used as a spice. However, in Wakayama Prefecture, the amount of fully ripened fruit harvested is extremely small. Therefore, ripened fruits were used in this study.
3.2. HPLC and LC/MS of Ethanol Extracts of Pericarps
A chromatogram of the ethanolic extract of pericarp is shown in Figure 2a. Several peaks were observed in the chromatogram. Of these, peaks A, B, C, and D were found to be of HεS, HαS, HβS, and HγS respectively, by comparison with the standard sanshools. In addition, MS analysis was performed for peaks B, E, and F. Peak B compound had the elemental composition of C16H26NO2 (M+H+) based on high-resolution LC-MS/MS (ESI, positive mode) data. The LC-MS data for peaks E and F indicated elemental compositions of C16H26NO (M+H+) and C18H28NO (M+H+), respectively. The molecular weights of the three major peaks B, E, and F were 263.1958, 247.3758, and 273.4131, respectively. Peaks B, E, and F were believed to be for HαS, α-sanshool (αS), and γ-sanshool (γS). From HPLC of references performed under the same conditions [10,11,12], we assumed that peaks E and F might be for αS and γS, respectively.
3.3. Effect of Alcohol Concentration on Extraction Amount of HαS and Its Stability
The pericarp powder (0.4 g) was suspended in aqueous ethanol solutions of various concentrations (20 mL). After vigorous mixing for 5 min at about 20 °C, the mixture was centrifuged at 2,000 ×g for 2 min and the supernatant was recovered. The amount of HαS in each supernatant was measured using HPLC. As shown in Figure 3, the amount of HαS extracted was constant when the ethanol concentration was up to 50% but decreased with a decrease in ethanol concentration to 50% or less. Furthermore, when these extracts were incubated at 70 °C, the instability of HαS increased with increasing proportion of water in the eluate (Figure 4). These results indicated that the HαS stabilizer, thought to be present in the pericarp, is soluble in ethanol.
3.4. Comparison of the Stability of HαS in Ethanolic Extracts of Pericarp and Segment Membranes
HαS stabilizer was found to be present in sansho pericarp, was apparently extracted with ethanol. Therefore, to search for stabilizing substances, we considered it necessary to build a system that minimized contamination of stabilizers derived from sansho. We collected segment membranes attached to the inside of the pericarps and prepared their ethanolic extract. The amount of HαS in the segment membrane was approximately 5 mg/g dry weight, which was equivalent to approximately 1/20 of that in the pericarp. As shown in Figure 2b, the HPLC chromatogram of the ethanolic extracts of segment membranes was similar to that of the ethanol extract of the pericarp.
The two ethanolic extracts were incubated at 60, 70, and 80 °C for 1 week and the residual amounts of HαS in them was compared (Figure 5). The amount of HαS in 95% ethanolic extract of the segmented membranes was reduced to 61.5 ± 4.65% in 7 days at 70 °C. On the contrary, the amount of HαS in the ethanolic extract of pericarps was maintained above 90% at 70 °C. Furthermore, because the segment membranes contain less chlorophyll and polyphenols, the influence of these substances on the assay system is believed to be weakened. Therefore, by adding various candidate antioxidants to the ethanolic extract of the segment membranes, we investigated their HαS-stabilizing activity.
3.5. HαS-Stabilizing Activity of Antioxidant Vitamin
Tocopherol is a fat-soluble antioxidant vitamin. Hisatomi et al. reported that the α-Toc content in the pericarp of sansho is 35 mg/100 g, which is approximately 11.7 times that in seeds [13]. In the pericarp of Budo sansho, we found that α-Toc content was 10.7 mg/g of dry weight and that β-, γ-, and δ-tocopherol were not present. Using a screening system, we attempted to examine the HαS-stabilizing activity of α-Toc. As shown in Figure 6, α-Toc exhibited a superior HαS-stabilizing activity even at a low concentration. As α-Toc contains a phenolic-chromanol ring linked to a saturated isoprenoid side chain (phytol), we also examined HαS-stabilizing activity of 2,2,5,7,8-pentamethyl-6-chromanol and phytol was examined. 2,2,5,7,8-Pentamethyl-6-chromanol showed excellent HαS-stabilizing activity, but phytol did not show any such activity (Figure 6). It is suggested that the antioxidant activity of 2,2,5,7,8-Pentamethyl-6-chromanol in α-Toc is related to the HαS-stabilizing activity. β-carotene is a red-orange pigment that is abundant in plants and has antioxidant properties. It is expected that β-carotene is also present in the sansho pericarp. However, because β-carotene is almost insoluble in ethanol, its effects were not confirmed in this study.
