Preprint
Article
Efficacy of Immune Checkpoint Inhibitors vs Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: In-direct Comparison of Disease-Free Survival
Altmetrics
Downloads
87
Views
28
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.pdf (372.79KB )
This version is not peer-reviewed
Submitted:
19 December 2023
Posted:
20 December 2023
You are already at the latest version
Alerts
Abstract
Background: The proven efficacy of mTOR inhibitors (mTORI), tyrosine kinase inhibitors (TKI) or immune checkpoint inhibitors (ICI) therapies in metastatic renal cell carcinoma (RCC), suggested that these agents should be investigated as adjuvant therapy with the aim of eliminating any residual undetectable microscopic disease after curative resection. Our study aimed to compare the efficacy of these treatments through the application of an innovative method that reconstructs individual patient data. Methods: Nine phase III studies describing different treatment options for adjuvant RCC were selected. Individual patient data were reconstructed from Kaplan–Meier (KM) curves through the “IPDfromKM method”. Disease-free survival (DFS) were compared among combination treatments and control arm (placebo). Results were summarized as multi-treatment KM curves. Standard statistical testing was used, including hazard ratio and likelihood ratio tests for heterogeneity. Results: In the overall population, this study showed that two ICIs (pembrolizumab and nivolumab plus ipilimumab) and one TKI (sunitinib) showed superiority over placebo, whereas both TKI and mTORI did not. As we assessed DFS as the primary endpoint for the adjuvant comparison, the overall survival benefit remains unknown.. Conclusion: This novel approach to investigating survival has allowed us to conduct all of the indirect head-to-head comparisons between these agents in a context where no "real" comparative trials have been conducted.
Keywords:
Subject: Medicine and Pharmacology - Oncology and Oncogenics
1. Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for about 2% of all cancer diagnoses worldwide. Approximately 80% of RCCs are clear cell tumors [1].
Over the last 20 years, the prognosis for patients with metastatic RCC has improved thanks to the results of clinical trials with mTOR inhibitors (mTORI), tyrosine kinase inhibitors (TKI) or immune checkpoint inhibitors (ICI). The most recent revolution is the treatment of metastatic RCC with ICI combinations or ICI-TKI combinations [2,3,4,5], which has resulted in a significant improvement in survival, although not a cure.
Prevention of metastatic disease remains a priority in the curative setting of early stage RCC. For patients with locoregional RCC, partial or radical nephrectomy is the standard of care, and adjuvant treatment is an option to reduce the risk of recurrence, considering that 40% of surgically resected patients with stage II-III disease will relapse [6,7,8,9,10].
The proven efficacy of ICI, TKI and mTORI therapies in metastatic RCC suggested that these agents should be investigated as adjuvant therapy with the aim of eliminating any residual undetectable microscopic disease after curative resection.
Numerous randomized phase III trials in the adjuvant treatment of patients with RCC have ended with conflicting results, but overall they seem to show a greater benefit of treatment with ICIs compared to TKIs and mTORI [11,12,13,14,15,16,17,18,19]. As a result of this uncertainty, adjuvant therapies have also had different regulatory pathways. In fact, sunitinib, the first adjuvant therapy approved by the Food and Drug Administration (FDA) for the adjuvant treatment of RCC, was not approved by the European Medicines Agency (EMA).
In this scenario, given the available results and the lack of head-to-head comparisons between the adjuvant treatment options studied to date, we conducted an analysis using a new artificial intelligence technique (called the "IPDfromKM" or "Shiny" method) to compare disease-free survival (DFS) in patients with resected primary RCC at risk of recurrence. Only results from phase III randomized clinical trials (RCTs) with adjuvant mTORI or ICI or TKI were included in the analysis.
The IPDfromKM method (also known as the Shiny method) is a new artificial intelligence tool that reconstructs individual patient data from the graph of Kaplan-Meier (KM) curves and allows cross-study comparisons to be made based on reconstructed patients [20,21]. This is a relatively new method for generating new original clinical evidence and is particularly suitable for indirect comparisons; thanks to this method, in the present report we provide a comparative overview of the main adjuvant treatments available for RCC patients and determine their place in therapy and their relative efficacy.
2. Materials and Methods
2.1. Study Design
In accordance with recent publications [5,21,22], we conducted a comprehensive literature review to identify primary treatment options for adjuvant treatment of RCC. Following the selection of relevant studies, we utilized the IPDfromKM method to reconstruct individual patient data from Kaplan-Meier graphs and consequently perform head-to-head indirect comparisons between treatments [20]. Our analysis focused on disease-free survival (DFS) as the primary endpoint. Results were shown through multi-treatment Kaplan-Meier curves.
