Preprint
Communication
Exploring FABP3 as a Potential Biomarker for Cardiac and Cerebral Injury Post-Stroke: Insights from Molecular Docking Studies with Natural Compounds
Altmetrics
Downloads
91
Views
48
Comments
0
This version is not peer-reviewed
Submitted:
17 December 2023
Posted:
19 December 2023
You are already at the latest version
Alerts
Abstract
Investigations are currently in progress to assess the potential of elevated FABP3 levels as biomarkers for assessing cardiac and cerebral injury post-stroke. Ongoing research endeavors are concentrated on elucidating the specific mechanisms by which FABPs, particularly FABP3, contribute to the complex processes involved in ischemic stroke. This study employs molecular docking techniques within a Virtual Screening framework to examine the interaction between various natural substances and the active site of human heart fatty acid-binding protein (PDB Code 4TKH). Utilizing Pyrx and Autodock Vina, the research aims to pinpoint compounds demonstrating robust binding interactions, characterized by high binding energy scores (kcal/mol) and the formation of multiple chemical bonds. The anticipated outcomes have the potential to yield valuable insights into the molecular interactions influencing human heart fatty acid-binding protein, suggesting possible implications for therapeutic interventions using these natural substances.From the docking and predicted toxicity results, Resistomycin has demonstrated superior characteristics compared to Morusin.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Cerebrovascular accidents, commonly known as strokes, are prevalent across diverse patient populations and carry a significant risk of morbidity and mortality. These occurrences can be categorized as ischemic, hemorrhagic, or subarachnoid in nature.Thrombosis involves an obstructive process that hinders blood flow to certain regions of the brain. Risk factors for thrombosis encompass atherosclerotic disease, vasculitis, and arterial dissection [2,3,4].
This work centers on Fatty Acid-Binding Proteins (FABPs), a protein family critically involved in the transport and intracellular trafficking of fatty acids. FABPs exhibit tissue-specific expression patterns, with distinct types found in various organs, including the heart. In the context of the heart, Fatty Acid-Binding Protein, heart (FABP3), also known as cardiac FABP, is a specific type of FABP that is predominantly found in cardiac muscle tissue. FABP3 is involved in the uptake and utilization of fatty acids by the heart for energy production. It facilitates the transport of fatty acids from the cell membrane to the mitochondria, where they undergo beta-oxidation to generate ATP, the energy currency of the cell [5,6,7].
The expression of FABP3 in the heart reflects its importance in the myocardial energy metabolism. Changes in FABP3 levels have been associated with various cardiovascular conditions, and it is considered a biomarker for myocardial injury.
Ischemic stroke is associated with Fatty Acid-Binding Proteins (FABPs), particularly FABP3, known as heart-type FABP. FABPs are crucial for transporting and utilizing fatty acids, impacting the energy metabolism in tissues like the heart and brain. In ischemic stroke, disrupted fatty acid metabolism and inflammation are implicated. FABP3, expressed in cardiac and brain tissues, is under investigation for its potential role in ischemic conditions. Elevated FABP3 levels are suggested to correlate with cardiac and cerebral injury, potentially serving as a biomarker for assessing damage post-stroke. Ongoing research aims to uncover the specific mechanisms through which FABPs, especially FABP3, contribute to the complex processes of ischemic stroke [8,9,10,11].
This computational study utilizes molecular docking techniques [12] within a Virtual Screening framework, employing the Pyrx program [13] with Autodock Vina as the algorithm [14]. The primary objective is to investigate the interaction of diverse natural substances with the active site of the human heart fatty acid-binding protein (PDB Code 4TKH). The study seeks to identify compounds that exhibit robust binding interactions, as evidenced by high binding energy scores (kcal/mol) and the formation of multiple chemical bonds. The findings of this investigation hold potential implications for understanding the molecular interactions influencing the human heart fatty acid-binding protein and may contribute to the exploration of these natural substances as candidates for therapeutic interventions.
2. Material and Methods
-Human heart fatty acid-binding protein (PDB Code 4TKH) : Binding site center (ångström) X( -8.3613714488), Y ( -21.5508188314) Z( 2.71964137179); size_X = 19.0939691723; size_Y = 19.0939691723; size_Z = 19.0939691723.