Ascorbic acid is a well-known water-soluble antioxidant vitamin normally present in plants. However, because it is insoluble in ethanol, the HαS-stabilizing activity of ascorbic acid could not be investigated using our assay system, and we believe that it is not a stabilizer of HαS. Ascorbic palmitate is an ester formed from ascorbic acid and palmitic acid, which is hydrolyzed into fat-soluble ascorbic acid and is used as a food additive. We investigated the HαS-stabilizing activity of ascorbic acid using ascorbic palmitate. It showed the highest HαS-stabilizing activity at approximately 2 mM, and the activity decreased at higher concentrations (Figure 6).
3.6. HαS-Stabilizing Activity of Phenolics
Many phenolic acids, including hydroxycinnamic acid, flavonoids, lignans, and coumarins, have been reported to be present in the genus Zanthoxylum [13,14,15,16,17,18,19]. We investigated the HαS-stabilizing activity of several of these compounds using our screening system. The HαS-stabilizing activities of mono-hydroxybenzoic acids (salicylic acid, PHBA, and vanillic acid), di-hydroxybenzoic acid (protocatechuic acid), and tri-hoxybenzoic acid (gallic acid, ethyl gallate, and sodium gallate) are shown in Figure 7.
Gallic acid and its related compounds showed high HαS-stabilizing activity at low concentrations (Figure 7). This was followed by protocatechuic acid, PHBA, and vanillic acid. As the number of phenolic hydroxy groups per molecule increased, the HαS-stabilizing activity increased. Among the mono-hydroxybenzoic acids, there was a difference in the HαS-stabilizing activity. The acidity of each of the three selected compounds differed; the pKa values of salicylic acid, PHBA, and vanillic acid are 2.97, 4.54, and 4.51, respectively. The acidity of mono-hydroxybenzoic acids appeared to affect the stability of HαS. Butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are synthetic lipophilic compounds, chemically derived from phenol. Owing to their antioxidant properties, they have been widely used as preservatives in edible oils. Because there is only one phenolic hydroxyl group in these two compounds, the sanshool-stabilizing effect is weak (data not shown).
Hydroxycinnamic acids are widely distributed in plants. The HαS-stabilizing activity of caffeic acid was found to be higher than that of p-coumaric and ferulic acids (Figure 7). Caffeic acid has two phenolic hydroxyl groups, whereas p-coumaric acid and ferulic acid have only one each. Flavonoids are also widely distributed in plants and have been reported from genus Zanthoxylum [7,13,14,15,16,17,18,19]. We examined the HαS-stabilizing activities of quercetin, naringenin, and hesperetin. Quercetin showed a high HαS-stabilizing activity at a concentration ≥0.1 mM (Figure 7). The HαS-stabilizing activity of quercetin, which has five hydroxyl groups in its structure, exceeded those of naringenin and hesperetin with two hydroxyl groups each. Arbutin, a sugar-containing hydroquinone, has been reported to be present in Zanthoxylum piperitum [14]. Considering the insolubility of arbutin in ethanol, we did not attempt to investigate its HαS-stabilizing activity. Hydroquinone, the aglycone portion of arbutin, has two hydroxyl groups attached to the benzene ring at the para-position and may act as a reducing agent. In preliminary tests, hydroquinone showed weak HαS-stabilizing activity (data not shown).
3.7. Effect of pH on the Stability of HαS
Ethanolic extracts of segmented membranes were mixed with the same volume of Britton–Robinson buffer (pH 2.2–12) [20] and the mixtures were incubated at 70 °C. On days 3 and 9, the HαS content in each mixture was assayed. A significant decrease in the HαS content was observed with sanshools degrading under acidic conditions (Figure 8).
4. Discussion
In this study, several compounds with stabilizing activity for HαS, which is the main pungent substance sansho, were found. Among these, α-Toc was found to substantially stabilize HαS in sansho pericarp. This appears to be one of the reasons for the abundance of α-Toc in sansho pericarp, which is an amphiphilic compound present in cell membranes. α-Toc, which is synthesized in chloroplasts and is present in cell membranes, plays an important role in preventing lipid oxidation [19,21]. The three-dimensional arrangement of α-Toc in lipid bilayer membranes in cell membranes has been studied [22]. Sanshools are amphiphilic compounds with fat-soluble fatty acid side chains and a hydrophilic amide moiety. The two compounds share common structural features. We speculate that sanshools may be present in the cell membrane or chloroplast lamellae of pericarp cells in close proximity to α-Toc. Further research on the interaction between α-Toc and sanshools and their locations in the cells is required.
