Preprint
Article
Trypanocidal Activity of Compounds Isolated From Parthenium incanum Kunth
Altmetrics
Downloads
113
Views
64
Comments
0
Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Alerts
Abstract
Parthenium incanum (Mariola) is used in Mexico to treat stomach and liver disorders. Reports have shown that the genus Parthenium contains biologically active metabolites. This work aimed to isolate and identify biologically active compounds with trypanocidal activity. The extracts were obtained through static maceration and extraction with successive reflux using different solvents. After that, they were fractionated with column chromatography, and the obtained fractions were compared by TLC; similar fractions were pooled and concentrated. A bioguided assay was performed with S. aureus ATCC 25923, using agar diffusion techniques and bioautography to determine preliminary biological activity. Active fractions were obtained and purified from a preparative TLC plate. The obtained compounds were identified as parthenin and coronopolin by TLC, FT-IR, and HPLC-MS. Finally, they were evaluated against the Ninoa strain of Trypanosoma cruzi, obtaining an IC50 of up to 45±2.5 ppm. It was concluded that P. incanum contains compounds with trypanocidal activity.

Keywords:
Subject: Biology and Life Sciences - Parasitology
1. Introduction
The genus Parthenium contains approximately 16 species of shrubs, herbaceous perennials, and annuals. P. incanum (also called Mariola) is a medicinal plant used in several states of Mexico to treat gastric disorders, such as constipation, diarrhea, poor digestion, and stomach pain. It is even used to treat liver conditions [1,2]. However, the importance of this genus is related to the fact that there is a significant number of reports of harmful effects in both humans and livestock, causing severe dermatitis upon contact and allergic respiratory problems [3]; also, it is known that it contains toxic bitter compounds called sesquiterpene lactones, of which different biological activities have been reported [2,4]. Despite its toxicity, P. incanum is commonly used in Mexico as a remedy for malaria, neuralgia, vermifuge, and other ailments [5,6]; furthermore, studies of hemolysis in erythrocytes and toxicity with brine shrimp show that extracts of P. incanum produce favorable results if they are subjected to Soxhlet-type defatting with non-polar solvents [7]. Because of this, P. incanum can be considered a source of metabolites with biological activity, mainly against Trypanosoma cruzi. Chagas disease, caused by the hemoflagellate parasite T. cruzi, is endemic in Mexico, Central America, and South America, affecting more than 6 million people; its limited treatment alternatives available on the market (benznidazole and nifurtimox) and their adverse side effects [8] are the reason why a search for new alternatives to treat this disease should be carried out. Diverse methods for isolating and bioguided identifying compounds with antimicrobial activity have been widely reported [9,10,11]. The procedure for obtaining these compounds is based on a series of steps. The first crucial step in investigating new compounds is determining the type of solvent used in the extraction [10] depending on the compound sought [9]. Likewise, a phytochemical screening assay must be performed; this is a quick, simple, and inexpensive procedure that provides results on the different phytochemicals of the whole plant extracts or a fraction obtained from them. Subsequently, different techniques such as column chromatography (CC), thin layer chromatography (TLC), bioautographic methods, high-performance liquid chromatography (HPLC), high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS), and Fourier transform infrared spectroscopy (FT-IR) can be used for the separation, isolation, and identification of active compounds [9,10,11,12,13,14].
The objective of this study is to determine, by using biodirected, chromatographic, and spectroscopic techniques and methodologies, if the isolated components of P. incanum have trypanocidal potential.
2. Results
2.1. Extraction of plant material
The methanolic extracts were obtained using two different techniques of extraction: static maceration and the Soxhlet extraction method. Regarding these, the maceration technique yielded 13.5%, while the Soxhlet extraction yielded 7.2%.
2.2. Preliminary phytochemical screening of the methanolic extracts
The qualitative phytochemical analysis showed several families of secondary metabolites for both methanolic extracts, emphasizing the presence of alkaloids, flavonoids, sesquiterpene lactones, sterols, triterpenes, and tannins (Table 1).
2.3. Chromatographic separation
After phytochemical analysis, the methanolic extracts were fractionated by column chromatography, and 40 fractions of each extract were obtained. Stepwise elutions were collected separately, concentrated, and subjected to TLC. All collected fractions showing a similar TLC pattern were pooled and tested for antimicrobial activity.
2.4. Bioguided assay
According to the disc-diffusion method and bioautographic results, the antimicrobial activity was evidenced in 5 fractions obtained from the maceration extract and 3 fractions from the Soxhlet extraction, all from the elution using chloroform: methanol 9.5:0.5 and 9:1 at the chromatographic column. Subsequently, the purity of the isolated compounds was evaluated through TLC as previously described; equal fractions were mixed to increase their concentration, resulting in two active fractions, named “macetarion-1” and “Soxhlet-1”. The weight of the active fractions was 90 mg to 105 mg, respectively. Phytochemical screening was repeated on isolated compounds resulting in a strong positive reaction to sesquiterpene lactones. Each fraction was subjected to PTLC to recover the biologically active compound.
