Preprint
Article
Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering
Altmetrics
Downloads
122
Views
71
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
20 October 2023
Posted:
23 October 2023
You are already at the latest version
Alerts
Abstract
Extensive damage to peripheral nerves is a health problem with few therapeutic alternatives. In this context, the development of tissue engineering seeks to obtain materials that can help recreate environments conducive to cellular development and functional repair of peripheral nerves. Different hydrogels have been studied and presented as alternatives for future treatments to emulate the morphological characteristics of nerves. Along with this, other research proposes the need to incorporate electrical stimuli into treatments as agents that promote cell growth and differentiation; however, no precedent correlates the simultaneous effects of the types of hydrogel and electrical stimuli. In this research, the neural differentiation of PC12 cells we are evaluated, relating the effect of collagen, alginate, GelMA, and PEGDA hydrogels with electrical stimulation modulated in four different ways. Our results show significant correlations for different culture conditions, allowing us to develop new experimental schemes for new materials in peripheral nerve engineering.

Keywords:
Subject: Chemistry and Materials Science - Biomaterials
1. Introduction
Tissue engineering has emerged as a promising field in regenerative medicine, especially in restoring and replacing damaged or degenerated tissues [1]. Among body tissues, peripheral nerve tissue (PNT) presents a unique set of challenges and opportunities. Unlike many other tissues, the PNT inherently has the capacity to regenerate; however, in extensive injuries, this regeneration is often insufficient, resulting in persistent loss of tissue function [2]. En este contexto, el desarrollo de biomateriales juega un papel fundamental al establecerse como matrices extracelulares sintéticas que proporcionan soporte físico y señales bioquímicas suficientes para el crecimiento y la diferenciación de las células [3,4].
Within this realm, hydrogels are desirable materials in peripheral nerve tissue engineering because, being highly hydrated and permeable, they closely mimic the viscoelastic nature of native tissues as the nerves [5]; in addition, such characteristics make them particularly apt for cell encapsulation and controlled delivery of bioactive factors, thus facilitating nerve tissue regeneration [6,7]. Specifically, hydrogels such as gelatin methacrylate (GelMA), polyethylene glycol diacrylate (PEGDA), collagen, and alginate have shown potential in nerve tissue engineering applications due to their biocompatibility and tunable mechanical properties [7,8,9,10,11].
However, the nerve cellular environment is not solely dictated by biochemical cues; electrical signals play a foundational role, where the physiological importance of two types of electrical signals is recognized: the low resistance intracellular signals propagated between two neurons through gap junctions and the electrical signals between neurons without contact between them derived from extracellular electric fields [12]. Numerous studies have demonstrated that electrical stimulation can promote cell proliferation, neurite elongation, and axonal growth direction [13,14,15]. Thus, combining biomaterials, such as the hydrogels mentioned above, with electrical stimuli might offer a synergistic strategy, enhancing PNT regeneration effectively.
The present research evaluates the interaction between electrical stimulation and neural differentiation of PC12 cells grown on different types of hydrogels. This approach aims to relate the surface topographical effects of hydrogels and the variation of electrical stimulation as relevant parameters for the generation of future therapeutic alternatives in peripheral nerve tissue engineering.
2. Materials and Methods
2.1. Reagents
The reagents used in this study were; Penicillin-streptomycin (AB), horse serum (HS), fetal bovine serum (FBS), collagen I, rat tail, phosphate buffer saline (PBS) and modified RPMI-1640 ATCC (Gibco TM); porcine skin gelatin, methacrylic anhydride, 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure), 2-hydroxy-2-methylpropiophenone (Darocur 1173), alginate, Poly(ethylene glycol) diacrylate (PEGDA) and Nerve Growth Factor (NGF) (Sigma-aldrich; merck KGaa, Germany). Other reagents were purchased for these experiments with sufficient quality.
2.2. PC12 Cell Culture, Differentiation and Viability
PC12 cells were cultured in RPMI-1640 ATCC modified medium supplemented with 10% HS, 5% FBS, 100 u/ml penicillin, and 100 mg/L streptomycin. PC12 cells were maintained at 37° C, in humidified incubator with 5% CO2. Differentiation medium consisted of RPMI-1640 ATCC modified medium supplemented with 2% HS, penicillin-streptomycin 100 u/ml and 50 ng/mL NGF. The differentiation cell concentration was 2x105 cell/ml. Cell viability was assessed with the WST1 assay in 96-well plate format following standard protocol [16]. Neurite extension and percentage of neurites per cell were measured was performed by photographic analysis using ImageJ single neurite tracer system®.
2.3. Preparation of Hydrogels and Surface Modification of Culture Systems
Collagen: For the modified protocol, the collagen solution was prepared by dissolving 1.5 mL of collagen (Collagen I) in 30 mL of 20 mM acetic acid. 2 mL of the solution was deposited in each well of the six-well plate, left to incubate for two hours and then washed three times with PBS pH 7.4 [17].
PEGDA: For the modified protocol, the hydrogel was prepared by mixing 1 g of PEGDA and 31 mg of Darocur 1173 (photoinitiator), dissolved in 10 mL of HEPES. 1.5 mL of solution was deposited in each well of a six-well culture plate and exposed to UV radiation (0.4 joules), the wells were washed three times with PBS pH 7.4 [18].
Alginate: For the modified protocol, the hydrogels were prepared by ionic polymerization by mixing 800 μL of an alginate solution and 1 mL of 100 μM CaCl2. 2 mL of the mixture is placed in each well of the six-well culture dish, allowed to rest for 30 minutes and washed 3 times with PBS pH 7.4. The alginate solution is prepared by dissolving 0.5 g of alginate in 50 mL of culture medium (RPMI 10%, HS 5% FBS, 1% AB) for a final concentration of 0.01% w/v [19].
