Preprint
Article
Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns
Altmetrics
Downloads
77
Views
30
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.zip (3.49MB )
This version is not peer-reviewed
Submitted:
17 November 2023
Posted:
21 November 2023
You are already at the latest version
Alerts
Abstract
Pregnancy at advanced maternal age is considered a risk factor for adverse maternal, fetal, and neonatal outcomes. Here we investigated whether maternal age could be associated with differences in the blood levels of newborn screening (NBS) markers for inborn metabolic disorders on the Recommended Universal Screening Panel (RUSP). Population-level NBS data from screen-negative singleton infants were examined, which included blood metabolic markers and covariates such as gestational age, birth weight, age at blood collection, infant sex, parent-reported ethnicity, and maternal age at delivery. Marker levels were compared between maternal age groups (age range: 15-44 years) using effect size analyses, which controlled for group size differences and potential confounding from other covariates. Our results showed that 13% of the markers had maternal age-related differences including newborn metabolites with either increased (C14, C16, C18, C18:1, C3DC) or decreased (C5OH) levels at advanced maternal age group (≥35 years, absolute Cohen’s d ≥ 0.2). The increased C3DC levels in this group correlated with a higher false-positive rate in newborn screening for malonic acidemia (p-value < 0.001), while no significant difference in screening performance was seen for other markers. Maternal age at delivery is associated with inborn metabolic differences and should be considered together with other clinical variables in genetic disease screening.

Keywords:
Subject: Biology and Life Sciences - Other
1. Introduction
The age of first-time mother’s has been increasing in the United States with the mean maternal age for the first childbirth increasing from 21.4 to 27.1 years from 1970 to 2020 [1,2]. In 2020, nearly 11% of women had their first child at the age of 35 and older compared to 0.25% in 1970 [2,3]. Similar trends have been found worldwide with demographic models predicting further increases in maternal age [4,5,6,7]. Observational research suggests that pregnancy later in life is a risk factor for adverse maternal, fetal, and neonatal outcomes [7,8]. For example, advanced maternal age has been associated with complications such as placenta previa, gestational diabetes mellitus, hypertensive disorders of pregnancy, and higher risk for intra-uterine growth restriction, prematurity, and chromosomal abnormalities [9,10,11]. Maternal medical conditions, complications during pregnancy, vaginal delivery versus cesarean section, and environmental stressors such as fetal tobacco exposure have previously been found to influence neonatal metabolism and adaptation [12,13,14,15,16].
In this study, we investigated whether maternal age (MA) could be associated with differences in the blood levels of newborn screening (NBS) markers for inborn metabolic disorders on the Recommended Universal Screening Panel (RUSP) [17]. Accurate detection of these disorders through newborn screening allows for rapid clinical diagnosis and management [18,19]. Importantly, as other covariates including gestational age, birth weight, age at blood collection, infant sex, and parent-reported ethnicity status may concurrently influence neonatal metabolism and development, we accounted for confounding by these covariates and stratified analyses across different maternal age groups. The identified maternal age-related differences in metabolite levels were correlated to false-positive screening cases for inborn errors of metabolism. Based on these findings, maternal age is suggested as an important covariate associated with metabolic differences in newborns that should be considered in the interpretation of newborn metabolic screening data and to support the development of algorithms for genetic disease screening.
2. Materials and Methods
2.1. Data Summary and Preprocessing
NBS data for 503,935 screen-negative singleton infants born between 2013-2017 were provided by the California NBS program. The data included 41 metabolites measured by MS/MS from newborn dried blood spots [20], 5 additional NBS markers including Galactose-1-phosphate uridyl tansferase (TRA), Thyroid-stimulation hormone (TSH), 17-hydroxyprogesterone (OHP), Immunoreactive trypsinogen (IRT), and T cell receptor excision circles (TREC), and 8 covariates of gestational age (GA), birth weight (BW), age at blood collection (AaBC), infant sex, parent-reported ethnicity, total parenteral nutrition (TPN), transfusion status, and maternal age (MA). Infants reported under the following criteria were removed from analysis: (1) BW less than 1000g or larger than 5000g; (2) GA smaller than 28 weeks or larger than 42 weeks; (3) AaBC unknown or before 12 hours or after 168 hours; (4) total parenteral nutrition (TPN) as unknown or positive; (5) red blood cell transfusion status as unknown or positive; and (6) MA under 15 years old (y) or older than 44 y, which resulted in 476,718 infants for analysis (Table S1). Infants with GA smaller than or equal to 36 weeks were classified as preterm birth, and those with GA larger than 36 weeks were classified as term birth. For the ethnicity-stratified analysis, infants with multiple parent-reported ethnicities (17.9%, n = 85,148) and those with unknown ethnicity (2.0%, n = 9,542) were further removed resulting in 382,028 (80.1%) newborns classified to only one of 17 racial/ethnic groups (Asian East Indian, Black, Cambodian, Chinese, Filipino, Guamanian, Hawaiian, Hispanic, Japanese, Korean, Laos, Middle Eastern, Native American, Other Southeast Asian, Samoan, Vietnamese, White) (Table S2). In addition, we analyzed data from first-tier NBS false-positive cases for 3 inborn metabolic disorders reported by the California NBS program, which included malonic acidemia (MAL, n=439), carnitine palmitoyltransferase type II deficiency (CPT-II, n=51), and 3-Methylcrotonyl-CoA carboxylase deficiency (3MCC, n=239). This study was overseen by the institutional review boards at Yale University (protocol #1505015917) and the State of California Committee for the Protection of Human Subjects (protocol #13-05-1236).
