Preprint
Article
Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori
Altmetrics
Downloads
99
Views
18
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.docx (123.25KB )
This version is not peer-reviewed
Submitted:
06 November 2023
Posted:
06 November 2023
You are already at the latest version
Alerts
Abstract
Trehalose is a non-reducing disaccharide and participates in physiological activities such as or-gan formation, energy metabolism, and stress resistance of insects. In the present study, phylo-genetic analysis divided 21 Bmtret1 orthologs into three clades. These genes are equally distrib-uted on the nine chromosomes. Cis-elements in the promoter regions of Bmtret1s indicated the possible function of Bmtret1s in response to hormones and environmental stimulus. The qPCR analysis showed the significantly different expression levels of Bmtret1s in different tissues and organs, indicating possible functional divergence. In addition, most Bmtret1s showed disturbed expression levels in response to BmNPV stresses. Our results provide a foundation for further functional dissection of Tret1s in Bombyx mori and implicate them as potential regulators for an-tiviral responses.
Keywords:
Subject: Biology and Life Sciences - Insect Science
1. Introduction
The silkworm, considered a model invertebrate creature, was the first insect used for silk production in human history and was widely used throughout domestication [1]. However, many viral diseases can pose a serious threat to the growth and development of silkworm [2,3]. Silkworm nuclear polyhedrosis virus (BmNPV) infection is a major threat to sericulture and can cause serious economic losses [4,5,6]. When BmNPV infects a host, two types of virus particles are produced: early budding virions (BVS), which are transmitted primarily between cells, and late occlusion-derived virions (ODVs), which are transmitted primarily between hosts [7,8]. ODV virus particles are packaged in polyhedra of a highly symmetric covalent crosslinked lattice [9]. BmNPV mainly infects silkworm larvae through the mouth. The polyhedron is alkaline lysed by the host intestinal environment, and the enteric membrane is destroyed by viruses to form pores [10]. The nucleocapsid protein of the virus enters the columnar epithelial cells of the host midgut through envelope-mediated membrane fusion, triggering primary infection. The nucleocapsid protein enters the nucleus under the traction of actin, undergoes transcription, and completes the assembly of the progeny viral nucleocapsid in the nucleus [11]. The mature progeny nucleocapsid enters the cytoplasm through the nuclear pore, obtains the host cell membrane structure under the traction of capsid protein, completes the growth process, and forms a new progeny virus. Late during infection, the progeny ODV is re-embedded in the polyhedron and released into the environment after the death and disintegration of the host. In recent decades, extensive research has been conducted to enrich our understanding of the molecular mechanisms of silkworm resistance to BmNPV infection [12,13]. However, the molecular mechanism of its antiviral activity has not been fully elucidated.
Trehalose, also known as fungose, is a non-reducing disaccharide formed by connecting two glucose molecules via an α, α-1, 1-glucoside bond. It is found in a wide variety of organisms, including bacteria, fungi, insects, plants, and invertebrates [14,15]. Due to its unique chemical properties, trehalose has the advantage of protecting organisms from a variety of environmental stresses such as cold, oxidation, hypoxia, and drying [16]. Trehalose is the main hemolymph sugar of most insects [17], accounting for 80%-90% of the total hemolymph sugar content. It is synthesized in the adipose body, an organ similar to the mammalian liver and adipose tissue, and is released into the hemolymph [18,19]. Trehalose plays an important role in the growth and stress resistance of organisms, so some people call trehalose "the sugar of life".
Trehalose metabolic pathways (synthesis, transport, and decomposition) have been extensively studied in insects. After feeding insects, sucrose can be hydrolyzed into fructose and glucose in the gut [20]. Insects tend to ingest excessive amounts of sucrose, most of which is converted into long-chain oligosaccharides and excreted as honeydew [21]. The remaining sucrose is used for energy metabolism and maintenance of osmotic balance [22]. Glucose is transported to the fat body via GLUT and participates in the synthesis of trehalose by TPS/TPP [23]. Trehalose cannot directly cross the cell membrane [24], but depends on the specific trehalose transporter TRET1 for facilitated diffusion into the cell [25]. Kanamori et al. showed that the Tret1 gene family is relatively conserved in insects, encoding proteins with different dynamic properties and participating in the release of trehalose from adipose bodies and its introduction into other tissues [26]. The Tret1 gene has been cloned from Polypedilum vanderplanki, Anopheles gambiae, and Nilaparvata lugens. Kikawada et al. isolated and characterized Tret1 from insects and found that trehalose synthesized in fat bodies was transported into hemolymph [27]. Trehalose is hydrolyzed into two glucose monomers by alginase in hemolymph and transported to tissues in the blood to meet energy requirements [28]. Studies on trehalose transporters have mainly focused on energy metabolism and stress resistance, but there are few studies on the antiviral mechanisms underpinning insect trehalose transport [29].
