Preprint
Case Report
The Role of Early Functional Electrostimulation in Partial Traumatic Brachial Plexus Injury Cats: A Pilot Study
Altmetrics
Downloads
134
Views
59
Comments
0
Débora Gouveia,Ana Cardoso,Carla Carvalho,Marina Moisés,Bernardo Trovão Do Rosário,Ana Catarina Oliveira,Inês Rijo,Inês Loureiro, Cátia Silva,António Almeida,Óscar Gamboa,
Cátia Silva,António Almeida,Óscar Gamboa, Ana Catarina Sousa,
Ana Catarina Sousa, Bruna Lopes,
Bruna Lopes, Patrícia Sousa,
Patrícia Sousa, Alícia Moreira,
Alícia Moreira, André Filipe Coelho,
André Filipe Coelho, Alexandra Rêma,Maria João Simões,
Alexandra Rêma,Maria João Simões, Carla Sofia Meireles,Maria Manuel Balça,
Carla Sofia Meireles,Maria Manuel Balça, António José Salgado,
António José Salgado, Rui Alvites,
Rui Alvites, Artur Severo P. Varejão,
Artur Severo P. Varejão, Ana Colette Maurício *,
Ana Colette Maurício *, António Ferreira,
António Ferreira, Ângela Martins
Ângela Martins














Débora Gouveia,Ana Cardoso,Carla Carvalho,Marina Moisés,Bernardo Trovão Do Rosário,Ana Catarina Oliveira,Inês Rijo,Inês Loureiro, Cátia Silva,António Almeida,Óscar Gamboa,
Cátia Silva,António Almeida,Óscar Gamboa, Ana Catarina Sousa,
Ana Catarina Sousa, Bruna Lopes,
Bruna Lopes, Patrícia Sousa,
Patrícia Sousa, Alícia Moreira,
Alícia Moreira, André Filipe Coelho,
André Filipe Coelho, Alexandra Rêma,Maria João Simões,
Alexandra Rêma,Maria João Simões, Carla Sofia Meireles,Maria Manuel Balça,
Carla Sofia Meireles,Maria Manuel Balça, António José Salgado,
António José Salgado, Rui Alvites,
Rui Alvites, Artur Severo P. Varejão,
Artur Severo P. Varejão, Ana Colette Maurício *,
Ana Colette Maurício *, António Ferreira,
António Ferreira, Ângela Martins
Ângela Martins














This version is not peer-reviewed
Submitted:
12 September 2023
Posted:
14 September 2023
You are already at the latest version
Alerts
Abstract
This prospective cohort pilot study included 22 cats diagnosed with partial traumatic brachial plexus injury (PTBPI), aiming to explore the response of an early intensive neurorehabilitation protocol. This protocol included functional electrical stimulation (FES), locomotor treadmill training and kinesiotherapy exercises, starting at the time with highest probability of nerve repair. The synergetic benefits of this multimodal approach were based on the structural and protective role of proteins and release of neurotrophic factors. Furthermore, parametrization of FES was according to presence or absence of deep pain. Results have shown 72.6% (16/22) cats that achieved ambulation, with 9 cats within 15 days, 2 cats until 30 days and 5 cats until 60 days, however with persistent knuckling position. During the 4 years follow-up, there was evidence of improvement on both muscle mass and muscle weakness, in addition to the disappearance of neuropathic pain. Notably, after the 60 days of neurorehabilitation, 3 cats improved ambulation after arthrodesis of the carpus. Thus, early rehabilitation, with FES applied at the first weeks after injury and accurate parametrization according to deep pain perception, may improve functionality and ambulation, reducing probability of amputation of the affected limb.
Keywords:
Subject: Biology and Life Sciences - Neuroscience and Neurology
1. Introduction
The brachial plexus is a complex anatomic structure that is formed by a network of ventral roots of the spinal cord segments of C6 to T2, in the cat, located between the intervertebral foramen and the thoracic limb [1].
Partial traumatic brachial plexus injury (PTBPI) is one of the most common peripheric nervous system lesions that affects the brachial plexus [2,3,4], most likely due to road traffic accidents [5]. This type of lesion may occur due to excessive traction of the thoracic limb or severe abduction of the scapula [5,6,7].
The diagnosis of PTBPI is usually based on history, clinical signs and findings of both clinical and neurological examination [2,6]. However, electrodiagnostic assessment can be useful as a precise tool for identifying damaged nerves trough the evoked compound muscle action potentials (CMAPs) that are a reflection of muscle force in normal and re-innervated muscles [8]. These tests were already used in cats and may be enough to localize injury of the brachial plexus, but are still not enough to discover the exact location of specific injured nerves [9].
Previous studies based on nerve conduction and electromyograms (EMG), which are not invasive, showed that these tools appeared to have a delayed diagnostic role but not immediately after injury, being more effective, for example six weeks later when fibrillations in the de-innervated muscles occur [10,11,12]. In addition, magnetic resonance imaging (MRI) has been described as a possible complementary exam to identify brachial plexus masses [9].
Each peripheral nerve is formed by a net of nervous fibers that give rise to the fascicles, covered by three layers of connective tissue: the epinerium, perinerium and inner layer, the endonerium. These support the fascicles and provide blood vessels [13].
Seddon (1943) [14] was the first to implement a classification for nerve injuries. They were classified into three categories based on presence of demyelination and damage extension to the axons and/or the connective tissues around the nerve [15,16]. Thus, from 1947, it was considered: neuropraxia, in the presence of focal demyelination without damage to the axons and/or connective tissues, mostly due to mild compression or nerve traction, leading to a transitory interruption or decrease in velocity conduction and muscle weakness; axonotmesis (crush injury), when there is direct damage to the axons and focal demyelination, while maintaining the nerves connective tissue continuity; and neurotmesis, when there is a full transection of the axons and connective tissue [10,14,15,16,17].
Later in 1951, Seddon’s classification has been adapted and expanded. According to Sunderland´s criteria, that is commonly used in a clinical setting of human medicine, neuropraxia is considered a type I nerve injury [10,15,18], whereas axonotmesis could be classified from type II to type IV injury. There is no diagnostic test that allows to distinguish between these intermediary grades, only histological methods could help in this assessment [10,15,16,17,19]. Regarding neurotmesis, it is considered a Sunderland´s criteria type V, usually with the need to resort to surgical intervention [15,16,17]. Aditionallty, Mackinnon and Dellon described a mixed type or Grade VI injury to the Sunderland grading classification [20].
In human medicine, a more aggressive approach with acute repair leads to better functional recover, avoiding the “wait and see” diagnosis that could be much more costly [10,19]. Also, early nerve repair may result in improved functional outcome with less muscle fibrosis and secondary atrophy, which begins soon after denervation occurs. Thus, the time needed for re-innervation of sensory receptors is much longer when compared to motor nerves, but with better outcomes when early repair occurs [10].
The PTBPI is mostly seen in cats, particularly in road traffic collisions, when an impact force promotes excessive medial or caudal movement of the limb. The lesions between C8-T1 are the most common, however C6-C7 could also be involved [5].
Clinical signs reveal a gait alteration compatible with proximal radial nerve paralysis, inability to support weight and to extend the carpus/digits, resulting in pain and dragging the limb on the floor [3,21]. In addition, the elbow could be ventrally positioned, acquiring a dropped elbow posture due to shoulder plexus paralysis, namely the latissimus dorsi and triceps long head, which are innervated by the caudal roots of the plexus [1,21,22].
Therefore, avulsion of the C8 rootlets affects the cutaneous trunci reflex on that side, regardless of the intensity of sensory stimulation. If the cranial roots of the plexus are preserved (C6-C7), with intact musculocutaneous nerve, the elbow stays in a flexion position, resulting in lameness score V [1,3,21,22].
Cases of total brachial plexus avulsion may result in absence of superficial pain, distally to the elbow and lateral surface of the arm, but if the cranial portion of the brachial plexus is preserved, this pain could be lost only distally to the elbow and sensation on the medial side of the thoracic limb will be intact [1,3,22].
In cats with PTBPI there could also be lesions of contusion, hemorrhage, and edema, with possible recovery that should be expected to occur in the first two to three weeks after injury, however in most cases this does not occur [21].
Thus, electrical stimulation (ES) in peripheral nerve injury is a treatment modality that could allow reinnervation of affected muscles and enhance functional outcome, promoted by daily stimulation. Upregulation of intramuscular neurotrophic factors is the base of this mechanism and may be essential for long term functional muscle alterations, something that already changed the paradigm in rats [23,24,25].
The therapeutical approach based on ES, aims to improve post-traumatic neuromuscular recovery and could be applied either to the damaged nerve [26,27,28] or to the denervated muscles [29,30,31], which was already applied in rat models of axonotmesis [26,32,33,34]. The degree of recovery regarding tissue healing is highly variable [22,35,36], but early neurorehabilitation protocols may help improve the final outcome.
In human medicine, neurorehabilitation protocols based on single sessions of ES associated with physical exercise, muscle stretching and passive movements for treatment of denervated muscles, leads to enhanced contraction without achieving muscle fatigue [26,30].
Research in this field, also revealed that applying low intensity ES directly to the nerve, trough implanted electrodes, increased functional and morphological regeneration of the nerve due to a possible delay in axonal degeneration, stimulation of nerve sprouting and myelin sheath regeneration [24,37]. Thus, stimulation with low-frequencies could increase myelinated fibers, axons density, revealing a higher ratio of blood vessels/total nerve area, when compared to non-stimulated and injured nerves. Furthermore, other studies have shown that ES is a strong source that delays degeneration in denervated muscle [26,38] and regulates molecular alterations in skeletal muscles [25,30]. In addition, it could be also related to the possibility of delaying macrophages recruitment to the injury site, inhibiting Wallerian degeneration following nerve damage [26]. Wallerian degeneration promotes axons disintegration, breakdown of myelin sheaths that surround axons, presence of numerous macrophages, fewer neutrophils, and Schwann cells scavenge myelin debris. Although, an efficient Wallerian degeneration may lead to an efficient peripheral nerve regeneration, where Schwann cells play an essential role [39].