2.2. Analysis of Maternal Age
To account for the influence of different covariates on newborn metabolite levels, we first studied the correlation between MA and infant sex, gestational age, and parent-reported ethnicity. We then analyzed newborn metabolic profiles across six MA groups with five years per age group. Blood levels of 46 markers in the first MA group (15-19 y) were used as the baseline to explore the gradual changes in marker levels with increasing MA (Figure 1). Effect size analysis was performed for each of the 46 markers using Cohen's d [21] to calculate marker level differences for the remaining five MA groups in comparison with the baseline group. We used absolute Cohen's d values larger than 0.2 as threshold [21,22] for significant differences between the comparison and the baseline group. We also compared MA-related metabolic differences between the MA group of 35 years or older and the baseline group (15-19 y) [7,15,23].
2.3. Analysis of Maternal Age in Relation to Other Variables
Two representative markers with increasing (C16, C3DC) and one marker with decreasing (C5OH) levels between MA of 20-44 y were selected to study the influence of other covariates on metabolite levels (Figure 2). The three NBS markers were among the metabolites found with the largest changes related to MA in Figure 1. We first excluded all infants under the criteria (1) BW less than 1000g or larger than 5000g; (2) GA smaller than 28 weeks or larger than 42 weeks; (3) AaBC unknown or before 12 hours or after 168 hours; (4) total parenteral nutrition (TPN) as unknown or positive; (5) red blood cell transfusion status as unknown or positive; and (6) MA under the age of 15 or older than 44 y, and then conducted stratified analysis by infant sex, gestational age, and parent-reported ethnicity. Specifically, we compared the changes in metabolite levels related to MA between (1) female and male infants; (2) term and preterm infants; and (3) infants belonging to the four major ethnicity groups including Asian, Black, Hispanic, and White.
2.4. Analysis of Maternal Age-Related Differences and False Positive Results
The three metabolic disorders studied (MAL, CPT-II, and 3MCC) are detected in NBS by elevated marker levels (C3DC, C16, and C5OH). Here we studied whether MA could impact NBS performance in detecting these diseases. In addition to the filtering criteria (1)-(6) described above, this analysis only included infants (n= 405,968) born with a normal birth weight (2500–4000g) and within the range of a term pregnancy (from 37 to 42 weeks) in order to mitigate the confounding from preterm births. The filtering criteria were consistently applied to the false positive data, except for the status of red blood cell transfusion, which was not available. We conducted an effect size analysis for all 46 metabolites to compare the MA ≥35 y group (n = 90,191 infants) to the baseline group (15-19 y, n = 17,063 infants) (Figure 3). For each of the three diseases, the proportion of false positive cases was compared to the proportion of screen negative infants in the MA ≥35 y group using Chi-squared test.
2.5. Statistical Analyses and Software
All statistical analyses and visualizations were conducted in R software 4.1.2 with packages: dplyr [24], effsize [25], ComplexHeatmap [26], ggplot2 [27], and ggpubr [28]. Distributions of MA -related to different clinical variables were visualized in boxplots. The pattern of signature metabolites was visualized using smoothed lines estimated from a generalized additive model [22,29]. Two sample t-tests were performed to check the difference in mean MA across groups. Comparisons of means in more than two groups were conducted using ANOVA [30]. Effect size analyses were conducted using Cohen's d values [21]. Patterns of all metabolites with the increase of MA were visualized using heatmaps. Hierarchical clustering was used to classify MA-related profiles across metabolites. Kolmogorov–Smirnov test [31] was performed to check the enrichment of acylcarnitine (AC) metabolites in the hierarchical clustering results. Proportion tests [32] were performed to check if the proportion of clinical variables in MA groups was the same.