In this study, we conducted transcriptomic profiling and bioinformatics analysis of the silkworm trehalose transporter Bmtret1 gene family and found it a candidate key gene family for silkworm BmNPV resistance in BmNPV susceptible species (Baiyu, BY). This information prompted us to analyze the expression of the sugar transporter gene in susceptible cultivars and its relationship to viral susceptibility. We also analyzed the homologous genes of Bmtret1 and their phylogenetic relationships to investigate their function in Bombyx mori. Through bioinformatic approaches, this study explores the functions of the silkworm Bmtret1 family, and provides a data reference for studying the molecular mechanisms behind insect virus resistance.
2. Results
2.1. Genome-wide identification and phylogenetic analysis of the Bmtret1s in B. mori
Based on the silkworm genome information, 21 Bmtret1 homologs were identified. These Bmtret1 homologs encode proteins of 204 to 591 amino acids with molecular weights of 23.18 to 65.47 kDa and theoretical isoelectric points of 4.83 to 9.48. Based on WoLFPSORT prediction of subcellular localization, most of them (18/21) likely localize to the plasma membrane (PM) (Table 1). According to the amino acid sequence, each sample is divided into three clades, and the samples within the same clade are highly related (Figure 1).
2.2. Chromosomal localization of Bmtret1s
The target genes were mainly distributed on chromosomes 5, 7, 13, 14, 17, 20, 26, 27 and 28. Chromosome 27 has the most target genes, followed by chromosomes 20 and 26 (Figure 2). No active tandem genes and gene replication pairs were found in the preliminary screening, while the results need to be further verified.
2.3. Sequence analysis of the Bmtret1s
Conserved regions in the Bmtret1 proteins were identified by multiple sequence alignment analysis of the amino acid sequence. The alignment and conserved motif analysis showed that the tret1 gene family retained four conserved sites, indicated by red highlighting (Figure 3).
2.4. Gene organization and promoter analysis of Bmtret1s
Cis-acting elements are present in the peripheral sequences of genes that affect gene expression. Cis-acting elements include promoters, enhancers, regulatory sequences, and inducible elements, which are involved in the regulation of gene expression. The cis-acting element itself does not encode any protein, but merely provides an action site that interacts with trans-acting factors. According to the annotation information of the silkworm genome, the molecular characteristics of the Bmtret1 genes were analyzed, and a phylogenetic tree was constructed to identify possible functional elements in each gene. Tret1s exon was located, and the sample sequence was basically located in the exon region, suggesting that it was involved in the regulation of this gene expression. As can be seen in the distribution map of cis-elements in the Tret1s promoter region (Figure 4), stress response-related elements were identified in multiple samples.
2.5. Expression profile of Bmtret1s in different tissues of silkworm
To determine the tissue and organ expression profile of Bmtret1s, the relative expression levels of Bmtret1 genes in silkworm blood (Hemocyte, HE), midgut (Midgut, MG), fat body (Fat Body, FB), posterior silk gland (Posterior Silk Gland, PS), and head (Head, HD) were measured by qRT-PCR with silkworm actin 3 as the internal reference. The expression of BMSK0011446 in the phylogenetic branch in these tissues was relatively low and is not shown (Figure 6 same). Gene expression patterns in phylogenetic Branch I varied greatly, with BMSK0011410 having the highest expression in the midgut (MG), BMSK0011573 having the highest expression in the posterior silk gland (PS), blood (HE), and posterior midgut (MG), and BMSK0011404 having the highest expression in the head (HD) (Figure 5a–c).
While the expression pattern of Bmtret1 in the phylogenetic clade is more diverse, the highest expressed Bmtret1s in the midgut (MG) were BMSK0003818, BMSK0015729, BMSK0015638 and BMSK0015673, where BMSK0015729 and BMSK0015638 had similar expression patterns, The expression levels of each tissue were ordered from high to low: midgut (MG), posterior silk gland (PS), head (HD), fat body (FB) and blood (HE) (Figure 5d,q,s,t); BMSK0015120, BMSK0002683 and BMSK0002685 were the highest in fat body (FB), with the latter two having similar expression patterns (Figure 5i–k). Four genes (BMSK0015774, BMSK0015674, BMSK0015633 and BMSK0015627) had the highest expression level in the posterior silk glands (PS). With the exception of high expression of BMSK0015627 in the midgut (MG), the expression patterns were very similar. All other tissues had relatively low expression levels (Figure 5g,n,p,r). The remaining genes have the highest expression in the head (HD). There are two patterns of expression. The expression pattern of BMSK0009966, BMSK0015122 and BMSK0007748 was similar to others in the phylogenetic branch I, except for having higher expression in the head (HD) and fat body (FB) (Figure 5e,f,o,c). The other type of Bmtret1s (BMSK0015118, BMSK0008304, and BMSK0012519) were the highest expressed in the fat body (FB) and Midgut (MG) (Figure 5h,l,m).
