Preprint
Article
Experimental Study on Photodegradation and Leaching of Typical Pesticides in Greenhouse Soil from Shouguang, Shandong Province, Northern China
Altmetrics
Downloads
111
Views
9
Comments
0
supplementary.docx (180.26KB )
This version is not peer-reviewed
Submitted:
06 August 2023
Posted:
08 August 2023
You are already at the latest version
Alerts
Abstract
Abstract: The migration and transformation of pesticides in the environment will have an impact on the ecosystem. This study collected greenhouse soil from Shouguang, Shandong Province, and studied the photodegradation and leaching of 17 common pesticides in the soil. The results of photodegradation experiments showed that the degradation rate of certain pesticides was increased in the light environment, compared with that in the dark controls. The light half-lives of emamectin benzoate, pyraclostrobin, and metalaxyl were all shorter than their respective dark half-lives, indicating that their residues in soil were greatly affected by light. The leaching experiment showed that the leaching potential of the leachable pesticides was: nitenpyram ≫ metalaxyl > acetamiprid > carbendazim > diethofencarb ≈ chlorantraniliprole > isoprothiolane > oxadixyl > boscalid ≈ tebuconazole > hexaconazole. Pesticides that are easy to leach but not easy to degrade, such as chlorantraniliprole and metalaxyl, have a high potential risk of groundwater pollution, and more degradation technologies should be used to reduce their pollution risk. The study on photodegradation and vertical migration behavior of various pesticides in this study was conducive to providing references for the agricultural use and pollution control of pesticides.
Keywords:
Subject: Environmental and Earth Sciences - Pollution
1. Introduction
Pesticides, as a type of artificially synthesized chemicals, have wide applications in reducing the loss of pests and diseases in food production. China is a traditional agricultural power and also the world's largest producer and user of pesticides. The production of chemical pesticide raw materials in China reached a peak of 3.778 million tons in 2016. Chinese production of herbicides, insecticides, and fungicides in 2016 was 1.773, 0.507, and 0.199 million tons, respectively [1]. Subsequently, in response to the “Action Plan for Zero Growth in Pesticide Use” by 2020, the production of pesticides decreased year by year, with only 2.148 million tons produced in 2020, a decrease of about 43%. The usage of pesticides in China was above 1.5 million tons from 2010 to 2018, while the usage decreased to below 1.4 million tons in the following two years. Due to the stable physical and chemical properties of certain pesticides, their accumulation continuously in the soil causing environmental pollution problems, or the transmission of crops containing pesticide residues through the food chain, which may have an impact on human health [2,3]. Therefore, the use of pesticides has gradually developed from organochlorine pesticides, which are highly toxic to humans, to low-toxicity, high-efficiency and easily degradable pesticides, such as organic nitrogen phosphorus, carbamate, neonicotinoid and pyrethroid pesticides.
The highly dependent greenhouse vegetable planting mode in China has high yield and economic benefits, and pesticides are often sprayed to prevent plant diseases and insect pests and improve crop yields [4]. However, due to the input of fertilizers and pesticides in the greenhouse, the soil properties of the greenhouse had undergone significant changes, such as degradation, acidification, salinization, imbalance, and severe pesticide residues [5], and even some easily leachable pesticides could pollute groundwater [6]. A study detected 76 pesticide residues in 317 agricultural topsoils across the European Union, and found that tebuconazole and boscalid were two of the most common and highest residual pesticides in soil, with median and maximum concentrations of 0.02 and 0.19 mg/kg, 0.04 and 0.41 mg/kg, respectively [7]. A pesticide monitoring of 75 cultivated lands in the Czech Republic found that 11% of the soil contained tebuconazole exceeding the limit of 0.01 mg/kg [8]. Tao et al. monitored 206 pairs of wheat field soil samples in the suburban of Beijing, and found that the detection rates of carbendazim and neonicotinoids were high [9]. The mean concentrations of acetamiprid, carbendazim, tebuconazole and hexaconazol were 4.5, 10.3, 36.8, and 114.2 μg/kg, with maximum values of 121.5, 179, 476.5, and 1035.6 μg/kg, respectively, and the maximum value of difenoconazole was 19 μg/kg [9]. It has been confirmed that high levels of pesticide residues exist in multiple agricultural regions.
The residual free dissolved pesticides in greenhouse soil undergo environmental behaviors such as migration and transformation under degradation and leaching [10]. Pesticides are easy to migrate under conditions such as rainfall and irrigation [11]. Wu et al. found that the impact of rainfall leaching into deep soil was greater than that of degradation on the dissipation of paclobutrazol and uniconazole [11]. Pesticides in soil were prone to degradation under the influence of light [12]. For example, the degradation rate of triazophos in moist soil was faster than that in dry soil, and the increase in light intensity increased the degradation rate of triazophos [13]. Plastic films covered in greenhouses could effectively reduce light intensity and thus reduce the photolysis rate of photosensitive pesticides. In addition, irrigation conditions also promoted the vertical migration of pesticides in the soil. Considering that current researches on pesticides mainly focus on efficacy and residues [14,15,16], and there was little research on their environmental behavior characteristics, and the types of pesticides are constantly increasing. This study conducted environmental behavior studies on multiple pesticides to provide a basis for improving food safety and reducing the risk of ecological pollution.
The purpose of this study was to analyze the influence of light on pesticide degradation in soil through photodegradation experiment, and clarify whether the vertical migration behavior of target pesticides may have an impact on groundwater pollution according to the content of pesticides leached from different volumes and polarities of leaching solution. What's more, the key points of environmental behavior were explored based on the classification characteristics of pesticides with different properties.
2. Materials and methods
2.1. Chemicals
There are 17 pesticides concerned in this study, including 6 insecticides and 11 fungicides. The target pesticide standards used in this study were purchased from AccuStandard (America), Chem Service (America), Dr. Ehrenstorfer (Germany), Anpel (China), O2si (America) and Toronto Research Chemicals (Canada), all with purity higher than 99%. The isotopelabeled internal standards including imidacloprid-d4, clothianidin-d3 and thiamethoxam-d3 with purity of 99.5%, were purchased from C/D/N Isotopes (Canada). The registered quantity, physicochemical properties and structural formulas of target pesticides are shown in Table S1, Table 1 and Figure S1, respectively. Acetonitrile, formic acid and ammonium formate were chromatographically purity and purchased from Fisher (America) and CNW (China). The ultrapure water was prepared by HHitech ultrapure water purifiers. Centrifuge using Shanghai Anting® low speed refrigerated multi-tube centrifuge.
2.2. Soil Collection
The research location of this study was located in Shouguang, Shandong Province. Shandong Province is located in the mid-latitude zone, belonging to the continental climate of the warm temperate monsoon region. Tomatoes are one of the main varieties of greenhouse vegetables in Shouguang, with a long history of cultivation. The soil samples were collected from the tomato greenhouses in Shouguang from September 2018 to early January 2019. In order to eliminate spatial differences in soil sample collection, surface soil at a depth of 10 cm was collected by five-point sampling method and then mixed. Put the evenly mixed samples into sample bags and stored them in a refrigerator at -20 ℃.
2.3. Photodegradation experiment
The impurities (gravel and plant tissue) were removed from the collected soil samples before the photodegradation experiment. After the samples were air-dried at room temperature, the soil samples screened with a 40-mesh sieve were mixed evenly and used for soil degradation experiments. Degradation of target pesticides in the soil was simulated in the laboratory artificial climate chamber under dark and light condition (a dark/light intensity of 0/2000 lux with a cycle of 24/12 h, temperature of constant 25°C, a relative humidity of 60%) for 6 and 14 days, respectively. Weigh 150 mg of soil into 1.5 mL centrifuge tube, add 100 μL 1.5 mg/L pesticide mixture standard (pesticide concentration in soil: 1000 ng/g), respectively, and add 100 μL deionized water for blank control. The photodegradation experiment was preceded by time zeroing to rule out background concentrations of pesticides, i.e., soils that may contain previously applied pesticides. A total of 31 samples were collected for dark controls and 71 samples for light degradation. Three spiked parallel samples and one blank control sample were taken for each sampling. Added 1 mL acetonitrile to each sample for extraction, rotated and mixed for 10 min, then centrifuged at the speed of 10000 r/min. 10 ng internal standard was added to about 1 mL supernatant removed, passed over 0.22 μm organophilic PTFE filter, put into a 1.8 mL brown bottle for testing.