3. Results
3.1. Identification of Metabolic Differences Related to Maternal Age
For MA and infant sex, there was no significant difference in the mean MA between male and female infants (p-value = 0.98) (Figures S1 and S2). For MA and parent-reported ethnicity, we found a significant difference between MA across the major ethnic groups (Asian, Black, Hispanic, White, and Others/Unknown) as well as the 17 detailed parent-reported ethnicity groupings (p-values < 0.001) (Figures S3 and S4). We observed that the Asian and White groups had higher MA compared with Hispanic and Black groups, with the Korean and Japanese subgroups having the highest mean MA (33.9 y and 35.1 y) among all 17 groups. We also identified a significant difference in MA between term and preterm birth (p-value < 0.001) (Figures S5 and S6). To further check the relationship between MA and GA, we visualized the proportion of preterm births and found a significant difference in the proportion across the six MA groups (p-value < 0.001; Figure S7). In addition, we found a significant difference in the proportion of preterm births across MA groups in the Asian, Black, and Hispanic newborn groupings (Figure S8).
The 46 NBS markers were found to cluster into three major groups, according to their changing blood levels in relation to MA (Figure 1). The top cluster includes metabolites with increasing levels compared with the baseline group (positive Cohen's d values; e.g., C16); the bottom cluster includes metabolites with decreasing levels compared with the baseline group (negative Cohen's d values; e.g., C5OH); while metabolites in between showed relative smaller absolute Cohen's d values (e.g., ARG). Several metabolites had non-monotonous patterns such as C0 with initially decreasing and then increasing levels in relation to the increase in MA. Overall, 7 of the 46 markers (C3, C14, C16, C18, C18:1, C3DC, and C5OH) showed significant differences between at least one of the seven MA groups and the baseline group (absolute Cohen’s d > 0.2). Additionally, a significant enrichment of acylcarnitines was found among the top-ranking metabolites in the hierarchical cluster analysis (p-value = 0.0088).
From the seven markers identified with significant differences compared with the baseline group in Figure 1 (absolute Cohen’s d > 0.2), we selected three markers (C16, C3DC, C5OH) to showcase the dynamic metabolic changes associated with MA and other clinical variables including sex, gestational age, parent-reported ethnicity (Figure 2) The three metabolites are NBS markers for the detection of three metabolic disorders (CPT-II, MAL, 3MCC) on the RUSP. For C16, mean blood levels initially increased for MA from 15 to 35 years and then plateaued. C16 levels were higher in males compared to female, while term infants exhibited higher C16 levels than preterm infants. White infants had higher C16 levels compared to other groups, while Asian infants showed a distinct trend of initially increasing levels from 15 to 28 years followed by decreasing levels from 28 to 44 years. For C3DC, mean levels showed similar patterns to C16, with the exception of notably higher C3DC levels in Black and White infants. For C5OH, mean levels monotonously decreased with increasing MA. Infant sex, gestational age, and parent-reported ethnicity all had an influence, with term Black male infants having the highest C5OH mean levels compared to the other groups. Notably, for all three markers, term and male infants had the highest mean levels compared to other groups.
3.2. Correlation of Maternal Age-Related Differences to False-Positive Results
We identified MA-related differences for 13% (6 of 46, Cohen’s d > 0.2) of the metabolites when comparing their levels between the MA group of ≥35 years and the baseline group (Figure 3). These markers (C14, C16, C18, C18:1, C3DC, C5OH) were also identified in our hierarchical clustering analysis (Figure 1) indicating consistency in results. Three of the metabolites identified are NBS markers for the detection of metabolic disorders on the RUSP (CPT-II, MAL, and 3MCC). Compared to the baseline group, the advanced MA group had elevated levels of the MAL marker C3DC (Cohen’s d= 0.22), the CPT-II marker C16 (Cohen’s d = 0.29), and decreased level of the 3MCC marker C5OH (Cohen’s d = -0.43). We reasoned that disease markers with an increased level in infants in the MA ≥35 y group could also lead to a higher number of false positives in this group. In turn, a marker with significantly lower levels could lead to a relatively lower number of false positives in this group. To test this hypothesis, we compared the proportion of healthy, screen-negative infants in the advanced MA group (22.2%) to the proportion of false-positive cases in that group for each of the three disorders. For malonic acidemia (MAL), a significantly higher number of false-positive cases was found in the advanced MA group (54 found, 35 expected, p-value < 0.001). No significant difference in the number of expected versus the number of identified false-positive cases was seen for CPT-II (9 identified, 9 expected) and 3MCC (31 identified, 33 expected).
4. Discussion
Advanced maternal age is associated with adverse pregnancy outcomes and yet little is known about the influences of maternal age on newborn metabolism. Here we used population-level newborn screening data to systematically examine the relationship between maternal age at delivery and newborn metabolite levels and whether maternal age could impact the performance of newborn screening for inborn metabolic disorders on the RUSP [17]. Considering the known influence of gestational age, birth weight, age at blood collection, and parent-reported ethnicity on newborn metabolism [22,33,34], we followed a stringent study design and controlled for the influence of these important covariates in the analysis of metabolite levels across different maternal age groupings.