2.6. Transcriptional level responses of Bmtret1s to BmNPV stress
The relative expression of Bmtret1 genes in the Hemocyte (HE), Midgut (MG), and Fat Body (FB) 24h after infection with BmNPV was determined by qRT-PCR. BmNPV is a viral disease caused by infection with polyhydrosis virus. All genes responded to infection with BmNPV. In the fat body (FB), the expression level of 16/20 Bmtret1s was increased and 4/20 was decreased. In the midgut (MG), 15/20 Bmtret1 genes were upregulated, 3/20 downregulated, and 2/20 unchanged. A different pattern was observed in the blood, where 12/20 Bmtret1s were downregulated. Only one gene (BMSK0015729) showed an upregulated level in the blood after infection (Figure 6).
Nine out of the 20 genes (BMSK0011410, BMSK0011404, BMSK0003818, BMSK0015122, BMSK0015774, BMSK0015120, BMSK0002683, BMSK0002685, and BMSK0008304) had a similar expression pattern, being upregulated in both the midgut (MG) and the fat body (FB). These genes are also closely related on the phylogenetic tree (Figure 6a,c,d,f,g,i–l, graph 1). With differences in their expression levels in the blood (HE), the nine genes can be divided into two expression pattern groups. Five genes (BMSK0011404, BMSK0003818, BMSK0015122, BMSK0015120, and BMSK0002685) showed an insignificant response in the blood (HE), while the remaining four genes were downregulated. Five other genes (BMSK0009966, BMSK0015118, BMSK0012519, BMSK0015627, and BMSK0015638) showed similar expression profiles to each other after BmNPV biological stress, with their expression levels being upregulated in the midgut and downregulated in the fat body (Figure 6e,h,m,r,s).
3. Discussion
Nuclear polyhedrosis virus disease of silkworm is highly infectious and harmful. It is the most common and most harmful silkworm disease in silkworm rearing and production, and causes serious economic losses every year [30,31]. Over the years, many researchers have been committed to screening and breeding resistant silkworm varieties and discovering resistance genes to elucidate the molecular mechanism of silkworm resistance to BmNPV [32]. With the development of biotechnology, more achievements have been made in the study of B. mori's resistance to BmNPV virus at the molecular level, and further studies have been made on genes or proteins that may be involved in the antiviral mechanism. After the completion of silkworm genome sequencing, gene chip technology has become an important gene expression analysis method, which has the advantages of large throughput and high accuracy. Zhou et al. detected 92 differentially expressed genes in the intestinal tissues of silkworm varieties BC8 and 306 after 12h of toxic treatment with nucleic acid probes. They further analyzed 10 up-regulated genes by fluorescent quantitative PCR. Fluorescence quantitative PCR technology can quickly compare and analyze the expression of all genes in the sample [33]. BmS3A is related to the inhibition of apoptosis of infected cells, which inhibits the replication of viruses [34]. SOP2 gene may promote actin polymerization process and affect virus replication [35]. We selected midgut tissues of a conventional susceptible strain of silkworm Baiyu infected with BmNPV for second-generation RNA-Seq transcriptome sequencing, for systematic screening of candidate differentially expressed genes involved in BmNPV infection resistance.
Trehalose, as a new type of natural sugar, can be used as a protective factor to protect the organism from external environmental stresses or internal metabolic disorders. TRET, the trehalose transporter, can transport trehalose from the fat body to the hemolymph, and plays an important role in insect stress resistance [36,37]. While TRET plays an important role in the resistance to numerous insect stresses, there are very few studies on the effects of TRET on virus infection. Some studies have speculated that trehalose transprotein-1 (Tret1) gene may be related to transport of the virus during the interaction between gray planthopper and rice stripe virus [38]. In addition, the trehalose transprotein-1 (BmTret1-like) gene of silkworm plays a specific role in the mechanism of BmNPV virus resistance [39]. Recent studies have shown that the expression of BmTret1-X1 gene has a clear inhibitory effect on the expression of viral genes in BmNPV [40]. The transcriptome results found that the expression level of Bmtret1s significantly responded to BmNPV biological stress, and we speculated that Bmtret1s may play an important role in the infection of BmNPV.
In this study, it was found that the Bmtret1 gene family varied greatly in different tissues with possible functional differences. Because trehalose is involved in the process of silkworm epidermis formation, it is speculated that the high expression level in the head may be associated with ecdysone and juvenile hormone. The higher expression in the two detoxifying organs of the midgut and fat body indicates that Bmtret1s may participate in the molecular mechanism of disease resistance. In BmNPV-susceptible varieties of white jade silkworm, the vast majority of Bmtret1 genes are downregulated in response to BmNPV oral infection. We speculate that the downregulation of the trehalose transporter gene in the blood allows for BmNPV invasion and is the cause for susceptibility. Furthermore, high expression of Bmtret1s in the midgut and fat body correlates with viral resistance in these two detoxification organs.