GelMA: The synthesis of methacrylated gelatin (GelMA) was prepared from a modified protocol by reaction of gelatin with methacrylic anhydride. From a 10% (w/v) gelatin solution in PBS (pH 7.5) at 50° C, methacrylic anhydride was added slowly (0.2 mL/min) under vigorous stirring until a 20% v/v gelatin solution was formed. The resulting solution was diluted 1:2 in PBS and dialyzed for 3 days in Milli-Q water at 40° C. The product was freeze-dried to obtain a powder in varying amounts [20]. Treatment of the culture surfaces was performed by dissolving 0.1 g of GelMA in 2 ml of PBS, plus 500 μL of culture medium and 100 μL of Irgacure (photoinitiator) 4:5:1, 1.5 ml of the mixture was deposited per well in six-well plates, and irradiated with UV 0.2 joule.
2.4. Electrical Stimulation
As shown in Figure 1, the electrical stimulation system consists of an arbitrary wave generator connected to a stimulation module composed of parallel-positioned carbon electrodes adapted for six-well plates. Plates previously treated with hydrogels were subjected to electrical stimulation by four types of stimuli, ES1 (200mV 50Hz), ES2 (400mV 50Hz), ES3 (200mV 100Hz), and ES4 (400mV 100Hz), for two hours per day of experimentation [21,22].
2.5. AFM Measurements
Two different AFMs were used to study the roughness and mechanical properties of four hydrogel solutions: Collagen, Alginate, PEGDA and GELMA. The collagen sample was measured using a Flex AFM (Nanosurf Inc., Switzerland), while alginate, PEGDA, and GelMA were measured with a NanoWizard3 (JPK, Germany). The AFM chips used for this research were qp-BioAC by NanoSensors (USA). These AFM chips have three different cantilevers with different elastic constants and dimensions. Each cantilever has a tip radius of around 10 nm and a Uniqprobe coating that consists of a reflective gold layer. This coating is applied to the backside of the cantilever, which faces the detector. This coating helps reduce the bending of the AFM cantilever and its chemical stability when measuring in liquid environments. In particular, the AFM measurements were performed in a PBS solution.
For the four samples, we use the same measurement protocol, which is described as follows. First, we use AFM tapping mode to make topographical images of the hydrogel surface [23]. Then, we perform force-volume spectroscopy to obtain the nanomechanical properties over the previously imaged region [24]. Calibration of the tip’s spring constants is made using the thermal noise method [25]. Force-indentation curves generated by this method are then fitted using the Hertz, model obtaining the surface’s Young’s modulus as a fitting parameter [26].
2.6. Statistical Analysis
The experimental design included 9 independent determinations per electrical stimulus condition and hydrogel type. The plotted results are represented with the mean and standard deviation (SD). ANOVA performed statistical tests plotted as (∗ p< 0.05; ∗∗ p< 0.01 and ∗∗∗ p<0.001). Post-hoc Tukey multiple comparisons tests were performed to determine interactions between factors affecting the dependent variable [27,28]. Analysis of variances and their ratios were calculated by Tukey post hoc tests using OriginLab® software.
3. Results and Discussion
3.1. PC12 Cells Viability on Hydrogels
The viability of PC12 cells, measured by WST1 assay, shows the effect of the surfaces coated with the different hydrogels in independent experiments three times: 24 hours, 48 hours, and 72 hours, shown in Figure 2A–C, respectively. In the assay, adjusted to the number of cells, it is delivered without significant differences in viability for plates coated with Collagen and GelMA concerning the control without hydrogel. In contrast, in a second group, experiments with plates coated with PEGDA and Alginate show a progressive reduction of viability (75.5± 3.8% and 72.7± 3.6% at 48 hours and 69.8± 4.5% and 67.5± 4.7% at 72 hours respectively). For these assays, the measurement after 4 hours of incubation of the WST1-treated cultures was used as time zero, which allows the establishment of an inference base better fitting the experimental conditions.
Behavior against differentiation where it has been used in combination with polypyrrole or graphite to improve its electrical conductivity [29,30], as an encapsulation medium [31,32] or with peptide modifications to improve the PC12 differentiation process [33], similar to what occurs with the use of PEGDA when been combined with polyaniline and carbon nanotubes to improve electrical conductivity [34,35] or with polylysine to improve cell adhesion [36]. On the other hand, the viability of PC12 cells on GelMA is similar to what occurs with collagen, which is the reference surface for culturing PC12 cells [17,37]
3.2. Differentiation of PC12 Cells: Topographical Hydrogel Characterization
Although there are antecedents of the use of these hydrogels as a substrate for PC12 cell growth, the difference in the behavior of the cultures and cell viability is remarkable; in this sense, Alginate shows the most significant reduction in viability, with Morphological characteristics associated with PC12 cell differentiation were measured based on the different hydrogels used. For this assay, the methodology commonly used for PC12 cell differentiation was homologized, which includes modification of the serum composition in the culture medium, addition of NGF, and treatment of the culture surfaces using collagen, whereas, for the assays, the latter was exchanged for the hydrogels studied. Morphological changes of PC12 cell differentiation associated with neurite outgrowth include neurite length and the percentage of cells expressing neurites. The results show a significant effect of the hydrogels used on neural differentiation. Specifically, regarding neurite length, in cell cultures where collagen was used, the lengths were greater on average 64.5± 7.1 mm, followed by GelMA 35.3± 5.7 mm, alginate 24.8± 3.4 mm and PEGDA 19.1± 4.5 mm (Figure 3A). This behavior was not correlatable with the measurement of the percentage of cells expressing neurite, and these results show that for cultures with PEGDA the percentage was 80.8± 3.8%, for collagen 76.3± 4.7%, for GelMA 64.5± 5.1%, and alginate 13.7± 2.8% (Figure 3B). Among the cultures analyzed, the significant differences are expressed in asterisks in the bar graphs as described in materials and methods. The microphotographs in Figure 3C–F show typical behaviors for the different cultures analyzed in this study.
