Preprint
Communication
Establishment of a Novel Anti-mouse CCR1 Monoclonal Antibody C1Mab-6
Altmetrics
Downloads
108
Views
54
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
27 December 2023
Posted:
27 December 2023
You are already at the latest version
Alerts
Abstract
C-C motif chemokine receptor 1 (CCR1/CD191) is a member of G-protein-coupled receptors, and is expressed on myeloid cells, such as neutrophils and macrophages. Because the CCR1 signaling promotes tumor expansion in the tumor microenvironment (TME), the modification of TME is an effective strategy for cancer therapy. Although CCR1 is an attractive target for solid tumors and hematological malignancies, anti-CCR1 therapeutic agents have not been approved. Here, we established a novel anti-mouse CCR1 (mCCR1) monoclonal antibody (mAb), C1Mab-6 (rat IgG2b, kappa), using the Cell-Based Immunization and Screening (CIBS) method. Flow cytometry and Western blot analyses showed that C1Mab-6 recognizes mCCR1 specifically. The dissociation constant of C1Mab-6 for mCCR1-overexpressed Chinese hamster ovary-K1 was determined as 3.9×10-9 M, indicating that C1Mab-6 possesses a high affinity to mCCR1. These results suggest that C1Mab-6 could be a useful tool for targeting CCR1 in preclinical mouse models.
Keywords:
Subject: Medicine and Pharmacology - Oncology and Oncogenics
1. Introduction
C-C motif chemokine receptor 1 (CCR1), also known as CD191, is a member of G-protein-coupled receptors for many chemokines, such as CCL3, CCL5, CCL7, CCL8, CCL13, CCL14, CCL15, CCL16, and CCL23 in humans.[1] CCR1, which is expressed on neutrophils or macrophages, is important for the infiltration of these cells. The leukocyte infiltration process consists of three steps: i) tethering, rolling, and arrest on the vessel wall, ii) crawling on the endothelium, and iii) transendothelial migration.[2] CCR1 contributes to stable adhesion of crawling neutrophils on the endothelium in the joints in the K/BxN serum transferred mice (a murine model for rheumatoid arthritis [RA]).[3,4]
CCR1 signaling promotes tumor invasion and metastasis. The cis-Apc(+/Delta716) Smad4(+/-) mutant mice develop spontaneous invasive colorectal cancers.[5] However, CCR1-knockout in the background decreases the invasiveness of these tumors in vivo.[6] CCR1-positive myeloid cells accumulate at the tumor invasive front and drive tumor invasion by producing the matrix metalloproteinases such as MMP9 and MMP2.[7,8] Liver metastasis is observed in 25% of patients with colorectal cancers and is the main cause of death.[9,10] Analysis of disease-free survival (DFS) after curative liver resection showed that the DFS of patients with CCL15 (a CCR1 ligand)-positive liver metastases is shorter than that of CCL15-negative.[8] More CCR1-positive cells accumulated around the CCL15-positive metastases than CCR15-negative ones.[8] CCR1 signaling also plays a critical role in the development of a carcinogen-induced hepatocellular carcinoma model. The CCR1 deficiency in mice reduced the tumor development through suppression of neovascularization and intratumoral Kupffer cell accumulation.[11]
TME influences the tumor progression and responsiveness to therapies.[12] Modification of TME is a strategy for improving the effect of antitumor therapies.[13] Although CCR1 is an attractive target for modifying TME and suppressing tumor progression, few preclinical studies using anti-mouse CCR1 (mCCR1) monoclonal antibodies (mAbs) have been reported.[14]
2. Materials and Methods
2.1. Antibodies
The anti-mouse CCR1 mAb (clone S15040E)[26] was purchased from BioLegend (San Diego, CA). We previously developed RcMab-1 against isocitrate dehydrogenase 1.[27] An anti-DYKDDDDK mAb (clone 1E6) was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Alexa Fluor 488-conjugated anti-rat IgG was purchased from Cell Signaling Technology, Inc. (Danvers, MA). HRP-conjugated anti-rat IgG (A9542) and HRP-conjugated anti-mouse IgG (P0260) were purchased from Sigma-Aldrich (St. Louis, MO) and Agilent Technologies, Inc. (Santa Clara, CA), respectively.
2.2. Animals
A five-week-old Sprague–Dawley rat was purchased from CLEA Japan (Tokyo, Japan). The animal was housed under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001).
