Preprint
Article
Overnutrition during Pregnancy and Lactation Induces Gender-Dependent Dysmetabolism in the Offspring Accompanied by Heightened Stress and Anxiety
Altmetrics
Downloads
110
Views
68
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Alerts
Abstract
Maternal obesity and gestational diabetes predispose to metabolic disturbances in the next generation. Moreover, the lactation phase also stands as critical for metabolic programming. Nevertheless, the precise mechanisms originating these changes remain unclear. Here we investigate the consequences of maternal diet lipid-rich diet during gestation and lactation, and its impact on metabolism and behavior in the offspring. Two experimental groups of Wistar female rats were used: a control group (NC) that fed a standard diet during gestation and lactation periods and an overnutrition group that fed a high-fat diet (HF, 60% lipid-rich) during the same phases. Offspring were analyzed at postnatal days 21, 28, and 2 months-old (PD21, PD28, PD60) for metabolic profile (weight, fasting glycemia insulin sensitivity, glucose tolerance) and euthanized for brain collection to evaluate by western blot markers of synaptic dynamics, metabolism and inflammation in the hypothalamus, hippocampus and prefrontal cortex. At 2 months-old, behavioral tests for anxiety, stress, cognition, and food habits were conducted. We observe that female offspring born from HF mothers exhibited increased weight gain and decreased glucose tolerance that attenuates with age. In offspring males, weight gain increased at P21 and worsened with age, while glucose tolerance remained unchanged. Offspring of HF mothers exhibited elevated levels of anxiety and stress during behavioral tests, displaying decreased predisposition for curiosity compared to the NC group. In addition, offspring from mothers with HF showed increased food consumption and a lower tendency towards food-related aggression. We conclude that exposure to HF diet during pregnancy and lactation induces dysmetabolism in the offspring accompanied by heightened stress and anxiety. There was sexual dimorphism in metabolic traits but not behavioral phenotypes.

Keywords:
Subject: Biology and Life Sciences - Endocrinology and Metabolism
1. Introduction
Metabolic syndrome (MS) is a serious public health problem affecting about 25% of the general population worldwide [1]. The definition of this syndrome has recently evolved to include a group of at least three of five cardio-metabolic abnormalities: high blood pressure, central obesity, insulin resistance (IR), elevated blood triglycerides, and atherogenic dyslipidemia, which together lead to an increased risk of cardio-metabolic pathologies [1]. The presence of MS-related disorders also has an impact on the central nervous system, causing neurological, and neurodegenerative diseases [2]. The intricate relationship between dysmetabolism and neurodegeneration has been described to be relevant not only to aging-associated comorbidities but also in mild cognitive impairment in younger ages and during the initial steps of organisms’ development. In fact, it is known that the nutritional status of the mother can influence prematurely the onset of diseases in the offspring, and there is significant research focused on understanding its impact on the metabolic status and nervous system of the offspring [3] Studies in animals have shown that maternal obesity can increase the likelihood of metabolic and neurodevelopmental diseases in the offspring [2,3,4]. Nevertheless, the precise mechanisms responsible for these outcomes are not thoroughly understood.
Recent evidence indicates that many components of a mother’s diet are pivotal in shaping aspects of offspring health, including the microbiome and the neonatal immune system [5]. Research suggests that maternal diet can influence the development of the offspring’s brain, endocrine system, and long-term behavior [6]. Therefore, maintaining a balanced and nutritious diet during pregnancy is crucial to meet the increased demands on the mother’s body, as adequate nutrient intake is essential to support the offspring’s growth and ensure a healthy birth weight.
During the perinatal period, mother’s obesity can elevate the risk of gestational diabetes and hypertension, potentially affecting placental function and fetal energy metabolism [7]. Obesity during pregnancy is also associated with complex alterations in neuroendocrine, metabolic, and inflammatory processes, which can potentially influence fetal hormonal exposure and nutrient supply [8,9,10].
Throughout pregnancy, the mother’s body undergoes significant metabolic changes which, if left untreated, can contribute to certain health disparities. In early pregnancy, there is an increase in maternal insulin secretion to facilitate the transportation of glucose and amino acids to the developing fetus [9,10]. However, this increased insulin secretion, combined with high levels of circulating lipids and the consequent increase in visceral adiposity, often results in the development of insulin resistance in maternal cells [7,8,9,10]. When lactation begins, a metabolic shift occurs, redirecting resources from storage milk synthesis, being this process intricately regulated by lactogenic hormones such as insulin, prolactin, and glucocorticoids, as well as by cytokines, growth factors, and substrate availability [11]. The alterations promoted by lactation causes several positive changes in metabolic processes. It leads to better glucose regulation, reducing insulin production, improving insulin sensitivity, and decreasing β-cell proliferation. In addition, lipid metabolism becomes less active in certain tissues and stored lipids are mobilized to facilitate the transport of lipids to the mammary gland for milk production [8,10,11]. As a result of these metabolic changes during lactation, there is a reduction in postpartum adiposity, potentially decreasing the risk of obesity. Based on this evidence, Stuebe and Rich-Edwards proposed the “reset hypothesis”, which suggests that lactation plays a crucial role in resetting the metabolic processes that take place during labor [12].
However, when mother overnutrition continues, offspring metabolism may be impacted as well as neurological development. Knowing the mechanisms behind the expected impact of hypercaloric diet fed mothers on the offspring metabolism and neurological development is the main goal of this study. Additionally, being well known that sex influences both metabolic [13]and neurodegenerative disorders [14] development and progression we will also focus our attention on the differences of the impact of maternal overnutrition on male vs female offspring metabolism and stress and anxiety behavior.
2. Materials and Methods
2.1. Animals
Experiments were performed in 12-week female, and 24-week male Wistar rats (200–400 g), obtained from Charles River Laboratories (Barcelona, Spain) and maintained at the NOVA Medical School animal facility. Animals were kept under temperature and humidity control (21 ± 1 °C; 55 ± 10% humidity) and a regular light (08.00–20.00 h) and dark (20.00–08.00 h) cycle, with food and water ad libitum. After randomization, animals were divided into a normal chow diet group (NC) or a high fat (HF) diet group. The NC group fed a standard diet (7.4% fat + 75% carbohydrates (4% sugar) + 17% protein, SDS diets RM1, Probiológica, Portugal), and the HF diet group fed a 60% lipid-rich diet (61.6% fat + 20.3% carbohydrate + 19.1% protein, TestDiet, Missouri, USA). After confirming that the females were pregnant, they stayed 1 per cage. During the breastfeeding period, the pup’s body weight was weekly motorized. On the weaning day—post-natal 21 days (PD21), both male and female offspring were separated from the mothers and kept 4 animals per cage. They were randomly divided and part of them were sacrificed at PD21, 28 postnatal days (PD28) and the other group were kept until 45 days post-natal- where metabolic and behavioral parameters were assessed. Metabolic evaluations were performed in the mothers by using the insulin tolerance test (ITT), and oral glucose tolerance test (OGTT) (Figure 1).
Pups were also tested for insulin sensitivity through the ITT and for glucose tolerance through the OGTT. Behavior was assessed by open field, y maze, elevated plus maze (EPM), light dark box, sucrose competition, food competition and novel object recognition (NOR) tests. At the time of sacrifice, PD21, PD28 and PD60 the animals were anesthetized and sacrificed with pentobarbital (60 mg/ kg i.p.) and the brains were dissected and frozen at −80 °C for further protein analysis.
All experiments and animal care were performed in accordance with the European Union Directive for Protection of Vertebrates Used for Experimental and Other Scientific Ends (2010/63/EU) and with the ARRIVE guidelines. Experimental protocols were approved by the Ethics Committee of NOVA Medical School/Faculdade de Ciências Médicas (nº194/2021/CEFCM) and by the Portuguese Authority for the animal Health (DGAV, Ref 0421/000/000/2021).
2.2. Metabolic Evaluation
2.2.1. Intravenous Insulin Tolerance Test (ITT)
Insulin sensitivity was assessed in conscious mothers using the intravenous ITT, which provides an estimate of overall insulin sensitivity [15]. In brief, following an overnight fast, an insulin bolus (0.1 U/Kg, Humulin Regular, Lilly) was administered in the tail vein, and the subsequent 15-minute decline in blood sugar levels was measured. Blood samples were collected by tail tipping, and glycemia was assessed using a glucometer (Precision Xtra Meter, Abbott Diabetes Care, Portugal) and test strips (Abbott Diabetes Care, Portugal). The constant rate of decline in plasma glucose (KITT) was calculated as previously outlined [16].
2.2.2. Intraperitoneal Insulin Tolerance Test (ipITT)
In the offspring, insulin sensitivity was assessed using the intraperitoneal ITT. Pups were fasted for approximately 6 hours with free access to water. Basal blood glucose was measured, and 0.1 U/Kg of insulin (Humulin Regular, Lilly) was administered intraperitoneally. Glucose was measured from blood collected from the tip of the tail vein at 5, 10, 15, 30, 45, 60, 120 minutes after the injection with a glucometer (Precision Xtra Meter, Abbott Diabetes Care, Portugal) and test strips (Abbott Diabetes Care, Portugal). Glucose excursion curves from the ITT were used to calculate the area under the curve and therefore to evaluate insulin sensitivity.
2.2.3. Oral Glucose Tolerance Test (OGTT)
Glucose tolerance was assessed through the OGTT. Overnight fasted animals, both mothers and pups, for approximately 12-15 hours were administered with a glucose solution (2g/kg in a 10 ul/g body weight volume) by gavage after the measurement of basal glycemia. Glucose levels were measured at 0 and 15, 30, 60 and 120 minutes after the oral gavage by tail tipping using a glucometer (Precision Xtra Meter, Abbott Diabetes Care, Portugal) and test strips (Abbott Diabetes Care, Portugal). The glucose excursion curves were used to calculate the area under the curve.
2.3. Behavioral Assessment
2.3.1. Open Field (OF)
The OF test is used to assess gross motor activity, anxiety, and willingness to explore [17]. In this test, animals are placed for 5 minutes in a square arena (70 cm x 70 cm x 75 cm). Their behavior is recorded and analyzed using Bonsai software (version 7.0). For that, an inner and central zone in the maze is defined (40 cm x 40 cm) and it is measured: the total distance covered, distance covered in the inner zone, total immobility time, immobility time in the inner zone, average velocity, and average velocity in the inner zone. It was considered that the animal is in the inner zone when more than half of its body is in the 40 cm area. Immobility is considered when the animal is stationary. Data presented result from a single trial for each rat.