2.4. Leaching experiment
A leaching experiment was conducted using fresh soil from the tomato greenhouse before planting in the autumn and winter seasons. Setted up two parallel samples and one blank sample, added 1 mL of 1 mg/L pesticide mixed standard to each parallel sample and added 1 mL of deionized water to the blank sample. Considering that irrigation is carried out after a period of time after pesticide application in the greenhouse, this study taken about 1 mL of surface soil and filled into a 6 mL solid phase extraction empty column after the equilibrium between pesticide and soil, and a vacuum filter device was used. Each milliliter of aqueous solution was added to the column to collect the eluent immediately. When the elution efficiency of the last milliliter of aqueous solution for all pesticides was less than 3%, we considered that the water-soluble pesticides were close to eluting completely at this time. Organic reagents with different elution capacities (methanol, acetonitrile and isopropyl alcohol) were then used to elute the pesticides and the elution effects were compared. Each milliliter of effluent was collected, and 10 ng internal standard was added, and then put into 1.8 mL brown bottle after passing a 0.22 μm hydrophilic or organophilic PTFE filter.
Table 1.
Physicochemical properties of 17 typical pesticides commonly used in Shouguang tomato greenhouses.
Table 1.
Physicochemical properties of 17 typical pesticides commonly used in Shouguang tomato greenhouses.
| Category | Pesticide | CAS number | Molecular formula |
Molecular weight |
Water solubility* (mg/L) |
Vapor pressure# (Pa) |
Henry constant* (Pa·m3/mol) |
logKow* (pH=7) |
GUS | logKoa | Koc (mL/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insecticide | Nitenpyram | 150824-47-8 | C₁₁H₁₅ClN₄O₂ | 270.72 | 5.90E+05 | 1.10E-06 | 3.54E-13 | -0.66 | 2.01 | 7.83 | 60 |
| Acetamiprid | 135410-20-7 | C₁₀H₁₁ClN₄ | 222.67 | 2.95E+03 | 1.73E-07 | 5.30E-08 | 0.8 | 0.94 | 6.35 | 343 | |
| Chlorantraniliprole | 500008-45-7 | C₁₈H₁₄BrCl₂N₅O₂ | 483.15 | 8.80E-01 | 6.30E-12 | 3.20E-09 | 2.86 | 3.51 | - | 330 | |
| Emamectin benzoate | 155569-91-8 | C56H81NO15 | 1008.24 | 2.40E+01 | 4.00E-10 | - | - | - | - | 283 | |
| Buprofezin | 69327-76-0 | C₁₆H₂₃N₃OS | 305.44 | 4.60E-01 | 4.20E-05 | 2.80E-02 | 4.93 | 0.45 | 8.7 | - | |
| Avermectin B1A | 65195-55-3 | C48H72O14 | 873.08 | 1.00E-02 | 2.00E-07 | - | 4.48 | - | 29.75 | 5638 | |
| Fungicide | Carbendazim | 10605-21-7 | C₉H₉N₃O₂ | 191.19 | 8.00E+00 | 9.00E-05 | 3.60E-03 | 1.48 | 2.21 | 10.54 | 350 |
| Oxadixyl | 77732-09-3 | C14H18N2O4 | 278.30 | 5.96E+02 | 2.70E-05 | 5.86E-07 | 1.4 | 3.89 | 10.3 | 169 | |
| Metalaxyl | 57837-19-1 | C₁₅H₂₁NO₄ | 279.33 | 8.40E+03 | 7.50E-04 | 1.60E-05 | 1.75 | 2.06 | 8.67 | 163 | |
| Diethofencarb | 87130-20-9 | C₁₄H₂₁NO₄ | 267.32 | 2.76E+01 | 9.94E-06 | 9.12E-05 | 2.89 | 1.09 | 7.36 | 271 | |
| Boscalid | 188425-85-6 | C₁₈H₁₂Cl₂N₂O | 343.21 | 4.60E+00 | 7.20E-07 | 5.18E-05 | 2.96 | 2.68 | 12.72 | 772 | |
| Tebuconazole | 107534-96-3 | C₁₆H₂₂ClN₃O | 307.82 | 3.60E+01 | 1.30E-06 | 1.00E-05 | 3.7 | 1.86 | 11.93 | 1000 | |
| Isoprothiolane | 50512-35-1 | C₁₂H₁₈O₄S₂ | 290.40 | 5.40E+01 | 1.88E-02 | 1.00E-01 | 3.3 | - | 8.69 | 1352 | |
| Hexaconazole | 79983-71-4 | C₁₄H₁₇Cl₂N₃O | 314.21 | 1.80E+01 | 1.80E-05 | 3.33E-04 | 3.9 | 2.31 | 10.77 | 1040 | |
| Difenoconazole | 119446-68-3 | C₁₉H₁₇Cl₂N₃O₃ | 406.26 | 1.50E+01 | 3.33E-08 | 9.00E-07 | 4.36 | 0.83 | 13.88 | 6120 | |
| Pyraclostrobin | 175013-18-0 | C₁₉H₁₈CIN₃O₄ | 387.82 | 1.90E+00 | 2.60E-08 | 5.31E-06 | 3.99 | 0.05 | 17.32 | 9300 | |
| Trifloxystrobin | 141517-21-7 | C₂₀H₁₉F₃N₂O₄ | 408.37 | 6.10E-01 | 3.40E-06 | 2.30E-03 | 4.5 | 0.15 | 9.86 | 2377 |
Notes: Above data are from PPBD database (https://sitem.herts.ac.uk/aeru/ppdb/index.htm); * represents the defined value of the parameter at 20℃; # represents the defined value of the parameter at 25℃; GUS represents groundwater ubiquity score, GUS < 1.8 indicates difficulty in leaching, GUS between 1.8-2.8 indicates possible leaching, GUS > 2.8 indicates easy leaching; GUS and Koc data were collected from https://www.pesticideinfo.org/; logKoa from EPI Suite software; “-” indicates that no valid data was found.
2.5. Determination of target pesticides
The target pesticides were determined by Xevo TQ-XS/Acquity UPLC-Class (Waters, USA) ultra-high pressure liquid chromatography-triple quadrupole mass spectrometry (UPLC-MS-MS) using the ACQUITY BEH C18 column (1.7 μm, 2.1 × 100 mm). ACQUITY BEH C18 VANGUARD (1.7 μm, 2.1 × 5 mm) protective column and online filter (model: 205000343) were used in front of the column. The liquid phase conditions were as follows: column temperature was 40℃, flow rate of mobile phase was 0.4 mL/min, injection volume was 2 μL. The mobile phase A was a 0.1% formic acid aqueous solution containing 5 mmol ammonium formate, the mobile phase B was 100% acetonitrile, and gradient elution conditions were shown in Table S2. The mass spectrum conditions were: electrospray ion source was in positive ion mode, the capillary voltage was 3200 V, the temperature of the ion source was 150℃, the desolvation gas and cone gas were both high-purity nitrogen gas, the flow rate of desolvation gas was 1000 L/hr, and that of cone gas was 150 L/hr. The mass spectrum parameters such as retention time, quantitative and qualitative ion pairs and voltage of the targets are shown in Table S3.
2.6. Quality assurance and quality control
The limits of quantitation (LOQs; signal-to-noise ratio of 10) ranged from 0.05 to 4.0 ng/g. The recovery (samples spiked with 20 ng standard solution) for most pesticides in soil samples was ranged from 74.4% (oxadixyl) and 105.8% (boscalid) (Table S4). Evaluation of matrix effects showed that the ionization enhancement of diethofencarb (100.1%), and the ionization suppression of the others (between 77.9% and 98.1%). During the sample pretreatment process, every 30 samples were considered as a batch, with two method blanks and two solvent blanks. A 20 ng/mL mixed pesticide standard was inserted into every 10 samples to ensure stability and reliability of analytical method during instrumental analysis.