Some phenolic compounds reported in Zanthoxylum exhibited high HαS-stabilizing activity. However, because these compounds are believed to be bound to sugars and organic acids, which makes them hydrophilic, they are difficult to access HαS. Therefore, we believe that they are not involved in the stabilization of HαS in the pericarp. Furthermore, the concentration of phenolic compounds, which are effective in stabilizing HαS, was much higher than that of α-Toc; it may, therefore, be difficult to practically use these phenolic compounds.
Capsaicin, present in red pepper (Capsicum annuum L.), and piperine, present in black pepper (Piper nigrum L.) are N-alkylamides [23]. These substances are responsible for the hot sensation of these peppers. Because these compounds are relatively stable, red and black peppers are used as spices and medicines worldwide. However, sanshools are easily oxidized when exposed to air and become extremely unstable, particularly when purified. Therefore, sansho and huajiao have been used only in a limited area of the world, although they provide another trigeminal sensation called tingling. Huajiao is often used in the form of chili oil, in which the pericarp is soaked in vegetable oil. The shelf life is relatively longer because the sanshools gradually elute from the pericarp, and when in the oil, they do not come in direct contact with air, or coexist with antioxidants, such as tocopherol derived from vegetable oils. However, further food processing is difficult in the presence of chili oil. If sanshools can be stabilized, sansho and huajiao could be used in a variety of processed and functional foods, and in pharmaceuticals.
Recent investigations have revealed various pharmacological activities of HαS, including stimulation of the digestive system, strong salivating effects through the activation of TRPV, and activation of somatosensory neurons through the inhibitory activity against two-pore potassium channels (KCNK3, KCNK9, and KCNK18) [24,25]. HαS is also being investigated as a local anesthetic. HαS has potential for the treatment of diseases and in healthcare. Therefore, for further pharmacological research or development of the medicinal uses of sanshools, it is important to identify the conditions or substances that stabilize sanshools. Methods for the synthesis of sanshools have also been reported [26,27,28], and the pure products, thus obtained, can be used; however, their stability is likely to be even more important.
Echinacea is a popular herbal medicine in Western countries that has been used to treat colds and influenza [29,30]. However, its usefulness in clinical trials has been questioned [30]. There are various reasons for this. Liu and Murphy suggested that the stability of N-alkylamide, which is one of the active ingredients of Echinacea, poses a problem, and its content is not guaranteed for each preparation [31]. Echinacea purpurea contains eight isobutylamides, all of which are susceptible to oxidative degradation and are very similar to sanshools. They found that phenolic acids in Echinacea prevented the degradation of these isobutylamides depending on the experimental conditions [31]. It is believed that the degradation of N-alkylamides is prevented to some extent by the antioxidant action of these phenolic acids. However, because N-alkylamides are vulnerable to acids, further stability can be expected using α-Toc.
5. Conclusions
We constructed a system for stabilizing substances in sanshools and found that α-Toc exhibits excellent activity. Stabilization of sanshools can be employed for developing new sansho products that exploit their excellent functionality.
6. Patents
TM is named an inventor on a patent (Takahiko Mitani and Takashi Tsuchida, Patent JP, 6630880, B).
Author Contributions
"Conceptualization, T.M.; Methodology, T.M.; Software, Y.Y.; Validation, S.K. and M.N.; Formal Analysis, Y.Y.; Investigation, T.M., Y.Y., N.Y. and Y.O.; Resources, T.M.; Data Curation, T.M., Y.Y., N.Y. and Y.O.; Writing – Original Draft Preparation, T.M.; Writing – Review & Editing, H.S.; Visualization, T.M.; Supervision, S.K.; Project Administration, H.S.; Funding Acquisition, T.M.” All the authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wakayama Industry Promotion Foundation (Wakayama, Japan) and the Yamazaki Spice Promotion Foundation (Tokyo, Japan).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors wish to acknowledge M.D. Shuhei Ito, chairman of Wakayama Bioscience Liaison Council, and Dr. Chikayoshi Kitamura, coordinator of NPO Kinki Agri-Hi-Tech, for their encouragement and advice. We received generous support from Ms. Yoshie Yamamoto and Mr. Takashi Tsuchida of Yamamoto Katsunosuke Shoten Ltd., Kainan, Wakayama. We would also like to thank Ms. Sayuri Nakano, and Ms. Hisa Mimura for helping with the conduct of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kono, T.; Shimada, M.; Yamamoto, M.; Kaneko, A.; Oomiya, Y.; Kubota, K.; Kase, Y.; Lee, K.; Uezono, Y. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of daikenchuto. Front. Pharmacol. 2015, 6, 159. [CrossRef]