A cluster analysis of 46 NBS markers reported for 476,718 screen-negative infants (Table S1) in relation to maternal age showed two large groups of metabolites characterized by either decreasing or increasing levels shortly after birth (Figure 1). We identified significant differences for seven newborn metabolites (absolute Cohen's d > 0.2) in an effect size analysis of metabolite levels between five maternal age groups (range 20-44 y) in comparison to the baseline group (15- 19 y). Six of the seven markers identified were confirmed in a separate analysis comparing newborn metabolite levels between the two groups of advanced maternal age (≥ 35 y) and teenage maternal age (15-19 y). The six newborn metabolites identified included two short-chain (C3DC, C5OH) and four long-chain (C14, C16, C18, C18:1) acylcarnitines. The enrichment of acylcarnitines in relation to maternal age at delivery (p-value = 0.0088) sheds new light on early postnatal metabolic differences. In previous work, ethnicity-related metabolic differences in infants showed larger differences in blood levels of acylcarnitines than of amino acids [35]. In addition to their use in NBS for inborn errors of fatty acid oxidation and energy metabolism, acylcarnitines are increasingly being recognized as biomarkers for a range of diseases such as diabetes, cardiovascular disorders, cancer, and as pharmaceutical agents [36].
To investigate these results, we performed covariate-stratified analyses of maternal age in relation to newborn metabolic profiles. We first considered that metabolic profiles could differ between male and female infants. Infant sex-stratified analyses showed similar cluster patterns for male (Figure S9) and for female infants (Figure S10) and confirmed the same seven acylcarnitine markers identified in the sex-combined analysis (Figure 1). We then considered that metabolic profiles could differ between different parent-reported ethnicity groupings. Ethnicity-stratified analyses revealed distinct metabolic clusters for Asian, Black, Hispanic, and White newborn groups (Figures S11–S14), however, each analysis identified six of the seven markers found in the ethnicity-combined analysis (Figure 1), which supported the robustness of the global analysis.
We then examined the influence on newborn metabolites for several clinical variables (infant sex, gestational age, and ethnicity) in relation to maternal age. Figure 2 shows results from a covariate-stratified analysis of selected metabolites with increasing (C16, C3DC) and with decreasing (C5OH) levels in relation to maternal age (Figure 2). Term infants and male infants had a tendency for higher levels for all three metabolites, while the major ethnicity groups showed distinct metabolite patterns in relation to maternal age. These examples illustrate the variable influences from the different covariates on newborn metabolite levels. Additionally, our analysis identified an overall association between maternal age and prematurity (Figure S5, p-value < 0.001), which was found to be significant for the Asian, Black, and Hispanic but not for the White sub-groups (Figure S6). Interestingly, the proportion of preterm to term births in relation to maternal age varied between different ethnicity groups (Figure S8). The lowest preterm birth rates for Black and Hispanic infants were seen at the maternal age of 20-24 y, while it was shifted to the right for the Asian and White groups (25-29 y). These findings highlight the complex relationship between maternal age, gestational age, infant sex, and parent-reported ethnicity, and motivate development of novel data mining algorithms that incorporate all screening metabolites and covariates in the analysis of newborn screening data.
We reasoned that the maternal age-related differences identified for 13% of the metabolites (Figure 3) could lead to false-positive newborn screens. We selected three diseases detectable using these screening markers and associated with frequent false-positive results. Analysis of false-positive cases for one of these diseases indicated maternal-related differences, which correlated with differences in marker levels discovered in the respective collection groups. Infants in the advanced maternal age group (≥ 35 y) were more likely to be false positive for malonic acidemia (MAL), which correlated with the elevated C3DC levels in screen-negatives in this group. In contrast, we did not find significantly more false positives for CPT-II (marker C16) and 3MCC (marker C5OH) in this group which was likely due to the much smaller sample size of false positives for these two disorders.
Our study had several limitations. First, metabolite levels are influenced by a number of factors such as gestational age, birth weight, and age at blood collection [22,37]. To investigate the relation between maternal age and newborn metabolites required a stringent approach that separates the influence from other covariates. In turn, such an approach resulted in a significant decrease in sample size and statistical power. For example, only 35% of the MAL false-positive cases (156 of 439) were available in this analysis. Thus, it could be possible that this covariant-stratified analysis has led to an underestimate of the true maternal-age-related effects on newborn metabolism. Second, significant differences in maternal age were found in both the major (N=4) and the detailed (N=17) parent-reported ethnicity groupings (Figures S3 and S4). However, the analysis of maternal age and newborn metabolic differences was limited to the four major ethnicity groupings due to the small sample size in some of the detailed sub-groups. The significantly higher maternal age in some Asian-ancestry groups (Korean, Japanese [both p-value<0.001]) could be associated with differences in newborn metabolic patterns. Third, although infants reported with multiple ethnicity categories were removed from the analysis (~18%, Table S1), this approach is highly limited as population admixture is often unknown. Additionally, many families do not identify themselves as belonging to predefined ethnicity categories and/or may affiliate with other ancestries [35]. Future studies could explore metabolic differences in cohorts with multiple parent-reported ethnicities to increase statistical power. Fourth, additional factors not studied here could be related to newborn metabolism. Smoking during pregnancy has been shown to increase the risk of preterm birth and low birth weight [38,39], while breastfeeding and variability in neonatal protein catabolism could influence blood metabolite levels [40]. Additionally, our study could be confounded by other risk factors in pregnancies of advanced maternal age such as placenta praevia, hypertensive complications, gestational diabetes mellitus and other maternal medical history. For example, Bass and Taylor found that combining prenatal screening of maternal serum with maternal age could help with detecting fetal disorder (trisomy 18) [41].