2.3.2. Y Maze
To assess short-term spatial memory, animals are challenged to a Y-shaped maze with 120° between each arm with 10 cm wide and 30 cm high. First, animals are submitted to a training session in which the rat is placed in the start point (A arm) of the Y-maze with a closed arm and allowed to freely explore it for 5 min. The experimental session occurred 1 h after training where the “novel” arm is open. The animal freely explores the maze for 5 min. The exploratory capacity of the animal is evaluated as well as the time spent both in the novel arm and in the arm that is always open. The first arm choice when both arms are open are evaluated, as well as the number of triads (i.e., ABC, CAB, or BCA) and entries. The spontaneous alternative behavior score (%) for each rat is calculated as the ratio of the number of alternations to the possible number (total number of arm entries minus two) multiplied by 100. Videos were recorded as explained in Section 2.4.1.
2.3.3. Elevated Plus Maze (EPM)
EPM test is used to characterize anxiety-related behavior. The apparatus is composed of a small central platform with four arms angled 90° from each other radiating outwards and is raised above the ground to a height of 112 cm and 50 cm in length and 12 cm in width. Alternating arms are enclosed by high opaque walls of 41,5 cm in height, with open tops. Animals are placed in the center of the maze and allowed to freely explore for 5 min for a single trial and recorded by an overhead camera. The time spent on open arms and the number of entries is measured. Data presented result from a single trial for each rat. Videos were recorded as explained in Section 2.4.1.
2.3.4. Light Dark Box (LDB)
The LDB test is used to evaluate anxiety responses in rodents. The apparatus is composed of two compartments. The large light compartment (2/3 of the box) is brightly lit and open, while the small dark compartment (1/3 of the box) is covered and dark. These two compartments are connected by a door of 10 cm. Rats are placed in the corner of the brightly illuminated chamber and are allowed to freely explore the maze for 5 minutes. Parameters as the time spent in the light/dark compartment, and the number of transitions is evaluated. Data presented result from a single trial for each rat. Videos were recorded as explained in Section 2.4.1.
2.3.5. Novel Object Recognition (NOR)
NOR test is commonly used in rodents to evaluate cognition, particularly recognition memory. This test is performed in the same maze as the OF test, with two different objects. The objects are different in shape and appearance. This test is composed by a train where the animals are allowed to explore the maze with two identical objects placed at an equal distance. One hour later, rats are allowed to explore the arena in the presence of the familiar object and a novel object to test long-term recognition memory. The time spent exploring each object and the ratio between the novel/novel+ familiar (interaction time) are analyzed. Data presented result from a single trial for each rat. Videos were recorded as explained in Section 2.4.1.
2.3.6. Block Test
Block test is used to assess olfactory function. This test evaluates sensitivity to social smells, an ability in rats [18]. Housed animals were exposed to five wood blocks (7cm x 2cm x 2cm) (Ultragene, Portugal) placed inside each cage for 1 week. Each rat underwent two training sessions. On the trial test, one block was replaced by a block that was originally in a cage with a different set of animals. Rat was videotaped for 1 minute. Parameters as the time that animals take to recognize the novel block and the time spent to sniff the novel block were evaluated. The animal was only considered to be sniffing the block when its nose was touching/very close to the block. Data presented result from a single trial for each rat.
2.3.7. Food Competition
Food competition is a test that not only measures the antagonistic and dominance behaviors of offspring but also assesses voracity and food preferences. The animals were fasted for 24-hour. Following the fasting period, the animals were randomly paired with a same-sex partner and placed in the maze (44 cm wide, 49 cm long, and 20 cm high box). Inside this maze, on one of its sides, there is an integrated dispenser with a retractable door, where the food was stored. The pair is placed inside the maze, and for 2 minutes, the door is kept closed. After 2 minutes, the dispenser’s door is opened to allow the rats access to the food. During the following 8 minutes, observations are made regarding the amount of food consumed, the occurrence of antagonistic behaviors, their duration, as well as the frequency of grooming behaviors exhibited by the rats. Data presented result from a single trial for each pair.
2.3.8. Water/Sucrose Competition
Similarly, to the food competition, the water/sucrose competition measures antagonistic and dominance behaviors in offspring, as well as their liquid consumption preferences.
The animals were subjected to a 24-hour liquid deprivation. During this period, only food was available ad libitum. After the fasting period, the animals are randomly paired as described in Section 2.3.7. Inside this maze, on one of its faces, there is a dispenser set at a 45º angle capable of holding a water bottle. This dispenser features a removable door, controlling the rats’ access to the liquid. The dispenser closed for 2 minutes. After that, for 8 minutes, the bottles become available, and the rats have access to it. Observations were made regarding the liquid intake, the occurrence of antagonistic behaviors, their duration, as well as the frequency of grooming behaviors exhibited by the rats.
The antagonistic behaviors included stealing pellets from another individual’s mouth or paws (Food Competition) or pushing the other’s head while drinking from the water bottle (Water/Sucrose Competition) and aggression, such as biting, moving the other away or just fighting.
The data presented result from a single trial for each pair. After the water trial, the same pair was exposed to the sucrose trial, with the same characteristics, however with sucrose is 1g/L.
2.4. Ex Vivo Analysis
After 21-, 28- and 60-days, animals were sacrificed and the following brain areas - frontal cortex, hypothalamus and hippocampus were dissected and harvested for protein analysis.
2.4.1. Tissue Lysate Preparation and Western Blot Analysis
For western blot analysis, brain samples (20 mg) were homogenized in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.1 % SDS, 0.25 % sodium deoxycholate) and sonicated 3 times in cycle of 30 seconds. Protein extracts were centrifuged for 10 minutes at 13.000 rpm at 4ºC, and the supernatant collected.
Proteins (8 μg) were separated by electrophoresis in 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transference to a nitrocellulose membrane (BioRad, Germany). After transference, the membrane was incubated with blocking solution composed by milk or bovine serum albumin (BSA) at room temperature for 1 hour. Primary antibody incubations were carried out overnight at 4ºC, using the following concentrations: Phosphorylated Insulin Receptor (anti- IR-p, rabbit 1: 500, abcam), Total Insulin Receptor (anti-IR T, mouse 1:1000, Santa Cruz); phosphorylated mitogen activated protein kinase (AMPK) (anti-pAMPK 1:500 rabbit Cell Signalling; and total AMPK (anti-AMPK rabbit 1:1000 Cell Signalling); Synaptosomal-Associated Protein (SNAP25) (anti-SNAP25 1:500, Santa Cruz); Postsynaptic density protein (PSD95) (anti-PSD95 1:1000, Invitrogen); Vesicular glutamate transporter 1 (vGLuT) (anti-vGLuT 1:1000, MilliporeSigma); Glial fibrillary acidic protein (GFAP) (anti-GFAP 1:2000, Palex); Tumor necrosis factor alpha (TNF-α) (anti-TNF-α 1:200, Sicgen). Secondary antibody was incubated at room temperature for 1.5 hours. Detection procedures were carried on according to ECL system (GE Healthcare, Life Sciences; Little Chalfont, UK), and the signal detected using a ChemiDocTM Imaging Systems (Bio-Rad, Hercules, CA, USA). Membranes were re-probed for the β-actin (anti-β-actin 1:2000, Sicgen).
2.5. Data Analysis
Data were analyzed using GraphPad Prism Software, version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA) and presented as mean values with the standard error of the mean (SEM). The significance of the differences between the groups was calculated by Students’ t test and by one- and two-way ANOVA with Dunnett’s, and Bonferroni and Sidak’s multiple comparison tests. Differences were considered significant as p ≤ 0.05. Experimental groups are constituted by 8-10 animals.
3. Results
3.1. Effect of Overnutrition during Pregnancy and Lactation on Metabolic Parameters in the Mothers
Overnourished mothers for 6 weeks under HF diet were evaluated for body weight, glucose metabolism, assessed by basal glycemia and OGTT, insulin action, evaluated by the ipITT, and body composition, assessed by liver and AT depots weight measurement.
As expected, HF diet feeding of mothers for 6 weeks promoted an increase in basal glycemia (Basal glycemia: NC Mothers= 69.14 ± 1.792; HF Mothers= 78.88 ± 3.573, mg/dL, p<0.05) and a drastic decrease in insulin sensitivity (kITT: NC Mothers= 4.864 ± 0.3957; HF Mothers= 1.849 ± 0.3188, % glucose/min, p<0.0001), without affecting significantly glucose tolerance (Table 1). Moreover, HF diet did not alter body weight or liver and AT depots weight in mothers, maybe due to the pregnancy and offspring delivery during that period (Table 1).
3.2. Effect of Overnutrition during Pregnancy and Lactation on Metabolic Function in the Offspring
3.2.1. Insulin Action and Glucose Homeostasis
Female and male offspring born from overnourished mothers during pregnancy and lactation periods were evaluated for body weight, basal glycemia, insulin sensitivity and glucose tolerance (Figure 2).
HF feeding to mothers promoted a significant increase in body weight of both female and male descendants during all the postnatal periods analyzed (Figure 2A). Female offspring from HF mothers increased 65.1%, 33.3% and 9.6% at PD 21, 28 and 60, respectively (Figure 2A, left panel). Male offspring from overnourished mothers showed a significant increase in 63.9%, 45.6% and 20.5% at PD 21, 28 and 60, respectively (Figure 2A, right panel).
Maternal overnutrition during pregnancy and lactation significantly increased basal glycemia of female offspring at PD21 and 28 in comparison to female offspring from NC mothers (basal glycemia: NC PD21= 74.5±6.5, HF PD21=95.6±7.8, p<0.01; NC PD28= 61.0±2.4, HF PD28= 78.8±2.6, p<0.05) without changing basal glycemia at PD60 (Figure 2B, left panel). Interestingly, we found that during litter development, NC female offspring showed an increase in basal glycemia that is indeed significant at PD60 (basal glycemia: NC PD21= 81.3±2.6, p<0.01) when compared to PD28 values and that, in contrast, HF female offspring decreased basal glycemia from PD21 to PD28 (p<0.05), a tendency maintained at PD60 (basal glycemia: HF PD60= 85.6±2.0, p<0.01). Basal glycemia was not altered by HF maternal feeding in male offspring in the period of development analyzed (Figure 2B, right panel).
Figure 2.
Effect of overnutrition during pregnancy and lactation on metabolic phenotype of the offspring, evaluated by weight gain (A), insulin sensitivity (B) and glucose tolerance (C), at postnatal day 21 (PD21), 28 (PD28) and 60 (PD60). A) from the left to the right represents body weight in g during offspring development for females and males. B) from the left to the right represents basal glycemia in mg/dL during early development for females and males. C) Insulin sensitivity is represented by glycemia profiles during ipITT (C1) and the area under the curve of those glycemia curves during early descendants’ development for females and males, from the left to the right, respectively (C2). D) glucose tolerance is represented by glycemia profiles during OGTT (D1) and the area under the curve of those glycemia curves during early descendants’ development for females and males, from the left to the right, respectively (D2). PD21 – postnatal day 21; PD28 – post-natal day 28; PD60 – post-natal day 60. Data represents mean ± SEM. Two-Way ANOVA with with Bonferroni and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.
