Preprint
Article
Role of Curing Agents in the Adaptive Response of the Bioprotective Latilactobacillus Curvatus CRL 705 from a Physiologic and Proteomic Perspective
Altmetrics
Downloads
97
Views
28
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.docx (2.28MB )
This version is not peer-reviewed
Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Alerts
Abstract
During meat processing, lactic acid bacteria (LAB) have to competitively adapt to the hostile en-vironment produced by curing additives (CA). The objective of this study was to investigate the ability of Latilactobacillus curvatus CRL 705, a bioprotective strain of meat origin, to adapt to CA. A physiological and proteomic approach was performed. CRL 705 was grown in a chemically de-fined medium (CDM) containing specific concentrations of CA (NaCl, nitrite, sucrose and ascor-bic acid). The results showed minor differences in growth kinetics in the presence of CA. Glucose consumption, present in CDM, and production of lactic acid and bacteriocins were not signifi-cantly affected. Proteomic analyses indicated that most of the identified proteins (36 out of 39) mainly related to carbohydrate metabolism (18%), posttranslational modifications (15.6%), energy production and conversion (11.1%), translation (11.1%) and nucleotide metabolism (8.9%), were under expressed. In response to the studied CA, CRL 705 slowed down its general metabolism, achieving slight changes in physiological and proteomic parameters. The observed performance is another characteristic that extends the well-known competitive profile of CRL 705 as a meat starter- and -bioprotective culture. This is the first report dealing with the impact of CA on LAB proteomics.

Keywords:
Subject: Biology and Life Sciences - Food Science and Technology
1. Introduction
Lactic acid bacteria (LAB) contribute to the hygienic and sensory quality of fermented meat products primarily through their carbohydrate and protein catabolism, resulting in sugar depletion, pH reduction, production of antimicrobial agents and the generation of flavor compounds [1,2]. Based on these characteristics, LAB are the preferred bacteria for the formulation of starter cultures. Appropriate cultures of indigenous microorganisms must often be selected to be more competitive. This means that they are well adapted to a particular substrate and have high metabolic capacities to beneficially affect quality and safety while preserving product typicity [3]. Therefore, competitive functional cultures have gained increasing attention to naturally control the shelf-life and safety of meat products [4]. The existence of a sequence of hurdles either specifically included, such as preservative compounds, generally known as curing additives (CA) or indirectly created in the stuffed mixture (low Eh, pH and aw, and LAB bacteriocins) will regulate bacterial growth in this ecosystem [5,6].
In spontaneously fermented sausages, the facultative heterofermentative lactobacilli Latilactobacillus sakei, Latilactobacillus (L.) curvatus, and Lactiplantibacillus (L.) plantarum constitute the predominant microbiota throughout ripening. L. curvatus has shown ubiquity and characteristics to deal with meat environment [7,8]. The L. curvatus CRL 705 strain, isolated from an Argentinean artisanal fermented sausage [9], produces two bacteriocins, lactocin Lac705 and the antilisterial AL705. Indeed, it has great technological and bioprotective potential, and constitutes our study model; it had been the subject of detailed biochemical, molecular and technological studies [10,11,12,13]. In addition, it was the first strain of the L. curvatus species whose genome was sequenced and deposited in GenBank [14]. The adaptation of the CRL 705 strain to the meat environment may be related to the presence in its genome of the rbsUDKR gene cluster for ribose catabolism that encodes a rbsU ribose transporter [7,8,15] similar to that of L. sakei strains [16,17]. Another adaptive feature of CRL 705 related to meat competitiveness is the presence of the catabolic cluster of N-acetylglucosamine, which allows the use of this compound, present in the muscle, as an energy source. In addition, as in other strains of L. curvatus, CRL 705 has an additional gene that encodes D-lactyl ether N-acetylmuramic 6-phosphate acid etherase for the catabolism of N-acetylmurein, also present in meat [8].
As mentioned above, CA are included in meat products to ensure better preservation and color/flavor development. The CA most used in the processing of dry fermented sausages are sodium chloride (NaCl), which enhances the flavor, favors drying and hinders microbial growth; sodium nitrite (NaNO2), which acts as a preservative and, when it is reduced to nitric oxide, participates in color development; ascorbic acid, used as an antioxidant; and sucrose, which acts as an additional energy source to ensure dominance of the starter culture [18]. Thus, during the processing, drying and ripening of fermented meat products, a hostile environment develops that selectively limits the microbiota present and to which the lactic acid strains manage to competitively adapt.
In this context, the objective of this work was to evaluate the response of L. curvatus CRL 705 during its growth in the presence of curing additives, through a physiological and proteomic approach. This approach would allow us to explore the metabolic routes mainly affected by the curing mixture and the strategies and culture conditions that this strain needs to improve its performance during the fermentation of meat subjected to curing conditions.
2. Materials and Methods
2.1. Bacterial strains and culture conditions
L. curvatus CRL 705 was isolated from an Argentinean dry fermented sausage [9] and belongs to the CERELA-CONICET culture collection. This strain was stored at −70 °C in milk yeast extract medium (10% w/v skim milk, 0.5% w/v yeast extract) containing 10% (v/v) glycerol as a cryoprotectant. Lactiplantibacillus plantarum CRL 691 (CERELA-CONICET culture collection), used as a sensitive target organism for the bacteriocin lactocin 705, was cultured using similar procedures than CRL705; while Listeria monocytogenes FBUNT from the National University of Tucumán (Tucumán, Argentina) was used as indicator strain for the bacteriocin AL705. This indicator microorganism was stored in Brain Heart Infusion (BHI) medium (Britania, Buenos Aires, Argentina) and activated before use in the same medium.
2.2. Bacterial growth
A preculture of L. curvatus CRL 705 was activated for 24 h in MRS broth at 30°C, transferred to chemical defined medium (CDM) [19] and incubated for 16 h at 30°C. This subculture was used to inoculate 150 mL CDM containing CA (CDM+CA) to an initial OD= 0.05-0.1. A culture grown in CDM without CA was used as a control. Glucose, as a carbon source, was added at a final concentration of 0.75% in both culture media. CDM+CA also contained 0.75% sucrose (w:v), 3% sodium chloride (w:v), 0.02% sodium nitrite (w:v), and 10 mg mL-1 ascorbic acid. Growth of L. curvatus CRL 705 was monitored during 24 h at 25 ºC (temperature used for fermentation during dry fermented sausages processing) by measuring optical density (OD) at 600 nm and cell viability in samples taken at 0, 2, 4, 6, 8, 10, 12, 16, 20 and 24 h of incubation. For bacterial enumeration, decimal dilutions were prepared, plated on MRS agar (Britania, Buenos Aires, Argentina), and incubated at 30 ºC for 48 h. Results were expressed as colony forming units per milliliter (CFUmL-1). The acidification potential of L. curvatus during growth on CDM was determined by measuring pH using an Altronix TPX 1 (New York, USA) digital pH-meter. Three independent cultures were performed for each condition.
2.3. Determination of the activity of Lac705 and AL705 bacteriocins
For antimicrobial activity, culture samples of L. curvatus CRL 705 were centrifuged (12,000 ×g, 10 min) and cell free supernatants (CFS) were collected and heated at 80 ºC for 20 min. The lactocin Lac705 activity was evaluated by the well-diffusion assay, according to Salvucci et al. [20] with some modifications. Briefly, on a Petri dish with 10 mL of MRS agar (1.2%), 10 mL of MRS soft agar (0.7%) containing 70 μL of the culture of the indicator strain L. plantarum CRL 691 was poured. After solidification, 5 µL of a two-fold serial dilution of each CFS at different sampling times (from 0 to 72 h) were seeded. After incubation at 30°C for 24 h, the presence or absence of a zone of growth inhibition (halo) around the seeded spot was observed. Results were expressed in arbitrary units per milliliter (AUmL-1) (AU mL-1= 1 / seeded vol × dilution factor). To test the activity of AL705, the same protocol was carried out but using Listeria monocytogenes FBUNT as the indicator strain in BHI medium.
