Preprint
Review
A Journey To Reach Ovary Using Next-Generation Technologies
Altmetrics
Downloads
145
Views
42
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
04 October 2023
Posted:
06 October 2023
You are already at the latest version
Alerts
Abstract
Although effective in term of chance of future live birth, the current methods for fertility preservation such as oocytes, embryo or ovarian tissue cryopreservation cannot be offered to all cancer patients and in all clinical contexts. Expanding options for fertility preservation is crucial to address the need to encompass all situations. One emerging strategy is pharmacoprotection, which is non-invasive and has the potential to fill existing gaps in fertility preservation. Besides the identification of the most efficient therapeutic agent itself, the potential off-target effect re-mains one of the main limitation of this strategy for clinical application, particularly when healthy ovarian tissue is targeted. This review focuses on the advances in pharmacoprotective approaches and the challenge of targeting the ovaries to deliver these agents. The unique properties of gold nanoparticles (AuNPs) make them an attractive candidate for this purpose. We discuss how AuNPs meet many of the requirements for an ideal drug delivery system, as well as the existing limitations that have hindering the progression of AuNPs research into more clinical trials. Additionally, the review highlights microRNA (miRNA) therapy as a next-generation approach to address the issues of fertility preservation and discusses the obstacles that currently impede its clinical availability.
Keywords:
Subject: Medicine and Pharmacology - Reproductive Medicine
1. Introduction
Fertility preservation (FP) for women cancer patients should be considered before initiating chemotherapies, radiotherapies or other anti-cancer therapies. Unfortunately, several chemotherapeutic agents and radiation exposure have side effects, including potential long-term impact on the fertility, by inducing premature ovarian insufficiency (POI). It is crucial to improve the quality of life after recovery from the cancer, given that the five-year cancer survival increased in recent decades [1], thanks to the constant advances in cancer treatments and personalized theragnostic medicine. Cryopreservation of oocytes [2], embryos [3], or ovarian tissue [4] are considered as the standard methods for FP. Although these techniques have been proven efficiency for women undergoing the procedure, they are invasive and have limitations such as the risk of reinsertion of cancer cells after transplantation of cryopreserved ovarian tissue, which remains the only option for pre-pubertal cancer patients [5].
Interest has arisen in alternative methods for FP to overcome the limitations of current techniques, including ovarian tissue engineering [6], in vitro ovarian tissue reconstitution through stem cells differentiation [7] and pharmacoprotective agents. The main advantage of this last approach is its non-invasive aspect, leading to the study of numerous molecules for their protective effects on fertility. These molecules can be categorized into two classes: those aimed to protect from DNA damage [8], which induced apoptosis in ovarian follicles, and those aimed to protect from the burn-out effect [9], characterized by excessive activation of primordial follicles. Both phenomena lead to POI by reducing ovarian reserve. Anti-apoptotic molecules included Imatinib, GNF-2, sphingosine-1-phosphate (S1P). Imatinib and GNF-2 are ABL kinase inhibitors and interfere with the process of DNA damage, ultimately leading to TAp63 activation and finally apoptosis [10,11]. Studies have demonstrated the protection of the ovarian reserve against cisplatin and cyclophosphamide-induced apoptosis in mouse models for imatinib and GNF-2 respectively. S1P is an inhibitor of ceramide-induced death pathways and shows prevention of ovarian follicles apoptosis after in vitro exposure of cyclophosphamide and doxorubicin in mice and human models [12].
The second main mechanism of follicular depletion after chemotherapy exposure is called the “burn-out” effect. It is defined by a massive activation of the quiescent follicles due to the direct damage on growing follicles, leading to a dramatic reduction of a key regulator of the follicular activation, the Anti-Mullerian Hormone (AMH) and activation of PI3K-AKT-mTOR pathway inducing granulosa cells (GCs) proliferation [13,14]. Example of molecules studied to prevent the burn-out effect include AS101, Rapamycin and Ghrelin, that all act on the PI3K-AKT-mTOR pathway, known to be involved in follicles activation. Those agents are inhibitors of PI3K, mTOR and FOXO3a respectively [15,16,17].
The most advanced research among protective molecules focuses on gonadotropin-releasing hormone agonists (GnRHa). While the above-mentioned molecules are in pre-clinical studies, GnRHa is currently used in clinical practice, although its efficiency remains controversial. A main proposed mechanism of action is that co-treatment of GnRHa with chemotherapeutic agent would maintain low level of follicle-stimulating hormones (FSH) to keep follicles at quiescent stage, as in pre-pubertal hormonal environment. However, this hypothesis remains not fully understood, knowing that follicular activation is gonadotropins independent [18]. Additionally, studies yield opposing results depending the type of cancer treated. While breast cancer patients seem to benefit from co-treatment with a better recovery of ovarian function [19], this advantage is not observed in lymphoma patients [20].
Among innovative ovarian protective approach, microRNA (miRNA) therapies represent another emerging strategy for FP and could be classified as pharmacoprotective agents with a particular state due to their mechanism of action on gene expression [21]. The goal is to inhibit or restore the expression of specific mRNAs that have been identified as either overexpressed or downregulated and play a role in the chemotherapy-induced ovarian damage. However, this growing interest and research for protective molecules raise to the question of how to deliver these chemical agents. Finding the right delivery system to target the ovary could open a new frontier in reproductive medicine and FP treatment. The ideal delivery system should meet the following requirements: (1) Safety and stability: not inducing toxicity and immunogenicity to allow circulation in the blood without degradation by nucleases or detection by the immune system; (2) Specificity: capable of reaching the targeted tissue by controlling renal clearance while having the size to cross the capillary endothelium; (3) Cellular uptake: able to enter cell targets, penetrate cell membrane, and reach the correct intracellular site of action; (4) Release: delivering the molecule, escaping the endolysosomal system, and avoiding exocytosis before the initiation of the action of the molecule carried; (5) Efficiency: carrying the necessary amount of payload that allow the molecule to initiate its action and function; (6) Deliverability: feasible manufacture with scalable and affordable possibilities.
Current delivery systems studied are typically classified into two categories: viral and non-viral carriers. Viral vectors, including lentiviral, adenoviral and adeno-associated viral vectors are already employed in clinical applications, mainly for virus vaccine, due to their inherent high delivery efficiency [22]. Unfortunately, pre-existing immunity toward these viruses can exist in human, and achieving organ specificity apart from their initial tissue affinity can be challenging. The non-viral category includes system based on lipidic, inorganic materials (such as silver, porous silica, gold), polymeric molecules (including chitosan, PEI, PAMAM), or extracellular vesicles (exosomes) (Figure 1). Among these non-viral vectors, the most advanced one for nucleic acid delivery, in terms of administration to a broad range of individuals, is the lipid nanoparticle (LNP), as demonstrated in mRNA COVID-19 vaccine technologies developed and commercialized by Moderna Therapeutics and Pfizer/BioNtech [23]. Although this recent outbreak opens the road for RNA therapeutics, achieving specificity through active targeting remains a challenge. Among the non-viral vectors mentioned, gold nanoparticles (AuNPs) stand out as one of the most tunable systems, a required propriety for the functionalization of the carrier to overcome various biological obstacles, in addition to their minimal toxicity and cost-effective synthesis.
This review will explore how AuNPs can meet these expectations and why they are a strong candidate for drug delivery of protective molecules, particularly miRNA therapy. It will highlight the advantages, current applications, as well as the limitations and gaps in this field. Furthermore, it will focus on the potential role miRNA therapy to be part of the next-generation medicine.
2. Gold Nanoparticles as next-generation delivery systems
AuNPs are interesting for their properties of biocompatibility, thanks to their inert nature, and for their high ability to be tunable in size, shape, and charge. Their main characteristic lies in their capacity to be multi-functionalized by binding molecules that provide the desired function (Figure 1). In fact, their surface chemistry is relatively easy to modify due to the quantity of functional groups that have an affinity for gold, such as carboxyl, thiols and amines [24]. Based on this property, any molecules that bind those functional groups can theoretically be loaded onto the surface of the AuNPs. The assembly of all different bioconjugations on the same AuNP is possible due to their high surface-volume ratio. Thus, in order to add stability to the system and hide it from the immune system, different polymers can attach to AuNPs, such as polyethylene glycol (PEG), which is extensively used for this purpose, including in the SARS-CoV vaccine on the surface of LNPs. PEG coating has the property to increase the half-life time of AuNPs in blood by providing a steric barrier to reduce their interaction with the plasma proteins, known as opsonization, and thus avoiding their recognition and uptake by the reticuloendothelial system (RES), leading to phagocytic clearance [25].
Then, as delivery system, AuNPs can carry a large variety of cargos, including proteins, drugs and nucleic acids, either individually or in co-delivery. Concerning peptides delivery, some AuNPs constructions are in clinical trials, such as CYT-6091 (phase I started in 2006) and C19-A3 AuNPs (Phase I in 2016). CYT-6091 is a AuNPs functionalized with PEGs that carry recombinant human tumor necrosis factor alpha (rhTNFa). This cytokine is known for its anti-tumor effects, and treatment with it prior to chemotherapy reduces tumor growth to a greater extent. In the phase I of the clinical study, patients selected had various types of cancer, such as adenocarcinoma of the colon, pancreas, lung, rectum, as well as ocular melanoma and ductal carcinoma of the breast. The AuNPs were well tolerated by the patients at the dose necessary to target the tumor through systemic administration [26]. Following these positive results, phase I was completed in 2009 and will lead to the phase II, where a combination of CYT-6091 and chemotherapy is planned to enhance the anti-tumor effect. This make CYT-6091 the first AuNPs therapy to enter clinical study and the most advanced one. C19-A3 AuNPs carry the human proinsulin peptide to improve antigen-specific immunotherapy for the treatment of type I diabetes. Phase I is still ongoing, but first results showed good tolerability after intradermal administration [27].