Figure 2.
Effect of overnutrition during pregnancy and lactation on metabolic phenotype of the offspring, evaluated by weight gain (A), insulin sensitivity (B) and glucose tolerance (C), at postnatal day 21 (PD21), 28 (PD28) and 60 (PD60). A) from the left to the right represents body weight in g during offspring development for females and males. B) from the left to the right represents basal glycemia in mg/dL during early development for females and males. C) Insulin sensitivity is represented by glycemia profiles during ipITT (C1) and the area under the curve of those glycemia curves during early descendants’ development for females and males, from the left to the right, respectively (C2). D) glucose tolerance is represented by glycemia profiles during OGTT (D1) and the area under the curve of those glycemia curves during early descendants’ development for females and males, from the left to the right, respectively (D2). PD21 – postnatal day 21; PD28 – post-natal day 28; PD60 – post-natal day 60. Data represents mean ± SEM. Two-Way ANOVA with with Bonferroni and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.

In Figure 2C is represented the curves of glycemia during the ipITT performed to the offspring (Figure 2C1) and the correspondent AUC of those curves (Figure 2C2). From the curves it can be seen that at PD21 male offspring from HF mothers present a significant increase in glycemia levels throughout all the ipITT (Figure 2C1, left panel) demonstrating a decrease in insulin sensitivity. At PD28 neither HF maternal feeding, nor sex, impacted glycemia profile during ipITT (Figure 2C1, central panel). At PD60, glycemia profiles during ipITT showed that male offspring have a significant increase in glycemia throughout the test, independently to the mothers’ diet during pregnancy and lactation (Figure 2C1, right panel). By the analysis of glycemia AUC from the ipITT during offspring development in the initial 60 days, insulin sensitivity significantly increased in the female descendants of NC mothers (AUC glycemia during ipITT: NC PD21=900.4±36.2; NC PD28=677.2±41.6, p<0.01; NC PD60=724.8±22.5, p<0.05) (Figure 2C2, left panel). In male offspring AUC glycemia during ipITT decreased from PD21 to PD28 and increased from PD28 to PD60 in both NC and HF descendants (AUC glycemia during ipITT: NC PD21=807.8±66.4; NC PD28=677.6±23.9, p<0.01; NC PD60=862.6±19.5, p<0.05; HF PD21=1022.2±81.6; HF PD28=663.9±41.2, p<0.01; HF PD60=878.6±34.9, p<0.05) (Figure 2C2, right panel), showing a significant increase in insulin sensitivity from PD21 to PD28 and a significant decrease in insulin sensitivity from PD28 to PD60 independent of maternal diet.
Finally, the impact of maternal diet and offspring sex on descendants’ glucose tolerance was evaluated by the OGTT (Figure 2D). The glycemia profiles showed that neither mother diet nor offspring sex impacted glucose tolerance at each PD evaluated (Figure 2D1). However, female offspring from HF mothers showed a significant decrease in glucose tolerance in comparison to female offspring of NC mothers at PD21 (AUC glycemia during OGTT: NC PD21=19955.2±1033.4; HF PD21=23999.4±857.5; p<0.05), an effect that is attenuated during offspring development (AUC glycemia during OGTT: HF PD21=23999.4±857.5; HF PD28=20866.1±345.6; HF PD60=20076.8±598.9; p<0.05) (Figure 2D2, left panel). Male offspring glucose tolerance was not altered during development by the diet fed to the mothers (Figure 2D2, right panel).
3.2.2. Liver and Adipose Tissue Depots Weight
In Table 2 are depicted the liver and adipose tissue depots weight of descendants of mothers submitted to NC or HF diet during gestation and lactation periods. Maternal overnutrition during pregnancy and lactation impacted both male and female offspring AT depots weight mainly at PD60.
In female offspring, overnutrition in mothers increased significantly visceral and perinephric AT at PD60 (visceral AT weight: NC Females PD60= 0.406 ± 0.038; HF Females PD60= 0.527 ± 0.055, p<0.001; perinephric AT weight: NC Females PD60= 0.196± 0.017; HF Females PD60= 0.374 ± 0.068, p<0.01, mg).
Regarding male offspring, maternal overnutrition promoted an increase in liver weight at PD21 (NC Males PD21= 2.286 ± 0.086; HF Males PD21= 2.717 ± 0.126, p<0.01) and at PD60 (NC Males PD60= 6.377 ± 0.326; HF Males PD60= 7.218 ± 0.465, p<0.05). Also, HF diet in mothers during pregnancy and lactation promoted an increase in the weight of visceral AT (NC Males PD60= 1.216 ± 0.182; HF Males PD60= 1.864 ± 0.152, p<0.01) and genital AT (NC Males PD60= 2.324 ± 0.375; HF Males PD60= 3.642 ± 0.503, p<0.01) in male descendants at PD60.
Brown AT weight did not alter significantly in both females and males descendants of HF mothers, at least till PD60.
Table 2.
Impact of overnutrition in mothers during pregnancy and lactation on the weight (mg) of the liver and of the different adipose tissue (AT) depots in male and female descendants at postnatal day 21, 28 and 60.
Table 2.
Impact of overnutrition in mothers during pregnancy and lactation on the weight (mg) of the liver and of the different adipose tissue (AT) depots in male and female descendants at postnatal day 21, 28 and 60.
| Liver | Visceral AT | Perinephric AT | Genital AT | Brown AT | |
|---|---|---|---|---|---|
| NC Females PD21 | 0.963 ± 0.066 | 0.262 ± 0.026 | 0.105 ± 0.017 | 0.128 ± 0.015 | 0.087 ± 0.010 |
| HF Females PD21 | 1.646 ± 0.139 | 0.278 ± 0.039 | 0.222 ± 0.026 | 0.318 ± 0.098 | 0.130 ± 0.021 |
| NC Females PD28 | 0.978 ± 0.056 | 0.257 ± 0.021 | 0.139 ± 0.012 | 0.114 ± 0.012 | 0.103 ± 0.009 |
| HF Females PD28 | 1.965 ± 0.115 | 0.344 ± 0.042 | 0.303 ± 0.053 | 0.286 ± 0.050 | 0.143 ± 0.015 |
| NC Females PD60 | 1.892 ± 0.118 | 0.406 ± 0.038 | 0.196± 0.017 | 0.214 ± 0.033 | 0.135 ± 0.009 |
| HF Females PD60 | 2.670 ± 0.158 | 0.527 ± 0.055 *** | 0.374 ± 0.068 ** | 0.472 ± 0.075 | 0.139 ± 0.010 |
| NC Males PD21 | 2.286 ± 0.086 | 0.376 ± 0.042 | 0.376 ± 0.059 | 0.305 ± 0.033 | 0.146 ± 0.016 |
| HF Males PD21 | 2.717 ± 0.126 ** | 0.561 ± 0.043 | 0.472 ± 0.042 | 0.528 ± 0.054 | 0.144 ± 0.012 |
| NC Males PD28 | 4.872 ± 0.405 | 1.029 ± 0.135 | 1.109 ± 0.212 | 1.993 ± 0.405 | 0.249 ± 0.025 |
| HF Males PD28 | 4.566 ± 0.178 | 1.472 ± 0.065 | 1.879 ± 0.281 | 2.183 ± 0.386 | 0.226 ± 0.026 |
| NC Males PD60 | 6.377 ± 0.326 | 1.216 ± 0.182 | 2.599 ± 0.381 | 2.324 ± 0.375 | 0.269 ± 0.025 |
| HF Males PD60 | 7.218 ± 0.465 * | 1.864 ± 0.152 ** | 3.162 ± 0.422 | 3.642 ± 0.503 ** | 0.284 ± 0.026 |
*p<0.05, **p<0.01; ***p<0.001 Two-way ANOVA with Sidak’s multicomparison test comparing animals submitted to normal chow (NC) and animals submitted to high-fat diet (HF) of the same age. PD21 – post-natal day 21; PD28 – post-natal day 28; PD60 – post-natal day 60.
3.3. Effect of Overnutrition during Pregnancy and Lactation on Behaviour Phenotype in the Offspring
In order to assess the impact of overnutrition during pregnancy and lactation on the offspring, a series of behavioral tests were conducted on offspring at PD60. These tests were categorized into three main groups: 3.2.1. Anxiety and Stress; 3.2.2. Memory and Learning; and 3.2.3. Food/Drink behavior.
3.3.1. Anxiety and Stress
In this battery of tests (Figure 3), we incorporated the OF, EPM, and the LDB test, all designed to measure anxiety and stress levels in the offspring [19].
Regarding the OF results, significant differences (p<0.05) were evident among the female offspring groups concerning the distance covered within the inner zone. This suggests that female offspring from mothers on a HF diet spend notably less time in the inner zone compared to those from control mothers. However, no significant differences were observed among the other groups for the remaining three parameters: total distance, total immobility/total test time, and immobility within the inner zone/total test time (Figure 3A).
In other hand, concerning the results of the Elevated Plus Maze (EPM) test, there were no significant differences detected between the groups (NC vs. HF) for the five parameters assessed: ratio (open arm/open+closed arm), ratio (closed arm/open+closed arm), number of poops, number of pees, and the frequency of alternations between arms (Figure 3B).
Finally, when it comes to the outcomes of the light-dark box test, there were no significant differences observed between the groups (NC vs. HF) in the three parameters analyzed: the proportion of time spent in the light zone relative to the total test time, the proportion of time spent in the dark zone relative to the total test time, and the number of alternations (Figure 3C).
Figure 3.
Effect of overnutrition during pregnancy and lactation on anxiety and stress, evaluated by the Open Field test (A), elevated plus maze test (B) and light-dark box test (C), in the offspring at postnatal day 60. A) from the left to the right the panels shows a schematic representation of the open field maze, the distance in the inner zone (cm), total distance covered (cm), total immobility (s), and the immobility in the inner zone (s); B) from left to right the panel shows a schematic representation of the elevated plus maze, the time in the open arms in comparison to the time in all arms (s), the time in the closed arms in comparison to the time in all arms (s), the number of poos in the maze, the number of pees, and the number of alternations between and the and the closed arms. Data represents mean ± SEM. Two-Way ANOVA with with Bonferroni and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals.
Figure 3.
Effect of overnutrition during pregnancy and lactation on anxiety and stress, evaluated by the Open Field test (A), elevated plus maze test (B) and light-dark box test (C), in the offspring at postnatal day 60. A) from the left to the right the panels shows a schematic representation of the open field maze, the distance in the inner zone (cm), total distance covered (cm), total immobility (s), and the immobility in the inner zone (s); B) from left to right the panel shows a schematic representation of the elevated plus maze, the time in the open arms in comparison to the time in all arms (s), the time in the closed arms in comparison to the time in all arms (s), the number of poos in the maze, the number of pees, and the number of alternations between and the and the closed arms. Data represents mean ± SEM. Two-Way ANOVA with with Bonferroni and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals.