2.4. Consumption of carbon sources and production of lactic acid, acetic acid and ethanol
The sugars and the end-products were determined in the Special Analyses Laboratory of CERELA-CONICET, Tucumán, Argentina. Sugar consumption and metabolite production were assessed at 0, 4, 12 and 24 h of cell growth in CDM with or without additives, as well as at the time of cell harvest for proteomic analyses. Lactic and acetic acids were measured by HPLC, using an Aminex HPX-87H ion exclusion column (ISCO 2350 model, 300 × 7.8 mm2, Bio-Rad Laboratories Inc., CA, USA), as previously described by Gerez et al. (2010) [21]. End-products concentrations were expressed as g L-1. Sugar consumption was also evaluated by HPLC, using an Aminex HPX-87P column (Bio-Rad), according to Ortiz et al. (2012) [22]. All data were analyzed using Eurochrom Basic Edition software for Windows.
2.5. Differential protein expression analysis
2.5.1. L. curvatus CRL705 cells recovery
For the proteomic assay, cells in late exponential growth phase in CDM+CA (6 h) or without CA- (6 h) were collected by centrifugation at 8,000 ×g (10 min , 20°C) washed and the pellets stored at −20°C until lysis to obtain CRL705 proteome. The experiment was replicated independently three times for each condition.
2.5.2. Cell-free protein extraction
Lysis of cells were carried out with glass beads (150 ± 212 μm in diameter, Sigma-Aldrich Co., St. Louis, MO, USA) and resuspended in 0.1 mol L-1 Tris-HCl buffer pH 7.5 in a ratio 1:2:1 (cell:buffer:bead) as described by Orihuel [23]. After lysis, samples were centrifuged (14,500 ×g, 10 min, 15 °C) to recover cell-free extract supernatant containing the proteome of L. curvatus CRL 705. The protein concentration analysis (Bradford assay) was determined. Aliquots of 600 μg of protein were finally stored at −80 °C, until further analysis.
2.5.3. Two-Dimensional Gel Electrophoresis (2DE)
Sample preparation and 2DE gels were carried out according to Bustos [24]. Isoelectrofocusing (IEF) was carried out in IPGphor (GE Healthcare, Uppsala, Sweden) at 53,500 Vh, with immobilized pH gradient (IPG) strips (ImmobilineDryStrip Gels, linear pH 4-7, 18 cm; GE Healthcare, Uppsala, Sweden). After IEF, strips were equilibrated at room temperature in 6 mol L-1 urea, 2% (w/v) SDS, 30% (w/v) glycerol, 50 mmol L-1Tris-HCl, pH 8.0 and the SDS-PAGE step was performed on homogeneous 12.5% (w/v) polyacrylamide gels (25 x 20.5 cm) at the constant current of 15 mA/gel at 15 °C (~16 h) using an Ettan DALTsix Large Vertical System (GE Healthcare). Colloidal Coomassie Blue Stain according to Candiano [25] was used for gel staining. The 2DE gels were digitalized using Image Scanner III LabScan 6.0 (GE Healthcare).
2.5.4. Image Acquisition and Data Analysis
Prodigy SameSpots version 1.0.3400.25570 (TotalLab, Newcastle, UK) was used for volume spot quantification using digitized gel images. A protein was considered differentially abundant if the normalized mean spot volume varied by at least 1.2-fold between compared spots. Student test and analysis of variance at a significance level of p < 0.05 was applied for unpaired samples.
2.5.5. Mass Spectrometry Protein Identification
Mass spectrometry analysis was carried out at CEQUIBIEM QB-FCEN-UBA/IQUIBICEN-CONICET (Buenos Aires, Argentina) and at Institut Pasteur (Montevideo, Uruguay). Selected spots were excised from the gels and submitted to tryptic digestion and mass spectrometry analyses as previously [26]. The samples were processed at CEQUIBIEM and analysed with an Ultraflex II Bruker Daltonics UV-MALDI-TOF-TOF mass spectrometer, equipped with a Nd:YAG laser (λem 355 nm). Flex Analysis 3.3 software was applied for visualization and comparison of the generated spectra. The peak list generated was based on signal-to-noise filtering and a contaminant exclusion list. Two high S/N MS peaks per sample were selected for MALDI TOF-TOF fragmentation. MS and MS/MS spectra for each spot were combined using BioTools software (Bruker Daltonics). Peptide samples analysed at Institut Pasteur (Montevideo, Uruguay) were subjected to analysis on an ABI 4800 (Sciex, Foster City, USA) mass spectrometer. The resulting file was then searched using Mascot (Matrix Science, Boston, MA) against the NCBInr database (20160618), taxonomy: L. curvatus CRL 705 (https://www.ncbi.nlm.nih.gov/nuccore/AGBU00000000.1). All proteins were identified using BLASTp in the NCBI database [27]. The database search parameters were: peptide mass tolerance of 100 ppm, fragment mass tolerance of 0.5 Da, one missed cleavage, methionine oxidation as variable modifications, and cysteine carbamidomethylation as fixed modification. Only matched proteins with significant scores (p < 0.05) were considered.
2.6. Functional analysis and interaction of the differentially expressed proteins
Functional analysis of the identified proteins that were assigned to the different Clusters of Orthologous Groups (COGs) [28] was performed using COGNITOR. Interacting protein networks were obtained using STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) version 10.05 [29]. Interacting Proteins (represented by nodes) are linked by edges. Prediction methods for interactions available in STRING were used with a confidence level of 0.4 (medium) [29].
2.7. Statistical analyses
Three biological replicates were performed for all experiments (growth kinetics, growth inhibition, and differential protein expression assays), and values and standard error were calculated. In proteomic analysis, one-way analysis of variance with t-test was conducted, and a p < 0.05 was considered to indicate a statistically significant difference. The hypergeometric distribution was tested to evaluate the enrichment of COG categories that were determined with COGNITOR on the Operon Mapper web platform [28,30]. This allowed evaluating the enrichment of the COG categories of the proteins encoded by L. curvatus CRL705 related to those differentially expressed by this strain in CDM- and in CDM+CA.
3. Results
3.1. Growth of L. curvatus CRL 705 in CDM with and without curing additives
Although similar growth of L. curvatus CRL 705 cells was observed in both media (Figure 1), the lag phase of CRL 705 was 2 h longer when cultured in the presence of the curing mixture (Figure 1A). After exponential growth started, both cultures showed similar growth rates and reached maximum cell density (4.44 x 107 and 5.07 x 107 CFU mL-1 in CDM+CA and CDM-, respectively) after 10-12 h of incubation. It was observed that the OD increased between 10-12 h while the viability values remained without significant variation. This discrepancy can be explained by the density of dead cells, which contribute to the turbidity of the culture. After 16 h, a slight decrease in growth was observed in CA+, although the final CFU mL-1 values, at 24 h of incubation, were similar in both conditions (1.62 x 107 CFU mL-1 in CDM+CA and 2.64 x 107 CFU mL-1 in CDM-). Cell growth was accompanied by a concomitant pH decrease, reaching minimal values of 4.23 at 10 h. Nevertheless, the acidifying potential of L. curvatus CRL 705 was not affected by the curing additives, showing similar kinetics and pH values, with regard to the Control, during the 24 h (Figure 1C).