5. Conclusions
In conclusion, using population-level newborn screening data, we found that blood markers for newborn metabolic disease were associated with maternal age at delivery. In accordance with findings from previous studies [22,34], maternal age did not have a linear relationship with metabolite levels and the association between metabolite levels and maternal age was also dependent on other covariates. Development of novel data mining models that incorporate newborn metabolic profiles, maternal age, and other clinical variables could further our understanding of metabolite-covariate relationships for improved genetic disease screening and diagnostics.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Text S1.
Author Contributions
Y.X. and C.S. designed the study and wrote the manuscript. Y.X. performed the statistical analysis. G.P., H.Z. and C.S. provided input on data analysis and interpretation. All authors edited and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by a grant from the National Institute of Child Health and Human Development (R01HD102537).
Institutional Review Board Statement
This study was overseen by the institutional review boards at Yale University (Protocol #1505015917) and the State of California Committee for the Protection of Human Subjects (Protocol #13-05-1236).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this study are subject to the following licenses/restrictions: The data used in this study were obtained from the California Biobank Program (CBP) under SIS Request 886. The California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication. The data can be obtained by others after submitting a new request to the CBP coordinator. Requests to access these datasets should be directed to [email protected].
Acknowledgments
We thank Robin Cooley, Hao Tang, Steve Graham, Stanley Sciortino, Lisa Feuchtbaum, and Robert Currier at the Genetic Disease Screening Program (GDSP) for support of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathews, T.J.; Hamilton, B.E. Mean Age of Mother, 1970-2000. Natl. Vital Stat. Rep. 2002, 51, 1–13.
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020. Natl. Vital Stat. Rep. 2021, 70, 1–50.
- Matthews, T.J.; Hamilton, B.E. First Births to Older Women Continue to Rise. NCHS Data Brief 2014, 1–8.
- Laopaiboon, M.; Lumbiganon, P.; Intarut, N.; Mori, R.; Ganchimeg, T.; Vogel, J.P.; Souza, J.P.; Gülmezoglu, A.M.; WHO Multicountry Survey on Maternal Newborn Health Research Network Advanced Maternal Age and Pregnancy Outcomes: A Multicountry Assessment. BJOG 2014, 121 Suppl 1, 49–56.
- Walker, K.F.; Bradshaw, L.; Bugg, G.J.; Thornton, J.G. Causes of Antepartum Stillbirth in Women of Advanced Maternal Age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 86–90. [CrossRef]
- Claramonte Nieto, M.; Meler Barrabes, E.; Garcia Martínez, S.; Gutiérrez Prat, M.; Serra Zantop, B. Impact of Aging on Obstetric Outcomes: Defining Advanced Maternal Age in Barcelona. BMC Pregnancy Childbirth 2019, 19, 342. [CrossRef]
- Pregnancy at Age 35 Years or Older: ACOG Obstetric Care Consensus No. 11. Obstet. Gynecol. 2022, 140, 348–366.
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Womens Health 2021, 13, 751–759.
- Egan, J.F.X.; Smith, K.; Timms, D.; Bolnick, J.M.; Campbell, W.A.; Benn, P.A. Demographic Differences in Down Syndrome Livebirths in the US from 1989 to 2006. Prenat. Diagn. 2011, 31, 389–394. [CrossRef]
- Who Is at Risk for Down Syndrome? Available online: https://www.nichd.nih.gov/health/topics/down/conditioninfo/Risks (accessed on 20 July 2023).
- Frederiksen, L.E.; Ernst, A.; Brix, N.; Braskhøj Lauridsen, L.L.; Roos, L.; Ramlau-Hansen, C.H.; Ekelund, C.K. Risk of Adverse Pregnancy Outcomes at Advanced Maternal Age. Obstet. Gynecol. 2018, 131, 457–463. [CrossRef]
- Ryckman, K.K.; Shchelochkov, O.A.; Cook, D.E.; Berberich, S.L.; Copeland, S.; Dagle, J.M.; Murray, J.C. The Influence of Maternal Disease on Metabolites Measured as Part of Newborn Screening. J. Matern. Fetal. Neonatal Med. 2013, 26, 1380–1383. [CrossRef]
- Martin, F.-P.; Rezzi, S.; Lussu, M.; Pintus, R.; Pattumelli, M.G.; Noto, A.; Dessì, A.; Da Silva, L.; Collino, S.; Ciccarelli, S.; et al. Urinary Metabolomics in Term Newborns Delivered Spontaneously or with Cesarean Section: Preliminary Data. Journal of Pediatric and Neonatal Indivi 2018, 7.