2.5. Identification of isolated compounds
Functional groups of the isolated compounds were determined through FT-IR. Figure 1 (maceration-1 compound) revealed the presence of the following groups; 3396 cm-1 for hydroxyl (OH), 2924 cm-1 and 2854 cm-1 for C-H (stretching alkane), 1707 cm-1 for the carbonyl group (C=O), 1458 cm-1 for C=C (double bond), 1377, cm-1 for C-H (stretching alkane), and 1104±5 cm-1 for C-O (lactone ring).
On the other hand, Figure 2 (Soxhlet-1 compound) shows the presence of functional groups: 3,327cm-1 (OH), 2,942 and 2,831 cm-1 (CH2), 1,741 cm-1 (C=O), 1,449 cm-1 (C=C), 1,337 cm-1 (CH3) and 1,021 cm-1 (C-O).
UHPLC-MS analyses of both compounds were operated to allow the selection of two pseudo molecular ions in positive mode, parthenin and coronopolin (m/z 263 and 265), and the scanning of product ions. To form the maceration sample, the main pseudo molecular ion with m/z 263 has given 4 peaks at various retention times 26.67, 28.10, 41.35, and 57.63 min, and several fragments (m/z 249, 245, 235, 231, 227, 221, 217, 207, 203, 201, 199, 189, 185, 179, 175, 163, 161, 149, 135, 123 and 109) (Figure 3).
While the main pseudo molecular ion with m/z 265 has given 4 peaks at various retention times 21.11, 26.96, 27.92 and 30.84 min and several fragments (m/z 247, 245, 235, 229, 219, 205, 201, 191, 187, 173, 159 and 139) (Figure 4).
On the other hand, from the Soxhlet sample, the main pseudo molecular ion with m/z 263 has given 4 peaks at various retention times, 23.46, 27.54, and 29.06 min, and several fragments (m/z 245, 227,217, 209, 201, 199, 189, 181, 173, 171 and 157) (Figure 5).
While the main pseudo molecular ion with m/z 265 has given 2 peaks at various retention times (26.27 and 27.87 min) and several fragments (m/z 247, 237, 229, 219, 205, 201, 187, 183, 173, 159, 147 and 105) (Figure 6).
2.6. Identification of isolated compounds using Analysis FT-IR and Analysis UHPLC-MS
FT-IR general spectrum showed the characteristic peaks for a sesquiterpene lactone. UHPLC-MS analyzed both samples, and the presence of two molecules with remarkable stereochemical similarity was confirmed, parthenin (Figure 7a) and coronopolin (Figure 7b).
It´s important to mention that both compounds presented trypanocidal potential against T. cruzi, with favorable results.
2.7. Evaluation of the trypanocidal activity
Crude extracts were first evaluated for trypanocidal activity and showed an IC50 of less than 100 ppm; the extract obtained by maceration presented an IC50 of 54.63 ppm ± 3.5 ppm, while the IC50 of the extract obtained by Soxhlet was 63.09 ppm ± 4.3 ppm. On the other hand, the isolated compounds obtained from maceration and Soxhlet presented activity at lower concentrations, showing an IC50 of 50.5 ppm ± 3 ppm and 45±2.5 ppm, respectively.