3.2. Bacteriocin activity
The production of the two bacteriocins, Lactocin 705 and AL705 by CRL 705 cells was evaluated using the corresponding sensitive strains, L. plantarum CRL 691 and Listeria monocytogenes FBUNT, respectively (Table 1). Both bacteriocins were produced during cell growth in the absence and presence of CA. Lactocin Lac705 was detected in the supernatants of both CDM cultures after 8 h of incubation, up to 24 h of growth, while the antilisteria bacteriocin AL705 was produced from two hours of the growth. Similar production kinetics of both bacteriocins is observed when the CRL 705 cells grow in culture medium rich in nutrients, such as MRS.
3.3. Consumption of carbon sources and production of acids
The effects of CA on the consumption of sugars and the concomitant production of acids by L. curvatus CRL 705 are shown in Figure 2. Initially, both media (CDM- and CDM+CA) contained glucose (0.75% equivalent to 7.5 g L-1) while CDM+CA also contained 7.5 g L-1 sucrose. CRL 705 cells have a higher glucose consumption in CDM without additives, especially after 8 h of growth, reaching approximately 4.7 g L-1 at 24 h, while in CDM+CA glucose consumption reached 2.9 g L-1 at the same time point. Sucrose added to CDM+CA, as a secondary carbon source, maintained the initial concentration throughout the incubation period, indicating the absence of sucrose consumption by CRL 705 in CDM+CA (results not shown). Regarding the production of lactic acid, it was equivalent in both media during the first 8 h, thereafter lactic acid production by CRL 705 became higher in absence of CA; 2.5 g L-1 of this metabolite was observed in CDM-CA at 24 h, respect to 1.7 g L-1 in CDM+CA, this being related to the higher consumption of glucose. Finally, a similar content of acetic acid coming from the CDM composition was observed from T0 throughout the study (Figure 2).
3.4. Differential protein expression by L. curvatus CRL 705 in the presence of curing additives
The differential protein expression of L. curvatus CRL 705 cultured in CDM with or without CA was evaluated by 2DE. Figure 3 shows representative 2DE proteome maps of CRL 705 cells grown under both conditions. The results of two-dimensional electrophoresis showed 44 differential protein spots (p < 0.05, fold > 1.2), which were subjected to MS/MS identification. Thirty-nine proteins were successfully identified (Table S2). All the identified proteins were assigned to different functional categories; five of them were included in more than one category. Most of the identified proteins (36 out of 39) showed lower expression levels in cells cultured in CDM with CA with fold changes between 1.2 and 2.8, as shows Table S2. Figure 4 represents the abundance (%) of the 36 proteins under-expressed in CDM+CA, grouped according to their functional COG category: 15.0% of the identified proteins were related to carbohydrate metabolism (G), 5.0% to amino acid metabolism (E), 10.0% to nucleotide metabolism (F), and 10.0% to energy production and conversion (C); translation, ribosomal structure and biogenesis (J) account for 10.0%. In addition, six proteins (15.0%) corresponded to posttranslational modification, protein turnover, and chaperones (O). Only three proteins were differentially overexpressed in CDM+CA, including the spot LT10 that correspond to the ATP-binding protein UgpC of the sn-glycerol-3-phosphate ABC transporter; spot LT19, identified as pyruvate oxidase; and spot LT41, corresponding to L-lactate oxidase. These proteins are related to carbohydrate metabolism, amino acid/coenzymes metabolism and energy production/conversion, respectively (Table S2).
Furthermore, according to hypergeometric distribution analysis, certain COG categories could be enriched, such as cell wall/membrane/envelope biogenesis (M), posttranslational modification (O), transcription (K), nucleotide metabolism (F), carbohydrate metabolism (G) and energy conversion and production (C). This suggest that among the differentially expressed proteins, there were more proteins from those specific categories than expected based on their coding relationship in the L. curvatus CRL 705 genome (Figures S1A and S1B). In fact, although four proteins related to carbohydrate metabolism and transport are most likely to be found in the genome, eight proteins have been identified in our study. In contrast, for the translation (J), replication, recombination and repair (L) and general function prediction categories (R), more proteins are more likely to be found than were found in our analysis, so it could imply an impoverishment of these categories as seen in Figure S1B.
3.5. Functional analysis and protein interaction
A protein-protein interaction network was constructed to find relationships with the performance of CRL 705 under the influence of CA from this proteomic perspective. As shown in Figure 5, 5 of the 39 identified proteins have no interactions with each other. The remaining 34 proteins interact through 71 edges, with different strengths, which represent the magnitude of their interactions. Seven proteins belong to the metabolism of carbohydrates, one of the categories that could be more represented in the sample. Also, the translational and posttranslational modification of proteins is represented by seven proteins. Categories related with redox processes, cell wall biosynthesis and nucleotide metabolism and transport are each represented by four proteins (Figure 5).
4. Discussion
The ability of L. curvatus CRL 705 to grow in a CDM containing the most used CA in meat processing was investigated. This is the first study that evaluates the adaptation of LAB to the curing conditions used in the meat industry through a physiological and proteomic approach.
In the present work, the presence of CA produced a longer lag phase compared to the control, probably due to the more stressful environment established by the curing mixture. Orihuel et al. (2018) [31] also reported optimal growth of a bioprotective LAB strain (Enterococcus mundtii CRL 35) under curing conditions in a meat-based medium. On the contrary, sodium chloride and sodium nitrite negatively affected the growth of another bioprotective LAB, L. sakei CTC 494 in an in vitro fermentation model [32]. Other studies demonstrated the ability of L. curvatus CRL 705 to grow at 5.3% NaCl in MRS and the tolerance of L. curvatus and L. sakei to high concentrations of sodium chloride (10-18%) [33,34]. Torriani et al. [33] showed that L. curvatus strains were not able to grow in the presence of sodium chloride in concentrations greater than 10%. Furthermore, no marked variation in pH decrease, or glucose consumption, due to CA was recorded during CRL 705 growth, also indicating that CA did not highly affect metabolic activity. On the other hand, the ability to produce both types of bacteriocins was not affected by curing conditions. On the contrary, Orihuel et al. [31] reported increases in Enterocin CRL35 production and activity when CRL 35 cells were grown under similar curing conditions, suggesting a stabilizing effect of curing additives, specifically attributed to ascorbic acid, on the antimicrobial peptide. A different impact of the curing conditions on the production of sakacin K by L. sakei CTC 494 was reported; a decrease in biomass due to NaCl resulted in a drop in bacteriocin production. In contrast, although sodium nitrite did not specifically affect bacteriocin production, this CA increased the toxic effect of lactic acid on bacterial growth [32].
During adaptation to different growth conditions, microorganisms react by modifying/ regulating the expression of proteins involved in DNA replication and repair, metabolism, and protein biosynthesis. In fact, the effect of numerous stressful stimuli was studied from a proteomic perspective on different LAB, such as the response to ethanol, acid or temperature [35,36,37,38,39,40,41,42,43]. In our study, CRL 705 cultured in the presence of a mixture containing NaCl, sodium nitrite, ascorbic acid and sucrose in the concentrations usually used for the production of fermented sausages, showed statistically significant differences in protein expression. Mainly under the expression of those involved in carbohydrate metabolism, posttranslational modifications, energy production, translation and nucleotide metabolism were evidenced, with variations between 1.2 and 2.8-fold. Even statistically significant, these differences reflected slight changes in the growth kinetics and acidification rates of the strain, showing the robust nature of CRL 705 to withstand curing conditions.
