Preprint
Article
Sterile Fecal Microbiota Transplantation Boosts Anti-Inflammatory T-Cell Response in Ulcerative Colitis Patients
Altmetrics
Downloads
106
Views
45
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
07 December 2023
Posted:
07 December 2023
You are already at the latest version
Alerts
Abstract
Ulcerative colitis is a chronic immune-mediated disease of unclear etiology, affecting people of different ages and significantly reducing the quality of life. Modern methods of therapy are mainly represented by anti-inflammatory drugs and are not aimed at a specific pathogenetic factor. In this study, we investigated the effect of transplantation of sterile stool filtrate from healthy donors on the induction of anti-inflammatory immune mechanisms. It was shown that performing such a procedure in patients with ulcerative colitis caused the appearance of T helper cells in the blood, which reacted to the content of sterile stool filtrates in an antigen-specific manner and produced IL10. At the same time, cells of the same patients before therapy in response to the addition of sterile stool filtrates were less reactive and predominantly produced IL4, indicating its pro-inflammatory skewing. The obtained data demonstrated the effect of an anti-inflammatory shift in the T-helper response after transplantation of sterile stool filtrate, which increased and persisted for at least three months after the procedure.
Keywords:
Subject: Medicine and Pharmacology - Immunology and Allergy
1. Introduction
Ulcerative colitis (UC) is a multifactorial disease, primarily driven by immune system hyperactivation. However, neither the particular pathogen, nor the antigen set has been described as a strong driving factor. The pathogenesis of UC is mainly immune-related inflammation, caused by hypersensitivity reaction in the sub-epithelial layers of the colon. UC in contrast with Crohn’s disease is primarily driven by Th2 type immune response making it more similar to hypersensitivity type 4 response rather than typical bacteria-specific response that is primarily caused by Th17 type of cells. However, Th2-typse response also is a hallmark of any type of long-lasting chronical inflammation, including both bacteria-specific or autoummune type response.
To the date the only available therapy is a spectrum of anti-inflammatory medications, varying from moderately light non-steroid anti-inflammatory drugs to the severe hormonal and cytostatic drugs. Despite the progress has been made with the introduction of a novel anti-inflammatory immunotherapy, the overall remission net rate remains low [1]. One of the potential explanations is that the state of immune system activity is not the primer for the inflammation, and blocking one or the other immune pathway does not play a long-term role, as another type of response may be activated towards unknown factor or a group of factors. In any case, it has been shown that Inflammatory Bowel Diseases (IDB) are associated with relative deficiency of regulatory T-cell response [2,3], making it an autoimmune spectrum diseases that is characterized by the lack of immune suppression towards self-antigens. However, no prognostic or actionable autoantigen has been proposed yet as a strong clinically significant biomarker.
A novel view to the problem implies that microbiota alteration may be a driver of the observed alterations. The intestinal microbiota itself serves as a relevant immune-training compartment [4] providing both innate (PAMP’s, MAMP’s) and specific (bacteria-derived antigens) [5]. The pattern of microbiota alteration characterized by the increase of the relative abundance of the Firmicutes phylum compared to the Bacteroidota phylum represents the typical finding during clinical examination of patients gut microbiome using 16S ribosomal RNA [6]. Also, the direct cause-effect relationship has been shown to the Bacteroidotes-mediated Treg upregulation [7], while bacteria of the Firmicutes phylum are recognized to be associated with autoimmune spectrum diseases, such as rheumatoid arthritis [8,9]. However, other studies do not support the Firmucited/Bacteroidotes ratio as a significant marker of the particular inflammatory bowel disease (IBD) [10].
As a method of treating patients with IBD, the Fecal Microbiota Transplantation (FMT) has been suggested. The concept was initially relying on the idea that the simple transfer of bacterial communities from healthy donors to patients with IBD may shift the inflammatory state because of induction of the right bacteria bringing immune-suppressive capacity. Nevertheless, to date no clinical trial has shown unambiguous outcomes for that type of therapy of patients with UC and Crohn’s disease [11]. It should be noted that microbiota transplantation being the method under investigation is not applicable to the patients with the severe inflammatory state that limits the data available on the potential treatment outcomes. The major source of negative outcomes of FMT may be the fact that the microbiota compositions may differ drastically, and the patient-specific metabolic profiles are not appropriate for the donor microbiota community «engraftment» [12]. Overall, the FMT was prone to be more successful in patients with non-severe onset or in prolongation of remission duration in chronic UC patients [10].
One of the promising possibilities is that bacterial transfer itself is not that important for FMT to be efficient, as metabolite profile or bacteriophage content [13,14,15]. In contrast to the idea that non-sterile FMT may bear the bacteria that directly influence immune response, the idea of bacteria-free FMT is based on its regulatory rather than bacterial-engraftment effect. Thus, FMT has been shown to alter the ratio of helper T cells in favor of regulatory T cells and other IL10-producing T cells in a murine model of dextran sulfate-induced colitis [16]. Applied to humans, FMT in patients with inflammatory bowel diseases resulted in increased IL10 production and a decrease in IL17 blood level [17]. Some researchers attribute these effects to colonization of healthy donor intestinal microbiota, accompanied by increased production of a number of metabolites that promote the formation of regulatory T cells. At the same time, other studies have shown that intestinal colonization in FMT is not a key factor in the beneficial effects of therapy [11].
The aim of this study was to investigate the potential of sterile stool samples containing no bacterial cells to influence anti-inflammatory helper T cell activation in patients after an experimental Sterile Fecal Microbiota Transplantation (SFMT).
