Preprint
Article
Prognostic Factors Associated with Tumor Recurrence and Overall Survival in Soft Tissue Sarcomas of the Extremities in a Colombian Reference Cancer Center
Altmetrics
Downloads
92
Views
34
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
22 January 2024
Posted:
22 January 2024
You are already at the latest version
Alerts
Abstract
Introduction:
Soft tissue sarcomas (STS) are low-incidence tumors whose clinical and histopathological factors are associated with adverse oncological outcomes. This study evaluated prognostic factors (PF) associated with tumor recurrence and overall survival (OS) in patients diagnosed with STS of the extremities, treated at the Instituto Nacional de Cancerología (INC), Bogotá, Colombia.
Materials and methods:
An analytical observational study of a historical cohort was carried out, including patients diagnosed with STS and managed surgically in the Functional Unit for Breast and Soft Tissue Tumors of the INC from January 2008 to December 2018.
Results:
A total of 227 patients were included; 74.5% had tumors greater than 5 cm. Most patients (29.1%) were in stage IIIB at diagnosis. Age was associated with higher mortality (HR=1.01; CI95%: 1–1.02; p=0.048); tumor persistence at admission to the INC (HR=2.34; CI95%: 1.25–4.35; p=0.007), and histologic grade III (HR=5.36; CI95%: 2.29–12.56; p=<0.001) showed statistical significance in the multivariate analysis for recurrence of any type, as were the PFs associated with a higher risk of local recurrence (HR=2.85; CI95%: 1.23–6.57; p=0.014 and HR=6.09; CI95%: 2.03–18.2; p=0.001), respectively. Tumor size (HR=1.03; CI95%: 1–1.06; p=0.015) and histologic grade III (HR=4.53; CI95%: 1.42–14.49; p=0.011) were associated with a higher risk of distant recurrence.
Conclusion:
This cohort showed that in addition to histologic grade and tumor size, tumor persistence at the time of admission has an impact on disease recurrence, so STS should be managed by a multidisciplinary team with experience in this pathology in high-volume reference centers.
Keywords:
Subject: Medicine and Pharmacology - Surgery
1. Introduction
Soft tissue sarcomas (STS) are a rare type of cancer, accounting for 1% of malignant tumors in adults (1). According to data from the American Cancer Society, by 2022, 13,190 new cases were expected in the United States, with an estimated mortality of 5,130 cases from this cause (2). In Colombia, according to data from the statistical yearbook of the Instituto Nacional de Cancerología (INC), in 2020, there were 72 new cases found in the extremities and 40 in the retroperitoneum (3). According to records of the database of the Functional Unit for Breast and Soft Tissue Tumors at the INC from July 2020 to July 2022, 125 new cases of STS were reported, 62 of them located in the extremities.
Although most sarcomas arise de novo, some risk factors have been identified for their appearance, such as exposure to radiation, environmental exposure to hydrochlorides and herbicides, consumption of immunosuppressive and antineoplastic drugs, chronic lymphedema, infection by Herpes virus type 8 and Epstein-Barr virus, hereditary syndromes such as Gardner syndrome, Li-Fraumeni syndrome, neurofibromatosis type 1, Bloom syndrome, Werner syndrome, Rothmund-Thomson syndrome, familial adenomatous polyposis, among others (4,5).
Location is a factor influencing cancer treatment and oncological outcomes. Most STS are in the extremities (43%), trunk (10%), intra-abdominal (19%), and retroperitoneum (15%) (4).
At present, there are more than 100 histologic subtypes (5), each of them with a variable clinical behavior and presentation according to age. Thus, in children, the most common is rhabdomyosarcoma; in young adults, synovial sarcoma; and in the elderly, undifferentiated pleomorphic sarcoma (formerly malignant fibrous histiocytoma). Lymph node metastases are rare; however, they occur more frequently in epithelioid sarcoma, rhabdomyosarcoma, clear cell sarcoma, synovial sarcoma, and angiosarcoma (6). The most common histologic types in the extremities are undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumors (7).
Staging is performed using the AJCC eighth edition system, which classifies STS according to tumor size (T), lymph node involvement (N), presence of metastasis (M), and histologic grade (G). It is most often estimated according to the French system (Fédération Nationale des Centres de Lutte Contre le Cancer, FNCLCC), which has demonstrated a greater ability to predict oncological outcomes. This system includes tumor differentiation, mitotic count, and necrosis (8,9).
The prognosis of these tumor types is variable, and it depends on clinicopathological factors, the main one being histologic grade, followed by tumor size, depth of the lesion, histologic type, proximal location, state of the resection margins, and the patient’s age, among others (5).
STS have high local recurrence rates that can reach up to 50% at 5 years and a 5-year overall survival ranging from 12% to 70% depending on location and histologic type (10).
Treatment should be multidisciplinary in high-volume centers, which has shown a significant impact on the prognosis and survival of these patients (11). Surgery is the mainstay of treatment, and its main objective is to achieve negative oncological margins to reduce the risk of local recurrence and, therefore, positively impact overall survival (5). For decades, amputation was the most accepted surgical intervention in managing sarcomas of the extremities. Rosenberg et al. (12) demonstrated how limb-sparing surgery followed by radiotherapy was equivalent to radical surgery in terms of overall survival, with adequate local control. For this reason, limb-sparing procedures are the standard for the treatment of STS of the extremities, achieving local control rates of 90% and a 5-year overall survival of 70% (10).