3. Discussion
The present investigation demonstrated that the compounds of P. incanum present trypanocidal activity. In addition, it was shown that the bioactive compounds, even from different extractions, maintained their biological activity. The plant material under study was sought according to the physical factors of its distribution. It refers to the geographical location of P. incanum with shrubs, mesquites, and sandy soil, with a fine texture and eroded by the action of the wind, in rocky places along roads; these characteristics correspond to the collection point. The presence of P. incanum has been recorded in different states of Mexico, such as Aguascalientes (municipality of Asientos, Tepezalá and the community of Juan El Grande), Chihuahua, Coahuila, Durango, Hidalgo, Guanajuato, State of Mexico, Nuevo León, Michoacán, San Luis Potosí, Querétaro, Sonora, Tamaulipas, and Zacatecas, finding P. incanum around 2 km from the municipality of Asientos [6,26]. Regarding the methanolic extractions, these were selected for the search of metabolites with antimicrobial activity because it has been reported that methanol is commonly used for the extraction of polar bio-active components since it can dissolve and extract most of the active compounds of interest in search of phytochemicals in medicinal plants [27]. The difference between the extraction yield may be due to the successive extraction using the Soxhlet apparatus since nonpolar compounds were previously eliminated from methanolic extract with hexane and chloroform [28]; this did not occur in the extraction by maceration since only a single solvent was used. Notably, the yield of the total Soxhlet extraction (hexane + chloroform + methanol) was 12.2% (data not shown). Qualitative phytochemical analyses of both methanolic extracts from P. incanum showed similar results to two previously reported methanolic extracts from Parthenium hysterophorus leaves [28,30]. Fractionation by column chromatography and their subsequent comparison of the fractions obtained by TLC allows us to obtain some specific regions and the compound of the plant under study [16]. Regarding this, column chromatography techniques have been widely used as purification techniques for successful fractionation and isolation of desired bioactive compounds from complex extract material; this is due to their simplicity, convenience, specificity, economy, and availability to be used in multiple mobile phases with different polarity. They are used to isolate and purify the active compounds that are responsible for the bioactivity, such as antimicrobial, antioxidant or cytotoxicity [31,32]. Following the previously mentioned processes and according to the results of the active fractions identified by disc-diffusion and bioautographic methods, we decided to carry out the detection of parthenin by eluting the compounds using TLC with a mixture of chloroform:acetone (3:1). When sprayed with the vanillin reagent with sulfuric acid, they showed a violet-blue band with an Rf value of around 0.6 after heating at 70 °C for 5 min, which is something characteristic of parthenin [21]. Subsequently, parthenin was recovered from the active fractions by PTLC with the help of a vanillin reagent. This was carried out because this methodology is used to isolate some compounds of interest, as in the case of isolated sterols of Lipidium meyenii Walp [22], obtained by PTLC. The high antimicrobial potential of parthenin has been previously demonstrated [33]; because of this, the antimicrobial activity was used as a biological indicator for the isolation of parthenin. The data obtained by the FT-IR analysis for the isolated compounds showed the presence of the main absorbance peaks (cm-1) of parthenin, the hydroxyl group (OH), carbonyl group (C=O), and the C-O that belongs to the lactone ring; these data agree with those obtained for different samples (seeds and leaves) of Parthenium hysterophorus [34,35,36]. Fourier Transform Infrared Spectroscopy offers a fast, valuable tool to identify and characterize the chemical constituents or functional groups present in the sample to elucidate the structural compounds [10,33], as was the case in identifying the isolated compounds. Our results of UHPLC-MS analyses showed the presence of major pseudo molecular ions in both samples, m/z 263 and 265, corresponding to parthenin and coronopolin, respectively; the molecular mass of the compounds of interest was used (262.31 g/mol for parthenin and 264.32 g/mol for coronopolin), along with an analysis in positive mode using electrospray ionization [M+H]+ for identification [21,37,38]. The product ions from both samples of main pseudo molecular ions with m/z 263 show the fragments 245 [M+H-18]+, 227 [M+H-36]+, 209 [M+H-54]+, 199 [M+H-64]+, 181 [M+H-82]+ and 149 m/z [M+H-114]+, which have been reported for the fragmentation of the parthenin by electrospray ionization in positive mode [38]. Meanwhile, main pseudo molecular ions with m/z 265 from both samples were obtained; these were the fragments 247 [M+H-18]+, 229 [M+H-36]+, 219 [M+H-46]+, 205 [M+H-60]+, 201 [M+H-64]+, 187 [M+H-78]+, 173 [M+H-92]+, 159 [M+H-106]+ and 139 [M+H-126]+. These types had not been previously reported. Loss of H2O [M+H-18]+ and loss of H2O with CO (carbonyl) [M+H-36]+ were observed for all analyses [39], giving characteristic ions for the compounds. The data obtained from the trypanocidal activity suggest that the isolated compounds need a lower concentration to inhibit T. cruzi than the crude extracts. However, our results are higher than those obtained by Muñoz et al. (2012), which determined, in vitro, the effect of the antiparasitic drugs Nifurtimox and Benznidazole; those results showed an IC 50 of 2.34 ppm ± 0.72 ppm and 13.12 ppm ± 2.45 ppm, respectively [40]. Furthermore, the data obtained by Acosta et al. (2020), in which the same antiparasitic agents were evaluated against several clones of T. cruzi, obtained a maximum IC50 of 15.22 ± 3.10 ppm [41]. The drugs mentioned above are approved and recognized as trypanocidal by PAHO/WHO. Nifurtimox is produced only in El Salvador, and benznidazole is produced only in Brazil and Argentina; unfortunately, both have a series of adverse effects [41]. These differences between data may be due to the type of strain used and the concentrations of parasites used.
4. Materials and Methods
4.1. Plant material
The aerial parts of P. incanum were collected in August of 2021 in the municipality of Real de Asientos, Aguascalientes, México (22°13´47.2” N, 102°06´20.9” W). The specimen was identified by Dr. Marco Antonio Guzmán Lucio and was stored in the Herbarium of Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Mx.