3.3.2. Memory and Learning
For this set of assessments (Figure 4), we used the Y maze, the novel object recognition (NOR) and the block test, designed to assess memory and learning [19]
The Y-maze test was used to evaluate spatial learning and memory capacity in the offspring of both NC and HF animals. There were no significant differences observed in the four parameters analyzed: the time spent by the animals in the new arm, the ratio of time spent in the novel arm to the sum of time spent in both the novel and home arms, the number of entries into the novel arm, and alternative behaviors. The last parameter calculates the ratio of the number of alternations to the possible number (total number of arm entries minus two) (Figure 4A).
Regarding NOR results, there were no significant differences observed between the NC and HF groups in the three parameters measured: the time required to identify the new object, the ratio of time spent interacting with the novel object over the total time spent interacting with both the novel and familiar objects, and the ratio of time spent interacting with the familiar object over the total time spent interacting with both the novel and familiar objects. Nevertheless, while not statistically significant, there is an observable trend in the time taken to identify the new object (Figure 4B).
Finally, the animal’s olfactory working memory was assessed using the block test that assesses the ability of the animals to discriminate between familiar and novel, innocuous scents [18]. Results are presented in Figure 3C. No significant differences were observed between the experimental groups for the three parameters analyzed: the ratio of time spent sniffing the novel block over the total time spent sniffing both the novel and own blocks, the ratio of time spent sniffing the own block over the total time spent sniffing both the novel and own blocks, and the time required to identify the new block. However, it is worth noting that there is a noticeable trend in the last parameter, where the offspring of HF animals tend to take more time to complete this task.
Figure 4.
Effect of overnutrition during pregnancy and lactation on memory and learning, evaluated by the Y-maze test (A), novel object recognition test (B) and block test (C), in the offspring at postnatal day 60. A) from the left to the right panel shows a schematic representation of the y maze, the first arm choice (%), the ratio between the novel arm vs the time in both novel and familiar, the % of novel arm entries, and the alternative behavior (%); B) from the left to the right the panel presents a schematic representation of novel object recognition apparatus, the time to identify the novel object (s), the ration between the time the animals spent interact with the novel object vs the total interaction time (s), and the time the animals spent interact with the familiar object vs the total interaction time (s); (C) from left to right the panel present a schematic representation of the block test, the time to identify the novel block (s), the time the animals spent sniffing the novel block vs the total time sniffing all the blocks, and the time the animals spent sniffing the own blocks vs the total time sniffing all the blocks. Data represents mean ± SEM. Two-Way ANOVA with Bonferroni and Sidak’s multicomparison tests; *p<0.05, **p<0.01 comparing NC animals.
Figure 4.
Effect of overnutrition during pregnancy and lactation on memory and learning, evaluated by the Y-maze test (A), novel object recognition test (B) and block test (C), in the offspring at postnatal day 60. A) from the left to the right panel shows a schematic representation of the y maze, the first arm choice (%), the ratio between the novel arm vs the time in both novel and familiar, the % of novel arm entries, and the alternative behavior (%); B) from the left to the right the panel presents a schematic representation of novel object recognition apparatus, the time to identify the novel object (s), the ration between the time the animals spent interact with the novel object vs the total interaction time (s), and the time the animals spent interact with the familiar object vs the total interaction time (s); (C) from left to right the panel present a schematic representation of the block test, the time to identify the novel block (s), the time the animals spent sniffing the novel block vs the total time sniffing all the blocks, and the time the animals spent sniffing the own blocks vs the total time sniffing all the blocks. Data represents mean ± SEM. Two-Way ANOVA with Bonferroni and Sidak’s multicomparison tests; *p<0.05, **p<0.01 comparing NC animals.

3.3.3. Food/Drink Behavior
To assess competition for food and drink, the respective two tests were used: Food Competition and Water/Sucrose Competition (Figure 5).
The food competition test was used to assess both competition for food, the voracity of feeding, as well as the dominance in offspring in both NC and HF animals. No significant differences were observed in any of the five analyzed parameters: time eating, food intake, antagonistic behavior, time of antagonistic behavior, and grooming. However, a trend can be observed for two parameters: time eating and food intake. HF animals tend to feed for a longer period and consume a higher amount of food compared to NC.
In these tests, antagonistic behaviors indicating dominance over the other were taken into account (see methods 2.3.7 and 2.3.8.).
Like the previous test, the Water/Sucrose Competition is used to determine if there is a preference for drinking between sucrose and water. To do so, the amount of liquid ingested, the speed at which animals consume it, as well as the dominance of this resource among NC and HF animals are measured. When comparing significant differences within the same trial (when the liquid is the same) it is clear that when they compete for water, that males born from HF mothers drink water for more time, although without significant differences in the amount of liquid intake. Note also, that no differences were observed in females in the time or amount of water intake. Moreover, animals when exposed to water do not exhibit significant differences on antagonistic behavior, time of antagonistic behavior and grooming. When animals compete for sucrose no significant differences are observed among the five parameters previously mentioned. When comparing the Water trial vs. the Sucrose trial, it seems that the animals spent more time-consuming water than sucrose, exhibit a greater quantity of antagonistic behaviors when exposed to water compared to sucrose, and also engage in a higher amount of grooming during the water trial.
3.4. Effect of Overnutrition during Pregnancy and Lactation on Hypothalamic, Hippocampal and Prefrontal Markers of Synaptic Transmission, Metabolic Signaling and Inflammation
To assess the impact of excessive nutrition during pregnancy and lactation in some areas of the brain related with stress and anxiety - the hypothalamus, hippocampus, and prefrontal cortex - a Western blot technique was employed to examine the levels of some proteins and receptors involved in synaptic dynamics (3.3.1), metabolism (3.3.2), and inflammation (3.3.3).
3.4.1. Protein Markers of Synaptic Transmission on the Hypothalamus, Hippocampus and Prefrontal Cortex
Stress, anxiety and cognition has been associated with altered synaptic transmission and plasticity [20,21]. To investigate synaptic dynamics in the three designated areas, we access three distinct proteins: the glutamate vesicular transporter (vGLUT), the synaptosomal-associated protein 25kDa (SNAP-25), that contributes to the SNARE complex involved in exocytosis and the postsynaptic density protein 95 (PSD-95), a member of the membrane-associated guanylate kinase family that with PSD-93 is recruited to NMDA receptor and potassium channel clusters.
In the hypothalamus, development induced an increase of 37.5% and of 44.7% (p<0.001) in levels of vGLUT and females showed a non-significant increase of PD28 and PD60. Male HF offspring do not exhibit significant differences with NC offspring, but female offspring at PD21 show a significant increase of 57.5% that attenuates with age (Figure 6A1). Both males and females showed an increase in SNAP-25 levels from PD21 onwards in NC animals, this being statistically significant only for females at PD60 (increase of 99.3%). Moreover, both males and females born from HF mothers exhibit altered levels of SNAP-25 at the hypothalamus, with males exhibiting always higher levels of protein that are significant at PD28 and females exhibiting significant higher levels at PD21 that are maintained at PD28 and PD60 (Figure 6A2). In relation to PSD-95 it is clear that males exhibit a tendency to progressively increase the levels of this protein with age, an effect that is not observed in females. Overnutrition during pregnancy and lactation did not change these results (Figure 6A3).
In the hippocampus, no significant differences were observed regarding age, diet and sex for vGLUT (Figure 6B1) or PSD-95 (Figure 6B3). However, notable differences are evident in SNAP-25 in males and females. Note that there is a clear decrease in the levels of SNAP-25 between PD21 and PD28 and PD60 in NC animals (males: decrease of 32.3% PD28 and 52.6% at PD60; females: decrease of 52.2% at PD28 and 39.9% at PD60). Interestingly, overfeeding of mothers during pregnancy and lactation did not change the levels of SNAP-25 in males at PD21 and PD28, but increased its levels by 37.5% at PD60. Moreover, females born from HF mothers showed significantly increased levels at PD21 and PD60 of 47.7% and 59.8% respectively.
The prefrontal cortex development in males and females produced significant changes in the levels of vGLUT, but not on the other proteins studied where no differences were observed between PD21 and PD28 and PD60 (Figure 6C). Note that vGLUT levels were significantly higher at PD28 (increase of 70.3%) and PD60 (increase of 51.1%) in males and at PD60 (increase of 37.4%%) in females. Interestingly, only male offspring from HF mothers show differences at PD21 that are attenuated with age (Figure 6C1). SNAP-25 levels were increased in males and females born from HF mothers, an effect that was attenuated with age, but there was more marked in males. Note that, at PD21 and PD28 males born from HF mothers show high levels of SNAP-25 that were attenuated at PD60. Interestingly, this increase, although non-significant, was only seen in females at PD21 (increase by 51.1%%) (Figure 6C2). HF diet during pregnancy and lactation did not change the levels of PSD-95 in males or females (Figure 6C3).
Figure 6.
Effect of overnutrition during pregnancy and lactation on the levels of proteins involved in synaptic transmission on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs shows the mean levels of the ratio between vGLUT and β-actin, the ratio between SNAP-25 and β-actin and the ratio between PSD-95 and β-actin in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control. Data represents mean ± SEM. Two-Way ANOVA with with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.
Figure 6.
Effect of overnutrition during pregnancy and lactation on the levels of proteins involved in synaptic transmission on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs shows the mean levels of the ratio between vGLUT and β-actin, the ratio between SNAP-25 and β-actin and the ratio between PSD-95 and β-actin in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control. Data represents mean ± SEM. Two-Way ANOVA with with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.

3.4.2. Protein Markers of Metabolism on the Hypothalamus, Hippocampus and Prefrontal Cortex
Figure 7 depicts the impact of hypercaloric diets consumed by mothers in some metabolic markers – insulin receptor and AMPK - in some specific brain areas of the offspring.
Figure 7.
Effect of overnutrition during pregnancy and lactation on the levels of insulin receptor and AMPK on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs represent mean levels of the ratio between phosphorylated insulin receptor/total insulin receptor (P-IR/T-IR) and β-actin, and the ratio between phosphorylated AMPK and total AMPK (p-AMPK/T-AMPK) and β-actin in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control. Data represents mean ± SEM. Two-Way ANOVA with with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.
Figure 7.
Effect of overnutrition during pregnancy and lactation on the levels of insulin receptor and AMPK on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs represent mean levels of the ratio between phosphorylated insulin receptor/total insulin receptor (P-IR/T-IR) and β-actin, and the ratio between phosphorylated AMPK and total AMPK (p-AMPK/T-AMPK) and β-actin in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control. Data represents mean ± SEM. Two-Way ANOVA with with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.