In STS of the extremities, oncological outcomes are similar using neoadjuvant vs. adjuvant radiotherapy (RT); however, neoadjuvant RT is preferred since this approach significantly reduces chronic complications (13).
Neoadjuvant and adjuvant systemic therapy and isolated limb perfusion (ILP) may be considered in patients at high risk of metastatic disease or if tumor volume reduction is required to facilitate surgical resection and limb sparing (14).
This study aimed to establish the prognostic factors (PF) associated with tumor recurrence and overall survival (OS) in patients diagnosed with STS of the extremities managed in the Functional Unit for Breast and Soft Tissue Tumors of the INC from January 2008 to December 2018.
2. Materials and Methods
An observational, analytical, historical cohort-type study was conducted, which included patients diagnosed with STS of the extremities managed in the Functional Unit for Breast and Soft Tissue Tumors of the INC from January 1, 2008, to December 31, 2018, who met the following inclusion criteria: over 18 years of age at diagnosis, diagnosis of primary or recurrent extremity STS without distant disease, and surgical management performed by one of the specialists of the Functional Unit during the described time period. Information on sociodemographic and clinicopathological characteristics was taken from the Functional Unit’s database and the electronic medical record system. Data were collected by one of the authors and then compiled in an electronic platform designed for the storage of clinical study information (REDCap). The quality and fidelity of the information were evaluated by an assigned supervisor from the Research Division of the INC. The study was approved by the Ethics Committee of the INC (Minute No. IX-023644).
For the descriptive statistical analysis, absolute and relative frequencies, medians, and interquartile ranges (IQR) were estimated for qualitative variables.
As oncological outcomes of interest in the study, overall survival (OS) was evaluated, defined as the time between admission to the Functional Unit and the time of death from any cause. Recurrence-free survival (RFS) was defined as the time between the date of admission to the Functional Unit and the date of diagnosis of local, regional, or systemic recurrence. For statistical analysis, cases of loss or termination of follow-up, without information on the outcomes of interest (recurrence or death), were taken as right censoring. The frequency of acute complications (occurring during the first 30 postoperative days) and chronic complications (occurring after 30 days) were considered as safety outcomes.
Survival functions estimated with the Kaplan-Meier method were used to describe OS and RFS. Cox proportional hazards models were developed to analyze the association between variables and outcomes taken as time to event. Binomial logistic regression models were used for the outcome of chronic complications (yes or no), and Poisson regression models were used for acute complications (number of complications). Hypothesis testing for the statistical models used 5% significance levels. Stata 16® statistical software was used for statistical analysis.
3. Results
During the mentioned study period, 424 patients with tumors of the extremities and trunk were admitted to the Functional Unit of the INC; 127 of them were excluded for having a diagnosis other than STS, and 70 patients for having a location other than the extremities or due to distant metastatic disease. In the end, 227 patients met the inclusion criteria for the study (Figure 1).
The median age at diagnosis was 53 years (IQR: 18-89); 51.3% (n=117) of the patients were men. Most of the patients (62.5%, n=142) were admitted to the Functional Unit without prior treatment, 27.8% (n=63) with persistent tumors after surgical management at another institution, and 9.7% (n=22) with recurrence of a pre-existing disease. The predominant anatomical location was the lower limb (77%, n=175), especially thigh (47.7%, n=108); 17.2% (n=39) of the tumors corresponded to well-differentiated liposarcoma, with the same percentage representing undifferentiated pleomorphic sarcoma. The predominant histologic grade was III in 64.8% (n=147). Most of the patients (29.2%, n=66) were in stage IIIB at diagnosis. In relation to tumor size, 74.5% (n= 169) of the patients presented with a size larger than 5 cm at diagnosis. The set of clinicopathological characteristics evaluated in the cohort is summarized in Table 1.
Regarding the therapeutic strategies used, 74.9% (n=170) underwent initial surgical treatment, with wide local resection being the most performed procedure (57.4%, n=130), followed by amputation (23.8%, n=54). In relation to neoadjuvant treatment, 11.9% of the patients (n=27) received RT, 10.1% (n=23) underwent ILP, and 3.1% (n=7) received neoadjuvant chemotherapy (CHT).
Of the patients, 7% (n=16) underwent lymph node dissection during the primary tumor surgery due to lymph node chain involvement in the affected limb; 8.3% (n=19) had sentinel lymph node biopsy. Sentinel lymph node involvement was found in 2 of these patients, and they underwent lymph node dissection.
There were acute postoperative complications in 26.9% (n=61) of the patients; the most frequent one was surgical wound dehiscence (39.3%, n=24). Of these patients, 19.7% (n=12) had received neoadjuvant RT. The second most frequent complication was infection of the superficial surgical site in 8.4% (n=19). There were 14 chronic complications (6.1%), the main one being functional limitation of the limb (n=4), followed by fibrosis (n=3).
Positive margins were reported in surgical pathology in 14.9% (n=34) of the patients; 6 of them underwent resection with planned positive margins and received RT (neoadjuvant in 2 and adjuvant in 4 cases). Eleven (4.8%) patients underwent surgery to widen the margins to achieve definitive negative pathology; 6 of them received neoadjuvant and 5 adjuvant RT.
It was not possible to perform margin widening in 12 patients; 5 of them had already received neoadjuvant RT and 7 were referred to adjuvant RT. Only 5 patients did not accept any other type of treatment and had not received neoadjuvant RT either.