4.2. Extraction
Two different extraction methods were carried out: static maceration and reflux by the Soxhlet apparatus. For maceration, 100g of dried and powdered plant material were extracted with 500 mL of methanol (CTR, Scientific) for 24 h at 25 °C, without shaking; they were kept in dark conditions. After the maceration time, the solvent was filtered using a Whatman No. 1 filter paper; right after that, it was completely evaporated using a rotary evaporator (Yamato Scientific CO. LTD RE 200). Finally, the extract was resuspended in 10 mL of the extraction solvent, mixed, and completely evaporated for dry weight determination. On the other hand, to obtain the extract by reflux (Soxhlet), 200 g of the dried and crushed plant were heated at 45 ºC to reflux for 48 h with 500 mL of hexane (CTR, Scientific), chloroform (CTR, Scientific), and methanol (CTR, Scientific) separately and in sequence. Non-polar extracts were discarded, and the methanolic extract was evaporated entirely under reduced pressure to determine its dry weight. The extracts (obtained through maceration and reflux) were suspended in 15 mL of absolute methanol, aliquoted in amber vials, and stored at 4 ºC until needed [7].
4.3. Phytochemical screening
The qualitative phytochemical screening was carried out using specific reactions to demonstrate the presence of secondary metabolites. The presence of unsaturated groups such as triterpenes, sterols, coumarins, alkaloids, sesquiterpene lactones, quinones, carboxyl group, tannins, flavonoids, saponins, and carbohydrates on the extracts was proven [15].
4.4. Chromatographic separation
The extracts (5 g) were fractionated through column chromatography using silica gel technical grade (Ca ~ 0.1%) 60Å of 200-400 mesh (Sigma Aldrich, St Louis, MO). The extracts were eluted using 2 L of different combinations of chloroform (CTR, Scientific) and methanol (CTR, Scientific), with increasing polarity. Fractions were collected in 50 mL volumes. All the obtained fractions were concentrated in a rotary evaporator (Yamato Scientific CO. LTD RE 200). Once focused, the dried fractions were resuspended in 10 mL of the appropriate solvent and placed in 25 mL beakers. Similarities between fractions were evaluated by thin layer chromatography (TLC), using aluminum foils with silica gel 60 F254 (Merck, Darmstadt, Germany). Similar fractions were combined [16].
4.5. Bioguided assay: detection, isolation, and identification of active compounds
Fractions with antimicrobial activity were selected by the disc-diffusion method, using 6 mm filter paper discs impregnated with approximately 5 mg of each fraction and placed on Mueller Hinton agar plates, previously seeded with S. aureus ATCC 25923 (1x106 CFU/mL). After the incubation period (overnight), antimicrobial activity was detected due to the presence of an inhibition zone surrounding the disc [17,18,19]. The detection of antibacterial compounds present in selected active fractions was made using the agar overlay bioautography method. For this, 10 X 20 cm pre-activated TLC plates (silica gel type G; 5-15 µm F254; Sigma-Aldrich, St Louis MO) were used as stationary phase. TLC plates were loaded with 10 µl of each selected active fraction in a narrow band; afterwards, the plates were developed in a solvent chamber using chloroform:acetone (3:1) as a mobile solvent system. Developed plates were air-dried overnight at 35 °C to remove the residual solvent. After this, the chromatogram was covered with molten MH Agar (Difco, Detroit, MI), maintained in a water bath at 45 ºC, previously seeded with a suspension of S. aureus 25923 at a final concentration of 1X108 CFU/mL; after solidification, the plates were incubated overnight at 37 °C in a humidity chamber. The active fraction was visualized through the appearance of clear zones after the incubation period [20,21]. The Rf value of the chromatogram was calculated and recorded.
4.6. Preparative thin-layer chromatography (PTLC)
Once the antimicrobial band was detected through bioautography, the active fraction was purified through preparative thin-layer chromatography (PTLC). This was performed on 20 cm X 20 cm glass plates precoated (1 mm) with Silica Gel 60 G (Sigma-Aldrich, St Louis, MO). Active fraction (50 µL) was applied as a narrow band, about 1.5 cm in height from the lower edge of the plates, leaving a 1 cm border on the sides of the plate. The plates were developed in a chromatographic chamber after conditioning for 20 min with mobile phase vapor, using the appropriate solvents previously described. After development, the plates were removed from the system and dried in a fume hood. The bands responsible for the antimicrobial activity were scraped out and recovered with methanol, according to the Rf previously obtained [22]. The compounds were placed in amber vials and were completely dried for further analysis. Once antimicrobial compounds were obtained, phytochemical screening was performed, as previously mentioned, to determine the identity of the isolated compounds.
