Preprint
Article
Plasma mir-203a-3p as a Novel Diagnostic Biomarker in Patients with Parkinson’s Disease Dementia
Altmetrics
Downloads
182
Views
115
Comments
1
A peer-reviewed article of this preprint also exists.
supplementary.zip (472.21KB )
This version is not peer-reviewed
Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Alerts
Abstract
The early detection of cognitive decline and timely non-pharmacological management or drug therapy are extremely important in providing care for Parkinson’s disease with dementia (PDD). In this study, we first conducted a discovery study to identify six plasma microRNAs that may allow for the differentiation of PD with or without mild cognitive impairment via NGS. A total of 120 participants were further recruited in a validation cohort and divided into four subgroups, namely, normal controls (HC), PD with no dementia (PDND), PD with mild cognitive impairment (PD-MCI) and PDD. Among the six candidates, miR-203a-3p was successfully detected in the plasma of the validation cohort using droplet digital PCR (ddPCR). Our results show that the ratio of miR-203a-3p/miR-16-5p observed in PDD was significantly increased compared to in PD-MCI (p < 0.001) and PDND (p = 0.041). Moreover, the ratio of miR-203a-3p/miR-16-5p showed a significant correlation with MoCA scores (r = -0.237, p = 0.024) in patients with PD (PwP). The ROC curve of the logistic regression model, consisting of the variables of age, the ratio of miR-203a-3p/miR-16-5p and the UPDRS III score, also demonstrated an average AUC of 0.883 via 5-fold cross-validation. In conclusion, the ratio of miR-203a-3p/miR-16-5p may serve as a potential biomarker for distinguishing cognitive dysfunction from PwP.
Keywords:
Subject: Biology and Life Sciences - Neuroscience and Neurology
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the elderly population [1]. The clinical features of Parkinson’s disease include not only motor symptoms, such as bradykinesia, tremor and postural rigidity, but also non-motor symptoms, such as fatigue, apathy and cognitive dysfunction [2]. It was reported that approximately 26.7% of PD patients were diagnosed with mild cognitive impairment (PD-MCI) [3], and around 20%-60% of PD-MCI may convert to PD with dementia (PDD) within five years after diagnosis [4]. The incidence rate of developing dementia is higher in patients with PD (PwP) than in non-PD controls [5]. The neuronal transmission of fibril alpha-synuclein (α-syn), followed by the formation of Lewy bodies in the substantia nigra pars compacta, is a well-known biomarker for PwP [2]. However, the histological and molecular markers for PDD remain inconclusive. Although alpha-synuclein (α-syn), tau neurofibrillary tangles (NFTs) and amyloid-β (Aβ) plaques have been found to be widespread in most PDD patients, the findings are not consistent [6]. Motor dysfunctions with cognitive dementia could increase the economic and psychological burden for caregivers [7]. PwP with dementia will gradually lose their basic living ability and may have a shorter lifespan than individuals with PD without dementia [5]. In addition, the efficacies of the approved drugs for treating PDD or PD-MCI are still limited [8]. This could become a serious issue for individuals and our society with an aging population [9,10]. Fortunately, a recent study has suggested that impaired cognitive function may be sustained or even rescued after treatment [1]. Therefore, it is important to identify PD patients with cognitive dysfunction at an early stage.
The diagnosis of PD with cognitive impairment and dementia, including executive and visuospatial deficits [11], is generally based on a level II neuropsychological assessment. Apart from neuropsychological tests, regular examinations include blood testing and brain imaging techniques, such as magnetic resonance imaging (MRI) [12,13]. However, the entire procedure of cognitive examination is often time-consuming and requires the involvement of multiple medical personnel [12,13]. Hence, reliable biomarkers that could facilitate diagnosis and enable rapid distinction between PD patients with and without cognitive impairments are an unmet need. Recently, emerging molecular biomarkers regarding inherited genetic mutants or toxic proteomic markers for PDD have attracted attention and have been recommended for deeper validation in human studies [1]. Compared with tissue biopsy or biofluid collection methods, the non-invasive collection method of plasma has made it become one of the most commonly studied resources of human biomarkers [14].
MicroRNA (miRNA) is a single-stranded non-coding RNA with an average length of 22 nucleotides [15]. MiRNA can mediate post-transcriptional expression via binding with target messenger RNAs (mRNAs) [16] and cease the transcription of the encoded gene. It has been reported that miRNAs exist not only in the cytoplasm but also in extracellular areas, such as cerebrospinal fluid, blood and other biofluids [16,17]. Circulating miRNAs may travel across the blood–brain barrier (BBB) due to their short sequence length [13]. Various studies have shown an association between miRNAs and neurodegenerative diseases, including Alzheimer’s disease (AD) and PD [18-20], and patients with cognitive decline [21,22]. This suggest that miRNAs may be a potential biomarker for PD with cognitive impairment.
It is widely accepted that an miRNA candidate exploration study should include both a discovery study phase and a validation study phase [23]. Therefore, we first conducted a discovery phase using next generation sequencing (NGS) and screened for miRNA candidates. Then, we conducted a validation phase using droplet digital PCR (ddPCR) on a different cohort. We aimed to determine whether the miRNA candidates could distinguish between PD with and without cognitive impairment.
2. Results
2.1. Using small RNA-seq to explore the potential miRNA cluster that may allow for differentiation of PD with or without cognitive impairment
Analyzing miRNA candidates from the NGS profiling in the discovery cohort
We firstly performed a discovery study phase using NGS (small RNA-seq) to explore the potential miRNA candidates associated with cognitive decline. The demographic variables of the discovery cohort are summarized in Table 1.
The NGS results consisted of approximately 2600 miRNAs and were filtered through a series of data processing steps and statistical analyses via the Biomedical Oriented Logistic Dantzig (BOLD) selector. The BOLD selector was used for miRNA candidate selection in consideration of the supersaturated data, and it analyzed thousands of miRNA profiles obtained from the relatively small sample size. Eventually, six miRNA candidates, namely, miR-203a-3p, miR-626, miR-662, miR-3182, miR-4274 and miR-4295, were clustered as potential biomarkers for identifying PDND from PD-MCI, with an average AUC of 0.8 (Figure 1). This finding encouraged us to carry out a follow-up investigation of these plasma miRNAs in a new PwP cohort with a larger sample size, and we validated the differential power of the miRNA expression level in PD with a different cognitive status.
2.2. Validation of the miRNA candidates in another PwP cohort
2.2.1. Measurement of the selected miRNA candidates
We then aimed to validate the previous findings by recruiting another PwP cohort. We examined plasma miRNAs using real-time PCR (RT-PCR) to study the expression levels of the six miRNAs in the new PwP cohort. The six miRNA candidates were first quantified using LNA-based RT-PCR (Qiagen, Germany), and the possible false-positive outcomes were ruled out via a melting curve analysis. However, except for miR-203a-3p, the detection of the remaining five miRNA candidates via RT-PCR (Ct>36) or non-overlapped dissociation curves (data not shown) failed. This outcome may have resulted from the low concentration of the total extracted plasma RNA, which was lower than the detection limit of the Bioanalyzer RNA Nano system (data not shown).