Regarding adjuvant treatment, 37.4% (n=85) of the patients received adjuvant RT, with doses ranging between 30 and 66 Gy, while intraoperative radiotherapy (IORT) was applied in 6.2% (n=14), with doses between 12 and 15 Gy. Adjuvant CHT was used in 22.4% (n=51), with the MAI scheme (mesna, doxorubicin, ifosfamide) being the most frequently administered combination in 78.2% (n=43) of the cases. Treatment types are described in Table 2.
Regarding the OS analysis, at the time of study closure, 37% (n=84) of the patients were alive with no evidence of disease, 26.4% (n=60) had died from the disease, 17.6% (n= 40) had died from another cause, and 2.2% (n=5) remained alive with clinical or imaging evidence of the disease.
There was a total of 100 deaths during follow-up, representing a mortality rate of 8.5 deaths per 100 patient-years (CI95%: 7–10.3).
The 227 patients included in the study provided a total of 1179.2 years of follow-up, with a median follow-up of 4.5 years (IQR: 6.1 years). The median OS was 10 years (25th percentile = 2.5 years; 75th percentile not reached) (Figure 2).
The Cox proportional hazards model, performed by combining various clinical and pathological variables related to OS, found a higher risk of mortality at an older age (HR=1.01; CI95%: 1–1.02; p=0.048). Histologic grade II (HR=0.69; CI95%: 0.007–0.67; p=0.021) and wide local resection (HR=0.48; CI95%: 0.24 –0.96; p=0.038) were factors associated with better OS (Table 3).
In relation to RFS, 33% (n=75) of the cohort patients presented with disease recurrence; 50.6% (n=38) at the local level. Of these patients, 65.7% (n=25) underwent new surgical procedure to control the disease, which included wide local resection (n=13), amputation (n=11), and compartmental resection (n=1); 34.2% (n=13) did not accept additional treatments. When analyzing the group of patients with local recurrence, it was found that 9 of them (23.6%) had positive margins, 2 planned and 7 unplanned. Two patients who had received IORT presented with local recurrence; 19.8% (n=45) had systemic progression, the most frequently involved organs being lung 80% (n=36) and bone 13.3% (n=6). Twenty percent (n=9) underwent pulmonary metastasectomy and 66.6% (n=30) received primary CHT.
For RFS of any type (local, regional, or distant), the 227 patients provided a total of 945 years of follow-up. The median follow-up was 2.9 years (IQR: 0.1-14.2). The median survival could not be estimated because less than 50% of the patients had this outcome (Figure 3).
The median RFS of any type, as well as the specific median for local, regional, or distant recurrence, could not be estimated since less than 50% of the patients presented this type of outcome.
The Cox proportional hazards model for RFS of any type (local, regional, or distant) showed that tumor persistence at the time of admission to the INC (HR=2.34; CI95%: 1.25–4.35; p=0.007) and histologic grade III (HR=5.36; CI95%: 2.29–12.56; p<0.001) were factors associated with higher disease recurrence. On the contrary, surgery to widen the margins of previous non-oncological surgery performed outside the INC (HR=0.16; CI95%: 0.045–0.6; p=0.006) was considered a protective factor. Neither neoadjuvant nor adjuvant CHT or RT treatment had an impact on the oncological outcomes measured in this cohort (Table 3).
When analyzing the variables related to local recurrence in the Cox proportional hazards model, it was found that tumor persistence at the time of admission to the INC (HR=2.85; CI95%: 1.23–6.57; p=0.014) and histologic grade III (HR=6.09; CI95%: 2.03–18.2; p=0.001) were factors associated with higher local recurrence.
Regarding distant recurrence, an unfavorable association was found with tumor size (HR=1.03; CI95%: 1.007–1.067; p=0.015) and histologic grade III (HR=4.53; CI95%: 1.42–14.49; p=0.01). Wide local resection (HR=0.37; CI95%: 0.14–0.94; p=0.03) and widening of margins of previous extra-institutional surgery (HR=0.07; CI95%: 0.008–0.64; p=0.019) behaved as protective factors of distant recurrence (Table 4).
It was not possible to develop the Cox logistic regression model to estimate the variables related to regional recurrence since few of these events occurred in this cohort.
Acute and Chronic Complications
According to the Poisson regression model for acute complications, widening the margins of extra-institutional surgery increases the number of acute complications by 69% (β=1.69; CI95%: 0.34–3.05; p=0.014), wide local resection increases it by 38% (β=1.38; CI95%: 0.43–2.33; p=0.004), compartmental resection by 37% (β=1.37; CI95%: 0.1–2.63; p= 0.03), and tumor relapse by 9.5%, (β=0.95; CI95%: 0.32–1.59; p=0.003). The number of acute complications also increases with older age (β=0.017; CI95%: 0.002–0.031; p=0.022) and larger tumor size (β=0.036; CI95%: 0.007–0.065; p= 0.015) (Table 5).
In the logistic regression model, the odds ratio (OR) of chronic complications in patients who received adjuvant RT was 4.36 (CI95%: 0.98–19.26; p=0.052). No relationship was found between this type of complications and the histopathological characteristics or treatment received (Table 5).
4. Discussion
To date, this is the first study that examines treatment experience results in patients with STS of the extremities managed in a Colombian reference cancer center, where most of the patients are admitted with advanced disease.