2.2. Literature Search
We searched the ClinicalTrials.gov databases to identify randomised controlled trials (RCTs) that were eligible for our analysis (last search on 15 November 2023). The search term was (adjuvant) AND ("renal cell carcinoma" OR "RCC"). Only phase III randomised interventional clinical trials were selected. The main inclusion criteria were: (a) RCC treatment (not diagnostic, imaging or surgical trials, not other diseases or solid tumours of different origin); (b) adjuvant treatment (not neoadjuvant/adjuvant treatment, not perioperative treatment, not locally advanced or metastatic treatment); (c) ICI or TKI or mTORI treatment; (d) inclusion of non-metastatic patients; (e) DFS endpoint; (f) publication of results as a KM curve. The selection of articles from our literature search was based on the PRISMA algorithm, which recorded the reasons for inclusion and exclusion of each trial [23]; the final list of included trials was determined in the last step of the PRISMA flow.
For each trial included, we recorded the number of patients enrolled and the number of events (defined as first documented local or distant recurrence of RCC, secondary systemic malignancy, or death from any cause, whichever occurred first). To avoid duplicate inclusion of patients from the same trial, only the most recent publication was included.
2.3. Reconstruction of Individual Patient Data
As previously described [21], patient-level data were reconstructed from KM curves using the IPDfromKM method [20]. The curves were digitized using Webplotdigitizer (version 4.5 online; url https://apps.automeris.io/wpd/) and then entered into the individual patient data reconstruction function of Shiny software (version: 1.2.3.0; last update: 22 March 2002).
The reconstructed individual patient data included each patient's observation period (defined as the difference between the dates of enrolment and last follow-up) and the patient's outcome at the last follow-up date (alive, dead or censored).
2.4. Statistical Analysis
Restricted mean survival times and DFS were assessed for each experimental treatment and compared to placebo using Cox statistics for time-to-event end-points; hazard ratio (HR) with a 95% confidence interval (95%CI) was estimated; medians with 95%CI were estimated as well. Heterogeneity in outcomes across control groups of different RCTs was assessed through the likelihood ratio test. Indirect comparisons between treatments were performed using the Cox model under the R-platform (version 4.2.1). Overall, our statistical analyses used four packages of the R-platform, namely "survival", "survRM2", "survminer", and "ggsurvplot" (2020; https://www.R-project.org/).
3. Results
Nine trials met the criteria for inclusion in our analysis (see Figure 1 for the PRISMA flowchart and Table 1 for RCT characteristics). Three trials involved an experimental treatment with parenteral ICIs while six trials were based on oral therapies such as TKIs (n=5) or mTORI (n=1). In all 9 trials, the control arm was placebo. In the application of the IPDfromKM method, we reconstructed 20 patient cohorts which represented the clinical material to perform our indirect comparisons.
To conduct the indirect comparisons between the three ICI treatments, the DFS Kaplan-Meier curves from the reconstructed patients of the ICI trials were plotted individually and reported in a single multi-treatment graph, with the three placebo cohorts pooled into a single graph. In this way, a total of 4 curves were generated (Figure 2A). Our DFS analysis on these reconstructed patients demonstrated, in comparison with placebo, superiority as adjuvant therapy for both pembrolizumab (HR 0.62; 95%CI 0.50-0.78; p<0.001) and nivolumab plus ipilimumab (HR 0.74; 95%CI 0.60-0.93; p=0.008). Atezolizumab showed no advantage in DFS compared to placebo (HR 1.04; 95%CI 0.86-1.26; p=NS).
The likelihood ratio test carried out on control arms showed no heterogeneity between these cohorts (likelihood ratio test, 5.01 on 2 df, p<0,08; Figure 2B).
Detailed results of indirect comparisons of the three ICIs treatments in all head-to-head combinations are reported as Forest plots of HRs with 95%CI (Figure 3 and Table S1). This analysis shows that pembrolizumab (HR=0.60; 95%CI 0.45-0.81; P<0.001) and nivolumab plus ipilimumab (HR=0.72; 95%CI 0.54-0.96; P=0.024) were superior to atezolizumab; on the other hand, pembrolizumab was not significantly superior to nivolumab plus ipilimumab (HR=0.83; 95%CI 0.61-1.14; P=NS).
To conduct indirect comparisons across oral treatments, the DFS Kaplan-Meier curves from reconstructed patients of TKI and mTORI trials were plotted individually and reported as a single multi-treatment graph. The six control arms of oral therapy treatments demonstrated significant heterogeneity and our head-to-head indirect comparisons were designed as follows (Figure S1). Firstly, we identified two non-heterogenous subgroups among these trials because patients in the ATLAS trial were comparable to those in SORCE and EVEREST trials (subgroup#1) while patients in the ASSURE trial were comparable to those in PROTECT and S-TRAC trials (subgroup #2). Likewise, e, patients treated with placebo in each of these two subgroups were combined to form two separate control cohorts. Finally, the DFS of the three active arms within each subgroup was compared to that of the respective pooled controls (Figure 4).