4. Materials and Methods
4.1. Sericulture breeding and virus preparation
Baiyu was acquired from the Resources Center of Silviculture Research Institute, Chinese Academy of Agricultural Sciences. All silkworm larvae were raised with fresh mulberry leaves. The worms were raised at 27 ± 1℃ at 75 ± 5% relative humidity under a 12h day and night cycle. The BmNPV strain was maintained in our laboratory and purified according to the protocol reported by Rahman et al. [8]. After the starvation treatment, the experimental group was fed 7ul of BmNPV suspension (2×108 polyhedra / ml), and the control group was fed normally. The blood, midgut, and fat body of larvae from both groups were taken 24h after infection. After 72h, the hemocyte, midgut, fat body, posterior silk gland, and head of the control group were collected. Three biological replicates were taken and stored at -80℃ after infection.
4.2. Identification of the Bmtret1 gene family in B. mori
Sequences homologous to the Bmtret1 genes were downloaded from the silkworm genome database. Their chromosomal distribution and homology relationships were analyzed. The Biological Toolbox v1.098774 was used to analyze the sequence length, molecular weight, and theoretical isoelectric point (PI) values for each homologous gene. The distribution of TM helices was determined by TMHMM Server v.2.0 Forecast. Subcellular localization of the Bmtret1s protein was predicted using the online tool WoLF PSORT.
4.3. Chromosomal localization and homology analysis of Bmtret1s
The chromosomal location information of the Bmtret1 gene family was extracted from the silkworm genome annotation file. This operation was performed and visualized in Tbtools v1.098774.
4.4. Sequence alignment
Bmtret1 protein sequences were aligned using clustalW and assembled in MEGA11.0.
4.5. Phylogenetic analysis of Bmtret1s
A neighbor-joining (NJ) phylogenetic tree was constructed using full-length Bmtret1 protein sequences from B. mori and using MEGA11.0 and JTT + G models, with bootstrap tests with 1000 replicates.
4.6. RNA extraction and quantitative Real-Time PCR (qRT-PCR) analysis
According to the manufacturer's instructions, EASYspin Tissue/Cell RNA Rapid Extraction Kit (Aidlab, China) and HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) were used for RNA extraction and cDNA synthesis.
RT-qPCR was performed using ABI StepOnePlus™ Real-Time PCR System (USA) to verify the expression patterns of Bmtret1 in different tissues at different times. The primers used are shown in Supplemental Table S1.
5. Conclusions
We conducted transcriptome and phylogenetic analysis of the silkworm Bmtret gene family and performed expression profiling and transcript level analysis after infection with BmNPV. The Bmtret1 gene family has been implicated in silkworm resistance against BmNPV, and the high expression of most Bmtret1s in the midgut and fat body may inhibit the gene transcription of BmNPV and DNA replication, and thus reduce the assembly efficiency of virions to resist BmNPV infection. The Bmtret1 gene family of silkworm trehalose transporter was preliminarily identified as a key candidate gene family of silkworm BmNPV resistance, and the specific mechanism needs further study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
G.D. Zhao and H.Y. Qian conceived and designed the experiments; M.J. Lin, Y.X. Qian, E.X. Chen and M.J. Wang performed the experiments. G. Ouyang and Y. Xu analyzed the data. M.J. Lin and G.D. Zhao wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation of Post Scientist in National Sericultural System (CARS-18-ZJ0101) and the Natural Science Foundation of Jiangsu Province (BK20201229).
Acknowledgments
We thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, K.; Dong, Z.; Pan, M. Common strategies in silkworm disease resistance breeding research. Pest Manag Sci 2023, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Luan, JB.; Li, JM.; Varela, N.; et al. Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J Virol 2011, 85, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Chen, HQ.; Yao, Q.; Bao, F.; et al. Comparative proteome analysis of silkworm in its susceptibility and resistance responses to Bombyx mori densonucleosis virus. Intervirology 2012, 55, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fei, S.; Xia, J.; et al. Sirt5 Inhibits BmNPV Replication by Promoting a Relish-Mediated Antiviral Pathway in Bombyx mori. Front Immunol 2022, 13, 906738. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, ZQ.; Dong, FF.; et al. Gene editing the BmNPV inhibitor of apoptosis protein 2 (iap2) as an antiviral strategy in transgenic silkworm. Int J Biol Macromol 2021, 166, 529-537. [CrossRef]