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Chi Guo, A.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The Urinary Metabolome of Healthy Newborns. Metabolites 2020, 10, 165. [CrossRef]
- Anand, S.T.; Ryckman, K.K.; Baer, R.J.; Charlton, M.E.; Breheny, P.J.; Terry, W.W.; Kober, K.; Oltman, S.; Rogers, E.E.; Jelliffe-Pawlowski, L.L.; et al. Metabolic Differences among Newborns Born to Mothers with a History of Leukemia or Lymphoma. J. Matern. Fetal. Neonatal Med. 2022, 35, 6751–6758. [CrossRef]
- Cajachagua-Torres, K.N.; Blaauwendraad, S.M.; El Marroun, H.; Demmelmair, H.; Koletzko, B.; Gaillard, R.; Jaddoe, V.W.V. Fetal Exposure to Maternal Smoking and Neonatal Metabolite Profiles. Metabolites 2022, 12. [CrossRef]
- American College of Medical Genetics Newborn Screening Expert Group Newborn Screening: Toward a Uniform Screening Panel and System--Executive Summary. Pediatrics 2006, 117, S296-307.
- Newborn Screening Available online: https://www.nichd.nih.gov/health/topics/factsheets/newborn (accessed on 20 July 2023).
- Rose, N.C.; Dolan, S.M. Newborn Screening and the Obstetrician. Obstet. Gynecol. 2012, 120, 908–917. [CrossRef]
- McHugh, D.M.S.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A.; Åhlman, H.; Allen, J.J.; Antonozzi, I.; Archer, S.; et al. Clinical Validation of Cutoff Target Ranges in Newborn Screening of Metabolic Disorders by Tandem Mass Spectrometry: A Worldwide Collaborative Project. Genet. Med. 2011, 13, 230–254. [CrossRef]
- Lachenbruch, P.A.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences (2nd Ed.). J. Am. Stat. Assoc. 1989, 84, 1096.
- Peng, G.; Tang, Y.; Cowan, T.M.; Zhao, H.; Scharfe, C. Timing of Newborn Blood Collection Alters Metabolic Disease Screening Performance. Front. Pediatr. 2020, 8, 623184. [CrossRef]
- Hook, E.B. Rates of Chromosome Abnormalities at Different Maternal Ages. Obstet. Gynecol. 1981, 58, 282–285.
- Wickham H, François R, Henry L, Müller K, Vaughan D Dplyr: A Grammar of Data Manipulation Available online: https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr.
- Maintainer, M. Package “Effsize” Available online: https://cran.r-project.org/web/packages/effsize/effsize.pdf (accessed on 20 July 2023).
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [CrossRef]
- Villanueva, R.A.M.; Chen, Z.J. Ggplot2: Elegant Graphics for Data Analysis (2nd Ed.). Measurement (Mahwah NJ) 2019, 17, 160–167. [CrossRef]
- Kassambara, A. “ggplot2” Based Publication Ready Plots [R Package Ggpubr Version 0.6.0]. 2023.
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563.
- Analysis of Variance – ANOVA. In Statistics and Data Analysis for Microarrays Using R and Bioconductor; Chapman and Hall/CRC, 2016; pp. 441–478 ISBN 9780429130588.
- Massey, F.J., Jr The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78.
- Newcombe, R.G. Interval Estimation for the Difference between Independent Proportions: Comparison of Eleven Methods. Stat. Med. 1998, 17, 873–890.
- Peng, G.; de Fontnouvelle, C.A.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Elevated Methylmalonic Acidemia (MMA) Screening Markers in Hispanic and Preterm Newborns. Mol. Genet. Metab. 2019, 126, 39–42. [CrossRef]
- Peng, G.; Tang, Y.; Gandotra, N.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Ethnic Variability in Newborn Metabolic Screening Markers Associated with False-Positive Outcomes. J. Inherit. Metab. Dis. 2020, 43, 934–943. [CrossRef]
- Peng, G.; Pakstis, A.J.; Gandotra, N.; Cowan, T.M.; Zhao, H.; Kidd, K.K.; Scharfe, C. Metabolic Diversity in Human Populations and Correlation with Genetic and Ancestral Geographic Distances. Mol. Genet. Metab. 2022, 137, 292–300. [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [CrossRef]
- Peng, G.; Tang, Y.; Cowan, T.M.; Enns, G.M.; Zhao, H.; Scharfe, C. Reducing False-Positive Results in Newborn Screening Using Machine Learning. Int. J. Neonatal Screen. 2020, 6, 16. [CrossRef]
- Soneji, S.; Beltrán-Sánchez, H. Association of Maternal Cigarette Smoking and Smoking Cessation with Preterm Birth. JAMA Netw. Open 2019, 2, e192514. [CrossRef]
- Tatsuta, N.; Asato, K.; Anai, A.; Suzuki, T.; Sakurai, K.; Ota, C.; Arima, T.; Sugawara, J.; Yaegashi, N.; Nakai, K.; et al. Timing of Maternal Smoking Cessation and Newborn Weight, Height, and Head Circumference. Obstet. Gynecol. 2023, 141, 119–125. [CrossRef]
- Porta, F.; Mussa, A.; Ponzone, A. Breastfeeding Effects on Newborn Screening. J. Pediatr. 2010, 156, 1033; author reply 1033-4. [CrossRef]
- Bass, H.N.; Taylor, J.B. Perinatal Screening for Congenital Malformations and Genetic Disorders: Current Status and Future Directions. Perm. J. 2002, 6, 15–20.