The delivery of drugs is extensively study for chemotherapeutic agents including doxorubicin [28], cyclophosphamide [29] and cisplatin [30]. Studies of these classic agents delivered by AuNPs are all in the pre-clinical stage, while a combination of phytochemicals including mangiferin and curcuma, conjugated to AuNPs, has reached a pilot clinical investigation step to treat breast cancer. After demonstrating that this AuNPs treatment, called Nano Swarna Bhasma (NSB) drug, reduces the tumor size in SCID female mice bearing human breast cancer, treatment administrated to six patients show no side effects [31]. It the area of RNA therapeutics, where the treatment consists in the use of RNA for gene expression modulation, the delivery of nucleic acids is the highly exploited.
The first RNA interference (iRNA) therapy using AuNPs tested in human is known as NU-0129. This system consists of a gold core carrying small interference RNA (siRNA) coated with a protective layer of oligoethylene glycol. SiRNAs are short RNA sequences, ranging from a size of 20 to 25 nucleotides, synthetized to perfectly match a fragment of the messenger RNA (mRNA) sequence targeted to downregulate the expression of a specific gene at the transcript level. The siRNA is delivered as a duplex with a passenger strand and a guide strand, which are taken into the RNA-Induced Silencing Complex (RISC) to degrade the targeted mRNA and silence the gene. In the case of NU-0129, the siRNA interferes with the expression of the oncogene Bcl2Like12 (Bcl2L12) to treat patients with glioblastoma. NU-0129 was administrated intravenously to eight patients and successfully crossed the blood brain barrier to reach the tumor. A correlation was observed between the accumulation of NU-0129 and the downregulation of Bcl2L12, along with the upregulation of its target genes, caspase-3 (cleaved caspase 3) and p53, at the protein level. The treatment was globally well tolerated, although two severe adverse events, rated as grade 3 out of 5, were observed. These adverse events were considered to be a consequence of the oncologic treatment. Phase 0, completed in 2020, is expected to be followed by a larger cohort study, with a particular focus on examining the consequences of a long-term accumulation of gold. Notably, more than 40% of the total gold content remained detectable up to 174 days after the trial enrollment, following a single dose administration [32]. Table 1 summarizes these AuNPs used as drug delivery that reached in clinical trials.
Finally, AuNPs functionalized with target molecules is essential for achieving specific organ delivery, especially when active targeting is required, in opposition with passive targeting. Passive targeting refers to the natural route taken by a delivery system when administrated in the body. This includes organs such as liver, spleen and kidney.
An enhanced permeability and retention (EPR) effect develops following the high neovascularization of tumor and the large gaps formed between endothelial cells that composed capillary blood vessels. This effect is also exploited for passive drug delivery to tumors (Figure 2). However, it has shown to be not always enough selective to avoid severe potential off-target toxicity when systemically administered, as it has led to death in a miRNA therapeutics phase 1 trial based on PH-dependent delivery strategy [33]. Local injection can also be applied to target tissues when they are accessible such as the skin. Meanwhile, active targeting is also necessary to reach organ that are not easily accessible, such as the ovary, or to improve the specificity for a specific type of cell within a tumor. To make it possible, appropriate molecule can be complexed to the AuNPs, such as peptides, antibodies, aptamers, or vitamins (Figure 2) [34]. Such precise targeting allows the decrease of side effect toxicity while enhancing therapeutic efficiency. In 2008, Patra et al. were the first to design an antibody-conjugated AuNPs to target the overexpression of epidermal growth factor receptor (EGFR) in pancreatic adenocarcinoma, using cetuximab to deliver the anticancer drug gemcitabine [35]. Since then, Patra team has applied their AuNPs-cetuximab to target ovarian cancer, which also overexpressed EGFR, and loaded it with p53 plasmid DNA to restore protein expression and reduce tumor progression in vivo [36]. The team also synthetized AuNPs functionalized with a bio-inspired fusion protein carrier to target human epidermal growth factor receptor -2 (HER2) for ovarian cancer and simultaneously co-deliver doxorubicin and siRNA against erbB2. In the three studies, they demonstrated a higher accumulation of gold in the tumor site of xenograft mice compared to the liver, kidney, or spleen, along with the reduction of the tumor volume compared with AuNPs not complexed with the antibody. They also showed better delivery with AuNPs compared to antibodies attached to the cargo without AuNPs [37].
Once the AuNPs reach their target cells, various mechanisms of internalization can occur, including clathrin-, caveolae-dependent or independent endocytosis and macropinocytosis. The choice of mechanism depends on the functionalization and proprieties of the AuNP system [38]. In cases where AuNPs are trapped within the endolysosomal system and are unable to access to their site of action, strategies for escaping this degradation system have been developed. Once the AuNPs reach their target cells, various mechanisms of internalization can occur, including clathrin-, caveolae-dependent or independent endocytosis and macropinocytosis.
Once the AuNPs reach their target cells, various mechanisms of internalization can occur, including clathrin-, caveolae-dependent or independent endocytosis and macropinocytosis. The choice of mechanism depends on the functionalization and proprieties of the AuNP system [38]. In cases where AuNPs are trapped within the endolysosomal system and are unable to access to their site of action, strategies for escaping this degradation system have been developed. The most widely employed is known as the proton sponge effect. This involves the use of a cationic molecule, such as polyethylenimine, to attract protons inside the lysosome compartment. This mediates the change of pH, creating an acidic environment that triggers the disruption of the lysosomal membrane, allowing the release of the AuNPs in the cytoplasm [39]. Another interesting study highlighting the potential for co-delivery in gene therapy, along with an escaping strategy, involved the technology called CRISPR-Cas9 for “clustered regularly interspaced short palindromic repeats and its associated protein 9”. CRISPR-Cas9 is a revolutionary gene editing therapy that utilized a prokaryote-derived immune system to correct mutations responsible for genetic diseases. This therapy employs Cas9 ribonucleoprotein, the enzyme responsible for cutting the targeted gene, along with an RNA guide, similar to siRNA therapy, and a donor DNA to replace the mutated gene with the corrected sequence. Currently, the main delivery system for the CRISP-Cas9 system is AAV viral vector. However, this vector has limited gene-packaging capacity, necessitating the use of multiple virus for efficient gene modulation. This is where AuNPs are interesting. In this study, they designed AuNPs with a dense multilayer of attached DNA to link the gold core with the DNA donor where Cas9 are adsorbed with the RNA guide. Finally, the entire system was coated with the polymer poly(N-(N-(2-aminoethyl)-2-aminoethyl) aspartamide) (PAsp(DET)) to disrupt the endosomal membrane. The study demonstrated an absence of cellular uptake in HEK293 cells when the polymer was not present, compared to the 80% of internalization when the polymer was complexed to the AuNPs. In vivo results indicated gene editing induction without causing an increase of inflammatory plasma cytokines or a reducing in body weight [40].
The final obstacle in cargo delivery could be failure to release the load, and its impossibility to join the site of action. To address this challenge, research is ongoing to develop linker molecule that are cleavable by internal or external stimuli, such as changes in pH or exposure to light. For instance, photolabile bond, such as o-Nitrobenzyl, was utilized in a study where a near-infrared (NIR) radiation applied to the AuNPs allowed the cleavage and the release of 5-fluorouracil [41]. Further research is required to assess the efficiency of this concept for AuNPs in vitro. However, it is important to note that, for most of the studies detailed in this review and in the literature, this problem is not systematically encountered.
Additionally, other AuNPs-based technologies in clinical trials exploited the unique optic, electronic and physicochemical properties of gold for diagnostic, treatment, imaging and sensing. These distinctive optical proprieties are attributed to their surface plasmon resonance (SPR), a physical phenomenon in which light excites the electrons of gold causing them to resonate, absorb and reflect light. This SPR enables the detection and sensing of AuNPs and their utilization in photothermal therapy (PTT). In this therapy, AuNPs have to accumulate in the tumor, which is then irradiated with near infrared (NIR) radiation. AuNPs absorb the radiation and convert it into heat inside the tumor, leading to cell death. This therapy, also called photothermal ablation, involves several AuNPs in clinical trials, including AuroShell, a silica-gold NPs coated with PEG, for the treatment of various solid tumor. Nanospectra Biosciences, Inc. initiated phase I in 2015 and described the safety of AuroShell, with two adverse events – an allergic reaction and epigastric pain [42]. These safety results allow the study the application to 16 prostate cancer patients, revealing that 62.5% of the patients were free of cancer after 3 months and 87.5% after 12 months [43]. Following these encouraging results, two other pilot studies were conducted with head and neck cancer patients (NCT00848042) and lung cancer patients (NCT01679470). A second gold nanoshell was developed to treat atherosclerotic plaques, reporting significant regression of coronary atherosclerosis with acceptable level of safety [44]. Another therapy using AuNPs and its SPR properties is photodynamic therapy (PDT). As the name suggests, this therapy relies on light to trigger a reaction of AuNPs. In this case, a photosensitizer agent, such as porphyrin, is added. When excited by light, a transfer of energy will produce reactive oxygen species, inducing apoptosis of cancer cells only in the presence of oxygen in the tissue. Many projects working on this therapy are still in pre-clinical stage, as well as the combination of PTT and PDT [45]. Their electro-optical properties can also be exploited for imaging. Their high density enables AuNPs to serve as contrast agent and to absorb X-ray radiation used for photoimaging. The sensing application utilize both physical properties to be detected and to be functionalized [46]. Lastly, a gold nanocrystal in suspension, called CNM-Au8, has entered phase II of clinical trials for the treatment of amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder. This drinkable drug suspended in bicarbonate solution, use the newly discovered catalytic property of gold to reduce disease-associated oxidative stress [47].