Cell behavior depends on the substrate surfaces' physical, chemical, and topographical characteristics, creating a favorable interaction dependent on the prior binding of specific serum proteins to the surfaces. [38,39]. In the differentiation of PC12 cells, as in other similar cell types, the extension of neurites is closely related to the mechanical stress and stiffness of the substrates where they proliferate and differentiate, affecting axonal strain. [40,41], however, these conditions show complex correlations where several factors influence in an indeterminate and difficult-to-predict way the behavior of neural development [37,42], which ends up affecting the interaction between the neural growth cone and the surface, which induces actin polymerization in the cytoskeleton. [43]. In our results, the mechanical properties determined by AFM show Young's modulus for the different hydrogels shown in Table 1 and Figure 4.
Considering these results, it is impossible to infer that the differences in the neural differentiation behavior shown in Figure 3 are significantly due to the stiffness of the different hydrogels, whose values are in similar ranges to those reported for using hydrogels. [44,45].
Roughness is another critical parameter in defining the topographical characteristics of the surfaces used for cell growth [46]. Roughness affects the adsorption of surface-binding proteins, which play an elemental role in cell adhesion [47]; however, the significant magnitude of the effects of changes in roughness is often evaluated in a particular way, mainly because changes in cell behavior respond to multiple variables [48]. There could be a threshold or limit of roughness dimensions that affect the magnitude of cellular responses; in this sense, this limit would depend on cell types and culture conditions [49].
Roughness is usually measured to determine two highly relevant parameters, the average surface roughness (Ra) and mean-square-roughness (RMS). Ra represents the arithmetic mean of the absolute values of the profile height deviations from the mean line recorded within the evaluation length. In contrast, RMS represents the mean-square-mean of the profile height deviations from the mean line recorded within the evaluation length [50].
3.3. Characterization of the Electrical Conductivity of Hydrogels
The hydrogels used in the present investigation are recognized biocompatible polymers used in tissue engineering and PC12 cell culture; however, there needs to be more background information on their comparative behavior concerning electrical conductivity. Electrical stimulation is considered a central element in developing biomaterials due to the different precedents demonstrating the relationship between the activation of different intracellular signaling pathways and the positive effect on cell behavior [61]. One of the critical aspects is the determination and modulation of the electrical conductivity of the biomaterials [62].
In the present investigation, the electrical conductivity was determined by measuring the conductivity variations in a circuit connected to the culture medium as a control. The results show a significant reduction in electrical conductivity for all hydrogels evaluated concerning the control. However, the conductivity was reduced to a lesser extent in PEGDA, which showed significant differences concerning alginate, GelMA, and collagen hydrogels (Figure 5). Hydrogels generally tend to have poor electrical conduction compared to culture media. Due to this, several alternatives have been evaluated to improve these properties through different strategies, such as chemical modification and doping of polymers, the addition of metallic nanoparticles, or the addition of carbon nanostructures. However, these alternatives modify the biological and toxicological properties, which could limit the use of these hydrogels [63,64,65,66,67].
3.4. Differentiation of PC12 Cells: Effect of Electrical Stimulation
In the present research for the different culture systems with GelMA, collagen, alginate, and PEGDA hydrogels, the effect of electrical stimulation was evaluated in independent experiments based on described methods. The stimulation conditions correspond to; ES1 (200mV 50Hz); ES2 (400mV 50Hz); ES3 (200mV 100Hz) and ES4 (400mV 100Hz). The results are shown separately for each hydrogel.
Figure 6 shows the effect of electrical stimulation on neural differentiation of PC12 cells cultured in collagen. The results show that the length of neurites concerning the control are significantly lower for conditions ES1, ES2, and ES3, while for ES4, the levels are comparable (Figure 6A). Regarding the percentage of cells expressing neurites (Figure 6B), electrical stimulation does not show significant differences in the ES1 condition. At the same time, it is significantly lower for the ES2, ES3, and ES4 conditions for the control. The effects on neural differentiation in PC12 cells related to electrical stimulation are insignificant or appear harmful when cultures are grown on collagen. This situation seems to be related to the low electrical conductivity of collagen, so several reports indicate the need to modify the hydrogel to improve this property [68,69]
On the other hand, the electrical stimulation results on cultures in alginate hydrogels show a significant increase in the length of neurites for the control, mainly associated with conditions ES3 and ES4 (Figure 7A). Regarding the percentage of cells expressing neurites, the results show that electrical stimulation conditions significantly favor neural differentiation, mainly associated with ES2 and ES3 conditions (Figure 7B).
The results show a significant increase in neural differentiation in alginate hydrogels without the need to modify the conductive properties of the hydrogel. Other investigations show results under other stimulation conditions but with the need to modify the electrical conduction characteristics, for example, by generating hybrids with polypyrrole or carbon nanotubes, which ostensibly improves the capabilities of hydrogels to promote neural differentiation using electrical stimulation [70,71].
The results of experiments performed on PEGDA show a significant effect of electrostimulation on neural differentiation (Figure 8). Graph 8A indicates that electrical stimulation, especially under the ES4 condition, increases neurite length compared to the control. On the other hand, graph 8B provides insight into the proportion of cells that are differentiating and expressing neurites. The ES2 and ES4 stimulation conditions show a high level of neurite expression, similar to the control. Under these conditions, neural differentiation is subject to a specific frequency independent of voltage. Several precedents demonstrate the dependence of electrical frequency on cellular activity, which promotes proliferation and differentiation. These investigations suggest establishing a delicate balance between electrical conditions to promote differentiation, which depends on several factors in the culture, such as cell type. [72,73,74].
Finally, the electrical stimulation results on GelMA cultures show increased neural differentiation (Figure 9). Regarding neurite length, for ES2 and especially ES4 conditions, higher levels of neurite length are shown, a trend similar to that observed with PEGDA and electrical frequency dependence. Regarding the percentage of cells expressing neurites, all electrical stimulation conditions significantly increase concerning the control (Figure 9A). Our results show the capacity of GelMA as a hydrogel capable of promoting neural differentiation efficiently in conjunction with electrical stimulation without the need to modify the polymer, unlike some investigations that include copolymerization with polypyrrole or the inclusion of carbon structures [75,76].