2.7. Statistical analysis
Zero-order, first-order and second-order reaction fitting on the degradation data of pesticides in soil were performed to adjust R2 and select the optimal reaction kinetics model. The zero-order (Eq. 1,2), first-order (Eq. 3,4) and second-order (Eq. 5,6) reaction rate constants and half-lives of pesticides photodegradation were calculated as follows [17]:
where k is the reaction rate constant of pesticide photodegradation (h−1), C0 and Ct are the concentrations (μg/g) of pesticides in soil samples before and after exposure time, respectively.
In this study, IBM® SPSS Statistics 26 software was used to analyze the normality and variability of data. The relevant images were drawn using MicrocalTM Origin 2020 software.
3. Results and discussion
3.1. Degradation kinetics in soil and half-lives
The photodegradation experiment of pesticides was carried out on the tomato greenhouse soil in Shouguang City, Shandong Province. The changes of pesticide residual concentration in the soil with time in dark and light conditions are shown in Figure 1 and Figure 2, and the results of half-life and residual rate after degradation are shown in Table 2. No matter in the dark or in the light, the degradation of trifloxystrobin in greenhouse soil followed the first-order kinetic equation, with the photodegradation half-life of 3.0 days and dark degradation half-life of 4.8 days. This result shows that the degradation of trifloxystrobin in soil is fast and its half-life is short. This is consistent with the research results of Wang et al., the half-life of trifloxystrobin in soil ranges from 0.54 to 8.8, and photolysis may be an important factor affecting the dissipation of trifloxystrobin in soil [18]. The degradation rate of trifloxystrobin is faster under dark controls. It is inferred from the EE configuration acid metabolites of trifloxystrobin that hydrolysis is the main way for dark degradation of trifloxystrobin [19]. Similarly, the degradation of emamectin benzoate, chlorantraniliprole, buprofezin in insecticides and difenoconazole, pyraclostrobin, boscalid, tebuconazole, isoprothiolane, metalaxyl and oxadixyl in fungicides all follow the second-order kinetics, and their photodegradation half-lives were 16.1, 56.3, 22.8, 61.1, 23.3, 41.1, 99.1, 80.3, 104 and 17.9 days, and their dark reaction half-lives are 18.6, 53.5, 18.8, 46.1, 34.0, 30.4, 29.8, 44.4, 110 and 9.8 days, respectively. The light half-lives of emamectin benzoate in insecticides, pyraclostrobin and metalaxyl in fungicides were 86.6%, 68.5% and 94.5% of the dark half-lives, respectively, which indicated that their residues in soil were greatly affected by light. As an active site of emamectin benzoate, the diene chromophore easily leads to photodegradation of emamectin benzoate [20]. Pyraclostrobin is prone to photolysis [21], and photolysis is the primary degradation of pyraclostrobin in natural environments [22]. Similarly, photolysis plays a more important role in the dissipation of metalaxyl than the adsorption between pesticides and soil particles [23].
Among the remaining pesticides with different levels of photodegradation and dark reaction kinetics, the photodegradation half-lives of avermectin B1A and diethofencarb were lower than their dark half-lives, indicating that light may promote the degradation of pesticides. The half-lives of two neonicotinoid pesticides, nitenpyram (light: 4.1 days; dark: 4.1 days) and acetamiprid (light: 2.5 days; dark: 2.6 days), were similar and shorter under different light conditions, indicating that neonicotinoid pesticides degrade more easily in soil than most pesticides. In addition, the half-lives of biogenic pesticides (avermectin B1A) and benzimidazole fungicides (carbendazim) were both less than 10 days, indicating that their degradation rate was relatively fast under light conditions. But different from carbendazim, the half-life of avermectin B1A was 58.4 days in the dark, and its half-life in the light was 10.4% of that in the dark, illustrating that light could accelerate the degradation of avermectin B1A in soil.
The half-lives of avermectin B1A, emamectin benzoate, buprofezin, nitenpyram, and acetamiprid in insecticides and trifloxystrobin, pyraclostrobin, diethofencarb, carbendazim, and oxadixyl in fungicides were all less than 30 days, hence they were classified as easily degradable pesticides [24]. Chlorantraniliprole, difenoconazole, boscalid, hexaconazole, tebuconazole, isoprothiolane, and metalaxyl were difficult to degrade pesticides (half-life greater than 30 days). After 14 days of photodegradation, the residue rates of nitenpyram and acetamiprid in insecticides, trifloxystrobin and diethofencarb in fungicides were lower than 10%, which were 8.8%, 2.9%, 5.9%, and 5.5%, respectively. It can be reasonably believed that these four pesticides could achieve good degradation effect under light conditions. The pesticide residue rates of hexaconazole, tebuconazole, isoprothiolane and metalaxyl after photodegradation were all higher than 90% (92.7%, 90.1%, 90.0%, and 91.7%, respectively), and the residue rates were also very high in the dark. This finding was highly consistent with the results of determining the difficulty of pesticide degradation based on their half-lives, indicating that the residues of these four fungicides in soil are persistent.
Figure 1.
Dark reaction kinetic curves of 17 pesticides in greenhouse soils. The horizontal axis represents time (days), and the vertical axis represents concentration (mg/g). The degradation of avermectin B1A follows a zero order fitting, the degradation of trifloxystrobin follows a first order fitting, and the degradation of other pesticides follows a second order fitting.
Figure 1.
Dark reaction kinetic curves of 17 pesticides in greenhouse soils. The horizontal axis represents time (days), and the vertical axis represents concentration (mg/g). The degradation of avermectin B1A follows a zero order fitting, the degradation of trifloxystrobin follows a first order fitting, and the degradation of other pesticides follows a second order fitting.

Figure 2.
Photodegradation kinetic curves of 17 pesticides in greenhouse soils. The horizontal axis represents time (days), and the vertical axis represents concentration (mg/g). The degradation of diethofencarb and hexaconazole follows a zero order fit, the degradation of nitenpyram, acetamiprid, carbendazim, and trifloxystrobin follows a first order fit, and the degradation of other pesticides follows a second order fit.
Figure 2.
Photodegradation kinetic curves of 17 pesticides in greenhouse soils. The horizontal axis represents time (days), and the vertical axis represents concentration (mg/g). The degradation of diethofencarb and hexaconazole follows a zero order fit, the degradation of nitenpyram, acetamiprid, carbendazim, and trifloxystrobin follows a first order fit, and the degradation of other pesticides follows a second order fit.
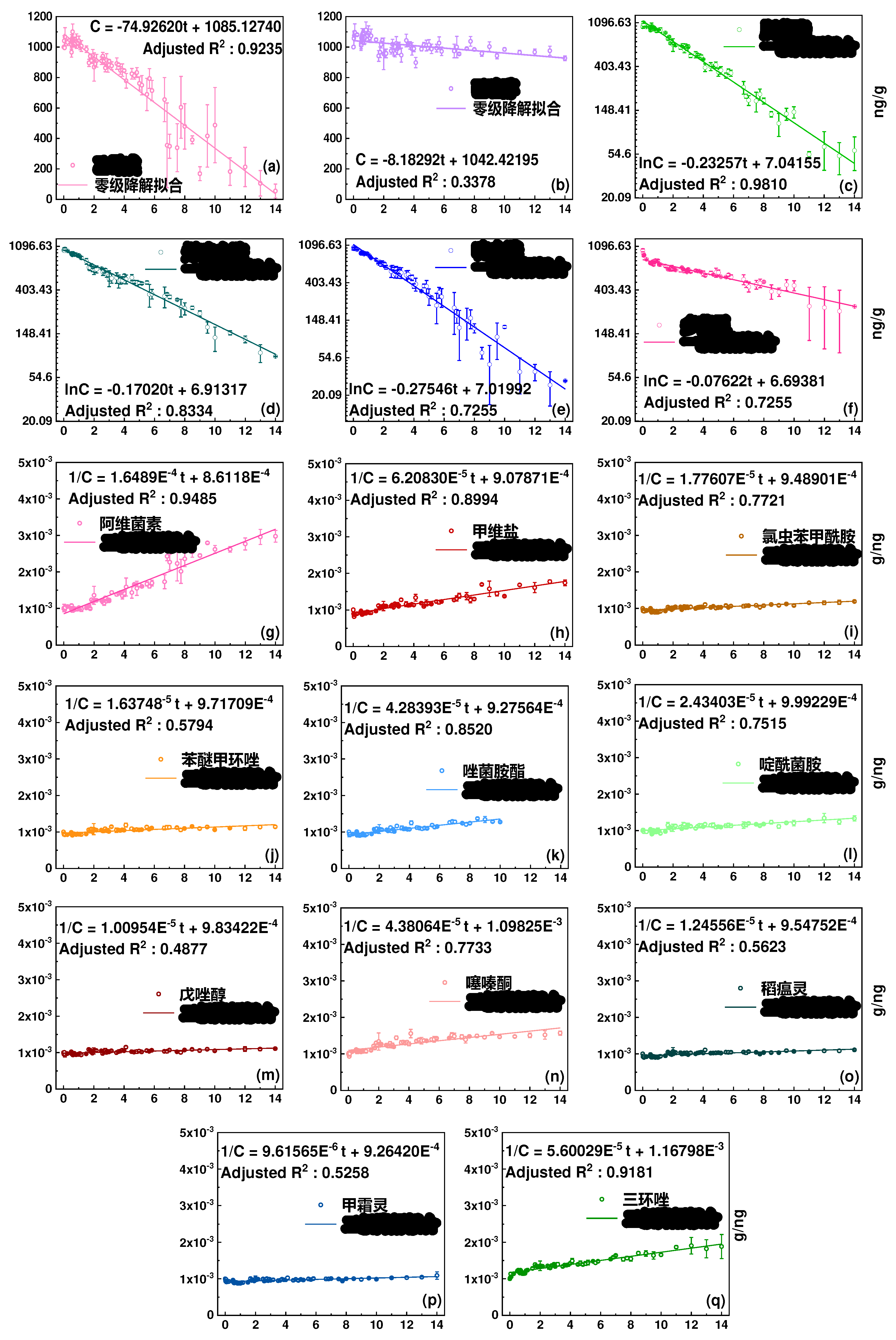
Table 2.