2.2.2. Motor function deterioration was associated with poor cognitive status
In Table 2, demographic variables such as the gender, age, education, UPDRS III, MoCA score and LEDD values of each study group are summarized. The percentage of males in HC and PDND was 56.67%, while the percentage of males in PD-MCI and PDD was 53.33% and 46.67%, respectively. Those in PDD were older than those in HC (p-value<0.0001) and PDND (p-value=0.025), while no significant difference was observed for ages among HC, PDND and PD-MCI. Although the duration of disease and the age of onset showed no significant difference among the PD patients, PDD had a significantly higher Hoehn–Yahr stage value than PDND (p-value<0.0001) and PD-MCI (p-value=0.0048). Correspondingly, PDD also had a higher UPDRS III score than PDND (p-value<0.0001) and PD-MCI (p-value=0.0002). This suggests that motor and cognitive functions may deteriorate more rapidly in PDD, given that the disease durations were similar across PDND, PD-MCI and PDD. No significant difference was observed in the years of formal education among the groups of HC, PDND and PD-MCI. PDD had significantly less years of education than HC (p-value=0.0256) and PDND (p-value=0.0327). Moreover, the value of LEDD was estimated according to the anti-Parkinson drug prescribed to the PD patients. The results show no significant difference in LEDD among PDND, PD-MCI and PDD. Although anticholinergic drugs were excluded from the LEDD estimation, it was estimated that five patients in PDND, seven patients in PD-MCI and one patient in PDD received a low-dose anticholinergic drug (2-4mg/d) for the management of severe tremor.
2.3. The expression level of plasma miR-203a-3p/miR-16-5p validated using ddPCR
Because the total amount of circulating miRNA may differ from person to person and usually presents a very low concentration, and because quantification with high sensitivity and specificity was required, we used ddPCR for quantifying the expression level of plasma miR-203a-3p in each fixed-volume sample [24]. Additionally, an endogenous reference miRNA was required for normalization since miR-203a-3p expression may be affected by intrinsic factors [25]. Endogenous miR-16-5p, which is abundant across intracellular and intercellular regions and relatively consistent in biofluids at different ages, has been used for normalizing miRNA in studies of Parkinson’s disease and multiple system atrophy [26-28]. Therefore, we combined the detection of our target miRNA and the endogenous reference miRNA, as the ratio of miR-203a-3p/miR-16-5p, to reduce the bias from individual intrinsic factors that may not relate to PD pathologies. An exogenous synthetic oligonucleotide, UniSp6, was used as a reference for RNA extraction. The ratio of miR-16-5p/UniSp6 showed no significant difference among the study groups, suggesting the robust extraction efficiency of each sample (Figure S1). After performing outlier identification, four patients in PD-MCI were excluded from further analysis (data not shown). Examples of the ddPCR results and the expression level of miR-203a-3p/miR-16-5p among each study group are visualized in Figure 2.
To elucidate whether the ratio of miR-203a-3p/miR-16-5p was different among the PD patients with and without cognitive decline, a non-parametric one-way ANOVA test, the Kruskal–Wallis test, was conducted (Figure 2D). The result shows that there was a significant difference in the ratio of miR-203a-3p/miR-16-5p among the four groups (p-value=0.0009). The mean ratio of miR-203-3p/miR-15-5p was 12.1 (SD, ±6.42), 10.31 (SD, ±5.89), 8.66 (SD, ±5.65) and 16.83 (SD, ±10.12) in the HC, PDND, PD-MCI and PDD groups, respectively. PDD showed a statistically significant increased ratio of miR-203a-3p/miR-16-5p compared to PD-MCI (p-value=0.0006) and PDND (p-value=0.0409). However, the ratio of miRNA had no significant difference in a comparison of PDND with PD-MCI. This finding may result from the minimal cognitive decline from PDND to PD-MCI, which may be insufficient for detecting the altered expression of plasma miRNA.
2.4. Correlation of miRNA expression and cognitive performance
To determine whether the selected miRNAs are associated with cognitive impairment, the correlations of the ratio of miR-203a-3p/miR-16-5p and the total score and individual domain scores of MoCA were analyzed using a Spearman correlation analysis (Table 3). No significant correlation was observed between the ratio of miR-203a-3p/miR-16-5p and gender, age, education, duration, Hoehn–Yahr stage, UPDRS III or LEDD (p-value>0.05) (data not shown). After the Spearman correlation analysis, the ratio of miR-203a-3p/miR-16-5p showed a significant negative correlation with the total MoCA score (r=-0.237, p-value=0.024) in PD patients (Table 3). To determine the specific cognitive domains that were closely related to the miRNA ratio, different cognitive domains of MoCA, such as the visuospatial, naming, attention, language, abstraction, memory and orientation domains, were analyzed. The results show that the ratio of miR-203a-3p/miR-16-5p had a significant correlation with three MoCA domains, namely, the visuospatial, language and orientation domains (Table 3).
2.5. Using the ratio of miR-203a-3p/miR-16-5p as variable for building regression model
An ROC analysis was performed to show the diagnosis power of PD with cognitive decline in PD patients using the ratio of miR-203a-3p/miR-16-5p.
The ROC plots for each comparison group analyzed using logistic regression with a 5-fold cross-validation are summarized in Table 4. The 95% confidence intervals of the sensitivity, specificity and accuracy of each set of compared groups were estimated, followed by an ROC analysis (Table 4). The ROC curve analysis discriminating between PD-MCI and PDD showed an average AUC of 0.716 (95% CI, 0.432-0.951). In addition, the ROC curve analysis discriminating between PDND and PDD showed an average AUC of 0.741 (95% CI, 0.482-0.951). Both ROC analyses support the notion that the ratio of miR-203a-3p/miR-16-5p could be used for predicting the cognitive status (total MoCA score ≦21).
To determine whether the demographic variables may also serve as confounding factors for differentiating PDD (total MoCA score ≤21) from PwP, multivariate logistic regression models were used. A full regression model was developed with the predictor variables, including age, gender, onset age, years of formal education, UPDRS III score, and the ratio of miR-203a-3p/miR-16-5p, presented as N203 for short. The total MoCA score was defined as the response variable. The AIC value of each regression model was estimated for the goodness of fit.
The results show that the reduced model with three variables, namely, the ratio of miR-203a-3p/miR-16-5p, age and the UPDRS III score, presented the best performance for predicting PDD (Table S1). The results show that the ratio of miR-203a-3p/miR-16-5p, age and the UPDRS III score all have positive associations with PDD. In other words, an older age and more severe motor symptoms with a higher ratio of miR-203a-3p/miR-16-5p could contribute to a higher risk of PDD (Table S1). After the variable selection, an ROC analysis of the test dataset was conducted using a five-fold cross-validation to examine the power of the reduced model; the corresponding ROC curve is shown in Figure 3. Apart from the abovementioned model, other ROC analyses were also performed for the regression models without the N203 variable to examine whether N203 may serve as a predictive parameter for PDD. The ROC analysis and the 95% confidence intervals of the AUC, specificity, sensitivity and accuracy of the reduced logistic regression model for the test dataset are summarized in Table S2. As a result, the reduced logistic regression model for predicting PDD showed an AUC of 0.8827 (95%CI, 0.7282-0.9938), a sensitivity of 0.7778, a specificity of 0.8889 and an accuracy of 0.8519 (Table S2). The result shows that the regression model with three variables, namely, N203, age and UPDRS III, had a higher prediction performance (AUC=0.8827) than the regression model with two variables, namely, age and UPDRS III (AUC=0.8272).
2.6. MiR-203a-3p associated with cognition-related KEGG pathways
To elucidate the role of miR-203a-3p in the cognition-related pathological mechanisms of PD, the target genes and the involved molecular pathways were filtered according to experimental evidence (Table S3). Five non-cancer-related pathways and the predicted target genes of miR-203a-3p are summarized in Table 5. As a result, a KEGG analysis of miR-203a-3p revealed several possible pathways that may be associated with the pathology of PD with cognitive dysfunction, including the dopaminergic synapse, apoptosis, thyroid hormone signaling, cholinergic synapse and NF-kappa B signaling pathways.