Proteins involved in translation, such as the trigger factor, glutamyl-tRNA(Gln) amidotransferase subunit A, 50S ribosomal proteins L10 and L7/L12, elongation factor Tu and molecular chaperone GroEL, were moderately repressed with 1.2-1.5 fold change in expression in the presence of CA. It is important to highlight that the high degree of interactions observed between differentially downregulated proteins mainly involved in translation. This under expression could be related to the elapsed growth observed during the first hours. In this sense, Fadda et al. [44] observed a slight degree of repression on an elongation factor (tuf) in L. sakei growing in a CDM supplemented with myofibrillar meat proteins.
As mentioned above, carbohydrate metabolism was downregulated and showed 6 proteins with close interactions with each other (STRING analysis). The under expression of glycolytic enzymes was also reported by others in L. sakei cells growing under salt stressing conditions [45,46]. Coincidentally, glycolytic enzymes were repressed by Lactiplantibacillus plantarum growing on hydrolyzed soybean [47]. The under expression of proteins involved in nucleotide synthesis when CRL 705 was grown in CDM+CA could indicate that the de novo nucleotide synthesis is repressed in the presence of CA [48]. On the other hand, the underexpression of proteins related to redox processes such as the ATP-binding subunit of the ATP-dependent protease Clp (spot LT08) or thioredoxin (LT15) could be explained by the presence of ascorbic acid in the curing mixture; this compound is a powerful antioxidant that participates in reduction-oxidation processes and could reduce the biological requirement of these enzymes [49].
5. Conclusion
L. curvatus is a ubiquitous species with a metabolism adapted to grow in different niches. Specifically, the performance of strain CRL 705 during growth in the presence of the curing mixture was slightly affected. This would indicate that the additives have exerted a mild depression in certain metabolic pathways reflected in the growth delay during the first hours and in the moderate repression of protein expression.
Taken together, the physiological and proteomic results could indicate that L. curvatus CRL 705, in response to the more stressful environment produced by curing additives, slowed down its overall metabolism to adapt and maintain its viability, achieving only minor modifications in growth/metabolic parameters and protein expression. Considering the meat origin of the strain, these are positive results and constitute another trait that extends the well-known competitive profile of L. curvatus CRL705 as a meat starter/bioprotective culture, definitively postulating the suitability of this strain to be used in fermentation of cured meat products.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1, Figure S1: A) Hypergeometric distribution for the probabilities (Prob) of finding a certain COG functional category a certain number of times “x” of the differentially expressed proteins of L. curvatus CRL 705 while growing in CDM with and without additives at 25 °C. The highest value of the y axis (Prob) for each of the curves represents the higher probability of the times of occurrence of proteins from a certain COG. B) Differences between measured and expected occurrence for the proteins of each COG category, where 0 represents no differences in occurrence and separates potentially enriched categories (positive values) from the potentially impoverished categories (negative values). Table S2: Differentially expressed proteins by L. curvatus CRL 705 during growth in CDM with (CDM+CA) and without (CDM-) the presence of curing additives at 25°C.
Author Contributions
Conceptualization, Lucrecia Teran, Raúl Raya and Silvina Fadda; Data curation, Lucrecia Teran and Alejandra Orihuel; Formal analysis, Lucrecia Teran, Alejandra Orihuel, Emilse Bentencourt, Raúl Raya and Silvina Fadda; Funding acquisition, Silvina Fadda; Investigation, Lucrecia Teran, Alejandra Orihuel, Emilse Bentencourt, Raúl Raya and Silvina Fadda; Methodology, Lucrecia Teran, Alejandra Orihuel, Emilse Bentencourt and Silvina Fadda; Project administration, Silvina Fadda; Resources, Silvina Fadda; Software, Lucrecia Teran and Alejandra Orihuel; Supervision, Silvina Fadda; Validation, Lucrecia Teran and Emilse Bentencourt; Visualization, Lucrecia Teran, Alejandra Orihuel and Silvina Fadda; Writing – original draft, Lucrecia Teran and Silvina Fadda; Writing – review & editing, Lucrecia Teran, Raúl Raya and Silvina Fadda.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PUE-2017-N°0035; PICT-2018-02249).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Lic. Elena Bru for her statistical assistance and Dr. Gaston Pourrieux for his technical assistance in HPLC analyses. Also CONICET for the doctoral scholarship of LCT to support the completion of her doctoral degree is fully acknowledged.
Conflicts of Interest
No conflict of interest declared.
References
- Lücke, F.-K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Leroy-Sétrin, S.; Fadda, S. Bacterial starters involved in the quality of fermented meat products. In Research advances in the quality of meat and meat products; Toldrá, F., Ed.; Trivandrum: Research Signpost, India, 2002; pp. 175–191. [Google Scholar]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G. Monitoring the bacterial population dynamics during fermentation of artisanal Argentinean sausages. Int. J. Food Microbiol. 2005, 103, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L. Stable and safe fermented sausages world-wide. In Fermented meats; Campbell-Platt, G., Cook, P.E., Eds.; Springer: New York, NY, USA, 1995; pp. 160–175. [Google Scholar]
- Vignolo, G.; Castellano, P.; Fadda, S. Starter cultures: bioprotective cultures. In Handbook of Fermented Meat and Poultry, 2nd ed.; Todrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J., Talon, R., Eds.; Blackwell Publishing Inc.: Malden, MA, 2015; pp. 129–137. [Google Scholar]
- Eisenbach, L.; Janßen, D.; Ehrmann, M.A.; Vogel, R.F. Comparative genomics of Lactobacillus curvatus enables prediction of traits relating to adaptation and strategies of assertiveness in sausage fermentation. Int. J. Food Microbiol. 2018, 286, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Terán, L.C.; Coeuret, G.; Raya, R.; Zagorec, M.; Champomier-Vergès, M.-C.; Chaillou, S. Phylogenomic analysis of Lactobacillus curvatus reveals two lineages distinguished by genes for fermenting plant-derived carbohydrates. Genome Biol Evol. 2018, 10, 1516–1525. [Google Scholar] [CrossRef]
- Vignolo, G.M.; Suriani, F.; Holgado, A.P.d.R.; Oliver, G. Antibacterial activity of Lactobacillus strains isolated from dry fermented sausages. J. Appl. Microbiol. 1993, 75, 344–349. [Google Scholar]
- Castellano, P.; Gonzalez, C.; Carduza, F.; Vignolo, G. Protective action of Lactobacillus curvatus CRL705 on vacuum-packaged raw beef. Effect on sensory and structural characteristics. Meat Sci. 2010, 85, 394–401. [Google Scholar]
- Castellano, P.; Raya, R.; Vignolo, G. Mode of action of lactocin 705, a two-component bacteriocin from Lactobacillus casei CRL705. Int. J. Food Microbiol. 2003, 85, 35–43. [Google Scholar] [CrossRef]
- Castellano, P.; Vignolo, G. Inhibition of Listeria innocua and Brochothrix thermosphacta in vacuum-packaged meat by addition of bacteriocinogenic Lactobacillus curvatus CRL705 and its bacteriocins. Lett. Appl. Microbiol. 2006, 43, 194–199. [Google Scholar] [CrossRef]
- Cuozzo, S.A.; Castellano, P.; Sesma, F.J.; Vignolo, G.M.; Raya, R.R. Differential roles of the two-component peptides of lactocin 705 in antimicrobial activity. Curr. Microbiol. 2003, 46, 180–183. [Google Scholar] [CrossRef]