- Saroh, K.; Kase, Y.; Hayakawa, T.; Murata, P.; Ishige, A.; Sasaki, H. Dai-kenchu-to accelerated small intestinal movement. Biol. Pharm. Bull. 2001, 24, 1122. [CrossRef]
- Yasuda, I.; Takeya, K.; Itokawa, H. Distribution of unsaturated aliphatic acid amides in Japanese Zanthoxylum species. Phytochemistry. 1982, 21, 1295-1298. [CrossRef]
- Mizutani, K.; Fukunaga, Y.; Tanaka, O.; Takasugi, N.; Saruwatari, Y.-i.; Fuwa, Tohru; Yamauchi, T.; Wang, J.; Jia, M.-R.; Li, F.-Y.; et al. Amides from huajiao, pericarps of Zanthoxylum bungeanum MAXIM. Chem. Pharm. Bull. 1988, 36, 2362–2365. [CrossRef]
- Sugai, E.; Morimitsu, Y.; Kubota, K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics bioscience. Biosci. Biotechnol. Biochem. 2005, 69, 1958–1962. [CrossRef]
- Zhang, M.; Wang, Jiaolong; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [CrossRef]
- Chruma, J.J.; Cullen, D.J.; Bowman, L.; Toy, P.H.. Polyunsaturated fatty acid amides from the Zanthoxylum genus – from culinary curiosities to probes for chemical biology. Nat. Prod. Rep. 2018, 35, 54–74. [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [CrossRef]
- Tan, F.; Xu, L.; Liu, Y.; Li, H.; Zhang, Dahan; Qin, C.; Han, Y.; Han, J. Design of hydroxy-a-sanshool loaded nanostructured lipid carriers as a potential local anesthetic. Drug Deliv. 2022, 29, 743–753. [CrossRef]
- Iseli, V.; Potterat, O.; Hagmann, L.; Egli, J.; Hamburger, M. Characterization of the pungent principles and the essential oil of Zanthoxylum schinifolium pericarp. Pharmazie. 2007, 62, 396–400. [CrossRef]
- Zhao, Z.F.; Zhu, R.-X.; Zhong, K.; He, Q.; Luo, A.-M.; Gao, H. Characterization and comparison of the pungent components in commercial Zanthoxylum bungeanum oil and Zanthoxylum schinifolium oil. J. Food Sci. 2013, 78, C1516–C1522. [CrossRef]
- Bader, M.; Stark, T.D.; Dawid, C.; Lösch, S.; Hofmann, T. All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J. Agric. Food Chem. 2014, 62, 2479–2488. [CrossRef]
- Hisatomi, E.; Matsui, M.; Kubota, K.; Kobayashi, A. Antioxidative activity in the pericarp and seed of Japanese pepper (Xanthoxylum piperitum DC). J. Agric. Food Chem. 2000, 48, 4924–4928. [CrossRef]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007, 100, 171–177. [CrossRef]
- Hur, J.M.; Park, J.G.; Hwang, Y.H. Aromatic acid and flavonoids from leaves of Zanthoxylum piperitum. Nat. Prod. Sci. 2001, 7, 23–26.
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. Grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity research. PLOS ONE. published 2014, 9, article |. [CrossRef]
- Zhong, K.; Li, X.-J.; Gou, A.-N.; Huang, Y.-N.; Bu, Q.; Gao, H. Antioxidant and cytoprotective activities of flavonoid glycosides-rich extract from the leaves of Zanthoxylum bungeanum. J. Food Nutr. Res. 2014, 2, 349–356. [CrossRef]
- Soll, J.; Schultz, G. 2-Methyl-6-phytylquinol and 2,3-dimethyl-5-phytylquinol as precursors of tocopherol synthesis in spinach chloroplasts. Phytochemistry. 1980, 19, 215–218. [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [CrossRef]
- Marquardt, D.; Williams, J.A.; Kučerka, N.; Atkinson, J.; Wassall, S.R.; Katsaras, J.; Harroun, T.A. Tocopherol activity correlates with its location in a membrane: a new perspective on the antioxidant vitamin E. J. Am. Chem. Soc. 2013, 135, 7523–7533. [CrossRef]
- Marquardt, D.; Kučerka, N.; Katsaras, J.; Harroun, T.A. α-Tocopherol’s Location in Membranes Is Not Affected by Their Composition. Langmuir. 2015, 31, 4464–4472. [CrossRef]
- Elufioye, TaiwoO.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Revista Brasileira de Farmacognosia (2020) 30:622–640. [CrossRef]
- Koo, J.Y.; Jang, Yongwoo; Cho, Hawon; Lee, C.-H.; Jang, K.H.; Ha Chang, Y.H.; Shin, Jongheon; Oh, Uhtaek. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur. J. Neurosci. 2007, 26, 1139–1147. [CrossRef]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772–779. [CrossRef]
- Igarashi, Y.; Aoki, K.; Nishimura, H.; Morishita, I.; Usui, K. Total synthesis of hydroxy-α- and hydroxy-β-sanshool using Suzuki-Miyaura coupling. Chem. Pharm. Bull. (Tokyo). 2012, 60, 1088–1091. [CrossRef]
- Zhou, J.; Xiao, Y.; Chen, T.; Gao, Jiyu; Huang, W.; Li, Z. Synthesis of hydroxy-α-sanshool. J. Chem. Res. 2021, 45, 310–314. [CrossRef]
- Nakamura, A.; Mimaki, K.; Tanigami, K.I.; Maegawa, T. An improved and practical method for synthesizing of α-sanshools and spilanthol. Front. Chem. 2020 Sec. Organic Chemistry Volume 8 - 2020, 8, 187. [CrossRef]
- Bauer, R. Chemistry, pharmacology and clinical applications of Echinacea products. In Herbs, Botanicals & Teas, Mazza, G.O., Ed.; Technomic Publishing: Lancaster, U.K., 2000; pp. 45–73.