3. Discussion
The common biomarkers for differentiating PDD comprise genetic and proteomic biomarkers. It has been suggested that genetic mutations such as GBA, MAPT, LRRK2 and ApoE may contribute to an increased risk of developing PD or rapid cognitive decline from PDND to PDD [29]. However, the prediction power of genetic mutations alone remains elusive because the onset age of PwP with known genetic mutations is uncertain, and the genetic marker itself may not serve as a prognosis indicator. Hence, proteomic markers, such as circulating pathological proteins, including α-syn, β-amyloid, tau and NfL, have been considered to be progress indicators for the motor and cognitive performance of PwP in recent studies [30,31,32]. A one-year follow-up study also showed that an elevated expression of plasma EV-derived alpha-synuclein, tau and β-amyloid was correlated with motor and cognition decline in PD [30]. The general techniques for examining CSF or plasma proteomic targets are based on high-affinity protein purifying columns or immunostaining kits, such as ELISA assays [33]. However, compared to genetic markers, examining proteomic markers is expensive and requires calibrated management. Additionally, the controversy of the protein expression level in different motor and cognition severities of PwP remains an unsolved problem. However, plasma miRNA may provide benefits as a state-specific biomarker for the dynamic motor and cognitive status. The alteration of plasma miRNA may also be considered a prognosis indicator for evaluating the predicted pharmacological changes in treatment [34].
miR-203a-3p has been suggested to bind to the 3’UTR of human DJ-1, which is a Parkinson’s disease-related gene and may prevent neurons from cytotoxic oxidative damage [34,35,36]. Overexpressed miR-203a-3p has been suggested to cause a deficiency of DJ-1 and further result in oxidative-stress-induced cell death [34,35], microglia-regulated neuronal injury [37] and the promotion of the neurodegenerative phenotype in vivo [38]. MiR-203a-3p has also been assumed to bind to the 3’UTR of SNCA, encoding α-syn, which is well known for elevating the risk of developing PD [39,40]. The aforementioned findings may support our hypothesis for selecting miR-203a-3p as a biomarker for PDD. However, reported miRNAs for PD with dementia are limited due to the poor prognosis and the loss of follow-up patient numbers. The lack of an experimental standardized protocol for examining plasma miRNA in human biopsies also remains an unsolved problem.
In the current study, our results indicate that PDD may correlate with severe motor and cognitive dysfunctions with a similar age of onset and duration of disease after diagnosis (Table 2). This is consistent with a previous study in which a later age of onset was associated with rapid progression from PD without dementia to PDD [41]. The ddPCR detection showed that the ratio of miR-203a-3p/miR-16-5p was significantly increased in PDD compared to in PD-MCI and PDND. In addition, the ratio of miR-203a-3p/miR-16-5p had a significant correlation with the total score and the three MoCA domains, namely, the visuospatial, language and orientation domains. According to previous studies, these three cognitive domains are associated with frontal lobe functions, which correspond to the pathological brain region of PD with cognitive dysfunction [41]. Apart from poor executive function, the diminished visuospatial and language functions in PD-MCI and PDD have also been highlighted as features of motor and cognitive decline symptoms [11,42]. Overall, the findings support our hypothesis that miR-203a-3p may serve as a dynamic biomarker for recognizing global and domain-specific cognitive decline in PwP.
MiR-203a-3p belongs to the miRNA family of miR-203. The underlying genes regulated by miR-203a-3p have been proposed to be related to cognitive decline in PwP. The KEGG analysis showed that multiple target genes of miR-203a-3p consisted of pathways, including the apoptosis and NF-kappa B signaling pathways. It is noteworthy that PDD has been characterized not only by the aggregation of fibril α-syn but also by tau and amyloid plaque pathologies [6]. The correlation between upregulated miR-203 and the activation of the apoptotic pathway was first reported by Swarup et al. [38]. Evidence suggested that miR-203 dysregulation was correlated with tauopathy, such as frontal temporal dementia (FTD), AD and progressive supranuclear palsy (PSP). The authors suggested that the downregulation of the neurodegeneration-associated synaptic (NAS) module and the upregulation of the apoptotic pathway detected via caspase-8 protein expression resulted from overexpressed miR-203 in both primary cortical mouse neuronal cultures and Tg4510 tau transgenic mice. Li et al. also reported that overexpressed miR-203 in both BV2 cells and the mouse hippocampus resulted in a reduced protein expression of 14-3-3θ. As the inhibitor of NF-κB signaling and the target of miR-203, 14-3-3θ may inhibit TLR2-induced NF-κB signaling [43]. Overexpressed miR-203 may also result in neuroinflammation and neuronal cell death in the hippocampus of mice; this led to spatial learning and memory dysfunction in the Barnes maze test. Taken together, upregulated miR-203 may cause the activation of inflammation and the apoptotic pathway, whereas the decreased expression of miR-203a-3p may provide the neuron-protecting effect in neurodegenerative disorders.
In addition to the genes involved in the regulation of apoptosis and inflammation, the KEGG analysis also revealed that the dopaminergic synapse, thyroid hormone signaling and cholinergic synapse pathways were associated with miR-203a-3p. The loss of dopaminergic synapses in the substantia nigra is assumed to be a hallmark of progressed motor symptoms in PwP [2]. The evidence may support our findings suggesting the upregulation of miR-203a-3p/miR-16-5p in PDD compared to in PDND, which indicates a relationship between increased miR-203a-3p and worse dopaminergic neuron loss. Dysregulated thyroid hormone signaling is also considered one of the potential causes of cognitive dysfunction in PD. Thyroid disturbance combined with an age above 70 is assumed to be a potential risk factor for developing PD according to the interconnection of the hypothalamic–pituitary–thyroid axis [44]. Additionally, patients with subclinical hypothyroidism generally have difficulty in gait, and a similar clinical motor symptom has been observed in PwP [45,46]. The degeneration of the cholinergic system is assumed to play an important role in multiple neurodegenerative disorders. The dysfunction of cholinergic synapses is generally recognized in Alzheimer’s disease, and the loss of a basal forebrain cholinergic system has also been reported in human post-mortem evidence of PDD [47]. The alteration of the cholinergic system may change not only motor functions but also non-motor symptoms [48]. The severe loss of cholinergic synapses or cholinergic receptors has been reported in PD with cognitive decline compared to that with intact cognition [49,50]. Based on the prior finding, cholinergic drugs were developed to treat cognitive decline in PD. Notably, cholinergic inhibitors, such as the cholinesterase inhibitor rivastigmine, were approved to treat dementia and other related cognitive dysfunctions in PD and AD [51,52], whereas subtype-specific muscarinic acetylcholine receptors (mAChR) antagonists were proposed as an alternative treatment for cognitive impairment [49].
There are some limitations to this study. The sample size was limited, and it has been observed that the mood, medical therapies and surgical therapies of participants may interfere with plasma examinations [53]; hence, a larger sample size and a follow-up study are needed. Furthermore, since PDD was associated with a higher age in our study (Table 2 and Table 4), the number of older HC and PDND individuals should be increased to rule out the aging effect. Although a level II neuropsychological assessment has been suggested to achieve a better sensitivity and accuracy when diagnosing PD-MCI, the global cognitive test MoCA may provide the benefit of prediction for the conversion of PD-MCI to PDD [54]. Moreover, this study only measured plasma miRNA, so exosome-derived miRNA may not be detected via our extraction method. Exosome-derived miRNA extraction normally requires more preparation than plasma miRNA extraction due to the low yields and extra separation and purification steps [55]. Hence, cell-free plasma miRNA is preferred when the amount of sample is limited.
4. Materials and Methods
4.1. Plasma miRNA profiling in the discovery cohort
4.1.1. Recruitment of participants
All patients with PD met the inclusion criteria proposed in UK Parkinson’s Disease Society Brain Bank Criteria. A total of 174 participants, comprising 40 HCs, 51 with MSA, 37 with PDND, 23 with PD-MCI and 23 with PDD, were recruited. The current study focused on the findings of patients with PD; the analysis of patients with MSA will be discussed in another publication.