2.3. Cell lines
LN229, Chinese hamster ovary (CHO)-K1, and P3X63Ag8U.1 (P3U1) cells were obtained from the American Type Culture Collection (Manassas, VA). pCMV6neo-myc-DDK vector with mCCR1 (Accession No.: NM_009912) was purchased from OriGene Technologies, Inc. (Rockville, MD). The plasmid was transfected into the cell lines using a Neon transfection system (Thermo Fisher Scientific, Inc., Waltham, MA). Subsequently, LN229 and CHO-K1, which stably overexpressed mCCR1 with C-terminal myc-DDK tags (hereinafter described as LN229/mCCR1 and CHO/mCCR1, respectively) were established using a cell sorter (SH800; Sony Corp., Tokyo, Japan), following cultivation in a medium containing 0.5 mg/mL G418 (Nacalai Tesque, Inc., Kyoto, Japan).
CHO-K1, P3U1, and CHO/mCCR1 were also cultured in a Roswell Park Memorial Institute (RPMI) 1640 medium (Nacalai Tesque, Inc.) that was supplemented with 10% heat-inactivated fetal bovine serum (FBS, Thermo Fisher Scientific Inc.), 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Nacalai Tesque, Inc.). LN229 and LN229/mCCR1 were cultured in a Dulbecco’s Modified Eagle Medium (DMEM; Nacalai Tesque, Inc.) that was supplemented as shown above. Then, cells were grown in a humidified incubator, which was supplied with 5% CO2 and 95% air at 37°C.
2.4. Hybridoma production
For developing anti-mCCR1 mAbs, a six-week-old female Sprague-Dawley rat (CLEA Japan, Tokyo, Japan) was immunized intraperitoneally with 1 × 109 cells of CHO/mCCR1. The immunogen was harvested after brief exposure to 1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). We added Alhydrogel adjuvant 2% (InvivoGen, San Diego, CA) as an adjuvant in the first immunization. Three additional injections of 1 × 109 cells of CHO/mCCR1 were performed without an adjuvant every week. We performed a final booster immunization of 1 × 109 cells of CHO/mCCR1 intraperitoneally two days before harvesting splenocytes. We fused the harvested splenocytes with P3U1 cells using polyethylene glycol 1500 (PEG1500; Roche Diagnostics, Indianapolis, IN). Hybridoma cells were cultured in the RPMI1640 medium, supplemented as shown above. We further added hypoxanthine, aminopterin, and thymidine (HAT; Thermo Fisher Scientific, Inc.), 5% Briclone (NICB, Dublin, Ireland), and 5 μg/mL of plasmocin into the medium. The hybridoma supernatants were screened by flow cytometry using LN229/mCCR1 and parental LN229. The culture supernatants of hybridomas were filtrated and purified using Ab-Capcher Extra (ProteNova, Kagawa, Japan).
2.5. Flow cytometry
CHO-K1, CHO/mCCR1, LN229, and LN229/mCCR1 cells were harvested by exposure to 1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque). The cells were washed with 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and treated with anti-mCCR1 mAbs for 30 min at 4oC. After washing, the cells were treated with Alexa Fluor 488-conjugated anti-rat IgG. Flow cytometric analysis was performed using the SA3800 Cell Analyzer (Sony, Tokyo, Japan).
2.6. Determination of the binding affinity by flow cytometry
The dissociation constants (KD) were suspended in 100 μL serially diluted anti-mCCR1 mAbs (100 µg/mL to 6 ng/mL), after which Alexa Fluor 488-conjugated anti-rat IgG (1:200) were added. Fluorescence data were subsequently collected, using an SA3800 Cell Analyzer (Sony Corp.), following the calculation of the dissociation constant (KD) by fitting the binding isotherms into the built-in; one-site binding model in GraphPad PRISM 10 (GraphPad Software, Inc., La Jolla, CA).