- Hebert, E.M.; Saavedra, L.; Taranto, M.P.; Mozzi, F.; Magni, C.; Nader, M.E.; Font de Valdez, G.; Sesma, F.; Vignolo, G.; Raya, R. Genome sequence of the bacteriocin-producing Lactobacillus curvatus strain CRL705. J. Bacteriol. 2012, 194, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Terán, L.C.; Raya, R.; Zagorec, M.; Champomier-Vergès, M.-C. Genetics and genomics of Lactobacillus sakei and Lactobacillus curvatus. In Lactobacillus Genomics and Metabolic Engineering; Ruzal, S., Ed.; Caister Academic Press, 2019; pp. 19–30. [Google Scholar]
- Chaillou, S.; Champomier-Vergès, M.-C.; Cornet, M.; Crutz-Le Coq, A.-M.; Dudez, A.-M.; Martin, V.; Beaufils, S.; Darbon-Rongère, E.; Robert Bossy, V.; Zagorec, M. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 2005, 23, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Stentz, R.; Zagorec, M. Ribose utilization in Lactobacillus sakei: analysis of the regulation of the rbs operon and putative involvement of a new transporter. J. Mol. Microbiol. Biotechnol. 1999, 1, 165–173. [Google Scholar] [PubMed]
- Talon, R.; Leroy-Sétrin, S.; Fadda, S. Dry fermented sausages. In Handbook of Food & Beverage Fermentation Technology. Volumen Series: 134; Hui, Y.H., Toldra, F., Eds.; Marcel Decker Inc: New York, NY, USA, 2004; 1016; pp. 397–416. [Google Scholar]
- Lauret, R.; Morel-Deville, F.; Berthier, F.; Champomier-Verges, M.; Postma, P.; Ehrlich, S.D.; Zagorec, M. Carbohydrate utilization in Lactobacillus sake. Appl. Environ. Microbiol. 1996, 62, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, E.; Saavedra, L.; Sesma, F. Short peptides derived from the NH2-terminus of subclass IIa bacteriocinenterocin CRL35 show antimicrobial activity. J. Antimicrob. Chemother. 2007, 59, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.; Carbajo, M.; Rollán, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Fornaguera, M.J.; Raya, R.R.; Mozzi, F. Lactobacillus reuteri CRL 1101 highly produces mannitol from sugarcane molasses as carbon source. Appl. Microbiol. Biotechnol. 2012, 95, 991–999. [Google Scholar] [CrossRef]
- Orihuel, A.; Terán, L.; Renaut, J.; Vignolo, G.M.; De Almeida, A.M.; Saavedra, M.L.; Fadda, S. Differential proteomic analysis of lactic acid bacteria—Escherichia coli O157: H7 interaction and its contribution to bioprotection strategies in meat. Front. Microbiol. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Raya, R.; de Almeida, A.M.; Fadda, S.; Taranto, M.P. Proteomic analysis of the probiotic Lactobacillus reuteri CRL1098 reveals novel tolerance biomarkers to bile acid-induced stress. Food Res. Int. 2015, 77, 599–607. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Righetti, P.G. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Nally, J.E.; Grassmann, A.A.; Planchon, S.; Sergeant, K.; Renaut, J.; Seshu, J.; McBride, A.J.; Caimano, M.J. Pathogenic leptospires modulate protein expression and post-translational modifications in response to mammalian host signals. Front. Cell. Infect. Microbiol. 2017, 7, 362. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafu, K.P.; Kuhn, M.; Bork, P.; Jensen, L.J.; von Mering, C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–452. [Google Scholar] [CrossRef]
- Taboada, B.; Estrada, K.; Ciria, R.; Merino, E. Operon-mapper: a web server for precise operon identification in bacterial and archaeal genomes. Bioinformatics 2018, 34, 4118–4120. [Google Scholar] [CrossRef]
- Orihuel, A.; Bonacina, J.; Vildoza, M.J.; Bru, E.; Vignolo, G.; Saavedra, L.; Fadda, S. Biocontrol of Listeria monocytogenes in a meat model using a combination of a bacteriocinogenic strain with curing additives. Food Res. Int. 2018, 107, 289–296. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Temperature and pH conditions that prevail during the fermentation of sausages are optimal for the production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 1999, 65, 974–981. [Google Scholar] [CrossRef]
- Torriani, S.; Van Reenen, C.; Klein, G.; Reuter, G.; Dellaglio, F.; Dicks, L. Lactobacillus curvatus subsp. curvatus subsp. nov. and Lactobacillus curvatus subsp. melibiosus subsp. nov. and Lactobacillus sake subsp. sake subsp. nov. and Lactobacillus sake subsp. carnosus subsp. nov., new subspecies of Lactobacillus curvatus Abo-Elnaga and Kandler 1965 and Lactobacillus sake Katagiri, Kitahara, and Fukami 1934 (Klein et al. 1996, emended descriptions), respectively. Int. J. Syst. Evol. 1996, 46, 1158–1163. [Google Scholar]
- Belfiore, C.; Raya, R.R.; Vignolo, G.M. Identification, technological and safety characterization of Lactobacillus sakei and Lactobacillus curvatus isolated from Argentinean anchovies (Engraulis anchoita). SpringerPlus 2013, 2, 257. [Google Scholar] [CrossRef]
- De Angelis, M.; Calasso, M.; Cavallo, N.; Di Cagno, R.; Gobbetti, M. Functional proteomics within the genus Lactobacillus. Proteomics 2016, 16, 946–962. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Desmond, C.; Fitzgerald, G.F; Stanton, C.; Ross, R.P. Improved stress tolerance of GroESL-overproducing Lactococcus lactis and probiotic Lactobacillus paracasei NFBC 338. Applied and Environmental Microbiology 2004, 70, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.; Deane, S.; Smit, S.; Dicks, L.M. Proteomic profiling of the acid stress response in Lactobacillus plantarum 423. J. Proteome Res. 2014, 13, 4028–4039. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.G.; Pi, K.; Choi, Y.J. The effect of low pH on protein expression by the probiotic bacterium Lactobacillus reuteri. Proteomics 2008, 8, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, K.W.; Park, T.H.; Park, J.Y.; Han, N.S.; Kim, J.H. Proteomic analysis of proteins increased or reduced by ethanol of Lactobacillus plantarum ST4 isolated from Makgeolli, traditional Korean rice wine. J. Microbiol. Biotechnol. 2012, 22, 516–525. [Google Scholar] [CrossRef]
- Liu, S. Proteomic analyses of ethanol tolerance in Lactobacillus buchneri NRRL B-30929. Proteomics 2014, 14, 2540–2544. [Google Scholar] [CrossRef]
- Suokko, A.; Poutanen, M.; Savijoki, K.; Kalkkinen, N.; Varmanen, P. ClpL is essential for induction of thermotolerance and is potentially part of the HrcA regulon in Lactobacillus gasseri. Proteomics 2008, 8, 1029–1041. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, W.; Sun, T.; Wu, J.; Yue, X.; Meng, H.; Zhang, H. Proteomic analysis of responses of a new probiotic bacterium Lactobacillus casei Zhang to low acid stress. Int. J. Food Microbiol. 2011, 147, 181–187. [Google Scholar] [CrossRef]
- Fadda, S.; Anglade, P.; Baraige, F.; Zagorec, M.; Talon, R.; Vignolo, G.; Champomier-Vergès, M.C. Adaptive response of Lactobacillus sakei 23K during growth in the presence of meat extracts: a proteomic approach. Int. J. Food Microbiol. 2010, 142, 36–43. [Google Scholar] [CrossRef]
- Marceau, A.; Zagorec, M.; Chaillou, S.; Méra, T.; Champomier-Verges, M.-C. Evidence for involvement of at least six proteins in adaptation of Lactobacillus sakei to cold temperatures and addition of NaCl. Appl. Environ. Microbiol. 2004, 70, 7260–7268. [Google Scholar] [CrossRef]
- Belfiore, C.; Fadda, S.; Raya, R.; Vignolo, G. Molecular basis of the adaption of the anchovy isolate Lactobacillus sakei CRL1756 to salted environments through a proteomic approach. Food Res. Int. 2013, 54, 1334–1341. [Google Scholar] [CrossRef]
- Siragusa, S.; De Angelis, M.; Calasso, M.; Campanella, D.; Minervini, F.; Di Cagno, R.; Gobbetti, M. Fermentation and proteome profiles of Lactobacillus plantarum strains during growth under food-like conditions. J. Proteomics 2014, 96, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Israel, L.; Gänzle, M.G.; Vogel, R.F. Proteomic approach for characterization of hop-inducible proteins in Lactobacillus brevis. Appl. Environ. Microbiol. 2007, 73, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.; Ross, P.; Stanton, C.; Turroni, F.; van Sinderen, D.; Varmanen, P.; Ventura, M.; Zúñiga, M.; Tsakalidou, E.; Kok, J. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Growth kinetics A) CFU mL-1, B) pH and C) OD of L. curvatus CRL 705 in CDM+CA (continue line) and CDM- (dashed line) at 25°C during 24 h.