4.1.2. Plasma collection
Blood in the amount of 10 ml was collected in BD Vacutainer® K2E (EDTA) Plus Blood Collection Tubes (Becton Dickinson, USA) and centrifuged at 2200xg for 15 minutes at room temperature (swinging bucket, KUBOTA 4000, Japan) within 3 hours of collection. The plasma layer was transferred, mixed via pipetting and stored at -80℃ until follow-up experiments.
4.1.3. Plasma miRNA sequencing
Samples containing small RNAs (<200 nucleotides) derived from 200-400 μl human plasma were prepared using the Qiagen miRNeasy Mini kit (Qiagen, #217004). A small RNA-Seq library was constructed using the QIAseq miRNA Library Kit (Qiagen, #331502). Single-ended small RNA-Seq was performed on Illumina NextSeq. Small RNA-Seq datasets were generated. The sequencing reads of each microRNA from two batches of subjects (75 and 99, respectively) were normalized using the trimmed mean of M-values (TMM) for each batch.
4.1.4. BOLD Selector included data analytic scheme
In the miRNA data analysis, we carried out a three-stage process. The initial stage involved data preprocessing. The missing value was approximated as 0. To merge the two datasets (containing 75 and 99 reads), a surrogate variable analysis in R (SVA; V.3.48) was used to normalize and remove batch effects; the union of the lowest expressed 10% microRNAs in both batches was trimmed before the statistical analysis. The BOLD selector included a data analytic scheme [56], narrowing down the ranking of miRNAs with adjusted parameter δ, suitable for clustering biomarker selection in supersaturated data. The second stage focused on the cross-validation of the dataset using the BOLD selector. Prior to this stage, we standardized expression matrix X and centered response variable Y, originally coded as 0 and 1. We chose the best δ from 15 uniformly spaced cut points within an interval from 0 to the maximum absolute value of max|X^T Y|. Data were split into 5 parts for cross-validation, with 80% being used for training and the remaining being used for testing in each fold. We applied the BOLD selector to the training data and constructed a logistic regression formula based on the selected microRNA candidates for predicting the testing data. The model's fit across 5 testing folds was assessed using the average area under the receiver operating characteristic curve (AUC), enabling us to select the optimal tuning parameter with the highest average AUC. The final stage entailed performing a full data analysis on the tuning parameters falling between the best δ and max|X^T Y|. During this phase, we ranked and identified the most important factors, which were subsequently employed to construct a final logistic regression formula.
4.2. Validating plasma miRNA candidates in new PD cohort
4.2.1. Sample size estimation
Two miRNAs were measured in the 4 study groups, namely, the Parkinson’s disease with no dementia (PDND), Parkinson’s disease with mild cognitive impairment (PD-MCI), Parkinson’s disease with dementia (PDD) and healthy control (HC, as a control group) groups. Prior sample size estimation was performed using G-power 3.1.9.4 [57], with the statistic F test of ANOVA (fixed effects, special effects, main effects and interactions), effect size=0.5, power=0.8 and α=0.05. The total sample size was suggested to be 48 or above. Considering the greater power of the statistic and to avoid heterogeneity within each study group, a total of 120 participants were recruited and examined.
4.2.2. Recruitment of participants
All PD patients met the same inclusion and exclusion criteria used in the recruitment for the discovery phase. Participants who received treatments or had a history of the following criteria were excluded from this study: (1) cancer; (2) server cardiovascular disease, renal disease, or brain injury; (3) autoimmune disease; (4) psychiatric disorders, such as schizophrenia; (5) deep brain stimuli surgery; (6) known genetic mutations associated with Parkinson’s disease; (7) difficulty in blood clotting; and (8) other possible cognition-affected neurological and musculoskeletal disorders or secondary and atypical Parkinsonism, including multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration and dementia with Lewy bodies. In addition, unified Parkinson’s disease rating scale (UPDRS) part III was used to evaluate the motor functions of PDND, PD-MCI and PDD. PD-MCI and PDD should achieve the criteria of the Montreal Cognitive Assessment (MoCA), a level I cognitive assessment proposed by the MDS Task Force. The levodopa equivalent daily dose (LEDD) was calculated via the LEDD conversion factor for PD patients [58]. Moreover, demographic variables, including gender, age, the duration of disease, UPDRS III score, total MoCA score, years of formal education and LEDD, were collected for each participant. The daily dose of anticholinergic drugs, including Akinfree and Biperiden, was estimated for each PD patient if used.
Eventually, 30 patients with PDND, 30 patients with PD-MCI and 30 patients with PDD were recruited from the Parkinson’s disease center in National Taiwan University Hospital. Moreover, 30 HCs were recruited from National Taiwan University Hospital and the Shixiang Community in Taiwan. All subjects gave and signed informed consent for inclusion before they participated in the study.
4.2.3. Cognitive assessments
All participants underwent the Montreal Cognitive Assessment (MoCA) [4] to quickly determine their cognitive performance, and this was carried out by Y.F. Hsu. The cognitive domains, including visuospatial, naming, attention, language, abstraction, memory and orientation domains, were evaluated via MoCA, and the total score was used for grouping. HC and PDND should meet a total MoCA score equal to or above 26. PD-MCI should meet a total MoCA score ranging from 22 to 25. PDD should meet a total MoCA score equal to or below 21.
4.2.4. Plasma collection
Plasma collection followed the protocol mentioned for the discovery cohort.
4.2.5. RNA extraction
Small RNAs were extracted from 200 μl plasma using the miRNeasy Serum/Plasma Advanced kit (Qiagen, Germany). The extraction process generally followed the guidelines of the manufacturer’s instructions, with the several modifications listed below. To test whether the extraction efficiency was robust, the exogenous synthetic spike-in UniSp6 (Qiagen, Germany) was added to the lysis buffer (Figure S1). However, UniSp6 is recommended for measuring extraction efficiency and is not suitable for the normalization of miRNA expression levels according to the manufacturer’s instructions. Thawed plasma samples underwent a series of centrifugation procedures: first, they were centrifuged at 12000xg at 4℃ for 3 minutes (fixed-angled, KUBOTA 6200, Japan), and then they were further centrifuged at 12000xg (fixed-angled, KUBOTA 3300T, Japan) at room temperature for 30 seconds, 30 seconds, 30 seconds, 2 minutes and 5 minutes. Twenty-two microliters of 55℃ pre-warmed RNase-free water (Invitrogen, Thermo Fisher) was added for RNA elution. Additionally, the eluted RNA was transferred and incubated on a UCP MiniElute column (Qiagen, Germany) again for 10 minutes at room temperature. After centrifugation at 12000xg for 1 minute (fixed-angled, KUBOTA 3300T, Japan), the final RNA was immediately placed on ice for reverse transcription (RT). The cDNA synthesis was manipulated following the instructions of the miRCURY LNA miRNA SYBR Green kit (Qiagen, Germany). A mixture prepared for cDNA synthesis contained 10 μl extracted RNA, 2μl 10x miRCURY RT Enzyme Mix, 4 μl 5x miRCURY SYBR Green RT Reaction Buffer and 4 μl RNase-free water (Invitrogen, Thermo Fisher). After the thermal reaction cycle was complete, the cDNA sample was stored at -20℃ until ddPCR examination.