3. Results and Discussion
Ischemic strokes, resulting from disruptions in blood flow to the brain, are a prevalent and significant health concern associated with elevated morbidity and mortality. These strokes are primarily linked to thrombotic or embolic events, where blood clots obstruct cerebral arteries or travel from other parts of the circulatory system to impede blood vessels in the brain. Risk factors contributing to ischemic strokes include atherosclerotic disease, vasculitis, and arterial dissection [1,2,3,4].
Investigations are underway to explore elevated FABP3 levels as potential biomarkers for evaluating cardiac and cerebral injury following a stroke. Current research endeavors are focused on uncovering the specific mechanisms through which FABPs, particularly FABP3, contribute to the intricate processes of ischemic stroke.
This study employs molecular docking techniques [12] within a Virtual Screening framework to analyze the interaction between various natural substances and the active site of human heart fatty acid-binding protein (PDB Code 4TKH). Utilizing Pyrx [13] and Autodock Vina [14], the research aims to identify compounds that demonstrate robust binding interactions, characterized by high binding energy scores (kcal/mol) and the formation of multiple chemical bonds. The outcomes have the potential to provide valuable insights into the molecular interactions influencing human heart fatty acid-binding protein, suggesting possible implications for therapeutic interventions using these natural substances. Based on the docking results, Morusin demonstrated a binding energy of -9.8 kcal/mol, while Resistomycin exhibited an even higher binding energy of -10.7 kcal/mol. These findings suggest that Morusin and Resistomycin have shown excellent binding affinities to the active site of the human heart fatty acid-binding protein (PDB Code 4TKH) compared to other natural compounds analyzed in this study. The considerable negative binding energies imply strong interactions between these compounds and the target protein, indicating their potential as promising candidates for further investigation and development in the context of therapeutic applications.
