Preprint
Article
Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence
Altmetrics
Downloads
102
Views
40
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
08 October 2023
Posted:
10 October 2023
You are already at the latest version
Alerts
Abstract
The Surgical Complexity Score (SCS) has been widely used to describe the surgical effort during advanced stage epithelial ovarian cancer (EOC) cytoreduction. Referring to a variety of multi-visceral resections, it best combines the numbers with the complexity of the sub-procedures. Nevertheless, not all potential surgical procedures are described by this score. Lately, the European Society for Gynaecological Oncology (ESGO) has established standard outcome quality indicators pertinent to achieving complete cytoreduction (CC0). There is a need to define what weight all these surgical sub-procedures comprising CC0 would be given. Explainable Artificial Intelligence (XAI) could explain the impact of real-time features on the CC0 prediction. We analyzed prospectively collected data from 560 consecutive patients with FIGO-stage III-IV who underwent cytoreductive surgery between Jan 2014 and Dec 2019 at a UK tertiary referral centre. Following adaptation of the structured ESGO ovarian cancer report template, we employed the eXtreme Gradient Boosting (XGBoost) algorithm to model an exhaustive list of surgical sub-procedures. We applied the Shapley Additive explanations (SHAP) framework to provide global (cohort) explainability. We used Cox regression for survival analysis and constructed Kaplan-Meier curves. The XGBoost model predicted CC0 with an acceptable accuracy (area under curve [AUC] = 0.70; 95% confidence interval [CI] = 0.63–0.76). Visual quantification of the feature importance for the prediction of CC0 identified upper abdominal peritonectomy (UAP) as the most important feature, followed by regional lymphadenectomies. The UAP best correlated with bladder peritonectomy and diaphragmatic stripping (Pearson’s correlations > 0.5). Clear inflection points were shown by pelvic and para-aortic lymph node dissection and ileocecal resection/right hemicolectomy, which increased the probability for CC0. When UAP was solely added to a composite model comprising of engineered features, it substantially enhanced its predictive value (AUC = 0.80, CI = 0.75-0.84). The UAP was predictive of poorer progression-free survival (HR=1.76, CI 1.14- 2.70, P:0.01) but not overall survival (HR=1.06, CI 0.56-1.99, P:0.86). The SCS did not have significant survival impact. Machine Learning allows for operational feature selection by weighting the relative importance of those surgical sub-procedures that appear to be more predictive of CC0. Our study identifies UAP as the most important procedural predictor of CC0 in surgically cytoreduced advanced-stage EOC women. The classification model presented here can potentially be trained with a larger number of samples to generate a robust digital surgical reference in high output tertiary centres. The upper abdominal quadrants should be thoroughly inspected to ensure that CC0 is achievable.
Keywords:
Subject: Medicine and Pharmacology - Oncology and Oncogenics
1. Introduction
In the western world, epithelial ovarian cancer (EOC) is the fifth most common cause of women’s cancer-related death [1]. Most women are diagnosed at an advanced stage mainly due to the lack of sufficient diagnostic tools (stage III or IV). The current gold standard treatment is cytoreductive surgery combined with carboplatin and paclitaxel chemotherapy and subsequent maintenance therapy [2,3]. Such complex treatment algorithms often require extensive radical cytoreductive surgery including multi-visceral resections [4,5]. Complete cytoreduction (CC0) and chemotherapy response appear to be the most critical prognostic factors [6].
Achieving CC0 frequently requires targeted maximal effort. Previous attempts to describe the extent of cytoreductive surgery led to the development of the surgical complexity score (SCS), which best combined the numbers with the complexity of the procedures [6]. Nevertheless, not all potential surgical procedures are described by this score. Lately, the European Society for Gynecologic Oncology (ESGO) has established ten quality indicators (QIs), based on the standards of practice to audit and improve advanced EOC surgery [7]. Three of these QIs were outcome indicators related to achievement of CC0. In the complex environment of the operating room, CC0 is not always realized. Inconsistency among surgeons in the interpretation of the size of residual disease has been reported, prompting accurate documentation of operative findings and outcomes in the surgical notes [8]. The QI8, a process indicator was related to prospective recorded information from an exhaustive list of structured surgical procedures as “minimum required elements in operative reports” [9]. There is a need to define what weight all these surgical procedures comprising CC0 would be given. Therefore, most surgeons should regularly seek objective but personalised strategies to evaluate their cytoreductive outcomes.
In the era of precision oncology, Artificial Intelligence (AI) could potentially support clinicians in making meaningful predictions of the surgical outcomes for quality improvement and delivery of modern ovarian cancer care [10]. We previously employed such innovative solutions to predict outcomes of cytoreductive surgery in advanced EOC [11,12]. Herein, we developed an AI algorithm to support the weighted importance of all surgical procedures performed at EOC cytoreductive surgery for CC0 forecasting. Using eXplainable Artificial Intelligence (XAI), we examined and interpreted the most salient procedural interactions to explain the overall model predictive performance.
2. Materials and Methods
The study was a single-center retrospective cohort study including patients treated at our ESGO accredited center of excellence for advance ovarian cancer surgery between 2014-2019. All consecutive incoming women with newly diagnosed advanced stage EOC who underwent surgery during their primary therapy were included in the study. Exclusion criteria included women < 18 years at first diagnosis, women with relapsed EOC or receiving palliative surgery, women with non- epithelial tumours, and those presenting at first diagnosis with early stage EOC. The patient cohort, the MDT consensus and the hospital setting have been previously described in detail [12,13]. All operations were carried out via a midline laparotomy by a team of gynaecological and, when necessary, hepatobiliary, or colorectal surgeons with an attempt to achieve total macroscopic clearance. Early intra-operative assessment of tumour dissemination was routinely performed and retrospectively documented in the operative notes prior to textual data entry in the ovarian cancer database. Ethics board approval was obtained through the Leeds Teaching Hospitals Trust (MO20/133163/18.06.20). The study was added to the UMIN/CTR Trial Registry (UMIN000049480).
The operative report was a frank adaptation of the structured ESGO ovarian cancer operative report template that included an exhaustive list of pelvic, lower abdomen and upper abdomen surgical procedures [8]. All the regions of the abdominal and pelvic cavity (ovaries, tubes, uterus, pelvic peritoneum, paracolic gutters, anterior parietal peritoneum, mesentery, peritoneal surface of the colon and bowel, liver, spleen, greater and lesser omentum, hepatic port hepatic, stomach, Morrison‘s pouch, lesser sac, surface of both hemi diaphragms, pelvic and para-aortic lymph nodes, and if applicable pleural cavity) was evaluated and described [13]. During the study years, systematic pelvic and para-aortic lymph node dissection or sampling was routinely performed, particularly in the presence of bulky lymph nodes. When applicable, the size and location of residual disease at the end of the operation, and the reasons for not achieving complete cytoreduction were reported. An ESGO-approved template was available on the ESGO website (https://guidelines.esgo.org/, accessed on 23 April 2023).
Two separate analyses were performed. Firstly, all cases were analysed to audit the trends of surgical procedures performed overtime in both the primary and interval debulking setting. Secondly, the most important predictive feature was interrogated against commonly used engineered features including the peritoneal carcinomatosis index (PCI) and the intra-operative mapping for ovarian cancer (IMO) score, in addition to the SCS. The PCI and IMO scores were calculated at the beginning of surgery to describe the intra-operative location of the disease [14,15]. We did not perform a propensity score matching, as recent evidence suggests the performance of these procedures does not significantly change in the interval cytoreductive surgery group [16].
Descriptive statistics were used to summarize the clinical characteristics of patients and their respectful cytoreductions. Continuous variables were summarized with means, standard deviations, medians, and ranges. The Kruskall-Wallis test was used to compare groups with respect to median values. Categorical variables were summarized with counts and percent. The Fisher’s exact test was used to compare groups with respect to categorical variables. Progression-free survival (PFS) was defined as the time (months) from the date of initial diagnosis to the date of progression or recurrence. Patients who were alive without progression or recurrence were censored on the date of last clinical assessment. Overall survival (OS) was defined as the time (months) from the date of initial diagnosis to the date of death. Patients who were alive were censored on the date of last follow-up. We used the Kaplan and Meier (K-M) method to estimate median PFS and OS stratified by various potential prognostic factors and the log-rank test to detect associations between variables and outcomes. Multivariate analysis using the Cox proportional hazards method was performed to identify potential independent risk factors for recurrence and mortality. Pearson’s correlation () was used to describe the associations amongst numerical variables and heatmaps were produced to illustrate the correlations. All tests were two-sided, and significance was determined at the 0.05 level.