- Cao, HH.; Zhang, SZ.; Zhu, LB.; et al. The digestive proteinase trypsin, alkaline A contributes to anti-BmNPV activity in silkworm (Bombyx mori). Dev Comp Immunol 2021, 119, 104035. [CrossRef]
- Katsuma, S.; Mita, K.; Shimada, T. ERK and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J Virol 2007, 81, 13700-13709. [CrossRef]
- Rahman, MM.; Gopinathan, Kp. Systemic and in vitro infection process of Bombyx mori nucleopolyhedrovirus. Virus Res 2004, 101, 109-118. [CrossRef]
- Braunagel, SC.; Summers, MD. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr Drug Targets 2007, 8, 1084-1095. [CrossRef]
- Blissard, GW. Baculovirus--insect cell interactions. Cytotechnology 1996, 20, 73-93. [CrossRef]
- Wang, Y.; Wang, Q.; Liang, C.; et al. Autographa californica multiple nucleopolyhedrovirus nucleocapsid protein BV/ODV-C42 mediates the nuclear entry of P78/83. J Virol 2008, 82, 4554-4561. [CrossRef]
- Bao, YY.; Tang, XD.; Lv, ZY.; et al. Gene expression profiling of resistant and susceptible Bombyx mori strains reveals nucleopolyhedrovirus-associated variations in host gene transcript levels. Genomics 2009, 82, 138-145. [CrossRef]
- Mei, X.; Li, C.; Peng, P.; et al. Bombyx mori C-Type Lectin (BmIML-2) Inhibits the Proliferation of B.mori Nucleopolyhedrovirus (BmNPV) through Involvement in Apoptosis. Int J Mol Sci 2022, 23, 8369. [CrossRef]
- Elbein, AD.; Pan, YT.; Pastuszak, I.; Carroll, D.; et al. New insights on trehalose: a multifunctional molecule. Glycobiology 2003, 13, 17-27. [CrossRef]
- Chen, A.; Tapia, H.; Goddard, JM.; Gibney, PA.; et al. Trehalose and its applications in the food industry. Compr Rev Food Sci Food Saf 2022, 21, 5004-5037. [CrossRef]
- Crowe, JH.; Carpenter, JF.; Crowe, LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol 1998, 60, 73-103. [CrossRef]
- Wyatt, GR.; Kale, GF. The chemistry of insect hemolymph.II.Trehalose and other carbohydrates. J Gen Physiol 1957, 40, 833-847. [CrossRef]
- Candy, DJ.; Kilby, BA. Site and mode of trehalose biosynthesis in the locust. Nature 1959, 183, 1594-1595. [CrossRef]
- Murphy, TA.; Wyatt, GR. The enzymes of glycogen and trehalose synthesis in silk moth fat body. J Biol Chem 1965, 240, 1500-1508. [CrossRef]
- Kikuta, S.; Nakamura, Y.; Hattori, M.; et al. Herbivory-induced glucose transporter gene expression in the brown planthopper, Nilaparvata lugens. Insect Biochem Mol Biol 2015, 64, 60-67. [CrossRef]
- Ashford, DA.; Smith, WA.; Douglas, AE. Living on a high sugar diet: the fate of sucrose ingested by a phloem-feeding insect, the pea aphid Acyrthosiphon pisum. J Insect Physiol 2000, 46, 335-341. [CrossRef]
- Fraga, A.; Ribeiro, L.; Lobato, M.; Santos, V.; Silva, J.R.; Gomes, H.; da Cunha Moraes, J.L.; de Souza Menezes, J.; de Oliveira, C.J.; et al. Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum. PloS one 2013, 8, e65125. [CrossRef]
- Tang, B.; Wang, S.; Wang, SG.; Wang, HJ.; et al. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front Physiol 2018, 9, 30. [CrossRef]
- García de Castro, A.; Tunnacliffe, A. Intracellular trehalose improves osmotolerance but not desiccation tolerance in mammalian cells. FEBS Lett 2000, 487, 199-202. [CrossRef]
- Stambuk, BU.; Panek, AD.; Crowe, JH.; Crowe, LM.; et al. Expression of high-affinity trehalose-H+ symport in Saccharomyces cerevisiae. Biochim Biophys Acta 1998, 1379, 118-128. [CrossRef]
- Kanamori, Y.; Saito, A.; Hagiwara-Komoda, Y.; et al. The trehalose transporter 1 gene sequence is conserved in insects and encodes proteins with different kinetic properties involved in trehalose import into peripheral tissues. Insect Biochem Mol Biol 2010, 40, 30-37. [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci U S A 2007, 104, 11585-11590. [CrossRef]
- Takiguchi, M.; Niimi, T.; Su, ZH.; Yaginuma, T. Trehalase from male accessory gland of an insect, Tenebrio molitor. cDNA sequencing and developmental profile of the gene expression. Biochem J 1992, 266, 19-22. [CrossRef]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S.; et al. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic Biochem Physiol 2017, 137, 81-90. [CrossRef]
- Harrison, RL.; Herniou, EA.; Jehle, JA.; et al. ICTV Virus Taxonomy Profile: Baculoviridae. J Gen Virol 2018, 99, 1185-1186. [CrossRef]
- Lange, M.; Wang, H.; Zhihong, H.; Jehle, JA. Towards a molecular identification and classification system of lepidopteran-specific baculoviruses. Virology 2004, 325, 36-47. [CrossRef]
- Buhroo, Z. A review: Disease resistance in mulberry silkworm Bombyx mori.L. Asian Journal of Science and Technology 2013, 4, 157-166.