Surgical treatment is the mainstay of treatment for patients with non-metastatic STS and for those with resectable metastases, independent of histologic subtype and location, with its main objectives being local control of the disease by achieving adequate surgical margins and preservation of limb function (2).
In STS, there are several clinical and histopathological factors associated with adverse oncological outcomes, which have allowed predicting the clinical course of the disease.
In this cohort, presentation type with persistent disease and high histologic grade were identified as the most important prognostic factors for RFS.
Patients admitted to the INC with tumor persistence after previous extra-institutional non-oncologic surgery had higher risk of recurrence of any type and higher risk of local relapse compared to patients who presented with primary disease, which relates to what has been described in series such as Blay et al. (15), who report lower rates of local recurrence, disease progression, and death in patients initially treated in high-volume centers by surgeons specialized in the management of STS.
Approximately 25% of patients with STS of the extremities develop distant metastases after surgical resection with negative margins (16). This incidence increases to 50% when high-risk factors are combined, such as tumor size > 5 cm, deep fascia tumors, and intermediate or high histologic grade (17,18). In this cohort, most patients (64.8%) had histologic grade III, which, in turn, was more related to worse RFS of any type and higher local and distant recurrence. This finding is similar to those described in series such as Coindre et al. (19) and Brennan et al. (20), where high histologic grade was related to worse distant metastasis-free survival rates, without being associated with worse OS rates.
The relationship between tumor size and local relapse has been controversial. In this cohort, 74.3% of the patients presented with tumor size larger than 5 cm, and no statistically significant association was found with local relapse and OS, but a statistical association was found with distant recurrence. Several series have found that tumor size does not have a significant influence on local control, but it does have a relationship with distant recurrence and disease-specific survival (21-24).
According to the World Health Organization (WHO) classification, there are more than 100 different histologic subtypes of STS, each of which has different clinical and prognostic characteristics. Any histologic subtype can develop in the extremities; in this cohort, the most frequent types were well-differentiated liposarcoma, undifferentiated pleomorphic sarcoma, and myxoid liposarcoma. Given the heterogeneity and multiple histologic subtypes described, it was not possible to include this variable in the multivariate analysis. Thus, it was difficult to establish a direct relationship between each of these subtypes and the different oncological outcomes described.
Microscopically positive margins are known to be associated with a higher rate of local recurrence. In this cohort, with a local recurrence rate of 16.7%, a weak association was found with marginal resection, but no statistically significant association was shown between positive surgical margins and local or distant recurrence. This relates to what is described in studies such as Gronchi et al. (25), where surgical margins had no impact on local and distant recurrence (p=0.179) in the first years of follow-up. However, they were associated with worse local recurrence rates after 5 years of follow-up, similar to that reported by Stojadinovic et al. (26), where an impact on local and distant recurrence was only found 2 years after resection of the primary tumor. In this cohort, 14.9% of the patients had positive surgical margins. It is likely that the lack of statistical significance with adverse oncological outcomes is due to some additional surgical intervention to achieve negative oncological margins. In addition, 2.6% of these patients had planned positive margins due to planned marginal resection because of neurovascular compromise, and all these patients received neoadjuvant or adjuvant RT.
Regarding the type of surgical procedure, an association close to statistical significance was found with marginal resection and local recurrence, but no differences were found between procedure type and the oncological outcomes analyzed. Most studies worldwide have failed to establish a relationship between the type of surgical procedure and survival (26). Series like Trovik et al. (27) and Zagars et al. (28) report intrinsic tumor characteristics, such as size, depth, histologic grade, and histologic subtype, as factors related to distant recurrence and disease-specific survival, rather than the type of procedure performed.
In this cohort, lymph node involvement was present in 7.9% of the patients, who underwent lymph node dissection during primary tumor surgery due to lymph node chain involvement in the affected limb or after a positive sentinel lymph node biopsy. Nodal involvement in STS is rare, with reported rates of 2 to 10% in all histopathological subtypes (29), which relates to what was found in the present study.
Although the role of radiation is well established in STS of the extremities, the optimal sequence of radiation surgery in terms of oncological outcomes has not yet been defined (30). In the study by O’Sullivan et al. (31) comparing preoperative and postoperative RT, neoadjuvant treatment revealed a slightly significant improvement in OS compared to postoperative treatment. In this cohort, no relationship was found between the time of administration of RT and OS, although a relationship was found between adjuvant RT and increased risk of chronic complications, which relates to what has been described in the study by Davis et al. (30), where postoperative RT was associated with a higher incidence of fibrosis, joint stiffness, and reduced limb functionality (31,32).
Isolated limb perfusion allowed performing limb-sparing surgery in 47.8% of the patients who underwent this type of intervention, being a therapeutic alternative in patients with STS whose tumors make conservative surgery difficult due to their extension (multifocal or multicompartmental) or volume.
This study showed that the only variable associated with higher mortality was age; OS could be affected by age not only in relation to the clinical course of the disease but also by unrelated concurrent morbidity.
As for acute complications, they occurred in 26.9% of patients in this cohort, which is similar to what is described in the literature with rates ranging from 11 to 29% (32). It was found that the widening of margins from previous extra-institutional surgery, wide local resection, and compartmental resection increase the risk of a greater number of acute complications, as well as tumor recurrence, which has been previously reported in series such as Schwartz et al. (33), where prolonged surgical time was related to a higher risk of infection and surgical wound dehiscence.
5. Study Limitations
One of the main limitations of this study is its retrospective nature; additionally, in some cases, the follow-up was short.