2.1. Model development
The eXtreme Gradient Boosting (XGBoost) algorithm was employed to model the features [17]. This combines all the generated hypotheses of weak learning algorithms into a single hypothesis to boost performance. The combined effect of eight parameters to maximize model performance was investigated by evaluating a grid of combinations of values using Scikit-learn’s GridSearchCV function.
The dataset was split into training and test cohorts (70%:30% ratio). A five-fold stratified cross-validation (CV) was performed and stratified folds were constructed to overcome data imbalance. The CV was iterated to decrease both variance and bias. Model performance was assessed by measuring the total area under the receiver-operating curve (AUC). Receiver operating characteristic (ROC) and Precision-Recall curves and state-of-art scores were used for performance metrics.
The artificial intelligence SHapley Additive exPlanations (SHAP) framework was employed to explain the cohort-level risk estimates and to define novel surgical risk phenotypes. The methodology enhances interpretability by explaining how much the presence of a feature contributes to the model’s overall prediction [18]. Visual quantification of the model prediction was demonstrated by producing (a) SHAP summary plots for the global (cohort) explanation of the results; (b) SHAP dependence plots of the critical risk features pertinent to the prediction. The Python’s SciPy library (version 2.7) (Python Software Foundation) Python Language Reference, version 2.7. available at http://www.python.org) was used for the analyses.
3. Results
A total of 560 EOC patients were enrolled in the study. The patient-specific descriptive statistics have been recently published [12]. The descriptive of the performed surgical sub-procedures is shown on Table 1 and Table 2. The patients were followed-up until April 2022. Several upper abdominal procedures including wedge liver resection, diaphragmatic stripping, splenectomy, UAP, cholecystectomy, stomach resection was statistically significant between the CC 0 and non-CC 0 groups.
The model performance for the above threshold prediction was moderate-to-high (AUC 0.63, 95% CI 0.60-0.67; AP 0.44, 95% CI 0.41-0.48) (Figure 1). To promote reproducibility, the optimal set of model parameters were the following: XGBoost: {"colsample_bylevel": 1, "gamma": 0.7, "learning_rate": 0.01, "max_delta_step": 1, "max_depth": 5, "min_child_weight": 2, "n_estimators": 250, "scale_pos_weight": 1.79, "subsample": 0.75}.
The feature importance based on SHAP values is shown in Figure 2. The feature order reflects their weighted importance, i.e., the sum of the SHAP value magnitudes across all the samples (global explainability). The position on the y-axis is determined by the feature and on the x-axis by the Shapley value. The colour represents the value of the feature from low (blue = CC 0) to high (red = non-CC 0). The top-3 features included para-aortic lymph node dissection, UAP and pelvic lymph node dissection. Their longer tails compared to other features demonstrate their importance for specific in not all patients (local explainability).
When the features were screened using random forest, UAP was the top feature for CC0 prediction (Figure 3A). A correlation heatmap demonstrated the pairwise associations amongst the surgical procedures. The highest correlations were observed between large bowel resection and stoma formation ( 0.8), followed by bladder peritonectomy and pelvic peritonectomy ( 0.7). Satisfactory correlations were demonstrated between UAP and other surgical procedures. The UAP best correlated with bladder peritonectomy and diaphragmatic stripping ( > 0.5) (Figure 3B).
The SHAP dependence plots reveal the impact of each feature on the prediction by plotting the value of the feature on the x-axis and the SHAP feature value on the y-axis. Certain surgical sub- procedures are clearly associated with higher likelihood of CC 0 including stomach resection, UAP, diaphragmatic stripping (upper abdomen) (Figure 4A-C); small bowel resection, right hemicolectomy, stoma formation (bowel-related) (Figure 4D-F); all lymph node dissections ranging from para-aortic to groin dissections (Figure 5).
3.1. Model comparison
When UAP was asked to predict solely CC 0, The ROC curve showed that UAP could effectively distinguish cytoreductive outcome (AUC = 0.78, CI:0.76-0.81). When UAP only was incorporated in a composite model comprising of engineered features, it substantially enhanced its predictive value (AUC = 0.80, CI:0.76-0.84) (Figure 6).
3.2. Survival data
The K-M analysis showed a difference between the CC 0 and non-CCO groups for both PFS and OS. The median PFS was 25 months for the CC 0 group (95% CI 22–29) and 18 months (95% CI 17–19) for the non-CC 0 group (p<0.05). The median OS was 58 months for the CC 0 group (95% CI 55–62) and 33 months (95% CI 32–34) for the non-CC 0 group (p<0.05).
In multivariate Cox analysis, performance of UAP was associated with poorer PFS (HR: 1.76; 95% CI: 1.14–2.70, p 0.001) (Figure 7A). There was a trend towards poorer OS (HR: 1.06; 95% CI: 0.56–1.99, p 0.86) (Figure 7B). Similar but very marginal worsening survival trend was observed for SCS on PFS (HR: 1.04, 95% CI: 0.95-1.13, p 0.430 and OS (HR: 1.01, 95% CI: 0.89-1.15).
4. Discussion
Surgeons are significantly challenged by EOC heterogeneity. There is an increasing need for tools to better tailor treatment strategies by improving the predictions of the surgical outcomes. By scrutinizing a validated but exhaustive list of surgical sub-procedures outside the “box standard” surgery for ovarian cancer, we aligned with the recently published NICE guidelines on maximal cytoreductive surgery [19] and successfully quantified the complexity of surgery, as highlighted in our proposed classification algorithm (Figure 8). By categorising critical procedures, we highlighted the potential key role of upper abdominal peritonectomy (UAP), a complex and technically demanding surgical procedure. Using a large dataset of women with advanced EOC who underwent cytoreductive surgery, we developed and validated an ML algorithm, which demonstrates satisfactory predictive performance but more importantly, identifies UAP as the most important procedural indicator of CC0 in surgically cytoreduced EOC women. In contrast to the Aletti SCS, whereas several abdominal procedures such as diaphragmatic stripping, splenectomy, bowel, or liver resections were arbitrarily allotted a higher score [6], our devised ML model supported the feature selection and weighted importance of all surgical sub-procedures irrespective of the individual practice. Nevertheless, if solely used, it did not yield any survival benefit. We found that UAP best correlated with bladder peritonectomy and diaphragmatic stripping. That said, the procedure should not be performed in isolation but as part of a “surgical package” for effective cytoreduction in selected patients.
The result is not surprising. The right upper quadrant of the abdominal cavity is generally the most affected by cancer metastases. Therefore, removal of upper abdominal disease remains one of the most challenging parts of cytoreductive surgery in advanced EOC. Fundamental anatomical knowledge and great expertise are needed to localize and dissect the critical vascular landmarks [20]. In one study, 42% of EOC patients had disease >1 cm involving the upper abdomen above the greater omentum [21]. A comprehensive approach to surgical cytoreduction should incorporate upper abdominal resection [22]. We acknowledge that adequate exposure is critical to allow for complete resection. In our centre, initiation of the paradigm shift towards more complex multi-visceral surgery in the years 2016 and 2017, allows for a more thorough early intra-operative examination by mobilizing the liver and other organs and exposing the pouch of Morrison [12]. Diaphragmatic involvement is estimated in up to 40% of these cases [23]. Various peritonectomy procedures that may be required for maximal surgical cytoreduction were originally described by Sugarbaker [24]. Of those, perhaps the most difficult peritonectomy is the lesser omentectomy with stripping of the omental bursa due to the presence of vital structures. Radical peritonectomies with en-bloc resection of extensive widespread diaphragmatic peritoneal carcinomatosis have also been described [25]. Centralised infrastructural support and collective knowledge of the entire team are paramount to achieve best possible oncologic outcomes with an acceptable morbidity profile, including even those patients with high burden disease [26].