- Zhou, Y.; Gao, L.; Shi, H.; et al. Microarray analysis of gene expression profile in resistant and susceptible Bombyx mori strains reveals resistance-related genes to nucleopolyhedrovirus. Genomics 2013, 101, 256-262. [CrossRef]
- Jiaping, Xu.; et al. Identification and characterization of Bms3a in Bombyx mori L. African Journal of Biotechnology 2008, 34, 24-30.
- Xu, JP.; Chen, KP.; Yao, Q.; et al. Identification and characterization of an NPV infection-related gene Bmsop2 in Bombyx mori L. Journal of Applied Entomology 2005, 129, 425-431. [CrossRef]
- Kanamori, Y.; Saito, A.; Hagiwara-Komoda, Y.; Tanaka, D.; Mitsumasu, K.; Kikuta, S.; et al. The trehalose transporter 1 gene sequence is conserved in insects and encodes proteins with different kinetic properties involved in trehalose import into peripheral tissues. Insect Biochem Mol Biol 2010, 40, 30-37. [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci U S A 2007, 104, 11585-11590. [CrossRef]
- Weidong, YU.; Biying, PAN.; Lingyu, QIU.; et al. The structure characteristics and biological functions on regulating trehalose metabolism of two NlTret1s in Nilaparvata lugens. Scientia Agricultura Sinica 2020, 53, 4802-4812. [CrossRef]
- Jianhua, Yang. Transcriptome analysis of midgut tissue infected by BmNPV and functional identification of BmTret1-like gene. MA thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2017.
- Qiuyun, Song. Identification of resistance of silkworm BmTret1-X1 gene to Bombyx mori nucleopolyhedrovirus (BmNPV). MA thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2022.
Figure 1.
Phylogenetic relationships of the TRET1 family genes in Bombyx mori. The sequences of the 21 TRET1 proteins from the above insect were aligned by Clustal Omega, and the phylogenetic tree was constructed by MEGA 11.0 using the NJ method with 1000 bootstrap replicates.
Figure 1.
Phylogenetic relationships of the TRET1 family genes in Bombyx mori. The sequences of the 21 TRET1 proteins from the above insect were aligned by Clustal Omega, and the phylogenetic tree was constructed by MEGA 11.0 using the NJ method with 1000 bootstrap replicates.

Figure 2.
Distribution of Bmtret1 genes in Bombyx mori chromosomes. The scale is provided in megabase (Mb).
Figure 2.
Distribution of Bmtret1 genes in Bombyx mori chromosomes. The scale is provided in megabase (Mb).

Figure 3.
Multiple sequence alignment of Bmtret1 proteins.

Figure 4.
Gene organization of Bmtret1s and cis-elements in promoter regions of Bmtret1s. (a) Phylogenetic tree using 21 Bmtret1s. (b) Exon/intron structures of Bombyx mori TRET1s. (c) Cis-element distribution in the promoter regions of Bmtret1s.
Figure 4.
Gene organization of Bmtret1s and cis-elements in promoter regions of Bmtret1s. (a) Phylogenetic tree using 21 Bmtret1s. (b) Exon/intron structures of Bombyx mori TRET1s. (c) Cis-element distribution in the promoter regions of Bmtret1s.

Figure 5.
Transcript levels of Bmtret1s in hemocyte, midgut, fat body, posterior silk gland, and head of Bombyx mori. Three technical replicates were analyzed. Different letters indicate statistically significant differences (Duncan's test, p < 0.05).
Figure 5.
Transcript levels of Bmtret1s in hemocyte, midgut, fat body, posterior silk gland, and head of Bombyx mori. Three technical replicates were analyzed. Different letters indicate statistically significant differences (Duncan's test, p < 0.05).