Most oral drug treatments did not show any DFS benefit over placebo. Only two studies with sorafenib or sunitinib demonstrated a DFS advantage, but the results were somewhat contradictory. In fact, in the SORCE trial, patients receiving sorafenib 400mg BID for 3 years showed a significantly longer DFS than the controls (HR=0.83; 95%CI 0.71-0.98; P=0.028), but not those treated for one year (HR=0.89; 95%CI 0.76–1.04; P=NS). Conversely, in the ASSURE study, patients treated with sorafenib for approximately one year (54 weeks) showed a significantly longer DFS than placebo (HR=0.85; 95%CI 0.74-0.99; P=0.036). In the S-TRAC study, sunitinib determined a significant advantage over placebo (HR=0.81; 95%CI 0.66-0.99; P=0.041), but in the ASSURE study the sunitinib treatment arm did not confirm this result, even though the dosage was the same.
Based on these intermediate results of our analysis, in the trials in which the active arm reported a DFS benefit over placebo (namely, KEYNOTE-564, CheckMate-914, S-TRAC, SORCE, based on the advantage at 3 years in sorafenib arm, and ASSURE, based on the advantage at 1-year in sorafenib arm), the treatment arms were indirectly compared with one another in all combinations. At the same time, we performed the heterogeneity test on the control arms (Figure S2). With a likelihood ratio test of 34.42 on 4 df, p<0,001), the SORCE trial proved to be an outlier compared with the other control arms and therefore was excluded from the indirect comparison. After this exclusion of the SORCE study, the analysis of the control arms showed no heterogeneity (likelihood ratio test= 3.68 on 3 df, p=0.3).
Figure 5 shows the results of our main analysis in which pembrolizumab (HR 0.67; 95%CI 0.54-0.83; p<0.001), nivolumab plus ipilimumab (HR 0.81; 95%CI 0.66-0.99; p=0.05) and sunitinib (HR 0.82; 95%CI 0.67-0.99; p=0.05) demonstrated superiority compared to placebo. These three treatments were indirectly compared with one another, and these comparisons showed that pembrolizumab was significantly superior to 1-year treatment with sorafenib (HR=0.76; 95%CI 0,60-0.98; P=0,038); no significant difference was observed in the remaining comparisons (Figure 6, Table S3).
In our estimation of absolute outcome parameters, only the S-TRAC trial had a sufficient follow-up to reach the median DFS; hence, we decided to calculate the Restricted Mean Survival Times (RMSTs) as an alternative to medians.
Our RMST analysis was truncated at 44 months; its results are reported in Table S3. All the five active treatments showed a DFS benefit compared to placebo. In terms of clinical relevance, pembrolizumab produced a three-month advantage over placebo (RMST of 36.09 vs 32.9 months, respectively), while it was only slightly superior to the other treatments.
4. Discussion
The present study investigated the main treatments for adjuvant RCC using an innovative method of indirect comparison of survival data, known as the "IPDfromKM" or "Shiny method". This technique is a valid alternative to network meta-analysis, mainly because of its ability to adjust for the different length of follow-up in the included trials. For this reason, the IPDFROMKM method is particularly suitable for studies in the field of oncology and haemato-oncology [22], where long follow-up is common. The use of the IPDFROMKM method has recently been extended to cardiology, where long follow-up is also common [24,25].
Our study was the first to indirectly compare the DFS of different drug classes in the adjuvant treatment of RCC. Our results showed that two ICIs (pembrolizumab and nivolumab plus ipilimumab) and one TKI (sunitinib) showed superiority over placebo, whereas both TKI and mTORI did not.
The pattern of heterogeneity estimated in the included trials is an interesting finding of our study. For the ICI trials, despite some differences in patient inclusion criteria (e.g., the KEYNOTE-564 and IMmotion010 trials enrolled 6% and 14% of M1 patients, respectively), the heterogeneity test showed substantial comparability between these populations. In contrast, the analysis of the TKI or mTORI trials showed significant heterogeneity in the control arms, which negatively affected our indirect comparisons. This heterogeneity was likely due to differences in patient selection criteria, such as the presence of a high proportion of patients with early-stage tumors, sarcomatoid features or specific factors that increase the risk of relapse.