The main effects of ES are still under investigation and there is a need for understanding how different frequencies may affect the regeneration of nerve fibers.
Findings have been shown that brief continuous stimulation with 20 Hz accelerates axon outgrowth in rats or mice [40,41], as animal models. Additionally, there is a possible effect in accelerating target reinnervation described in humans (and changes due to brain derived neurotrophic factor (BDNF) and its TrkB receptors upregulation [42].
Treatment of PTBPI could be seen from different perspectives, such as conservative, surgical or a mixed approach. Conservative management could be based on functional neurorehabilitation with prevention of self-mutilation and secondary wounds, which could be associated with carpal arthrodesis to prevent contracture and decreased carpus functionality [7,43,44,45].
The first aim of this prospective cohort pilot study was to describe the recovery of PTBPI in the cat after implementation of a mixed multidisciplinary protocol based on neurorehabilitation, including functional electrical stimulation (FES). The second aim was to determine the different parameters of FES, according to the presence or absence of deep pain perception (DPP). Lastly, to assess progression of the intensive neurorehabilitation protocol (INRP) throughout the time and its limitations.
2. Material and Methods
This prospective cohort pilot study in cats with PTBPI was performed at the Arrábida Animal Rehabilitation Center (CRAA, Portugal), between 2016 and 2023, after approval by the Lusófona Veterinary Medicine Faculty Ethics committee and a signed consent from the owners.
2.1. Participants
All cats diagnosed with PTBPI were recruited from the neurorehabilitation center, using the inclusion criteria described in Table 1.
According to the neurological clinical signs of the affected limb, localization of the brachial plexus injury was attributed to a caudal lesion (C8, T1, T2). These cats didn´t performed conduction studies and EMG, but their presentation was acute and non-progressive.
Exclusion criteria was based on all other diseases that could affect the brachial plexus, such as inflammatory, infectious, neoplastic or other trauma lesions but with different clinical presentation.
2.2. Study Design
All 22 cats with PTBPI were referred to the rehabilitation center, three to seven days after trauma, and were admitted in this prospective cohort study following hemodynamic stabilization.
All had a previous emergency setting consultation in different hospitals, that was followed by stabilization resorting to the restrictive resuscitation fluid therapy protocol, blood work and point of care ultrasound (POCUS). At admission in the rehabilitation center, all cats presented normal mental state, heat rate, respiratory rate, blood pressure and temperature. The neurorehabilitation examination consisted on the assessment of: passive standing posture; spinal reflexes of the thoracic limb (withdrawal reflex and extensor carpi radialis reflex); palpation of all possible muscles that are innervated by the brachial plexus nerves (Table 2) with specific palpation of the thoracic limb medial region as described in Figure 2.
For the remaining neurorehabilitation exam, and to understand mostly the C8-T2 injury, necessary key points are described in Table 3 [1,22].
The specific examination of the brachial plexus region was very important, given that changes on the EMG can only be noticed after seven to ten days of clinical onset. Therefore, it was essential to assess superficial pain and perform the thoracic limb dermatomes map, to evaluate the presence of deep pain in the first and fifth digits and to monitor the possibility of paresthesia with behavior changes, such as biting, liking or self-mutilation. In those specific cases, an Elizabethan collar was applied.
The evaluation of superficial and deep pain was performed with a 12 cm Halsted mosquito forceps and an ink marker was used to draw the cut-off point of the dermatomes map, for further evaluation (Figure 3).
2.3. Procedures
The neurorehabilitation protocol for PTBPI had multiple aims, namely: a) to maintain/restore joint range of motion (ROM)) and neuromuscular function, preventing pain, trauma and self-mutilation [46]; b) to prevent muscle contractures [44,47] and joint contractures, maintaining the elbow and carpus flexion/extension end-feel; c) to minimize neurogenic atrophy, promoting muscle strengthening and muscle flexibility; d) to reduce the possibility of paresthesia with recovery of sensory sensibility [48]; e) to achieve ambulation and improve quality of life.
All cats (n=22) were subjected to the same early INRP. Procedures took place in a controlled and quiet environment on the cat´s rehabilitation room 6 times/day, performing different modalities, such as laser therapy and ultrasounds, followed by a 30 min window to perform the electrotherapy protocols (functional electrostimulation of the radial nerve) associated to the locomotor training, according to each patient´s cardiorespiratory condition and the phase of neurological recovery.
All animals were under a hospitalization regimen and had clinical discharge when they achieved ambulation, although the maximum time of the implementation of this study´s protocol was of two months. Furthermore, all cats had to have their nails cut, an Elizabethan collar in case of possible self-mutilation and a harness. A positive reinforcement was given with treats, toys or playing with other friendly cats. Modalities and exercises were executed by three veterinarians, nowadays with the certified canine rehabilitation practitioner (CCRP) and two CCRP students. All evaluations were performed by a veterinarian diplomate of the European college of veterinary sports medicine and rehabilitation, with a PhD in neurorehabilitation.
2.3.1. Intensive neurorehabilitation protocol (INRP)
- 1st - 3rd week of INRP
In the first three weeks the protocol was similar (Table 4). It was mandatory to avoid active movements with limb stretching and the main focus was to increase depolarization of the radial, median and ulnar nerves. This was achieved using class IV laser therapy (LiteCure Companion Therapy Laser®, New Castle, USA), due to its regenerative role, applied on the anatomic path of the nerves. In addition, a 4-point technique of the same laser was implemented in the carpal, elbow, and shoulder joints, promoting analgesia, anti-inflammatory effects, and edema reduction [49].
Additionally, the ultrasound modality (BTL-4820 Smart®, Hertfordshire, GB) was prescribed to promote neural depolarization [50], beyond its primary role in muscle relaxation and ligament flexibility, reducing secondary muscle contractures [51] and allowing passive ROM (PROM) exercises for the shoulder, elbow and carpal joints (Figure 4). These exercises could be repeated 4-6 times/day (10-30 sets in each session), finishing with manipulation of all digits, individually or jointly [52].
FES (BTL-4820 Smart®, Hertfordshire, GB) was implemented based on the radial nerve anatomic pathway, with one electrode placed in C6, C7, C8, T1, T2 and the other in the motor point of the triceps brachialis muscle (Figure 5), and electrical parameters according to DPP (Table 4).
All rehabilitation modalities and exercises were immediately associated to the use of a corrective carpal splint (Buster Leg Splint, Kruuse, Denmark) applied with a vet-wrap tape (Vet-Flex, Kruuse, Denmark), that was placed 2 hours in the morning and afternoon (Figure 6), to avoid joint contracture.
Regarding frequency, the sequence of B + C+ D (Table 4) was repeated three times daily, whereas the other modalities were only performed once a day.
ES on the pelvic limb was also performed in cats with signs of proprioceptive deficits on the ipsilateral pelvic limb, to promote distal depolarization. This included the sciatic nerve FES, with one electrode placed on L7-S1 and the other on the motor point of the flexor muscle group (50 Hz; 6-24 mA in deep pain negative; 6-14 mA in deep pain positive), but also the peroneal nerve branch stimulation (one electrode L7-S1 and the other on the dorsal region of the paw, with the same parameters) (Figure 7). It is important to mention that all cats that had a doubtful manifestation after activation of C fibers by stimulating the periosteum receptors, required parameters (mA) similar to the referred for deep pain negative patients.
On the time period between the second and third week, some cats recovered joint motion of the elbow and were highly susceptible for stimulation of distal radial nerve depolarization, aiming mobility of the carpal joint and reducing the possibility of contracture. Thus, it was also performed radial nerve FES for carpal extension, with one electrode at C7, C8, T1, T2 and the other one on the dorsal region of the paw (Figure 8). It was important to take into consideration the electrode size, ensuring that the current would progress without concerns.
- 4th – 6th week of INRP
The laser therapy program was continued, the radial nerve FES (both triceps muscle and extension of the carpus), ROM exercises, postural standing, ultrasounds and, in some cases, FES of the pelvic limb. All these were followed by locomotor training on the land treadmill (10 – 20 min) with bicycle movements performed by a CCRP veterinarian or nurse, which was repeated twice a day during 6 days/week. Bicycle movements were performed without limb stretching and with direct contact of the digits to the treadmill belt for mechanoreceptors and proprioceptors stimulation (Figure 9). There was no inclination and speed increased until 1.8 km/h.
Near to the 4th week, considered a period of increased possible neural regeneration, the underwater treadmill (UWTM) training was introduced, once a day until a maximum of 10 min, with water level at the lateral epicondyle of the femur, temperature of 24-26ºC, no inclination and speed until a maximum of 2.2 km/h (Figure 10). UWTM training was performed after the rehabilitation modalities of laser, ultrasounds and FES, allowing a physiological muscle contraction based on the agonist/antagonist rule.
In this, as in all type of exercises, cats had to be in a controlled, easy and quiet environment, motivated by positive reinforcements. The rehabilitator should be inside the UWTM, performing rhythmic bicycle movements.
Furthermore, in this neurological phase, postural standing exercises on top of a balance board were added (2 min, 4 min rest), followed by walking in different floor surfaces (2 min, 6 min rest) and cavaletti rails (1-2 min), always with the corrective splint applied in order to facilitate elbow flexion.
In all cats that developed wounds, wound site cleaning was performed at the end of the day with a 3% chlorhexidine solution and class IV laser therapy was done for granulation tissue stimulation [53].