4.2.6. Droplet digital PCR
For the examination of miR-203a-3p and miR-16-5p, the cDNA samples were diluted by 1:10 and 1:320, respectively. One non-RT control (no transcriptase added during RT) and one non-template control (no cDNA template added during ddPCR amplification; NTC) were quantified to ensure that no genomic DNA remained and that no false-positive results would be detected. The miRNA was quantified using a ddPCR system (Bio-Rad, USA). First, a mixture with a total volume of 20 μl was prepared with 9 μl diluted cDNA, 10 μl digital PCR™ Eva-Green supermix (Bio-Rad, USA) and 1μl LNA miRCURY miRNA PCR Assay (for miR-203a-3p and miR-16-5p) (Qiagen, Germany). Second, 70 μl QX100 Droplet Generation oil (Bio-Rad, USA) and the 20 μl mixture were loaded into a cartridge. After processing in the QX200 Droplet Generator (Bio-Rad, USA), the droplet-containing liquid was gently transferred onto a 96-well plate, and cDNA amplification was initiated in a T100 thermal cycler (Bio-Rad, USA). The thermal cycling was modified from the manufacturer’s instructions (Bio-Rad, USA). The annealing temperature (Tm) was adjusted individually for miR-203a-3p (Tm=55℃) and miR-16-5p (Tm=56℃). The PCR products were quantified using the QX200 Droplet Reader (Bio-Rad, USA) and QuantaSoft software (Bio-Rad, USA). The quality control of the ddPCR result was suggested to meet the criteria of a total droplet number above 10000 copies/μl and total positive droplets above 3 copies/μl. The copy number per microliter of miR-203a-3p divided by that of miR-16-5p was then multiplied by 10000 to obtain the ratio of miRNA.
4.2.7. Pathway prediction
Based on a well-known database that integrates up-to-date genomic information, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis provides graphic maps for genes of interest to interpret and predict molecule cascades and potential functions [59,60,61]. Hence, the target genes of miR-203a-3p were predicted using the KEGG pathway database with miRPathDB 2.0, and the predicted pathways were filtered by the experimental (any) data [62].
4.2.8. Statistical analysis
The demographic variables and the ratio of miR-203a-3p/miR-16-5p were analyzed using the Kruskal–Wallis test via GraphPad Prism. The demographic variables analyzed using the non-parametric one-way ANOVA Kruskal–Wallis tests included gender, age, total MoCA score, duration of disease, UPDRS III score, years of formal education and LEDD. After performing the Kruskal–Wallis tests, Dunn's multiple comparisons tests were conducted for a post hoc analysis to reveal whether there were significant differences between the study groups. Additionally, outliers were identified and excluded from the analysis if they were larger than Q3 +1.5IQR or smaller than Q1 -1.5IQR. Spearman correlation tests, a non-parametric correlation analysis, were applied for the ratio of miR-203a-3p/miR-16-5p, different cognitive domains (visuospatial, naming, attention, language, abstraction, memory and orientation domains) and the demographic variables. To understand the diagnosis power of PD with or without cognition decline using the ratio of miR-203a-3p/miR-16-5p, the 4 study groups were compared and analyzed using a receiver operating characteristic (ROC) curve analysis with R software (version 4.3.2), package pROC (version 1.18.5) [63] and package caret (version 6.0-94) [64]. In addition, multivariate logistic regression models were developed consisting of the demographic variables and the ratio of miRNA factors. The demographic and miRNA data of 90 PD patients were divided by 70% for model training and 30% for model testing. A reduced logistic regression model was determined via the smallest Akaike Information Criteria (AIC). The predicted values of the average AUC obtained via 5-fold cross-validation were used in the test dataset evaluation. All coordinates of ROC curves, including the area under curve (AUC), sensitivity, specificity and accuracy, were estimated via the maximal sum (sensitivity + specificity). Additionally, 95% confidence intervals (CIs) were also calculated for AUC, sensitivity, specificity and accuracy using bootstrapping (boot runs = 2000).
5. Conclusions
In summary, plasma miR-203a-3p was significantly increased in PDD compared to in PD-MCI and PDND. Moreover, the ratio of miR-203a-3p/miR-16-5p was significantly correlated with the total MoCA score and multiple domains, such as the visuospatial, language and orientation domains. Combining age, the ratio of miRNA and UPDRS III, the logistic regression model (AUC = 0.883) may facilitate the differentiation of PDD from PwP. Therefore, the ratio of plasma miR-203a-3p/miR-16-5p may be a novel biofluid marker for patients with PD with cognitive dysfunction.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Y.-F.H., S.-P.L. and R.-M.W.; Methodology, Y.-F.H. and Y.-T.Y.; Formal Analysis, Y.-F.H., Y.-T.C. and F.K.H.P.; Investigation, Y.-F.H.; Resources, R.-M.W.; Writing—Original Draft Preparation, Y.-F.H. and R.-M.W.; Visualization, Y.-F.H.; Supervision, R.-M.W.; Funding Acquisition, S.-P.L. and R.-M.W. All authors have read and agreed to the published version of the manuscript before submission.
Funding
This research was funded by National Taiwan University and National Taiwan University Hospital, grant number 111-UN0007.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the National Taiwan University Hospital Research Ethics Committee, Taipei, Taiwan (NTUH-REC No. 201711054RINA, date: 7 February 2018; NTUH-REC No. 201912129RINB, date: 17 January 2020; NTUH-REC No. 201905113RINC, date: 18 September 2019; NTUH-REC No. 202011020RINC, date: 21 December 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the privacy of the participants.
Acknowledgments
We thank Prof. Takahiro Ochiya for technical support during the discovery phase. We thank Miss Jing-Wen Huang for developing the BOLD selector data analytic scheme and Mr. Yan-Ru Ju for statistically analyzing the NGS dataset. We are grateful for the support from the Centre for Parkinson and Movement Disorders during the participant recruitment process. We also thank the staff of the Second and the Fifth Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support during the study. We thank Dr. Sam Chi-Hao Liu for English editing of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.L.; Wu, R.M.; Tai, C.H.; Lin, C.H.; Cheng, T.W.; Hua, M.S. Neuropsychological profile in patients with early stage of Parkinson's disease in Taiwan. Park. Relat. Disord. 2012, 18, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Troster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Åström, D.O.; Simonsen, J.; Raket, L.L.; Sgarbi, S.; Hellsten, J.; Hagell, P.; Norlin, J.M.; Kellerborg, K.; Martinez-Martin, P.; Odin, P. High risk of developing dementia in Parkinson's disease: A Swedish registry-based study. Sci. Rep. 2022, 12, 16759. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson's disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease - repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Hsu, C.C.; Yang, Y.H. A Nationwide Survey of Dementia Prevalence in Long-Term Care Facilities in Taiwan. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Huang, C.-S. Aging in Taiwan: Building a Society for Active Aging and Aging in Place. Gerontol. 2015, 56, 176–183. [Google Scholar] [CrossRef]
- Huertas, I.; Jesús, S.; García-Gómez, F.J.; Lojo, J.A.; Bernal-Bernal, I.; Bonilla-Toribio, M.; Martín-Rodriguez, J.F.; García-Solís, D.; Gómez-Garre, P.; Mir, P. Genetic factors influencing frontostriatal dysfunction and the development of dementia in Parkinson's disease. PLoS ONE 2017, 12, e0175560. [Google Scholar] [CrossRef] [PubMed]
- Lanskey, J.H.; McColgan, P.; Schrag, A.E.; Acosta-Cabronero, J.; Rees, G.; Morris, H.R.; Weil, R.S. Can neuroimaging predict dementia in Parkinson's disease? Brain 2018, 141, 2545–2560. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Burn, D.; Goetz, C.; Aarsland, D.; Brown, R.G.; Broe, G.A.; Dickson, D.; Duyckaerts, C.; Cummings, J.; Gauthier, S.; et al. Diagnostic procedures for Parkinson's disease dementia: Recommendations from the movement disorder society task force. Mov. Disord. 2007, 22, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Htike, T.T.; Mishra, S.; Kumar, S.; Padmanabhan, P.; Gulyas, B. Peripheral Biomarkers for Early Detection of Alzheimer's and Parkinson's Diseases. Mol. Neurobiol. 2019, 56, 2256–2277. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Liu, S.C.; Hsu, Y.F.; Wu, R.M. The role of noncoding RNAs in Parkinson's disease: Biomarkers and associations with pathogenic pathways. J. Biomed. Sci. 2021, 28, 78. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Lu, J.; Cao, S.; Zhao, Q.; Yu, Z. Identification of aberrant circulating miRNAs in Parkinson's disease plasma samples. Brain Behav. 2018, 8, e00941. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.R.; Sulaiman, S.A.; Saperi, A.A.; Jamal, R.; Mohamed Ibrahim, N.; Abdul Murad, N.A. MicroRNAs and Target Genes As Biomarkers for the Diagnosis of Early Onset of Parkinson Disease. Front. Mol. Neurosci. 2017, 10, 352. [Google Scholar] [CrossRef]