2.7. Western blot analysis
Cell lysates (10 μg) were boiled in sodium dodecyl sulfate (SDS) sample buffer (Nacalai Tesque, Inc.), after which proteins were separated on 5%–20% polyacrylamide gels (FUJIFILM Wako Pure Chemical Corporation) and transferred to polyvinylidene difluoride membranes (Merck KGaA). After blocking with 4% skim milk (Nacalai Tesque, Inc.) in 0.05% Tween 20-containing PBS, membranes were incubated with 0.1 μg/mL of anti-DYKDDDDK or 1 μg/mL of the other primary mAbs. Then, they were incubated again with horseradish peroxidase (HRP)-conjugated anti-rat immunoglobulins (for anti-CCR1 mAbs and RcMab-1; diluted 1:10,000) or anti-mouse immunoglobulins (for anti-DYKDDDDK; diluted 1:1,000). Finally, protein bands were detected using ImmunoStar LD (FUJIFILM Wako Pure Chemical Corporation) with a Sayaca-Imager (DRC Co. Ltd., Tokyo, Japan).
3. Results
3.1. Establishment of anti-mCCR1 antibodies
We used the CBIS method to establish novel an anti-mCCR1 antibody (Figure 1). The CBIS method is a high-throughput method for the establishment of antibodies against membrane proteins. CBIS method consists of two main steps: immunization of antigen-overexpressing cells (Figure 1A) and screening of hybridoma supernatants using flow cytometry (Figure 1B). After immunization of CHO/mCCR1 and screening of hybridoma supernatants with LN229/mCCR1, C1Mab-6 (rat IgG2b, kappa) was finally developed.
3.2. Flow cytometry using anti-CCR1 mAbs
To check the specificity and reactivity of C1Mab-6, we performed flow cytometry against LN229/mCCR1, LN229, CHO/mCCR1, and CHO-K1. S15040E is a commercially available mAb[26] and was used as a positive control in this study. C1Mab-6 bound to LN229/mCCR1 and CHO/mCCR1 cells in a dose-dependent manner (Figure 2A and Figure 3A). In contrast, S15040E exhibited lower reactivity against both cells compared to C1Mab-6. C1Mab-6 and S15040E did not react with LN229 cells and CHO-K1 even at 10 µg/mL (Figure 2B and Figure 3B), indicating that both mAbs are specific to mCCR1.
3.3. Determination of dissociation constant of anti-CCR1 mAbs
Next, we determined the dissociation constant (KD) of anti-CCR1 mAbs against mCCR1, which is expressed on the cell surface using flow cytometry. The geometric mean of the fluorescence intensity at each concentration of C1Mab-6 and S15040E was plotted. By fitting one-site binding models, the KD values of C1Mab-6 and S15040E for LN299/mCCR1 were determined as 1.0×10-8 M and 1.7×10-7 M, respectively (Figure 4A and Figure 4B). Furthermore, the KD values of C1Mab-6 and S15040E for CHO/mCCR1 were calculated as 3.9×10-9 M and 3.5×10-8 M, respectively (Figure 4C and Figure 4D), indicating that C1Mab-6 possesses much higher affinity than S15040E.