3.5. Statistical Analysis
The results were analyzed using a multiple pairwise comparisons ANOVA test, considering two factors: type of hydrogel and type of electrical stimulus, each with four different levels. Figure 10 shows the ANOVA interaction plot using the percentage of cells expressing neurites as a dependent variable. Regarding the interaction between the hydrogel and electrical stimulus, Figure 10A, the cells in collagen show a relatively stable percentage of expression throughout the four electrical stimuli, approximately 80%. The cells have a markedly lower response to alginate than the other hydrogels. Although there is a slight increase in ES3, the percentage of expression is still considerably lower, below 40%. For PEGDA and GelMA, meanwhile, similar response patterns are shown in these two hydrogels across electrical stimuli, although GelMA generally has a slightly higher percentage of neurite expression. Notably, both hydrogels peak at ES3, indicating that this specific stimulus potentiates neurite expression in cells cultured on these hydrogels.
The results of the interaction between the hydrogel and dependent variable (Figure 10B) show how the dependent variable (percentage of cells expressing neurites varies as a function of the hydrogel. In this regard, collagen has the highest percentage, followed closely by GelMA, while alginate has the lowest percentage, consistent with its low response in the electrical stimulus plot. Finally, PEGDA shows an intermediate response. The overall interaction interpretation shows that the response of PC12 cells in terms of neurite expression depends not only on the type of hydrogel but also on the type of electrical stimulus to which they are subjected. While collagen appears to be the most conducive hydrogel for neurite expression under all conditions, GelMA and PEGDA show a particularly favorable response to the ES3 electrical stimulus. In contrast, alginate consistently shows a low ability to promote neurite expression, regardless of the electrical stimulus.
On the other hand, ANOVA interaction analysis considering neurite length as a dependent variable (Figure 11) shows that in the interaction between the hydrogel and electrical stimulus when cells are cultured on collagen, the neurite length fluctuates along the electrical stimuli, with a peak in ES3 and a valley in ES4. In alginate, the cells show a consistently low neurite length, regardless of electrical stimulus, and remain below the other hydrogels. For the experiments with PEGDA, a noticeable peak is shown at ES2 and a valley at ES3, while GelMA neurite length increases with ES2 and ES3, the latter being the highest peak among all hydrogels at ES3.
When analyzing the interaction between hydrogel and the dependent variable (Figure 11B), it is depicted how neurite length varies directly as a function of hydrogel without a specific electrical stimulus. Collagen and PEGDA have similar lengths, while alginate has the shortest length and GelMA has the longest. As an overall analysis, the length of neurites expressed by PC12 cells is influenced by the type of hydrogel in which they are cultured and the type of electrical stimulus to which they are subjected. GelMA is particularly conducive to promoting increased neurite length, especially under ES3 electrical stimulus. Alginate consistently shows a low ability to promote neurite extension under all electrical stimulus conditions. Cells in collagen and PEGDA show mixed responses depending on the electrical stimulus, but generally, they tend to have longer neurite lengths than alginate but shorter than GelMA.
4. Conclusions
The expression of neurites in PC12 cells is influenced by both the type of hydrogel and the type of electrical stimulus. Collagen consistently appears to be the most conducive hydrogel for promoting neurite expression across all conditions, with a stable high percentage of expression. GelMA and PEGDA also exhibit favorable responses, particularly in response to the ES3 electrical stimulus, with GelMA showing slightly higher neurite expression percentages. In contrast, alginate consistently demonstrates a low ability to promote neurite expression, regardless of the electrical stimulus. The interaction between the hydrogel and electrical stimulus reveals interesting patterns when considering neurite length as the dependent variable. Collagen shows fluctuations in neurite length across electrical stimuli. Alginate consistently maintains a lower neurite length compared to other hydrogels. PEGDA exhibits a notable peak at ES2 and a valley at ES3, while GelMA demonstrates an increase in neurite length with ES2 and ES3, with ES3 yielding the highest peak among all hydrogels. This inconsistent behavior could have its origin in surface or chemical phenomena, which were not considered in the present study, revealing the need to investigate these analyses further to understand the nature of these events. However, this research reveals significant, unknown results that help understand and direct future research..
Author Contributions
“Conceptualization, Yusser Olguín; methodology, Yusser Olguín, Mónica Selva, Diego Benavente, Diego Jaramillo, Nicole Orellana; validation, Tomas Corrales, Carolina Otero, Ivan Montenegro, Alejandro Madrid; formal analysis, Yusser Olguín, Cristian Acevedo, Tomas Corrales, Alejandro Madrid, Iván Montenegro, Carolina Otero; investigation, Yusser Olguín and Cristian Acevedo; writing Yusser Olguín et al.
Funding
This research was funded by FONDECYT 11201056.and ANID PIA/APOYO AFB220004.