- 6th – 8th week of INRP
The same protocol was executed, considering A+B+C+D+E (Table 4), as well as locomotor training on the land treadmill with 10% inclination and the application of TheraBand to increase muscle strength and reduce neurogenic atrophy. Time of performance was aimed for 30 min twice a day, however in patients that could not perform this prolonged training, a detailed circuit was done, including: standing on a central pad stimulation (2 min), rest (2 min), walking in different floors (2 min), rest (2 min), up/down stairs (2 min), rest (2 min), up/down ramps (2 min), rest (2 min), cavaletti (2 min), rest (2 min), walking in “8”, rest (2 min) and, lastly, free time (playing with other cats or toys).
Modalities, such as laser and ultrasound, were reduced in the last two weeks, for each 48 h and the laser applied on the joints was only done in the interphalangeal and carpal regions. FES protocols were decreased for once a day, 5-6 times/week.
- Neuropathic pain
In addition, cats with signs of neuropathic pain had a pharmacological support based on pregabalin (2 mg/kg BID) for 4 weeks, decreased for SID in the next 4 weeks. If any of these cats showed signs of pain, such as excessive liking, interferential transcutaneous electrical nerve stimulation (TENS) was prescribed for the carpal and digits region. This technique used two different channels crossed each other at a 90◦ angle: one channel with 80–150 Hz, 0.5–1 mA, pulse duration 2–50 µs, 10 min; the other channel with 1–10 Hz, 0.5–1 mA, pulse duration 100–400 µs, 10 min [54].
2.4. Data collection
Data collected from all 22 cats, included the continuous quantitative variables age (< 7 years or ≥ 7 years), weight (< 5kg or ≥ 5kg), time until medical discharge (30 days or 60 days) and time until DPP recovery (< 30 days or ≥ 30 days). Categorical nominal variables collected were sex, breed, DPP in the first four digits (present, absent, doubtful), and with particular attention DPP in the 5th digit (present, doubtful), withdrawal reflex (present, absent or diminished), extensor carpi radialis reflex (present, absent or diminished), cutaneous trunci reflex (present or absent), Horner syndrome (present or absent), knuckling on the thoracic and pelvic limb (present or absent), muscle atrophy of the triceps and extensor carpi radialis muscles (present, absent or mild), dermatomes until elbow, between elbow and carpus and between carpus and digits (present or absent), shoulder motion (present or absent), elbow motion (present or absent), carpus motion(present or absent), carpal contracture (present, absent or mild), standing position (present or absent), arthrodesis (performed or not performed), ambulation (present or absent), neuropathic pain (present or absent), and clinical occurrences (present or absent). At follow up time points the muscle mass (improved or not) and muscle weakness (improved or not) were considered for evaluation, besides comparison with the healthy contralateral limb for the same parameters.
2.5. Statistical analyses
Data was recorded using Microsoft Office Excel 365® (Microsoft Corporation, Redmond, WA, USA) and processed in IBM SPSS Statistics 25® (International Business Machines Corporation, Armonk, NY, USA) software. Shapiro-Wilk normality test (for n<50), arithmetic means, median, mode, variance, standard deviation (SD), minimum, maximum and standard error of mean (SEM), were recorded for continuous variables age and weight. Descriptive statistics with frequency analysis was made for all categorical nominal variables. Chi-square tests were also performed to verify relevant relations proven by a p-value <0.05.
3. Results
In this prospective cohort pilot study (n=22), for the binominal variable sex, 68.2% (15/22) males and 31.8% (7/22) females were identified. Descriptive analysis of the continuous variables, age and weight, are presented in Table 5, and showed a mean age of 4.86 years old and mean weight of 4.73 kg.
Additionally, descriptive statistics with frequency analysis of all categorical nominal variables are displayed in Table 6, considering time of admission (T0). There were no significant statistical relations obtained between age, weight, sex or breed and the outcome results at discharge.
At admission (T0), 86.4% (19/22) of all cats had positive dermatomes only until the elbow and movement of the shoulder joint. There were 13.6% (3/22) cats with absence of this and all dermatomes, as well as movement in all joints.
Regarding the presence of dermatomes at T0, there was no significant relation with ambulation in discharge, however the recovery of dermatomes during rehabilitation presented a strong significance [X2(1, n=22) = 9.263, p=0.002]. There were 72.7 % (16/22) of cats that recovered ambulation and all had positive dermatomes at medical discharge. These same 16 cats recovered movement off all three joints during the INRP, 11 cats had this recovery until 30 days, 3 cats within 45 days and only 2 cats recovered by 60 days.
The deep pain evaluation at T0 revealed absence of DPP in the first four digits on 45.5% (10/22) and the remaining 54.5% (12/22) had doubtful DPP. The relation between DPP at admission and ambulation achievement, obtained a strong significance [X2(1, n=22) = 9.900, p=0.002], with all doubtful DPP cats achieving ambulation.
Considering the evaluation of deep pain in the 5th digit, that was present in 40.9% (9/22) and doubtful in 59.1% (13/22), it was revealed no significant relation with ambulation recovery. However, a significance [X2(1, n=22) = 4.701, p=0.030] was obtained with development of carpal contractures, with most contractures seen on cats with doubtful DPP on the 5º digit (n=9).
Additionally, a strong significant relation between the development of carpal contractures and time until medical discharge [X2(1, n=22)=22.000, p <0.001] was shown, with all cats that presented this clinical sign obtaining medical discharge at 60 days.
Thus, from the total population 72.7% (16/22) achieved ambulation, whereas 27.3% (6/22) didn´t recover. For these 16 cats, median time for ambulation was 30 days, with 11 cats having medical discharge at day 30 and 5 cats at day 60.
There were 50% (11/22) cats with medical discharge at day 30, and all were ambulatory recovering DPP until 15 days (n=9) or until 30 days (n=2). All showed presence of knuckling position during the INRP, followed by total recovery of reflexes, dermatomes and joint movement, with absence of carpal contracture, although with diminished extension of the carpal joint.
Of the remaining 50% (11/22) that had medical discharge by day 60, only 5 cats recovered total DPP and developed knuckling position, followed by ambulation, although all presented positive evolution regarding reflexes and development of carpal contractures. There were 6 cats with incomplete recovery of DPP but with improvement in the 5th digit, and three of them recovered all dermatomes with joint movement until the elbow, resorting to surgical arthrodesis (Figure 11). The other three, only recovered dermatomes between shoulder and elbow and movement until the elbow, with amputation of the limb later on the follow ups (2 cats in F3 and 1 cat in F4).
Regarding neuropathic pain, there were 18.2% (4/22) cats with this clinical sign at admission. During the following time points of the INRP, 50% (11/22) cats showed development of neuropathic pain until day 15, associated to the development of carpal contracture’s [X2(1,n=22)=18.333, p <0.001]. They manifested progressive improvement and only 6 cats maintained this sign at medical discharge, the same that later required surgical arthrodesis or amputation.
The clinical occurrences during the study period were based on wounds located on the dorsal region of the digits and were registered in 40.9% (9/22) of the cats.
Horner syndrome was diagnosed in 13.6 (3/22) cats, with total recovery in two cats by day 30, one that achieved ambulation and one other needing arthrodesis. The cat that did not recovery from the Horner syndrome by day 60 ended up requiring limb amputation at F3.
Considering follow ups evaluation, there was an evident improvement on both muscle mass and muscle weakness (Figure 12). However, 3 cats only showed a slight improvement on the first follow up (3 months), always maintaining neuropathic pain, and later followed by limb amputation.
When comparing to the contralateral limb, there was always less muscle mass and more muscle weakness in all follow ups until one year (F3). Although after 2 years (F4), 7 cats showed no difference between both limbs, which was maintained until 4 years (F6) with resolution of neuropathic pain.
4. Discussion
In this prospective cohort pilot study, 22 cats were referred for the neurorehabilitation center after PTBPI diagnostic due to road traffic accidents, which are common in both human patients and small animals [5,15,16,17,57]. All had acute non-progressive injury of the peripheral nervous system, although submitted to early start of nerve repair to improve functional outcomes [10,20] and to avoid muscle fibrosis and neurogenic atrophy. Our INRP implementation timeline was similar to those practiced in human medicine and earlier than the median time of 14-13 days after injury that is described by Menchetti et al (2020) [57]. Furthermore, the same study revealed a median duration of 60 days until recovery, with owner´s reports of improvement in 5 cats. When comparing to our study, these 22 cats had a high probability of axonotmesis (equivalent to Sunderland grade II – IV) and presented half of the time of median duration until ambulation (30 days).
Human medicine protocols are usually based on the beneficial role of locomotor training, as one of the best options to treat peripheral nerve injury, associated with other physical exercises and modalities, such as ES. The volume and intensity of training with standardized and correctly selected frequencies of ES, could improve functionally and can be translated to veterinary medicine, considering the “one health” concept [13,58,59,60].
There was no significant relation between the final functional outcome and age, probably associated with our sample population that has similar ages, with a mean age of 4.86 years (SEM = 0.467). The same was observed with weight, with a mean of 4.73 kg (SEM 0.239), and with sex and breed.
Many factors could influence functional recovery following nerve injury. Beyond the time elapsed between trauma and treatment to repair, there is the distance from the injury site to the target muscle and the regeneration rate, that is usually around 1 mm per day, resulting in atrophy of both distal nerve and effector muscles, which may unable functional recovery. Also, in admission (T0), there was 45.5% (10/22) of cats without DPP in the first four digits and 54.5% (12/22) with doubtful DPP, an important fact given that literature points out the absence of DPP as the single neurologic sign associated to amputation of the affected limb [57,61,62].
In our results the significant relation between DPP and ambulation (p=0.002), showing that all cats with doubtful DPP became ambulatory, could be related to the INRP conservative treatment that ensured protection of the injury region, avoid other damage of nerve structure with pain control and management of sensory deficits between the first 15 to 21 days. The ES programs were probably an important tool that was early implemented since the beginning of treatment, assuring stimulation from proximal to distal, according to the neurological evaluation and joint ROM of the affected limb.
Total ambulation recovery was 72.7% (16/22), median time of 30 days, with 11 cats discharged at day 30 and 5 cats at day 60, thus in less time than that previously described [55], however in agreement with Santifort (2016) [63] in one cat.