Due to the heterogeneity of the histologic subtypes, it was not possible to include this variable in the multivariate analysis; thus, it was not possible to establish a relationship between this variable and the outcomes evaluated.
6. Conclusions
Soft tissue sarcomas are low-incidence tumors, comprising a heterogeneous group of neoplasms with diverse outcomes determined by several factors. In this cohort, tumor persistence was a determining prognostic factor, so these types of tumors should ideally be managed in referral centers by an experienced multidisciplinary team, seeking to improve oncological outcomes.
References
- Hui JYC. Epidemiology and Etiology of Sarcomas. Surg Clin North Am. 2016;96:901-14. [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020.
- Instituto Nacional de Cancerología. Anuario estadístico 2020. Vol. 18. Bogotá: INC; 2020.
- Pisters, P. W. , Weiss, M., & Maki, R. (2011). Soft-tissue sarcomas. Cancer Management: A Multidisciplinary Approach Medical, Surgical, & Radiation Oncology, 4th ed., UBM Medica LLC, Norwalk, CT.
- Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200-29. [CrossRef]
- Keung EZ, Chiang YJ, Voss RK, Cormier JN, Torres KE, Hunt KK, et al. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur J Surg Oncol. 2018;44(1):170-7. [CrossRef]
- Sbaraglia M, Dei Tos AP. The pathology of soft tissue sarcomas. Radiol Medica. 2019;124(4):266-81. [CrossRef]
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-9. [CrossRef]
- Voss RK, Chiang YJ, Torres KE, Guadagnolo BA, Mann GN, Feig BW, et al. Adherence to National Comprehensive Cancer Network Guidelines is Associated with Improved Survival for Patients with Stage 2A and Stages 2B and 3 Extremity and Superficial Trunk Soft Tissue Sarcoma. Ann Surg Oncol. 2017;24(11):3271-8. [CrossRef]
- Billingsley KG, Lewis JJ, Leung DHY, Casper ES, Woodruff JM, Brennan MF. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer. 1999;85(2):389-95.
- Wasif N, Smith CA, Tamurian RM, Christensen SD, Monjazeb AM, Martinez SR, et al. Influence of physician specialty on treatment recommendations in the multidisciplinary management of soft tissue sarcoma of the extremities. JAMA Surg. 2013;148(7):632-9. [CrossRef]
- Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305-15. [CrossRef]
- O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-41. [CrossRef]
- Tierney JF, Mosseri V, Stewart LA, Souhami RL, Parmar MK. Adjuvant chemotherapy for soft-tissue sarcoma: review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72(2):469-75. [CrossRef]
- Blay JY, Honoré C, Stoeckle E, Meeus P, Jafari M, Gouin F, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol. 2019;30(7):1143-53. [CrossRef]
- Demetri GD, Blay JY, Casali PG. Advances and controversies in the management of soft tissue sarcomas. Future Oncol. 2016;13(1S):3-11. [CrossRef]
- Weskamp P, Ufton D, Drysch M, Wagner JM, Dadras M, Lehnhardt M, et al. Risk Factors for Occurrence and Relapse of Soft Tissue Sarcoma. Cancers (Basel). 2022;14(5):1273. [CrossRef]
- Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200-29. [CrossRef]
- Coindre JM, Trojani M, Contesso G, David M, Rouesse J, Bui NB, et al. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer. 1986;58(2):306-9. [CrossRef]
- Brennan, M. F., Antonescu, C. R., Moraco, N., & Singer, S. (2014). Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Annals of surgery, 260(3), 416.
- Coindre J-M, Terrier P, Bui NB, Bonichon O, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Feredarion of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869-77. [CrossRef]
- Gaynor JJ, Tan CC, Casper ES, Collin CF, Friedrich C, Shiu M, et al. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: A study of 423 adults. J Clin Oncol. 1992;10(8):1317-29. [CrossRef]
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679-89. [CrossRef]
- Stefanovski PD, Bidoli E, De Paoli A, Buonadonna A, Boz G, Libra M, et al. Prognostic factors in soft tissue sarcomas: A study of 395 patients. Eur J Surg Oncol. 2002;28(2):153-64. [CrossRef]
- Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol. 2005;23(1):96-104. [CrossRef]
- Stojadinovic A, Leung DHY, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235(3):424-34. [CrossRef]
- Trovik CS, Bauer HCF, Alvegård TA, Anderson H, Blomqvist C, Berlin, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36(6):710-6. [CrossRef]
- Zagars GK, Ballo MT, Pisters PWT, Pollock RE, Patel SR, Benjamin RS, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer. 2003;97(10):2530-43. [CrossRef]
- Rosenthal J, Cardona K, Sayyid SK, Perricone AJ, Reimer N, Monson D, et al. Nodal metastases of soft tissue sarcomas: risk factors, imaging findings, and implications. Skeletal Radiol. 2020;49(2):221-9. [CrossRef]
- avis, A. M., O'Sullivan, B., Turcotte, R., Bell, R., Catton, C., Chabot, P., ... & Randomized, N. C. C. T. G. (2005). Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiotherapy and oncology, 75(1), 48-53.