Acknowledgments
We thank the Dr. Daniel Alcalay Biotechnology center, where most of this research was developed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H. , et al. Tissue engineering and regenerative medicine: Achievements, future, and sustainability in asia. Frontiers in bioengineering and biotechnology 2020, 8, 83. [Google Scholar] [CrossRef]
- Zhang, P.X.; Han, N.; Kou, Y.H.; Zhu, Q.T.; Liu, X.L.; Quan, D.P.; Chen, J.G.; Jiang, B.G. Tissue engineering for the repair of peripheral nerve injury. Neural regeneration research 2019, 14, 51–58. [Google Scholar]
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert review of neurotherapeutics 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Annals of biomedical engineering 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Materials today. Bio 2023, 20, 100668. [Google Scholar] [CrossRef]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J. , et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioactive materials 2021, 6, 638–654. [Google Scholar] [CrossRef]
- Ma, F.; Xiao, Z.; Chen, B.; Hou, X.; Dai, J.; Xu, R. Linear ordered collagen scaffolds loaded with collagen-binding basic fibroblast growth factor facilitate recovery of sciatic nerve injury in rats. Tissue engineering. Part A 2014, 20, 1253–1262. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, H.X.; Li, X.; Xu, W.J.; Yin, Y.X.; Dai, H.L.; Wang, X.B.; Huang, Z.J.; Xu, P.H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC advances 2018, 8, 10806–10817. [Google Scholar] [CrossRef]
- Chapla, R.; Alhaj Abed, M.; West, J. Modulating functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.S.; Pereda, A.E. Two forms of electrical transmission between neurons. Frontiers in molecular neuroscience 2018, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical stimulation promotes stem cell neural differentiation in tissue engineering. Stem cells international 2021, 2021, 6697574. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue engineering. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef] [PubMed]
- Trueman, R.P.; Ahlawat, A.S.; Phillips, J.B. A shock to the (nervous) system: Bioelectricity within peripheral nerve tissue engineering. Tissue engineering. Part B, Reviews 2022, 28, 1137-1150.
- Yung, H.S.; Lai, K.H.; Chow, K.B.; Ip, N.Y.; Tsim, K.W.; Wong, Y.H.; Wu, Z.; Wise, H. Nerve growth factor-induced differentiation of pc12 cells is accompanied by elevated adenylyl cyclase activity. Neuro-Signals 2010, 18, 32-42.
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. Pc12 cell line: Cell types, coating of culture vessels, differentiation and other culture conditions. Cells 2020, 9. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable rgd-modified peg hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rutten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal differentiation from induced pluripotent stem cell-derived neurospheres by the application of oxidized alginate-gelatin-laminin hydrogels. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nature protocols 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Ashok, A.; Tai, W.L.; Lennikov, A.; Chang, K.; Chen, J.; Li, B.; Cho, K.S.; Utheim, T.P.; Chen, D.F. Electrical stimulation alters DNA methylation and promotes neurite outgrowth. Journal of cellular biochemistry 2023. [Google Scholar] [CrossRef]
- Zhang, Q.; Beirne, S.; Shu, K.; Esrafilzadeh, D.; Huang, X.F.; Wallace, G.G. Electrical stimulation with a conductive polymer promotes neurite outgrowth and synaptogenesis in primary cortical neurons in 3d. Scientific reports 2018, 8, 9855. [Google Scholar] [CrossRef]
- Hansma, P.; Cleveland, J.; Radmacher, M.; Walters, D.; Hillner, P.; Bezanilla, M.; Fritz, M.; Vie, D.; Hansma, H.; Prater, C. , et al. Tapping mode atomic force microscopy in liquids. Applied Physics Letters 1994, 64, 1738–1740. [Google Scholar] [CrossRef]
- Hugel, T.; Seitz, M. The study of molecular interactions by afm force spectroscopy. Macromolecular Rapid Communications 2001, 22, 989–1016. [Google Scholar] [CrossRef]
- Hutter, J.; Bechhoefer, J. Calibration of atomic-force microscope tips. Review of Scientific Instruments 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Dintwa, E.; Tijskens, E.; Ramon, H. On the accuracy of the hertz model to describe the normal contact of soft elastic spheres. Granular Matter 2008, 10, 209–221. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Dolci, L.S.; Mangano, C.; Giardino, L.; Gualandi, C.; Focarete, M.L.; Calza, L. In vitro testing of biomaterials for neural repair: Focus on cellular systems and high-content analysis. BioResearch open access 2016, 5, 201–211. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Multiple comparison analysis testing in anova. Biochemia medica 2011, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Molino, B.Z.; Chung, J.H.Y.; Pannell, J.T.; Kuester, M.; Molino, P.J.; Hanks, T.W. Synthesis and 3d printing of conducting alginate-polypyrrole ionomers. Gels 2020, 6. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.Y.; Young, T.H.; Yang, H.J.; Ji, Y.R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydrate polymers 2019, 224, 115112. [Google Scholar] [CrossRef]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate composition effects on a neural stem cell-seeded scaffold. Tissue engineering. Part C, Methods 2009, 15, 541-550.
- Buyukoz, M.; Erdal, E.; Alsoy Altinkaya, S. Nanofibrous gelatine scaffolds integrated with nerve growth factor-loaded alginate microspheres for brain tissue engineering. Journal of tissue engineering and regenerative medicine 2018, 12, e707–e719. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.Y. Dual peptide-presenting hydrogels for controlling the phenotype of pc12 cells. Colloids and surfaces. B, Biointerfaces 2017, 152, 36-41.
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive pani/pegda macroporous hydrogels for nerve regeneration. Advanced healthcare materials 2013, 2, 218–227. [Google Scholar] [CrossRef]
- He, L.; Lin, D.; Wang, Y.; Xiao, Y.; Che, J. Electroactive swnt/pegda hybrid hydrogel coating for bio-electrode interface. Colloids and surfaces. B, Biointerfaces 2011, 87, 273-279.
- Cai, L.; Lu, J.; Sheen, V.; Wang, S. Promoting nerve cell functions on hydrogels grafted with poly(l-lysine). Biomacromolecules 2012, 13, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiang, Y.; Fang, J.; Li, X.; Lin, Z.; Dai, G.; Yin, J.; Wei, P.; Zhang, D. The influence of the stiffness of gelma substrate on the outgrowth of pc12 cells. Bioscience reports 2019, 39. [Google Scholar] [CrossRef]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The evolution of polystyrene as a cell culture material. Tissue engineering. Part B, Reviews 2018, 24, 359-372.
- Zuniga-Aguilar, E.; Olayo, R.; Ramirez-Fernandez, O.; Morales, J.; Godinez, R. Nerve cells culture from lumbar spinal cord on surfaces modified by plasma pyrrole polymerization. Journal of biomaterials science. Polymer edition 2014, 25, 729–747. [Google Scholar] [PubMed]
- Wen, Y.Q.; Gao, X.; Wang, A.; Yang, Y.; Liu, S.; Yu, Z.; Song, G.B.; Zhao, H.C. Substrate stiffness affects neural network activity in an extracellular matrix proteins dependent manner. Colloids and surfaces. B, Biointerfaces 2018, 170, 729-735.