4.7. Identification of isolated compounds using Analysis FT-IR and Analysis UHPLC-MS
Two mg of each isolated compound were used for the Fourier-Transform Infrared Spectroscopy (FT-IR) analysis. The FT-IR analysis was carried out using a Perkin Elmer Spectrum GX, 16 scans in the region of 4000-650 cm-1; chemical tests were carried out to confirm the type of secondary metabolite [23,24]. For analysis of UHPLC-MS, chromatographic separation was performed using an Ultra HPLC Ultimate 3000 chromatographic system consisting of a high-pressure gradient pump with an online degasser, an autosampler, a column oven, and a variable wavelength UV-Vis detector (Thermo Fisher Scientific Dionex). A Discovery HS F5 column (150 x 2.1 mm, 3 μm; Supelco) was used with a mixture of a 2 % (v/v) acetic acid aqueous solution and acetonitrile as a mobile phase. Gradient elution started with a 5-min period with 5 % acetonitrile and increased to 50 % in 45 min. After this, a 10-min washing step with 80 % acetonitrile was included, and then the mobile phase was returned to the initial composition, conditioning for 20 min before the next injection. The mobile phase flow was 200 µL/min, column temperature 50 ºC, and an injection volume of 10 µL. Detection was performed by mass spectrometry using an LCQ Fleet (Thermo Fisher Scientific) equipped with an electrospray ionization source (ESI) and an ion trap mass analyzer. Nitrogen was used as sheath and drying gas at a flow of 40 and 10 units, respectively; analyses were performed using the following parameters: spray voltage 5 kV, capillary voltage 10 V, capillary temperature 325°C, and lens tube voltage 60 V. Data acquisition was performed in positive mode and included a full scan of m/z 100 to 1000 and mass/mass experiments using the collision-induced dissociation (CID), with a normalized collision energy of 24.5%, an isolation width of 0.9 m/z, an activation Rf voltage (activation Q) of 0.25, an activation time of 30 ms and a scanning range of m/z 100 to 300. Samples were dissolved using methanol (LC-MS grade) and filtered through a 0.2 µm nylon membrane (Millipore).
4.8. Evaluation of the trypanocidal activity of extracts and isolated compounds
The trypanocidal effect of the extracts and isolated compounds on the Ninoa strain of T. cruzi was determined by a microdilution method. For this, 140 µl of epimastigotes (1x105 /mL) growing in the Novy-MacNeal-Nicolle (NNN) culture medium was placed in each well. Afterwards, 10 µl of the compound at different concentrations (1000-10 ppm) were added. The negative control was epimastigotes without treatment; 1% crystal violet was used for the positive control. Methanol was used as a blank; it was the vehicle of the compound to be evaluated. The assay was performed 2 times in triplicate. The plates were incubated for 24 h at 25 ± 1 °C in a humid chamber. Viability was determined by microscopic observations using a Neubauer chamber [25]. The inhibitory concentration 50 (IC50) was obtained using the Probit method with SPSS software ver. 23.
5. Conclusions
This study confirmed the trypanocidal potential against T. cruzi of two isolated Parthenium incanum compounds, parthenin, and coronopolin.
However, the antecedents indicate that parthenin, the main compound of the species of the Parthenium genus, presents toxicity. Therefore, the toxic and cytotoxic evaluation of parthenin and bioactive compounds of the Parthenium genus should be considered to clarify their possible therapeutic use.
Author Contributions
“Conceptualization, E.S.G., and D.A.H.M; methodology, R.I.C.G.; validation, E.S.G., M.H.M.O. and A.C.M.; formal analysis, R.C.R, and A.C.M..; investigation, D.A.H.M. and K.D.S.B.; resources, E.S.G.; writing—original draft preparation, D.A.H.M.; writing—review and editing, E.S.G. R.C.R.; supervision, E.S.G. and A.C.M..; project administration, E.S.G..; funding acquisition, E.S.G. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by Programa de Apoyo a la Investigación Científica y Tecnológica (PAICYT, UANL SA834-19).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The collaboration of Erick Lopez Macias in collecting plant material is appreciated. Likewise, we thank Norma Adela Carrasco Ruiz and Joselyn Georgina Flores Ibarra for maintaining and preserving the T. cruzi strain used.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study's design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kane CW. Herbal medicine of the American Southwest: A guide to the identification, collection, preparation, and use of medicinal and edible plants of the Southwestern United States. Lincoln Town Press. 2006; 277.
- Usharani B, Solomon Raju AJ. Reproductive ecology of the globally invasive Whitetop Weed, Parthenium hysterophorus (Asteraceae). Phytol. Balcan: Int. J Balk Flora Veg, 2018; 24:225-38.
- Patel S. Harmful and beneficial aspects of Parthenium hysterophorus: an update. Biotech 2011; 1:1–9. [CrossRef]
- Shah WA. Phytochemical review profile of sesquiterpene lactone parthenin. Int J Res Pharm Chem 2014; 4:217-21.