- Gingrich AA, Bateni SB, Monjazeb AM, Darrow M, Thorpe SW, Kirane AR, et al. Neoadjuvant Radiotherapy is Associated with R0 Resection and Improved Survival in Extremity Soft Tissue Sarcoma Patients Undergoing Surgery: An NCDB Analysis Alicia. Ann Surg Oncol. 2018;24(11):3252-63. [CrossRef]
- Broecker JS, Ethun CG, Monson DK, Lopez-Aguiar AG, Le N, McInnis M, et al. The Oncologic Impact of Postoperative Complications Following Resection of Truncal and Extremity Soft Tissue Sarcomas. Ann Surg Oncol. 2017;24(12):3574-86. [CrossRef]
- Schwartz A, Rebecca A, Smith A, Casey W, Ashman J, Gunderson L, et al. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res. 2013;471(11):3612-7. [CrossRef]
Figure 1.
Selection of cohort patients.
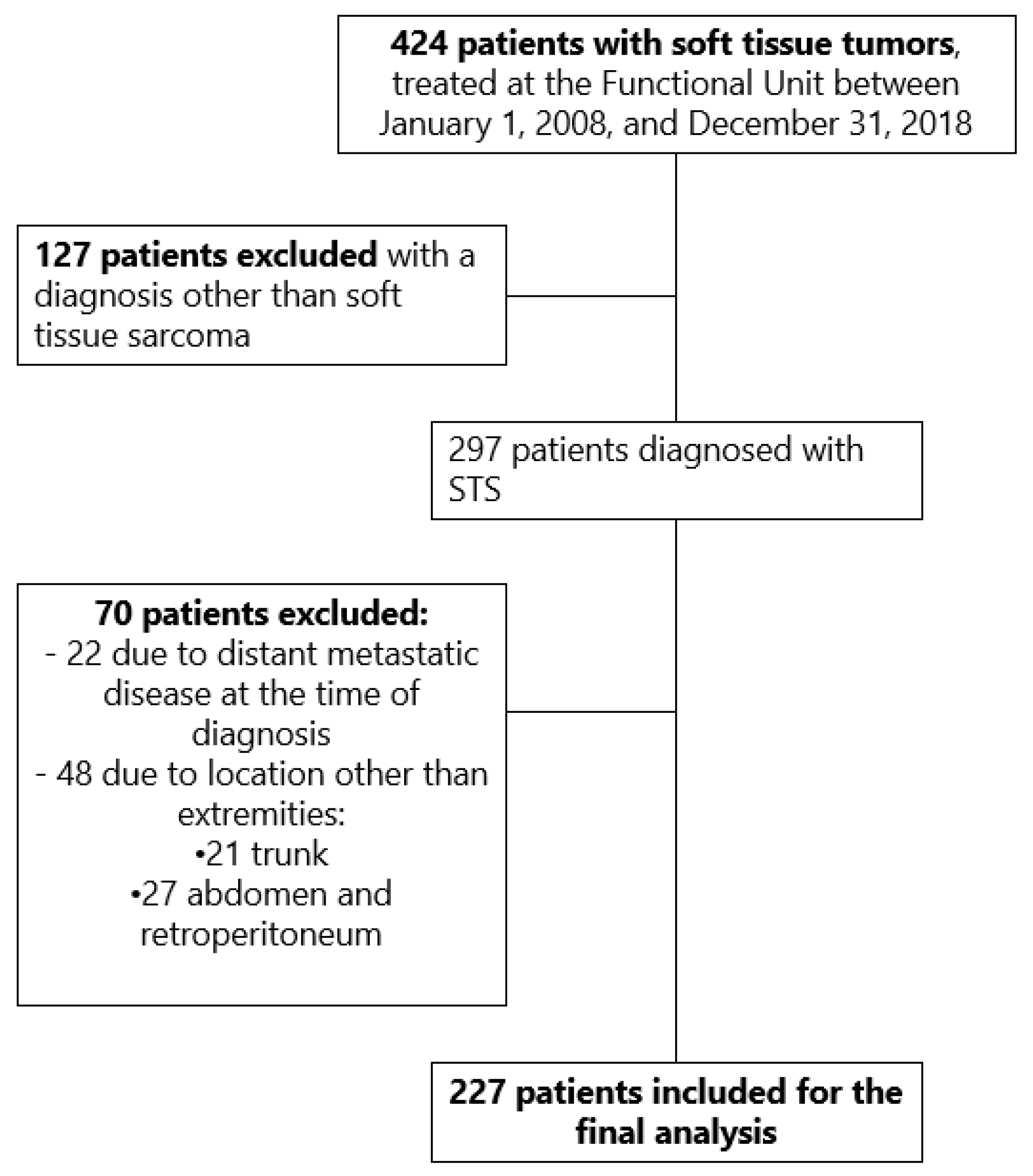
Figure 2.
Overall survival function estimated with the Kaplan-Meier method in patients with STS of the extremities.
Figure 2.
Overall survival function estimated with the Kaplan-Meier method in patients with STS of the extremities.

Figure 3.
Recurrence-free survival function estimated with the Kaplan-Meier method in patients with STS of the extremities.
Figure 3.
Recurrence-free survival function estimated with the Kaplan-Meier method in patients with STS of the extremities.

Table 1.