Figure 1.
displays of human heart fatty acid-binding protein complexed with crystal myristic acid within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and crystal myristic acid. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of crystal myristic acid.
Figure 1.
displays of human heart fatty acid-binding protein complexed with crystal myristic acid within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and crystal myristic acid. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of crystal myristic acid.
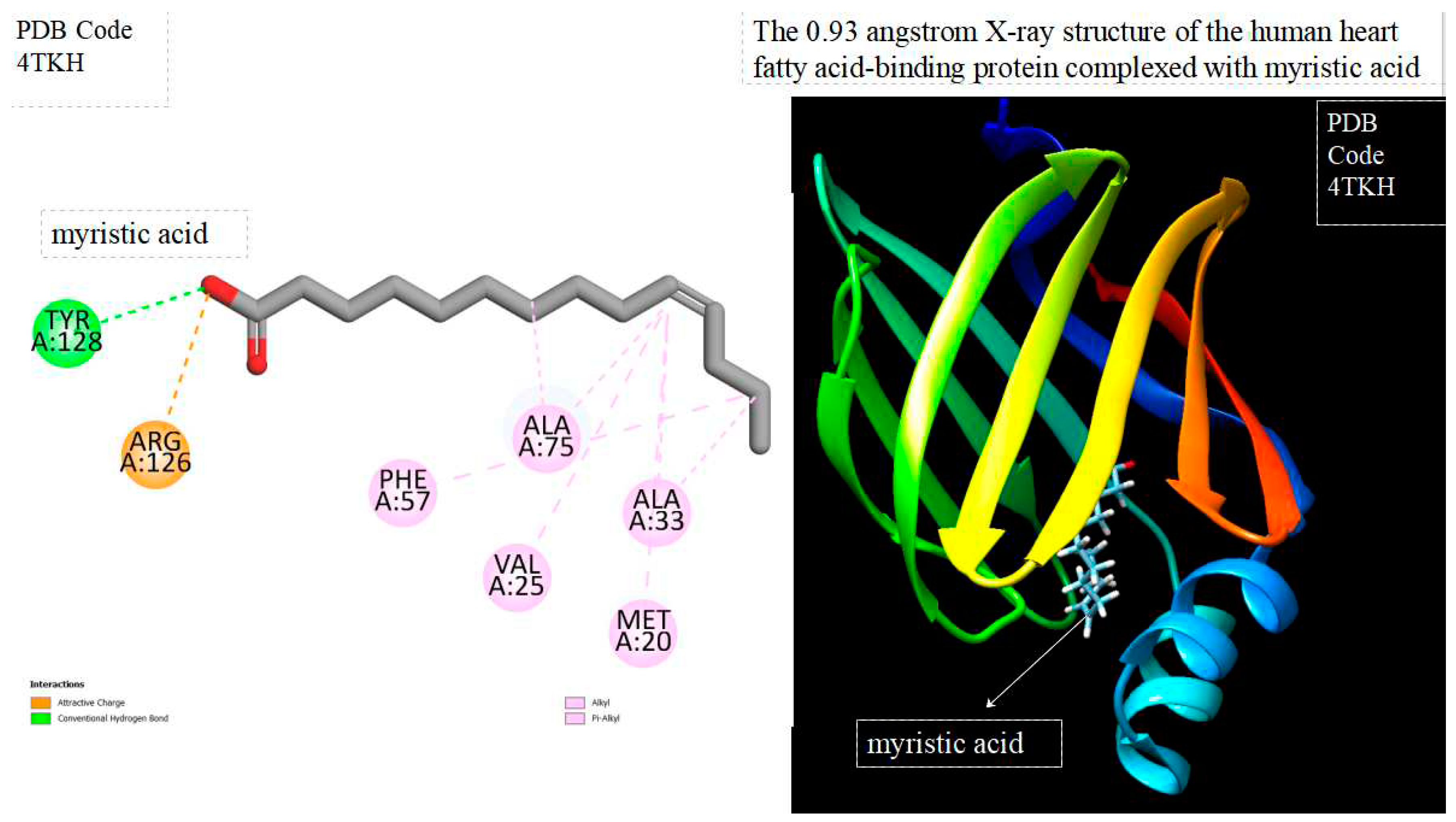
Figure 2.
displays of human heart fatty acid-binding protein complexed with docked myristic acid within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked myristic acid. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked myristic acid.
Figure 2.
displays of human heart fatty acid-binding protein complexed with docked myristic acid within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked myristic acid. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked myristic acid.
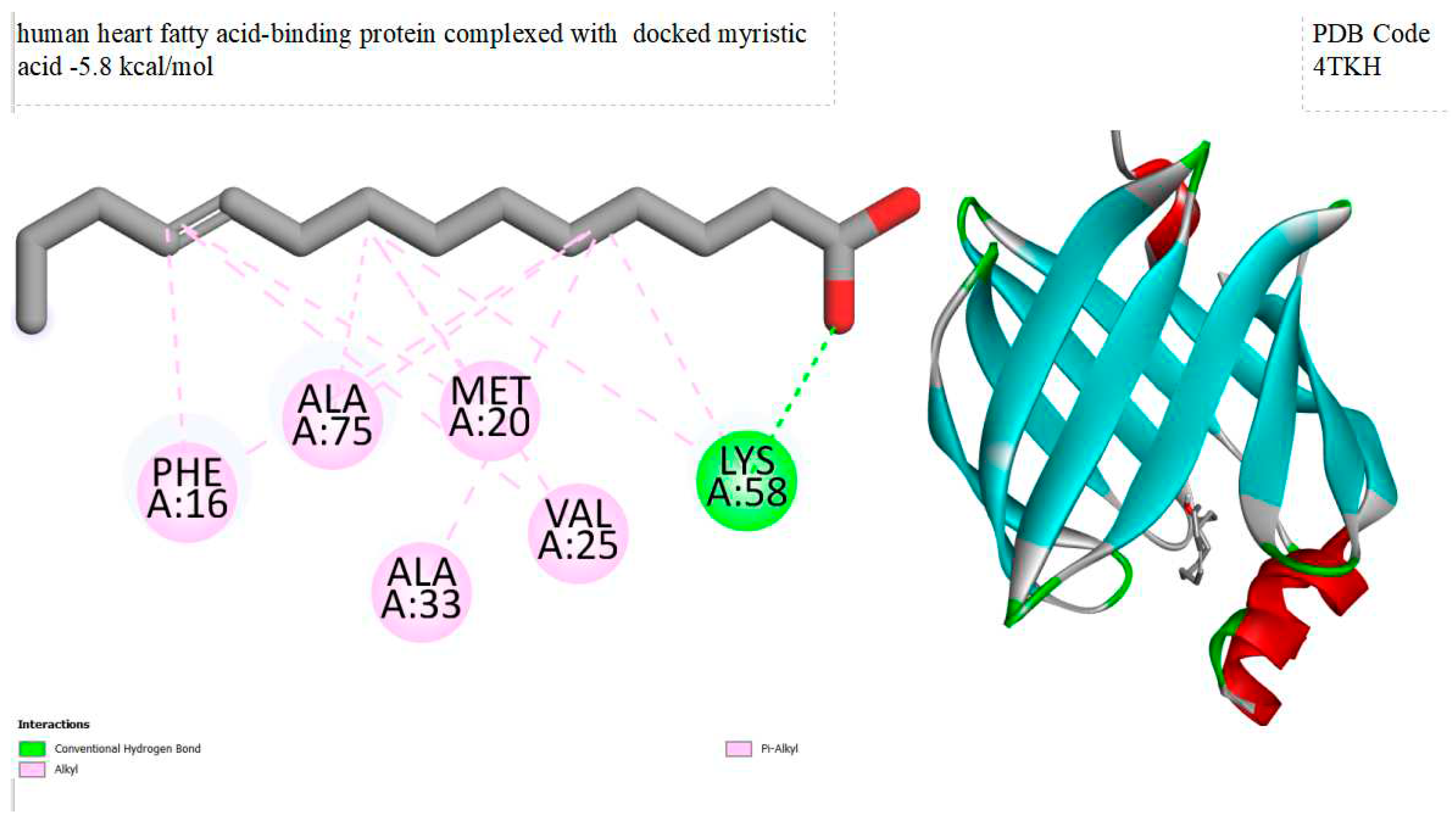
Figure 3.
displays of human heart fatty acid-binding protein complexed with docked Morusin within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked Morusin . Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked Morusin .
Figure 3.
displays of human heart fatty acid-binding protein complexed with docked Morusin within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked Morusin . Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked Morusin .

Figure 4.
displays of human heart fatty acid-binding protein complexed with docked Resistomycin within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked Resistomycin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked Resistomycin.
Figure 4.
displays of human heart fatty acid-binding protein complexed with docked Resistomycin within the Ligand Binding Site. On the left side, 2D diagrams illustrate the residue interactions between the protein and docked Resistomycin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of docked Resistomycin.