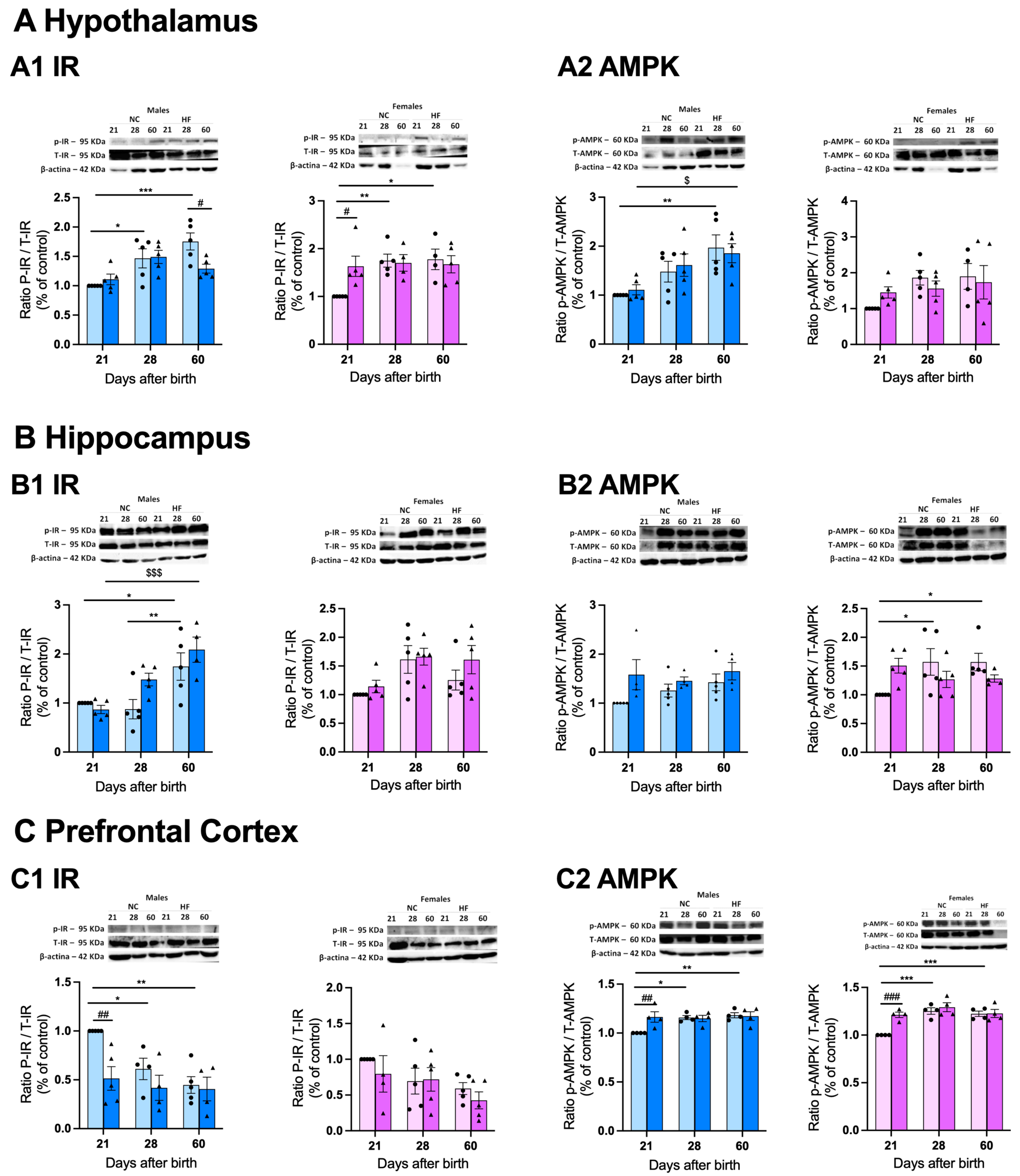
We can observe that in the hypothalamus development increased significantly and progressively the levels of the ratio IR-P/IR-T in males and females born from NC mothers (PD28 males 31.9% increase; PD60 males 43.0% increase; PD28 females 42.8% increase, PD60 females 44.4% increase), suggesting a higher phosphorylation of the IR with development. Interestingly, while no changes were observed in the levels of this ratio at PD21 and PD28 in males born from HF mothers, a significant reduction was seen at PD60 (49.3% decrease). In contrast, in females HF diet in mothers promoted a higher ratio of IR-P/IR-T at PD21 (40.2% increase) that was attenuated with age (Figure 7A1). Panel A2 shows the ratio between AMPK-P/AMPK-T, a metabolic sensor. Age increases the levels of the ratio AMPK-P/AMPK-T in both females and males, being these only significant in males (Figure 7A2), suggesting higher phosphorylation of AMPK with development. HF diet consumption in mothers did not change this ratio in both sexes (Figure 7A2). In the hippocampus, the levels of the ratio IR-P/IR-T follow more or less the same trend than in hypothalamus, with the phosphorylation of IR increasing by 49.8% at PD60 in males and progressively with development in females although without statistical significance (Figure 7B1). HF diet intake by mothers did not alter significantly the ratio of IR-P/IR-T in the offspring in both sexes (Figure 7B1). Interestingly, we observe sexual dimorphism in the ratio AMPK-P/AMPK-T at the hippocampus, with females NC showing a progressively and statistical significant increase in the phosphorylation of AMPK at PD28 (36.4%) and PD60 (36.3%), but no effect on males (Figure 7B2). HF diet in mothers did significantly change the ratio of AMPK-P/AMPK-T in the offspring in both sexes (Figure 7B2). Figure 7C depicts the levels of the same proteins in the prefrontal cortex. Note that there is a progressive decrease in the ratio IR-P/IR-T with development in the offspring from NC mothers, that is more accentuated and statistically significant in males (PD 28 38.8% decrease and PD60 55.4% decrease compared with PD21) than in females (Figure 7C1). Also note that HF diet intake in mothers promoted a decrease of 48.6% in the phosphorylation of IR at PD21 in males that was not altered with age. In females, no differences were observed (Figure 7C1). In contrast the ratio of AMPK-P/AMPK-T statistically increase in the offspring of NC mothers from PD21 onwards, both in males (PD28 13.6% increase, PD60 15.4% increase), and females (PD28 20.2% increase, PD60 18.3% increase). HF diet intake in mothers induced a higher phosphorylation of AMPK in the offspring at PD21 in both males (14.1% increase) and females (17.5% increase) that was maintained with age (Figure 7C2).
3.4.3. Protein Markers of Inflammation on the Hypothalamus, Hippocampus and Prefrontal Cortex
Inflammation has been implicated in several mood and anxiety disorders as well as in cognitive declive [22,23]. To explore the inflammation as being involved in the effects of overnutrition during pregnancy and lactation in stress and anxiety in the offspring we evaluated the levels of 3 inflammatory markers: GFAP, whose expression was shown to be increased in brain inflammatory states [23]; TNF-α, pro-inflammatory cytokine, expressed in many brain pathologies and associated with neuronal loss [24] and IL6-R, the receptor for IL6, a major cytokine that has anti- and pro-inflammatory in the brain and that has been linked with neurodegenerative diseases and with chronic stress contributing to neurobehavioral complications [25].
In the hypothalamus, no significant changes were observed in GFAP levels in males concerning diet or age. However, although GFAP levels in females did not change with age in NC offspring, they exhibited a 38.5% significant increase from PD21 to PD28 in HF offspring (Figure 8A1). Surprisingly, the levels of TNF-α decrease from PD21 to PD28 and PD60 both in males (PD28 32.7% , PD60 26.2%) and females (PD28 36.4% , PD60 52.4%) in NC offspring. Overnutrition during pregnancy and lactation in mothers did not alter the levels of TNF-α in the hypothalamus in the offspring (Figure 8A2). Considering IL6-R, there was an increase in the levels of this protein with development in both males (PD28 36.4%, PD60 91.8%) and females (PD28 32.5%)in NC offspring. Overnutrition during pregnancy and lactation in mothers did not alter significantly the levels of IL6-R in the hypothalamus in the offspring (Figure 8A3).
Figure 8.
Effect of overnutrition during pregnancy and lactation on the levels of proteins involved in inflammation on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs shows mean levels of the ratio between GFAP and β-actin, the ratio between TNF-α and β-actin and the ratio between IL6-R in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control Data represents mean ± SEM. Two-way ANOVA with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.
Figure 8.
Effect of overnutrition during pregnancy and lactation on the levels of proteins involved in inflammation on the hypothalamus (A), hippocampus (B) and prefrontal cortex (C). From the left to the right panel, graphs shows mean levels of the ratio between GFAP and β-actin, the ratio between TNF-α and β-actin and the ratio between IL6-R in males and females, respectively. On the top of the graphs are shown representative western blot membranes for the proteins of interest and the respective loading control Data represents mean ± SEM. Two-way ANOVA with Tukey’s and Sidak’s multicomparison tests; *p < 0.05, **p < 0.01 comparing NC animals with different ages; #p < 0.05, ##p < 0.01, and ###p < 0.001 comparing NC vs HF animals with the same age; and $p < 0.05, $$p < 0.01, and $$$$p < 0.0001 comparing HF animals with different ages.

In the hippocampus, when examining GFAP, no significant differences were concerning age and diet for both sexes in the offspring (Figure 8B1). In the analysis of the TNF-α, it becomes apparent that diet has a notable impact on males. While there was a decrease in TNF with age in male NC offspring, there was an increase in the levels IL6-R in HF offspring at PD28 and PD60 when compared to NC (35.8% and 51.2%, respectively), a trend not observed in females (Figure 8B2). When comparing IL6-R in males, HF diet in mothers increased by 35.8% at PD21, an effect that was attenuated at PD28 and PD60. In females, age did not change IL6-R levels in NC offspring, but HF diet intake in mothers increased significantly by 28.1% IL6-R levels at PD60 days (Figure 8B3).
In the prefrontal cortex GFAP levels increased significantly with age in males from NC offspring (PD28 39.6% and PD60 32.5%), an effect absent in females. Overnutrition during pregnancy and lactation in mothers did not alter significantly the levels of GFAP in the prefrontal cortex in the offspring (Figure 8C1). TNF-α decreases with age in both males and females born from NC mothers, however only in males the decrease is statistically significant ( PD28 41.5%, PD60 47.5% in comparison with PD21). HF diet intake in mothers did not change TNF-α levels in males or females (Figure 8C2). In the analysis of IL6-R, there was a 29.9% increase in IL6-R levels at PD60 in the male offspring from NC mothers, while in females no alterations were observed with age. Overnutrition during pregnancy and lactation in mothers did not alter significantly the levels of IL6-R in the prefrontal cortex in the offspring (Figure 8C3).