Degradation and residue of pesticides in soil under dark and light conditions.
| Category | Pesticide | Dark | Light | ||||
|---|---|---|---|---|---|---|---|
| Dynamic level | Half-life (day) | Residual rate after 6 days |
Dynamic level | Half-life (day) | Residual rate after 14 days |
||
| Insecticide | Nitenpyram | Second-order | 4.1 | 40.3% | First-order | 4.1 | 8.8% |
| Acetamiprid | Second-order | 2.6 | 31.5% | First-order | 2.5 | 2.9% | |
| Chlorantraniliprole | Second-order | 53.5 | 96.3% | Second-order | 56.3 | 84.1% | |
| Emamectin benzoate | Second-order | 18.6 | 79.1% | Second-order | 16.1 | 57.6% | |
| Buprofezin | Second-order | 18.8 | 75.8% | Second-order | 22.8 | 63.7% | |
| Avermectin B1A | Zero-order | 58.4 | 96.4% | Second-order | 6.1 | 33.6% | |
| Fungicide | Carbendazim | Second-order | 6.7 | 50.9% | First-order | 9.1 | 30.9% |
| Oxadixyl | Second-order | 9.8 | 61.1% | Second-order | 17.9 | 53.1% | |
| Metalaxyl | Second-order | 110 | 101.4% | Second-order | 104 | 91.7% | |
| Diethofencarb | Second-order | 19.8 | 82.0% | Zero-order | 6.7 | 5.5% | |
| Boscalid | Second-order | 30.4 | 88.4% | Second-order | 41.1 | 74.9% | |
| Tebuconazole | Second-order | 29.8 | 88.1% | Second-order | 99.1 | 90.1% | |
| Isoprothiolane | Second-order | 44.4 | 93.8% | Second-order | 80.3 | 90.0% | |
| Hexaconazole | Second-order | 42.4 | 92.9% | Zero-order | 61.1 | 92.7% | |
| Difenoconazole | Second-order | 46.1 | 92.3% | Second-order | 61.1 | 87.6% | |
| Pyraclostrobin | Second-order | 34.0 | 90.8% | Second-order | 23.3 | 78.5% | |
| Trifloxystrobin | First-order | 4.8 | 45.7% | First-order | 3.0 | 5.9% | |
Note: The degradation of pyraclostrobin under light conditions was only simulated for 10 days.
3.2. Leaching potential of pesticides in soil
Soil chromatography was used to simulate the leaching of pesticides in soil. In the elution process of aqueous solution, we observed that the elution efficiency of the seventh one-milliliter aqueous solution for each pesticide was less than 3%, at which point the water-soluble pesticides were close to completely elated. The pesticides in the column were then sequentially eluted with 1 mL of methanol, 1 mL of acetonitrile and 1 mL of isopropanol. The remaining pesticides were eluted using isopropanol.
More than 90% of all seven target pesticides, including chlorantraniliprole, metalaxyl, nitenpyram, diethofencarb, acetamiprid, carbendazim, oxadixyl, were leached by the aqueous solution (Table 3). Among them, only 25% of oxadixyl was leached by organic solvents, and the proportions of other six pesticides leached by organic solvents were less than 5%. This result is related to their higher water solubility or groundwater ubiquity score (GUS). These seven pesticides with a high leaching proportion of aqueous solution belong to neonicotinoid (nitenpyram and acetamiprid), o-formamide benzoamide (chlorantraniliprole), benzimidazole (carbendazim), phenylamide (oxadixyl), benzamide (metalaxyl) and carbamates (diethofencarb) pesticides. Neonicotinoid insecticides are widely used due to their highly water solubility (water solubility of nitenpyram: 5.90×105 mg/L; water solubility of acetamiprid: 2.95×103 mg/L) and rapid degradation characteristics (half-life: 2.5-4.1 days). The leaching amount of neonicotinoid insecticides generally increases with the increase of soil moisture content [25], and highly water-saturated soil is conducive to the leaching of neonicotinoid insecticides, which then migrate vertically and penetrate into the soil aquifers [15]. Metalaxyl also had high leaching mobility (GUS=2.06), but its degradation efficiency in soil was low (half-life: 104-110 days), indicating a high possibility of groundwater pollution [26].
For isoprothiolane, boscalid, tebuconazole and hexaconazole, more than 38% of the target compounds were leached out in aqueous solution. Except the aqueous leaching percentages of hexaconazole and tebuconazole were 38.1% and 41.9%, respectively, the aqueous leaching percentages of the other two pesticides were all over 60%. However, for the six pesticides that were difficult to leach in aqueous solution, avermectin B1A and emamectin benzoate were only leached less than 20% of the target compounds by organic solvents, while 32.6% of trifloxystrobin were leached, and more than half of difenoconazole, pyraclostrobin and buprofezin were leached. Therefore, we speculated that these six pesticides basically stayed on the surface of the soil after application, and would not enter deep soil. Avermectin B1A was strongly adsorbed on various types of soil, especially soil rich in organic matter and clay, illustrating a low probability of avermectin B1A leaching from soil and contaminating groundwater (GUS: -1.10-0.08) [27]. Similarly, the water solubility of emamectin benzoate (2.4 mg/L) is extremely low, and it also has the characteristic of being difficult to leach in soil [28]. The solvent extraction process of emamectin benzoate can be promoted by adding organic solvents (e.g., ether, acetone, ethyl acetate, or water) that are miscible with water [29].
In this study, the ratio of the volume of aqueous solution and organic solvents used for leaching was set as an indicator to evaluate the leaching potential of pesticides. The higher the ratio of aqueous solution and organic solvents, the easier infiltration and the stronger the transferability of pesticides. Among 17 pesticides, the leaching potential of avermectin B1A, emamectin benzoate, trifloxystrobin, difenoconazole, pyraclostrobin, and buprofezin, was not evaluated due to the difficulty of aqueous solution leaching. The leaching potential of the remaining 11 pesticides is: nitenpyram ≫ metalaxyl > acetamiprid > carbendazim > diethofencarb ≈ chlorantraniliprole > isoprothiolane > oxadixyl > boscalid ≈ tebuconazole > hexaconazole. The leaching potential of pesticides is closely related to their physicochemical properties.
Table 3.