3.4. Western blot using anti-CCR1 mAbs
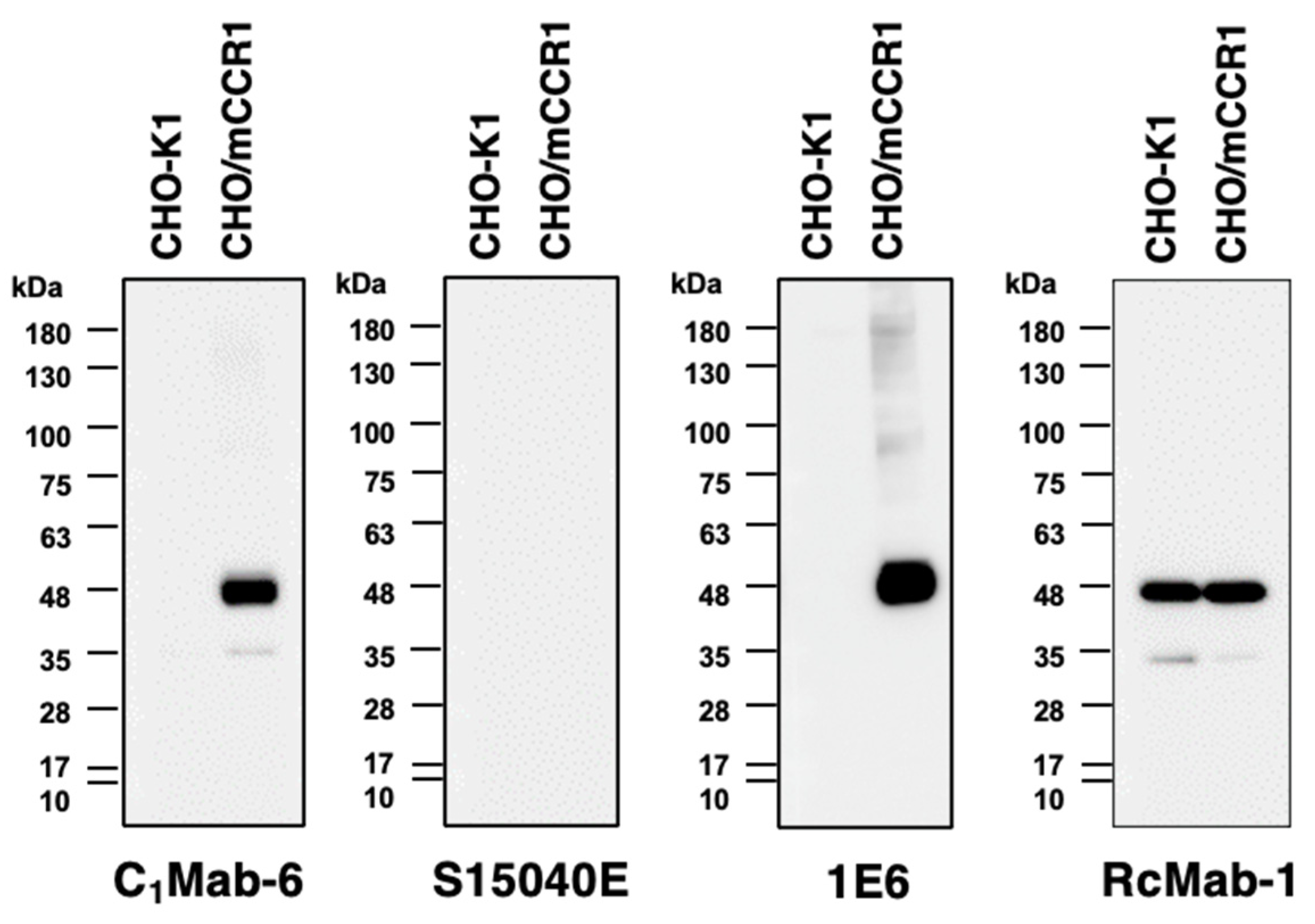
Finally, we performed Western blot analysis using anti-CCR1 mAbs. Lysates of CHO-K1 and CHO/mCCR1 were probed. The molecular weight of mCCR1-mycDDK is about 44,000. C1Mab-6 and an anti-DYKDDDDK mAb (clone 1E6) detected mCCR1 as about a 44-kDa band from lysates of CHO/mCCR1 (Figure 5) although S15040E could not detect mCCR1 in the lysates of CHO/mCCR1. C1Mab-6 did not detect any bands from the lysates of CHO-K1, indicating that C1Mab-6 can recognize mCCR1 specifically in Western blot analysis.
4. Discussion
Anti-CCR1 therapies are potential treatments to improve the quality of life of multiple myeloma (MM) patients. MM is an incurable plasma B-cell malignancy.[28] Systemic osteopenia is observed in most MM patients.[29] Osteopenia causes bone fractures and impacts their quality of life. Serum levels of CCL3 are elevated in MM patients and correlate with the extent of bone disease.[30] MM cells produce CCL3, which activates osteoclast.[31,32] Neutralization of CCL3 with anti-CCL3 antibodies blocks osteolysis in mice.[32] CCL3 is a ligand activating CCR1 and CCR5.[1] CCX721, a small molecule antagonist against CCR1 not CCR5, dramatically reduced the osteolysis induced by 5TGM1, a murine MM cell line.[33] Additionally, CCX721 also reduces tumor burden of 5TGM1 cells inoculated intravenously.[33] CCX721 does not block the proliferation of 5TGM1 cells in vitro or inoculated subcutaneously.[33] These results indicated that CCR1 blockage inhibits tumor burden and osteolysis by MM through modifying the bone marrow microenvironment.
RA is a chronic inflammatory disease characterized by massive infiltration of synovial tissue and synovial fluid with immune cells in affected joints.[34] CCR1 is abundantly expressed by RA monocytes/macrophages, which mediate the inflammation at these sites.[35] CCX354, an analog of CCX721, was developed for the treatment of RA.[36] CCX354 exhibited good safety and clinical activities to RA patients in a Phase II study.[37] However, once daily oral administration is needed because the half-life of CCX354 in the plasma of humans is approximately 6 h.[36] Antibodies are therapeutic drugs stable in serum (the half-life of antibodies is around 3 weeks).[38] C1Mab-6 can bind to mCCR1-expressed cells with a high affinity (Figure 4). C1Mab-6 could be useful tools for developing anti-CCR1 therapies in preclinical murine models.