Figure 1.
Growth kinetics A) CFU mL-1, B) pH and C) OD of L. curvatus CRL 705 in CDM+CA (continue line) and CDM- (dashed line) at 25°C during 24 h.
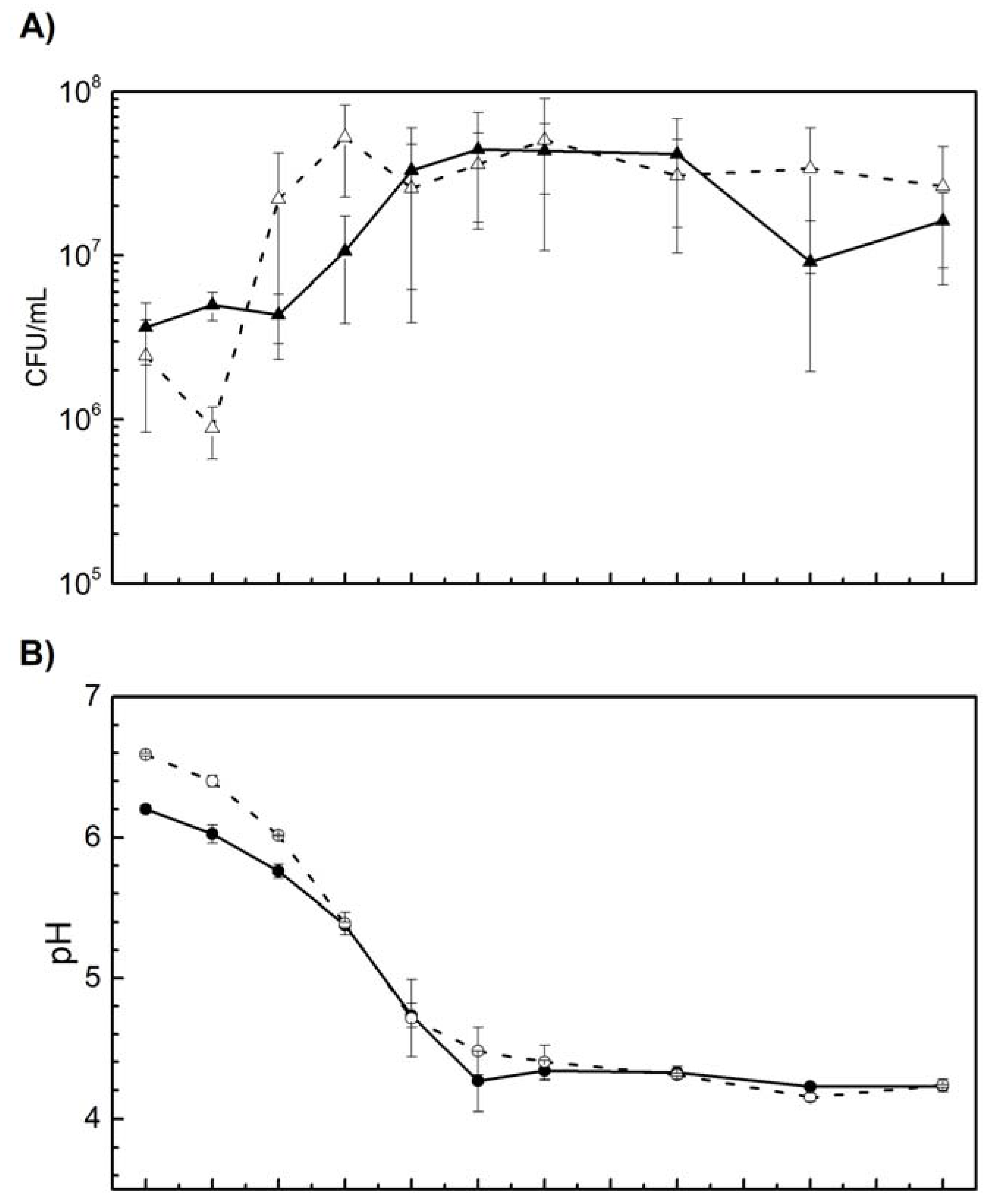
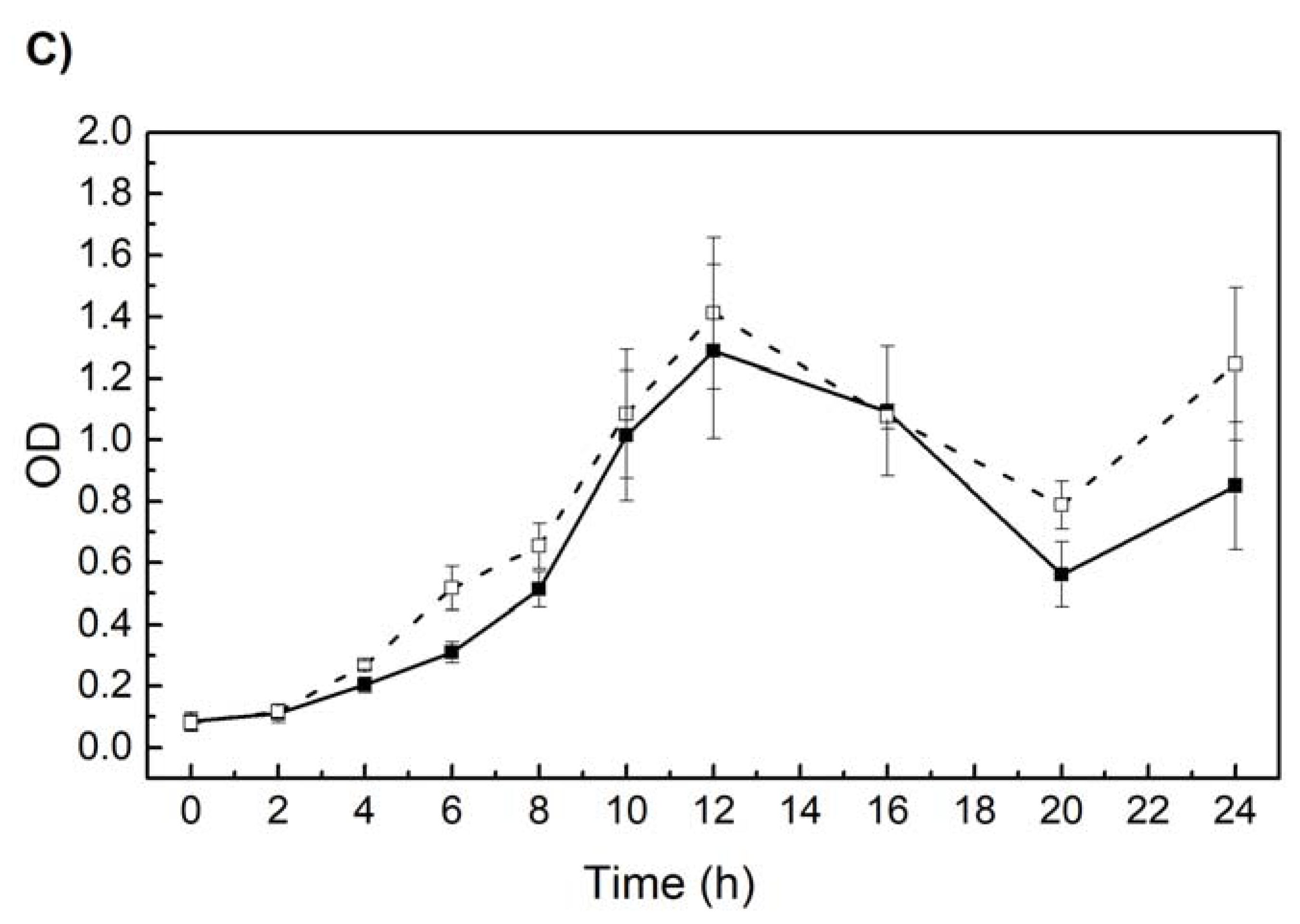
Figure 2.
Consumption of sugars and production of acids by L. curvatus CRL 705 during its growth in mediums CDM+CA (full bar) and CDM- (empty bar). The concentration (g L-1) of glucose, lactic acid and acetic acid was determined by HPLC.
Figure 2.
Consumption of sugars and production of acids by L. curvatus CRL 705 during its growth in mediums CDM+CA (full bar) and CDM- (empty bar). The concentration (g L-1) of glucose, lactic acid and acetic acid was determined by HPLC.

Figure 3.