Figure 5.
displays of chemical structures of Morusin and Resistomycin respectively.

Table 1.
comparison of predicted toxicity properties of Morusin and Resistomycin using the pkCSM Database.
Table 1.
comparison of predicted toxicity properties of Morusin and Resistomycin using the pkCSM Database.
| Compounds | AMES toxicity | Max. tolerated dose (human) (log mg/kg/day) |
Oral Rat Acute Toxicity (LD50) (mol/kg) | Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day) | Hepatotoxicity | Skin Sensitisation |
T. Pyriformis toxicity (log ug/L) |
Minnow toxicity (log mM) |
|---|---|---|---|---|---|---|---|---|
| Morusin | No | 0.231 | 2.361 | 2.017 | yes | no | 0.334 | -0.135 |
| Resistomycin | No | 0.408 | 2.236 | 2.026 | yes | no | 0.318 | 1.64 |
4. Conclusions
This study employed molecular docking techniques, utilizing the Pyrx program with Autodock Vina, to investigate the interaction of various natural substances with the active site of the human heart fatty acid-binding protein (PDB Code 4TKH). Among the screened compounds, Morusin and Resistomycin exhibited exceptional binding energies of -9.8 kcal/mol and -10.7 kcal/mol, respectively. Following the selection of these best-docked compounds, a comprehensive toxicity assessment was conducted using the pkCSM database. Predicted toxicity parameters for Morusin included a Max. Tolerated Dose (Human) of 0.231 log mg/kg/day, Oral Rat Acute Toxicity (LD50) of 2.361 mol/kg, and Oral Rat Chronic Toxicity (LOAEL) of 2.017 log mg/kg_bw/day. For Resistomycin, predicted values included a Max. Tolerated Dose (Human) of 0.408 log mg/kg/day, Oral Rat Acute Toxicity (LD50) of 2.236 mol/kg, and Oral Rat Chronic Toxicity (LOAEL) of 2.026 log mg/kg_bw/day. Resistomycin emerges as a particularly compelling candidate, exhibiting superior binding affinity and favorable toxicity aspects. Further research and clinical investigations are warranted to validate these results and explore the translational implications of these natural compounds.