4. Discussion
In this study we demonstrated that: 1) female offspring born from overfeeding mothers during pregnancy and lactation showed increased weight gain and decreased glucose tolerance that attenuates with age; 2) offspring males born from overfeeding mothers exhibited increased weight gain that worsened with age while glucose tolerance remained unchanged; 3) offspring from HF mothers exhibited increased levels of anxiety and stress during behavioral tests, displaying decreased predisposition for curiosity comparing to control group; 4) offspring born from overfeeding mothers exhibited alterations in exocytotic capacity in the hypothalamus, hippocampus and prefrontal cortex and in some inflammatory markers in the hippocampus that are different in males and females. As a whole we demonstrate that maternal HF diet feeding during pregnancy and lactation induces dysmetabolism in the offspring accompanied by heightened stress and anxiety and by alterations in synaptic dynamics and neuroinflammation. Moreover, we found that there was sexual dimorphism in metabolic traits but not in behavior phenotypes.
4.1. Effect of Overnutrition during Pregnancy and Lactation on Metabolic Function in the Offspring
Herein we showed, as expected, that throughout offspring development, distinctions arise concerning sex, diet, and age. As the pup develops, body weight undergoes fluctuations depending on age, consistently showing a tendency to increase, largely influenced by dietary factors. Sexual dimorphism becomes apparent from 21 days onwards, with males generally exhibiting larger size and consequent greater weight than females. Over time, particularly in males, there is an escalating weight disparity influenced by diet. Conversely, in females, who commence hormone production at day 28 [26] the impact of diet on weight variation gradually diminishes. The hormone estrogen plays a pivotal role in regulating food intake and body weight, extending its influence to the modulation of insulin receptor abundance and ultimately being responsible for these differences between genders in overnutrition-dependent body weight gain [27].
Sexual dimorphism observed in weight was also evident in basal glycemia levels, with female offspring exhibiting changes in basal glycemia while male offspring don’t. This increased basal glycemia in female offspring at PD21 and PD28, compared to NC animals, suggests a significant impact of maternal diet during early development that could be attributed to a phenomenon known as developmental programming [28], where maternal influencing during critical periods of fetal and neonatal development shape the long-term health of offspring. Maternal overnutrition induces changes in the intrauterine environment, influencing the release of hormones and leading to epigenetic modifications [29]. These epigenetic changes can subsequently impact gene expression, potentially contributing to the observed differences in basal glycemia [30]. Interestingly, at PD60, the basal glycemia in female offspring from HF mothers did not differ significantly from NC female offspring. This stabilization or resolution of metabolic effects over time suggests potential adaptive mechanisms or compensatory changes in response to early-life exposures and a possible contribution of hormonal effects [31]. Hormonal and genetic differences between male and female offspring may contribute to the observed variations in metabolic outcomes, and in this case on basal glycemia [32].
Sexual dimorphism was also observed at insulin sensitivity. At PD21, male offspring from HF mothers demonstrated a significant increase in the area under the curve of the insulin tolerance test, indicating a decreased insulin sensitivity. This early effect suggests that maternal overnutrition during critical developmental periods may induce alterations in insulin signaling pathways, contributing to reduced insulin sensitivity in male offspring during early postnatal life. Surprisingly, at PD28, neither HF maternal feeding nor sex seemed to significantly impact glycemia profiles during the ITT. This lack of effect at PD28 suggests potential adaptive responses or normalization of insulin sensitivity in the offspring, possibly involving compensatory mechanisms that counteract the initial impact of maternal overnutrition [33]. By PD60, male offspring, irrespective of maternal diet, displayed a significant increase in the area under the curve of the ipITT. This effect at PD60 suggests that the effects of age on insulin sensitivity that are clearly described in adulthood are already seen at early ages [34]. Regarding female offspring, a different pattern emerged. The glycemia during ipITT indicated a significant increase in insulin sensitivity from PD21 to PD28 and a subsequent decrease from PD28 to PD60 in both NC and HF female descendants. These dynamic changes suggest a time-dependent modulation of insulin sensitivity in females. Hormonal fluctuations, metabolic adaptation, or other sex-specific factors may contribute to these variations in insulin sensitivity during different developmental stages [34]. Moreover, the absence of effects of maternal dysmetabolism on the insulin sensitivity with development contrasts with the documented impact of maternal obesity on insulin resistance later in life in animals [35] and humans [36]. However, this lack of effect on insulin sensitivity with development might be attributable to the duration of the exposure of mothers to the high fat diet and their level of metabolic dysfunction.
The glycemia profiles during OGTT, reveal that at each PD evaluated, neither maternal diet nor offspring sex exerted a significant impact on glucose tolerance. This suggests a robustness or resilience in glucose tolerance to variations in maternal nutrition during the assessed developmental stages, indicating that the offspring can maintain a relatively stable glucose tolerance despite differences in maternal diet or offspring sex [37]. However, a notable exception emerged in female offspring at PD21. Female descendants of HF mothers exhibited a significant decrease in glucose tolerance compared to their counterparts from NC mothers. Interestingly, this effect in female offspring was attenuated during development. The area under the curve of the OGTT decreased in HF female offspring from PD21 to PD28 and remained relatively stable from PD28 to PD60. We have to take in account that females start hormonal production at PD28 [26], therefore at this age it must have a potential adaptive response or compensatory mechanism in HF female offspring as they mature, indicating a dynamic regulation of glucose tolerance, potentially with hormonal fluctuations in female development having an important impact [37]. Conversely, male offspring did not exhibit alterations in glucose tolerance during development based on the diet fed to the mothers. This lack of effect in males underscores a potential sex-specific response to maternal diet in the context of glucose tolerance during the assessed developmental period. Moreover, a recent meta analysis showed that the exposure to maternal hyperglycemia during pregnancy might be associated with offspring obesity and abnormal glucose tolerance, although the association depends on the duration and intensity of intrauterine exposure to hyperglycemia [38] which is in agreement with the contrasting effects of our data with some published literature.
4.2. Effect of Overnutrition during Pregnancy and Lactation on Behaviour and CNS Functions in the Offspring
In the present manuscript we show that maternal exposure to an HF diet correlates with a propensity for stress and anxiety-like behavior in the offspring without alterations in memory and learning and on food behaviour. Our study demonstrates that offspring from HF mothers exhibit diminished exploratory behaviors and reduced curiosity, reflecting outcomes observed in previous studies involving offspring from HF damms [40]. This behavioral trend is consistent with existing literature that shows that rats subjected to an HF diet exhibit prolonged dwell times in the shadowy corners of mazes and an increased frequency of defecation, aligning with patterns reported by other researchers [39] and with human studies that demonstrate that prenatal maternal obesity is associated with offspring anxiety disorders, and that these associations may be long-lasting [42]. Moreover it was shown that the severity of diabetes during pregnancy may increase offspring’s vulnerability for depression/anxiety during childhood and adolescence [40] which is in agreement with our data presented herein.
Trying to unveil the underlying neurobiological mechanisms by which maternal overnutrition induces stress and anxiety in the offspring we focused on the synaptic dynamics and transmission, on metabolism and on neuroinflammation. In the evaluation of synaptic dynamics, a notable observation is the neuroprotection observed in females, that could be primarily attributed to the presence of hormones, such as estrogen, which serve a crucial role in preserving and maintaining neuronal health [41]. This neuroprotective effect contributes to the manifestation of sexual dimorphism, particularly evident in the prefrontal cortex concerning markers like SNAP-25, a marker for exocytosis and PSD-95, a marker for postsynaptic glutamatergic transmission. Additionally, the increase of vGLUT in the early days of life, which is also linked to glutamatergic transmission, may be associated with age and, once again, is influenced by the presence of hormones, especially in females, as noted in the hypothalamus and hippocampus [42]. Regarding the impact of overnutrition in mothers in the offspring, this is mainly observed at the exocytosis level, with HF-exposed pups displaying an increased rate of exocytosis in the 3 regions studied – hypothalamus, hippocampus and prefrontal cortex. However, we can also see a sexual dimorphism in synaptic transmission since the increase in the exocytotic marker, SNAP-25 seen in males in not observed in females neither in the hypothalamus nor prefrontal cortex being even decreased at PD60 in females at the hippocampus. Conversely, in the post-synaptic region, no discernible changes are evident with respect to either diet or age on the glutamatergic marker. While there are some evidence reporting altered glutamatergic signalling in the amygdala in the offspring from obese mothers [41,43], in the 3 regions studied in the present manuscript,overnutrition in in mothers do modify clearly markers of glutamatergic signalling suggesting that the effects of high fat diet involve other neurotransmitters than glutamate. This agrees with some demonstrations of decreased levels of GABAergic and serotonergic neurotransmitters in the whole brain [44]. Moreover, since hypothalamic neurotransmitters like POMC and NPY are critical for energy balance and feeding [45], that POMC-originated circuit regulates stress [46], that the ablation of POMC neurons showed anxiety-like behavior [47] and that NPY knockouts are anxious [48] probably other neurotransmitters will be key in driving this stress/anxiety phenotype in the offspring of dysmetabolic mothers. As a whole our results clearly suggests a selective influence of dietary factors on specific aspects of synaptic function, underscoring the intricate interplay between nutrition, gender-related hormonal influences, and synaptic mechanisms.
Concerning metabolism molecular pathways, an intriguing pattern emerges as males exhibit an age-related increase in the activation of insulin signalling pathway, while females maintain a consistent level throughout early days development. Notably, in the prefrontal cortex of males, there is a decline in insulin receptor phosphorylation with age. This trend suggests a plausible scenario wherein the initial higher activation of these receptors to participate in the high-rate metabolism at birth diminishing as the pup matures [43,44,45]. Interestingly, no significant effect of HF diet in mothers was appreciated in insulin signalling cascade in the offspring except for the statistically decrease in activation at PD60 in males at the hypothalamus. Note that, at the hypothalamus insulin suppresses food intake, is involved in glucose and fat metabolism regulation [49,50] and controls sympathetic activity [51]. Therefore, a decrease activation of this pathway at PD60 can antecipate the late development of cardiometabolic complications. Regarding AMP-activated protein kinase (AMPK), the activation of this protein experiences an increase across various brain regions and age groups. This versatile protein not only facilitates the conversion of AMP into ATP but also responds to nutrients, metabolites, and hormones involved in energy balance [52], being also involved in the regulation of growth and reprogramming metabolism [53], which could explain the increase during development. Herein we observed slight increases in AMPK signalling at PD21 in the offspring of HF mothers, both in males and females, that are attenuated with age. Within the hormones that modulate AMPK-dependent metabolic control there is insulin, leptin, ghrelin, estrogens, etc. Therefore, hormonal maturation is a crucial step that will change AMPK pathway activation in the control of food intake and energy metabolism. Moreover, the distinct hormonal maturation between sexs will make AMPK modulation different in males and females due to the role of sex-specific hormones previously described [51]. Given its multifaceted role in various pathways, further investigations are warranted to pinpoint the specific pathways activated by AMPK, and its specific role in the context of brain function [46,47] and in the context of the transgenerationality of dysmetabolism.