2. Results
2.1. Immune Status of Biological Samples and Experimental Settings
The study included 8 patients who agreed to undergo sterile intestinal microbiota transplantation and met the inclusion criteria for patients in the study design. The hypothesis of this study suggests that the administration of sterile stool filtrates can cause an immunomodulatory effect due to antigenic and adjuvant factors of the microbiota of healthy donors. CD4+ lymphocytes and the concentration of cytokines IL4 and IL10, which play a role in the state of maintaining or switching off the inflammatory response, were studied. Biomaterial from patients was collected at three time points: before trans-plantation, 1 and 3 months after the procedure (Figure 1a).
In order to establish these effects, we carried out cross-cultivation of peripheral mononuclear cells obtained at different periods of the study with samples of donor and autologous stool filtrates obtained at the same time points (Figure 1b). Criteria for cell activation under conditions in vitro is the expression of the marker CD25 and CD127, corresponding to IL2 and IL7 receptors on the cell surface. It has been shown that CD25+CD127+ are the helper T-cells with anti-inflammatory properties. In particular, cells producing IL10 [18], while and CD25+CD127low cells are a full-fledged regulatory T cells [19].
When isolating cells and stool filtrates, it was necessary to evaluate the initial indicators of the immune status of patients, as well as the presence of the studied cytokines in stool filtrates, to exclude artifacts in subsequent measurements ex vivo.
We did not find any significant changes in the total content of CD4+ T lymphocytes in the blood of patients over the period after the therapy and compared with the group of healthy patients (Figure 2a). At the same time, analysis of the cell content phenotypically corresponding to regulatory T lymphocytes (CD3+CD4+CD25+CD127low) demonstrated a trend towards a decrease in the number of these cells in patients 3 months after therapy as compared with the state before therapy and compared to a group of healthy patients (Figure 2b). The content of Treg cells in the blood of patients before therapy did not significantly differ from that in healthy patients.
2.2. Influence of Sterile Fecal Filtrates from Donors on Helper T Cell Population in UC Patients
At the first stage, we assessed the effect of sterile filtrates of donor stool on phenotypic and physiological changes in cells in vitro. The introduction of these filtrates into cell cultures obtained before and after therapy demonstrated time-dependent dynamics in relation to the presence of both activated T cells and their production of cytokines. Analysis of cytokine production (Figure 3a,b) indicated that sterile filtrates of donor stool was unable to induce production of the cytokine IL4 that is the Th2 cell response marker in the culture fluid of patients, donors, and healthy volunteers. The production of IL10 was significantly increased in patients three months after therapy. One month after therapy, there was also a tendency for IL10 to increase, but only in some patients. The sterile filtrates of donor stool were unable to induce IL10 production in cells from patients before therapy, as well as in cells from donors and healthy volunteers.
Regarding the presence of CD4+ cells in culture indicated their increase in dynamics after therapy (Figure 3c). Analysis of the activation status of these cells in terms of expression of CD25 and CD127 markers showed a time-dependent increase in the content of activated lymphocytes with the CD25+CD127+ phenotype (Figure 3d). Normalizing their number to the total number of CD4+ cells reduced the level of the differences. Cells that were obtained before therapy responded to the addition of sterile filtrates of donor stool to a lesser extent than those that were obtained three months after therapy, which may indicate the formation of an antigen-specific immune response pattern. Cells of healthy volunteers responded to the addition of sterile filtrates of donor stool by increasing the content of CD4+ lymphocytes, and CD4+ CD25+ CD127+ cells at a level corresponding to the cells of patients before therapy. Apparently, the sterile filtrate of donor stool, having a minimal antigenic and adjuvant load, was able to form an environment that supports the viability of immune cells; however, it was not sufficient to activate proliferation. No CD25+ CD127low cells were detected.
Analysis of the subtypes of activated cells characterizing the memory phenotype according to the expression of CD45RA, CD45RO and CCR7 markers (Figure 4) demonstrated that the bulk of cells responding to the addition of the filtrate were TEMRA-cells (CD45RA+CD45RO-CCR7-), corresponding to maximally reactogenic cells, both when introducing sterile filtrate of donor stool into the cells of patients and into the culture of healthy volunteers. At the same time, the content of cells with a central memory phenotype increased in patients three months after therapy. This effect was not observed in cells from healthy volunteers.
2.3. Autologous Sterile Fecal Filtrates Drive Changes in IL4 and IL10 Production
The production of cytokines in the cultures of these cells is characterized by a noticeable dynamic of the production of IL 4 and IL 10 (Figure 5). Thus, in contrast to the sterile filtrate of donor stool, the Auto 0 and Auto 1 filtrate were able to induce the production of IL 4 in cells before therapy, and less pronounced in cells of the first month after therapy. The Auto 3 filtrate was unable to significantly stimulate IL4 production. At the same time, there was a decrease in sensitivity to stimulation of IL4 production depending on the period of cell receipt.
On the contrary, IL10 production resembled that during stimulation with sterile filtrates of donor stool, in which cells of the zero and first month did not respond to the addition of the filtrate, while cells of the third month significantly produced this cytokine. At the same time, there was a drop in the level of IL10 production depending on the time point the cells were obtained.
The ratio of the levels of these cytokines in different groups shows a statistically significantly increased IL 10/ IL 4 ratio in all groups, although its level decreased depending on the filtrate used. Unfortunately, the study design did not allow investigating the production of cytokine by individual cells, which does not provide insight into the cellular source of these cytokines.