3. MicroRNA therapies as innovative ovarian protection approach
MiRNAs are short non-coding RNA (ncRNA), typically consisting of 20-25 nucleotides, similar in size to previously described siRNAs. Both types of ncRNAs modulate gene expression at the post-transcriptional level. However, there is a key distinction between them: siRNAs are artificially designed sequences that perfectly match specific mRNAs, whereas miRNAs are endogenous and have the ability to target multiple mRNA gene expressions [48]. This versatility arises from the imperfect interaction between miRNAs and mRNA, typically involving complementary base pairing between the seed sequence of miRNAs (the first 2 to 7 nucleotides in the 5' region) and the 3'-UTR sequence of the target mRNA. The biogenesis of miRNAs starts with the transcription of a primary-miRNA (pri-miRNA) from the genome by RNA polymerase II. Pri-miRNA is a double-strand RNA with hairpin loop, capped at 5’ end, and with a polyadenosine (polyA) tail at the 3’ end. The nuclear proteins Drosha and DGCR8 remove the cap and polyA tail, forming the precursor-miRNA (pre-miRNA). The pre-miRNA is then exported to the cytoplasm by crossing the nuclear membrane protein Exportin-5. In the cytoplasm, the RNase III enzyme Dicer cleaves the hairpin structure, generating a miRNAs duplex. The Argonaute protein unwinds this duplex, and the mature single-strand miRNA can now incorporate the RNA-induced silencing complex (RISC) complex. This complex is responsible for inhibiting of mRNA translation or inducing its degradation (Figure 3A) [49].
The discovery of the first miRNA, lin-4, dates back to 1993 in the nematode Caenorhabditis elegans [50]. Since then, thousands of miRNAs have been identified across species, showing high level of conservation in both miRNA sequences and their target mRNA interaction [51]. An estimation based on a bioinformatic study suggest that the human genome contains around 2300 miRNAs, with 1115 of them identified in miRBase, a miRNA database [52]. In total, human miRNAs are capable of modulating the expression of up to 60% of protein-coding genes, resulting in a complex and powerful regulatory network. MiRNAs regulate genes involved in various cell process, including cell division, proliferation, apoptosis, and metabolism. It is not surprising that dysregulation of a crucial miRNAs can lead to pathologies such as neurological disorders, cardiovascular diseases, diabetes, fibrotic diseases, and, most notably, cancer. Their significant roles in both normal and pathological physiology make miRNAs interesting candidates for diagnosis as markers and therapeutic applications. In the context of cancer, miRNAs can be classified into two categories: tumor-suppressor miRNAs and oncogenic miRNAs. Tumor-suppressors miRNAs have their expression reduced while oncogenes are overexpressed. In cancer therapy, the use of miRNAs can involve the replacement of tumor-suppressor miRNAs using miRNA mimics or the inhibition of oncogenic miRNAs using anti-miRNAs (Figure 3B).
The growing interest in miRNA-based therapy is reflected in the number of clinical studies and their progress over the past decade (Table 2) [53,54,55,56]. In the first trials, modifications to the molecular structure enables direct injection of the drug without the need for a delivery system. This modification in the ribose of the nucleic acid is called Locked Nucleic Acid (LNA), enhances RNA stability and protects the molecule from degradation. The most advanced miRNA therapy study using a delivery system is TargomiRs. This treatment for patients with recurrent malignant pleural mesothelioma entered phase I in 2014 [57]. The delivery system used to encapsulate miR-16 mimics is a nanocell, a non-living vesicle derived from the asymmetric division of bacteria, called EnGenIC Dream Vector by the biotech company. MiR-16 targets genes such as BCL2 and JUN, involved in cancer progression. Additionally, the nanocells are coated with EGFR-specific antibodies on their surface to target the tumor overexpressing EGFR on mesothelioma cells. In pre-clinical studies, tumor growth inhibition was observed in mice when the nanocells delivering miR-16 were injected into the tail [58]. Phase I trial reported an acceptable safety profile, and further studies are planned to combinate TargomiRs with chemotherapy. However, issues related cardiac toxicity and other adverse events suggesting an immune reaction need to be addressed first. Another miRNA-based replacement therapy, which was the first to employ delivery system and to enter clinical trial, also induced immune reaction but was classified as severe adverse events and had to stop at the Phase I after four death patients were reported. In this study, miR-34a mimic was delivered using a LPN (MRX34) to treat patients with advanced solid tumors [59]. To date, no miRNA therapy has reached the market, although siRNA therapies have received approval from the US Food and Drug Administration and/or the European Medicines Agency. Patisiran, an siRNA targeting transthyretin and delivered by LNPs, is among these approved therapies. It is used to treat hereditary transthyretin-mediated amyloidosis. However, the clinical utility of Patisiran is limited due to the requirement for steroids and antihistamines before siRNA treatment to prevent immune reactions [60]. These clinical studies and therapies highlight the importance of targeting specific tissues and understanding the targeted mRNAs of a particular miRNA. Improving the specificity of delivery systems remains an ongoing challenge as already discussed. Research is still necessary to achieve the greatest specificity but the interest for this challenge is growing. Concerning the target mRNAs and the impact on molecular pathways, advancements in bioinformatics, which continuously develop new miRNA target prediction tools, should help to resolve this obstacle [61].
Cancer therapy can significantly impact the expression profiles of miRNAs, as shown by studies reporting significant changes in miRNA expression in cancer cells during and after chemotherapy exposure [62,63]. For instance, a study on breast cancer patients revealed upregulation of tumor suppressor miRNAs in patients who received neoadjuvant bevacizumab in combination with chemotherapeutic agents. Additionally, downregulation of oncogenic miRNAs was observed in patients treated with the neoadjuvant only. Importantly, these results were detected in responding patients. Among the downregulated oncogenic miRNAs, miR-4465 showed the strongest correlation with a reduction in tumor cells proliferation. Based on correlated and predicted target genes, miR-4465 was identified as regulator of genes associated to cell cycle regulation and DNA damage response (DDR) [64]. Conversely, miRNAs can affect chemotherapy by either promoting chemoresistance [65] or enhancing chemosensitivity [66]. MiRNAs can play a role in chemoresistance by regulating genes involved in DDR, drug efflux pump that maintain high drug concentrations inside the cells, and tumor survival mechanisms [67]. On the other hand, miRNAs can enhance chemosensitivity as reported in a study where let-7a downregulated genes involved in metabolic reprogramming crucial for cell cycle progression. The study also demonstrated that let-7a can induce the production of reactive oxygen species (ROS) in breast cancer cells [68]. Let-7a is known as tumor suppressor, as some of its target genes include oncogenes such as MYC, RAS and HMGA2 [69]. Based on this knowledge, a study attempted to overcome chemoresistance by co-delivering let-7a and doxorubicin using NPs composed of magnetic core and mesoporous silica. They reported higher tumor growth inhibition in mice bearing breast cancer cells and injected with both NPs co-delivering let-7a and doxorubicin compared to those treated with NPs co-delivering let-7a or doxorubicin alone [70]. Additionally, circulating miRNAs, which are detectable in biopsies and blood, have emerged as diagnostic biomarkers for cancer detection and evaluation of the response to cancer treatment [71]. There are few diagnostic tools based on miRNAs detection available on the market [72].
In the context of fertility preservation, miRNAs are gaining attention due to their endogenous nature and their ability to target multiple signaling pathways, in contrast with the other pharmacoprotective agents aforementioned. Since cancer treatments can induce damage to the ovaries at different biological levels, this ability of miRNAs to target multiple factors is a unique and advantageous feature. Furthermore, miRNAs are known to play a role in ovarian function, including follicular development, oocytes maturation, and steroidogenesis, in normal physiology but also in metabolic and gynecological diseases, as extensively detailed in this review [21]. However, it is only recently that miRNA-based therapy has been considered as potential future fertility preservation method. In a study of 2016, miR10a transfected using liposomes showed reduce apoptotic effects on mouse granulosa cells exposed to nitrogen mustard in vitro. Additionally, the study demonstrated that miR10-a prevented follicles from undergoing atresia after mice were injected with busulfan, a well-known gonadotoxic alkylating agent. The mechanism of action appeared to involve BIM, a regulator of apoptosis, targeted by miR-10a [73]. However, another study also focused on miR10a to prevent gonadotoxicity induced by 4-Hydroperoxycyclophosphamide (4-HC), the active metabolite of another alkylating agent (cyclophosphamide), did not show a rescue of damage using liposomes to deliver the miRNAs in post-natal day 3 (PND3) ovary in vitro [74]. However, the same research team analyzed the expression profile of 384 characterized miRNAs in ovaries before and after 4HC exposure in vitro and identified let-7a as promising candidate for preventing apoptosis of primordial follicles induced by chemotherapy. They reported reduced apoptotic effect on PND3 ovaries when transfected with mimic-let-7a delivered by liposomes [75]. Moreover, they evaluated the oocyte competence using in vitro treated PND3 ovaries transplanted under the kidney capsule of adult mice to observe further in vivo follicular development. An improvement of the follicular survival and oocytes quality was observed in the PND3 ovaries transfected with mimic-let-7a and exposed to 4-HC in vitro compared to the group exposed to chemotherapy alone prior transplantation [76]. Let-7a targets genes involved in cell cycle and apoptosis as already mentioned. Other studies evaluated miRNAs protective effect against gonadotoxicity induced by chemotherapy in rats by targeting PTEN using either miR-21 [77,78], miR-144-5p [79], or unidentified miRNAs [80], all through mesenchymal stem cell-derived exosomes. However, PTEN is a negative regulator of the PI3K/Akt pathway involved in follicular activation. Inhibiting PTEN could lead to the depletion of ovarian reserve by “over-activating” quiescent follicles through the activation of PI3K/Akt pathway. Another study focused on miR-144 but its 3p strand showed only partial protection of primordial follicles damaged by cisplatin exposure in adult mouse ovary in vivo. The mechanism of action was suggested to involve the targeting of MAP3K9 by miR-144-3p, which regulates apoptosis via the p38 MAPK pathway [81].