Overall, the study indicates that certain surgical procedures -and not the overall surgical load- are predictive of the likelihood for CC0. In addition to UAP, the top feature ranking was complemented by regional lymph node dissection, including pelvic, para—aortic or retroperitoneal lymphadenectomy. In the past, descriptions of extensive retroperitoneal nodal debulking and subsequent long-term survival justified performing these procedures in selected patients [27]. Between 2014 and 2019, the results of the LION trial were not available [28]. Therefore, during surgical cytoreduction either on the upfront or delayed setting, the bilateral pelvic and para-aortic regions were systematically assessed, and consequently, systematic lymphadenectomy was rather routinely performed. Following publication of the LION trial results, routine lymphadenectomy is not warranted, as it does not confer a survival benefit unless there is evidence of macroscopically or radiologically enlarged lymph nodes. Distribution of the disease in anatomical regions such as the omental bursa, surface of the pancreas, lesser omentum, caudate lobe, portal riad to mention but a few should not be an absolute contraindication for debulking, except when a deep infiltration of porta hepatis or celiac trunk are present [29].
The established benefit of upper abdominal cytoreduction in advanced EOC has been demonstrated even for optimal cytoreduction [30,31]. In the study by Ren et al comparing 116 patients undergoing radical surgery in the upper abdomen with 237 patients undergoing standard surgery in which only nodules larger than 1 cm were resected in the upper abdomen, the PFS and OS were significantly higher in the radical surgery group compared to the standard surgery group (PFS: 19.5 vs. 13.3 months, HR: 0.61; 95% CI: 0.46–0.80, p < 0.001; OS: not reached vs. 39.3 months, HR: 0.47; 95% CI: 0.30–0.72, p < 0.001) [30]. In our study, we failed to confer a survival benefit from the sole performance of UAP. At first glance, this looks odd. We explained why UAP should be offered as part of a “surgical package” to selected patients. It appears that any transient benefit is potentially outplayed by a high disease load in that cohort of patients. The benefits from an intervention (UAP) depend not only on the hazard ratio but also on the shape of the underlying probability distribution, which is disease related. Therefore, the hazard ratio must be interpreted judiciously in studies where the duration of events is the primary efficacy variable. Although it can be helpful for the purposes of statistical hypothesis testing the benefit from the procedure, other measures such as median times to the study endpoint are important, particularly useful when the event of interest i.e., OS may eventually occur across the entire cohort. Then the time-to-event curve drops to zero, such that risk at the end of follow-up is not an issue [32]. In our study, although UAP increased the hazard rate for PFS, the treatment effect was larger because >50% of the patients did not have a relapse at the time. Our complete cytoreduction rates are comparable to other high-volume specialized centers [33].
5. Strengths and limitations
The study supported the current paradigm shift for organised centralisation of services moving away from the traditional patterns of cytoreductive surgery. Strength of the study was the study design that allowed to weight the importance of the individual procedures as outcome indicators. The cohort has been extensively scrutinised [12,13]. We applied XAI frameworks to explain the modelling “black box”, but also quantitative results not essentially included under the XAI umbrella, such as Cox regression [34]. We did not assess the morbidity of the surgical procedures, but it is assumed to vary as others have demonstrated the wide range in complications rates [35]. The findings may not be generalizable to all providers caring for ovarian cancer patients. The outcome rates and potential complications associated with the incorporation of upper abdominal surgery into EOC cytoreduction may be influenced by the experience of the surgeon performing these procedures and the institutional capacity to manage these patients [36]. Indeed, within our own practice, we observed variations in the surgical effort extended at complete resection. Nevertheless, the study was designed in such way not to reflect individual practice. Data from pre-operative imaging were not included in the study because the miliary or plaque-like morphology of the peritoneal disease makes it often undetectable by imaging [37]. Disease in the upper abdomen does not come without involvement of the lower regions [38]. To achieve complete clearance, we stress out the need for thorough exploration and visual inspection of the upper abdominal cavity early at surgery to resect all disease sites. Finally, the classification model presented here can potentially be trained with a larger number of samples to generate a robust digital surgical reference in high output tertiary centres, through a free access inter-operability platform for gynaecologic oncology services.
6. Conclusions
Our work represents a significant advancement in the discussion about surgical effort at advanced EOC cytoreductive surgery. We provide surgical interpretable evidence for predicting the key intervention required to achieve CC0. In contrast to the widely used SCS, our ML methodology allows for operational feature selection by weighting the relative importance of those surgical procedures that appear to be more predictive of CC0. The upper abdominal quadrants should be thoroughly inspected to ensure that CC0 is achievable.
References
- Du Bois, A.; Quinn, M.; Thigpen, T.; Vermorken, J.; Avall-Lundqvist, E.; Bookman, M.; Bowtell, D.; Brady, M.; Casado, A.; Cervantes, A.; others. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Annals of oncology 2005, 16, viii7–viii12. [CrossRef]
- Monk, B.J.; Coleman, R.L.; Fujiwara, K.; Wilson, M.K.; Oza, A.M.; Oaknin, A.; O’Malley, D.M.; Lorusso, D.; Westin, S.N.; Safra, T.; others. ATHENA (GOG-3020/ENGOT-ov45): A randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA–MONO) and rucaparib in combination with nivolumab (ATHENA–COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer. International Journal of Gynecologic Cancer 2021, 31. [CrossRef]
- Dahm-Kähler, P.; Palmqvist, C.; Staf, C.; Holmberg, E.; Johannesson, L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival-A population-based cohort study. Gynecologic oncology 2016, 142, 211–216. [CrossRef]
- Palmqvist, C.; Staf, C.; Mateoiu, C.; Johansson, M.; Albertsson, P.; Dahm-Kähler, P. Increased disease-free and relative survival in advanced ovarian cancer after centralized primary treatment. Gynecologic oncology 2020, 159, 409–417. [CrossRef]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane database of systematic reviews 2011. [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. American journal of obstetrics and gynecology 2007, 197, 676–e1. [CrossRef]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; others. European Society of Gynaecologic Oncology quality indicators for advanced ovarian cancer surgery. International Journal of Gynecologic Cancer 2016, 26. [CrossRef]
- Préfontaine, M.; Gelfand, A.T.; Donovan, J.T.; Powell, J.L. Reproducibility of tumor measurements in ovarian cancer: a study of interobserver variability. Gynecologic oncology 1994, 55, 87–90. [CrossRef]
- Fotopoulou, C.; Concin, N.; Planchamp, F.; Morice, P.; Vergote, I.; du Bois, A.; Querleu, D. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. International Journal of Gynecological Cancer 2020, 30, 436–440. [CrossRef]
- Kawakami, E.; Tabata, J.; Yanaihara, N.; Ishikawa, T.; Koseki, K.; Iida, Y.; Saito, M.; Komazaki, H.; Shapiro, J.S.; Goto, C.; others. Application of artificial intelligence for preoperative diagnostic and prognostic prediction in epithelial ovarian cancer based on blood biomarkers. Clinical cancer research 2019, 25, 3006–3015. [CrossRef]
- Laios, A.; Gryparis, A.; DeJong, D.; Hutson, R.; Theophilou, G.; Leach, C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. Journal of Ovarian Research 2020, 13, 1–8. [CrossRef]
- Laios, A.; Kalampokis, E.; Johnson, R.; Thangavelu, A.; Tarabanis, C.; Nugent, D.; De Jong, D. Explainable artificial intelligence for prediction of complete surgical cytoreduction in advanced-stage epithelial ovarian cancer. Journal of personalized medicine 2022, 12, 607. [CrossRef]
- Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Nugent, D.; De Jong, D. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers 2023, 15, 966. [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Peritoneal carcinomatosis: principles of management 1996, pp. 359–374. [CrossRef]
- Sehouli, J.; Könsgen, D.; Mustea, A.; Oskay-Özcelik, G.; Katsares, I.; Weidemann, H.; Lichtenegger, W. „IMO”-Intraoperatives Mapping des Ovarialkarzinoms. Zentralblatt für Gynäkologie 2003, 125, 129–135. [CrossRef]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. Journal of computer and system sciences 1997, 55, 119–139. [CrossRef]
- Dottino, J.A.; He, W.; Sun, C.C.; Zhao, H.; Fu, S.; Rauh-Hain, J.A.; Suidan, R.S.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. National trends in bowel and upper abdominal procedures in ovarian cancer surgery. International Journal of Gynecologic Cancer 2020, 30. [CrossRef]
- Slack, D.; Hilgard, S.; Jia, E.; Singh, S.; Lakkaraju, H. Fooling lime and shap: Adversarial attacks on post hoc explanation methods. Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society, 2020, pp. 180–186. [CrossRef]
- NICE. Overview: Maximal cytoreductive surgery for advanced ovarian cancer: Guidance.