2.4. Autologous Sterile Fecal Filtrates Iinfluence on Helper T cell Population
To assess changes in the immunomodulatory properties of patient filtrates over time after therapy, we introduced patient filtrates into cultures of peripheral mononuclear cells. The experiment was organized in such a way that the filtrates obtained at each stage of the study were applied to cells of the same stage, as well as to cells obtained in subsequent stages (Figure 1b). If SFMT therapy leads to the formation of a new microbiota with different antigenic properties than before therapy, then we can assume a change in the ability of cells to be activated in the presence of the filtrate of the subsequent period.
The level of CD4+ lymphocytes in the culture fluid increased depending on the age of the cells, but not on the age of the filtrate that was added to the culture fluid. ANOVA analysis showed the presence of a statistically significant time factor for cell (F =67.01, p value < 0.0001), while the time factor of filtrates introduced into the culture medium had practically no role (F = 1.43, p value =0.27). This change can be traced both in paired analysis of cell dynamics (Figure 6b) and in the total dynamics of the content of CD4+ lymphocytes (Figure 6a).
The change in the number of CD25+CD127+ cells (Figure 6c) was also practically independent of what filtrate was added to the cell culture (ANOVA , F=1.2, pvalue =0.31); however, when autologous filtrates of months one and three were added to the culture, a sharp gap was observed between the number of these cells among PBMCs of months one and three. At the same time, a pronounced scatter in the level of CD25+CD127+ was observed among cells of the third month, regardless of the stimulation option.
Analysis of cellular memory markers indicated the following patterns (Figure 7). Just as in the case of stimulation of cells with sterile filtrate of donor stool, stimulation with autologous filtrates was accompanied by a high content of TEMRA-cells, the level of which, however, fell among the cells of the third month. In this case, there was a clear increase in the number of these cells when stimulated with filtrates of the third month, compared with filtrates before therapy (Kruskal - Wallis, p value < 0.001). When stimulated with Auto 0 and Auto 1 filtrates, the level of TEMRA-cells in the cells of the third month was significantly lower than in the cells of the first and second months. Two - way ANOVA demonstrated the dependence of the severity of changes on which cells were introduced (F =20.44, p-value <0.001), and on what filtrates were used for stimulation (F=6.49, p-value < 0.001).
The content of central and effector memory cells had a slight dependence on the type of filtrate introduced (corresponding ANOVA data: F = 4.89, pvalue =0.063 and F =4.03, p value =0.009), but much more dependent on the time point when cells were obtained (F =39.4 and F=71.17, respectively, p value <0.0001). It should be noted that in the cells of the third month there was a pronounced dispersion in the level of content of these cell types.
This pattern resembles the differences in cell composition during stimulation with sterile filtrate of donor stool, in which, depending on the time point the cells were obtained, displace-ment of the TEMRA cell population was observed in favor of memory cell populations. In this case, sterile fecal filtrates Auto 0 and Auto 1 similar shift to a greater extent.
3. Discussion
Sterile filtrates of the fecal microbiota from patients before and after therapy contain a factor that stimulates the formation of cells with the activation phenotype CD25+ CD127+. Moreover, cells before therapy tend to produce IL4, while cells after therapy and, in particular, cells of the third month, are more likely to produce IL10. The production of IL10 by these cells is most pronounced when stimulated with donor filtrate and patient filtrate before therapy. Sterile fecal filtrates from patients after three months do not cause a significant increase in IL4 production and stimulate IL10 production to the least extent. However, all sterile fecal filtrates were able to stimulate IL10 in cell after the SFMT procedure.
Based on the data presented, it is possible to suggest a model of the influence of sterile fecal microbiota transplantation on the state of the patient’s immune system. Model 1 suggests that the sterile filtrate of donor stool introduces bacteriophages into the intestines of patients that can change the ratio of the intestinal bacterial flora towards less pro-inflammatory. Model 2 states that the main effect of sterile filtrate of donor stool is to modify microbiome metabolites, which in turn affects the proportion of bacteria in the patients' gut. In any of these cases, the factor influencing the state of immune cells can have both antigenic and adjuvant properties. In the first case, main therapeutic factor is the decrease in the number of bacteria causing persistent activation of pro-inflammatory signals. In the second case, there is a modification of the response of cells with the same immune specificity that was present in the intestines of patients.
It should also be taken into account that the exposure time of the donor material on the intestinal epithelium does not exceed 2-3 hours, and therefore, the introduced factors either must be potent, or their short-term effects must be accompanied by long-standing consequences. From this point of view, model 1 seems to be more acceptable. Provided that a suitable host bacterium is found, and the corresponding bacteriophages express just moderate lytic properties, these phages are able to persist in the ecosystem for a long time, controlling the degree of growth of certain bacteria. The feasibility of such a model has been previously shown in a study of sterile microbiota transplantation in patients with clostridia infection [20], and is directed to a significant increase in bacteriophages of the Caudoviricetes class [20]. This effect is associated with a shift in the microbiota towards an increase in the representation of the Firmicutes phylum compared to Bacteroidetes [21].
While this model is capable of explaining the disease severity relief, it does not provide clues to understanding the helper T cell response skewness. Particularly, our data show that increased IL10 production can be observed independently of what type of filtrate is introduced to the culture, indicating that the antigenic load is not that necessary as the period post-therapy.