Percentage of target pesticides leached from aqueous and organic solvents.
| Solvent | Volume | Nitenpyram | Acetamiprid | Chlorantraniliprole | Emamectin benzoate |
Buprofezin | Avermectin B1A | Carbendazim | Oxadixyl | Metalaxyl |
|---|---|---|---|---|---|---|---|---|---|---|
| Water solution |
1 | 67±0.2 | 74.4±1 | 53.1±0.3 | 0±0 | 0±0 | 0±0 | 53.6±2.8 | 41.5±1.2 | 64±1.5 |
| 2 | 28.4±1.9 | 33.5±2.1 | 25.2±4.2 | 0±0 | 0±0 | 0±0 | 23.7±2.5 | 22.2±2.8 | 30.8±1.4 | |
| 3 | 5.1±2.6 | 8.5±3.4 | 7.9±3.2 | 0±0 | 0±0 | 0±0 | 8.2±2.7 | 11.6±1.1 | 6.9±3.8 | |
| 4 | 1.8±1.1 | 4±2.5 | 3.9±2.1 | 0±0 | 0±0 | 0±0 | 4.5±2.5 | 8±2.5 | 2.7±1.9 | |
| 5 | 0.6±0.3 | 1.7±0.8 | 1.9±0.9 | 0±0 | 0±0 | 0±0 | 2.3±1 | 5.2±1.4 | 1±0.6 | |
| 6 | 0.3±0.1 | 0.9±0.3 | 1.2±0.4 | 0±0 | 0±0 | 0±0 | 1.4±0.5 | 3.8±0.7 | 0.5±0.2 | |
| 7 | 0.2±0.1 | 0.6±0.2 | 0.8±0.3 | 0±0 | 0±0 | 0±0 | 1±0.3 | 3±0.4 | 0.3±0.1 | |
| Methanol | 8 | 0.1±0 | 0.5±0 | 1.1±0.3 | 3.3±0.7 | 24.3±9.8 | 6.6±3.3 | 0.8±0.2 | 5.5±2.4 | 0.2±0 |
| Acetonitrile | 9 | 0.1±0 | 0.8±0.2 | 1.3±0.4 | 2.5±0.5 | 17.7±6.4 | 4.4±2.1 | 1.1±0.4 | 6.6±2.7 | 0.2±0.1 |
| Isopropanol | 10 | 0±0 | 0.8±0.2 | 1.4±0.5 | 2±0.8 | 16.3±2.6 | 3.7±0.1 | 1.2±0.4 | 7.5±1.5 | 0.2±0 |
| 11 | 0±0 | 0.3±0.2 | 0.5±0.5 | 0.4±0.4 | 6.5±5.6 | 1.2±1 | 0.4±0.3 | 2.7±2.3 | 0.1±0.1 | |
| 12 | 0±0 | 0.2±0.2 | 0.3±0.3 | 0.1±0.1 | 3±3.3 | 0.6±0.7 | 0.2±0.2 | 1.5±1.7 | 0.1±0.1 | |
| 13 | 0±0 | 0.1±0.1 | 0.2±0.2 | 0±0 | 1.7±1.8 | 0.3±0.3 | 0.1±0.1 | 0.7±0.8 | 0±0 | |
| 14 | 0±0 | 0.1±0.1 | 0.1±0.2 | 0±0 | 1.6±1.9 | 0.4±0.4 | 0.1±0.1 | 0.6±0.7 | 0±0 | |
| Subtotal | Water solution | 103.4±2.1 | 123.8±6.2 | 94±2.4 | 0±0 | 0.1±0 | 0±0 | 94.5±1.6 | 95.3±2.1 | 106.1±6.5 |
| Organic solvent | 0.1±0 | 2.6±0.6 | 4.9±1 | 8.4±0.2 | 71.1±1 | 17.2±2.8 | 3.9±0.7 | 25±1.9 | 0.9±0.1 | |
| Total | 103.6±2.1 | 126.4±6.8 | 98.9±3.4 | 8.4±0.2 | 71.2±1 | 17.2±2.8 | 98.4±2.2 | 120.4±4 | 107±6.6 | |
| Water solution/organic solvent |
799.16 | 46.74 | 19.22 | 0 | 0 | 0 | 24.36 | 3.81 | 121 | |
Continued Table 3.
Percentage of target pesticides leached from aqueous and organic solvents.
| Solvent | Volume | Diethofencarb | Boscalid | Tebuconazole | Isoprothiolane | Hexaconazole | Difenoconazole | Pyraclostrobin | Trifloxystrobin |
|---|---|---|---|---|---|---|---|---|---|
| Water solution | 1 | 48.6±1 | 24.4±0.3 | 12.6±0.4 | 29.9±0.6 | 9±0.8 | 0±0 | 0±0 | 0±0 |
| 2 | 28.4±3.8 | 16.2±4.9 | 10.4±3.5 | 24.9±6.3 | 8.4±2.9 | 0±0 | 0±0 | 0±0 | |
| 3 | 10.3±4.3 | 9.3±1.5 | 7.5±2.2 | 14.5±3.7 | 7.7±1.4 | 0±0.1 | 0±0 | 0±0 | |
| 4 | 5±2.7 | 5.5±1.3 | 4.4±1 | 8.3±3.2 | 4.5±0.4 | 0±0 | 0±0 | 0±0 | |
| 5 | 2.3±1.1 | 3.7±0.7 | 3±0.6 | 5.1±1.9 | 3.5±0.2 | 0±0 | 0±0 | 0±0 | |
| 6 | 1.4±0.5 | 2.7±0.6 | 2.3±0.4 | 3.5±1.2 | 2.8±0.2 | 0±0 | 0±0 | 0±0 | |
| 7 | 1±0.4 | 2.2±0.4 | 1.7±0.4 | 2.5±0.9 | 2.3±0.4 | 0±0 | 0±0 | 0±0 | |
| Methanol | 8 | 1.2±0.3 | 6±2.6 | 4.9±1.4 | 4±0.6 | 7.8±2.7 | 16.7±7.8 | 15.4±7.8 | 11.3±4.3 |
| Acetonitrile | 9 | 1.4±0.3 | 6.1±2.2 | 4.9±1 | 4.2±0.4 | 7±1.4 | 13.8±5.2 | 12.5±5.6 | 8.1±2.1 |
| Isopropanol | 10 | 1.3±0.6 | 6.1±2.1 | 4.8±2.2 | 3.9±1.9 | 6.8±2.9 | 13.6±1.9 | 11.5±0.3 | 7.3±2 |
| 11 | 0.5±0.5 | 2.4±2.4 | 2.1±2.1 | 1.6±1.6 | 2.9±2.8 | 5.6±5.1 | 5.2±4.9 | 2.8±2.7 | |
| 12 | 0.3±0.3 | 1.4±1.6 | 1.2±1.4 | 0.9±1.2 | 1.5±1.8 | 2.7±3.1 | 2.8±3.3 | 1.5±1.7 | |
| 13 | 0.2±0.2 | 0.7±0.8 | 0.6±0.7 | 0.4±0.6 | 0.8±0.9 | 1.5±1.7 | 1.5±1.8 | 0.8±0.9 | |
| 14 | 0.1±0.1 | 0.6±0.7 | 0.5±0.7 | 0.4±0.5 | 0.7±0.9 | 1.4±1.8 | 1.5±2 | 0.8±1 | |
| Subtotal | Water solution | 97.1±4.2 | 64±0.8 | 41.9±1.4 | 88.7±3.9 | 38.1±0.5 | 0.1±0.1 | 0±0 | 0±0 |
| Organic solvent | 4.9±1 | 23.3±2.8 | 19±4.7 | 15.5±4.8 | 27.5±5.2 | 55.3±0.8 | 50.3±0.9 | 32.6±1.8 | |
| Total | 102±5.2 | 87.2±1.9 | 60.9±6.1 | 104.2±8.7 | 65.7±5.7 | 55.4±0.8 | 50.3±0.9 | 32.6±1.8 | |
| Water solution/organic solvent | 19.71 | 2.75 | 2.21 | 5.72 | 1.38 | 0 | 0 | 0 | |
3.3. Environmental behaviors of pesticides
Pesticides applied to fields are likely to be absorbed by soil particles, or dissipated through microbial degradation, chemical hydrolysis or photodegradation [30]. In this study, it was found that the residues of many pesticides in soil were greatly affected by light, such as avermectin B1A and diethofencarb. In addition, direct photolysis was found to be the main degradation mode of pyraclostrobin [31], and sunlight irradiation could improve the degradation rate of buprofezin [32]. The photodegradation rate of pesticides in soil is affected by various factors. For example, the dark half-life of avermectin B1A in four types of Brazilian soils with different textures (clay, sandy-clay, sandy, and sandy-clay-loam) is between 9 and 13 days, which may be related to soil texture [27]. In greenhouses with suitable temperature and moist soil, the role of microbial degradation is also extremely important. The maximum time required to reduce the content of avermectin B1A in non-sterile soil by 50% was 4 days, while the concentration of avermectin B1A in sterile soil did not decrease after 37 days in dark controls, suggesting that aerobic microbial degradation may be an important mechanism leading to the degradation of avermectin B1A in soil [33]. In addition to degradation, pesticides in greenhouse soil can evaporate into the air or leach into groundwater [34], where pesticide residues may pose a threat to drinking water sources and have adverse health effects [35]. There are many factors affecting whether pesticides will infiltrate groundwater, including the physical and chemical properties of pesticides, soil texture and organic matter content, soil permeability, microbial content, application methods and amounts, and climate change [36]. The potential risk of groundwater contamination increases when certain pesticide properties overlap, including high water solubility (>30 ppm), high persistence (half-life >3 months), and low soil adsorption (log Koc < 2) [37,38].