- Franze, K.; Janmey, P.A.; Guck, J. Mechanics in neuronal development and repair. Annual review of biomedical engineering 2013, 15, 227–251. [Google Scholar] [CrossRef]
- Athamneh, A.I.; Suter, D.M. Quantifying mechanical force in axonal growth and guidance. Frontiers in cellular neuroscience 2015, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. Journal of neurochemistry 2014, 129, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness sensing by cells. Physiological reviews 2020, 100, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Doron, G.; Levy, M.J.; Temenoff, J.S. Hydrogel culture surface stiffness modulates mesenchymal stromal cell secretome and alters senescence. Tissue engineering. Part A 2020, 26, 1259–1271. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef]
- Covani, U.; Giacomelli, L.; Krajewski, A.; Ravaglioli, A.; Spotorno, L.; Loria, P.; Das, S.; Nicolini, C. Biomaterials for orthopedics: A roughness analysis by atomic force microscopy. Journal of biomedical materials research. Part A 2007, 82, 723–730. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth - facile surface modification for enhanced cell culture. RSC advances 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.; Schon, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Meijome, J.M.; Lopez-Alemany, A.; Almeida, J.B.; Parafita, M.A. Surface afm microscopy of unworn and worn samples of silicone hydrogel contact lenses. Journal of biomedical materials research. Part B, Applied biomaterials 2009, 88, 75-82.
- Kontomaris, S.V.; Stylianou, A.; Malamou, A. Atomic force microscopy nanoindentation method on collagen fibrils. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano letters 2020, 20, 748–757. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.; Pretzer, R.; Montazami, R.; Montazami, N. Shear at fluid-fluid interfaces affects the surface topologies of alginate microfibers. Clean Technology 2019, 1, 265–272. [Google Scholar] [CrossRef]
- Munz, M. Microstructure and roughness of photopolymerized poly(ethylene glycol) diacrylate hydrogel as measured by atomic force microscopy in amplitude and frequency modulation mode. Applied Surface Science 2013, 279, 300–309. [Google Scholar] [CrossRef]
- Yu., X.; Zhu, X.; Li, J.; Wu, Z.; Wang, Y.; Liu, F. The recent developments and applications of the traceless-staudinger reaction in chemical biology study. RSC Advances 2015, 130, 107192-108066.
- Askari, F.; Zandi, M.; Shokrolahi, P.; Tabatabaei, M.H.; Hajirasoliha, E. Reduction in protein absorption on ophthalmic lenses by pegda bulk modification of silicone acrylate-based formulation. Progress in biomaterials 2019, 8, 169–183. [Google Scholar] [CrossRef]
- Lai, J.Y.; Luo, L.J.; Ma, D.H. Effect of cross-linking density on the structures and properties of carbodiimide-treated gelatin matrices as limbal stem cell niches. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta biomaterialia 2012, 8, 3080–3090. [Google Scholar] [CrossRef]
- Wandiyanto, J.V.; Linklater, D.; Tharushi Perera, P.G.; Orlowska, A.; Truong, V.K.; Thissen, H.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Juodkazis, S., et al. Pheochromocytoma (pc12) cell response on mechanobactericidal titanium surfaces. Materials 2018, 11.
- Simitzi, C.; Stratakis, E.; Fotakis, C.; Athanassakis, I.; Ranella, A. Microconical silicon structures influence ngf-induced pc12 cell morphology. Journal of tissue engineering and regenerative medicine 2015, 9, 424–434. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomaterials research 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Testore, D.; Zoso, A.; Kortaberria, G.; Sangermano, M.; Chiono, V. Electroconductive photo-curable pegda-gelatin/pedot:Pss hydrogels for prospective cardiac tissue engineering application. Frontiers in bioengineering and biotechnology 2022, 10, 897575. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S. Electrically conducting hydrogels for health care: Concept, fabrication methods, and applications. International journal of bioprinting 2020, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Lim, J.H.; Lee, J.M.; Chung, B.G. Electro-responsive conductive blended hydrogel patch. Polymers 2023, 15. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Pareta, R.; Harrison, B.S. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface focus 2014, 4, 20130050. [Google Scholar] [CrossRef]
- Kaklamani, G.; Kazaryan, D.; Bowen, J.; Iacovella, F.; Anastasiadis, S.H.; Deligeorgis, G. On the electrical conductivity of alginate hydrogels. Regenerative biomaterials 2018, 5, 293–301. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3d bioprinting of neural tissues. Advanced healthcare materials 2021, 10, e2001600. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jang, L.; Kim, S.; Yang, J.; Yang, K.; Cho, S.W.; Lee, J.Y. Polypyrrole/alginate hybrid hydrogels: Electrically conductive and soft biomaterials for human mesenchymal stem cell culture and potential neural tissue engineering applications. Macromolecular bioscience 2016, 16, 1653–1661. [Google Scholar] [CrossRef]
- Ma, H.; Yu, K.; Wang, H.; Liu, J.; Cheng, Y.Y.; Kang, Y.; Wang, H.; Zhang, J.; Song, K. Fabrication and detection of a novel hybrid conductive scaffold based on alginate/gelatin/carboxylated carbon nanotubes (alg/gel/mmwcnts) for neural tissue engineering. Tissue & cell 2023, 80, 101995.