Patients at high risk of relapse should be the focus of adjuvant trials, as currently recommended [27], and indeed the benefit of adjuvant TKI treatment may be greater in patients at higher risk, as demonstrated in the S-TRAC trial [18] with sunitinib. In addition to eligibility criteria, the efficacy of TKIs and mTORIs may be related to other factors that influence drug exposure, such as patient adherence, discontinuation due to adverse drug reactions, different dose reduction schedules and duration of treatment. For example, in the sunitinib arm of the ASSURE trial, the dose was allowed to be reduced to 25 mg, and midway through the trial the starting dose was changed from 50 mg to 37.5 mg (a dose level not allowed in the S-TRAC trial). In addition, the duration of oral treatment was quite heterogeneous, with ICI proposing 3 years of treatment in the ATLAS trial and the sorafenib arm in the SORCE trial, compared with 1 year in the other trials. Furthermore, grade 3-4 adverse events occurred in 46% of patients receiving everolimus and 49-72% in the TKI trials, which is more frequent than in the adjuvant ICI trials. The occurrence of adverse events may require discontinuation of treatment due to intolerance, but this may also be related to patient choice. Indeed, patients who have undergone nephrectomy are considered disease-free and may be less willing to accept serious adverse events and reduced quality of life [28]. Finally, it is possible that the vascular endothelial growth factor pathway, which is primarily targeted by TKIs, is less involved in the growth of early stage RCC, while remaining a hallmark of metastatic disease.
Given that life expectancy after nephrectomy is nearly 40% at 10 years [29], a limitation of the current analysis is that overall survival data were immature in some cases. We accepted disease-free survival as the primary endpoint for the adjuvant comparison, but the benefit in overall survival remains unknown. Overall survival is likely to be influenced by subsequent treatments in the metastatic setting, so it is an open question whether the DFS benefit is sufficient to support the financial burden of these therapies. In addition to these economic issues, further follow-up of these trials will show whether there are long-term survivors and whether the superiority of pembrolizumab over other TKIs is confirmed.
5. Conclusions
Conclusively, the "IPDfromKM method" made it possible to produce significant clinical data regarding novel treatments for adjuvant RCC and to analyze their effectiveness using unique indirect comparisons. Furthermore, as new treatments or longer follow-up become available, we will be able to promptly update the results of this research thanks to the “one to many approach” that our group just established [30].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Visual representation of indirect treatment comparisons of six control arms of oral therapy treatments; Figure S2: Visual representation of indirect treatment comparisons for DFS of control arms of KEYNOTE-564, CheckMate-914, S-TRAC, SORCE and ASSURE Trials; Table S1: HR of inter-treatment comparison between the three ICIs treatments; Table S2: HR of Inter-treatment comparison between adjuvant treatments for RCC; Table S3: Restricted Mean survival time (RMST) of the five active treatments that showed a DFS benefit compared to placebo.
Author Contributions
Conceptualization, A.O. and V.D.; Data curation, L.D.B. and V.D.; Formal analysis, A.O., L.D.B., A.M. and V.D.; Investigation, L.G.; Methodology, A.O., L.G., A.M. and V.D.; Software, A.M.; Writing – original draft, A.O. and V.D.; Writing – review & editing, L.G. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Materials.
Acknowledgments
Italian Society for Clinical Pharmacy and Therapeutics (SIFaCT), Turin, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P, Barsouk A. Epidemiology of Renal Cell Carcinoma. World J Oncol. 2020;11(3):79-87. [CrossRef]
- Calvo E, Escudier B, Motzer RJ, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Ravaud A, et al. Everolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer. 2012;48(3):333-9. [CrossRef]
- Xu JX, Maher VE, Zhang L, Tang S, Sridhara R, Ibrahim A, Kim G, Pazdur R. FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis. Oncologist. 2017;22(3):311-317. [CrossRef]
- Lalani AA, Heng DYC, Basappa NS, Wood L, Iqbal N, McLeod D, Soulières D, Kollmannsberger C. Evolving landscape of first-line combination therapy in advanced renal cancer: a systematic review. Ther Adv Med Oncol. 2022;14:17588359221108685. [CrossRef]
- Ossato A, Mengato D, Chiumente M, Messori A, Damuzzo V. Progression-Free and Overall Survival of First-Line Treatments for Advanced Renal Cell Carcinoma: Indirect Comparison of Six Combination Regimens. Cancers (Basel). 2023;15(7):2029. [CrossRef]
- Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, Schmidinger M, Suárez C, de Velasco G; ESMO Guidelines Committee. Electronic address: [email protected]. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol. 2021;32(12):1511-1519. [CrossRef]