Figure 1.
Newborn metabolite levels and maternal age. To explore newborn metabolic differences in relation to maternal age (MA), we selected six newborn groups based on MA at delivery with the first group (15–19 y, n = 19,528) being defined as a baseline for each metabolite. Effect size differences (Cohen’s d) for all 46 metabolites between each of the five MA groups (20–44 y) and the baseline (15–19 y) were calculated. Positive Cohen’s d (in red) indicates elevated metabolite levels, and negative Cohen’s d (in blue) indicates decreased levels compared to the baseline. Hierarchically clustering was used to group metabolites into two clusters of either decreasing (at the top) or increasing (at the bottom) levels compared with the baseline MA group. Seven markers in bold showed significant differences between at least one of the five MA groups and the baseline group (absolute Cohen’s d > 0.2), including NBS markers for metabolic disorders on the RUSP [17] (labeled with *). Acylcarnities (AC) were enriched in the top cluster of markers with increasing levels. (p-value = 0.0088). AA,Amino acid.
Figure 1.
Newborn metabolite levels and maternal age. To explore newborn metabolic differences in relation to maternal age (MA), we selected six newborn groups based on MA at delivery with the first group (15–19 y, n = 19,528) being defined as a baseline for each metabolite. Effect size differences (Cohen’s d) for all 46 metabolites between each of the five MA groups (20–44 y) and the baseline (15–19 y) were calculated. Positive Cohen’s d (in red) indicates elevated metabolite levels, and negative Cohen’s d (in blue) indicates decreased levels compared to the baseline. Hierarchically clustering was used to group metabolites into two clusters of either decreasing (at the top) or increasing (at the bottom) levels compared with the baseline MA group. Seven markers in bold showed significant differences between at least one of the five MA groups and the baseline group (absolute Cohen’s d > 0.2), including NBS markers for metabolic disorders on the RUSP [17] (labeled with *). Acylcarnities (AC) were enriched in the top cluster of markers with increasing levels. (p-value = 0.0088). AA,Amino acid.
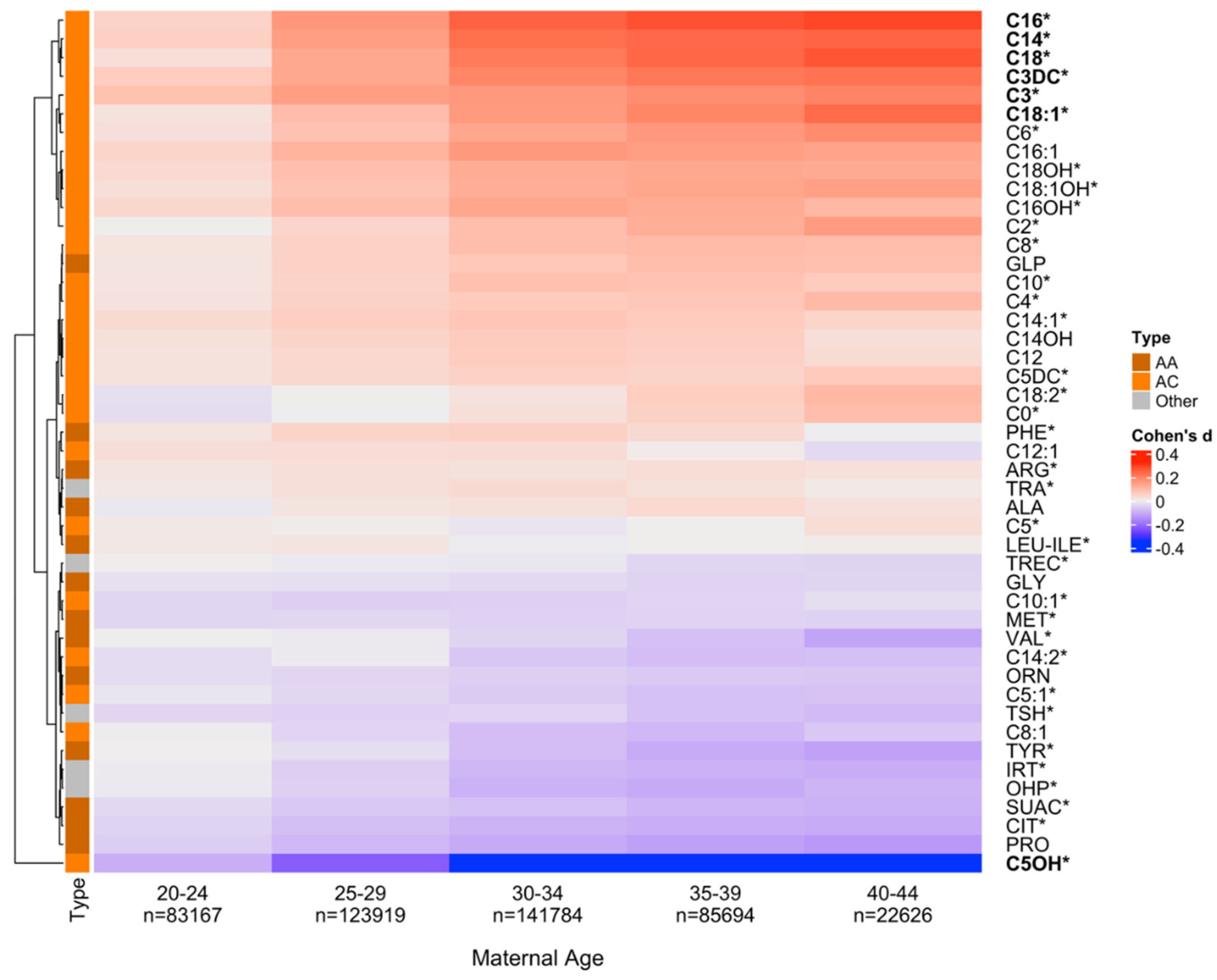
Figure 2.
Maternal age and clinical variables. The association between maternal age at delivery (15-44 years) and three representative metabolites (C16, C3DC, C5OH) is shown in a-c. The association between these metabolites, maternal age, and the covariates of infant sex, parent-reported ethnicity, and gestational age is shown in d-l. For each metabolite, relationships between different maternal ages are shown for male (n= 247,446) and female infants (n= 229,272) (d-f); preterm (n = 23,541) and term (n = 453,177) (g-i); Asian (n= 52,642), Black (n= 23,902), Hispanic (n= 184,595), and White infants (n= 120,362) (j-l). Solid smoothed lines are means estimated from generalized additive models with the shading showing the 95% confidence interval of the mean estimation.