4. Targeting the ovaries
The challenge of actively targeting specific tissues lies in the identification of the most appropriate markers, which should not only be specific to the tissue of interest but also be present on the surface of the most accessible cell type, and more precisely here, under normal physiological conditions. This specificity is more challenging than targeting tumor cells, which tend to overexpress proteins, including membrane proteins, due to their particular profiles that distinguish them from healthy cells. Moreover, tumors are highly vascularized, which induced the EPR effect previously mentioned, making it easier to target the tissue. Consequently, much of the research on targeting the ovaries has focused on ovarian cancer cells. This focus is also driven by the broader applications of ovarian cancer research, given the significant interest in nanomedicine for cancer and the accessibility of ovarian cancer cell lines, thanks to their natural immortality features. Hence, ovarian cancer cells can overexpress surface markers such as human epidermal growth factor receptor-2 (HER2) and folate receptors. These overexpressed receptors can be exploited for targeted drug delivery to enhance the efficiency of chemotherapy. For instance, one study utilized trastuzumab, an antibody targeting HER-2, to deliver cisplatin loaded on PLGA NPs. The results showed higher cytotoxicity in SKOV-3, a human ovarian cancer cell line, compared to free cisplatin, and better internalization in cells with HER2 receptors compared to HER2-negative cell lines [82]. Another application involved targeting folate receptors using nanocomplex system to deliver siRNA in SKOV3 cells [77]. GnRH, also known as luteinizing hormone-releasing hormone (LHRH), has its receptors overexpressed in ovarian cancer. In a study that employed AuNPs functionalized with LHRH peptide, selective uptake of the AuNPs was observed in vitro. They also demonstrated, in vivo, a preferential uptake of the AuNPs by organs in the abdominal cavity, primarily the ovaries, compared to other organs such as liver, kidney, spleen and pancreas [83]. Cancer stem cells (CSCs), which have the capacity of self-renewal, contribute to the heterogeneity of cancer cell population. These cells, as stem cells, also express specific surface markers such as CD44 and CD133.These markers have been employed as targeting proteins for the delivery of drug such as paclitaxel, in mouse model [84]. Additionally, they have been used as proof-of-concept markers [85].
To effectively target the healthy ovary, it is crucial to understand the cell population within the tissue and identify the cell types that are most accessible through blood vessels. The ovaries consist of the cortex, where follicle development occurs, and the medulla, composed mainly of fibroelastic connective tissue and blood vessels. As the objective is to protect the female gamete from cancer therapy-induced damage, we will focus on follicles-specific markers although the entire ovarian tissue should be protected from apoptosis. Ovarian follicles are the functional unit that contain the oocyte surrounded by protective layer(s) of granulosa cells (GCs) and theca cells, both somatic cells [86]. The Human Protein Atlas, a database of human proteomes, provides open access to tissue-specific proteomes maps, among other proteogenomic analysis [87,88]. The database reports 178 elevated genes and five enriched genes in the ovary, meaning that their expression is at least four-fold higher in the ovary compared to the tissue with the second highest mRNA expression level. Among these genes, only one encodes for a membrane protein, zona pellucida glycoprotein 4 (ZP4). This protein is specifically expressed by the oocyte and composed the zona pellucida, the extracellular matrix between oocytes and GCs. Recent research, driven by advancements in bioinformatic tools and technologies like single-cell sequencing, has focused on transcriptomics of human ovarian follicles. In a review by Zhang et al., human oocyte-specific markers such as ZP1-2-3-4, DDX4, SYCP3, SOX30, ZAR1, DAZL, YBX2, H1FOO and LHX8 were identified, along with and GCs-specific markers that included CYP11A1, STAR, INHBA and AMH [89]. In another review by Chen et al., which studied novel regulators of follicle activation in mice, known oocytes-specific genes BMP15, DAZL, DDX4, DPPA3, FIGLA, GDF9, LHX8, NOBOX, NPM2, OOG1, POU5F1, SOHLH1, SOX30, SUB1, SYCP3, TAF7L, YBX2, ZAR1, ZP2 and GCs-specific genes AARD, ALDH1A2, AMH, AMHR2, CYP11A, CYP19A1, FOXL2, FSHR, FST, GATM, GNG13, HMGCS2, INHA, INHBA, KITL, KRT8, KRT19, RSPO1, STAR, UPK3B, WNT6 were used to score single-cell gene expression [90]. While many of the genes mentioned in these reviews are involved in molecular pathways, they may not be the best candidates as markers for targeting the ovary. Notably, gonadotrophins receptors, FSHR and LHCGR are known as expressed on ovarian follicles and listed as mostly expressed in ovary and testis on NCBI protein-coding gene database. Another review on human transcriptome focus on oocytes, reported TGFBR1-2 and BMPR2 as expressed at the membrane of oocytes [91]. While spatial proteomic studies are necessary to determine how to exploit this list of cell-specific genes, the known specific receptors are promising (Table 3). For instance, the conjugation of the LHRH peptide to AuNPs has shown potential in cancer research and could be extended to other applications if similar results are observed in healthy mice.
5. Limitations and perspectives
In this review, we have addressed the question of how nanotechnology can offer new perspective for future fertility preservation strategy and expand restoration options. With the breakthrough of nanomedicine and innovative therapeutic approaches for cancer treatments, including miRNAs-based therapy, research into next-generation delivery systems is gaining momentum. We have illustrated how AuNPs can meet the expectations as a promising vector for transporting biomolecules and targeting specific tissues by examining the most advanced studies utilizing AuNPs in diagnostics, treatments, and other applications. As previously mentioned, AuNPs are remarkable in terms of biocompatibility, stability, tunability and functionality, and scalability due to their ease to synthetize. However, it is important to recognize that the size, shape and charge of AuNPs, resulting from the chemical surface composition, play a crucial role in determining their toxicity and pharmacodynamics. These properties can vary significantly between studies, making it challenging to summarize the best functionalization strategy, and have to be confirmed at each modification.
Furthermore, because of their great capacity to be modified (size, shape, charge) and functionalized (miRNAs, ligand, drugs), the lack of standardization in AuNPs has resulted in studies yielding conflicting results on the same research topic. For instance, size-dependent cytotoxicity may change based on biological parameters, such as the type of cell line studied [92], or the physiological moment when AuNPs are administrated. In fact, a study revealed that estrous cycle affect doxorubicin efficiency deliver by LNPs in the ovary with a higher accumulation during mouse ovulation. They also observed a size-depend accumulation for AuNPs in the ovary [93]. The variability of the results can also be due to the various techniques used to detect cytotoxicity or uptake [94]. Therefore, the development of more standardized methods of synthesis and analysis could improve the reliability of results concerning the properties and behavior of AuNPs.
Another critical limitation of the application of AuNPs is their accumulation in non-target organs. The natural biodistribution of AuNPs in certain organs upon administration is inevitable but can be diminished using target ligand as already discussed. Moreover, their efficient clearance from the target site once they have achieved their intended action is equally important. AuNPs are known be non-biodegradable, and they can persist in the body for extended periods, as reported during the clinical study of NU-0129 [32]. This issue becomes particularly relevant when the target organ is healthy, as opposed to cancerous tissue. However, a study has reported an unexpected intracellular biodegradation of AuNPs in primary human fibroblasts. They studied the biotransformation of AuNPs over a period of 6 months in vitro and observed a degradation of AuNPs by the lysosomes through oxidation of gold induced by ROS after two weeks of exposure to 4 nm AuNPs. This was followed by recrystallization through metallothionein, according to their transcriptomic study, forming structures resembling aurosomes [95]. Aurosomes are structures formed after the administration of gold salts, a treatment used historically to address conditions like rheumatoid arthritis before the development of more efficient solution. If AuNPs degradation are recrystallized into aurosomes in some particular cases, it means that different forms of gold could share the same metabolism and the one utilized by gold salts has already been more extensively studied [96]. For instance, some indicated an elimination of gold through urines several months after gold administration [97]. It should be noted that 7 nm and 12 nm AuNPs, also included in the study, showed longer degradation time, closer to several months rather than the two weeks for 4 nm AuNPs [95]. While these findings are promising, more extensive research is necessary to determine the specific conditions under which AuNPs degradation and recrystallization occur. This could also determine the best route of administration for AuNPs in clinical application.