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M, Klatte T, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022;82(4):399-410. [CrossRef]
- Martinez Chanza N, Tripathi A, Harshman LC. Adjuvant Therapy Options in Renal Cell Carcinoma: Where Do We Stand? Curr Treat Options Oncol. 2019;20(5):44. [CrossRef]
- Motzer RJ, Ravaud A, Patard JJ, Pandha HS, George DJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et al. Adjuvant Sunitinib for High-risk Renal Cell Carcinoma After Nephrectomy: Subgroup Analyses and Updated Overall Survival Results. Eur Urol. 2018;73(1):62-68. [CrossRef]
- Harshman LC, Xie W, Moreira RB, Bossé D, Ruiz Ares GJ, Sweeney CJ, Choueiri TK. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: A trial-level meta-analysis. Cancer. 2018;124(5):925-933. [CrossRef]
- Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Gurney H, Chang YH, Lee JL, et al. Investigators. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144. [CrossRef]
- Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, Albiges L, Rini B, Tomita Y, Kann AG, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2022;400(10358):1103-1116. [CrossRef]
- Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, Buti S, Barthélémy P, Goh JC, Ye D, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet. 2023;401(10379):821-832. [CrossRef]
- Gross-Goupil M, Kwon TG, Eto M, Ye D, Miyake H, Seo SI, Byun SS, Lee JL, Master V, Jin J, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol. 2018;29(12):2371-2378. [CrossRef]
- Eisen T, Frangou E, Oza B, Ritchie AWS, Smith B, Kaplan R, Davis ID, Stockler MR, Albiges L, Escudier B, et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results From the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol. 2020;38(34):4064-4075. [CrossRef]
- Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, Jewett M, Dutcher JP, Atkins MB, Pins M, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008-16. [CrossRef]
- Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, Lee JL, Melichar B, Rini BI, Choueiri TK, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol. 2017;35(35):3916-3923. [CrossRef]
- Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med. 2016;375(23):2246-2254. [CrossRef]
- Ryan CW, Tangen CM, Heath EI, Stein MN, Meng MV, Alva AS, Pal SK, Puzanov I, Clark JI, Choueiri TK, et al. Adjuvant everolimus after surgery for renal cell carcinoma (EVEREST): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2023;402(10407):1043-1051. [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [CrossRef]
- Messori, A. Synthetizing Published Evidence on Survival by Reconstruction of Patient-Level Data and Generation of a Multi-Trial Kaplan-Meier Curve. Cureus 2021, 13, e19422. [CrossRef]
- Messori, A.; Damuzzo, V.; Rivano, M.; Cancanelli, L.; Di Spazio, L.; Ossato, A.; Chiumente, M.; Mengato, D. Application of the IPDfromKM-Shiny Method to Compare the Efficacy of Novel Treatments Aimed at the Same Disease Condition: A Report of 14 Analyses. Cancers 2023, 15, 1633. [CrossRef]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [CrossRef]
- Messori A. Reconstruction of individual-patient data from the analysis of Kaplan-Meier curves: the use of this method has extended from oncology to cardiology (preprint). Open Science Framework. published 13 November 2023, url https://osf.io/qejus, accessed 15 November 2023. [CrossRef]
- de Sá Marchi MF, Calomeni P, Gauza MM, Kanhouche G, Ravani LV, Rodrigues CVF, Tarasoutchi F, de Brito FS Jr, Rodés-Cabau J, Van Mieghem NM, et al. Impact of periprocedural myocardial injury after transcatheter aortic valve implantation on long-term mortality: a meta-analysis of Kaplan-Meier derived individual patient data. Front Cardiovasc Med. 2023;10:1228305. [CrossRef]
- Serrano C, Rothschild S, Villacampa G, Heinrich MC, George S, Blay JY, Sicklick JK, Schwartz GK, Rastogi S, Jones RL, et al. Rethinking placebos: embracing synthetic control arms in clinical trials for rare tumors. Nat Med. 2023;29(11):2689-2692. [CrossRef]
- Agrawal S, Haas NB, Bagheri M, Lane BR, Coleman J, Hammers H, Bratslavsky G, Chauhan C, Kim L, Krishnasamy VP, et al. Eligibility and Radiologic Assessment for Adjuvant Clinical Trials in Kidney Cancer. JAMA Oncol. 2020;6(1):133-141. [CrossRef]
- Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol. 2013;40(4):482-91. [CrossRef]
- Giberti C, Oneto F, Martorana G, Rovida S, Carmignani G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur Urol. 1997;31(1):40-8. [CrossRef]
- Messori, A.; Rivano, M.; Cancanelli, L.; Damuzzo, V.; Ossato, A.; Chiumente, M.; Mengato, D. The “One-to-Many” Survival Analysis to Evaluate a New Treatment in Comparison with Therapeutic Alternatives Based on Reconstructed Patient Data: Enfortumab Vedotin Versus Standard of Care in Advanced or Metastatic Urothelial Carcinoma. Cureus 2022, 14, e28369. [CrossRef]
Figure 1.
PRISMA flowchart of the process of trial selection. Renal cell carcinoma (RCC); Disease-free survival (DFS).
Figure 1.
PRISMA flowchart of the process of trial selection. Renal cell carcinoma (RCC); Disease-free survival (DFS).