FMT has previously been shown to alter the ratio of helper T cells in favor of regulatory T cells and other IL 10 -producing T cells [16]. It has been shown that microbiota transplantation in patients with inflammatory bowel diseases leads to an increase in the production of IL10 and a decrease in IL17 in the blood [17]. This effect was directly attributed to the colonization of healthy donor intestinal microbiota, accompanied by an increase in the production of a number of metabolites that promote the formation of regulatory T cells. However, other studies have shown that intestinal colonization in FMT is not a key factor in the beneficial effects of therapy, and some small molecules produced by “healthy” microbiota is more important [11]. For example, it is now known that SCFA and bile acids make an environment to stimulate helper T cells towards regulatory phenotype independently on antigen. On the other hand, the influence of low-molecular compounds is limited to a single use, which does not allow the formation of a long-term pharmacological effect.
Perhaps, the combination of antigen-specific and non-specific factors is the clue to solve the task of switching helper T cell response pattern. However, more thorough studies are required to unravel this problem. Particularly, it should be uncovered which bacteriophages contribute to microbiota shift, and which of the representatives of micro-biota a capable of game changing in helper T cell phenotype definition. It is also to be disclosed whether the effect of SFMT is longstanding, or it has limited duration. It is also likely that no single defined formulation of SMFT content exists, and the therapy is about to be patient-specific. Nevertheless, SFMT shows promise as the therapy influencing on the mechanisms rather than just symptoms of UC.
As for the limitations of the study, the small sample size of patients is the most important one that is due to the experimental nature of the procedure and strict inclusion criteria with the exclusion of patients immediately before the planned procedure in case of deterioration or detection of systemic signs of an inflammatory response in clinical tests. Limitations also include the lack of a group of healthy volunteers who underwent a similar procedure. Subsequent studies are expected to take into account the above circumstances, including increasing the sample size of patients, including healthy volunteers in the study group, and also expanding the range of markers used to identify lymphocytic phenotypes.
In conclusion, our data indicated that SFMT in patients with UC caused an anti-inflammatory shift in the T-helper response. Notably, this effect increased and persisted for at least three months after the procedure.
4. Materials and Methods
4.1. Patients
Patients with UC of mild severity level (UCEIS score 2-4) were recruited in the Center for New Medical Technologies SB RAS during the period from December 2021 to January 2023. The patient’s age varied in range from 19 to 54 years old. The inclusion criteria were UC score 2-4, confirmed with clinical assessment and colonoscopy, ESR level less than 30 mm/hour. Exclusion criteria: in-creased stool frequency, ESR rate and blood signatures of exacerbation of inflammatory response (increased levels of CRP and WBC) at the day of procedure or early during the curation period. All patients were followed up during the period of 3 months after the procedure. The blood parameters and colonoscopy were performed.
The study was approved by the Local Ethics Committee of the Autonomous Non-Commercial Organization "Center of New Medical Technologies in Akademgorodok" (Protocol #2, date of approval 12 January 2019). Informed written consents were obtained from donors and patients before the enrollment as well as during follow up period.
4.2. Donors
All donors were young, healthy volunteers (20-25 years old), not suffering from chronic diseases, free of infections and have not been hospitalization incidents for at least the last two months. All potential donors were examined with biochemical and microbi-ological assays including general and biochemical blood tests, as well as blood ELISA for the presence of Giardia, Toxocara, Opisthorchid, Ascaris, Trichynella. Additionally do-nors were examined for the infections T. pallidum, HIV 1 and HIV 2, hepatitis B and C viruses. All donor fecal samples were assessed for the content and infection agents presence according (C. difficile, Campilobacter jejuni, Salmonella spp., Shigella spp., en-teinvasive Escherichia coli, Cryptosporidium spp., Cyclospora spp., Giardia spp., Iso-spora spp.), rotavirus A, noroviruses I and II and adenoviruses F, as well as helminths and their eggs. The pilot study was approved by Local ethical committee of the Auton-omous Non- Commercial organization "Center of New Medical Technologies in Akad-emgorodok".
4.3. Preparation of Sterile Fecal Filtrate
Donor stool samples were collected in clean specifically prepared environment. Part of the stool samples were used for clinical laboratory assessment, the other part was divided into samples of equal weights (over 100 g) and frozen at -84°C.
Donor samples were used for the preparation of the sterile filtrate the day before the procedure of sterile fecal transplantation. The procedures were conducted under the standard operational procedures developed locally in the Center for Novel Medicine Technologies in clean aseptic conditions. Briefly, thawed portion of donor stool was placed in 300 ml of sterile clinical grade solution of 0.9% NaCl, blended using tissue shredder, and sequentially filtered through sterile filter cascades of 0.45 and 0.22 mi-crometer filter cassettes into the GMP-grade sterile disposable flask. Filtrates were stored at +4°C overnight. The two hours before the procedure of Sterile Fecal Microbiota Transplantation the material were placed in aseptically processed box to equilibrate the temperature. The filtrates were introduced into to colon of patients during the fibrocolonoscopy procedure.
4.4. Patient’s Samples Collection
Blood and stool samples were collected before the procedure of Sterile Fecal microbiota transplantation, one and three months after the procedure. For the study, donors and patients were taken venous blood early in the morning. Blood was transported to the research laboratory in blood collection tubes with anticoagulant (K3 EDTA). Stool samples were frozen at -20°C until the analysis.
4.5. PBMC and Plasma Preparation from Patient Venous Blood
Cell fractionation was performed within 2 hours after sampling. At the first stage, it was centrifuged at 300 g for 10 minutes and a fraction of crystals was taken to a depth of 1.5-2 ml. Next, the rest of the sample was diluted twice in calcium-free phosphate-buffered saline and transferred to 15 ml conical tubes pre-filled with Ficoll Histopaque-1077 solution at a ratio of blood:Ficoll = 3:1. Next, the solutions were centrifuged by 400 g for 30 minutes in a centrifuge with a rotary bucket type at a reduced speed of acceleration and deceleration. After centrifugation, the upper fraction containing diluted plasma and platelets was removed, and then the interphase containing peripheral mononuclear cells was collected. This pool was washed three times in phosphate-buffered saline, after which the cells were counted. Some of the cells were immediately stained with marker antibodies for immunophenotyping.