- Stepanyan, A.; Malakyan, Z.; Alaverdyan, A.; Davtyan, H.; Hovhannisyan, T. Right upper quadrant peritonectomy. Answering frequently asked questions. International Journal of Gynecological Cancer 2021, 31, 1305–1306. [CrossRef]
- Zivanovic, O.; Eisenhauer, E.L.; Zhou, Q.; Iasonos, A.; Sabbatini, P.; Sonoda, Y.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecologic oncology 2008, 108, 287–292. [CrossRef]
- Kehoe, S.M.; Eisenhauer, E.L.; Chi, D.S. Upper abdominal surgical procedures: liver mobilization and diaphragm peritonectomy/resection, splenectomy, and distal pancreatectomy. Gynecologic oncology 2008, 111, S51–S55. [CrossRef]
- Bogani, G.; Ditto, A.; Martinelli, F.; Lorusso, D.; Chiappa, V.; Donfrancesco, C.; Di Donato, V.; Indini, A.; Aletti, G.; Raspagliesi, F. Surgical techniques for diaphragmatic resection during cytoreduction in advanced or recurrent ovarian carcinoma: a systematic Review and meta-analysis. International Journal of Gynecologic Cancer 2016, 26. [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Annals of surgery 1995, 221, 29. [CrossRef]
- Lago, V.; Domingo, S.; Matute, L.; Padilla-Iserte, P.; Gurrea, M.; others. Radical en bloc peritonectomy in advanced ovarian cancer 2018. [CrossRef]
- Fotopoulou, C.; Taskiran, C. The principles of safe and efficacious upper abdominal surgery. Gynecol Pelvic Med 2021, 4. [CrossRef]
- Spirtos, N.M.; Gross, G.M.; Freddo, J.L.; Ballon, S.C. Cytoreductive surgery in advanced epithelial cancer of the ovary: the impact of aortic and pelvic lymphadenectomy. Gynecologic oncology 1995, 56, 345–352. [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; others. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. New England Journal of Medicine 2019, 380, 822–832. [CrossRef]
- Straubhar, A.M.; Filippova, O.T.; Cowan, R.A.; Lakhman, Y.; Sarasohn, D.M.; Nikolovski, I.; Torrisi, J.M.; Ma, W.; Abu-Rustum, N.R.; Gardner, G.J.; others. A multimodality triage algorithm to improve cytoreductive outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer: a Memorial Sloan Kettering Cancer Center team ovary initiative. Gynecologic oncology 2020, 158, 608–613. [CrossRef]
- Ren, Y.; Jiang, R.; Yin, S.; You, C.; Liu, D.; Cheng, X.; Tang, J.; Zang, R. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC cancer 2015, 15, 1–12. [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecologic oncology 2009, 114, 26–31. [CrossRef]
- Spruance, S.L.; Reid, J.E.; Grace, M.; Samore, M. Hazard ratio in clinical trials. Antimicrobial agents and chemotherapy 2004, 48, 2787–2792. [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecologic oncology 2009, 114, 26–31. [CrossRef]
- Laios, A.; De Jong, D.; Kalampokis, E. Beauty is in the explainable artificial intelligence (XAI) of the “agnostic” beholder. Translational Cancer Research 2023, 12, 226. [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2021. [CrossRef]
- Chang, S.J.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. Role of aggressive surgical cytoreduction in advanced ovarian cancer. Journal of gynecologic oncology 2015, 26, 336–342. [CrossRef]
- Pannu, H.K.; Bristow, R.E.; Montz, F.J.; Fishman, E.K. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 2003, 23, 687–701. [CrossRef]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Shah, M.Y.; Shaikh, S.; Prabhu, A.; Mishra, S.; others. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? European Journal of Surgical Oncology 2020, 46, 582–589. [CrossRef]
Figure 1.
(A) Receiver Operator Characteristic (ROC) curve showing the diagnostic accuracy of all the surgical sub-procedures for the prediction of complete cytoreduction (AUC = 0.63) (B) Precision Recall curve and Average Precision performance value (AP = 0.44).
Figure 1.
(A) Receiver Operator Characteristic (ROC) curve showing the diagnostic accuracy of all the surgical sub-procedures for the prediction of complete cytoreduction (AUC = 0.63) (B) Precision Recall curve and Average Precision performance value (AP = 0.44).
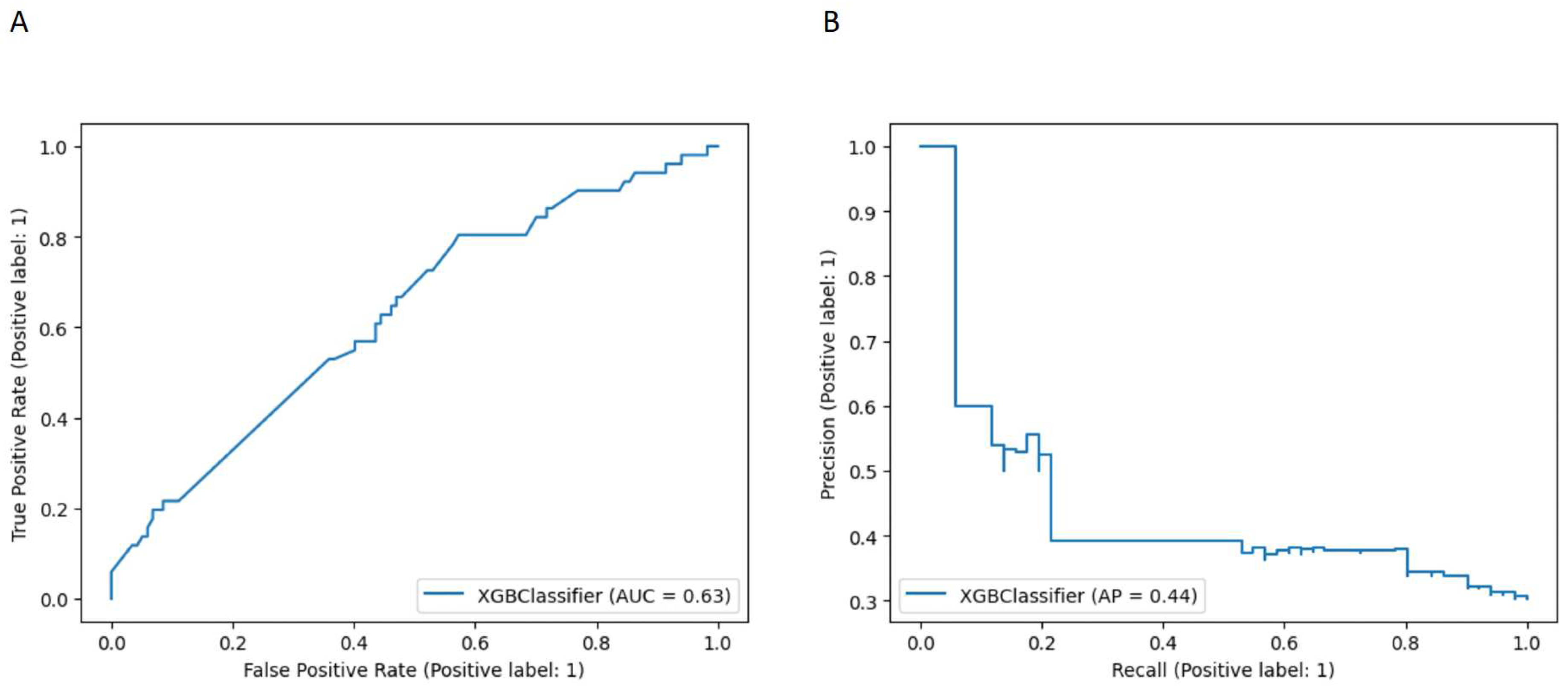
Figure 2.