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Advanced biomedical research 2016, 5, 97. [Google Scholar]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of osteogenesis in mc3t3-e1 cells by different frequency electrical stimulation. PloS one 2016, 11, e0154924. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; Roshankhah, S.; Mardani, M.; Hashemibeni, B.; Naghsh, E.; Kazemi, M.; Salahshoor, M. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iranian journal of basic medical sciences 2014, 17, 571–576. [Google Scholar] [PubMed]
- Dutta, S.D.; Ganguly, K.; Randhawa, A.; Patil, T.V.; Patel, D.K.; Lim, K.T. Electrically stimulated 3d bioprinting of gelatin-polypyrrole hydrogel with dynamic semi-ipn network induces osteogenesis via collective signaling and immunopolarization. Biomaterials 2023, 294, 121999. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liu, E.W.; Lin, Y.J.; Ng, H.Y.; Lee, J.J.; Hsu, T.T. The synergistic effect of electrical stimulation and dermal fibroblast cells-laden 3d conductive hydrogel for full-thickness wound healing. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef]
Figure 1.
Graphical representation of culture system and electrical stimulation in six-well plate format.
Figure 1.
Graphical representation of culture system and electrical stimulation in six-well plate format.

Figure 2.
Bar graph of cell viability levels by WST1 test. A-C represents the results of measurements at 24, 48 and 72 hours, respectively.
Figure 2.
Bar graph of cell viability levels by WST1 test. A-C represents the results of measurements at 24, 48 and 72 hours, respectively.

Figure 3.
Neural differentiation of PC12 cells according to topography. A. Bar graph of neurite length measurements. B. Bar graph of the percentage of cells expressing neurites. C-F. Representative microphotographs of PC12 cells in collagen, alginate, PEGDA, and GelMA, respectively.
Figure 3.
Neural differentiation of PC12 cells according to topography. A. Bar graph of neurite length measurements. B. Bar graph of the percentage of cells expressing neurites. C-F. Representative microphotographs of PC12 cells in collagen, alginate, PEGDA, and GelMA, respectively.

Figure 4.
A-D. AFM topographical image alginate, collagen, GelMA and PEGDA respectively. E-H. Young's modulus histograms of alginate, collagen, GelMA, and PEGDA, respectively.
Figure 4.
A-D. AFM topographical image alginate, collagen, GelMA and PEGDA respectively. E-H. Young's modulus histograms of alginate, collagen, GelMA, and PEGDA, respectively.
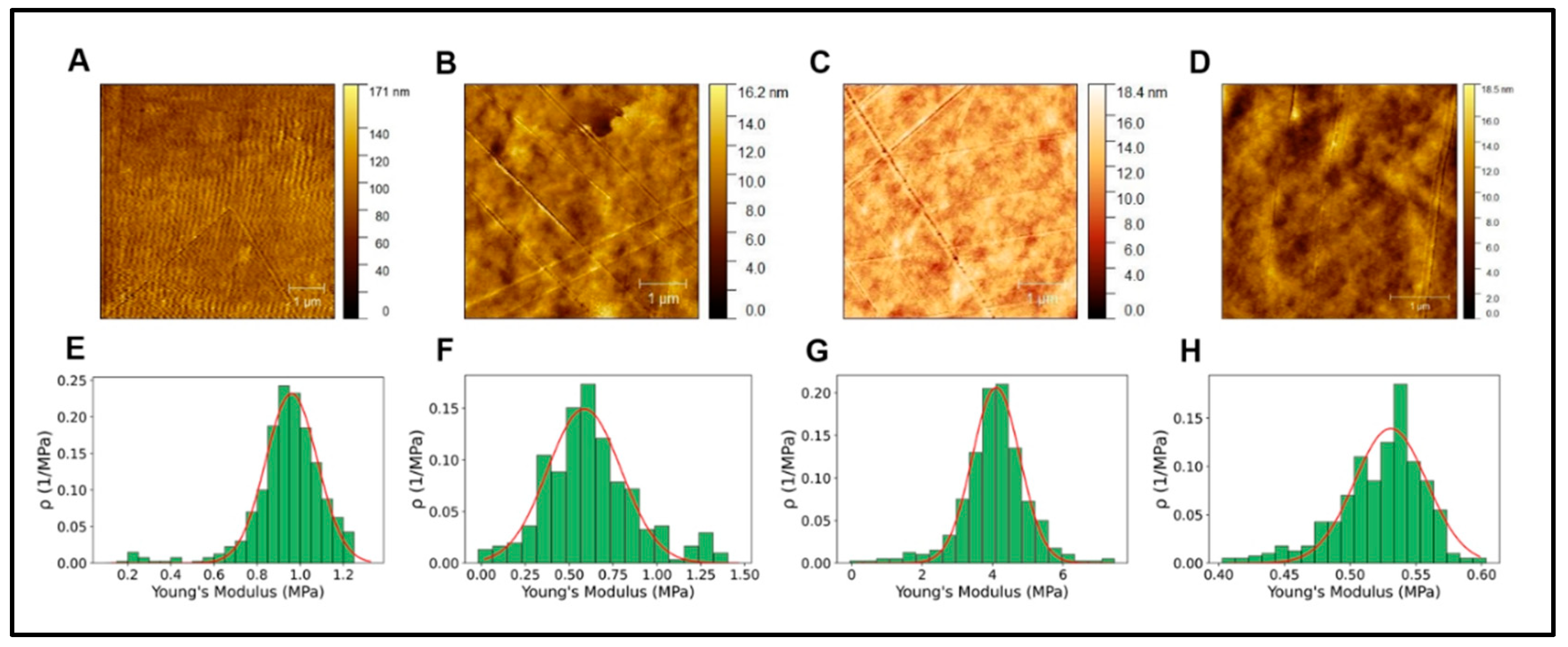
Figure 5.
Bar graph of the variation of electrical conductivity in different hydrogels.

Figure 6.
Neural differentiation in collagen. A. Bar graph of neurite length. B. Bar graph of the percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).
Figure 6.
Neural differentiation in collagen. A. Bar graph of neurite length. B. Bar graph of the percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).

Figure 7.
Neural differentiation in alginate. A. Bar graph of neurite length. B. Bar graph of percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).
Figure 7.