- Batistela, M.S.; Josviak, N.D.; Sulzbach, C.D.; de Souza, R.L. An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer's and Parkinson's Diseases. Int. J. Neurosci. 2017, 127, 547–558. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Yang, F.C.; Chiu, M.J.; Lin, W.C.; Lu, C.H.; Yang, S.Y. Relevance of plasma biomarkers to pathologies in Alzheimer's disease, Parkinson's disease and frontotemporal dementia. Sci. Rep. 2022, 12, 17919. [Google Scholar] [CrossRef]
- Fan, T.S.; Liu, S.C.; Wu, R.M. Alpha-Synuclein and Cognitive Decline in Parkinson Disease. Life (Basel Switz. ) 2021, 11. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Faraldi, M.; Gomarasca, M.; Banfi, G.; Lombardi, G. Free Circulating miRNAs Measurement in Clinical Settings: The Still Unsolved Issue of the Normalization. Adv. Clin. Chem. 2018, 87, 113–139. [Google Scholar] [CrossRef]
- Fehlmann, T.; Lehallier, B.; Schaum, N.; Hahn, O.; Kahraman, M.; Li, Y.; Grammes, N.; Geffers, L.; Backes, C.; Balling, R.; et al. Common diseases alter the physiological age-related blood microRNA profile. Nat. Commun. 2020, 11, 5958. [Google Scholar] [CrossRef]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Maniati, M.; Koros, C.; Simitsi, A.M.; Bozi, M.; Pachi, I.; Stamelou, M.; Paraskevas, G.P.; et al. Circulating Brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson's disease. Mov. Disord. 2020, 35, 457–467. [Google Scholar] [CrossRef]
- Marques, T.M.; Kuiperij, H.B.; Bruinsma, I.B.; van Rumund, A.; Aerts, M.B.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. MicroRNAs in Cerebrospinal Fluid as Potential Biomarkers for Parkinson's Disease and Multiple System Atrophy. Mol. Neurobiol. 2017, 54, 7736–7745. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Wang, X.; Zhang, L.; Jiang, S.; Yuan, Y.; Li, J.; Zhu, L.; Zhang, K. Relationship between the plasma levels of neurodegenerative proteins and motor subtypes of Parkinson's disease. J Neural Transm (Vienna) 2017, 124, 353–360. [Google Scholar] [CrossRef]
- Chan, L.; Chung, C.C.; Hsieh, Y.C.; Wu, R.M.; Hong, C.T. Plasma extracellular vesicle tau, beta-amyloid, and alpha-synuclein and the progression of Parkinson's disease: A follow-up study. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221150329. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, C.; Zhang, Q.; Shen, T.; Sun, J. Serum NFL discriminates Parkinson disease from essential tremor and reflect motor and cognition severity. BMC Neurol. 2022, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Shaw, J.S.; Cheng, F.Y.; Chen, P.H. Plasma total tau predicts executive dysfunction in Parkinson's disease. Acta Neurol. Scand. 2022, 145, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; Irwin, D.J. Fluid and Biopsy Based Biomarkers in Parkinson's Disease. Neurother. : J. Am. Soc. Exp. NeuroTherapeutics 2023, 20, 932–954. [Google Scholar] [CrossRef] [PubMed]
- Tsoporis, J.N.; Ektesabi, A.M.; Gupta, S.; Izhar, S.; Salpeas, V.; Rizos, I.K.; Kympouropoulos, S.P.; Dos Santos, C.C.; Parker, T.G.; Rizos, E. A longitudinal study of alterations of circulating DJ-1 and miR203a-3p in association to olanzapine medication in a sample of first episode patients with schizophrenia. J. Psychiatr. Res. 2022, 146, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Antipova, D.; Bandopadhyay, R. Expression of DJ-1 in Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2017, 1037, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Sandrelli, F.; Beltramini, M.; Greggio, E.; Bubacco, L.; Bisaglia, M. Recent findings on the physiological function of DJ-1: Beyond Parkinson's disease. Neurobiol. Dis. 2017, 108, 65–72. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, Z.; Yang, M.; Yu, W.; Luo, R.; Zhou, J.; He, J.; Chen, Q.; Song, Z.; Cheng, S. Non-Coding RNA in Microglia Activation and Neuroinflammation in Alzheimer's Disease. J. Inflamm. Res. 2023, 16, 4165–4211. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Hinz, F.I.; Rexach, J.E.; Noguchi, K.I.; Toyoshiba, H.; Oda, A.; Hirai, K.; Sarkar, A.; Seyfried, N.T.; Cheng, C.; et al. Identification of evolutionarily conserved gene networks mediating neurodegenerative dementia. Nat. Med. 2019, 25, 152–164. [Google Scholar] [CrossRef]
- Marchese, D.; Botta-Orfila, T.; Cirillo, D.; Rodriguez, J.A.; Livi, C.M.; Fernández-Santiago, R.; Ezquerra, M.; Martí, M.J.; Bechara, E.; Tartaglia, G.G. Discovering the 3' UTR-mediated regulation of alpha-synuclein. Nucleic Acids Res. 2017, 45, 12888–12903. [Google Scholar] [CrossRef]
- Nies, Y.H.; Mohamad Najib, N.H.; Lim, W.L.; Kamaruzzaman, M.A.; Yahaya, M.F.; Teoh, S.L. MicroRNA Dysregulation in Parkinson's Disease: A Narrative Review. Front. Neurosci. 2021, 15, 660379. [Google Scholar] [CrossRef]
- Phongpreecha, T.; Cholerton, B.; Mata, I.F.; Zabetian, C.P.; Poston, K.L.; Aghaeepour, N.; Tian, L.; Quinn, J.F.; Chung, K.A.; Hiller, A.L.; et al. Multivariate prediction of dementia in Parkinson's disease. NPJ Park. Dis. 2020, 6, 20. [Google Scholar] [CrossRef]
- Auclair-Ouellet, N.; Lieberman, P.; Monchi, O. Contribution of language studies to the understanding of cognitive impairment and its progression over time in Parkinson's disease. Neurosci. Biobehav. Rev. 2017, 80, 657–672. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Li, J.; Liang, X.; Song, C.; Zou, Y. miR-203, fine-tunning neuroinflammation by juggling different components of NF-kappaB signaling. J. Neuroinflammation 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.S.; Ahn, J.H.; Oh, J.K.; Chang, I.B.; Song, J.H.; Wee, J.H.; Min, C.Y.; Yoo, D.M.; Choi, H.G. Association Between Thyroid Diseases and Parkinson's Disease: A Nested Case-Control Study Using a National Health Screening Cohort. J. Park. Dis. 2021, 11, 211–220. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Darweesh, S.K.; Korevaar, T.I.; Mattace-Raso, F.U.; Dehghan, A.; Franco, O.H.; van der Geest, J.N.; Ikram, M.A.; Peeters, R.P. Gait patterns associated with thyroid function: The Rotterdam Study. Sci. Rep. 2016, 6, 38912. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Sanjari Moghaddam, H.; Zare-Shahabadi, A.; Rahmani, F.; Rezaei, N. Neurotransmission systems in Parkinson's disease. Rev. Neurosci. 2017, 28, 509–536. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Yarnall, A.J.; Weil, R.S.; Moro, E.; Moehle, M.S.; Borghammer, P.; Bedard, M.A.; Albin, R.L. Cholinergic system changes in Parkinson's disease: Emerging therapeutic approaches. Lancet Neurol. 2022, 21, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; Nathan, P.J.; Bakker, G.; Lawson, R.A.; Yarnall, A.J.; Burn, D.J.; O'Brien, J.T.; Taylor, J.P. Spatial Covariance of Cholinergic Muscarinic M(1) /M(4) Receptors in Parkinson's Disease. Mov. Disord. 2021, 36, 1879–1888. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Kandiah, N.; Pai, M.C.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson's disease dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef]
- Litvan, I.; Kieburtz, K.; Troster, A.I.; Aarsland, D. Strengths and challenges in conducting clinical trials in Parkinson's disease mild cognitive impairment. Mov. Disord. 2018, 33, 520–527. [Google Scholar] [CrossRef]
- Boel, J.A.; de Bie, R.M.A.; Schmand, B.A.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Goldman, J.G.; Troster, A.I.; Burn, D.J.; Litvan, I.; et al. Level I PD-MCI Using Global Cognitive Tests and the Risk for Parkinson's Disease Dementia. Mov. Disord. Clin. Pr. 2022, 9, 479–483. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Huang, J.W.; Lin, Y.H.; Phoa, F.K.H.; Lin, S.P.; Tsai, Y.T.; Kuo, M.C.; Ueda, K.; Wu, R.M. Differentiating Patient Group across Parkinsonism Spectrum via the Biomedical Oriented Logistic Dantzig Selector (BOLD Selector). (manuscript in submission).