MiRNAs therapy also has its limitations. As for AuNPs, off-target can be limited with the help of target ligand conjugated to the delivery system. However, once miRNAs reach their intracellular site of action, their regulation of multiple target genes, which is viewed as benefit compared to other molecules, could also lead to unanticipated biological responses. While bioinformatic tools have made significant strides in predicting miRNA target genes, these predictions remain speculative and must be validated through experimental studies. Additionally, the complex cellular environment, reactive to external and internal signals, cannot always be accurately simulated in in vitro models, which are not fully representative of physiological reality. Moreover, genetic diversity among human populations could explain the difficulty of translating preclinical findings to clinical application, not reach yet, almost systematically due to immune response in patients One key challenge in miRNA therapy is finding the right balance between toxicity and efficacy when determining the appropriate dose for administration. The ideal dose should reflect the physiological concentration of miRNAs lost in the disease state. In addition to the efficiency, this consideration is important in order to not saturate the RISC complex and induce lack in another miRNA action. Despite these limitations, it is crucial to remember that miRNAs were only discovered 30 years ago. Their potential for therapeutic use has been explored more recently, with the first miRNA therapy ending clinical phase I trials in 2012, just a decade ago. This rapid progress is noteworthy, especially considering that miRNA therapy research is still in its early stages of development in terms of historical timeframes.
Author Contributions
I.D. and T.T.A.N. conceptualized the design of the review. T.T.A.N. executed the data collection and the writing of the review. I.D. reviewed and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the P.D.R.-F.N.R.S.: T.00.70.20. I.D. is a senior research associate at the F.N.R.S.
Conflicts of Interest
T.T.A.N has no conflict of interest to report. ID reports research grants from Roche, speaker honoraria from Ferring, Novartis and travel grant from Theramex, Ferring.
Additional note
All figures were created with BioRender.com with the agreement number MU25VPRPQK for Figure 1, WN25VPRLH5 for Figure 2, and DE25VPRBWM for Figure 3.
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. The Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Pregnancy After Human Oocyte Cryopreservation. The Lancet 1986, 327, 884–886. [Google Scholar] [CrossRef]
- Brown, J.R.; Modell, E.; Obasaju, M.; Ying, Y.K. Natural Cycle In-Vitro Fertilization with Embryo Cryopreservation Prior to Chemotherapy for Carcinoma of the Breast. Human Reproduction 1996, 11, 197–199. [Google Scholar] [CrossRef]
- Demeestere, I.; Simon, P.; Emiliani, S.; Delbaere, A.; Englert, Y. Orthotopic and Heterotopic Ovarian Tissue Transplantation. Hum Reprod Update 2009, 15, 649–665. [Google Scholar] [CrossRef]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live Birth after Autograft of Ovarian Tissue Cryopreserved during Childhood. Human Reproduction 2015, 30, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A Review on Biomaterials for Ovarian Tissue Engineering. Acta Biomater 2021, 135, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Akahori, T.; Woods, D.C.; Tilly, J.L. Female Fertility Preservation through Stem Cell-Based Ovarian Tissue Reconstitution In Vitro and Ovarian Regeneration In Vivo. Clin Med Insights Reprod Health 2019, 13, 117955811984800. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How Do Chemotherapeutic Agents Damage the Ovary? Hum Reprod Update 2012, 18, 525–535. [Google Scholar] [CrossRef]
- Roness, H.; Gavish, Z.; Cohen, Y.; Meirow, D. Ovarian Follicle Burnout: A Universal Phenomenon? Cell Cycle 2013, 12, 3245–3246. [Google Scholar] [CrossRef]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the C-Abl-TAp63 Pathway Protects Mouse Oocytes from Chemotherapy-Induced Death. Nat Med 2009, 15, 1179–1185. [Google Scholar] [CrossRef]
- Bellusci, G.; Mattiello, L.; Iannizzotto, V.; Ciccone, S.; Maiani, E.; Villani, V.; Diederich, M.; Gonfloni, S. Kinase-Independent Inhibition of Cyclophosphamide-Induced Pathways Protects the Ovarian Reserve and Prolongs Fertility. Cell Death Dis 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-Phosphate Prevents Chemotherapy-Induced Human Primordial Follicle Death. Human Reproduction 2014, 29, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Hamish, W.; Wallace, B. Toxicity of Chemotherapy and Radiation on Female Reproduction.
- Grosbois, J.; Devos, M.; Demeestere, I. Implications of Nonphysiological Ovarian Primordial Follicle Activation for Fertility Preservation. Endocr Rev 2020, 41. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide Triggers Follicle Activation and "burnout "; AS101 Prevents Follicle Loss and Preserves Fertility. Sci Transl Med 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kimura, F.; Zheng, L.; Kaku, S.; Takebayashi, A.; Kasahara, K.; Tsuji, S.; Murakami, T. Protective Effect of a Mechanistic Target of Rapamycin Inhibitor on an in Vivo Model of Cisplatin-Induced Ovarian Gonadotoxicity. Exp Anim 2018, 67, 493–500. [Google Scholar] [CrossRef]
- Hoon Jang, Younghwa Na, Kwonho Hong, Sangho Lee, Sohyeon Moon, Minha Cho, Miseon Park, Ok-Hee Lee, Eun Mi Chang, Dong Ryul Lee, Jung Jae Ko, Woo Sik Lee, Y.C. Synergistic Effect of Melatonin and Ghrelin in Preventing Cisplatin-Induced Ovarian Damage via Regulation of FOXO3a Phosphorylation and Binding to the P27Kip1 Promoter in Primordial Follicles. J Pineal Res 2017, 63, e12432. [CrossRef]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian Protection with Gonadotropin-Releasing Hormone Agonists during Chemotherapy in Cancer Patients: From Biological Evidence to Clinical Application. Cancer Treat Rev 2019, 72, 65–77. [Google Scholar] [CrossRef]
- Lambertini, M.; Boni, L.; Michelotti, A.; Gamucci, T.; Scotto, T.; Gori, S.; Giordano, M.; Garrone, O.; Levaggi, A.; Poggio, F.; et al. Ovarian Suppression with Triptorelin during Adjuvant Breast Cancer Chemotherapy and Long-Term Ovarian Function, Pregnancies, and Disease-Free Survival a Randomized Clinical Trial. JAMA - Journal of the American Medical Association 2015, 314, 2632–2640. [Google Scholar] [CrossRef]
- Demeestere, I.; Brice, P.; Peccatori, F.A.; Kentos, A.; Dupuis, J.; Zachee, P.; Casasnovas, O.; Van Den Neste, E.; Dechene, J.; De Maertelaer, V.; et al. No Evidence for the Benefit of Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian Function and Fertility in Lymphoma Survivors Treated with Chemotherapy: Final Long-Term Report of a Prospective Randomized Trial. Journal of Clinical Oncology 2016, 34, 2568–2574. [Google Scholar] [CrossRef]
- Alexandri, C.; Daniel, A.; Bruylants, G.; Demeestere, I. The Role of MicroRNAs in Ovarian Function and the Transition toward Novel Therapeutic Strategies in Fertility Preservation: From Bench to Future Clinical Application. Hum Reprod Update 2020, 26, 174–196. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angewandte Chemie - International Edition 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Stealth Nanoparticles: High Density but Sheddable PEG Is a Key for Tumor Targeting. Journal of Controlled Release 2010, 145, 178–181. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-RhTNF Nanomedicine. Clinical Cancer Research 2010, 16, 6139–6149. [Google Scholar] [CrossRef]
- Tatovic, D.; Mcateer, M.A.; Barry, J.; Barrientos, A.; Rodríguez Terradillos, K.; Perera, I.; Kochba, E.; Levin, Y.; Dul, M.; Coulman, S.A.; et al. Safety of the Use of Gold Nanoparticles Conjugated with Proinsulin Peptide and Administered by Hollow Microneedles as an Immunotherapy in Type 1 Diabetes. Immunotherapy Advances 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Lee, C.; Kim, T.W.; Oh, D.E.; Bae, S.O.; Ryu, J.; Kong, H.; Jeon, H.; Seo, H.K.; Jeon, S.; Kim, T.H. In Vivo and In Vitro Anticancer Activity of Doxorubicin-Loaded DNA-AuNP Nanocarrier for the Ovarian Cancer Treatment 2020, 12. [CrossRef]
- Banu, H.; Stanley, B.; Faheem, S.M.; Seenivasan, R.; Premkumar, K.; Vasanthakumar, G. Thermal Chemosensitization of Breast Cancer Cells to Cyclophosphamide Treatment Using Folate Receptor Targeted Gold Nanoparticles. Plasmonics 2014, 9, 1341–1349. [Google Scholar] [CrossRef]
- Xiong, X.; Arvizo R., R. R.; Saha, S.; Robertson J., D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of Ovarian Cancer Cells to Cisplatin by Gold Nanoparticles. Oncotarget 2014, 5, 6453–6465. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Mohandoss, D.K.D.; Darshakumar, R.D.; Joshi, C.M.; Katti, K. V. New Approaches in Breast Cancer Therapy through Green Nanotechnology and Nano-Ayurvedic Medicine – Pre-Clinical and Pilot Human Clinical Investigations. Int J Nanomedicine 2020, 15, 181–197. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A First-in-Human Phase 0 Clinical Study of RNA Interference-Based Spherical Nucleic Acids in Patients with Recurrent Glioblastoma. Sci Transl Med 2021, 13. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br J Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem Soc Rev 2020, 49, 8774–8789. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted Delivery of Gemcitabine to Pancreatic Adenocarcinoma Using Cetuximab as a Targeting Agent. Cancer Res 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Vydiam, K.; Jeyalakshmi Srinivasan, D.; Mukherjee, S.; Roy, A.; Kuncha, M.; Rao, T.N.; Sistla, R.; Gopal, V.; Patra, C.R. Restoration of P53 Function in Ovarian Cancer Mediated by Gold Nanoparticle-Based EGFR Targeted Gene Delivery System. ACS Biomater Sci Eng 2019, 5, 3631–3644. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Srinivasan, D.J.; Mukherjee, S.; Haroon, M.M.; Dar, G.H.; Venkatraman, U.; Patra, C.R.; Gopal, V. Engineered Fusion Protein-Loaded Gold Nanocarriers for Targeted Co-Delivery of Doxorubicin and ErbB2-SiRNA in Human Epidermal Growth Factor Receptor-2+ Ovarian Cancer. J Mater Chem B 2017, 5, 7082–7098. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem Soc Rev 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Molecular Therapy 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat Biomed Eng 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Fedoryshin, L.L.; Tavares, A.J.; Petryayeva, E.; Doughan, S.; Krull, U.J. Near-Infrared-Triggered Anticancer Drug Release from Upconverting Nanoparticles. ACS Appl Mater Interfaces 2014, 6, 13600–13606. [Google Scholar] [CrossRef]
- Stern, J.M.; Kibanov Solomonov, V. V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int J Toxicol 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc Natl Acad Sci U S A 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-Gold Nanoparticles for Atheroprotective Management of Plaques: Results of the NANOM-FIM Trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V. V. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials 2022, 15, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem Rev 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M.C.; Menon, P.; Huynh, W.; Rynders, A.; Ho, K.S.; Glanzman, R.; Hotchkin, M.T. Study Protocol of RESCUE-ALS: A Phase 2, Randomised, Double-Blind, Placebo-Controlled Study in Early Symptomatic Amyotrophic Lateral Sclerosis Patients to Assess Bioenergetic Catalysis with CNM-Au8 as a Mechanism to Slow Disease Progression. BMJ Open 2021, 11, e041479. [Google Scholar] [CrossRef]
- Ambros, V. The Functions of Animal MicroRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat Rev Mol Cell Biol 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Feinbaum, R.; Ambros, V.; Lee, R. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 116, 843–854. [Google Scholar]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.P.; et al. An Estimate of the Total Number of True Human MiRNAs. Nucleic Acids Res 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Lindow, M.; Kauppinen, S. Discovering the First Microrna-Targeted Drug. Journal of Cell Biology 2012, 199, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ree, M.H.; Van Der Meer, A.J.; Van Nuenen, A.C.; De Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen Dosing in Chronic Hepatitis C Patients Results in Decreased MicroRNA-122 Levels without Affecting Other MicroRNAs in Plasma. Aliment Pharmacol Ther 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Inc., R.T. Inc., R.T. Regulus Announces Pipeline Updates and Advancements Available online: https://www.prnewswire.com/news-releases/regulus-announces-pipeline-updates-and-advancements-300472142.html.