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [CrossRef]
- Liu, Y.; Murphy, P.A. Alkamide stability in Echinacea purpurea extracts with and without phenolic acids in dry films and in solution. J. Agric. Food Chem. 2007, 55, 120–126. [CrossRef]
Figure 1.
Preparation of flavedos and segment membranes from dried pericarps. The flavedo and segment membranes were separated by holding the dried sansho pericarp in a mortar with a pestle. (a) flavedo; (b) segment membrane.
Figure 1.
Preparation of flavedos and segment membranes from dried pericarps. The flavedo and segment membranes were separated by holding the dried sansho pericarp in a mortar with a pestle. (a) flavedo; (b) segment membrane.

Figure 2.
HPLC and LC-MS of ethanolic extracts of pericarp and segment membranes. The retention times (RT) for peaks A–D and the sanshool standards were compared, and the respective compounds were identified. The molecular weights of the compounds represented by peaks B, E, and F were determined using LC-MS analysis.
Figure 2.
HPLC and LC-MS of ethanolic extracts of pericarp and segment membranes. The retention times (RT) for peaks A–D and the sanshool standards were compared, and the respective compounds were identified. The molecular weights of the compounds represented by peaks B, E, and F were determined using LC-MS analysis.
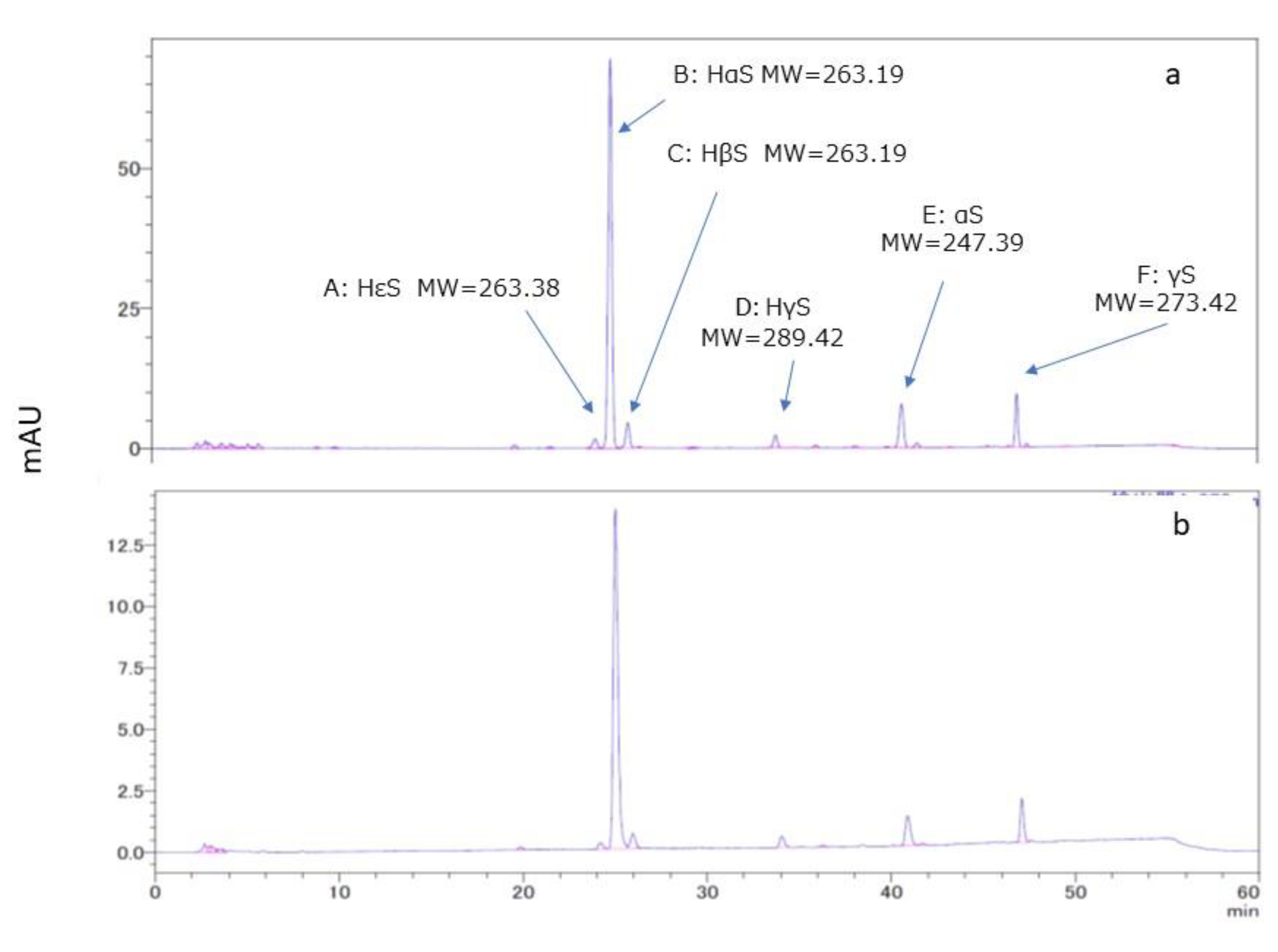
Figure 3.
Effects of differences in ethanol concentration on the extraction of HαS. Sansho pericarp powder (0.4 g) was suspended in solvent (20 mL) having different ethanol concentrations, mixed vigorously, and centrifuged. The content of HαS in the supernatant was analyzed using HPLC.
Figure 3.