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Jost, S.T.; Kaldenbach, M.A.; Antonini, A.; Martinez-Martin, P.; Timmermann, L.; Odin, P.; Katzenschlager, R.; Borgohain, R.; Fasano, A.; Stocchi, F.; et al. Levodopa Dose Equivalency in Parkinson's Disease: Updated Systematic Review and Proposals. Mov. Disord. 2023, 38, 1236–1252. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 1093. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. : A Publ. Protein Soc. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kehl, T.; Kern, F.; Backes, C.; Fehlmann, T.; Stöckel, D.; Meese, E.; Lenhof, H.P.; Keller, A. miRPathDB 2.0: A novel release of the miRNA Pathway Dictionary Database. Nucleic Acids Res. 2020, 48, D142–d147. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
Figure 1.
The miRNA candidates selected by BOLD selector statistical analysis. A. The top 6 miRNAs were filtered for differentiating PD-MCI (n=23) from PDND (n=37) when δ=8.68. B. The ROC analysis results using 6 miRNAs together for identifying PD-MCI from PDND in a 5-fold cross-validation.
Figure 1.
The miRNA candidates selected by BOLD selector statistical analysis. A. The top 6 miRNAs were filtered for differentiating PD-MCI (n=23) from PDND (n=37) when δ=8.68. B. The ROC analysis results using 6 miRNAs together for identifying PD-MCI from PDND in a 5-fold cross-validation.
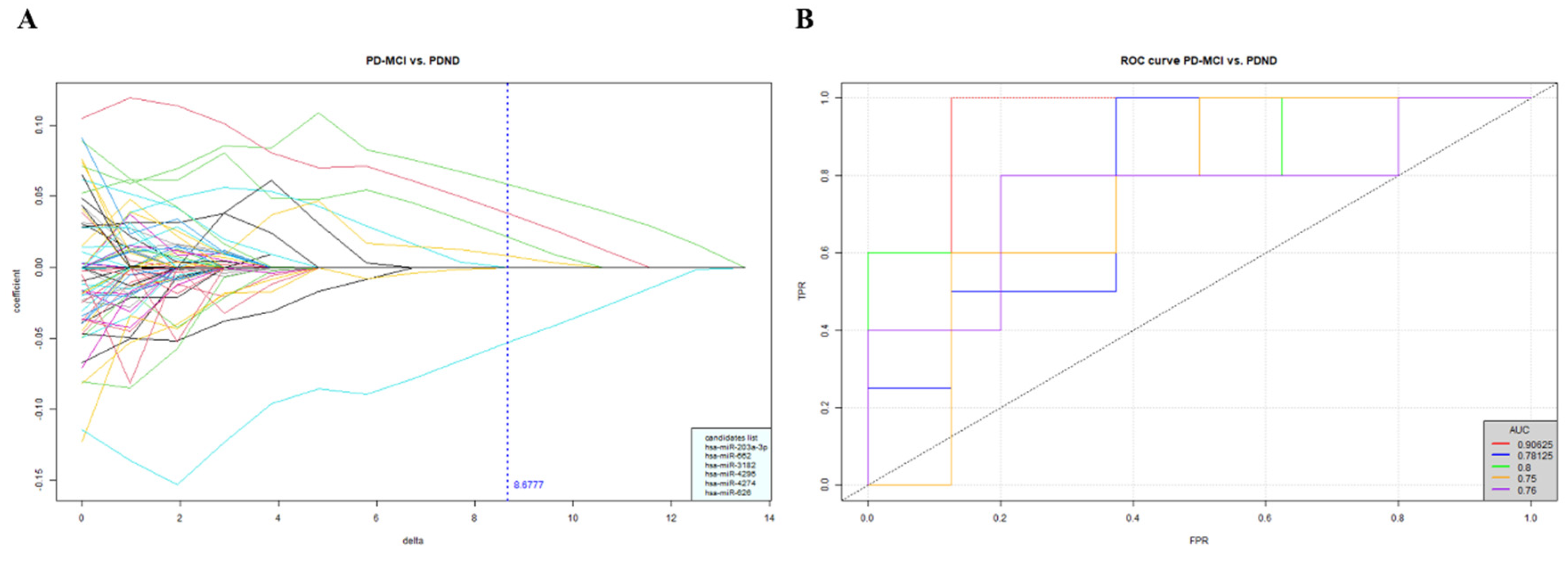
Figure 2.
The ddPCR examination of miR-203a-3p/miR-16-5p. Panels A–C show examples of ddPCR results (n = 1 in each group). A. The representative 1D amplitude plot shows the positive (blue) and negative (gray) droplets for examining miR-203a-3p. NTC, the non-template control (n=1), HC (n=1), PDND (n=1), PD-MCI (n=1) and PDD (n=1). The droplets above the amplitude threshold (pink horizontal line) were estimated as positive droplets, and the remaining detected droplets were classified as negative droplets. B. The estimated number of total (green bar; 18743, 16498, 15633, 16776 and 16599) and positive (blue bar; 0, 5, 11, 13 and 15) droplets for the examination of miR-203a-3p via ddPCR. Positive droplets with less than 3 droplets were considered negative results. C. The expression level of miR-203a-3p converted to copies per microliter. D. The ratio of miR-203a-3p/miR-16-5p of all samples in validation cohort was analyzed using Kruskal–Wallis test followed by Dunn's multiple comparisons test (post hoc test) among the study groups. HC (n=30), PDND (n=30), PD-MCI (n=30) and PDD (n=30). Data are shown as mean ± SD.
Figure 2.