- Ebadi M. Pharmacodynamic Basis of Herbal Medicine. CRC Press. Health Fit. 2006; 699.
- García Regalado G. Plantas Medicinales de Aguascalientes. Universidad Autónoma de Aguascalientes 2015; 296–97.
- Hernández Marín DA, Guevara Lara F, Rivas Morales C, Verduzco Martínez JA, Galindo Rodríguez SA, Sánchez García E. Biological activity of Nothoscordum bivalve (L.) Britton and Parthenium incanum Kunth extracts. Indian J Trad Knowl. 2018; 14:699-06.
- Acosta N, Yaluff G, López E, Bobadilla C, Ramírez A, Fernández I, Escobar P. In vitro susceptibility to benznidazole, nifurtimox and posaconazole of Trypanosoma cruzi isolates from Paraguay. Biomedica. 2010; 40:749-63.
- Van-Vuuren SF. Antimicrobial activity of South African medicinal plants. J ethnopharmacol 2008; 119: 462-72. [CrossRef]
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY. Extraction, Isolation, and characterization of bioactive compounds from plant extracts. Afr J Tradit Complement Alt M. 2011;8:1. [CrossRef]
- Al Saman MA, Hamouda RA, Abdella A, El Sabbagh SM, El Seoud GWA. TLC bioautographic detection and characterization of the antibacterial compound from the Cyanobacterium Anabaena oryzae. Asian J Biotechnol Bioresour Technol. 2018; 3:1-13. [CrossRef]
- Jesionek W, Móricz AM, Alberti A, Ott PG, Kocsis B, Horváth G, Choma IM. TLC-Direct Bioautography as a Bioassay Guided Method for Investigation of Antibacterial Compounds in Hypericum perforatum L. J AOAC Int. 2015; 98: 1013–20. [CrossRef]
- Shan Y. Chapter 2: Methods for Determining the Functional Components of Citrus Peel, In Comprehensive Utilization of Citrus By-Products. Academic Press. 2016; 15-30.
- Scigelova M, Hornshaw M, Giannakopulos A, Makarov A. Fourier transform mass spectrometry. Mol Cell Prot. 2011; 10. [CrossRef]
- Sánchez E, Rivas-Morales C, Castillo S, Leos Rivas C, García-Becerra L, Ortiz Martínez DM. Antibacterial and antibiofilm activity of methanolic plant extracts against nosocomial microorganisms. Evid Based Complement Alternat Med: eCAM 2016; págs. 1-8. [CrossRef]
- Ibarra Estrada E, Pacheco-Sánchez M, García Mateos R, San Miguel Chávez R., Ramírez Valverde G, Soto Hernández RM. Actividad antioxidante de alcaloides de Erythrina americana Miller. Rev Fitotec Mex 2011; 34:241. [CrossRef]
- Zeouk I, Ouedrhiri W, Sifaoui I, Bazzocchi IL, Piñero JE, Jiménez IA, Lorenzo Morales J, Bekhti K. Bioguided Isolation of Active Compounds from Rhamnus alaternus against Methicillin-Resistant Staphylococcus aureus (MRSA) and Panton-Valentine Leucocidin Positive Strains (MSSA-PVL). Mol 2011; 26:4352. [CrossRef]
- Saravanan, S, Thangaraj P. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Science and Human Wellness. 2014; 3.
- Khurm M, Chaudhry BA, Uzair M, Janbaz KH. Antimicrobial, Cytotoxic, Phytotoxic and Antioxidant Potential of Heliotropium strigosum Willd. Medicines (Basel). 2016; 3:20. [CrossRef]
- Kagan IA, Flythe MD. Thin-layer chromatographic (TLC) separations and bioassays of plant extract to identify antimicrobial compounds. J Visual Exp. 2014; 85. [CrossRef]
- Saucedo Hernández Y, Bravo Sánchez L, Gonzalez Bedia MM., Torres Gómez L, Jorge Rodríguez E, Gonzalez San Miguel HM, Gonzalez Mosquera D, Polín García L, Dhooghe L, Theunis M, Pieters L, Apers S. Determination of parthenin in Parthenium hysterophorus L. using HPLC-UV: Method development and validation. Phytochemistry letters. 2011; 4: 134-37. [CrossRef]
- Gutiérrez J, Montaño K, Bracho J, Rodríguez C, Chang A. Caracterización de esteroles en la fracción lipídica de la maca (walp.) mediante técnicas cromatográficas. Rev Soc Química de Perú. 2009; 75: 254-65.
- Basarkar UG, Saoji A. Isolation, characterization of sesquiterpene parthenin and its estimation from Parthenium hysterophorus pollen. Int J Emerg Technol Comput Appl Sci. 2013; 5: 364-68.