Model classification differences explained by the SHAP values. (A) Summary plot showing feature distribution plots based on the sum of SHAP value magnitudes over all samples. The color represents the feature value (red non-CC0, blue CC0 resection) and the x-axis represents the impact score according to binary output (B) Standard bar plot of the mean absolute SHAP values for each feature showing the average impact on the global model output. SHAP, Shapley Additive exPlanations.; CC, Complete Cytoreduction
Figure 2.
Model classification differences explained by the SHAP values. (A) Summary plot showing feature distribution plots based on the sum of SHAP value magnitudes over all samples. The color represents the feature value (red non-CC0, blue CC0 resection) and the x-axis represents the impact score according to binary output (B) Standard bar plot of the mean absolute SHAP values for each feature showing the average impact on the global model output. SHAP, Shapley Additive exPlanations.; CC, Complete Cytoreduction
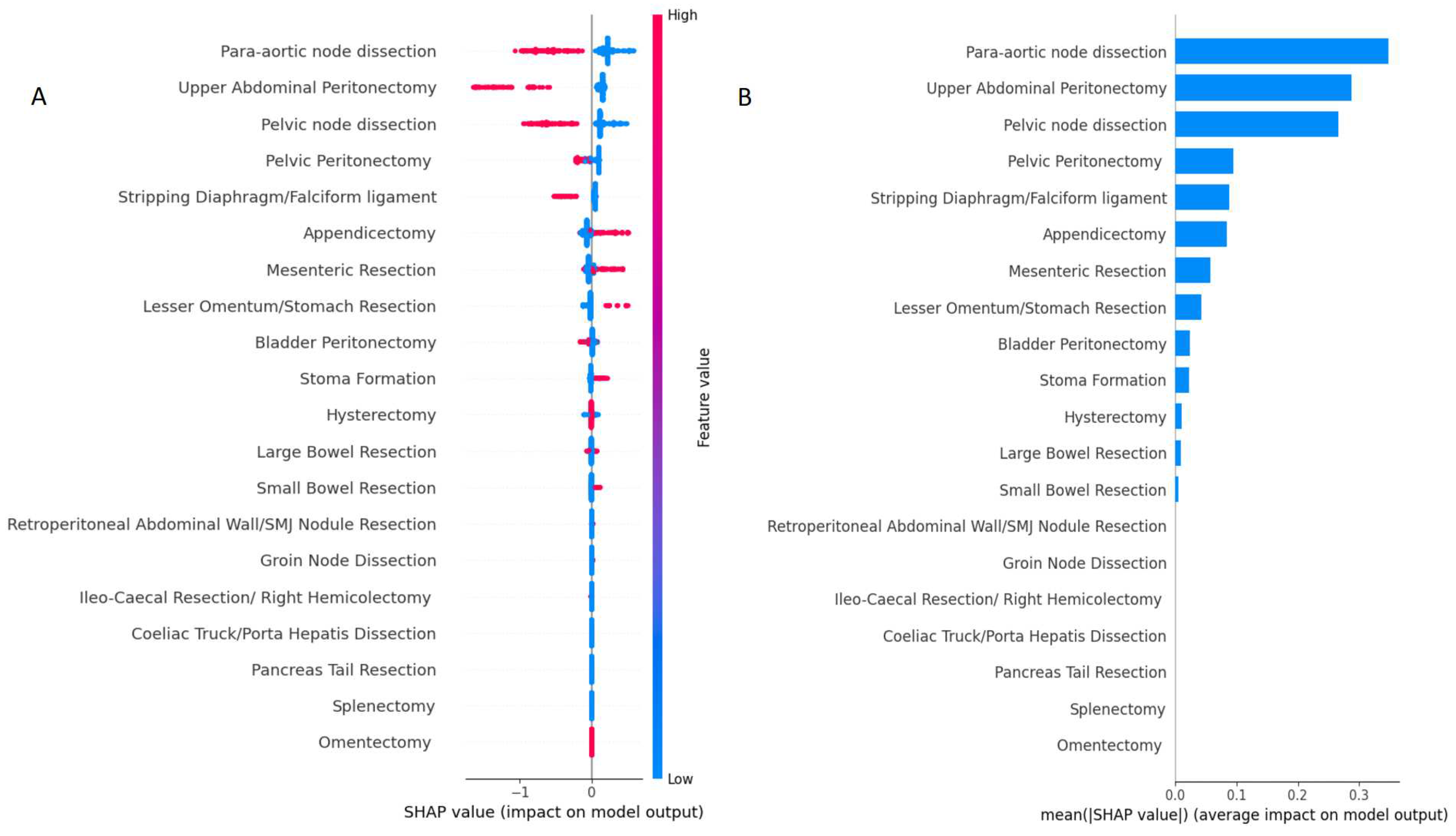
Figure 3.
(A) Feature importance plot showing the relevance of each variable to the CC0 prediction when screened using random forest. (B) Correlation heatmap demonstrating the pairwise correlations amongst the surgical procedures. The Pearson correlation () was used. CC); complete cytoreduction
Figure 3.
(A) Feature importance plot showing the relevance of each variable to the CC0 prediction when screened using random forest. (B) Correlation heatmap demonstrating the pairwise correlations amongst the surgical procedures. The Pearson correlation () was used. CC); complete cytoreduction
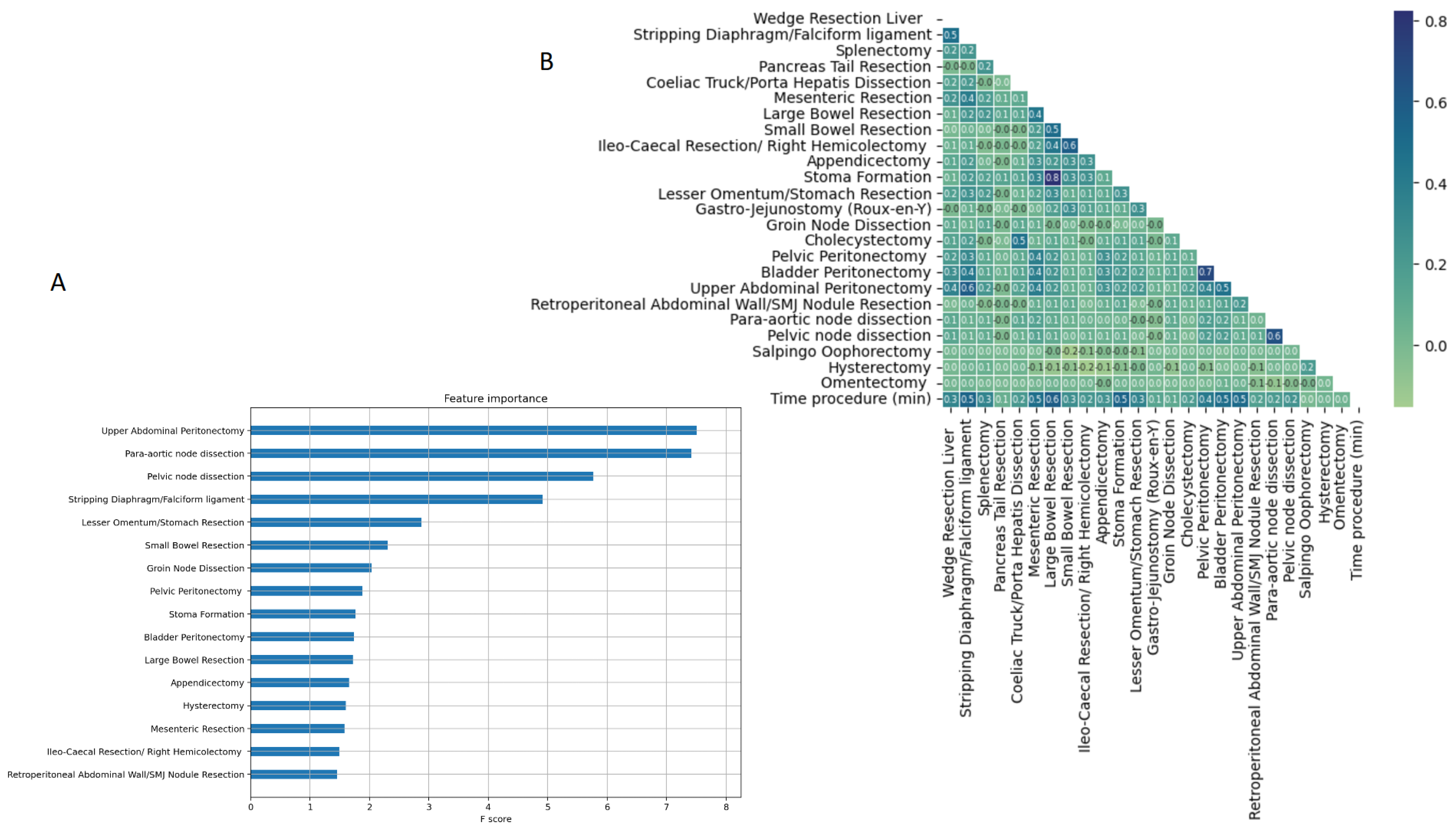
Figure 4.