Sensorimotor evaluation with dermatomes recovery showed a clear significance with ambulation throughout the study (p=0.002), related to joint contractures resolution with 11 cats recovering shoulder, elbow, and carpus motion in 30 days. Therefore, it is essential to avoid muscle and joint contractures, maintaining limb mobility with conservative treatment [63,64,65,66].
In human medicine, several non-surgical approaches that includes pharmacological, electrical, cell-based and laser therapies have been used to promote re-myelination and enhance recovery in peripheral nervous system diseases [67,68,69,70,71]. The implementation of ES, particularly between the second and third week, may help muscles passive contraction, normal morphology of electromyographic waves during treatment [72], reduce muscle atrophy, promoting muscle reinnervation by increasing expression of structural protective proteins and neurotrophic factors [71].
Different authors [73,74,75] suggests that it is possible to mimic the physiological wave of Ca2+ influx that generates a retrograde signal leading to subsequent activation of cell autonomous mechanisms, initiating regeneration [76,77]. Thus, FES may help promoting upregulation of BDNF, neurotrophins and TrK receptors [78,79], as well as glial cell like derived neurotrophic factor (GDNF) [71,80,81,82].
Additionally, studies have been demonstrating earlier axon and Schwann cell outgrowth with accelerated reconnection to the target injury site. This could be related to cell mechanisms that increase the adenosine monophosphate with Ca2+ influx, regulating BDNF and TrkB expression [27,40,76,83,84,85].
Early recovery of DPP in the 5th digit, shown significant relation to ambulation (p=0.03), and 9 cats that have developed carpus contractures due to a faster re-innervation of the flexor muscles and not the extensor muscles, demonstrated a knuckling persistent position during gait [86,87]. Experimental studies on rats with peripheral lesion on the sciatic nerve refer to the implementation of PROM and assisted active exercises, such as the use of a 45º net inside the cage to avoid prolonged muscle inactivation with wrong positioning [88,89,90]. Therefore, revealing the meaning of an early multimodal protocol with the application of a corrective splint for 2h twice a day.
In the same study, 50% of the cats recovered ambulation until day 30, but all had a progressive sensorimotor evolution, requiring different ES programs, adapted to each case and neurological evolution (e.g. presence of knuckling position). For example, FES for the extensor muscles of the carpus with a precise trapezoid current and parameters adjusted to DPP, was able to generate an action potential strong enough for nerve depolarization and consequent muscle contraction [91]. Also, an accurate placement of electrodes is crucial for opposing the low current that unable physiological contraction in these conditions [92]. Cats with evident neurogenic atrophy, need longer time to recover and, to avoid muscle fatigue, the programed duty cycle should be of 1:5 and ramp down of 1-2 sec [93,94]. The trapezoid modulation, which is a triangular current with a duration of 200 ms has shown to have better effects on denervated muscles [71,95].
Thus, in the study, FES was selected with specific parameters and methods according to the daily neurological situation of each cat, with perfect knowledge that high frequencies of ES could aggravate nerve damage [71,96], causing fatigue and neuropathic pain.
INRP included both ES and locomotor training, aiming to achieve the synergetic result of BDNF upregulation [30,40,41,97,98,99,100]. Furthermore, intensive locomotor exercise started after 21 days, when neural regeneration is higher without limitation of muscle neurogenic atrophy and strength improvement [72,73].
After 6 weeks, the starting of stretching maneuvers allowed to maintain muscles flexibility [72,101] and passive stretching promoted stimulation of mechanoreceptors, slowing down protein degradation [102] and increasing elbow and carpus ROM. From the other 50 % of cats with medical discharge at day 60, five recovered DPP and ambulation of the injured limb, however six only improved DPP on the 5th digit and three had dermatomes recovery with complete movement of the elbow, a perfect scenario for performing an arthrodesis, improving functionality, quality of life and decreasing neuropathic pain.
Some authors refer the need for more than 3 months to recovery (from 3 to 12 months) after PTBPI [57,67]. Our results showed that only 3 cats didn´t recover dermatomes in the forearm with joint movement until the elbow, maintaining neuropathic pain, wounds and discomfort throughout the long-term follow-ups, resulting in 13.6% of limb amputation in agreement with [57,61].
In human patients with brachial plexus injury, neuropathic pain is described with a frequency of 30 to 80% [103,104] and with highly refractory presentation [105,106,107,108,109]. Comparing to our sample population at admission, 18.2% of the cats had this clinical sign, increasing to 50% during the first 15 days, probably associated with carpal contracture (p<0.001), and in 40.9% related to wounds on the dorsal region of the digits due to knuckling position. Pain management associated to pregabalin administration and resolution of these occurrences improved the outcome.
In the current investigation, there was a high variability of sensory pattern and sympathetic innervation, consistent with the evolution of Horner syndrome and a weakness pattern, in some cases, that had a positive evolution. During the long-term follow ups, 7 cats showed improvements in muscle mass and muscle weakness at F4 (2 years), which was maintained until 4 years and without neuropathic pain.
Interferential TENS could, also, be associated with progressive improve of neuropathic pain, given their analgesic effects [72,110] to control allodynia and hyperalgesia, according to the type of injury or ability to recover [72,111]. In addition, to treat pain, inflammation and edema, the INRP included class IV laser therapy, acting on the upregulation of nitric oxide. This, as other free radicals that result from lipidic peroxidation of the central and peripheric nervous system, is associated to cell necrosis, and as an important role on neuropathic pain, when compared to other human medicine peripheral neuropathies (e.g. diabetic polyneuropathy, multiple sclerosis, stroke) [103,112].
In our study there was no observation of signs related to possible phantom limb pain, that is reported to occur in 54-85 % of amputees in human medicine [100,113,114,115]. This phenomenon, interpreted as a cortical reorganization [111], is estimated to happen in veterinary patients within the first 2 years after amputation and up to 10% could possibly persist through life [116,117]. However, this was not observed in long term follow ups until 4 years for the 3 amputated cats.
The main limitation in this study was the absence of EMG studies can localize injury and to provide functional information and follow-up of the re-innervation process, although these studies require a specific timing that must be considered. This is mostly because, in axonal lesion, reduction of CMAPs requires several days, electrical stimulation distally to the injury can be normal and the sign of Wallerian degeneration requires at least two to three weeks to appear. Subsequent research endeavors should prioritize the inclusion of EMG procedures as an integral component of INRP monitoring and early injury classification.
The small sample size is another limitation due to strict criteria for inclusion to reduce selection bias, as well as absence of control group in a clinical setting rehabilitation center.
5. Conclusion
Early INRP may have an important role in promoting ambulation of cats diagnosed with partial traumatic brachial plexus injury.
FES accurate parameters, depending on DPP, could be essential to improve sensory-motor recovery by allowing expression of structural protective proteins and neurotrophic factors, that increase with the synergic effect of locomotor training and that in this study started after 21 days. This prospective cohort pilot study had 72.7 % of ambulation with a median time of 30 days. This investigation should be continued with further studies.
Author Contributions
Conceptualization Â.M.; methodology D.G.; validation Â.M., A.F., A.C.M; formal analysis, Â.M., A.F., A.V, A.C.M; investigation D.G., A.C., C.C., M.M., B.T.R., A.C.O., I.R., Ó.G. A.A., B.L., P.S., A. M., A. C., A. R., M.J.S., C.S.M., M.M.B., R.A.; writing—original draft preparation, D.G.; writing—review and editing Â.M, A.F., A.V., R.A.; supervision Â.M., A.C.M.; project administration Â.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Prémios Santa Casa Neurociências–Prize Melo e Castro for Spinal Cord Injury Research (MC-04/17; MC-18-2021). The author Rui D. Alvites acknowledges the Centro de Estudos de Ciência Animal (CECA), Instituto de Ciências, Tecnologias e Agroambiente (ICETA), Porto University (UP), and Fundação para a Ciência e Tecnologia (FCT) for the funding and availability of all technical, structural, and human resources necessary for the development of this work. The work was supported through the project UIDB/00211/2020 funded by FCT/MCTES through national funds. The authors acknowledges FCT for funding the project 2022.04501.PTDC (Olfabionerve - Olfactory Mucosa Mesenchymal Stem Cells and Biomaterials Promoting Peripheral Nerve Regeneration ) and the PhD Scholarships Ana Catarina Sousa (SFRH/BD/146689/2019), Bruna Lopes (2021.05265.BD), Patrícia Sousa (2023.00246.BD), André Coelho (2023.00428.BD) and Alicia Moreira (2023.00544.BD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- E.E. Motor system. In Fundamentals of canine neuroanatomy and neurophysiology. Uemura, E.E., ed. Iowa, USA: John Wiley & Sons, 2015, pp 257-288.
- Troupel, T.; Caenegem, N.V.; Jeandel, A.; Thibaud, J.; Nicolle, A.; Blot, S. Epidemiological, clinical, and electrophysiological findings in dogs and cats with traumatic brachial plexus injury: a retrospectove study of 226 cases. J Vet Intern Med. 2021, 35, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A.; Glass, E.; Kent, M. Lower motor neuron: spinal nerve, general somatic efferent system. In Veterinary Neuroanatomy and clinical neurology. de Lahunta, A., Glass, E., Klent, M., eds. 4th ed, St. Louis, MO: Elsevier, 2014, pp. 102-161.
- Wheeler, S.J.; Jones, D.G.; Wright, J.A. The diagnosis of brachial plexus disoreders in dogs: a review of twenty-two cases. J Small Anim Pract. 1986, 27(3), 147–157. [Google Scholar] [CrossRef]
- Soens, I.V.; Struys, M.M.; Polis, I.E.; Bhatti, S.F.; Meervenne, S.A.; Martlé, V.A.; Nollet, H.; Tshamala, M.; Vanhaesebrouck, A.E.; Ham, L.M. Magnetic stimulation of the radial nerve in dogs and cats with brachial plexus trauma: a report of 53 cases. Vet J. 2009, 182, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, I.R.; Duncan, I.D.; Lawson, D.D. Avulsion of the brachial plexus-2. Clinical aspects. J Small Anim Pract. 1974, 15, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.S. Brachial plexus injuries and dysfunctions. Vet Clin of North Am: Small Anim Practic 1988, 18, 565–580.