The ddPCR examination of miR-203a-3p/miR-16-5p. Panels A–C show examples of ddPCR results (n = 1 in each group). A. The representative 1D amplitude plot shows the positive (blue) and negative (gray) droplets for examining miR-203a-3p. NTC, the non-template control (n=1), HC (n=1), PDND (n=1), PD-MCI (n=1) and PDD (n=1). The droplets above the amplitude threshold (pink horizontal line) were estimated as positive droplets, and the remaining detected droplets were classified as negative droplets. B. The estimated number of total (green bar; 18743, 16498, 15633, 16776 and 16599) and positive (blue bar; 0, 5, 11, 13 and 15) droplets for the examination of miR-203a-3p via ddPCR. Positive droplets with less than 3 droplets were considered negative results. C. The expression level of miR-203a-3p converted to copies per microliter. D. The ratio of miR-203a-3p/miR-16-5p of all samples in validation cohort was analyzed using Kruskal–Wallis test followed by Dunn's multiple comparisons test (post hoc test) among the study groups. HC (n=30), PDND (n=30), PD-MCI (n=30) and PDD (n=30). Data are shown as mean ± SD.

Figure 3.
The ROC curve of the reduced logistic regression model. The average AUC was estimated using a 5-fold cross-validation.
Figure 3.
The ROC curve of the reduced logistic regression model. The average AUC was estimated using a 5-fold cross-validation.

Table 1.
Demographic characteristics of each study group in the discovery cohort.
| HC (n=40) |
PDND (n=37) |
PD-MCI (n=23) |
PDD (n=23) |
p-value* | |
|---|---|---|---|---|---|
| Gender, % male | 40.00% | 54.05% | 73.91% | 52.17% | ns |
| Age, year | 69.08 ± 6.05 | 64.78 ± 12.51 | 67.70 ± 7.15 | 72.00 ± 5.52 | ns |
*p-value was estimated by performing Kruskal–Wallis test on the 4 groups. Data are shown as mean ± SD. HC, healthy control; PDND, Parkinson’s disease with no dementia; PD-MCI, Parkinson’s disease with mild cognitive impairment; PDD, Parkinson’s disease with dementia; ns, no significant difference.
Table 2.
Demographic characteristics of each study group in the validation cohort.
| HC (n=30) |
PDND (n=30) |
PD-MCI (n=30) |
PDD (n=30) |
p-value* | |
|---|---|---|---|---|---|
| Gender, % male | 56.67% | 56.67% | 53.33% | 46.67% | - |
| Age, year | 66.67 ± 5.14 | 69.67 ± 7.03 | 70.13 ± 6.75 | 75.20 ± 6.92 | <0.0001 |
| MoCA† | 28.00 ± 2.00 | 28.00 ± 1.25 | 23.00 ± 1.00 | 17.50 ± 7.00 | <0.0001 |
| Education, year | 14.13 ± 4.13 | 14.13 ± 2.79 | 11.47 ± 4.75 | 10.73 ± 4.64 | 0.0049 |
| Onset age, year | - | 63.53 ± 7.96 | 64.13 ± 7.96 | 67.37 ± 8.71 | ns |
| Duration, year | - | 7.10 ± 3.91 | 6.90 ± 3.07 | 7.23 ± 4.75 | ns |
| Hoehn–Yahr stage† | - | 2.00 ± 1.00 | 2.00 ± 1.00 | 3.00 ± 2.00 | <0.0001 |
| UPDRS III† | - | 13.00 ± 12.00 | 18.50 ± 9.00 | 27.00 ± 22.00 | <0.0001 |
| LEDD | - | 682.54 ± 438.75 | 747.78 ± 398.03 | 765.82 ± 419.36 | ns |
*p-value was estimated by performing Kruskal–Wallis test on the 4 groups. ns: no significant difference. Data for gender, age, education, onset age, duration and LEDD are shown as mean ± SD. †Data for MoCA, Hoehn–Yahr stage and UPDRS III are shown as median ± IQR.
Table 3.
Spearman correlation of the miRNA ratio and cognitive domain in PD patients.
| Cognitive domains of MoCA | Spearman r | p-value |
|---|---|---|
| Total score* | -0.237 | 0.024 |
| Visuospatial* | -0.207 | 0.050 |
| Naming | -0.117 | 0.272 |
| Attention | -0.112 | 0.292 |
| Language* | -0.208 | 0.049 |
| Abstract | -0.124 | 0.246 |
| Memory | -0.205 | 0.052 |
| Orientation* | -0.220 | 0.037 |
*The ratio of miR-203a-3p/miR-16-5p had significantly negative correlation (p-value<0.05).
Table 4.
The AUCs and 95% confidence intervals of specificity, sensitivity and accuracy for the ROC curve analysis for each comparison group.
Table 4.
The AUCs and 95% confidence intervals of specificity, sensitivity and accuracy for the ROC curve analysis for each comparison group.
| Comparison groups | AUC (95% CI) |
Specificity (95% CI) |
Sensitivity (95% CI) |
Accuracy (95% CI) |
|---|---|---|---|---|
| PD-MCI/PDD | 0.7160 (0.4321-0.9506) |
0.5556 (0.2222-0.8889) |
1.0000 (1.0000-1.0000) |
0.7778 (0.6111-0.9444) |
| PD-MCI/PDND | 0.5309 (0.2469-0.8025) |
0.8889 (0.6667-1.0000) |
0.4444 (0.1111-0.7778) |
0.6667 (0.4444-0.8333) |
| PDD/PDND | 0.7407 (0.4815-0.9506) |
0.5556 (0.2222-0.8889) |
1.0000 (1.0000-1.0000) |
0.7778 (0.6111-0.9444) |
| PDD/HC | 0.6420 (0.3333-0.9259) |
0.6667 (0.3333-1.0000) |
0.7778 (0.4444-1.0000) |
0.7222 (0.5000-0.8889) |
| PD-MCI/HC | 0.6667 (0.3824-0.9136) |
0.8889 (0.6667-1.0000) |
0.5556 (0.2222-0.8889) |
0.7222 (0.5556-0.8889) |
| PDND/HC | 0.7160 (0.4318-0.9383) |
0.8889 (0.6667-1.0000) |
0.6667 (0.3333-1.0000) |
0.7778 (0.6111-0.9444) |
Table 5.
The KEGG analysis pathway and the predicted target genes of miR-203a-3p.
| Database | Pathway | p-value | Targets |
|---|---|---|---|
| KEGG | Dopaminergic synapse | 3.00E-04 | AKT2,CLOCK,CREB1,GNAS,GSK3B,KIF5B,MAPK8,MAPK9,PPP1CB,PRKACB,PRKCA |
| KEGG | Apoptosis | 0.011 | AKT2, ATM,MYD88,PIK3CA,PRKACB,TNF |
| KEGG | Thyroid hormone signaling pathway | 0.014 | AKT2, GSK3B,PIK3CA,PRKACB,PRKCA,SRC,STAT1 |
| KEGG | Cholinergic synapse | 0.027 | AKT2, CREB1,KCNJ2,PIK3CA,PRKACB,PRKCA |
| KEGG | NF-kappa B signaling pathway | 0.041 | ATM, CXCL8,MYD88,SYK,TNF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Plasma mir-203a-3p as a Novel Diagnostic Biomarker in Patients with Parkinson’s Disease Dementia
Ya-Fang Hsu
et al.
,
2024
Exploring Circulating Long Non-Coding RNAs in Mild Cognitive Impairment Patients’ Blood
Bruna De Felice
et al.
,
2023
Quantification of Circulating Cell-Free DNA in Idiopathic Parkinson’s Disease Patients
Małgorzata Wojtkowska
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