Clinicopathological characteristics of cohort patients.
| Characteristics | Total of patients (n=227), n (%) |
|---|---|
| Median age (years) | 53 (18-89) |
|
Sex Men Women |
117 (51.5) 110 (48.5) |
|
Presentation type Primary without treatment Tumor persistence Tumor recurrence |
142 (62.5) 63 (27.8) 22 (9.7) |
|
Histologic type Well-differentiated liposarcoma Undifferentiated pleomorphic sarcoma Myxoid liposarcoma Synovial sarcoma Myxofibrosarcoma Leiomyosarcoma Malignant neural sheath tumor Others |
39 (17.2) 39 (17.2) 33 (14.5) 25 (11) 21 (9.3) 19 (8.4) 16 (7) 35 (15.4) |
|
Histologic grade I II III No data |
67 (29.5) 12 (5.3) 147 (64.8) 1 (0.4) |
|
Tumor size T1 T2 T3 T4 No data (Initial surgery outside the INC) |
53 (23.3) 64 (28.1) 40 (17.7) 65 (28.7) 5 (2.2) |
|
Clinical stage IA IB II IIIA IIIB IV (Lymph node involvement) Not applicable (Dermatofibrosarcoma protuberans) |
14 (6.2) 43 (18.9) 35 (15.4) 57 (25.1) 66 (29.1) 3 (1.3) 9 (3.9) |
|
Tumor location Thigh Leg Forearm Gluteus Foot Arm Shoulder Hand |
108 (47.7) 39 (17.2) 23 (10.1) 16 (7) 13 (5.7) 12 (5.3) 10 (4.4) 6 (2.6) |
Table 2.
Types of treatment administered to cohort patients.
| Administered treatment | Total of patients (n=228), n (%) |
|---|---|
|
Initial treatment type Surgical treatment Neoadjuvant RT ILP Neoadjuvant CHT |
170 (74.9) 27 (11.9) 23 (10.1) 7 (3.1) |
|
Type of primary tumor surgery Wide local resection Amputation Widening of margins of previous non-oncological surgery outside the INC Compartmental resection Marginal resection |
130 (57.4) 54 (23.8) 28 (12.3) 9 (3.9) 6 (2.6) |
|
Additional interventions Sentinel lymph node Lymph node dissection IORT |
19 (8.3) 18 (7.9) 14 (6.1) |
|
Positive margins in INC pathology No Yes Planned positive margins |
189 (83.3) 34 (14.9) 6 (17.7) |
|
Surgery to achieve negative margins Widening of margins |
11 (4.8) |
|
Adjuvant treatment Adjuvant CHT Adjuvant RT |
51 (22.4) 85 (37.3) |
RT: radiotherapy; ILP: Isolated limb perfusion; CHT: chemotherapy; IORT: intraoperative radiotherapy.
Table 3.
Adjusted Cox proportional hazards estimates for OS and RFS of all types (local, regional, and distant).
Table 3.
Adjusted Cox proportional hazards estimates for OS and RFS of all types (local, regional, and distant).
| Variable | Overall Survival Hazard Ratio (IC95%) | Recurrence-Free Survival Hazard Ratio (IC95%) | ||
|---|---|---|---|---|
| Age | 1.01 (1–1.02) | p=0.048 | 0.99 (0.983–1.015) |
p=0.92 |
| Tumor size | 1.01 (0.99–1.04) | p=0.18 | 1.03 (0.996–1.056) | p=0.08 |
|
Histologic grade I II III |
Ref. 0.69 (0.007–0.67) 0.13 (0.01–1.1) |
p= 0.021 p=0.062 |
Ref. 1.97 (0.49–7.9) 5.36 (2.29–12.56) |
p=0.33 p<0.001 |
|
Type of primary tumor surgery Amputation Compartmental resection Wide local resection Marginal resection Widening of margins of previous non-oncological surgery outside the INC |
Ref. 0.69 (0.2–2.23) 0.48 (0.24–0.96) 1.81 (0.47–6.9) 0.36 (0.12–1.1) |
p=0.56 p=0.038 p=0.38 p=0.075 |
Ref. 0.68 (0.16–2.83) 0.56 (0.26–1.21) 2.16 (0.41–11.3) 0.16 (0.045–0.6) |
p=0.6 p=0.14 p=0.36 p=0.006 |
|
Positive margins in INC pathology Yes No |
1.11 (0.54–2.26) Ref. |
p=0.76 |
1.56 (0.75–3.2) Ref. |
p=0.23 |
|
Presentation type Primary without treatment Tumor persistence Tumor recurrence |
Ref. 0.95 (0.51–1.77) 0.83 (0.39–1.76) |
p=0.88 p=0.63 |
Ref. 2.34 (1.25–4.35) 1.62 (0.74–3.53) |
p=0.007 p=0.21 |
|
Initial treatment type Surgical treatment Neoadjuvant CHT Neoadjuvant RT ILP |
Ref. 1.18 (0.43–3.24) 0.7 (0.3–1.65) 0.75 (0.36–1.54) |
p=0.73 p=0.42 p=0.44 |
Ref. 0.68 (0.15–3.03) 0.86 (0.35–2.09) 0.62 (0.25–1.52) |
p=0.61 p=0.75 p=0.75 |
|
Adjuvant treatment Adjuvant CHT Yes No Adjuvant RT Yes No |
0.66 (0.38–1.14) Ref. 0.7 (0.38–1.27) Ref. |
p=0.14 p=0.24 |
1.14 (0.3–1.2) Ref. 0.6 (0.3–1.2) Ref. |
p=0.15 p=1.2 |
Ref: Reference; CHT: chemotherapy; RT: radiotherapy; ILP: isolated limb perfusion.
Table 4.