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. Journal of Investigative Dermatology 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.B.; Vallely, M.P.; et al. Restoring Expression of MiR-16: A Novel Approach to Therapy for Malignant Pleural Mesothelioma. Annals of Oncology 2013, 24, 3128–3135. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br J Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Kristen, A. V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi Therapeutic for the Treatment of Hereditary Transthyretin-Mediated Amyloidosis. Neurodegener Dis Manag 2019, 9, 5–23. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. MiRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc 2021, 4, 1–20. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Lang, M.; Wehbe, H.; Maheshwari, S.; Mendell, J.T.; Jiang, J.; Schmittgen, T.D.; Patel, T. Involvement of Human Micro-RNA in Growth and Response to Chemotherapy in Human Cholangiocarcinoma Cell Lines. Gastroenterology 2006, 130, 2113–2129. [Google Scholar] [CrossRef]
- Hummel, R.; Wang, T.; Watson, D.I.; Michael, M.Z.; Van Der Hoek, M.; Haier, J.; Hussey, D.J. Chemotherapy-Induced Modification of MicroRNA Expression in Esophageal Cancer. Oncology Reports 2011, 26, 1011–1017. [Google Scholar] [CrossRef]
- Lindholm, E.M.; Ragle Aure, M.; Haugen, M.H.; Kleivi Sahlberg, K.; Kristensen, V.N.; Nebdal, D.; Børresen-Dale, A.L.; Lingjærde, O.C.; Engebraaten, O. MiRNA Expression Changes during the Course of Neoadjuvant Bevacizumab and Chemotherapy Treatment in Breast Cancer. Molecular Oncology 2019, 13, 2278–2296. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and Drug Resistance. Cancer Gene Therapy 2010, 17, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.E.; Chung, J.H.; Verducci, J.S.; Lin, S.; Park, J.K.; Dai, Z.; Liu, C.G.; Schmittgen, T.D.; Reinhold, W.C.; Croce, C.M.; et al. MicroRNAs Modulate the Chemosensitivity of Tumor Cells. Molecular Cancer Therapeutics 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, H.; Liu, X.; Evans, B.R.; Medina, D.J.; Liu, C.G.; Yang, J.M. Role of MicroRNA MiR-27a and MiR-451 in the Regulation of MDR1/P-Glycoprotein Expression in Human Cancer Cells. Biochemical Pharmacology 2008, 76, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Serguienko, A.; Grad, I.; Wennerstrøm, A.B.; Meza-Zepeda, L.A.; Thiede, B.; Stratford, E.W.; Myklebost, O.; Munthe, E. Metabolic Reprogramming of Metastatic Breast Cancer and Melanoma by Let-7a MicroRNA. Oncotarget 2015, 6, 2451–2465. [Google Scholar] [CrossRef]
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. Let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells. Cell 2007, 131, 1109–1123. [Google Scholar] [CrossRef]
- Yin, P.T.; Pongkulapa, T.; Cho, H.Y.; Han, J.; Pasquale, N.J.; Rabie, H.; Kim, J.H.; Choi, J.W.; Lee, K.B. Overcoming Chemoresistance in Cancer via Combined MicroRNA Therapeutics with Anticancer Drugs Using Multifunctional Magnetic Core-Shell Nanoparticles. ACS Applied Materials and Interfaces 2018, 10, 26954–26963. [Google Scholar] [CrossRef]
- De Guire, V.; Robitaille, R.; Tétreault, N.; Guérin, R.; Ménard, C.; Bambace, N.; Sapieha, P. Circulating MiRNAs as Sensitive and Specific Biomarkers for the Diagnosis and Monitoring of Human Diseases: Promises and Challenges. Clinical Biochemistry 2013, 46, 846–860. [Google Scholar] [CrossRef]
- Meiri, E.; Mueller, W.C.; Rosenwald, S.; Zepeniuk, M.; Klinke, E.; Edmonston, T.B.; Werner, M.; Lass, U.; Barshack, I.; Feinmesser, M.; et al. A Second-Generation MicroRNA-Based Assay for Diagnosing Tumor Tissue Origin. The Oncologist 2012, 17, 801–812. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.K.; Liu, I.H.; Wu, S.C. Exosomal MiR-10a Derived from Amniotic Fluid Stem Cells Preserves Ovarian Follicles after Chemotherapy. Scientific Reports 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Alexandri, C.; Stratopoulou, C.A.; Demeestere, I. Answer to Controversy: MiR-10a Replacement Approaches Do Not Offer Protection against Chemotherapy-Induced Gonadotoxicity in Mouse Model. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, C.; Stamatopoulos, B.; Rothé, F.; Bareche, Y.; Devos, M.; Demeestere, I. MicroRNA Profiling and Identification of Let-7a as a Target to Prevent Chemotherapy-Induced Primordial Follicles Apoptosis in Mouse Ovaries. Scientific Reports 2019. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, C.; Van Den Steen, G.; Demeestere, I. Let-7a Mimic Transfection Reduces Chemotherapy-Induced Damage in a Mouse Ovarian Transplantation Model. Scientific Reports 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of MiR-21 in Stem Cells Improves Ovarian Structure and Function in Rats with Chemotherapy-Induced Ovarian Damage by Targeting PDCD4 and PTEN to Inhibit Granulosa Cell Apoptosis. Stem cell research & therapy 2017, 8, 187. [Google Scholar] [CrossRef]
- Thabet, E.; Yusuf, A.; Abdelmonsif, D.A.; Nabil, I.; Mourad, G.; Mehanna, R.A. Extracellular Vesicles MiRNA-21: A Potential Therapeutic Tool in Premature Ovarian Dysfunction. Molecular Human Reproduction 2020, 26, 906–919. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MiR-144-5p Improves Rat Ovarian Function after Chemotherapy-Induced Ovarian Failure by Targeting PTEN. Laboratory Investigation 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Cao, R.C.; Lv, Y.; Lu, G.; Liu, H. Bin; Wang, W.; Tan, C.; Su, X.W.; Xiong, Z.; Ma, J.L.; Chan, W.Y. Extracellular Vesicles from IPSC-MSCs Alleviate Chemotherapy-Induced Mouse Ovarian Damage via the ILK-PI3K/AKT Pathway. Zoological Research 2023, 44, 620–635. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, B.; Zhu, Y.; Chen, M.; Huang, J.; Guo, H.; Wang, F. MicroRNA-144-3p Protects against Chemotherapy-Induced Apoptosis of Ovarian Granulosa Cells and Activation of Primordial Follicles by Targeting MAP3K9. European journal of medical research 2023, 28, 264. [Google Scholar] [CrossRef]
- Domínguez-Ríos, R.; Sánchez-Ramírez, D.R.; Ruiz-Saray, K.; Oceguera-Basurto, P.E.; Almada, M.; Juárez, J.; Zepeda-Moreno, A.; del Toro-Arreola, A.; Topete, A.; Daneri-Navarro, A. Cisplatin-Loaded PLGA Nanoparticles for HER2 Targeted Ovarian Cancer Therapy. Colloids and Surfaces B: Biointerfaces 2019, 178, 199–207. [Google Scholar] [CrossRef]
- Kumar, D.; Moghiseh, M.; Chitcholtan, K.; Mutreja, I.; Lowe, C.; Kaushik, A.; Butler, A.; Sykes, P.; Anderson, N.; Raja, A. LHRH Conjugated Gold Nanoparticles Assisted Efficient Ovarian Cancer Targeting Evaluated via Spectral Photon-Counting CT Imaging: A Proof-of-Concept Research. Journal of Materials Chemistry B 2023, 11, 1916–1928. [Google Scholar] [CrossRef]
- Khayrani, A.C.; Mahmud, H.; Ko Oo, A.K.; Zahra, M.H.; Oze, M.; Du, J.; Alam, M.J.; Afify, S.M.; Abu Quora, H.A.; Shigehiro, T.; et al. Targeting Ovarian Cancer Cells Overexpressing Cd44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef]
- Skubitz, A.P.N.; Taras, E.P.; Boylan, K.L.M.; Waldron, N.N.; Oh, S.; Panoskaltsis-Mortari, A.; Vallera, D.A. Targeting CD133 in an in Vivo Ovarian Cancer Model Reduces Ovarian Cancer Progression. Gynecologic Oncology 2013, 130, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Oktay, K. The Ovary: Anatomy and Function throughout Human Life. Annals of the New York Academy of Sciences 2008, 1127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Database, S. The Human Protein Atlas Available online: https://www.proteinatlas.org/.