In this study, nitenpyram, diethofencarb, acetamiprid, carbendazim and oxadixyl are easily leachable and degradable pesticides, and their degradation in soil may produce toxic chemicals. Density functional theory calculations and ecological risk evaluation of carbendazim showed that the acute toxicity of its degradation products oxidized by hydroxyl radicals in aqueous solution decreased in varying degrees compared with that of carbendazim [39]. A new nonthermal technology (dielectric barrier discharge cold plasma) was used to degrade carbendazim in aqueous solution, and the toxicity of its four degradation products (including one oxidation degradation product and three cleavage products of benzimidazole heterocyclic ring) estimated was much lower than that of carbendazim [40]. After prolonged irradiation by a solar-simulator, the toxicity of the photoproducts of carbendazim, carbendazim N-C5 dimer and other low molecular weight products, was lower than that of carbendazim [41]. Photodegradation can reduce the toxicity of carbendazim, but photodegradation products of many pesticides are more toxic. Approximately 50% of the degradation products of imidaclothiz (a neonicotinoid pesticide) were more toxic to aquatic organisms such as Daphnia magna and Danio rerio than the parent chemical [42]. Therefore, more attention should be paid to the toxicity of degradation products of easily leachable and degradable pesticides in soil.
Chlorantraniliprole and metalaxyl were pesticides that are easy to leach but not easy to degrade, while boscalid, hexaconazole, tebuconazole, and isoprothiolane were pesticides that are leachable but not easy to degrade, and their potential risk of contaminating groundwater was high. Among different drinking water sources (surface water, groundwater, water at public pumping stations, surface water chemically treated at household level, harvested rainwater, and bottled water) in rural areas of the Mekong River delta in Vietnam, isoprothiolane (maximum concentration: 8.49 μg/L) was detected in almost all water samples analyzed [43]. Monitoring on groundwater in Northwest of Italy found that the concentrations of seven monitored pesticides in 30% of wells were higher than the Environmental Quality Standard for groundwater (0.1 μg/L), which includes chlorantraniliprole and metalaxyl [44]. The most frequently detected pesticides in groundwater in a vineyard area of La Rioja (Spain) were metalaxyl, tebuconazole, and boscalid [45]. Metalaxyl had moderate mobile or mobile potential in 86% and 14% of natural soils, respectively, and tended to migrate to deeper soil layers, suggesting that continuous application of metalaxyl could result in its leaching from the soil to groundwater [46]. In shallow groundwater (<40 meter below the surface) collected from 54 monitoring wells in Long Island, New York, metalaxyl was found to be intensively used in agricultural environment [47]. The highest detected concentration of metalaxyl (89.58 ng/L) was found in different water samples (groundwater, Wenyu River, sewage treatment plants, and hospital) in Beijing, with a detection rate of 100% [48]. Fortunately, the pesticides found in groundwater in multiple regions around the world would not pose a potential health risk to humans at current concentration.
Avermectin B1A, emamectin benzoate, trifloxystrobin, pyraclostrobin and buprofezin were pesticides not easy to leach but easy to degrade, and their degradation processes (photolysis, hydrolysis, biodegradation) often occured in upper soil. Avermectin B1A and its metabolites do not migrate easily in soil due to their low solubility and strong adsorption with organic matter (Koc ≥ 4000). They were mostly degraded in the environment through photodegradation and aerobic decomposition of soil organisms [49]. The high partition coefficient of trifloxystrobin demonstrates its strong adsorption in all experimental soils originating from India and Germany [19]. Field leaching data showed that trifloxystrobin still existed in the top 80 cm after 16 weeks treatment, indicating that the possibility of trifloxystrobin leaching was extremely low [50]. And another field studies of trifloxystrobin in soil of different latitudes found that photolysis may be the primary dissipation route of trifloxystrobin, and sunshine hours may have a significant effect on the dissipation of trifloxystrobin [51]. Similarly, most residues of pyraclostrobin remained in the topsoil even under high rainfall conditions, therefore, the migration potential of pyraclostrobin reached to lower soil depth was almost negligible [52]. Many studies have focused on the biodegradation of these non-leachable but easily degradable pesticides in the topsoil. Trifloxystrobin, buprofezin and pyraclostrobin could be degraded by Hyphomicrobium sp. [53], Rhodococcus sp. (YL-1) [54] or Pseudomonas sp. (DFS35-4) [55], HI2 and HI6 [56], respectively. Among them, DFS35-4 strain was able to degrade 50 mg/L buprofezin by over 70% in three days (under the condition of pH 5.0-10.0 and temperature 20-30℃) [55], and HI2 and HI6 microorganisms degraded more than 99% of pyraclostrobin within five days (C0 = 100 mg/L) [56], exhibiting excellent microbial degradation effects. The toxic effects of degradation products formed by various degradation reactions of pesticides in the soil surface are also of concern. The results of field experiments in Beijing and Shandong Province showed that the main residual compound in tomato was trifloxystrobin, whereas it was its metabolite trifloxystrobin acid (CGA321113) in soil [57]. The final residual level of trifloxystrobin and its metabolite in tomato were lower than the EU maximum residue limit (0.5 mg/kg), and the residue of metabolites in soil samples was higher than its parent compound [57]. The simulation of molecular docking suggested that the CDOCKER interaction energy of pyraclostrobin (-44.71) was lower than its intermediate products (>-30.00), Methyl N-phenyl-carbamate and 1-(4-chlorophenyl)-3-hydroxy-1H-pyrzole, indicating that the intermediates were less toxic than pyraclostrobin [31]. Avermectin B1A in soil is not easily taken up by plants, and it will not be bioconcentrated by fish (calculated steady-state bioconcentration coefficient was 52, which had a rapid depuration) [58]. Overall, further research is needed on the degradation process of these non-leachable but easily degradable pesticides in soil and the physicochemical properties of the degradation products.
Difenoconazole was a pesticide that was difficult to leach and degrade, thus it may easily accumulate in plant crops. Difenoconazole could be absorbed and accumulated in rice plants (Oryza sativa L.) under soil-treated conditions, the concentrations of difenoconazole in roots (15.6 and 79.1 mg/kg dw) were much higher than that in leaves (0.23 and 3.4 mg/kg dw) [59]. The deposition amount of difenoconazole increased gradually with the increase of dosages, and its accumulation order was upper leaf > lower leaf > upper stem > lower stem > cultivated soil > fruit [60]. After excessive accumulation in plant tissues, difenoconazole could be rapidly degraded by glutathione S-transferase, or reduced oxidative damage by increasing the content of flavonoids and anthocyanins in leaves, thereby achieving self-protection [61].