Effects of differences in ethanol concentration on the extraction of HαS. Sansho pericarp powder (0.4 g) was suspended in solvent (20 mL) having different ethanol concentrations, mixed vigorously, and centrifuged. The content of HαS in the supernatant was analyzed using HPLC.

Figure 4.
Thermal stability of HαS in sansho pericarp extract obtained using a solvent with different ethanol concentrations. Sansho pericarp powder (0.4 g) was mixed in solvent (20 mL) having different ethanol concentrations, mixed vigorously, and centrifuged. The supernatant was incubated at 70 °C for 11 days. On days 1, 2, 3, 7, and 11, the amount of HαS in the samples was analyzed using HPLC.
Figure 4.
Thermal stability of HαS in sansho pericarp extract obtained using a solvent with different ethanol concentrations. Sansho pericarp powder (0.4 g) was mixed in solvent (20 mL) having different ethanol concentrations, mixed vigorously, and centrifuged. The supernatant was incubated at 70 °C for 11 days. On days 1, 2, 3, 7, and 11, the amount of HαS in the samples was analyzed using HPLC.
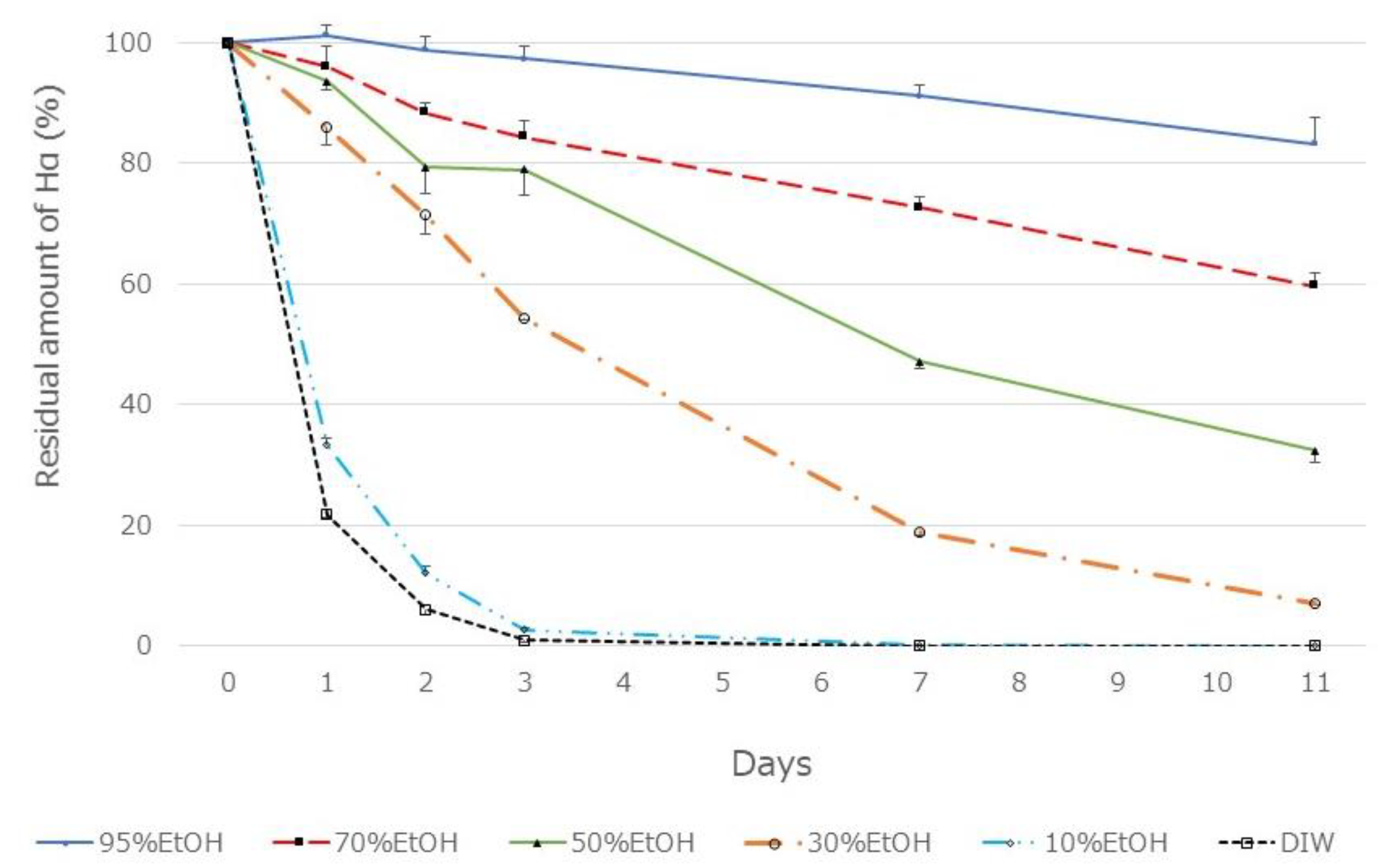
Figure 5.
Thermal stability of HαS in ethanolic extracts of pericarp and segment membranes. Ethanolic extracts of sansho pericarp and segment membranes were prepared as described in the Methods section. Each extract was incubated for 1 week at 60, 70, or 80 °C, and the amount of HαS in the samples was analyzed using HPLC. The solid line shows the ethanolic extract of the pericarp and the dashed line shows that of the segment membranes.
Figure 5.
Thermal stability of HαS in ethanolic extracts of pericarp and segment membranes. Ethanolic extracts of sansho pericarp and segment membranes were prepared as described in the Methods section. Each extract was incubated for 1 week at 60, 70, or 80 °C, and the amount of HαS in the samples was analyzed using HPLC. The solid line shows the ethanolic extract of the pericarp and the dashed line shows that of the segment membranes.