4.6. PBMC Freezing and Storage
Possible The cells were frozen in a solution containing fetal bovine serum and DMSO (in a ratio of 9:1) with a density of 5 million/ml. Freezing was carried out in two stages: 1) in containers with isopropanol up to -70°C; 2) transfer of frozen test tubes to liquid nitrogen vapor. The plasma samples were frozen at -70°C.
4.7. Cultivation of PBMC
Cultivation of isolated peripheral mononuclear cells was carried out in RPMI-1640 medium containing inactivated calf serum, glutamine 4 mM. On the day of the study, the cells collected at different periods of the study were thawed, washed from the freezing liquid, and placed in a prepared culture medium containing GM-CSF (at a final concentration of 40 ng/mL), which is necessary for the activation of monocytes and the presentation of antigens. The cells were seeded into the wells of a 96-well plate in the amount of 200 thousand cells per well in 100 μl of culture medium. Cells were supplemented with samples of sterile-filtered stool samples from donors or autologous. Cultivation was carried out for 4 days. During cultivation, the formation of cell clusters was observed and their number and size were photographed. At the end of the cultivation period, the culture liquid was collected for enzyme immunoassay. Cells were stained with surface antibodies to analyze the phenotypes involved in the activation process.
4.8. FACS Analysis of Fresh and Cultured PBMC
To determine lymphocyte subpopulations, peripheral mononuclear cells were stained with antibodies to surface markers (BD biosciences, NJ, USA). The following antibodies were used for the research panel: CD3-(FITC), CD4-(BV510), CD25-(PE), CD197-(BV421), CD45RA-(PerCP), CD45RO-(APC-Cy7). This panel aims to search for the presence of regulatory T cells, as well as their characterization in terms of memory phenotype and effector properties.
Staining was carried out in a phosphate-buffered saline solution containing 0.5% bovine albumin for 1 hour at +4°C. The cells were then fixed in 4% formalin for 15 minutes. Formalin was inactivated with 0.15 M solution of glycine. Analysis was per-formed on a Novocyte 3000 flow cytometer (ACEA biosciences Inc, CA, USA).
4.9. Study of Cytokines in Blood and Sterile Stool Samples
The study of cytokines in blood plasma was carried out by solid-phase ELISA using Vector-Best kits according to the instructions. The day before the study, the plasma was thawed at +4°C. For the analysis of cytokines in sterile stool samples, the volume of the sample added to the ELISA plate was normalized to the weight of the sample from which the filtrate was obtained.
4.10. Data Analysis and Statistical Inference
Flow Cytometry data analysis was performed using NovoExpress cytometry software. Data analysis and statistical inference were managed using GraphPadPrism v.8 and Python 3.7. For time-dependent paired analysis paired T-test with Tukey post-hoc was applied. For the comparison of stool filtrates, Kruskall-Wallis and Dunn pos-hoc was applied.
Author Contributions
Conceptualization, A.C. and N.T.; methodology, A.C.; formal analysis, A.C. and P.D.; investigation, A.C., P.D., T.Y., L.A.A., and E.S.; data curation, A.C., P.D. and N.T; writing—original draft preparation, A.C. and N.T; writing—review and editing, A.C. and N.T.; supervision, N.T.; project administration, A.C., V.M., and N.T; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
All immunological investigations were funded by the Russian Science Foundation; Project No. 21-14-00360. Blood and stool sampling was supported by the Ministry of Education and Science, Project No. 121031300043-8.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Ethics Committee of the Center for personalized medicine, Novosibirsk (protocol #2, date of approval 12 January 2019) including the written consents from patients and healthy volunteers to present their blood and fecal samples for scientific purposes (according to guidelines of the Helsinki ethics committee).
Informed Consent Statement
Informed consent was obtained from all healthy volunteers and patients involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
All co-authors have seen and agree with the contents of the manuscript and the order of authors, and there is no financial interest to report. All co-authors declare that they have no conflict of interest.