Neural differentiation in alginate. A. Bar graph of neurite length. B. Bar graph of percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).

Figure 8.
Neural differentiation in PEGDA. A. Bar graph of neurite length. B. Bar graph of percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).
Figure 8.
Neural differentiation in PEGDA. A. Bar graph of neurite length. B. Bar graph of percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).
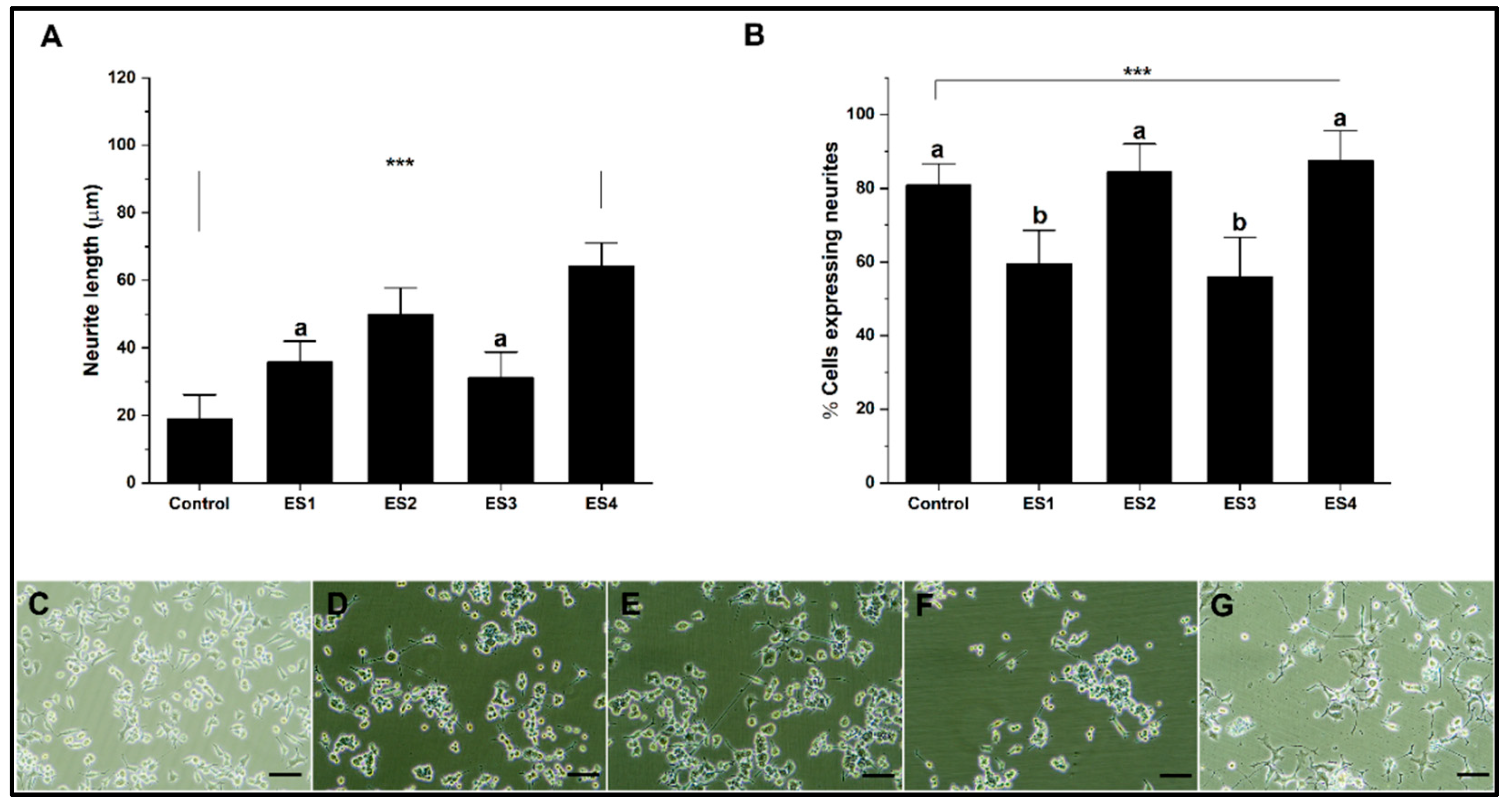
Figure 9.
Neural differentiation in GelMA. A. Bar graph of neurite length. B. Bar graph of the percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).
Figure 9.
Neural differentiation in GelMA. A. Bar graph of neurite length. B. Bar graph of the percentage of cells expressing neurites. C-G. Representative micrographs of control, ES1, ES2, ES3 and, ES4, respectively. (reference bar 50 μm).

Figure 10.
ANOVA Tukey interaction plot. Effect of factors on the percentage of cells expressing neurites. A. type of electrical stimulation. B. type of hydrogel.
Figure 10.
ANOVA Tukey interaction plot. Effect of factors on the percentage of cells expressing neurites. A. type of electrical stimulation. B. type of hydrogel.

Figure 11.
ANOVA Tukey interaction plot. Effect of factors on the percentage of cells expressing neurites. A. type of electrical stimulation. B. type of hydrogel.
Figure 11.
ANOVA Tukey interaction plot. Effect of factors on the percentage of cells expressing neurites. A. type of electrical stimulation. B. type of hydrogel.

Table 1.
AFM measurements of topographical parameters.
| Collagen | GelMA | PEGDA | Alginate | |
|---|---|---|---|---|
| Ra | 11.4 nm | 1.3 nm | 1.2 nm | 1.6 nm |
| RMS | 14.61 nm | 1.6 nm | 1.6 nm | 2.0 |
| Young’s modulus (MPa) | 0.96 ± 0.11 | 0.59 ± 0.21 | 4.10 ± 0.67 | 0.53 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering
Yusser Olguín
et al.
,
2023
Erectile Dysfunction Treatment using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model
Hyong Woo Moon
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated