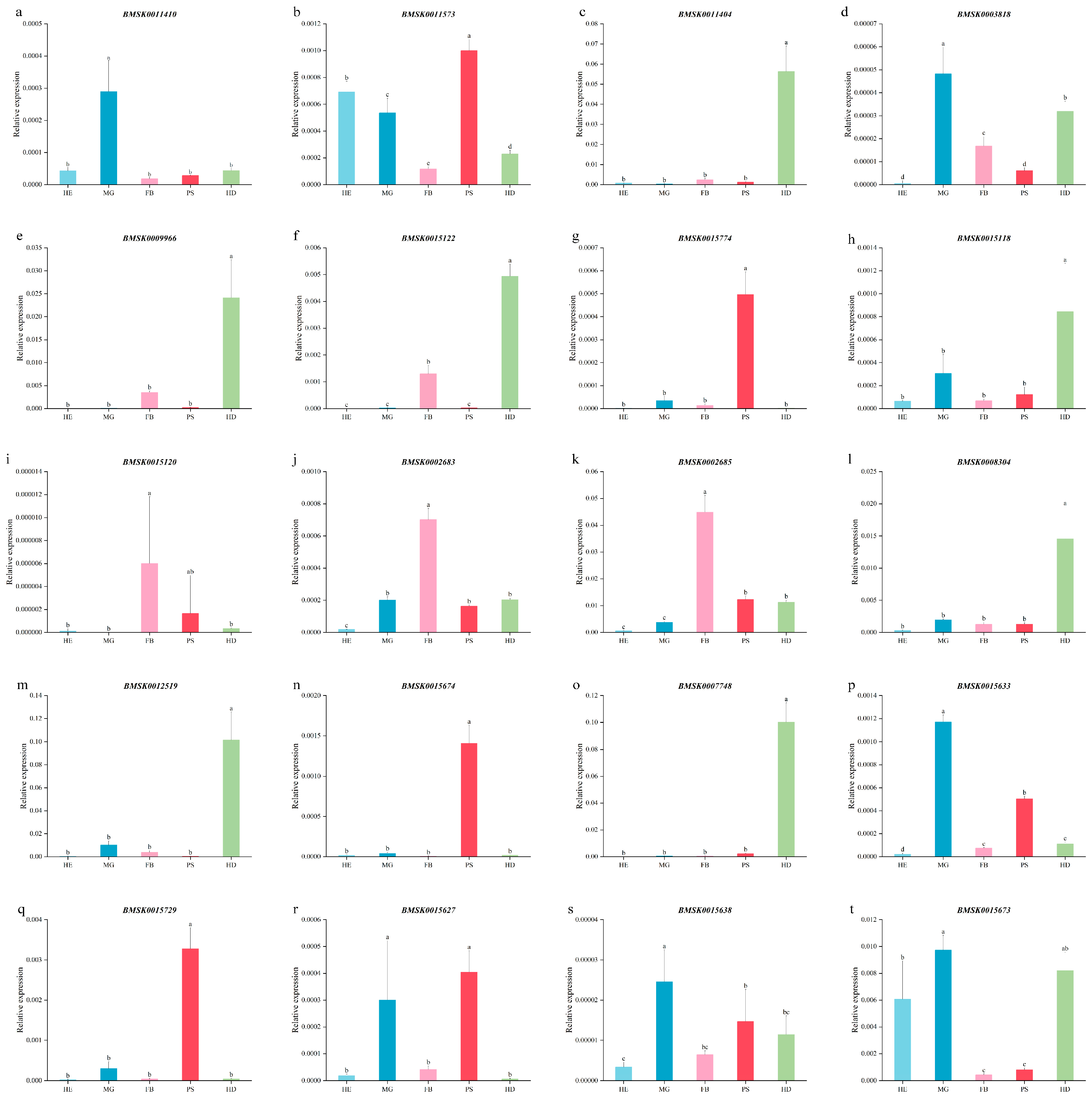
Figure 6.
Expression levels of 20 Bmtret1 genes in response to BmNPV stress conditions. Three technical replicates were analyzed. Asterisks indicate significant differences as determined by Student’s t-test (* p < 0.05; ** p < 0.01; *** p < 0.001).
Figure 6.
Expression levels of 20 Bmtret1 genes in response to BmNPV stress conditions. Three technical replicates were analyzed. Asterisks indicate significant differences as determined by Student’s t-test (* p < 0.05; ** p < 0.01; *** p < 0.001).
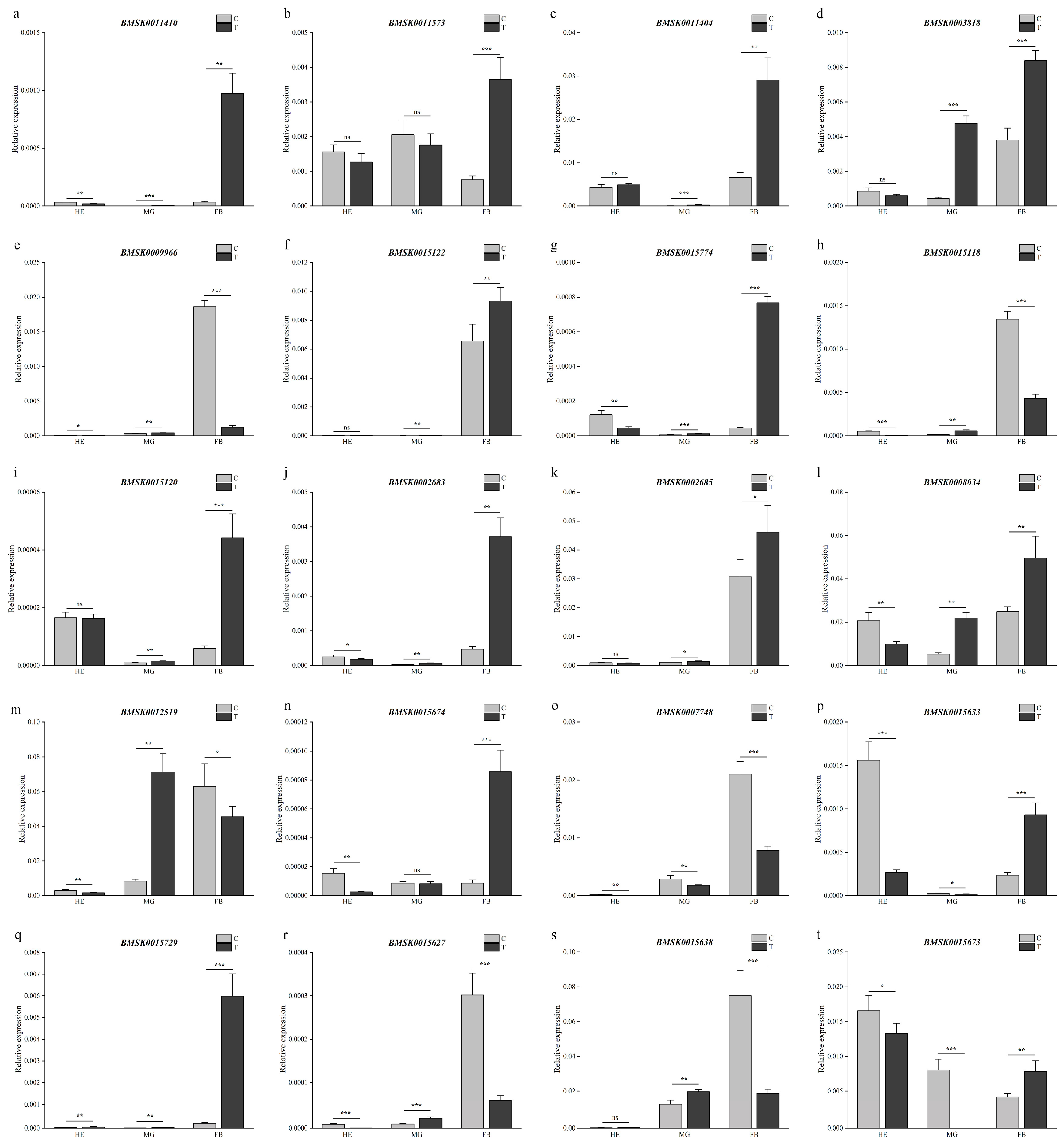
Table 1.
TRET1 gene family in Bombyx mori.
| Gene ID | CDS Size (bp) | Protein physicochemical characteristics | TMHs | Subcellular localization* |
|||
|---|---|---|---|---|---|---|---|
| Length (aa) | MW (kDa) | pI | Aliphatic index | ||||
| BMSK0011410 | 1443 | 480 | 51.74 | 9.31 | 116.67 | 12 | PM |
| BMSK0011573 | 1155 | 384 | 42.90 | 6.02 | 107.16 | 7 | PM |
| BMSK0011404 | 1401 | 466 | 50.77 | 8.31 | 111.33 | 12 | PM |
| BMSK0011446 | 1632 | 543 | 58.85 | 7.84 | 100.72 | 9 | PM |
| BMSK0003818 | 1524 | 507 | 56.44 | 7.55 | 112.76 | 11 | PM |
| BMSK0009966 | 615 | 204 | 23.18 | 8.28 | 101.18 | 4 | EX |