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Annals of Anatomy 2011, 193, 321-333.
- Anson, A.; Gil, F.; Laredo, F.G.; Soler, M.; Belda, E.; Ayala, M.D.; Agut, A. Correlative ultrasound anatomy of the feline brachial plexus and major nerves of the thoracic limb. Vet Radiol Ultrasound 2013, 54, 2, 185-193.
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Effron, C.R.; Beasley, R.W. Compression neuropathies in the upper limb and electrophysiological studies. In Grabb and Smith’s Plastic Surgery. Thorne, C.H., Bartlett, S.P., Beasley, R.W., Aston, S.J., Gurtner, G.C., Spear, S.L., eds. Philadelphia, USA: Lippincott Williams & Wilkins; 2006, pp. 86.
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve. 2000, 23(6), 863–87. [Google Scholar] [CrossRef]
- Alvites, R.; Rita Caseiro, A.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Colette Maurício, A.; Spurkland, A. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent. Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Seddon, H.J. Three types of nerve injury. Brain. 1943, 66, 237. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J. C. Peripheral nerve trauma: mechanisms of injury and recovery. Hand Clin. 2013, 29 3, 317–330. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J. , et al. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. Intern J Bio Sci 2020, 16 (1), 116-134.
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; Schoonhoven, J. The role of current techniques and concepts in peripheral nerve repair. Plastic Surg Intern. 2016, 4175293. [Google Scholar] [CrossRef]
- Smith, J.W. Microsurgery of peripheral nerves. Plastic Rec Surg. 1964, 33, 317–329. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: surgical application, atate of the art, and future challenges, Crit Rev Bio Eng. 2011, 39 (2), 81-124.
- Mackinnon, S.; Dellon, A.L. Surgery of the peripheral nerve. NY: Thieme; Diagnosis of nerve injury, 1988, pp. 74–8.
- De Lahunta, A. Feline neurology. Vet Clin North Am. 1976, 6(3), 433–451. [Google Scholar] [CrossRef] [PubMed]
- Dessal, F. Fundamentos de Neuroanatomia. In Neurologia Felina. Dessal, F., ed. Buenos Aires, Argentina: Editorial Inter-Médica, 2020.
- Willand, M.P. Electrical stimulation enhances reinnervation after nerve injury. Eur J Trans Myol. 2015, 25(4), 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Chen, J.; Hsu, Y.; Bau, D.; Yao, C.; Chen, Y. High-Frequency electrical stimulation can be a complementary therapy to promote nerve regeneration in diabetic rats. Plos One. 2013, 8(11), e79078. [Google Scholar] [CrossRef] [PubMed]
- Foecking, E.M.; Fargo, K.N.; Coughlin, L.M.; Kim, J.T.; Marzo, S.J.; et al. Single session if brief electrical stimulation immeadiately following crush injury enhances functional recovery of rat facial nerve. J Rehabil Res Dev. 2012, 49, 451–458. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Russo, T.L.; Geuna, S.; Domingues, N.; Salvini, T.F.; Parizotto, N.A. Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle & Nerve 2010, 685-693.
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, C. , Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef]
- Brushart, T.M.; Jari, R.; Verge, V. ; Rohde. C.; Gordon, T. Electrical stimula- tion restores the specificity of sensory axon regeneration. Exp Neurol 2005, 194, 221–229.
- Evans, G.R. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001, 263, 396–404. [Google Scholar] [CrossRef]
- Marqueste, T.; Alliez, J.R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation af- ter peripheral nerve injury and repair. J Appl Physiol. 2004, 96, 1988–1995. [Google Scholar] [CrossRef]
- Russo, T.L.; Peviani, S.M.; Durigan, J.L.; Salvini, T.F. Electrical stimulation increases matrix metalloproteinase-2 gene expression but does not change its activity in denervated rat muscle. Muscle Nerve. 2008, 37, 593–600. [Google Scholar] [CrossRef]
- Varejão, A.S.; Cabrita, A.M.; Meek, M.F.; Bulas-Cruz, J.; Melo-Pinto, P.; Raimondo, S.; et al. Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurotrauma. 2004, 21, 1652–1670. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier- Santos, M.A.; Martinez, A.M. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J Peripher Nerv Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Kerns, J.M.; Lucchinetti, C. Electrical field effects on crushed nerve regeneration. Exp Neurol 1992;117:71–80.
- Lundborg, G. Enhancing posttraumatic nerve regeneration. J Peripher Nerv Syst. 2002, 7, 139–140. [Google Scholar] [CrossRef]
- Samii, M.; Carvalho, G.A.; Nikkhah, G.; Penkert, G. Surgical reconstruc- tion of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997, 87, 881–886. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low inten- sity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods. 2003, 129, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dow, D.E.; Cederna, P.S.; Hassett, C.A.; Kostrominova, T.Y.; Faulkner, J.A.; Dennis, R.G. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve. 2004, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Kim, D.; Kim, D.; Huh, Y.; Park, C.; Chung, H.; Jung, J.; Jeong, N.Y. Heme Oxygenase 1 in Schwann cells regulates peripheral nerve degeneration against oxidative stress. ASN Neuro 2019 11:1759091419838949.
- Al-Majed, A.A.; Brushart, T.; Gordon, T. Electrical stimulation accelarates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Neumann, C.; Brushart, T.M.; Gordon, T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000, 29(7), 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel sundrome patients. Exp Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef]
- Denny, H.R; Butterworth, S.J. Peripheral nerve injury. In A guide to canine and feline orthopaedic surgery, Denny, H.R; Butterworth, S.J, eds. Oxford, UK: Blackwell science Lds, 2000; pp. 201–205. [Google Scholar]
- Añor, S. Monoparesis. In BSAVA Manual of canine and feline neurology. Platt, S.; Olby, N., eds. Cheltenahm, UK: Brithish Small Animal Veterinary Association, 2013; pp. 328–341. [Google Scholar]
- Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R. Therapeutic exercise and manual therapy. In Reabilitação e Fisioterapia na Prática de Pequenos Animais. Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R, eds. São Paulo, Brasil: Roca. 2008, pp. 447-463.
- Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R. Physical therapy for specific diagnoses. In Reabilitação e Fisioterapia na Prática de Pequenos Animais. Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R, eds. São Paulo, Brasil: Roca. 2008, pp. 609-627.
- Shores, A.; Pearce, L. Traumatic and Neoplastic Diseases of the Brachial Plexus. In Mechanisms of disease in Small Animal Surgery. Bojrab, M.J.; Monnet, E., eds. Jackson, WY: Teton New Media Inc. 2010.
- Knecht, C. D.; Raffe, M. R. Diseases of the Brachial Plexus. In Textbook of Small Animal Orthopaedics. Newton, C.D.; Nunamaker, D.M., eds. Filadélfia, PA: Lippincott, 1985. [Google Scholar]
- Marcolino, A. M.; Barbosa, R. I.; Fonseca, M. C.; Mazzer, N.; Ellui, V. M. Reabilitação fisioterapêutica na lesão do plexo braquial: relato de caso. Fisioterapia em Movimento 2008, 21, 53-60.
- Monte-Raso, V. V.; Barbieri, C. H.; Mazzer, N.; Fazan, V. P. Os efeitos do ultra- som terapêutico nas lesões por esmagamento do nervo ciático de ratos: análise funcional da marcha. Revista Brasileira de Fisioterapia 2006, 10, 113-119.
- Millis, D. L.; Levine, D. Basic science of veterinary rheabilitation. In Canine Rehabilitation and Physical Therapy. Millis, D. L.; Levine, D., eds. Filadélfia, PA: W. B. Saunders Co., Ltd, 2014; pp. 79–153. [Google Scholar]
- Bocksthler, B.; Wittek, K. Passive Range of Motion Exercises and Stretching. In Essential facts of physical medicine, rehabilitation and sports medicine in companion animals. Bocksthler, B.; Wittek K, eds. 2019, pp. 110.
- Gouveia, D.; Fonseca, S.; Carvalho, C.; Cardoso, A.; Almeida, A.; Gamboa, Ó.; Canejo-Teixeira, R.; Ferreira, A.; Martins, Â. Clinical occurrences in the neurorehabilitation of dogs with severe spinal cord injury. Animals (Basel), 2023, 13 (7), 1164.
- Martins, Â.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A comparison between body weight-supported treadmill training and conventional over-ground training in dogs with incomplete spinal cord injury. Front Vet Sci. 2021, 8, 597949. [Google Scholar] [CrossRef]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Silva, C.; Coelho, T.; Gamboa, Ó.; Ferreira, A. Functional neurorehabilitation in dogs with an incomplete recovery 3 months following intervertebral disc surgery: a case series. Animals. 2021, 11(2442), 1–21. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J. Ambulation in dogs with absent pain perception after acute thoracolumbar Spinal Cord Injury. Front Vet Sci. 2020, 7, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, M.; Gandini, G.; Bravaccini, B.; Dondi, M.; Gagliardo, T.; Bianchi, E. Clinical and electrodiagnostic findings and quality of life of dogs and cats with brachial plexus injury. Vet Sci. 2020, 7(3), 101. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon. 2021, 7, e08281. [Google Scholar] [CrossRef]
- Franzblau, L.E.; Shauver, M.J.; Chung, K.C. Patient satisfaction and self-reported outcomes after complete brachial plexus avulsion injury. J. Hand Surg. 2014, 39, 948–955. [Google Scholar] [CrossRef]
- Welch, J.A. Peripheral nerve injury. Semin. Vet. Med. Surg. (Small Anim.) 1996, 11, 273–284.