Figure 6.
Effect of antioxidant vitamins on the stability of HαS at 70 °C. The ethanolic extract of sansho segment membranes was prepared as described in Materials and Methods. The candidate antioxidant compounds were dissolved in ethanol at various concentrations. Both the ethanolic solutions were mixed and incubated for 1 week at 70 °C. The amount of HαS in the samples was analyzed using HPLC.
Figure 6.
Effect of antioxidant vitamins on the stability of HαS at 70 °C. The ethanolic extract of sansho segment membranes was prepared as described in Materials and Methods. The candidate antioxidant compounds were dissolved in ethanol at various concentrations. Both the ethanolic solutions were mixed and incubated for 1 week at 70 °C. The amount of HαS in the samples was analyzed using HPLC.

Figure 7.
Effect of phenolics on the stability of HαS at 70 °C. The ethanolic extract of sansho segment membranes was prepared as described in Materials and Methods. The candidate compounds were dissolved in ethanol at various concentrations. Both the ethanolic solutions were mixed and incubated for 1 week at 70 °C. The amount of HαS in the samples was analyzed using HPLC.
Figure 7.
Effect of phenolics on the stability of HαS at 70 °C. The ethanolic extract of sansho segment membranes was prepared as described in Materials and Methods. The candidate compounds were dissolved in ethanol at various concentrations. Both the ethanolic solutions were mixed and incubated for 1 week at 70 °C. The amount of HαS in the samples was analyzed using HPLC.