References
- Stone, M.J.; Hayward, J.A.; Huang, C.; Z, E.H.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, Y.; Miyabe, C.; Murooka, T.T.; Kim, E.Y.; Newton, G.A.; Kim, N.D.; Haribabu, B.; Luscinskas, F.W.; Mempel, T.R.; Luster, A.D. Complement C5a Receptor is the Key Initiator of Neutrophil Adhesion Igniting Immune Complex-induced Arthritis. Sci Immunol 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.D.; Haase, C.; Cook, A.D.; Hamilton, J.A. K/BxN Serum-Transfer Arthritis as a Model for Human Inflammatory Arthritis. Front Immunol 2016, 7, 213. [Google Scholar] [CrossRef]
- Takaku, K.; Oshima, M.; Miyoshi, H.; Matsui, M.; Seldin, M.F.; Taketo, M.M. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell 1998, 92, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Kometani, K.; Hashida, H.; Matsunaga, A.; Miyoshi, H.; Hosogi, H.; Aoki, M.; Oshima, M.; Hattori, M.; Takabayashi, A.; et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet 2007, 39, 467–475. [Google Scholar] [CrossRef]
- Hirai, H.; Fujishita, T.; Kurimoto, K.; Miyachi, H.; Kitano, S.; Inamoto, S.; Itatani, Y.; Saitou, M.; Maekawa, T.; Taketo, M.M. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis 2014, 31, 977–989. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, P.; Fujii, C.; Nakamoto, Y.; Gao, J.L.; Kaneko, S.; Murphy, P.M.; Mukaida, N. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer 2006, 118, 1869–1876. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Wang, S.B.; Liu, J.H.; Jin, L.; Liu, Y.; Li, C.Y.; Su, Y.R.; Liu, Y.R.; Sang, X.; Wan, Q.; et al. Modifying the tumour microenvironment and reverting tumour cells: New strategies for treating malignant tumours. Cell Prolif 2020, 53, e12865. [Google Scholar] [CrossRef]
- Kiyasu, Y.; Kawada, K.; Hirai, H.; Ogawa, R.; Hanada, K.; Masui, H.; Nishikawa, G.; Yamamoto, T.; Mizuno, R.; Itatani, Y.; et al. Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice. Cancer Lett 2020, 487, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Suzuki, H.; Tanaka, T.; Saito, M.; Li, G.; Goto, N.; Nanamiya, R.; Kaneko, M.K.; Kato, Y. C(3)Mab-3: A Monoclonal Antibody for Mouse CC Chemokine Receptor 3 for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022, 41, 74–79. [Google Scholar] [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Sano, M.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 65–70. [Google Scholar] [CrossRef]
- Ouchida, T.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Cx(4)Mab-1: A Novel Anti-Mouse CXCR4 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2023. [Google Scholar] [CrossRef]
- Hosono, H.; Asano, T.; Takei, J.; Sano, M.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of an Anti-Elephant Podoplanin Monoclonal Antibody PMab-265 for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 141–145. [Google Scholar] [CrossRef]
- Takei, J.; Asano, T.; Nanamiya, R.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Harada, H.; et al. Development of Anti-human T Cell Immunoreceptor with Ig and ITIM Domains (TIGIT) Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Asano, T.; Sano, M.; Takei, J.; Hosono, H.; Nanamiya, R.; Nakamura, T.; Yanaka, M.; Harada, H.; Fukui, M.; et al. Development of Monoclonal Antibody PMab-269 Against California Sea Lion Podoplanin. Monoclon Antib Immunodiagn Immunother 2021, 40, 124–133. [Google Scholar] [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Monoclonal Antibody PMab-292 Against Ferret Podoplanin. Monoclon Antib Immunodiagn Immunother 2022, 41, 101–109. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-EpCAM Monoclonal Antibody for Various Applications. Antibodies (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Nanamiya, R.; Suzuki, H.; Takei, J.; Li, G.; Goto, N.; Harada, H.; Saito, M.; Tanaka, T.; Asano, T.; Kaneko, M.K.; et al. Development of Monoclonal Antibody 281-mG(2a)-f Against Golden Hamster Podoplanin. Monoclon Antib Immunodiagn Immunother 2022, 41, 311–319. [Google Scholar] [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; Ishikawa, K.; Ouchida, T.; Kaneko, M.K.; Kato, Y. EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tanaka, T.; Kudo, Y.; Tawara, M.; Hirayama, A.; Kaneko, M.K.; Kato, Y. A Rat Anti-Mouse CD39 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2023. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation. Signal Transduct Target Ther 2021, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kato Kaneko, M.; Ogasawara, S.; Kato, Y. Establishment of a multi-specific monoclonal antibody MsMab-1 recognizing both IDH1 and IDH2 mutations. Tohoku J Exp Med 2013, 230, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Puertas, B.; Gonzalez-Calle, V.; Sobejano-Fuertes, E.; Escalante, F.; Queizan, J.A.; Barez, A.; Labrador, J.; Alonso-Alonso, J.M.; Garcia de Coca, A.; Cantalapiedra, A.; et al. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, M.; Harada, T.; Tanaka, E.; Abe, M. Pathogenesis and treatment of multiple myeloma bone disease. Jpn Dent Sci Rev 2021, 57, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Politou, M.; Szydlo, R.; Goldman, J.M.; Apperley, J.F.; Rahemtulla, A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol 2003, 123, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.; Pozzi, S.; Patel, K.; Vaghela, N.; Fulciniti, M.T.; Veiby, P.; Hideshima, T.; Santo, L.; Cirstea, D.; Scadden, D.T.; et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 2011, 25, 1174–1181. [Google Scholar] [CrossRef]
- Oyajobi, B.O.; Franchin, G.; Williams, P.J.; Pulkrabek, D.; Gupta, A.; Munoz, S.; Grubbs, B.; Zhao, M.; Chen, D.; Sherry, B.; et al. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 2003, 102, 311–319. [Google Scholar] [CrossRef]
- Dairaghi, D.J.; Oyajobi, B.O.; Gupta, A.; McCluskey, B.; Miao, S.; Powers, J.P.; Seitz, L.C.; Wang, Y.; Zeng, Y.; Zhang, P.; et al. CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood 2012, 120, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Haringman, J.J.; Ludikhuize, J.; Tak, P.P. Chemokines in joint disease: the key to inflammation? Ann Rheum Dis 2004, 63, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Katschke, K.J., Jr.; Rottman, J.B.; Ruth, J.H.; Qin, S.; Wu, L.; LaRosa, G.; Ponath, P.; Park, C.C.; Pope, R.M.; Koch, A.E. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum 2001, 44, 1022–1032. [Google Scholar] [CrossRef]
- Dairaghi, D.J.; Zhang, P.; Wang, Y.; Seitz, L.C.; Johnson, D.A.; Miao, S.; Ertl, L.S.; Zeng, Y.; Powers, J.P.; Pennell, A.M.; et al. Pharmacokinetic and pharmacodynamic evaluation of the novel CCR1 antagonist CCX354 in healthy human subjects: implications for selection of clinical dose. Clin Pharmacol Ther 2011, 89, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Balanescu, A.; Tseluyko, V.; Bojin, S.; Drescher, E.; Dairaghi, D.; Miao, S.; Marchesin, V.; Jaen, J.; Schall, T.J.; et al. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. Ann Rheum Dis 2013, 72, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Morell, A.; Terry, W.D.; Waldmann, T.A. Metabolic properties of IgG subclasses in man. J Clin Invest 1970, 49, 673–680. [Google Scholar] [CrossRef]
Figure 1.
The scheme of establishment of C1Mab-6 by CBIS method. (A) CHO/mCCR1-mycDDK cells were injected intraperitoneally into a Sprague–Dawley rat. The splenocytes of the rat were fused with P3U1 cells and seeded in 96 well plates. (B) The culture supernatants of each well were screened through flow cytometry to distinguish anti-mCCR1 mAb-producing hybridomas. C1Mab-6 was established by limiting dilution and some additional screenings.
Figure 1.
The scheme of establishment of C1Mab-6 by CBIS method. (A) CHO/mCCR1-mycDDK cells were injected intraperitoneally into a Sprague–Dawley rat. The splenocytes of the rat were fused with P3U1 cells and seeded in 96 well plates. (B) The culture supernatants of each well were screened through flow cytometry to distinguish anti-mCCR1 mAb-producing hybridomas. C1Mab-6 was established by limiting dilution and some additional screenings.