When inflammatory markers were analyzed we showed a general trend where females exhibit lower levels of these markers compared to males. This distinction is notably apparent from the age of 28 days onward, coinciding with the onset of hormone production in females, which may act as a protective mechanism against external stressors, thereby mitigating the risk of elevated inflammation [54]. Note also that in contrast, males, on average, display an upswing in inflammatory markers, namely GFAP and IL6-R, when being descendants of NC mothers. Notably, all the regions studied in the present manuscript exhibit a higher amount of the TNF-α molecule at PD21 that decreases with development. Throughout uterine development, pupps are constantly protected from external agents, benefiting from the protective environment provided by their mother’s uterus, including a regulated temperature. Consequently, it is expected that their inflammatory levels remain low during this stage of development [56]. However, after birth, the newborn is exposed to a variety of external agents and environmental conditions that can trigger an initial increase in inflammatory levels during the first few days [57]. Afterwards, the newborn gradually acclimatizes to these external factors, leading to a reduction in inflammation levels [58]. The tendency for inflammation levels to decrease with age reflects the continuous acclimatization of the newborn to the external environment. Regarding the effect of overnutrition of mothers in the offspring while no alterations in GFAP levels, which have been associated previously with neuroinflammation [22,23] were seen in the present study, we observe increased levels of TNF-α particularly on the hippocampus of males and of IL6-R at the hypothalamus and at the hippocampus in both males and females suggesting that neuroinflammation in a neurobiological process that underlies the development of stress and anxiety behavior. This agrees with previous studies that demonstrated that anxiety-like behavior was associated with increased mRNA expression of proinflammatory markers including IL6, TNFα, NFkB and MCP-1 in the hypothalamus and amygdala [59,60,61]. However, more information will be needed and more markers of inflammation should be tested and even in different models (different exposure times to diets and different diets) to definitely define an association between neuroinflammation and stress and anxiety behavior in the offspring.
5. Conclusions
Taken together this work demonstrates that, as demonstrated before, exposure to HF diet during pregnancy and lactation induces dysmetabolism in the offspring, and adds information related with behavior impact of maternal overnutrition, showing that it induces heightened stress and anxiety. Finally, we have shown that most of these effects of maternal overnutrition during pregnancy and lactation show sexual dimorphism on metabolic traits but not in behavioral phenotypes.
Author Contributions
Conceptualization, S.V.C, J.F.S. and F.O.M..; methodology, G.M.M., A.M.C., J.F.S., J.P., M.V.F., I.F.A., S.V.C.; resources, S.V.C., F.O.M., J.F.S:; data curation, G.M.M., A.M.C. F.O.M, S.V.C.; writing, A.M.C, G.M.M, F.O.M., S.V.C; writing—review and editing, J G.M.M., A.M.C., J.F.S., J.P., M.V.F., I.F.A., F.O.M., S.V.C..; funding acquisition, F.O.M., J.F.S., S.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology with PhD grants to G.M.M (Ref 2022.12291.BD), A.M.C. (Ref 2022.11376.BD) and I.F.A (Ref UI/BD/154298/2022) and CEEC contracts to F.O.M (CEEC IND/02428/2018) and J.F.S. (2021.03439.CEECIND).
Institutional Review Board Statement
The animal study was reviewed and approved by NOVA Medical School Ethics Committee (Ref nº 194/2021/CEFCM) and by Direção Geral Agricultura e Veterenária (Ref.ª 0421/000/000/2021 DGAV).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alberti, K. G. M. M. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Rojas, M. Metabolic syndrome: Is it time to add the central nervous system? Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Barker, D. J. P. The origins of the developmental origins theory. Journal of Internal Medicine 2007, 412–417. [Google Scholar] [CrossRef]
- Marciniak, A. , Patro-Małysza, J., Kimber-Trojnar, Ż., Marciniak, B., Oleszczuk, J., and Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwanese Journal of Obstetrics and Gynecology 2017, 56, 2133–138. [Google Scholar] [CrossRef]
- Mulligan, C. M. and Friedman, J. E. Maternal modifiers of the infant gut microbiota: Metabolic consequences. Journal of Endocrinology 2017, 235, R1–R12. [Google Scholar] [CrossRef]
- Jašarević, E. Howard, C. D. Misic. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Sousa, D. et al., “Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Patro, B. , Liber, A., Zalewski, B., Poston, L., Szajewska, H. and Koletzko, B. Maternal and paternal body mass index and offspring obesity: A systematic review. Annals of Nutrition and Metabolism 2013, 63, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A. Baptista, F. I. and Matafome, P. Programming of future generations during breastfeeding: The intricate relation between metabolic and neurodevelopment disorders. Life Sciences 2022, 298. [Google Scholar] [CrossRef] [PubMed]
- Leddy, M. A. , Power, M. L. and Schulkin, J. The Impact of Maternal Obesity on Maternal and Fetal Health. 2008.
- Hyatt H., W. Zhang, Y. Hood, W. R. and Kavazis, A. N. Lactation has persistent effects on a mother’s metabolism and mitochondrial function. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Mezei, G. C. Ural, S. H. and Hajnal, A. Differential effects of maternal high fat diet during pregnancy and lactation on taste preferences in rats. Nutrients 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Gautier, A. et al. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol (Oxf) 2013, 78, 373–378. [Google Scholar] [CrossRef]
- Kokras, N. and Dalla, C. Preclinical sex differences in depression and antidepressant response: Implications for clinical research. Journal of Neuroscience Research 2017, 95, 731–736. [Google Scholar] [CrossRef]
- Monzillo, L. U. and Hamdy, O. Evaluation of Insulin Sensitivity in Clinical Practice and in Research Settings. Nutrition Reviews 2003, 61, 397–412. [Google Scholar] [CrossRef]
- Ribeiro, M. J. , Sacramento, J. F. Gonzalez, C. Guarino, M. P., Monteiro, E. C. and Conde, S. V. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 2013, 62, 2905–2916. [Google Scholar] [CrossRef]
- Seibenhener M., L. and Wooten, M. C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments 2015. [Google Scholar] [CrossRef]
- Lehmkuhl, A. M. E. , Dirr, R. and Fleming, S. M. Olfactory assays for mouse models of neurodegenerative disease. Journal of Visualized Experiments 2014. [Google Scholar] [CrossRef]
- Wolf, A. , Bauer, B., Abner, E. L., Ashkenazy-Frolinger, T. and Hartz, A. M. S. A comprehensive behavioral test battery to assess learning and memory in 129S6/ Tg2576 mice. PLoS One 2016, 11. [Google Scholar] [CrossRef]
- Kennedy, M. B. Synaptic signaling in learning and memory. Cold Spring Harb Perspect Biol 2016, 8, 1–16. [Google Scholar] [CrossRef]
- Borrow, A. P. , Stranahan, A. Suchecki, M. D. and Yunes, R. Neuroendocrine Regulation of Anxiety: Beyond the Hypothalamic-Pituitary-Adrenal Axis. Journal of Neuroendocrinology 2016, 28. [Google Scholar] [CrossRef]
- Sun, Y. , Koyama, Y., and S. Shimada, “Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Frontiers in Aging Neuroscience 2022, 14, 16. [Google Scholar] [CrossRef]
- Won, E. and Kim, Y. K. Neuroinflammation-associated alterations of the brain as potential neural biomarkers in anxiety disorders. International Journal of Molecular Sciences 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Neniskyte, U. , Vilalta, A. and Brown, G. C. Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. FEBS Lett 2014, 588, 2952–2956. [Google Scholar] [CrossRef]
- McGregor B., A. , et al., “Corrigendum to ‘Alpha-Synuclein-induced DNA Methylation and Gene Expression in Microglia’ [Neuroscience 468 (2021) 186–198] (Neuroscience (2021) 468 (186–198), (S0306452221002748), (10.1016/j.neuroscience.2021.05.027)). Neuroscience 2022, 492, 108. [Google Scholar] [CrossRef]
- Boberg, J. et al., “Comparison of female rat reproductive effects of pubertal versus adult exposure to known endocrine disruptors. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Sanchez-Garrido M., A. , et al., “Early overnutrition sensitizes the growth hormone axis to the impact of diet-induced obesity via sex-divergent mechanisms. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Şanlı, E. and Kabaran, S. Maternal Obesity, Maternal Overnutrition and Fetal Programming: Effects of Epigenetic Mechanisms on the Development of Metabolic Disorders. Curr Genomics 2019, 20, 419–42. [Google Scholar] [CrossRef]
- Bianco M., E. and Josefson, J. L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Current Diabetes Reports 2019, 19. [Google Scholar] [CrossRef]
- Rosen E., D. et al., “Epigenetics and epigenomics: Implications for diabetes and obesity. Diabetes 2018, 67, 1923–1931. [Google Scholar] [CrossRef]
- Shou, J. , Chen, P. J., and Xiao, W. H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetology and Metabolic Syndrome 2020, 12, 11. [Google Scholar] [CrossRef]
- Nicholas, L. M. , Nagao, M., Kusinski, L. C., Fernandez-Twinn D. S., Eliasson, L., and Ozanne S. E. Exposure to maternal obesity programs sex differences in pancreatic islets of the offspring in mice. Diabetologia 2020, 63, 324–337. [Google Scholar] [CrossRef]
- “MCN-16-e13031”.