Conflicts of Interest
Authors declare that they do not have any conflict of interest
Funding
None
Author Contributions
Protocol designed by IVF . All authors read and approved the final manuscript.
References
- Hui, C., Tadi, P., & Patti, L. (2018). Ischemic stroke.
- Rosendaal, F. R. (1999). Venous thrombosis: a multicausal disease. The Lancet, 353(9159), 1167-1173. [CrossRef]
- Kyrle, P. A., & Eichinger, S. (2005). Deep vein thrombosis. The Lancet, 365(9465), 1163-1174. 9465. [CrossRef]
- Esmon, C. T. (2003). Inflammation and thrombosis. Journal of Thrombosis and haemostasis, 1(7), 1343-1348. [CrossRef]
- Storch, J., & McDermott, L. (2009). Structural and functional analysis of fatty acid-binding proteins. Journal of lipid research, 50, S126-S131. [CrossRef]
- Haunerland, N. H., & Spener, F. (2004). Fatty acid-binding proteins–insights from genetic manipulations. Progress in lipid research, 43(4), 328-349. [CrossRef]
- Hanhoff, T., Lücke, C., & Spener, F. (2002). Insights into binding of fatty acids by fatty acid binding proteins. Cellular Lipid Binding Proteins, 45-54. [CrossRef]
- Wunderlich, M. T., Hanhoff, T., Goertler, M., Spener, F., Glatz, J. F., Wallesch, C. W., & Pelsers, M. M. (2005). Release of brain–type and heart–type fatty acid–binding proteins in serum after acute ischaemic stroke. Journal of neurology, 252, 718-724. [CrossRef]
- Guo, Q., Kawahata, I., Cheng, A., Jia, W., Wang, H., & Fukunaga, K. (2022). Fatty acid-binding proteins: their roles in ischemic stroke and potential as drug targets. International journal of molecular sciences, 23(17), 9648. [CrossRef]
- Tu, W. J., Zeng, X. W., Deng, A., Zhao, S. J., Luo, D. Z., Ma, G. Z., ... & Liu, Q. (2017). Circulating FABP4 (fatty acid–binding protein 4) is a novel prognostic biomarker in patients with acute ischemic stroke. Stroke, 48(6), 1531-1538. [CrossRef]
- Tso, A. W. K., Lam, T. K. Y., Xu, A., Yiu, K. H., Tse, H. F., Li, L. S. W., ... & Lam, K. S. L. (2011). Serum adipocyte fatty acid–binding protein associated with ischemic stroke and early death. Neurology, 76(23), 1968-1975. [CrossRef]
- Fan, J., Fu, A., & Zhang, L. (2019). Progress in molecular docking. Quantitative Biology, 7, 83-89. [CrossRef]
- Dallakyan, S., & Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Chemical biology: methods and protocols, 243-250. [CrossRef]
- Trott, O., & Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry, 31(2), 455-461. [CrossRef]
- Pires, D. E., Blundell, T. L., & Ascher, D. B. (2015). pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of medicinal chemistry, 58(9), 4066-4072. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
African Natural Products for Multitarget-Based Treatment of Alzheimer’s Disease: An In Silico Study
Alkhansa Altaher Ahmed
et al.
,
2023
In silico tool for predicting and designing Blood-Brain Barrier Penetrating Peptides
Vinod Kumar
et al.
,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