Figure 2.
Maternal age and clinical variables. The association between maternal age at delivery (15-44 years) and three representative metabolites (C16, C3DC, C5OH) is shown in a-c. The association between these metabolites, maternal age, and the covariates of infant sex, parent-reported ethnicity, and gestational age is shown in d-l. For each metabolite, relationships between different maternal ages are shown for male (n= 247,446) and female infants (n= 229,272) (d-f); preterm (n = 23,541) and term (n = 453,177) (g-i); Asian (n= 52,642), Black (n= 23,902), Hispanic (n= 184,595), and White infants (n= 120,362) (j-l). Solid smoothed lines are means estimated from generalized additive models with the shading showing the 95% confidence interval of the mean estimation.

Figure 3.
Newborn metabolic difference in relation to advanced maternal age (≥ 35 years old). To identify metabolic differences related to advanced maternal age, 405,968 screen-negative term infants (37–42 weeks) with BW range of 2,500 g to 4,000 g were grouped into the advanced (≥ 35 years old) and the baseline (15–19 years old) maternal age groups. Effect size differences (Cohen’s d) for each of the 46 metabolites were calculated between the advanced and the baseline MA group. Positive Cohen’s d values indicate relatively higher metabolite levels in the advanced MA group. Metabolites are ranked from top to bottom based on Cohen’s d values.
Figure 3.
Newborn metabolic difference in relation to advanced maternal age (≥ 35 years old). To identify metabolic differences related to advanced maternal age, 405,968 screen-negative term infants (37–42 weeks) with BW range of 2,500 g to 4,000 g were grouped into the advanced (≥ 35 years old) and the baseline (15–19 years old) maternal age groups. Effect size differences (Cohen’s d) for each of the 46 metabolites were calculated between the advanced and the baseline MA group. Positive Cohen’s d values indicate relatively higher metabolite levels in the advanced MA group. Metabolites are ranked from top to bottom based on Cohen’s d values.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns
Yuhan Xie
et al.
,
2023
Excessive Gestational Weight Gain Alters the DNA Methylation and Influences Foetal and Neonatal Body Composition
Perla Pizzi Argentato
et al.
,
2023
COVID-19 Infection during Pregnancy: Disruptions in Lipid Metabolism and Implications for Newborn Health
Natalya A. Frankevich
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