- Melo, B. F. , Sacramento, J. F., Capucho, A. M., Sampaio-Pires, D., Prego, C. S., and Conde, S. V. Long-Term Hypercaloric Diet Consumption Exacerbates Age-Induced Dysmetabolism and Carotid Body Dysfunction: Beneficial Effects of CSN Denervation. Front Physiol 2022, 13. [Google Scholar] [CrossRef]
- Desai, M. , Han, G., Mossayebi, E., Beall, M. H., and Ross, M. G., “552: Programmed insulin resistance of offspring of obese mothers. Am J Obstet Gynecol 2017, 216, S325–S326. [Google Scholar] [CrossRef]
- Mingrone, G. et al., “Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 2008, 31, 1872–1876. [Google Scholar] [CrossRef]
- van den Beld, A. W. , Kaufman, J. M., Zillikens, M. C., S. Lamberts, W. J., Egan, J. M. and van der Lely, A. J. The physiology of endocrine systems with ageing. The Lancet Diabetes and Endocrinology 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Kawasaki, M. et al., “Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Aslani, S. , Vieira, N., Marques, F., Costa, P. S., Sousa, N. and Palha, J. A. The effect of high-fat diet on rat’s mood, feeding behavior and response to stress. Transl Psychiatry 2015, 5. [Google Scholar] [CrossRef]
- XIANG, A. et al., “251-OR: Maternal Diabetes during Pregnancy and Incidence of Depression and Anxiety in Offspring from Childhood to Young Adulthood. Diabetes 2022, 71. [Google Scholar] [CrossRef]
- Sohrabji, F. On: Wed. Gene Expr 2018, 13, 311–319, Available: www.cognizantcommunication.com. [Google Scholar] [CrossRef]
- Daniels, R. W. , Miller, B. R., and DiAntonio, A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis 2011, 41, 415–420. [Google Scholar] [CrossRef]
- Glendining, K. A. , Fisher, L. C. and Jasoni, C. L. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 2018, 96, 132–141. [Google Scholar] [CrossRef]
- Erbas, O. et al., “Neurobehavioral effects of long-term maternal fructose intake in rat offspring. International Journal of Developmental Neuroscience 2018, 69, 68–79. [Google Scholar] [CrossRef]
- Qi,Y. et al., “Agrp-negative arcuate NPY neurons drive feeding under positive energy balance via altering leptin responsiveness in POMC neurons. Cell Metab 2023, 35, 979–995. [CrossRef]
- Qu, N. et al., “A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatry 2020, 25, 1006–1021. [Google Scholar] [CrossRef]
- Greenman, Y. et al. Postnatal Ablation of POMC Neurons Induces an Obese Phenotype Characterized by Decreased Food Intake and Enhanced Anxiety-Like Behavior. Molecular Endocrinology 2013, 27, 1091–1102. [Google Scholar] [CrossRef]
- Reichmann, F. and Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int J Mol Sci 2019, 20, 1317. [Google Scholar] [CrossRef]
- ESLER, M. , “Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 2001, 14, S304–S309. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. and Cheng, K. 2018. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. International Journal of Molecular Sciences 2018, 19, 3552. [Google Scholar] [CrossRef] [PubMed]
- Kola, B. Role of AMP-Activated Protein Kinase in the Control of Appetite. J Neuroendocrinol 2008, 20, 942–951. [Google Scholar] [CrossRef]
- Mihaylova, M. M. , and Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Liu, Y. Z. , Wang, Y. X. and Jiang, C. L. Inflammation: The common pathway of stress-related diseases. Frontiers in Human Neuroscience 2017, 11, 20. [Google Scholar] [CrossRef]
- Burch, K. E. , McCracken, Buck, K. D. J., Davis, R. L., Sloan, D. K., and Curtis, K. S. Relationship Between Circulating Metabolic Hormones and Their Central Receptors During Ovariectomy-Induced Weight Gain in Rats. Front Physiol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C. P. , Bennett, J. M., Derry, H. M., and Kiecolt-Glaser, J. K. House. 2001. [Google Scholar] [CrossRef]
- Humberg, A. et al., Preterm birth and sustained inflammation: consequences for the neonate Network, German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Semin Immunopathol 2020, 42, 451–468. [Google Scholar] [CrossRef]
- Schultz, C. et al., Immature anti-inflammatory response in neonates. Clin Exp Immunol 2004, 135, 130–136. [Google Scholar] [CrossRef]
- Sasaki, A. , Vega, W., Sivanathan, de S., St-Cyr, S. and McGowan, P. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef]
- Martin, H. L. et al., A peroxisome proliferator-activated receptor-δ agonist provides neuroprotection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience 2013, 240, 191–203. [Google Scholar] [CrossRef]
- Jakovac, H. , Grubić Kezele, T. and Radošević-Stašić, B. Expression Profiles of Metallothionein I/II and Megalin in Cuprizone Model of De- and Remyelination. Neuroscience 2018, 388, 69–86. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Schematic illustration of the protocol of the study.
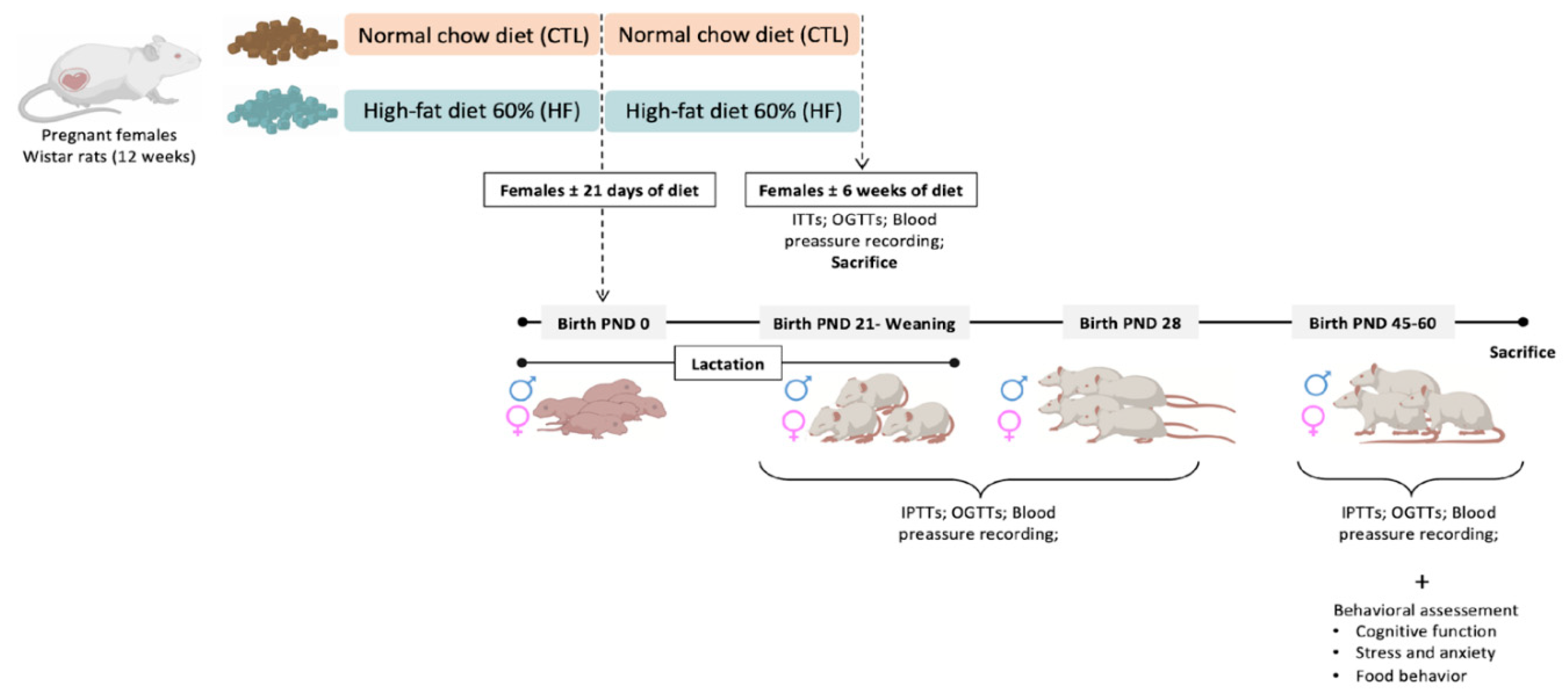
Figure 5.
Effect of overnutrition during pregnancy and lactation on food behavior, evaluated by food competition tests (A) and water-sucrose competition tests (B). A) from the left to the right the panel shows a schematic representation of the food competition test, the time spent eating (s) and the food intake (g) (A1), the antagonistic behavior and the time of antagonistic behavior (s) (A2), and the time of grooming (s) (A3); B1-B3) from the left to the right the panel represents a schematic representation of water-sucrose competition, time drinking (s) and the liquid intake (ml) (B1), the antagonistic behavior and the time of antagonistic behavior (s) (B2), and time of grooming (s) (B3) in response to water competition; B4-B6) from the left to the right the panel represents shows the time drinking (s) and the liquid intake (ml) (B4), the antagonistic behavior and the time of antagonistic behavior (s) (B5), and time of grooming (s) (B6) in presence of sucrose. Data represents mean ± SEM. Two-way ANOVA with Bonferroni and Sidak’s multicomparison test; *p < 0.05, **p < 0.01 comparing NC animals.
Figure 5.
Effect of overnutrition during pregnancy and lactation on food behavior, evaluated by food competition tests (A) and water-sucrose competition tests (B). A) from the left to the right the panel shows a schematic representation of the food competition test, the time spent eating (s) and the food intake (g) (A1), the antagonistic behavior and the time of antagonistic behavior (s) (A2), and the time of grooming (s) (A3); B1-B3) from the left to the right the panel represents a schematic representation of water-sucrose competition, time drinking (s) and the liquid intake (ml) (B1), the antagonistic behavior and the time of antagonistic behavior (s) (B2), and time of grooming (s) (B3) in response to water competition; B4-B6) from the left to the right the panel represents shows the time drinking (s) and the liquid intake (ml) (B4), the antagonistic behavior and the time of antagonistic behavior (s) (B5), and time of grooming (s) (B6) in presence of sucrose. Data represents mean ± SEM. Two-way ANOVA with Bonferroni and Sidak’s multicomparison test; *p < 0.05, **p < 0.01 comparing NC animals.

Table 1.
Impact of high-fat diet on metabolic parameters in mothers. .
| NC MOTHERS | HF MOTHERS | |
|---|---|---|
| Body Weight (g) | 243.2 ± 5.122 | 236.8 ± 2.577 |
| Basal Glycaemia (mg/dL) | 69.14 ± 1.792 | 78.88 ± 3.573 * |
| kITT (% glucose/min) | 4.864 ± 0.3957 | 1.849 ± 0.3188 **** |
| AUC Glycaemia (mg/dL*min) | 18138 ± 417.4 | 19612 ± 594.9 |
| Liver (g/kg) | 0.0280 ± 0.001 | 0.0289 ± 0.001 |
| Visceral Adipose Tissue (g/kg) | 0.0057 ± 0.002 | 0.0066 ± 0.002 |
| Perinephric Adipose Tissue (g/kg) | 0.0153 ± 0.002 | 0.0171 ± 0.004 |
| Genital Adipose Tissue (g/kg) | 0.0232 ± 0.002 | 0.0254 ± 0.002 |
| Brown Adipose Tissue (g/kg) | 0.0007 ± 0.000 | 0.0016 ± 0.000 |
* p<0.05, **** p<0.0001 Students’ t test comparing values in animals submitted to normal chow (NC) and animals submitted to high-fat diet (HF).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Overnutrition during Pregnancy and Lactation Induces Gender-Dependent Dysmetabolism in the Offspring Accompanied by Heightened Stress and Anxiety
Gonçalo M Melo
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated