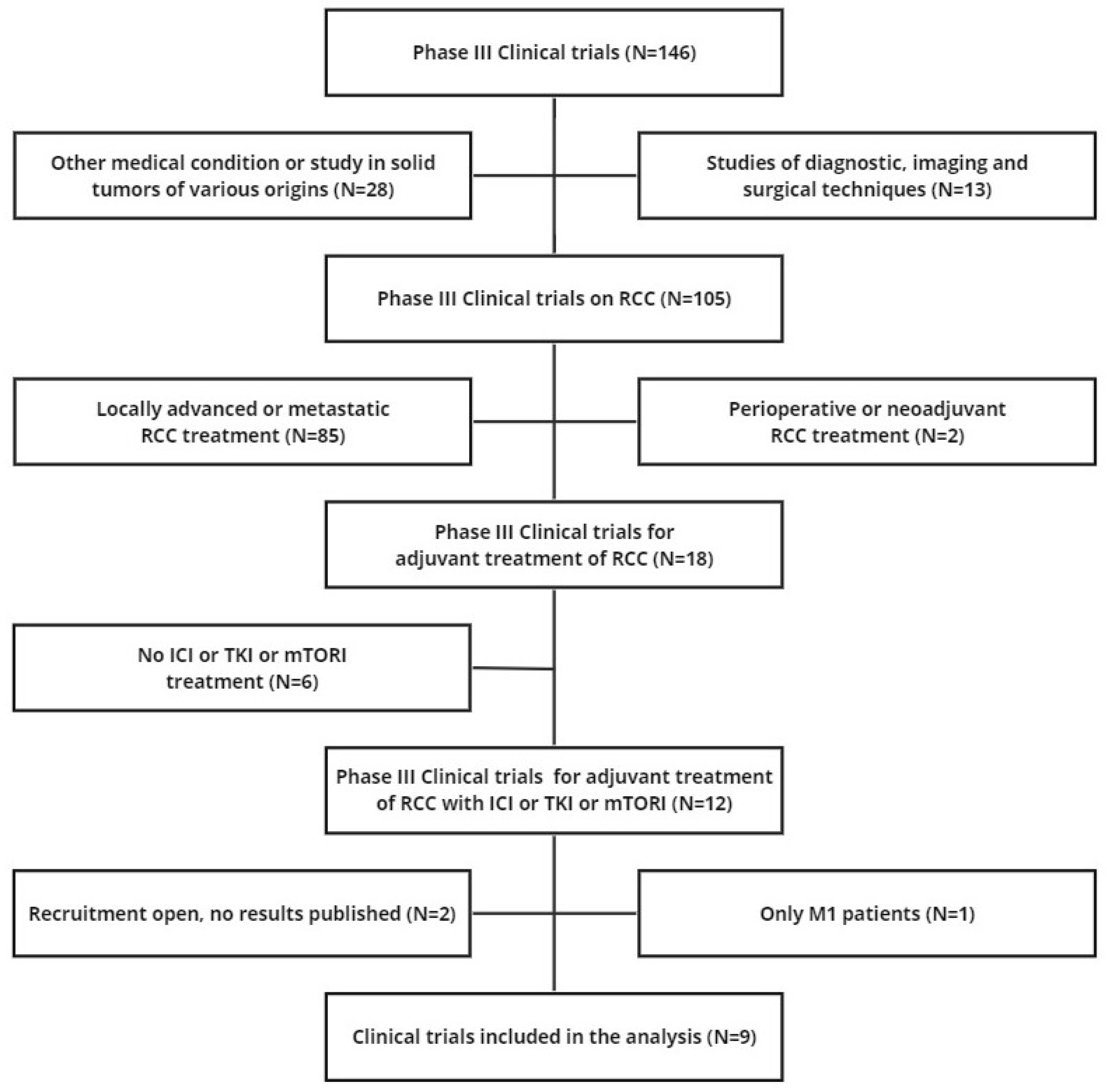
Figure 2.
Panel A. Application of the Shiny method to placebo (n=1297 from 3 trials; in red) and to 3 combination treatments: (a) pembrolizumab 200 mg (n=496; in green); (b) atezolizumab 12000 mg (n=390; in light blue); (c) nivolumab 240 mg + ipilimumab 1 mg/kg (n=405; in purple); (d) placebo (n=1297; in red). Panel B. After reconstruction of individual patient data from control arms of included trials (placebo treatment), the following Kaplan-Meier curves were generated from: Powles et al., 2022 (n=498; in red [11]); Pal et al., 2022 (n=388; in green [12]); Motzer et al., 2023 (n=411; in blue [13]). N: number of enrolled patients. Endpoint: disease-free survival (DFS), Time in months.
Figure 2.
Panel A. Application of the Shiny method to placebo (n=1297 from 3 trials; in red) and to 3 combination treatments: (a) pembrolizumab 200 mg (n=496; in green); (b) atezolizumab 12000 mg (n=390; in light blue); (c) nivolumab 240 mg + ipilimumab 1 mg/kg (n=405; in purple); (d) placebo (n=1297; in red). Panel B. After reconstruction of individual patient data from control arms of included trials (placebo treatment), the following Kaplan-Meier curves were generated from: Powles et al., 2022 (n=498; in red [11]); Pal et al., 2022 (n=388; in green [12]); Motzer et al., 2023 (n=411; in blue [13]). N: number of enrolled patients. Endpoint: disease-free survival (DFS), Time in months.

Figure 3.
Forest plot showing the disease-free survival of adjuvant renal cell carcinoma patients treated with ICI treatment.
Figure 3.
Forest plot showing the disease-free survival of adjuvant renal cell carcinoma patients treated with ICI treatment.

Figure 4.
Application of the Shiny method in Subgroups analysis. Panel A comparison of DFS of placebo (n=1535 from ATLAS, SORCE and EVEREST trials; in red) with treatment arms of ATLAS (axitinib n=363; in gold); [14]), SORCE (sorafenib for three years; n=639; in turquoise) and (sorafenib for one year; n=642; in blue); [15]) and EVEREST RCT (everolimus; n=755; in purple); [19]). Panel B comparison of DFS of placebo (n=1517 from ASSURE, PROTECT and S-TRAC trials; in red) with treatment arms of ASSURE (sorafenib for 1 year (n=649; in gold) and (sunitinib; n=647; in light green); [16]), PROTECT (pazopanib (n=571; in blue); [17]) and S-TRAC RCT (sunitinib; n=309; in purple); [18]). N: number of enrolled patients. End-point: disease-free survival (DFS), Time in months.
Figure 4.
Application of the Shiny method in Subgroups analysis. Panel A comparison of DFS of placebo (n=1535 from ATLAS, SORCE and EVEREST trials; in red) with treatment arms of ATLAS (axitinib n=363; in gold); [14]), SORCE (sorafenib for three years; n=639; in turquoise) and (sorafenib for one year; n=642; in blue); [15]) and EVEREST RCT (everolimus; n=755; in purple); [19]). Panel B comparison of DFS of placebo (n=1517 from ASSURE, PROTECT and S-TRAC trials; in red) with treatment arms of ASSURE (sorafenib for 1 year (n=649; in gold) and (sunitinib; n=647; in light green); [16]), PROTECT (pazopanib (n=571; in blue); [17]) and S-TRAC RCT (sunitinib; n=309; in purple); [18]). N: number of enrolled patients. End-point: disease-free survival (DFS), Time in months.

Figure 5.
Comparison between DFS of placebo (n=1862 from 4 trials; in red) and of 4 treatments that showed survival benefit compared to control: a) pembrolizumab (n=496; in gold); b) nivolumab + ipilimumab (n=405; in light green); c) sorafenib for one year (n=649; in blue) and d) sunitinib (n=309; in purple). N: number of enrolled patients. Endpoint: disease-free survival (DFS), Time in months.
Figure 5.
Comparison between DFS of placebo (n=1862 from 4 trials; in red) and of 4 treatments that showed survival benefit compared to control: a) pembrolizumab (n=496; in gold); b) nivolumab + ipilimumab (n=405; in light green); c) sorafenib for one year (n=649; in blue) and d) sunitinib (n=309; in purple). N: number of enrolled patients. Endpoint: disease-free survival (DFS), Time in months.

Figure 6.
Forest plot showing the disease-free survival of adjuvant renal cell carcinoma patients treated with ICI and TKI treatments.
Figure 6.
Forest plot showing the disease-free survival of adjuvant renal cell carcinoma patients treated with ICI and TKI treatments.