Dependence plots demonstrating clear inflection points for several surgical sub-procedures at cytoreduction (A-C):Upper abdomen, (D-F) Bowel resections
Figure 4.
Dependence plots demonstrating clear inflection points for several surgical sub-procedures at cytoreduction (A-C):Upper abdomen, (D-F) Bowel resections

Figure 5.
Dependence plots demonstrating clear inflection points for various regional lymph node dissections
Figure 5.
Dependence plots demonstrating clear inflection points for various regional lymph node dissections

Figure 6.
Performance metrics of devised models for the prediction of complete cytoreduction (A) UAP (B) Composite model comprised of UAP and commonly used engineered features.
Figure 6.
Performance metrics of devised models for the prediction of complete cytoreduction (A) UAP (B) Composite model comprised of UAP and commonly used engineered features.
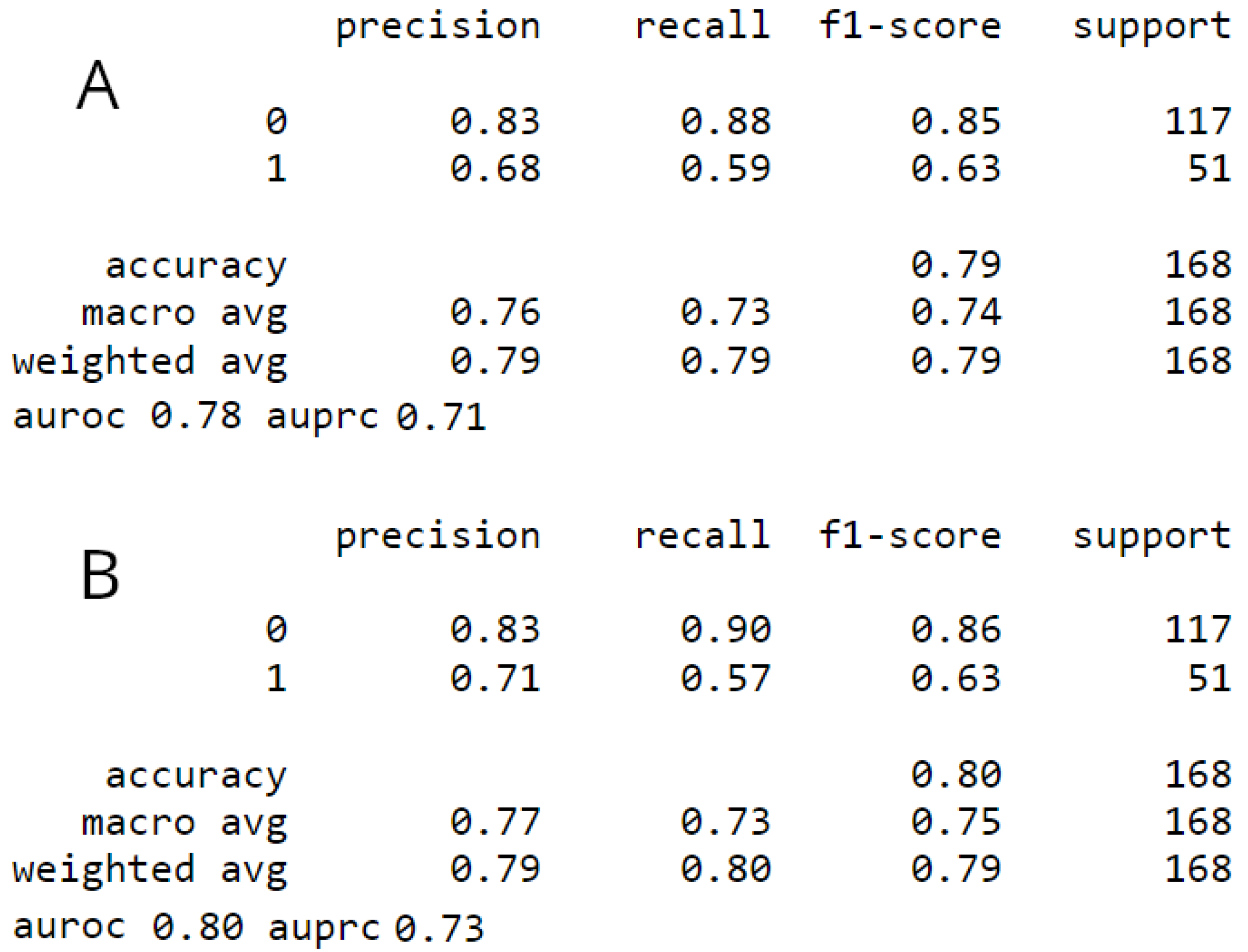
Figure 7.
Cohort survival outcomes analyzed according to the occurrence of UAP (blue= UAP cohort; orange=non-UAP cohort) (A) progression-free-survival (B) overall-survival. Note the shape difference between the concave (UAP group) and the sinusoidal (non-UAP group) curves. Hazard ratio (HR) and 95% confidence interval (CI) for prospective log-linear associations (Cox regression) between (C) recurrence and non-recurrence (D) fatal and non-fatal outcomes including the UAP and commonly used engineered features. The hazard ratio can be misleading if used to quantify the benefit from the intervention. A relatively large hazard ratio can yield small treatment effects (sinusoidal curves). In contrast, a relatively small hazard ratio (concave curves) can yield large intervention effects reflected by longer median survival times for 50% of patients.
Figure 7.
Cohort survival outcomes analyzed according to the occurrence of UAP (blue= UAP cohort; orange=non-UAP cohort) (A) progression-free-survival (B) overall-survival. Note the shape difference between the concave (UAP group) and the sinusoidal (non-UAP group) curves. Hazard ratio (HR) and 95% confidence interval (CI) for prospective log-linear associations (Cox regression) between (C) recurrence and non-recurrence (D) fatal and non-fatal outcomes including the UAP and commonly used engineered features. The hazard ratio can be misleading if used to quantify the benefit from the intervention. A relatively large hazard ratio can yield small treatment effects (sinusoidal curves). In contrast, a relatively small hazard ratio (concave curves) can yield large intervention effects reflected by longer median survival times for 50% of patients.