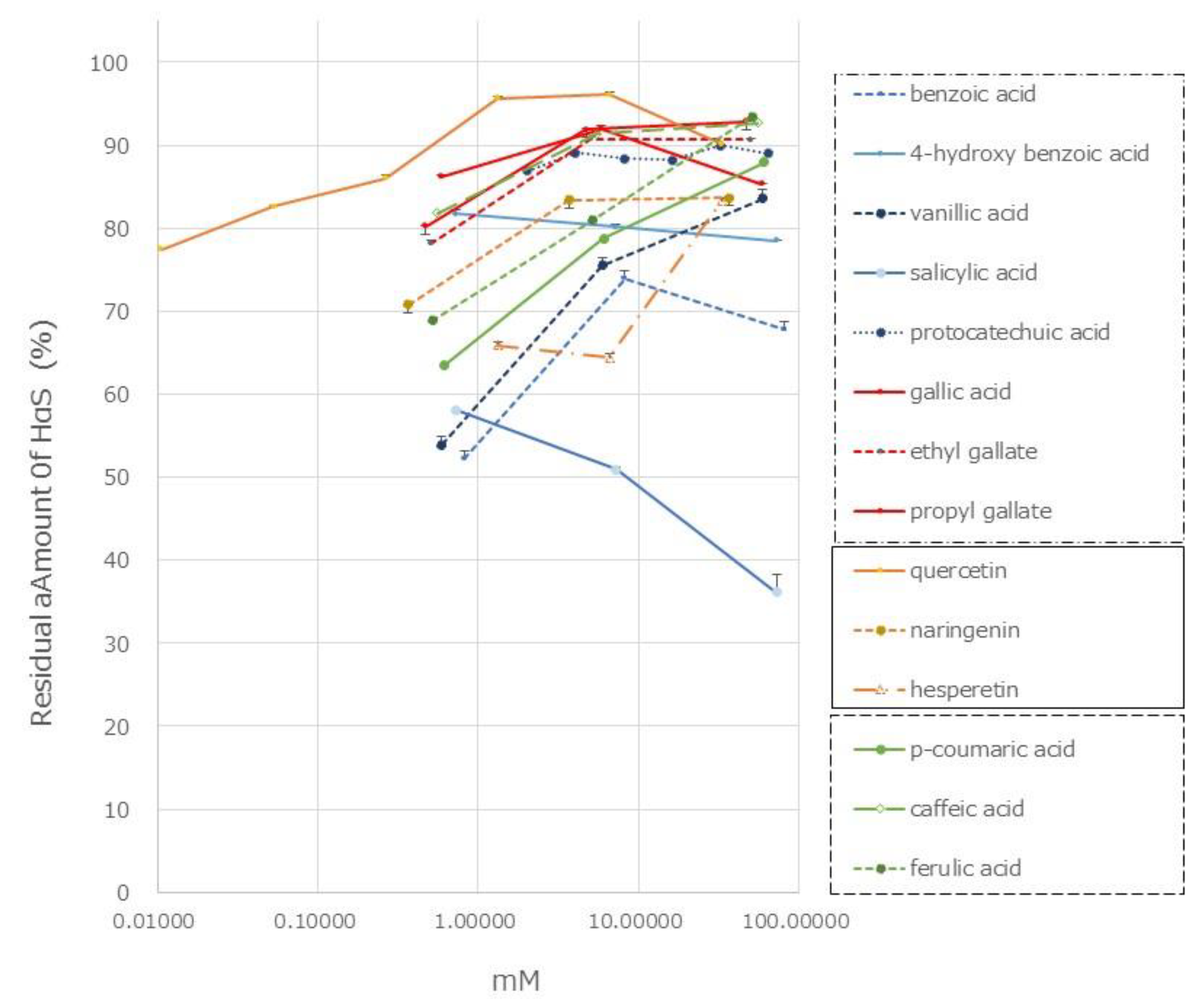
Figure 8.
Effect of pH on the stability of HαS. Ethanolic extracts of segmented membranes were mixed with the same volume of Britton–Robinson buffer (pH 2.2–12), and the mixtures were incubated at 70 °C. On days 3 and 9, the HαS content of each mixture was assayed.
Figure 8.
Effect of pH on the stability of HαS. Ethanolic extracts of segmented membranes were mixed with the same volume of Britton–Robinson buffer (pH 2.2–12), and the mixtures were incubated at 70 °C. On days 3 and 9, the HαS content of each mixture was assayed.

Table 1.
Features of sansho fruits at different maturation stages.
| Stage | Harvest Time | Color of the Pericarp | Seed |
|---|---|---|---|
| Unripe fruits | May | Green | Unmatured, soft, pale green |
| Ripe fruits | Late July to Early August | Pale green | Mature, black |
| Fully ripened fruits | September onward | Pale red to red | Mature, black |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Anti-melanogenic and Anti-oxidant Activities of an Ethanolic Extract of Kummerowia striata and its active compounds, p-coumaric acid and quercetin
Ju-Hyoung Park
et al.
,
2018
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