In this study, the photodegradation and leaching of typical pesticides in tomato greenhouse soil were studied, which are located in Shouguang, Shandong Province, northern China. The impacts of light on pesticide degradation were explored by comparing the half-lives of pesticides under light and dark controls, and the degradation degrees of pesticides in the soil were analyzed based on the residual rate after treatment. Aqueous solution and organic solvents were used to leach pesticides from greenhouse soil. According to the leaching content of pesticides in different volumes and types of leaching solutions, the vertical migration ability of various pesticides in the soil was analyzed, and the potential risk of groundwater pollution for pesticides applied in tomato greenhouses was explored. In addition, this study combined the degradation and migration of pesticides in soil to analyze the environmental behavior and toxicity of different types of pesticides, providing a scientific basis for the agricultural application of pesticides. However, there are also some limitations in this study. First, only the effects of the dark and natural light environment on pesticide degradation were considered when conducting photodegradation research, UV photodegradation experiments were not included. However, the design of natural light was also consistent with the reality of conventional agricultural production in tomato greenhouses. Second, the half-lives of certain pesticides in the dark were shorter than that in the light, which may be due to the simulation time under dark controls was shorter, which could not better reflect the long-term changes of pesticides in soil. Further experiments should extend the dark reaction time, better fit the kinetic equation of pesticide degradation, and provide more accurate data support for photodegradation of pesticides in greenhouse soil. Last but not least, as mentioned before, there are many factors that affect the degradation and leaching of pesticides, such as soil texture and organic matter content, among others. The lack of these soil analyses in this study makes it impossible to determine the extent to which pesticide degradation and leaching processes are affected by them. However, the samples used for intercomparison in this study were all from the same well-mixed soil samples, which strictly maintains the consistency of the variables other than the independent variables. Therefore, the results of this study are informative for the study of the environmental processes of pesticides in greenhouse soils.
4. Conclusions
In order to study the migration and degradation behavior of pesticides, photodegradation and leaching experiments were carried out with 17 common pesticides in tomato greenhouse soil in Shandong Province, northern China. The half-lives of avermectin B1A, emamectin benzoate, buprofezin, nitenpyram, acetamiprid, trifloxystrobin, pyraclostrobin, diethofencarb, carbendazim and oxadixyl were less than 30 days, which were easily degradable pesticides. The residue rates of nitenpyram, acetamiprid, trifloxystrobin and diethofencarb were all lower than 10% after 14 days of photodegradation, indicating that these pesticides could achieve good degradation effects under light conditions. The light half-lives of emamectin benzoate, pyraclostrobin and metalaxyl were 86.6%, 68.5% and 94.5% of their respective dark half-lives, indicating that their photodegradation rates in soil were high. More than 90% of chlorantraniliprole, metalaxyl, nitenpyram, diethofencarb, acetamiprid, carbendazim and oxadixyl, can be leached under aqueous solution leaching. Avermectin B1A, emamectin benzoate, trifloxystrobin, difenoconazole, pyraclostrobin and buprofezin, which were difficult to leach in aqueous solution, may not enter deep soil. The leaching potential of leachable pesticides was: nitenpyram ≫ metalaxyl > acetamiprid > carbendazim > diethofencarb ≈ chlorantraniliprole > isoprothiolane > oxadixyl > boscalid ≈ tebuconazole > hexaconazole. The environmental behavior analysis of pesticides indicated that the toxicity of degradation products of pesticides that are easily leachable and degradable should be paid more attention. For pesticides that are leachable but not easily degradable, the potential risk of groundwater pollution should be explored. For pesticides that are difficult to leach and degrade, further research should be conducted on their accumulation in crops.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: List of commonly used pesticides and registered quantities of pesticides in vegetable areas under Shouguang facilities; Table S2: Elution gradient of mobile phase in liquid chromatography; Table S3: Instrumental analysis parameters of target pesticides; Table S4: Recovery of typical pesticides in soil; Figure S1: Structural formulas of 17 target pesticides.
Author Contributions
Li-Ting Hua: Formal analysis, Writing-original draft & editing. Rui-Lin Wu: Sampling, Methodology, Experimentation, Formal analysis. Chao-Nan Wang: Sampling. Yi-Long Li: Sampling. Fu-Liu Xu: Writing - review & editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This study was funded by the National Key Research and Development Plan of China (2016YFD0201204) and by the National Natural Science Foundation of China - Shandong United Fund (U2006214).
Conflicts of Interest
The authors declare no conflict of interest.
References
- CCPIA, Chinese Pesticide Industry Yearbook 2017. 2017, Beijing: ChinaCrop Protection Industry Association.
- Singh, S., et al., Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett., 2016. 14(3): p. 317-329. [CrossRef]
- Wang, Y., et al., Accumulation and toxicity of thiamethoxam and its metabolite clothianidin to the gonads of Eremias argus. Sci. Total Environ., 2019. 667: p. 586-593. [CrossRef]
- Song, Y., et al., Long-term plastic greenhouse cultivation changes soil microbial community structures: A case study. J. Agric. Food Chem., 2018. 66(34): p. 8941-8948. [CrossRef]
- Hu, W., et al., Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere, 2017. 170: p. 183-195. [CrossRef]
- Li, Y., et al., Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere, 2018. 207: p. 708-714. [CrossRef]
- Silva, V., et al., Pesticide residues in European agricultural soils - A hidden reality unfolded. Sci. Total Environ., 2019. 653: p. 1532-1545. [CrossRef]
- Hvezdova, M., et al., Currently and recently used pesticides in Central European arable soils. Sci. Total Environ., 2018. 613-614: p. 361-370. [CrossRef]
- Tao, Y., et al., Occurrence and dietary risk assessment of 37 pesticides in wheat fields in the suburbs of Beijing, China. Food Chem., 2021. 350: p. 129245. [CrossRef]
- Rani, S. and D. Sud, Degradation of dimethoate pesticide in soil: Impact of soil moisture and enhanced sunlight intensity. Water. Air. Soil Pollut., 2022. 233: p. 24. [CrossRef]
- Wu, C., et al., Dissipation and enantioselective degradation of plant growth retardants paclobutrazol and uniconazole in open field, greenhouse, and laboratory soils. Environ. Sci. Technol., 2013. 47(2): p. 843-849. [CrossRef]
- Konstantinou, I., A. Zarkadis, and T. Albanis, Photodegradation of selected herbicides in various natural waters and soils under environmental conditions. J. Environ. Qual., 2001. 30(1): p. 121–130. [CrossRef]
- Rani, S. and D. Sud, Role of enhanced solar radiation for degradation of triazophos pesticide in soil matrix. Sol. Energy., 2015. 120: p. 494-504. [CrossRef]
- Houbraken, M., et al., Multi-residue determination and ecological risk assessment of pesticides in the lakes of Rwanda. Sci. Total Environ., 2017. 576: p. 888-894. [CrossRef]
- Menon, M., R. Mohanraj, and W. Sujata, Monitoring of neonicotinoid pesticides in water-soil systems along the agro-landscapes of the Cauvery delta region, South India. Bull. Environ. Contam. Toxicol., 2021. 106(6): p. 1065-1070. [CrossRef]
- Sultana, T., et al., Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere, 2018. 202: p. 506-513. [CrossRef]
- Xi, N., et al., Elevated temperatures decrease the photodegradation rate of pyrethroid insecticides on spinach leaves: Implications for the effect of climate warming. Environ. Sci. Technol., 2021. 55(2): p. 1167-1177. [CrossRef]
- Wang, C., et al., Field dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in soil and apples. Environ. Monit. Assess., 2015. 187(1): p. 4100. [CrossRef]
- Banerjee, K., A. Ligon, and M. Spiteller, Environmental fate of trifloxystrobin in soils of different geographical origins and photolytic degradation in water. J. Agric. Food Chem., 2006. 54: p. 9479-9487. [CrossRef]
- Shang, Q., et al., Pesticide-conjugated polyacrylate nanoparticles: novel opportunities for improving the photostability of emamectin benzoate. Polym. Adv. Technol. , 2012. 24: p. 137-143. [CrossRef]
- Gao, Y., et al., Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. J Hazard Mater, 2021. 414: p. 125513. [CrossRef]
- Zeng, L., et al., Evaluation of photolysis and hydrolysis of pyraclostrobin in aqueous solutions and its degradation products in paddy water. J. Environ. Sci. Health, Part B, 2019. 54: p. 317-325. [CrossRef]
- Bermúdez-Couso, A., et al., Influence of different abiotic and biotic factors on the metalaxyl and carbofuran dissipation. Chemosphere, 2013. 90(10): p. 2526-2533. [CrossRef]
- Li, Y., Residue dynamics of typical pesticides in the water-soil-crop system of paddy field and their effects on soil microorganisms, in College of Urban and Environmental Sciences. 2020, Peking University: Beijing. p. 253.