- Sharmin H, Misbahuddin M. Compound isolated from the cock’s comb and its effect on palmar arsenical keratosis. Bangabandhu Sheikh Mujib Medical University Journal. 2020; 13: 1-8. [CrossRef]
- Rodríguez Garza NE, Molina Garza ZJ, Galaviz Silva L, Quintanilla Licea R. Evaluación in vitro de extractos de plantas medicinales contra Trypanosoma cruzi, agente causal de la enfermedad de Chagas. 2019.
- Díaz Reyes C, Granados Sánchez D, Uribe Gómez M, Rodríguez Trejo DA, Granados Victorino RL. Ordenación De La vegetación De Las Sierras Y Llanuras Occidentales Municipio De Catorce, San Luis Potosí. Rev Mex Cienc Agric. 2020; 11: 713. [CrossRef]
- Pandey A, Tripathi S. Concept of standardization, extraction and pre-phytochemical screening strategies for the herbal drug. J Pharmacogn Phytochem 2014; 2: 115-19.
- Raaman N. Phytochemical Techniques. New India Publishing. 2006; p. 10.
- Sinha SN, Paul D. Antioxidant potentials of Parthenium hysterophorus. L leaf extracts. Sci Res J India. 2014; 3: 80-6.
- Deshpande B, Sharma D, Pandey B. Phytochemicals and antibacterial screening of Parthenium hysterophorus. Indian J. Sci. Res. 2017; 13: 199-02.
- Kenkel JV. Analytical Chemistry for Technicians. (3rd ed.). CRC Press 2001; p. 584. [CrossRef]
- Harvey D. Modern analytical chemistry. Boston, McGraw-Hill 2000; P. 798.
- Siddhardha B, Ramakrishna G, Basaveswara Rao MV. In vitro antibacterial efficacy of a sesquiterpene lactone, parthenin from Parthenium hysterophorus L (Compositae) against enteric bacterial pathogens. Int J Pharm, Chem Biol Sci. 2012; 2: 206-09.
- Polín García L. Aislamiento, caracterización y determinación cuantitativa de la proteína en los sólidos pulverulentos de Parthenium hysterophorus, Linn. Universidad Central “Marta Abreu” de las Villas. Facultad Química-Farmacia. Departamento de Farmacia. Santa Clara. 2006.
- Ashokkumar R, Ramaswamy M. Phytochemical screening by FTIR spectroscopic analysis of the extracts of selected Indian medicinal plants. Int J Curr Microbiol Appl Sci. 2014;3: 395-06.
- Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017; 6: 42. [CrossRef]
- Roy DC. Shaik MM. Toxicology, phytochemistry, bioactive compounds and pharmacology of Parthenium hysterophorus. J Med Plants Studies. 2013; 1: 126-41.
- Niranjan A, Mishra S, Lehri A, Amla DV, Upadhyay RS, Nautiyal CS. Identification and quantification of heterologous compounds parthenin and organic acids in Parthenium hysterophorus L. Using HPLC-PDA-MS-MS. Anal lett. 2012; 46: 48-59. [CrossRef]
- Brent LC, Reiner JL, Dickerson RR, Sander LC. Method for characterization of low molecular weight organic acids in atmospheric aerosols using ion chromatography mass spectrometry. Anal Chem. 2014; 86: 7328-36. [CrossRef]
- Muñoz Calderón A, Santaniello A, Pereira A, Yannuzzi J, Díaz-Bello Z, Alarcón de Noya B. Susceptibilidad in vitro a Nifurtimox y Benznidazol de aislados de Trypanosoma cruzi obtenidos de pacientes venezolanos con enfermedad de Chagas infectados por mecanismos de transmisión oral y vectorial. Rev Ibero-Latinoam Parasitol. 2012; 71: 14-22.
- Sosa Estani S, Altcheh J, Riarte A, Freilij H, Fernandez M, Lloveras S, Pereiro A, Castellano LG, Salvatella R, Nicholls RS. Lineamientos básicos del tratamiento etiológico de enfermedad de Chagas. Medicina (Buenos Aires). 2015; 75: 270-72.
Figure 1.
FT-IR spectrum of maceration-1 compound.

Figure 2.
FT-IR spectrum of soxhlet-1 compound.

Figure 3.
Fragmentation of maceration-1 compound (pseudo molecular ion m/z 263).

Figure 4.
Fragmentation of maceration-1 compound (pseudo molecular ion m/z 265).

Figure 5.
Fragmentation of soxhlet-1 compound (pseudo molecular ion m/z 263).