| BMSK0015122 | 1635 | 544 | 58.66 | 9.48 | 113.86 | 11 | PM |
| BMSK0015774 | 1233 | 410 | 44.81 | 8.17 | 108.24 | 10 | PM |
| BMSK0015118 | 1275 | 424 | 46.07 | 4.83 | 115.26 | 11 | PM |
| BMSK0015120 | 1374 | 457 | 49.34 | 6.59 | 117.13 | 12 | PM |
| BMSK0002683 | 1353 | 450 | 49.19 | 8.61 | 116.42 | 11 | PM |
| BMSK0002685 | 1776 | 591 | 65.47 | 9.15 | 98.65 | 10 | PM |
| BMSK0008304 | 1359 | 452 | 49.90 | 8.74 | 102.23 | 10 | PM |
| BMSK0012519 | 1368 | 455 | 49.99 | 9.42 | 100.26 | 10 | PM |
| BMSK0015674 | 1494 | 497 | 54.72 | 9.05 | 107.95 | 10 | MT |
| BMSK0007748 | 1398 | 465 | 51.74 | 9.05 | 108.04 | 12 | PM |
| BMSK0015633 | 1608 | 535 | 58.76 | 8.92 | 103.20 | 12 | PM |
| BMSK0015729 | 684 | 227 | 26.00 | 5.21 | 92.69 | 2 | CY |
| BMSK0015627 | 1368 | 455 | 50.32 | 9.08 | 125.34 | 11 | PM |
| BMSK0015638 | 1512 | 503 | 56.54 | 9.28 | 104.10 | 12 | PM |
| BMSK0015673 | 1515 | 504 | 55.87 | 9.08 | 113.57 | 10 | PM |
* The subcellular localizations were predicted by WoLFPSORT. MT, Mitochondrial; PM, Plasma Membrane; EX, Extracellular; CY, Cytoplasmic.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori
Mingjun Lin
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