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Molecular Cell 2018, 72, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Russo, D.D.; Drake, R.S.; Duncan, F.E.; Shalek, A.K.; Goods, B.A.; Woodruff, T.K. Single-Cell Transcriptomics of Staged Oocytes and Somatic Cells Reveal Novel Regulators of Follicle Activation. Reproduction 2022, 164, 55–70. [Google Scholar] [CrossRef]
- Kocabas, A.M.; Crosby, J.; Ross, P.J.; Otu, H.H.; Beyhan, Z.; Can, H.; Tam, W.L.; Rosa, G.J.M.; Halgren, R.G.; Lim, B.; et al. The Transcriptome of Human Oocytes. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 14027–14032. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochemistry and Biophysics Reports 2021, 26, 100991. [Google Scholar] [CrossRef]
- Poley, M.; Mora-Raimundo, P.; Shammai, Y.; Kaduri, M.; Koren, L.; Adir, O.; Shklover, J.; Shainsky-Roitman, J.; Ramishetti, S.; Man, F.; et al. Nanoparticles Accumulate in the Female Reproductive System during Ovulation Affecting Cancer Treatment and Fertility. ACS Nano 2022, 16, 5246–5257. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles : What We Have Learned so Far ? Perspective 2010, 2313–2333. [Google Scholar] [CrossRef]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected Intracellular Biodegradation and Recrystallization of Gold Nanoparticles. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F.; Murphy, C.J. Gold-Based Therapy: From Past to Present. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 22639–22648. [Google Scholar] [CrossRef] [PubMed]
- Krusius, F.E.; Markkanen, A.; Peltola, P. Plasma Levels and Urinary Excretion of Gold during Routine Treatment of Rheumatoid Arthritis. Annals of the rheumatic diseases 1970, 29, 232–235. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Different types of delivery systems: virus vector, polymeric-based, lipid-based, inorganic-based and extracellular vesicle-based vectors. Gold nanoparticles functionalization with various ligands, molecules and cargos.
Figure 1.
Different types of delivery systems: virus vector, polymeric-based, lipid-based, inorganic-based and extracellular vesicle-based vectors. Gold nanoparticles functionalization with various ligands, molecules and cargos.

Figure 2.
Gold nanoparticles conjugated with target ligands (as specific), microRNAs and PEG. Two types of targeting phenomena: (1) Passive targeting of AuNPs exploiting EPR effect of tumor or natural route after administration; (2) Active targeting of AuNPs requires functionalization with target ligands such as antibody, peptide, vitamin (e.g. folic acid), and aptamer (DNA, RNA or peptide molecules that bind to a specific target molecule). Once the appropriate target ligand is selected, AuNPs could reach specific cells by utilizing receptor-ligand recognition. PEG: Polyethylen glycol, EPR: Enhanced permeability and retention.
Figure 2.
Gold nanoparticles conjugated with target ligands (as specific), microRNAs and PEG. Two types of targeting phenomena: (1) Passive targeting of AuNPs exploiting EPR effect of tumor or natural route after administration; (2) Active targeting of AuNPs requires functionalization with target ligands such as antibody, peptide, vitamin (e.g. folic acid), and aptamer (DNA, RNA or peptide molecules that bind to a specific target molecule). Once the appropriate target ligand is selected, AuNPs could reach specific cells by utilizing receptor-ligand recognition. PEG: Polyethylen glycol, EPR: Enhanced permeability and retention.

Figure 3.
A. MicroRNAs biogenesis started from the transcription of the pri-miRNAs in the nucleus. The pri-miRNAs are processed into pre-miRNAs, and into mature miRNAs. Finally, miRNAs can act at post-transcriptional level to inhibit target mRNAs. B. MicroRNA-based therapy: miRNA replacement to rescue downregulated miRNAs using mimic miRNAs and miRNA inhibition therapy to inhibit overexpressed miRNAs using anti-miR, miRNA mask or miR sponge. MiRNA: microRNA, DROSHA: Class 2 ibonuclease III enzyme, DGCR8: DiGeorge syndrome critical region 8, pri-miRNA: primary microRNA, pre-miRNA: precursor microRNA, DICER: Double-stranded RNA-specific endoribonuclease, RISC: RNA-induced silencing complex.
Figure 3.
A. MicroRNAs biogenesis started from the transcription of the pri-miRNAs in the nucleus. The pri-miRNAs are processed into pre-miRNAs, and into mature miRNAs. Finally, miRNAs can act at post-transcriptional level to inhibit target mRNAs. B. MicroRNA-based therapy: miRNA replacement to rescue downregulated miRNAs using mimic miRNAs and miRNA inhibition therapy to inhibit overexpressed miRNAs using anti-miR, miRNA mask or miR sponge. MiRNA: microRNA, DROSHA: Class 2 ibonuclease III enzyme, DGCR8: DiGeorge syndrome critical region 8, pri-miRNA: primary microRNA, pre-miRNA: precursor microRNA, DICER: Double-stranded RNA-specific endoribonuclease, RISC: RNA-induced silencing complex.
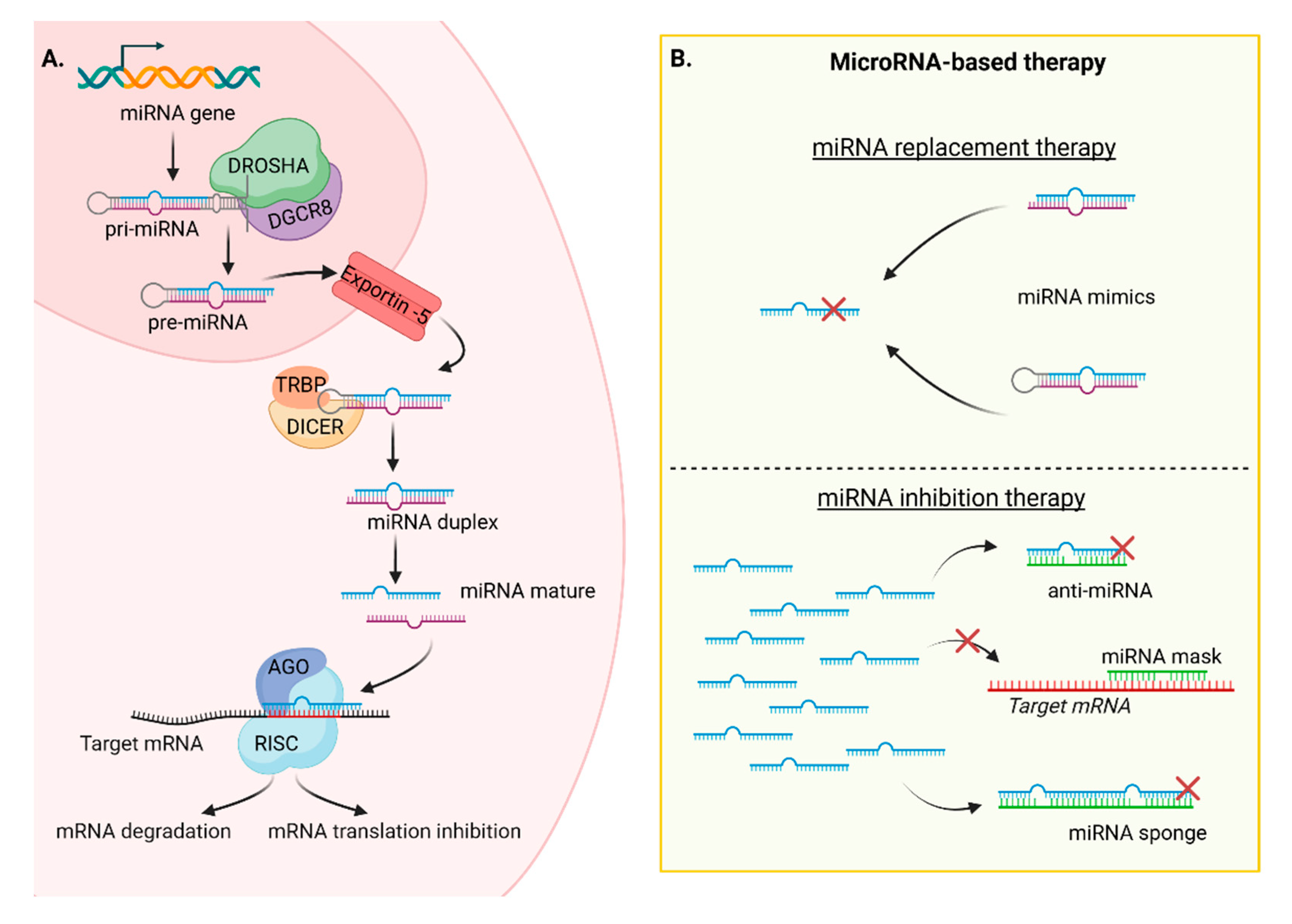
Table 1.