References
- AlAmeel, T.; AlMutairdi, A.; Al-Bawardy, B. Emerging Therapies for Ulcerative Colitis: Updates from Recent Clinical Trials. Clin. Exp. Gastroenterol. 2023, 16, 147–167. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schäffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory Bowel Disease and Mutations Affecting the Interleukin-10 Receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Kammermeier, J.; Elawad, M.; Glocker, E.-O. Interleukin-10 and Interleukin-10–Receptor Defects in Inflammatory Bowel Disease. Curr. Allergy Asthma Rep. 2012, 12, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Basha, O.M.; Hafez, R.A.; Salem, S.M.; Anis, R.H.; Hanafy, A.S. Impact of gut Microbiome alteration in Ulcerative Colitis patients on disease severity and outcome. Clin. Exp. Med. 2022, 23, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3 + regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Q.; Zeng, B.; Fang, Y.; Wei, H. Analysis of Fecal Lactobacillus Community Structure in Patients with Early Rheumatoid Arthritis. Curr. Microbiol. 2013, 67, 170–176. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2. [Google Scholar] [CrossRef]
- Wu, R.; Xiong, R.; Li, Y.; Chen, J.; Yan, R. Gut microbiome, metabolome, host immunity associated with inflammatory bowel disease and intervention of fecal microbiota transplantation. J. Autoimmun. 2023, 103062. [Google Scholar] [CrossRef]
- Chu, N.D.; Crothers, J.W.; Nguyen, L.T.T.; Kearney, S.M.; Smith, M.B.; Kassam, Z.; Collins, C.; Xavier, R.; Moses, P.L.; Alm, E.J. Dynamic Colonization of Microbes and Their Functions after Fecal Microbiota Transplantation for Inflammatory Bowel Disease. MBio 2021, 12. [Google Scholar] [CrossRef]
- Jaramillo, A.P.; Awosusi, B.L.; Ayyub, J.; Dabhi, K.N.; Gohil, N. V; Tanveer, N.; Hussein, S.; Pingili, S.; Makkena, V.K. Effectiveness of Fecal Microbiota Transplantation Treatment in Patients with Recurrent Clostridium Difficile Infection, Ulcerative Colitis, and Crohn’s Disease: A Systematic Review. Cureus 2023. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Round, J.L. Immune–bacteriophage interactions in inflammatory bowel diseases. Curr. Opin. Virol. 2021, 49, 30–35. [Google Scholar] [CrossRef]
- Federici, S.; Kviatcovsky, D.; Valdés-Mas, R.; Elinav, E. Microbiome-phage interactions in inflammatory bowel disease. Clin. Microbiol. Infect. 2023, 29, 682–688. [Google Scholar] [CrossRef]
- Draper, L.A.; Ryan, F.J.; Smith, M.K.; Jalanka, J.; Mattila, E.; Arkkila, P.A.; Ross, R.P.; Satokari, R.; Hill, C. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 2018, 6, 220. [Google Scholar] [CrossRef]
- Burrello, C.; Garavaglia, F.; Cribiù, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Shaheen, W.; Oo, Y.H.; Iqbal, T.H. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin. Exp. Immunol. 2019, 199, 24–38. [Google Scholar] [CrossRef]