2DE gels showing the differentially expressed proteins by L. curvatus CRL 705 during its growth in CDM. without (A) or with (B) a mix of curing agents (44 spots). The differentially expressed and identified proteins (39 spots) are numbered according to the spot number shown in Table 1. For clarity, the 39 spots are numbered in two gels: a) LT1 to LT22 and b) LT23 to LT43.
Figure 3.
2DE gels showing the differentially expressed proteins by L. curvatus CRL 705 during its growth in CDM. without (A) or with (B) a mix of curing agents (44 spots). The differentially expressed and identified proteins (39 spots) are numbered according to the spot number shown in Table 1. For clarity, the 39 spots are numbered in two gels: a) LT1 to LT22 and b) LT23 to LT43.
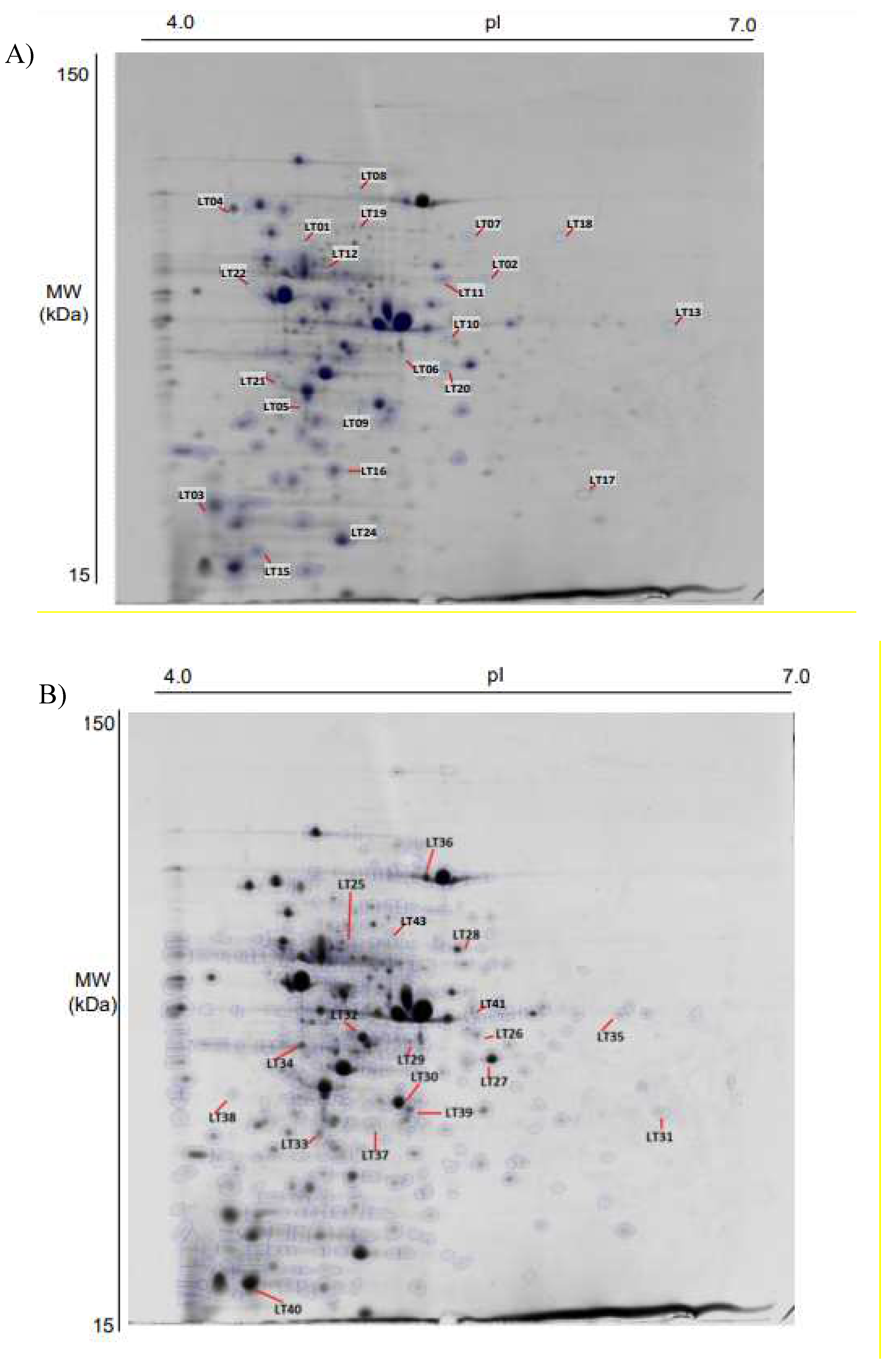
Figure 4.