Gold nanoparticles as drug delivery system in clinical trials.
| Name | Molecule | AuNPs | Treatment | Clinical Phase |
|---|---|---|---|---|
| NU-0129 | RNAi for Bcl2L12 | 13 nm Thiolated PEG |
Gliobastoma | Phase 0 (NCT03020017) 2017-2020 |
| CYT-6091 | rhTNF | 27 nm PEGylated |
Various solid tumor |
Phase I (NCT00356980) 2006-2009 |
| C19-A3 | Proinsuline peptide | 5 nm | Type I diabetes | Phase I (NCT02837094) 2016- |
| Nano Swarna Bhasma |
Combination of phytochemicals | 35 nm | Breast cancer | Phase 0 DNA_SPN_B001_17 AYUSH |
| DengueTcP (EMX-001) |
Synthetic T cell-selective multivalent with dengue virus peptide antigens (vaccine) | 5 nm | Dengue fever | Phase I (NCT04935801) 2021- |
| Corona TcP | Betacoronavirus T cell-priming immune Vaccine |
5 nm | SARS-COV2 | Phase I (NCT05113862) 2022- |
Table 2.
Micro-based therapy in clinical trials.
| Name | Molecule | Delivery system | Treatment | Target | Clinical Phase |
|---|---|---|---|---|---|
| Miravirsen (RG101) |
Anti-miRNA-122 | LNA-antisense | Chronic hepatitis C |
Liver | Phase II (NCT01727934) 2012-2014 Unknown |
| MRX34 | miR-34 mimic | LNPs | Advanced solid tumors |
Tumor | Phase I (NCT02862145) 2016-2017 Withdrawn |
| MesomiR-1 | miR-16 mimic | EnGeneIC Dream Vectors | Malignant pleural mesothelioma |
Tumor expressing EGFR |
Phase I (NCT02369198) 2014-2017 Completed |
| Lademirsen (RG-012) |
Anti-miR-21 | Oligonucleotides modification |
Alport Syndrome | Kidney | Phase II Suspended |
| Cobomarsen (MRG-106) |
Anti-miR155 | LNA-antisense | Mycosis fungoides | Skin | Phase II (NCT03713320) 2019-2020 Terminated1 |
| TLV-associated adult T-cell lymphoma/leukemia, diffuse large B-cell lymphoma and chronic lymphocytic leukemia | Lymphatic system |
Phase I (NCT02580552) 2016-2020 Completed |
|||
| Remlarsen (MRG-201) |
miR-29 mimic | LNA-mimic | Fibrotic diseases | Phase II (NCT03601052) 2018-2020 Completed |
|
| Obefazimob (ABX464) |
miR-124 mimic | Capsule (oral administration) |
Active Rheumatoid Arthritis |
Immune system |
Phase II (NCT05177835) 2021- Recruiting |
| Ulcerative Colitis | Phase III (NCT05507203) 2022- Recruiting |
1 Terminated for business reasons.
Table 3.
Ovarian markers mostly based on human and mouse transcriptomic studies.
| Name | Abbreviation | Cell- Specificity supposed |
Model | Ovary RNA Expression1 |
Location |
|---|---|---|---|---|---|
| Alanine And Arginine Rich Domain Containing Protein |
AARD | GCs | Human | N/M | Intracellular |
| Aldehyde Dehydrogenase 1 Family Member A2 |
ALDH1A2 | GCs | Human | Endometrial stromal cells | Intracellular |
| Anti-Mullerian Hormone | AMH | GCs | Mouse and human | Granulosa cells | Secreted |
| Anti-Mullerian Hormone Receptor Type 2 |
AMHR2 | GCs | Human | Granulosa cells | Membrane, Intracellular |
| Bone Morphogenetic Protein 15 |
BMP15 | Oocytes | Human | N/M | Secreted |
| Bone morphogenetic protein receptor type 2 |
BMPR2 | Oocytes | Human | N/M | Membrane |
| Cytochrome P450 Family 11 Subfamily A Member 1 |
CYP11A1 | GCs | Mouse and human | N/M | Intracellular |
| Cytochrome P450 Family 19 Subfamily A Member 1 | CYP19A1 | GCs | Human | N/M | Membrane, Intracellular |
| Deleted In Azoospermia Like | DAZL | Oocytes | Mouse and human | Oocytes | Intracellular |
| DEAD-Box Helicase 4 | DDX4 | Oocytes | Mouse and human | Oocytes | Intracellular |
| Developmental Pluripotency Associated 3 |
DPPA3 | Oocytes | Human | Oocytes | Intracellular |
| Folliculogenesis Specific Bhlh Transcription Factor |
FIGLA | Oocytes | Human | Oocytes | Intracellular |
| Forkhead Box L2 | FOXL2 | GCs | Human | Granulosa cells, Ovarian stromal cells, Endometrial stromal cells | Intracellular |
| Follicle Stimulating Hormone Receptor |
FSHR | GCs | Human | Granulosa cells | Membrane, Intracellular |
| Follistatin | FST | GCs | Human | Granulosa cells | Secreted, Intracellular |
| Glycine Amidinotransferase | GATM | GCs | Human | Granulosa cells | Intracellular |
| Growth Differentiation Factor 9 | GDF9 | Oocytes | Human | Oocytes | Secreted, Intracellular |
| G Protein Subunit Gamma 13 | GNG13 | GCs | Human | N/M | Intracellular |
| H1.8 Linker Histone | H1FOO | OocytesGCs | HumanMouse | Oocytes | Intracellular |
| 3-Hydroxy-3-Methylglutaryl- Coa Synthase 2 |
HMGCS2 | GCs | Human | N/M | Intracellular |
| Inhibin Subunit Alpha | INHA | GCs | Mouse and human | Granulosa cells Ovarian stromal cells |
Secreted |
| Inhibin Subunit Beta A | INHBA | GCs | Mouse and human | N/M | Secreted |
| KIT Ligand | KITL | GCs | Human | N/M | Membrane, Intracellular |
| Keratin 8 | KRT8 | GCs | Human | N/M | Intracellular |
| Keratin 19 | KRT19 | GCs | Human | N/M | Intracellular |
| Luteinizing Hormone/ Choriogonadotropin Receptor |
LHCGR | GCs | Human | Ovarian stromal cells | Membrane, Intracellular |
| LIM Homeobox 8 | LHX8 | Oocytes | Mouse and human | Oocytes | Intracellular |
| NOBOX Oogenesis Homeobox | NOBOX | Oocytes | Human | Oocytes | Intracellular |
| Nucleophosmin/ Nucleoplasmin 2 |
NPM2 | Oocytes | Human | Oocytes | Intracellular |
| Oocyte-Specific Gene | OOG1 | Oocytes | Human | Oocytes | Intracellular |
| POU Class 5 Homeobox 1 | POU5F1 | Oocytes | Human | N/M | Intracellular |
| RNA Polymerase I Subunit A | RPO1 | GCs | Human | N/M | Intracellular |
| Spermatogenesis And Oogenesis Specific Basic Helix-Loop-Helix 1 |
SOHLH1 | Oocytes | Human | Oocytes | Intracellular |
| SRY-Box Transcription Factor 30 |
SOX30 | Oocytes | Mouse and human | N/M | Intracellular |
| Steroidogenic Acute Regulatory Protein |
STAR | GCs | Mouse and human | Ovarian stromal cells |
Intracellular |
| SUB1 Regulator Of Transcription |
SUB1 | Oocytes | Human | N/M | Intracellular |
| Synaptonemal Complex Protein 3 |
SYCP3 | Oocytes | Mouse and human | Oocytes | Intracellular |
| TATA-Box Binding Protein Associated Factor 7 Like |
TAF7L | Oocytes | Human | N/M | Intracellular |
| Transforming growth factor beta receptor 1 |
TGFBR1 | Oocytes | Human | N/M | |
| Uroplakin 3B | UPK3B | GCs | Human | N/M | Membrane, Intracellular |
| Y-Box Binding Protein 2 | YBX2 | Oocytes | Mouse and human | N/M | Intracellular |
| Zygote Arrest 1 | ZAR1 | Oocytes | Mouse and human | Oocytes | Intracellular |
| Zona Pellucida Glycoprotein 2 |
ZP-2 | Oocytes | Mouse and human | N/M | Secreted, Membrane |
1 The RNA specificity category is based on mRNA expression levels in the analyzed cell types based on scRNA-seq data from normal tissues on Human Protein Atlas. N/M: not mentionned.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
A Comparative Analysis of Naïve Exosomes and Enhanced Exosomes with a Focus on the Treatment Potential in Ovarian Disorders
Mohammad Mousaei Ghasroldasht
et al.
,
2024
Oocyte Vitrification for Fertility Preservation in Women with Benign Gynecologic Disease: National Clinical Practice Guidelines Developed by a Modified Delphi Consensus Process
Blandine Courbiere
et al.
,
2021
Intravenous Infusion of Nucleated Peripheral Blood Cells Restores Fertility in Mice with Chemotherapy-Induced Premature Ovarian Failure
Abdeljabar El Andaloussi
et al.
,
2018
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