Figure 2.
Flow cytometry of anti-CCR1 mAbs against LN229/mCCR1. LN229/mCCR1 (A) and LN229 (B) cells were treated with 0.1–10 µg/mL of C1Mab-6 or S15040E, followed by treatment with Alexa Fluor 488-conjugated anti-rat IgG antibodies. The red lines show the cells treated with each mAb. The black lines show the cells treated with blocking buffer and Alexa Fluor 488-conjugated anti-rat IgG antibodies (negative control).
Figure 2.
Flow cytometry of anti-CCR1 mAbs against LN229/mCCR1. LN229/mCCR1 (A) and LN229 (B) cells were treated with 0.1–10 µg/mL of C1Mab-6 or S15040E, followed by treatment with Alexa Fluor 488-conjugated anti-rat IgG antibodies. The red lines show the cells treated with each mAb. The black lines show the cells treated with blocking buffer and Alexa Fluor 488-conjugated anti-rat IgG antibodies (negative control).

Figure 3.
Flow cytometry of anti-CCR1 mAbs against CHO/mCCR1. CHO/mCCR1 (A) and CHO-K1 (B) cells were treated with 0.1–10 µg/mL of C1Mab-6 or S15040E, followed by treatment with Alexa Fluor 488-conjugated anti-rat IgG antibodies. The red lines show the cells treated with each mAb. The black lines show the cells treated with blocking buffer and Alexa Fluor 488-conjugated anti-rat IgG antibodies (negative control).
Figure 3.
Flow cytometry of anti-CCR1 mAbs against CHO/mCCR1. CHO/mCCR1 (A) and CHO-K1 (B) cells were treated with 0.1–10 µg/mL of C1Mab-6 or S15040E, followed by treatment with Alexa Fluor 488-conjugated anti-rat IgG antibodies. The red lines show the cells treated with each mAb. The black lines show the cells treated with blocking buffer and Alexa Fluor 488-conjugated anti-rat IgG antibodies (negative control).

Figure 4.
Kinetic analyses of anti-CCR1 mAbs against mCCR1-overexpressed cells. The binding affinity of C1Mab-6 and S15040E against LN229/mCCR1 cells (A and B) and CHO/mCCR1 cells (C and D) were determined by flow cytometry. The dots show the geometric mean of fluorescence intensity at each concentration. The solid lines are the fitting curve calculated by GraphPad PRISM 6.
Figure 4.
Kinetic analyses of anti-CCR1 mAbs against mCCR1-overexpressed cells. The binding affinity of C1Mab-6 and S15040E against LN229/mCCR1 cells (A and B) and CHO/mCCR1 cells (C and D) were determined by flow cytometry. The dots show the geometric mean of fluorescence intensity at each concentration. The solid lines are the fitting curve calculated by GraphPad PRISM 6.

Figure 5.
Kinetic analyses of anti-CCR1 mAbs against mCCR1-overexpressed cells. The binding affinity of C1Mab-6 and S15040E against LN229/mCCR1 cells (A and B) and CHO/mCCR1 cells (C and D) were determined by flow cytometry. The dots show the geometric mean of fluorescence intensity at each concentration. The solid lines are the fitting curve calculated by GraphPad PRISM 6.
Figure 5.
Kinetic analyses of anti-CCR1 mAbs against mCCR1-overexpressed cells. The binding affinity of C1Mab-6 and S15040E against LN229/mCCR1 cells (A and B) and CHO/mCCR1 cells (C and D) were determined by flow cytometry. The dots show the geometric mean of fluorescence intensity at each concentration. The solid lines are the fitting curve calculated by GraphPad PRISM 6.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Determination of the Binding Epitope of an Anti-mouse CCR9 Monoclonal Antibody (C9Mab-24) using the 1 × alanine and 2 × alanine-substitution Method
Hiyori Kobayashi
et al.
,
2022
Epitope Mapping of Anti-mouse CCR3 Monoclonal Antibodies using Flow Cytometry
Nami Tateyama
et al.
,
2022
Epitope Mapping of an Anti-mouse CCR8 Monoclonal Antibody C8Mab-2 Using Flow Cytometry
Hiyori Kobayashi
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