- Santifort, K.M. Return of function in a feline thoracic limb after suspected traumatic brachial plexus injury with loss of nociception. Vet. Rec. Case Rep. 2016, 4(1), e000334. [Google Scholar] [CrossRef]
- Smith, B.W.; Daunter, A.K.; Yang, L.J.S.; Wilson, T.J. An Update on the Management of Neonatal Brachial Plexus Palsy—Replacing Old Paradigms: A Review. JAMA Pediatr. 2018, 172, 585. [Google Scholar] [CrossRef]
- Rich, J.A.; Newell, A.; Williams, T. Traumatic brachial plexus injury rehabilitation using neuromuscular electrical muscle stimulation in a polytrauma patient. BMJ Case Rep. 2019, 12, e232107. [Google Scholar] [CrossRef]
- Helgeson, K.; Stoneman, P. Shoulder injuries in rugby players: Mechanisms, examination, and rehabilitation. Phys. Ther. Sport. 2014, 15, 218. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res. 2020, 98(5), 780–795. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016, 17(12).
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J. , Reid, A.J. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 2015, 82–83, 160–167.
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int Rev Neurobiol 2009, 87, 295–315. [Google Scholar] [PubMed]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T. ; Wang, J; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front Neurol 2023, 14, 1-13.
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial plexus injuries in sport medicine: Clinical evaluation, diagnostic approaches, treatment options and rehabilitative interventions. J Funct Morphol Kinesiol. 2020, 5(2), 22. [Google Scholar] [CrossRef] [PubMed]
- Javeed, S.; Faraji, A.H.; Dy, C.; Ray, W.Z.; MacEwan, M.R. . Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg.: Adv. Tech. Case Manag. 2021, 24, 101117.
- Gordon, T.; Udina, E.; Verge; V. M.K.; de Chaves, E.I.P. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009, 13(4), 412–441. [Google Scholar] [CrossRef]
- Aglah, C.; Gordon, T.; de Chaves, E.I.P. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008, 55(1), 8–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Xu, Q.-G.; Franz, C.K.; Zhang, R.; Dalton, C.; Gordon, T.; Verge, V.M.K.; Midha, R.; Zochodne, D.W. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm: Laboratory investigation. J. Neurosurg. 2012, 116(3), 498–512. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met. Front. Cell. Neurosci. 2019, 12, 522. [Google Scholar] [CrossRef]
- Hoffman, H. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust. J. Exp. Biol. Med. Sci. 1952, 30, 541–566. [Google Scholar] [CrossRef]
- Nix, W.A.; Hopf, H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Research. 1983, 272(1), 21–25. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013, 38, 3691–701. [Google Scholar] [CrossRef]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; et al. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials. 2021, 275, 120982. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013, 240, 157–67. [Google Scholar] [CrossRef]
- English, A.W.; Meador, W.; Carrasco, D.I. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005, 21, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.R.; Meek, M.; Robinson, P.H.; Gramsbergen, A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Met. 2000, 96, 89–96. [Google Scholar] [CrossRef]
- Meek, M.F.; Van Der Werff, J.F.A.; Nicolai, J.P.A.; Gramsbergen, A. Biodegradable p (DLLA-e-CL) Nerve guides versus autologous nerve grafts: electromyographic amd video analysis. Muscle & Nerve 2001, 24 753-759.
- Watson, N.C.; Jejurikar, S.; Kalliainen, L.K.; Calderon, M.S.; URbanchek, M.G.; Eguchi, T.; Kuzon, J.R. Range of motion physiotherapy reduces the force deficit in antagonists to denervated rat muscles. J Surg Res. 2001, 99, 156–160. [Google Scholar] [CrossRef]
- Siegel, S.G.; Patton, B.; English, A.W. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol. 2000, 166, 205–212. [Google Scholar] [CrossRef]
- Varejão, A.S.P.; Cabrita, A.M.; Geuna, S.; Melo-Pinto, P.; Filipe, V.M.; Gramsbergen, A.; Meek, M.F. Toe out angle: a functional index for the evaluation of sciatic nerve recovey in the rat model. Exp Neurol. 2003, 183. [Google Scholar] [CrossRef]
- Richard, M.A.; Spaich, E.G.; Serrao, M.; Andersen, O.K. Stimulation site and phase modulation of the withdrawal reflex during gait initiaion. Clin Neurophysiol 2015, 126(12), 2282–2289. [Google Scholar] [CrossRef]
- Pilkar, R.B.; Yarossi, M.; Forrest, G. Empirical mode decomposition as a tool to remove the function electrical stimulation artifact from surface electromyograms: preliminary investigation. In: 2012 annual international conference of the IEEE engineering in medicine and biology society. San Diego, CA: IEEE, 2012, pp. 1847–50.
- Martins, A.; Gouveia, D.; Cardoso, A.; Gamboa, Ó.; Millis, D.; Ferreira, A. Nervous system modulation through electrical stimulation in companion animals. Acta Vet Scand. 2021, 63, 22. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Bockstahler, B. Electrical stimulation. In: Canine rehabilitation and physical therapy; Millis, D., Levine D, Eds.; Elseviers: Philadelphia, 2014, pp. 342–56.
- Haastert-Talini, K.; Schmitte, R.; Korte, N.; Klode, D.; Ratzka, A.; Grothe, C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. 2011, 28, 661–74. +. [CrossRef] [PubMed]
- Pieber, K.; Herceg, M.; Paternostro-Sluga, T.; Schuhfried, O. Optimizing stimulation parameters in functional electrical stimulation of denervated muscles: a cross-sectional study. J Neuroeng Rehabil. 2015, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, R.; Pavone, V.; Testa, G.; Pesce, I.; Scaturro, D.; Musumeci, G.; Mauro, G.; Vecchio, M. The role of physical exercise and rehabilitative implication in the process of nerve repair in peripheral neuropathies: a systematic review. Diagnostics (Basel). 2023, 13(3), 364. [Google Scholar] [CrossRef]
- Pachter, B.R.; Eberstein, A. Passive Exercise and Reinnervation of the Rat Denervated Extensor Digitorum Longus Muscle after Nerve Crush. Am. J. Phys. Med. Rehabilitation. 1989, 68, 179–182. [Google Scholar] [CrossRef]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef]
- Hartley, R.A.; Kordecki, M.E. Rehabilitation of chronic brachial plexus neuropraxia and loss of cervical extension in a high school football player: A case report. Int. J. Sports Phys Ther. 2018, 13, 1061–1072. [Google Scholar] [CrossRef]
- Cui, J.; Blaha, C.; Moradkhan, R.; Gray, K.S.; Sinoway, L.I. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J. Physiol. 2006, 576, 625. [Google Scholar] [CrossRef]
- Teixeira, M.J.; da Paz, M.G.D.S.; Bina, M.T.; Santos, S.N.; Raicher, I.; Galhardoni, R.; Fernandes, D.T.; Yeng, L.T.; Baptista, A.F.; de Andrade, D.C. Neuropathic pain after brachial plexus avulsion-central and peripheral mechanisms. BMC Neurol. 2015, 15, 73. [Google Scholar] [CrossRef]
- Treede, R.-D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Vannier, J.L.; Belkheyar, Z.; Oberlin, C.; Montravers, P. Management of neuropathic pain after brachial plexus injury in adult patients: A report of 60 cases. Ann. Fr. Anesth. Reanim. 2008, 27, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, S.; Ghaleb, A.H. Cervical Spinal Cord Stimulation for the Management of Pain from Brachial Plexus Avulsion. Pain Med. 2014, 15, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D.; et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, P.; Rui, J.; Zhao, X.; Lao, J. The clinical characteristics of neuropathic pain in patients with total brachial plexus avulsion: A 30-case study. Injury. 2016, 47, 1719–1724. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Meng, C.; Zhou, Y.; Lao, J.; Zhao, X. A new model for the study of neuropathic pain after brachial plexus injury. Injury. 2017, 48, 253–261. [Google Scholar] [CrossRef]
- Lovaglio, A.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India. 2019, 67, 32. [Google Scholar] [CrossRef]
- Emamhadi, M.; Andalib, S. Successful recovery of sensation loss in upper brachial plexus injuries. Acta Neurochir. (Wien). 2018, 160, 2019–2023. [Google Scholar] [CrossRef]
- Sadosky, A.; McDermott, A. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008, 8(1), 45–56. [Google Scholar] [CrossRef]
- Ehde, D.M.; Czerniecki, J.M.; Smith, D.G.; Campbell, K.M.; Edwards, W.T.; Jensen, M.P.; et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000, 81(8), 1039–44. [Google Scholar] [CrossRef]
- Melzack, R. Phantom limbs. Sci Am. 1992, 266(4), 120–6. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Krebs, B.; Nielsen, J.; Rasmussen, P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985, 21, 267–78. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, M.; Gandini, G.; Gallucci, A.; Della Rocca, G.; Matiasek, L.; Matiasek, K.; Gentilini, F.; Rosati, M. Approaching phantom complex after limb amputation in the canine species. J. Vet. Behav. 2017, 22, 24–28. [Google Scholar] [CrossRef]
- Probstner, D.; Thuler, L.C.; Ishikawa, N.M.; Alvarenga, R.M. Phantom limb phenomena in cancer amputees. Pain Pract. 2010, 10, 249–256. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Cat with ipsilateral monoplegia and proprioceptive deficits, showing knuckling posture. .

Figure 2.
Medial aspect of the thoracic limb. N.: Nerve.

Figure 3.
Thoracic limb cut-off point of the dermatomes map marked with ink for further evaluation.

Figure 4.
Passive range of motion (PROM) exercises on the carpal joint of a cat.