- Narsale, A.; Lam, B.; Moya, R.; Lu, T.; Mandelli, A.; Gotuzzo, I.; Pessina, B.; Giamporcaro, G.; Geoffrey, R.; Buchanan, K.; et al. CD4+CD25+CD127hi cell frequency predicts disease progression in type 1 diabetes. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Gołąb, K.; Krzystyniak, A.; Marek-Trzonkowska, N.; Misawa, R.; Wang, L.J.; Wang, X.; Cochet, O.; Tibudan, M.; Langa, P.; Millis, J.M.; et al. Impact of culture medium on CD4+ CD25highCD127lo/neg Treg expansion for the purpose of clinical application. Int. Immunopharmacol. 2013, 16, 358–363. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2017, gutjnl-2017-313952. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Anderco, P.; Birsan, S. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Biomedicines 2023, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Experimental design. a). Sterile filtrates of donor stools were obtained and collected at -84°C. A day before the SFMT procedure, donor samples were thawed and processed under clean-room conditions. b) Patient’s samples (blood and stool) were collected right before the SMFT and in the follow-up periods of 1 and 3 months.
Figure 1.
Experimental design. a). Sterile filtrates of donor stools were obtained and collected at -84°C. A day before the SFMT procedure, donor samples were thawed and processed under clean-room conditions. b) Patient’s samples (blood and stool) were collected right before the SMFT and in the follow-up periods of 1 and 3 months.
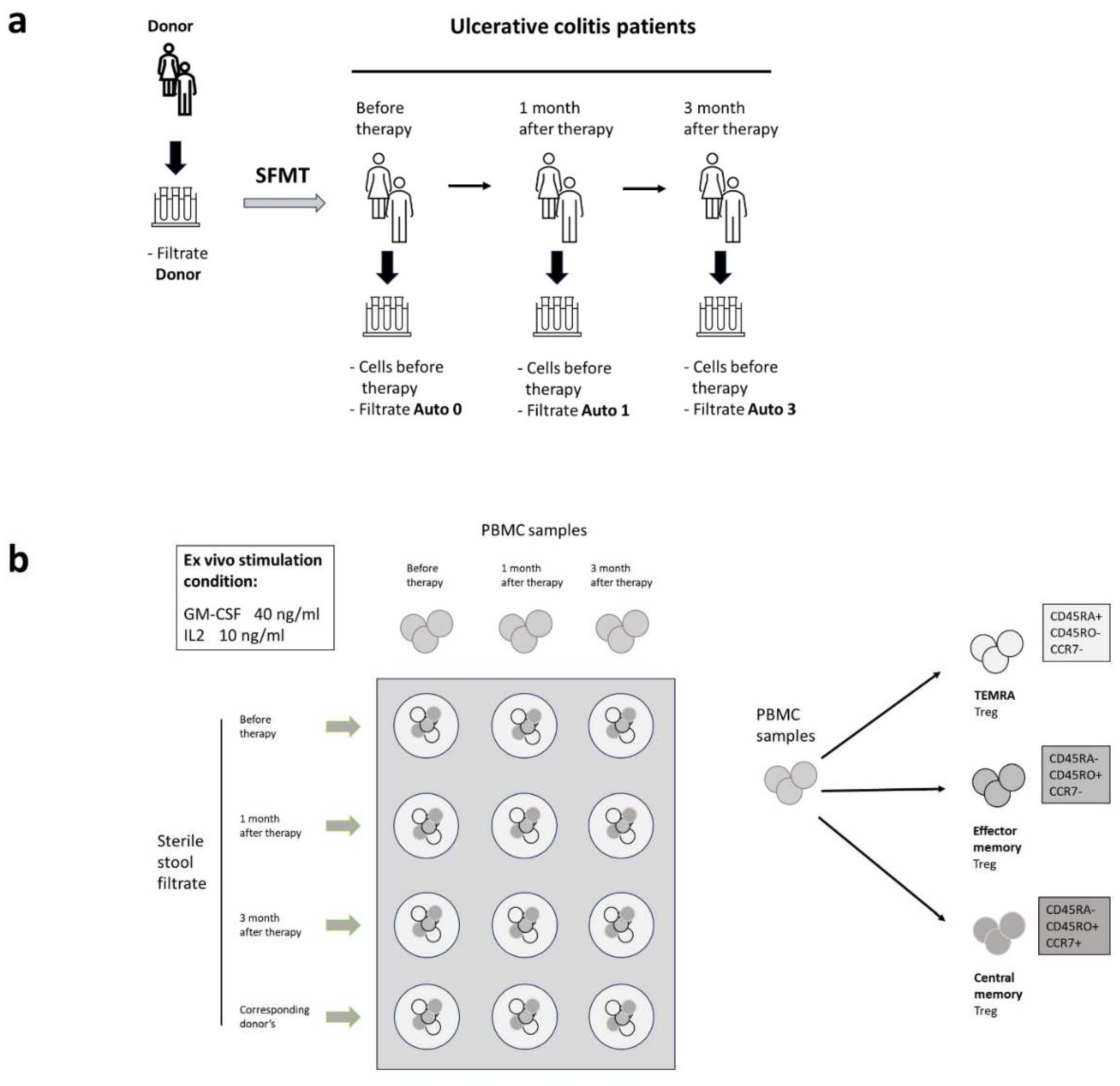
Figure 2.
Patient’s and healthy control group CD4+ cells and cytokine levels. Plots description: a) Overall CD4+ T-cell content among all CD3+ cells. b) presence of T-helper cells with activation phenotype CD25+CD127+; c) IL4 plasma concentration plot; d) IL10 plasma concentration plot. DESIGNATIONS: Healthy – healthy control volunteers; Colitis 0 – Blood samples from patients before the SFMT; Colitis 1 and Colitis 3 – Blood samples from patients after 1 and 3 months post SFMT respectively.
Figure 2.
Patient’s and healthy control group CD4+ cells and cytokine levels. Plots description: a) Overall CD4+ T-cell content among all CD3+ cells. b) presence of T-helper cells with activation phenotype CD25+CD127+; c) IL4 plasma concentration plot; d) IL10 plasma concentration plot. DESIGNATIONS: Healthy – healthy control volunteers; Colitis 0 – Blood samples from patients before the SFMT; Colitis 1 and Colitis 3 – Blood samples from patients after 1 and 3 months post SFMT respectively.

Figure 3.
Influence of sterile fecal filtrates from donors on patient’s cells. Cells were stimulated in presence of GM-CSF and IL2 for two days. Pairwise and box plots are shown to illustrate the intra-individual dynamics of the parameter. a) IL4 production in culture medium; b) IL10 production in culture medium; c) presence of CD4+ T cells among the population survived after two days on ex vivo activation; d) level of CD4+CD25+CD127+ cells capable of producing effector cytokines.
Figure 3.
Influence of sterile fecal filtrates from donors on patient’s cells. Cells were stimulated in presence of GM-CSF and IL2 for two days. Pairwise and box plots are shown to illustrate the intra-individual dynamics of the parameter. a) IL4 production in culture medium; b) IL10 production in culture medium; c) presence of CD4+ T cells among the population survived after two days on ex vivo activation; d) level of CD4+CD25+CD127+ cells capable of producing effector cytokines.
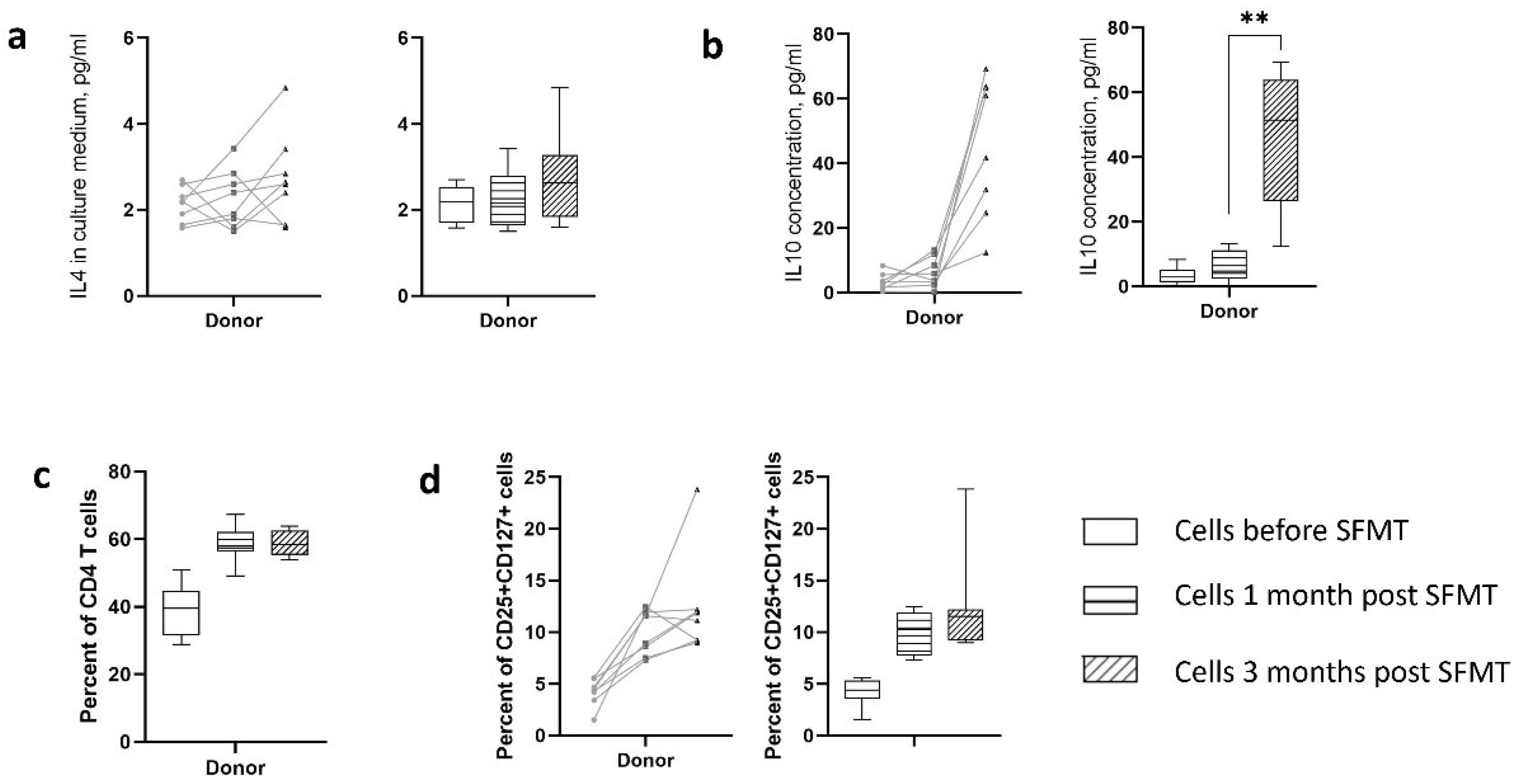
Figure 4.
Memory phenotypes of CD25+CD127+ T cells in ex vivo cultured conditions under stimulation with donor-derived stool filtrate. Pairwise plot aimed at demonstrate individual patient’s dynamics. Plots description: a) percent of activated T cells with phenotype of Central Memory cells (CD45RA-CD45RO+CCR7+); b) Percent of cells with phenotype of TEMRA-Cells’+5; c) Subset of cells with phenotype of Effector Memory cells. Effector memory cells percent of * - designate significance level p <0.01.