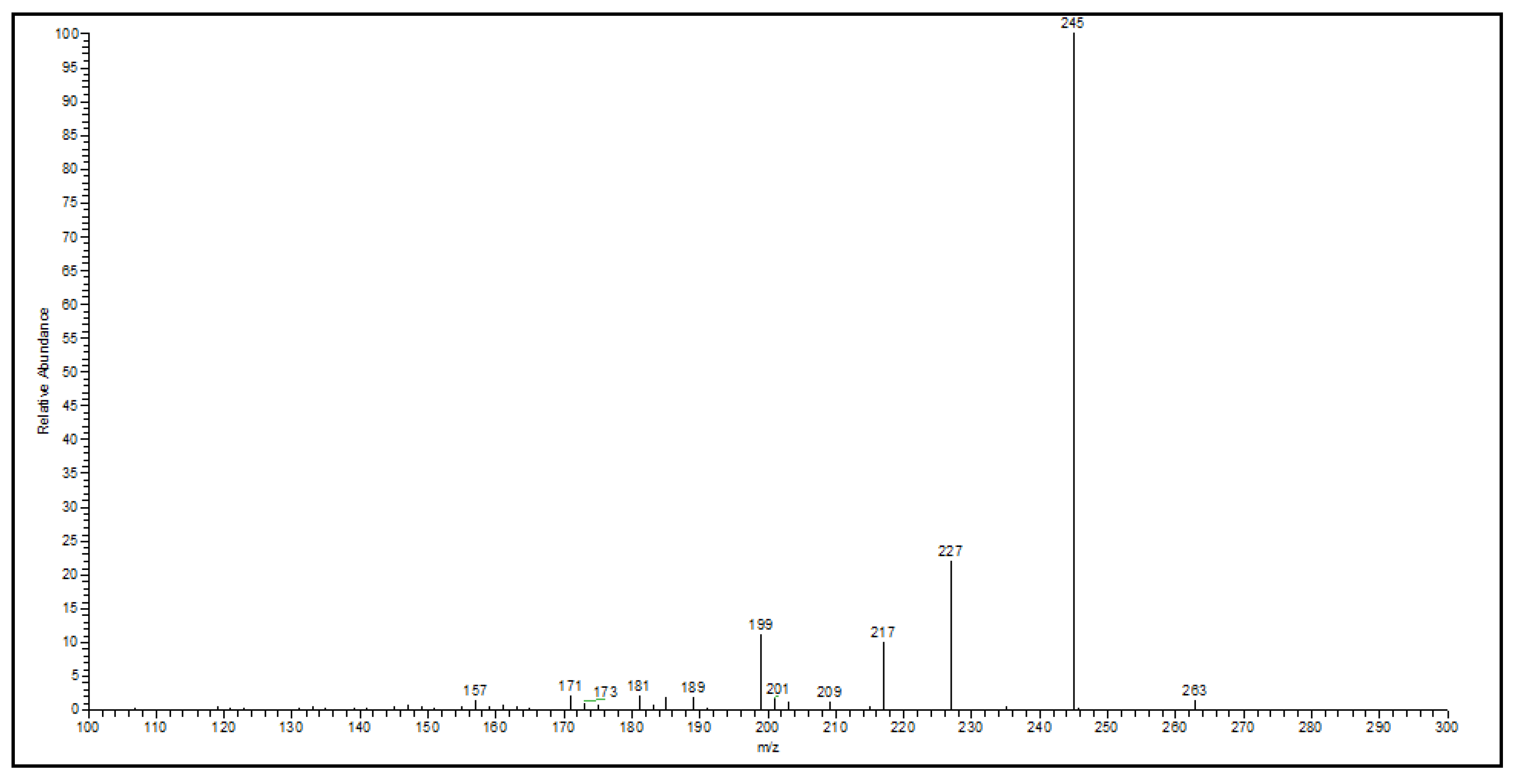
Figure 6.
Fragmentation of soxhlet-1 compound (pseudo molecular ion m/z 265).

Figure 7.
Identified compounds (a) parthenin; (b) coronopolin.

Table 1.
Preliminary phytochemical screening of methanolic extracts.
| Test | Maceration | Soxthlet |
|---|---|---|
| Flavonoids | + | + |
| Alkaloids | - | + |
| Tannins | + | + |
| Sesquiterpene lactones | + | + |
| Coumarins | + | + |
| Carbohydrates | + | + |
| Sterols | - | + |
| Terpenes | - | + |
| Saponins | - | - |
| Quinones | + | - |
| Carboxil group | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Trypanocidal Activity of Compounds Isolated From Parthenium incanum Kunth
David Alejandro Hernández-Marín
et al.
,
2023
Phytochemical Characterization and Biological Activities of Stenomesson miniatum Bulb Extract, a Medicinal Plant of the Andes.
Mariacaterina Lianza
et al.
,
2023
Antimalarial Activity of Aqueous Extracts of Nasturtium (Tropaeolum majus L.) and Benzyl Isothiocyanate
Ana Maria Pintão
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