Relative abundance (%) of the 36 proteins under expressed by L. curvatus CRL 705 in CDM+CA, grouped according to their functional COG category. The COG categories are represented by one letter as follows: O: Molecular chaperones and related functions; J: Translation, including ribosome structure and biogenesis; G: Carbohydrate metabolism and transport; C: Energy production and conversion; T: Signal transduction mechanisms; K: Transcription; E: Amino acid transport and metabolism; F: Nucleotide transport and metabolism; H: Coenzyme transport and metabolism; M: Cell wall structure and biogenesis and outer membrane; L: Replication, recombination and repair; R: General functional prediction only.
Figure 4.
Relative abundance (%) of the 36 proteins under expressed by L. curvatus CRL 705 in CDM+CA, grouped according to their functional COG category. The COG categories are represented by one letter as follows: O: Molecular chaperones and related functions; J: Translation, including ribosome structure and biogenesis; G: Carbohydrate metabolism and transport; C: Energy production and conversion; T: Signal transduction mechanisms; K: Transcription; E: Amino acid transport and metabolism; F: Nucleotide transport and metabolism; H: Coenzyme transport and metabolism; M: Cell wall structure and biogenesis and outer membrane; L: Replication, recombination and repair; R: General functional prediction only.
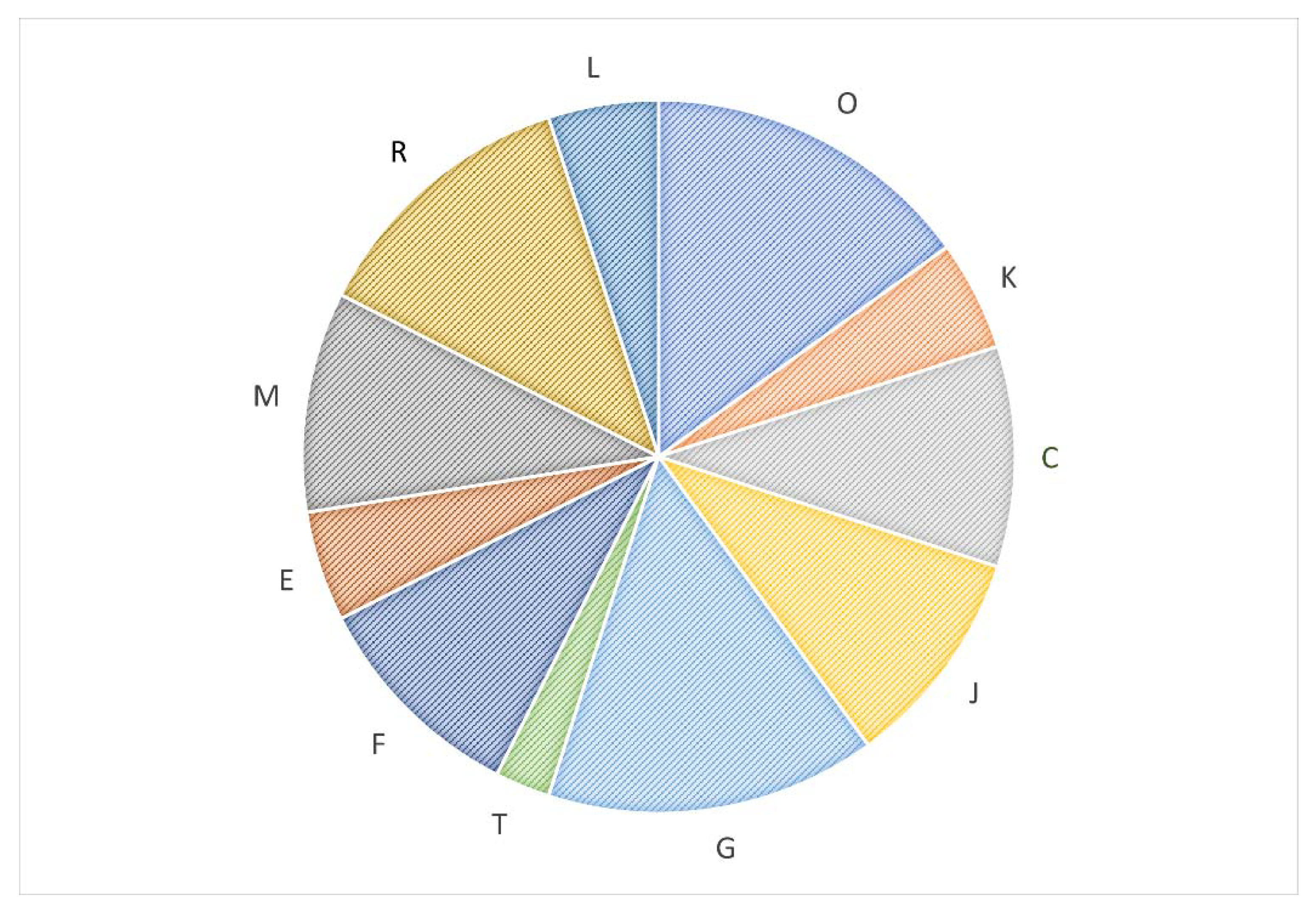
Figure 5.
Protein-protein interaction network of the differentially expressed proteins of L. curvatus CRL 705 during growth in CDM with and without additives. The circles highlight the proteins related with carbohydrate metabolism, redox process, cell wall biosynthesis, nucleotide metabolism and translation & posttranslational modifications. Proteins are represented by nodes while the interactions between them by edges. Strength of the different interactions is represented by the thickness of the lines. The network was constructed with STRING v10.05.
Figure 5.
Protein-protein interaction network of the differentially expressed proteins of L. curvatus CRL 705 during growth in CDM with and without additives. The circles highlight the proteins related with carbohydrate metabolism, redox process, cell wall biosynthesis, nucleotide metabolism and translation & posttranslational modifications. Proteins are represented by nodes while the interactions between them by edges. Strength of the different interactions is represented by the thickness of the lines. The network was constructed with STRING v10.05.

Table 1.
L. curvatus CRL 705 bacteriocin activity in MRS, MCD- and CDM+CA during 24 h at 25 °C.
| Lactocin 705* | AL 705** | |||||||
| Time (h) | 2 | 8 | 16 | 24 | 2 | 8 | 16 | 24 |
| MRS | - | + | +++ | ++ | + | ++ | +++ | +++ |
| CDM- | - | + | +++ | ++ | + | ++ | +++ | +++ |
| CDM+CA | - | ++ | +++ | ++ | + | +++ | +++ | +++ |
MRS: MRS broth; CDM- : Chemically defined medium without curing additive supplementation inoculated with CRL705 at 25°C; CDM+CA: Chemically defined medium with curing additive supplementation inoculated with CRL705 at 25°C; *: Lactocin 705 activity (AU mL-1): - <100 UA; +: 100-200; ++: 201-400; +++: >400; **: AL 705 activity (AU mL-1): - <5000; +: 6800-12800; ++: 12801-25600; +++: >25600.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Role of Curing Agents in the Adaptive Response of the Bioprotective Latilactobacillus Curvatus CRL 705 from a Physiologic and Proteomic Perspective
Lucrecia Cecilia Terán
et al.
,
2023
Genetic Identification and Technological Potential of Indigenous Lactic Acid Bacteria Isolated from Alheira, A Traditional Portuguese Sausage
Nathália Fernandes
et al.
,
2023
Novel Probiotic for Peppers Fermentation with Safe and Health-Promoting Potential
Micaela Ivana Nuñez
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