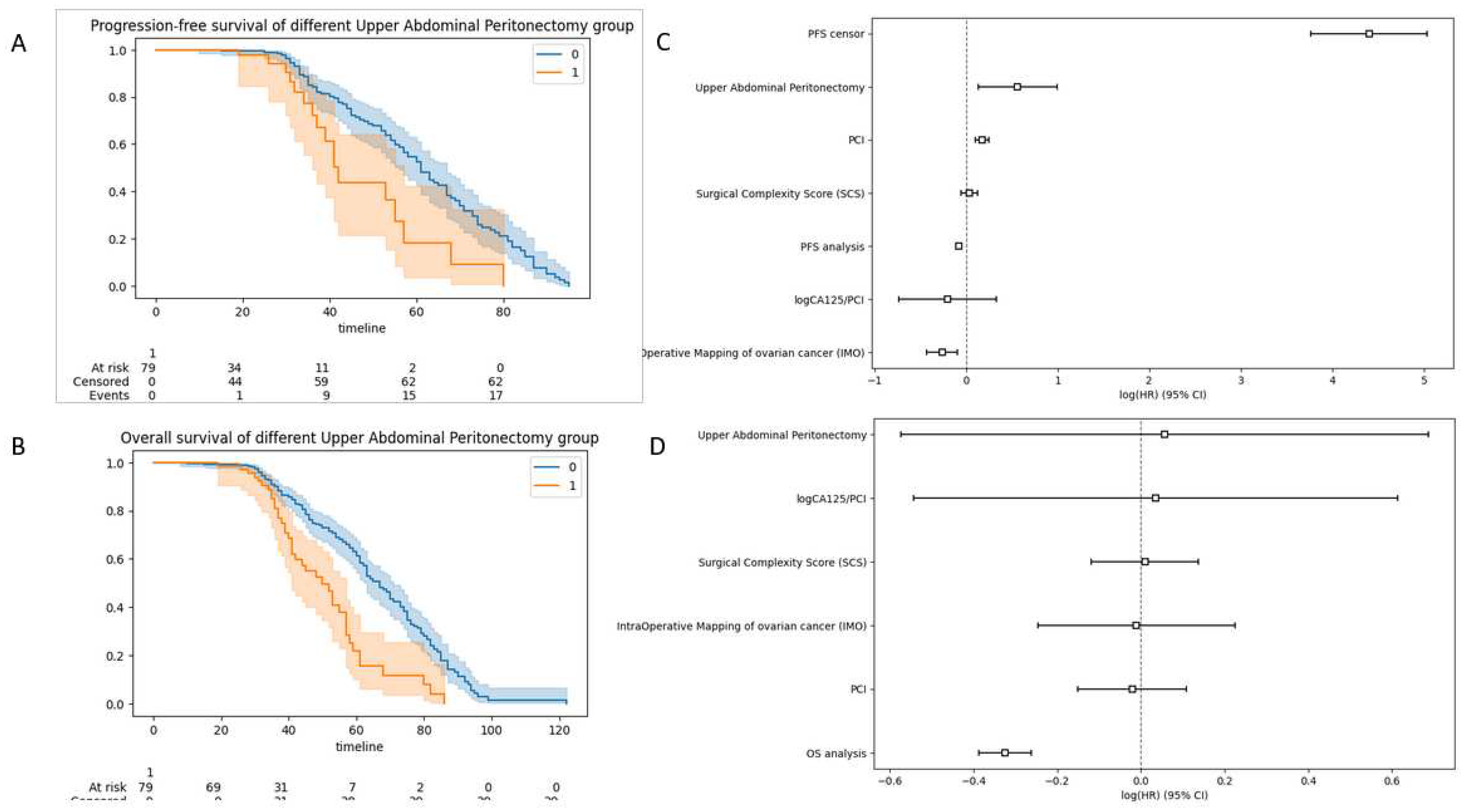
Figure 8.
Study flowchart. The probability to achieve complete cytoreduction (CC0) can be well quantified by a ML-driven model inclusive all surgical sub-procedures. Upper abdominal peritonectomy is the most important predictive feature. A “surgical package” of maximal effort targeted cytoreduction including upper abdominal peritonectomy should be offered in selected patients. Thorough inspection of upper abdominal quadrants to ensure that CC0 is achievable reflects good clinical practice. ML; Machine Learning.
Figure 8.
Study flowchart. The probability to achieve complete cytoreduction (CC0) can be well quantified by a ML-driven model inclusive all surgical sub-procedures. Upper abdominal peritonectomy is the most important predictive feature. A “surgical package” of maximal effort targeted cytoreduction including upper abdominal peritonectomy should be offered in selected patients. Thorough inspection of upper abdominal quadrants to ensure that CC0 is achievable reflects good clinical practice. ML; Machine Learning.
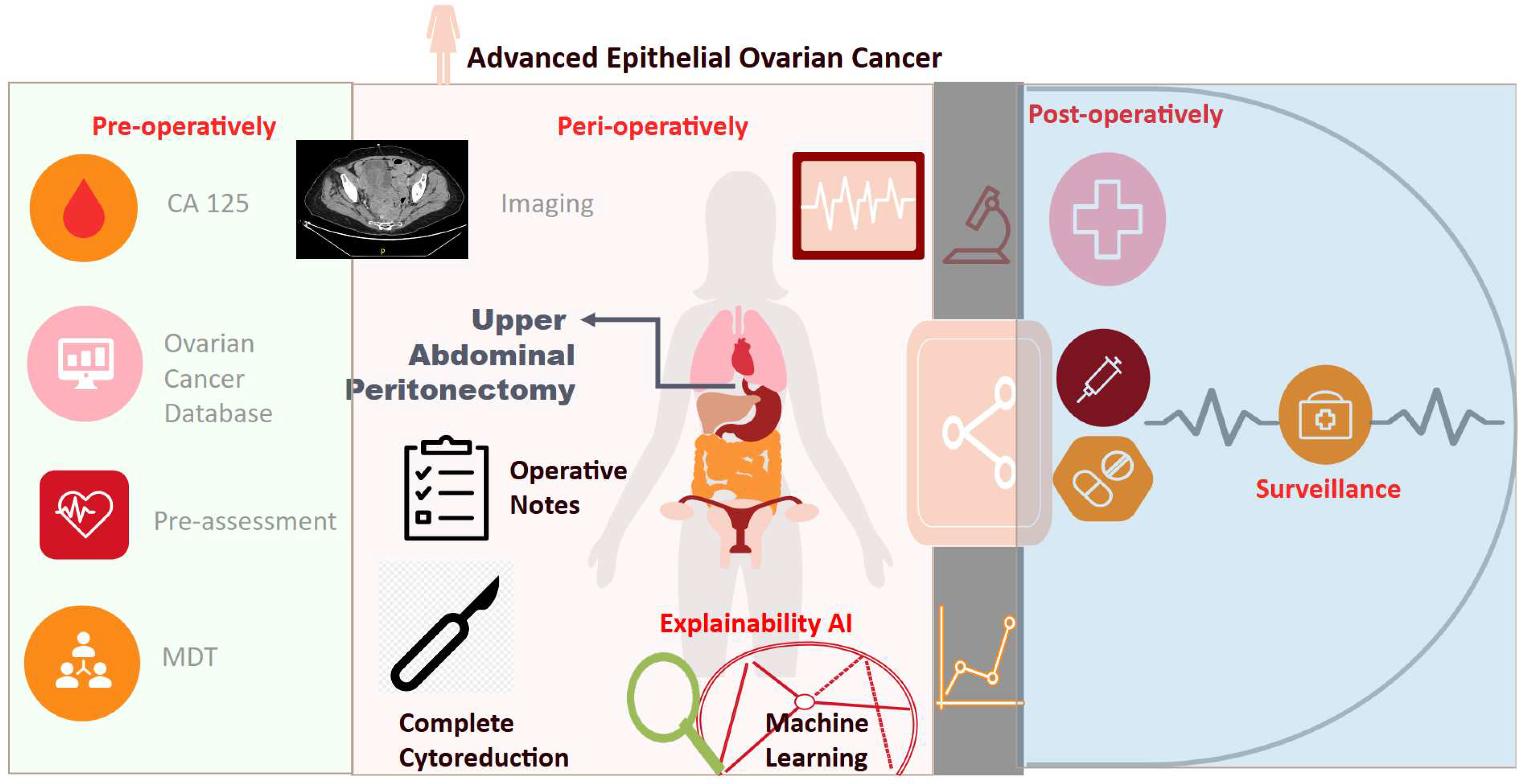
Table 1.