- Radolinski, J., et al., Transport of a neonicotinoid pesticide, thiamethoxam, from artificial seed coatings. Sci. Total Environ., 2018. 618: p. 561-568. [CrossRef]
- Kookana, R., H. Di, and L. Aylmore, A field study of leaching and degradation of nine pesticides in a sandy soil Aust. J. Soil Res., 1995. 33: p. 1019-1030. [CrossRef]
- de Oliveira Ferreira, F., R. Porto, and S. Rath, Aerobic dissipation of avermectins and moxidectin in subtropical soils and dissipation of abamectin in a field study. Ecotoxicol. Environ. Saf., 2019. 183: p. 109489. [CrossRef]
- Takai, K., et al., Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci., 2001. 57(5): p. 463-466. [CrossRef]
- Zhang, S., et al., Preparation and physicochemical characteristics of polylactide microspheres of emamectin benzoate by modified solvent evaporation/extraction method. J. Agric. Food Chem., 2013. 61(50): p. 12219-12225. [CrossRef]
- Kaur, R., et al., Pesticide residues degradation strategies in soil and water: a review. Int. J. Environ. Sci. Technol., 2021. 20(3): p. 3537-3560. [CrossRef]
- Fan, L., et al., Photolysis and photo-induced toxicity of pyraclostrobin to Vibrio fischeri: Pathway and toxic mechanism. Aquat. Toxicol., 2020. 220: p. 105417. [CrossRef]
- Fenoll, J., et al., Rate of loss of insecticides during soil solarization and soil biosolarization. J. Hazard. Mater., 2011. 185(2-3): p. 634-638. [CrossRef]
- Dionisio, A. and S. Rath, Abamectin in soils: Analytical methods, kinetics, sorption and dissipation. Chemosphere, 2016. 151: p. 17-29. [CrossRef]
- Liu, J., et al., Physiochemical assessment of environmental behaviors of herbicide atrazine in soils associated with its degradation and bioavailability to weeds. Chemosphere, 2021. 262: p. 127830. [CrossRef]
- Swartjes, F. and M. Van der Aa, Measures to reduce pesticides leaching into groundwater-based drinking water resources: An appeal to national and local governments, water boards and farmers. Sci. Total Environ., 2020. 699: p. 134186. [CrossRef]
- Li, Z., A health-based regulatory chain framework to evaluate international pesticide groundwater regulations integrating soil and drinking water standards. Environ. Int., 2018. 121: p. 1253-1278. [CrossRef]
- Aliste, M., et al., Mobility of insecticide residues and main intermediates in a clay-loam soil, and impact of leachate components on their photocatalytic degradation. Chemosphere, 2021. 274: p. 129965. [CrossRef]
- Gavrilescu, M., Fate of Pesticides in the Environment and its Bioremediation. Eng. Life Sci., 2005. 5(6): p. 497-526. [CrossRef]
- Liu, W., et al., DFT insights into the degradation mechanism of carbendazim by hydroxyl radicals in aqueous solution. J. Hazard. Mater., 2022. 431: p. 128577. [CrossRef]
- Wang, J., et al., Degradation of carbendazim in aqueous solution by dielectric barrier discharge cold plasma: Identification and toxicity of degradation products. Food Chem., 2023. 403: p. 134329. [CrossRef]
- Jornet, D., et al., Photodegradation of carbendazim sensitized by aromatic ketones. J. Photochem. Photobiol. A Chem., 2013. 256: p. 36-41. [CrossRef]
- Ma, C., et al., Kinetics, mechanisms and toxicity of the degradation of imidaclothiz in soil and water. J. Hazard. Mater., 2021. 403: p. 124033. [CrossRef]
- Chau, N., et al., Pesticide pollution of multiple drinking water sources in the Mekong Delta, Vietnam: evidence from two provinces. Environ. Sci. Pollut. Res. Int., 2015. 22(12): p. 9042-9058. [CrossRef]
- Zambito Marsala, R., et al., First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Total Environ., 2020. 736: p. 139730. [CrossRef]
- Manjarres-Lopez, D., et al., Assessment of pesticide residues in waters and soils of a vineyard region and its temporal evolution. Environ. Pollut., 2021. 284: p. 117463. [CrossRef]
- Andrades, M., M. Sa´nchez-Martı´n, and M. Sa´nchez-Camazano, Significance of soil properties in the adsorption and mobility of the fungicide metalaxyl in vineyard soils. J. Agric. Food Chem., 2001. 49: p. 2363-2369. [CrossRef]
- Fisher, I., et al., Pesticides and their degradates in groundwater reflect past use and current management strategies, Long Island, New York, USA. Sci. Total Environ., 2021. 752: p. 141895. [CrossRef]
- Zhang, Y., et al., Occurrence and risk evaluation of organophosphorus pesticides in typical water bodies of Beijing, China. Environ. Sci. Pollut. Res. Int., 2021. 28(2): p. 1454-1463. [CrossRef]
- Wratten, S. and A. Forbes, Environmental assessment of veterinary avermectins in temperate pastoral ecosystems. Ann. appl. Biol., 1996. 128: p. 329-348. [CrossRef]
- Dusek, J., et al., Field leaching of pesticides at five test sites in Hawaii: study description and results. Pest Manag. Sci., 2010. 66(6): p. 596-611. [CrossRef]
- Wang, C., et al., Field dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in soil and apples. Environ. Monit. Assess., 2015. 187: p. 4100. [CrossRef]
- Reddy, S., S. Gupta, and V. Gajbhiye, Adsorption-desorption and leaching of pyraclostrobin in Indian soils. J. Environ. Sci. Health, Part B, 2013. 48(11): p. 948-959. [CrossRef]
- Jiang, W., et al., Esterase TriH responsible for the hydrolysis of trifloxystrobin in Hyphomicrobium sp. B1. Int. Biodeterior. Biodegrad., 2022. 174: p. 105465. [CrossRef]
- Li, C., et al., Biodegradation of buprofezin by Rhodococcus sp. strain YL-1 isolated from rice field soil. J. Agric. Food Chem., 2012. 60(10): p. 2531-2537. [CrossRef]
- Chen, K., et al., Isolation of a buprofezin co-metabolizing strain of Pseudomonas sp. DFS35-4 and identification of the buprofezin transformation pathway. Biodegradation, 2011. 22(6): p. 1135-1142. [CrossRef]
- Chen, X., et al., Biodegradation of pyraclostrobin by two microbial communities from Hawaiian soils and metabolic mechanism. J. Hazard. Mater., 2018. 354: p. 225-230. [CrossRef]
- Wang, L., et al., Residues and dissipation of trifloxystrobin and its metabolite in tomatoes and soil. Environ. Monit. Assess., 2014. 186(11): p. 7793-7799. [CrossRef]
- Halley, B., W. VandenHeuvel, and P. Wislocki, Environmental effects of the usage of avermectins in livestock. Vet. Parasitol., 1993. 48: p. 109-125. [CrossRef]
- Ge, J., et al., Uptake and translocation of imidacloprid, thiamethoxam and difenoconazole in rice plants. Environ. Pollut., 2017. 226: p. 479-485. [CrossRef]
- Chen, S., et al., Deposition distribution, metabolism characteristics, and reduced application dose of difenoconazole in the open field and greenhouse pepper ecosystem. Agr. Ecosyst Environ., 2021. 313: p. 107370. [CrossRef]
- Li, J., et al., Transcriptomic and physiological properties reveal the tolerance mechanism to difenoconazole toxicity in wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf., 2023. 255: p. 114787. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Experimental Study on Photodegradation and Leaching of Typical Pesticides in Greenhouse Soil from Shouguang, Shandong Province, Northern China
Li-Ting Hua
et al.
,
2023
Assessing the Dissipation of Pesticides of Different Polarities in Soil Samples
Carlos Eduardo Rodríguez-Palma
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