Figure 4.
Memory phenotypes of CD25+CD127+ T cells in ex vivo cultured conditions under stimulation with donor-derived stool filtrate. Pairwise plot aimed at demonstrate individual patient’s dynamics. Plots description: a) percent of activated T cells with phenotype of Central Memory cells (CD45RA-CD45RO+CCR7+); b) Percent of cells with phenotype of TEMRA-Cells’+5; c) Subset of cells with phenotype of Effector Memory cells. Effector memory cells percent of * - designate significance level p <0.01.

Figure 5.
Dynamics of cytokine production in cells of different time points stimulated with autologous filtrates of different time points. * designates p-value <0.01, ** p-value <0.001. Auto0, Auto1 and Auto3 stand for Autologous stool sample right before therapy, 1 month and 3 months after therapy.
Figure 5.
Dynamics of cytokine production in cells of different time points stimulated with autologous filtrates of different time points. * designates p-value <0.01, ** p-value <0.001. Auto0, Auto1 and Auto3 stand for Autologous stool sample right before therapy, 1 month and 3 months after therapy.

Figure 6.
Dynamics of patient’s cells stimulated with autologous filtrates of different time points. a) Content of CD4+ T-cells in cell culture population depending on time the cells were obtained or the sterile stool filtrate applied to the cell culture. b) The same data presented pairwise to demonstrate patient-specific dynamics. c) Activation of CD4 T-cells based on CD25 and CD127 surface expression profile. d) The same data presented pairwise to demonstrate patient-specific dynamics.
Figure 6.
Dynamics of patient’s cells stimulated with autologous filtrates of different time points. a) Content of CD4+ T-cells in cell culture population depending on time the cells were obtained or the sterile stool filtrate applied to the cell culture. b) The same data presented pairwise to demonstrate patient-specific dynamics. c) Activation of CD4 T-cells based on CD25 and CD127 surface expression profile. d) The same data presented pairwise to demonstrate patient-specific dynamics.

Figure 7.
Memory phenotypes of CD25+CD127+ T cells in ex vivo cultured conditions under stimulation with donor-derived stool filtrate. Auto 0, Auto 1 and Auto 3 correspond to patient’s sterile stool filtrate derived before therapy and 1, and 3 months in the following period. * - designate significance level p <0.01.
Figure 7.
Memory phenotypes of CD25+CD127+ T cells in ex vivo cultured conditions under stimulation with donor-derived stool filtrate. Auto 0, Auto 1 and Auto 3 correspond to patient’s sterile stool filtrate derived before therapy and 1, and 3 months in the following period. * - designate significance level p <0.01.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Sterile Fecal Microbiota Transplantation Boosts Anti-Inflammatory T-Cell Response in Ulcerative Colitis Patients
Anton Chechushkov
et al.
,
2023
Banana Lectin: A Novel Immunomodulatory Strategy for Mitigating Inflammatory Bowel Disease
Radmila Miljkovic
et al.
,
2024
Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention
Krasimira Aleksandrova
et al.
,
2017
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