Descriptive statistics of the performed surgical sub-procedures.
| Variable | Overall | Training Set | Testing Set | pvalue (training) | No UAP | UAP | pvalue (UAP) | |
|---|---|---|---|---|---|---|---|---|
| Wedge Resection Liver | 0 | 537 (95.89) | 428 (95.54) | 109 (97.32) | 0.558 | 475 (98.75) | 62 (78.48) | <0.001 |
| 1 | 23 (4.11) | 20 (4.46) | 3 (2.68) | 0.558 | 6 (1.25) | 17 (21.52) | <0.001 | |
| Stripping Diaphragm/Falciform ligament | 0 | 484 (86.43) | 385 (85.94) | 99 (88.39) | 0.6 | 455 (94.59) | 29 (36.71) | <0.001 |
| 1 | 76 (13.57) | 63 (14.06) | 13 (11.61) | 0.6 | 26 (5.41) | 50 (63.29) | <0.001 | |
| Splenectomy | 0 | 543 (96.96) | 435 (97.1) | 108 (96.43) | 0.951 | 474 (98.54) | 69 (87.34) | <0.001 |
| 1 | 17 (3.04) | 13 (2.9) | 4 (3.57) | 0.951 | 7 (1.46) | 10 (12.66) | <0.001 | |
| Pancreas Tail Resection | 0 | 559 (99.82) | 447 (99.78) | 112 (100.0) | 1.0 | 480 (99.79) | 79 (100.0) | 1 |
| 1 | 1 (0.18) | 1 (0.22) | 0 (0.0) | 1.0 | 1 (0.21) | 0 (0.0) | 1 | |
| Coeliac Truck/Porta Hepatis Dissection | 0 | 554 (98.93) | 443 (98.88) | 111 (99.11) | 1.0 | 479 (99.58) | 75 (94.94) | 0.002 |
| 1 | 6 (1.07) | 5 (1.12) | 1 (0.89) | 1.0 | 2 (0.42) | 4 (5.06) | 0.002 | |
| Mesenteric Resection | 0 | 427 (76.25) | 340 (75.89) | 87 (77.68) | 0.785 | 399 (82.95) | 28 (35.44) | <0.001 |
| 1 | 133 (23.75) | 108 (24.11) | 25 (22.32) | 0.785 | 82 (17.05) | 51 (64.56) | <0.001 | |
| Large Bowel Resection | 0 | 496 (88.57) | 399 (89.06) | 97 (86.61) | 0.572 | 440 (91.48) | 56 (70.89) | <0.001 |
| 1 | 64 (11.43) | 49 (10.94) | 15 (13.39) | 0.572 | 41 (8.52) | 23 (29.11) | <0.001 | |
| Small Bowel Resection | 0 | 537 (95.89) | 430 (95.98) | 107 (95.54) | 1.0 | 464 (96.47) | 73 (92.41) | 0.168 |
| 1 | 23 (4.11) | 18 (4.02) | 5 (4.46) | 1.0 | 17 (3.53) | 6 (7.59) | 0.168 | |
| Ileo-Caecal Resection/ Right Hemicolectomy | 0 | 539 (96.25) | 432 (96.43) | 107 (95.54) | 0.868 | 465 (96.67) | 74 (93.67) | 0.326 |
| 1 | 21 (3.75) | 16 (3.57) | 5 (4.46) | 0.868 | 16 (3.33) | 5 (6.33) | 0.326 | |
| Appendicectomy | 0 | 439 (78.39) | 352 (78.57) | 87 (77.68) | 0.939 | 398 (82.74) | 41 (51.9) | <0.001 |
| 1 | 121 (21.61) | 96 (21.43) | 25 (22.32) | 0.939 | 83 (17.26) | 38 (48.1) | <0.001 | |
| Stoma Formation | 0 | 509 (90.89) | 407 (90.85) | 102 (91.07) | 1.0 | 449 (93.35) | 60 (75.95) | <0.001 |
| 1 | 51 (9.11) | 41 (9.15) | 10 (8.93) | 1.0 | 32 (6.65) | 19 (24.05) | <0.001 | |
| Lesser Omentum/Stomach Resection | 0 | 534 (95.36) | 427 (95.31) | 107 (95.54) | 1.0 | 468 (97.3) | 66 (83.54) | <0.001 |
| 1 | 26 (4.64) | 21 (4.69) | 5 (4.46) | 1.0 | 13 (2.7) | 13 (16.46) | <0.001 | |
| Gastro-Jejunostomy (Roux-en-Y) | 0 | 558 (99.64) | 447 (99.78) | 111 (99.11) | 0.859 | 480 (99.79) | 78 (98.73) | 0.658 |
| 1 | 2 (0.36) | 1 (0.22) | 1 (0.89) | 0.859 | 1 (0.21) | 1 (1.27) | 0.658 | |
| Groin Node Dissection | 0 | 549 (98.04) | 440 (98.21) | 109 (97.32) | 0.819 | 473 (98.34) | 76 (96.2) | 0.407 |
| 1 | 11 (1.96) | 8 (1.79) | 3 (2.68) | 0.819 | 8 (1.66) | 3 (3.8) | 0.407 | |
| Cholecystectomy | 0 | 553 (98.75) | 442 (98.66) | 111 (99.11) | 1.0 | 479 (99.58) | 74 (93.67) | <0.001 |
| 1 | 7 (1.25) | 6 (1.34) | 1 (0.89) | 1.0 | 2 (0.42) | 5 (6.33) | <0.001 |
Table 2.
Descriptive statistics of the performed surgical sub-procedures (Cont.).
| Variable | Overall | Training Set | Testing Set | pvalue (training) | No UAP | UAP | pvalue (UAP) | |
|---|---|---|---|---|---|---|---|---|
| Pelvic Peritonectomy | 0 | 277 (49.46) | 216 (48.21) | 61 (54.46) | 0.281 | 273 (56.76) | 4 (5.06) | <0.001 |
| 1 | 283 (50.54) | 232 (51.79) | 51 (45.54) | 0.281 | 208 (43.24) | 75 (94.94) | <0.001 | |
| Bladder Peritonectomy | 0 | 358 (63.93) | 284 (63.39) | 74 (66.07) | 0.676 | 351 (72.97) | 7 (8.86) | <0.001 |
| 1 | 202 (36.07) | 164 (36.61) | 38 (33.93) | 0.676 | 130 (27.03) | 72 (91.14) | <0.001 | |
| Upper Abdominal Peritonectomy | 0 | 481 (85.89) | 383 (85.49) | 98 (87.5) | 0.693 | 481 (100.0) | 0 (0.0) | <0.001 |
| 1 | 79 (14.11) | 65 (14.51) | 14 (12.5) | 0.693 | 0 (0.0) | 79 (100.0) | <0.001 | |
| Retroperitoneal Abdominal Wall/SMJ Nodule Resection | 0 | 537 (95.89) | 432 (96.43) | 105 (93.75) | 0.312 | 470 (97.71) | 67 (84.81) | <0.001 |
| 1 | 23 (4.11) | 16 (3.57) | 7 (6.25) | 0.312 | 11 (2.29) | 12 (15.19) | <0.001 | |
| Para-aortic node dissection | 0 | 381 (68.04) | 303 (67.63) | 78 (69.64) | 0.768 | 333 (69.23) | 48 (60.76) | 0.172 |
| 1 | 179 (31.96) | 145 (32.37) | 34 (30.36) | 0.768 | 148 (30.77) | 31 (39.24) | 0.172 | |
| Pelvic node dissection | 0 | 414 (73.93) | 335 (74.78) | 79 (70.54) | 0.427 | 363 (75.47) | 51 (64.56) | 0.056 |
| 1 | 146 (26.07) | 113 (25.22) | 33 (29.46) | 0.427 | 118 (24.53) | 28 (35.44) | 0.056 | |
| Salpingo Oophorectomy | 0 | 6 (1.07) | 3 (0.67) | 3 (2.68) | 0.182 | 6 (1.25) | 0 (0.0) | 0.683 |
| 1 | 554 (98.93) | 445 (99.33) | 109 (97.32) | 0.182 | 475 (98.75) | 79 (100.0) | 0.683 | |
| Hysterectomy | 0 | 56 (10.0) | 41 (9.15) | 15 (13.39) | 0.245 | 49 (10.19) | 7 (8.86) | 0.871 |
| 1 | 504 (90.0) | 407 (90.85) | 97 (86.61) | 0.245 | 432 (89.81) | 72 (91.14) | 0.871 | |
| Omentectomy | 0 | 7 (1.25) | 4 (0.89) | 3 (2.68) | 0.296 | 7 (1.46) | 0 (0.0) | 0.594 |
| 1 | 553 (98.75) | 444 (99.11) | 109 (97.32) | 0.296 | 474 (98.54) | 79 (100.0) | 0.594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence
Alexandros Laios
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