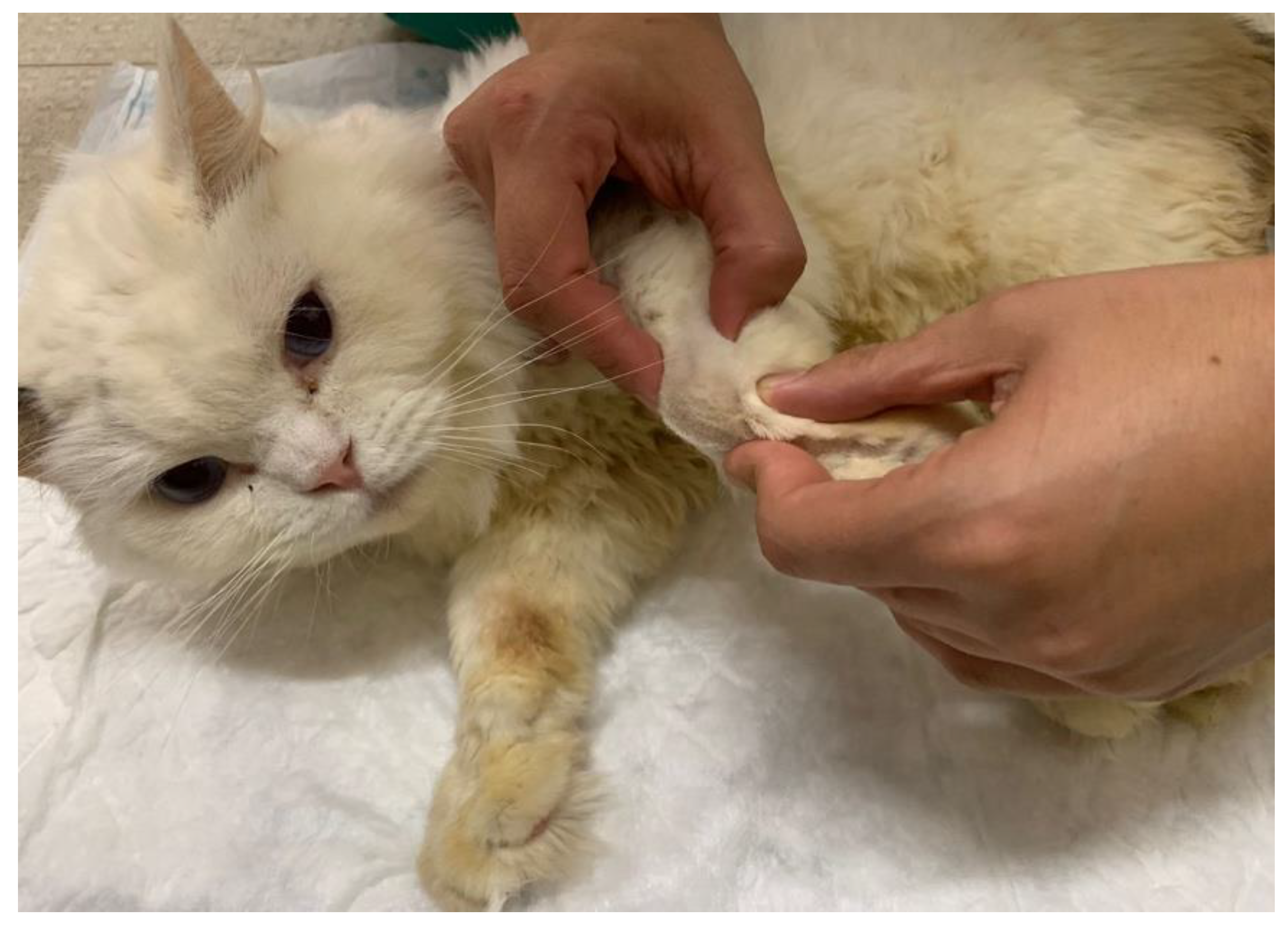
Figure 5.
Functional electrical stimulation of the radial nerve (one electrode placed in C6, C7, C8, T1, T2 and the other in the motor point of the triceps brachialis muscle).Figure 6. Corrective carpal splint. A) Necessary material for the splint. B) Splint applied on the carpus of a cat.
Figure 5.
Functional electrical stimulation of the radial nerve (one electrode placed in C6, C7, C8, T1, T2 and the other in the motor point of the triceps brachialis muscle).Figure 6. Corrective carpal splint. A) Necessary material for the splint. B) Splint applied on the carpus of a cat.

Figure 6.
Corrective carpal splint. A) Necessary material for the splint. B) Splint applied on the carpus
of a cat.
Figure 6.
Corrective carpal splint. A) Necessary material for the splint. B) Splint applied on the carpus
of a cat.

Figure 7.
Functional electrical stimulation of the hindlimb. A) Sciatic nerve stimulation (one electrode placed on L7-S1 and the other on the motor point of the flexor muscle group); B) Peroneal nerve branch stimulation (one electrode L7-S1 and the other on the dorsal region of the paw).
Figure 7.
Functional electrical stimulation of the hindlimb. A) Sciatic nerve stimulation (one electrode placed on L7-S1 and the other on the motor point of the flexor muscle group); B) Peroneal nerve branch stimulation (one electrode L7-S1 and the other on the dorsal region of the paw).

Figure 8.
Functional electrical stimulation of the radial nerve for carpal extension (one electrode at C7, C8, T1, T2 and the other one on the dorsal region of the paw).
Figure 8.
Functional electrical stimulation of the radial nerve for carpal extension (one electrode at C7, C8, T1, T2 and the other one on the dorsal region of the paw).
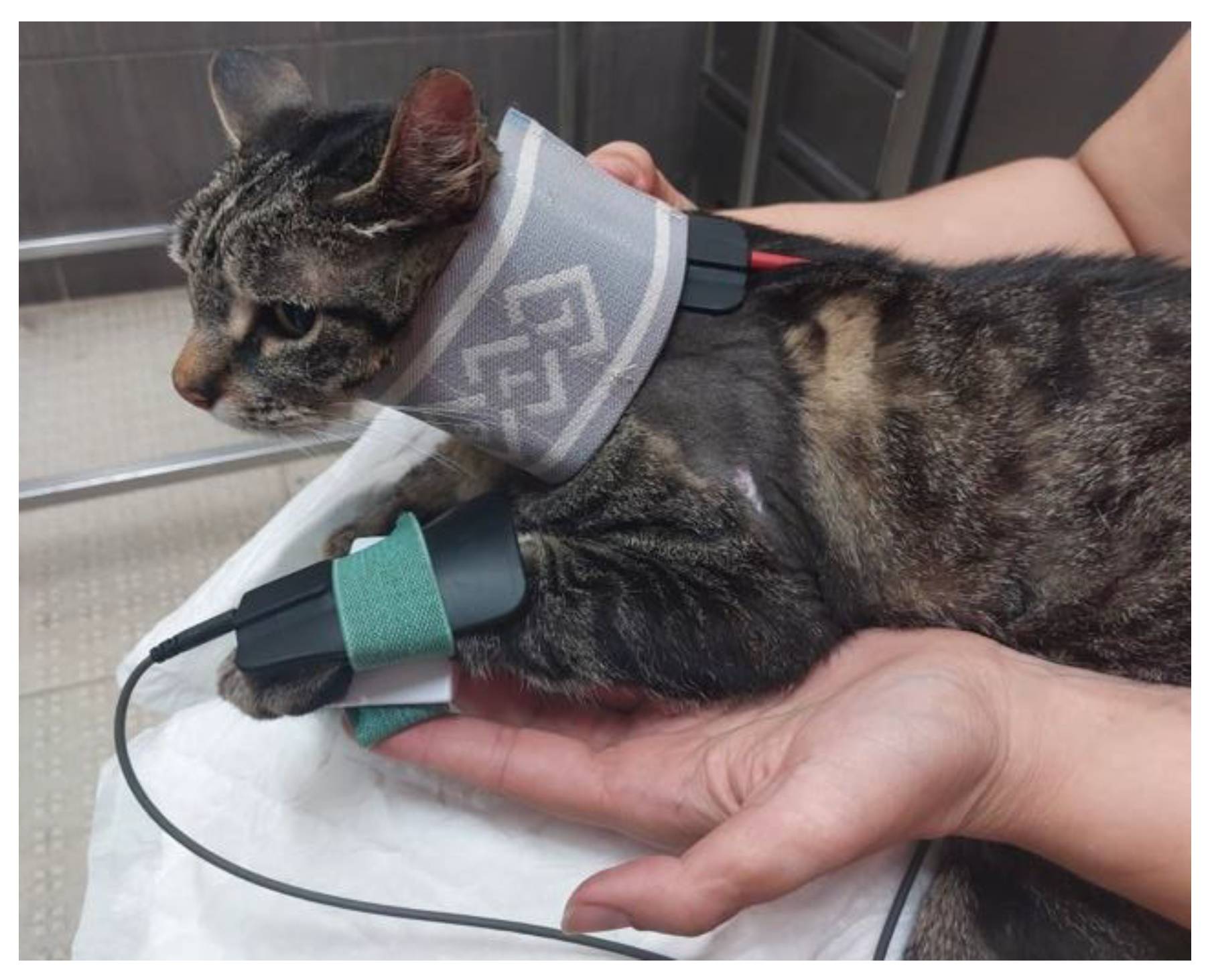
Figure 9.
Locomotor training in a land treadmill with bicycle movements performed on the affected limb.
Figure 9.
Locomotor training in a land treadmill with bicycle movements performed on the affected limb.

Figure 10.
Locomotor training in the underwater treadmill with bicycle movements performed on the affected limb.
Figure 10.
Locomotor training in the underwater treadmill with bicycle movements performed on the affected limb.

Figure 11.
Radiographic projections of a surgical arthrodesis performed on a cat. A) Anteroposterior projection. B) Mediolateral oblique projection. C) Mediolateral projection.
Figure 11.
Radiographic projections of a surgical arthrodesis performed on a cat. A) Anteroposterior projection. B) Mediolateral oblique projection. C) Mediolateral projection.