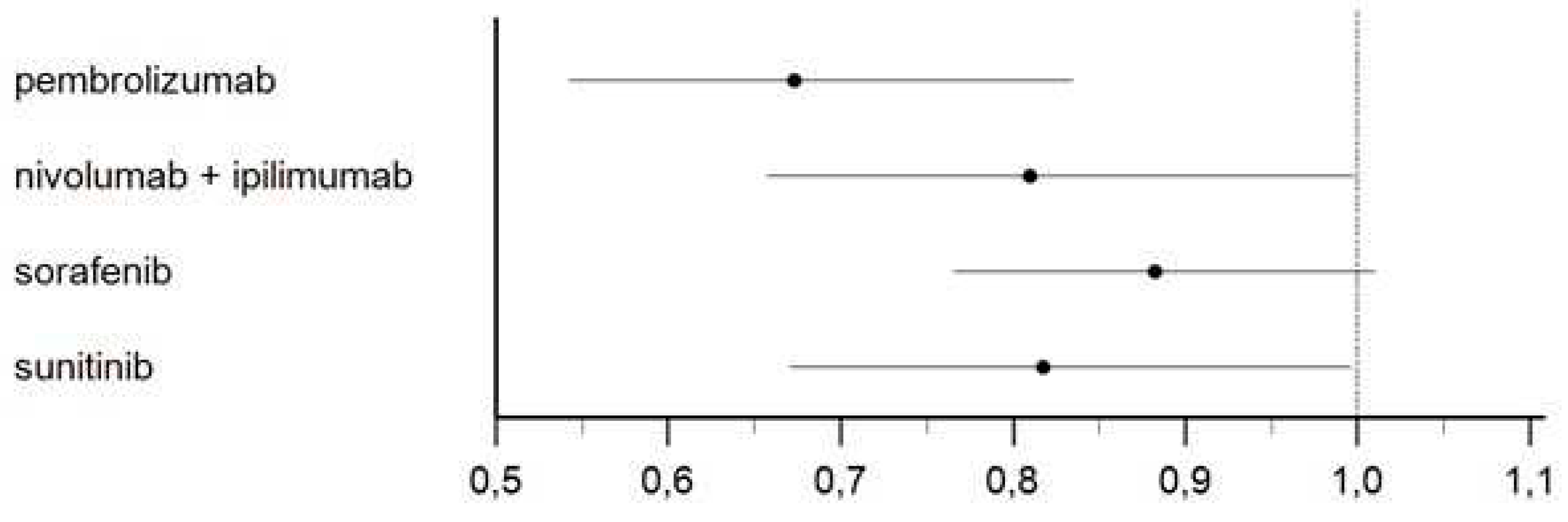
Table 1.
Main information about the 9 RCTs included in the analysis.
| # | Trial | Reference | Treatments under Comparison | DFS | |
|---|---|---|---|---|---|
| Treatment Group (Events/Patients) | Controls (Events/Patients) | ||||
| ARCC01 | KEYNOTE-564 (two-arm) | Powles et al. [11] | Pembrolizumab (200mg) Q3W (max 17 cycles); Placebo Q3W. | 114/496 | 169/498 |
| ARCC02 | IMmotion010 (two-arm) | Pal et al. [12] | Atezolizumab (1200mg) Q3W (max 16 cycles); Placebo Q3W. | 164/390 | 168/388 |
| ARCC03 | CheckMate914 (two-arm) | Motzer et al. [13] | Nivolumab (240mg) Q2W (max 12 cycles) + Ipilimumab (1mg/kg) Q6W (max 4 cycles); Placebo Q2W+Q6W. | 110/405 | 118/411 |
| TKI01 | ATLAS (two-arm) |
Gross -Goupil et al. [14] | Axitinib (5mg) BID (max 3 years); Placebo BID (max 3 years). | 96/363 | 107/361 |
| TKI02 | SORCE (three-arm) |
Eisen et al. [15] | Sorafenib (400mg) BID for 3 years | 245/639 | 167/430 |
| Sorafenib (400mg) BID for 1 year plus placebo for 2 years; Placebo BID for 3 years. | 242/642 | ||||
| TKI03 | ASSURE (three-arm) |
Haas et al. [16] | Sunitinib (50mg) (4weeks on/2off) for 54 weeks; | 284/647 | 287/647 |
| Sorafenib (400mg) BID for 54 weeks; Placebo for 54 weeks. | 284/649 | ||||
| TKI04 | PROTECT (two-arm) |
Motzer et al. [17] | Pazopanib (600mg) for 1 years; Placebo for 1 years. | 194/571 | 202/564 |
| TKI05 | S-TRAC (two-arm) |
Ravaud et al. [18] | Sunitinib (50mg) (4weeks on/2off) for 52 weeks; Placebo for 52 weeks. | 113/309 | 144/306 |
| TKI06 | EVEREST (two-arm) |
Ryan et al. [19] | Everolimus (10mg) for 54 weeks; Placebo for 54 weeks. | 262/755 | 294/744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Efficacy of Immune Checkpoint Inhibitors vs Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: In-direct Comparison of Disease-Free Survival
Andrea Ossato
et al.
,
2023
Comparative Efficacy of First-Line Immune-Based Combination Therapies in Metastatic Renal Cell Carcinoma. A Systematic Review and Network Meta-Analysis.
Reza ELAIDI
et al.
,
2020
Combined anti-PD-1 and anti-CTLA-4 Treatment in Stage IV Melanoma Patients: Bicentric Analysis of Real-world Data
Alexandru Dorin Adrian Silași
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