Figure 12.
Outcomes evaluation, regarding muscle mass and muscle weakness, in the follow ups consultations. F1: 3 months; F2: 6 months; F3:1 year; F4: 2 years; F5: 3 years; F6: 4 years.
Figure 12.
Outcomes evaluation, regarding muscle mass and muscle weakness, in the follow ups consultations. F1: 3 months; F2: 6 months; F3:1 year; F4: 2 years; F5: 3 years; F6: 4 years.
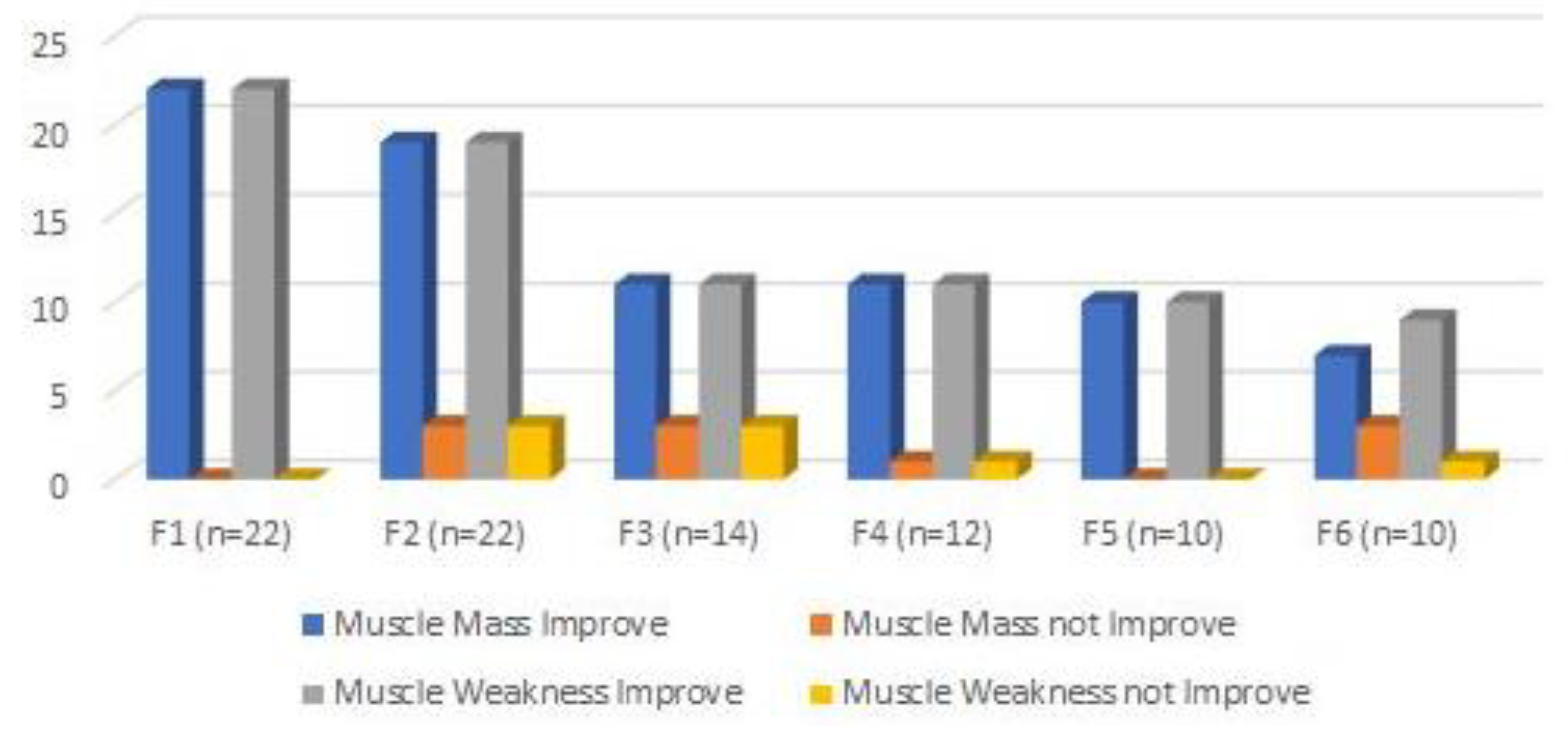
Table 1.
Inclusion criteria for the study population.
| Clinical signs |
|
|
|
|
|
|
|
|
| ||
| Diagnosis |
|
|
Table 2.
Muscles innervated by the brachial plexus nerves.
| Nerve | Origin | Innervated muscles |
|---|---|---|
| Radial | C6, C7, C8, T1, T2 | Extensor carpi ulnaris; Triceps brachialis; Extensor carpiradialis; Lateral and common digital extensor |
| Ulnar | C8, T1, T2 | Deep digital flexor; Flexor carpi ulnaris |
| Median | C7, C8, T1 | Superficial digital flexor; flexor carpi radialis |
| Lateral thoracic | C8, T1 | Cutaneous trunci |
| Nerve | ||
|---|---|---|
| Radial |
|
|
| Ulnar |
|
|
| Median |
|
|
Table 4.
Intensive neurorehabilitation protocol in the 1st, 2nd and 3rd week.
| Rehabilitation modality/exercise | Parameters | Implementation |
|---|---|---|
| Laser Therapy (A) |
18-22 J Class IV Laser Radial nerve pathway; SID |
Regenerative Role |
| 5-10 J Class IV Laser 4-point joint technique; SID (Shoulder, Elbow, Carpus) |
Analgesia, Anti-inflammatory effects | |
| FES of the radial nerve (B) |
30 – 40 Hz; 6-16 mA Trapezoidal modulation 1:4 duty cycle 2-4 s ramp up; 8 s plateau; 1-2 s ramp down; 10 min; TID |
In Deep pain positive |
| 30 – 40 Hz; 6-24 mA Trapezoidal modulation 1:4 or 1:5 duty cycle 2-4 s ramp up; 8 s plateau; 1-2 s ramp down; 10 min; TID |
In Deep pain negative | |
| Range of motion exercises (C) |
10-30 sets 4-6 times/day; |
All joints: shoulder, elbow, carpal and digits |
| Postural standing position (D) |
2-3 min 4-6 times/day |
|
| Ultrasound (E) |
1 MHz; 1.5 w/cm2; 10 min Pulsed mode; Duty cycle of 20%; Pulse ratio 1:4; 5 cm transduced head; |
Muscles: Triceps brachialis; Biceps brachialis; Extensor carpi radialis; Lateral and Common digital extensor |
| FES: Functional electrical stimulation; J: Joule; Hz: Hertz; mA: Miliampere; s: seconds; MHz: Megahertz. | ||
Table 5.
Descriptive analysis of age and weight in the study population.
| Total (n=22) | ||
| Age | Mean | 4,86 |
| Median | 5 | |
| Mode | 2 | |
| Variance | 4,790 | |
| SD | 2,189 | |
| Minimum | 2 | |
| Maximum | 8 | |
| SEM | 0,467 | |
| Shapiro-Wilk Normality Test | 0,021 | |
| Weight | Mean | 4,73 |
| Median | 5 | |
| Mode | 4 | |
| Variance | 1,255 | |
| SD | 1,120 | |
| Minimum | 3 | |
| Maximum | 7 | |
| SEM | 0,239 | |
| Shapiro-Wilk Normality Test | 0,071 | |
SD: Standard Deviation; SEM: Standard Error of Mean
Table 6.
Sample characterization of study population (n=22) at time of admission.
|
Total (n=22) |
||
| Age | < 7 years old: 72.7% (16/22) ≥ 7 years old: 27.3% (6/22) |
|
| Weight | < 5 kg: 45.5% (10/22) ≥ 5 kg: 54.5% (12/22) |
|
| Sex | Male: 68.2% (15/22) Female: 31.8% (7/22) |
|
| Breed | Mixed breed: 86.4% (19/22) Persian: 13.6% (3/22) |
|
| Knuckling | Forelimb | Absent: 100% (22/22) |
| Hindlimb | Absent: 90.9% (20/22) Present: 9.1% (2/22) |
|
| DPP | 1-4th Digits | Absent:45.5% (10/22) Doubtful: 54.5% (12/22) |
| 5th Digit | Present:40.9% (9/22) Doubtful: 59.1% (13/22) |
|
| Reflexes | Withdrawl reflex | Absent: 100% (22/22) |
| Extensor carpi radialis reflex | Absent: 100% (22/22) | |
| Cutaneous Trunci reflex | Absent: 27.3% (6/22) Present: 72.7% (16/22) |
|
| Horner Syndrome | Absent: 86.4% (19/22) Present: 13.6% (3/22) |
|
| Dermatomes | Until elbow | Absent: 13.6% (3/22) Present: 86.4% (19/22) |
| Between elbow and carpus | Absent: 100% (22/22) | |
| Between carpus and digits | Absent: 100% (22/22) | |
| Joint motion | Shoulder | Absent: 13.6% (3/22) Present: 86.4% (19/22) |
| Elbow | Absent: 100% (22/22) | |
| Carpus | Absent: 100% (22/22) | |
| Muscle Atrophy | Triceps brachialis | Absent: 86.4% (19/22) Present: 13.6% (3/22) |
| Extensor carpi radialis | Absent: 100% (22/22) | |
| Carpal contracture | Absent: 100% (22/22) | |
| Standing position | Absent: 100% (22/22) | |
DPP: Deep Pain Perception; ROM: Range of Motion.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Comparison of the Short-term Surgical Outcomes in Patients with Closed Traction Injuries to Brachial Plexus
Vladimir Ulyanov
et al.
,
2022
Determining the algorithm of rehabilitation procedures in patients with brachial plexus injuries based on the prospective clinical neurophysiology studies
Kinga Lewczuk
et al.
,
2024
Restoration of Over-Ground Walking via Non-invasive Neuromodulation Therapy
Monzurul Alam
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