Adjusted Cox proportional hazards estimates for local and distant recurrence.
| Variable | Local Recurrence Hazard Ratio (IC95%) | Distant Recurrence Hazard Ratio (IC95%) | ||
|---|---|---|---|---|
| Age | 0.99 (0.77–1.02) | p=0.77 | 0.99 (0.983–1.019) |
p=0.93 |
| Tumor size |
1 (0.95–1.05) |
p=0.89 |
1.03 (1.007–1.067) |
p=0.015 |
|
Histologic grade I II III |
Ref. 1.1 (0.11–10.5) 6.09 (2.03–18.2) |
p=0.92 p=0.001 |
Ref. 2.21 (0.37–13.08) 4.53 (1.42–14.49) |
p=0.38 p=0.011 |
|
Type of primary tumor surgery Amputation Compartmental resection Wide local resection Marginal resection Widening of margins of previous non-oncological surgery outside the INC |
Ref. 3.43 (0.48–24.63) 1.69 (0.46–6.1) 8.88 (0.75–104.4) 0.51 (0.08–3.06) |
p=0.21 p=0.42 p=0.08 p=0.46 |
Ref. 0.48 (0.086–2.74) 0.37 (0.14–0.94) 0.78 (0.08–7.52) 0.07 (0.008–0.64) |
p=0.41 p=0.038 p=0.83 p=0.019 |
|
Positive margins in INC pathology Yes No |
1.74 (0.69–4.34) Ref. |
p=0.23 |
1 (0.37–2.72) Ref. |
p=0.98 |
|
Presentation type Primary without treatment Tumor persistence Tumor recurrence |
Ref. 2.85 (1.23–6.57) 1.6 (0.5–5.07) |
p=0.014 p=0.42 |
Ref. 1.49 (0.65–3.41) 1.42 (0.55–3.64) |
p=0.33 p=0.46 |
|
Initial treatment type Surgical treatment Neoadjuvant CHT Neoadjuvant RT ILP |
Ref. 0.81 (0.09–6.84) 0.77 (0.21–2.65) 1.02 (0.32–3.24) |
p=0.85 p=0.65 p=0.96 |
Ref. 1.35 (0.28–6.44) 0.98 (0.32–3.03) 0.50 (0.14–1.78) |
p=0.7 p=0.98 p=0.29 |
|
Adjuvant treatment Adjuvant CHT Yes No Adjuvant RT Yes No |
0.75 (0.33–1.69) Ref. 0.55 (0.22–1.4) Ref. |
p=0.49 p=0.21 |
1.25 (0.62–2.54) Ref. 0.82 (0.33–2.01) Ref. |
p=0.52 p=0.66 |
Ref.: Reference; CHT: chemotherapy; RT: radiotherapy; ILP: isolated limb perfusion.
Table 5.
Poisson regression model for acute complications and logistic regression model for chronic complications.
Table 5.
Poisson regression model for acute complications and logistic regression model for chronic complications.
| Variable | Acute Complications Coefficient (β) (IC95%) | Chronic Complications Odds Ratio (IC95%) | ||
|---|---|---|---|---|
| Age | 0.017 (0.002–0.031) | p=0.022 | 1 (0.96–1.03) |
p=0.89 |
| Tumor size | 0.036 (0.007–0.065) | p=0.015 | 0.96 (0.87–1.07) | p=0.52 |
|
Histologic grade I II III |
Ref. 0.19 (0.82–1.22) 0.41 (0.19–1.02) |
p=0.7 p=0.18 |
Ref. 1.1 (0.07–15.76) 1.55 (0.3–7.9) |
p=0.94 p=0.59 |
|
Type of primary tumor surgery Amputation Compartmental resection Wide local resection Marginal resection Widening of margins of previous non-oncological surgery outside the INC |
Ref. 1.37 (0.1–2.63) 1.38 (0.43–2.33) 1.47 (-0.12–3.06) 1.69 (0.34–3.05) |
- p=0.03 p=0.004 p=0.07 p=0.014 |
Ref. - 2.49 (0.23–26.46) 7.33 (0.24–219.49) 0.77 (0.027–21.82) |
p=0.44 p=0.25 p=0.88 |
|
Positive margins in INC pathology Yes No |
Ref. -0.07 (-0.754–0.596) |
p=0.81 |
Ref. 0.62 (0.11–3.37) |
p=0.58 |
|
Presentation type Primary without treatment Tumor persistence Tumor recurrence |
Ref. -0.24 (-1.01–0.52) 0.95 (0.32–1.59) |
p=0.53 p=0.003 |
Ref. 1.53 (0.32–7.25) 3.35 (0.68–16.33) |
p=0.59 p=0.13 |
|
Initial treatment type Surgical treatment Neoadjuvant CHT Neoadjuvant RT ILP |
Ref. -0.14 (-1.63–1.34) 0.16 (-0.05–0.88) -0.21 (-1.18–0.76) |
p=0.84 p=0.65 p=0.67 |
Ref. 2.31 (0.18–29.12) 3.53 (0.44–28.42) 2.41 (0.37–15.68) |
p=0.51 p=0.23 p=0.35 |
|
Adjuvant treatment Adjuvant CHT Yes No Adjuvant RT Yes No |
0.02 (-0.57–0.62) Ref. -0.03 (-0.6–0.53) Ref. |
p=0.93 p=0.9 |
0.55 (0.11–2.64) Ref. 4.36 (0.98–19.26) Ref. |
p=0.45 p=0.052 |
Ref: Reference; CHT: chemotherapy; RT: radiotherapy; ILP: isolated limb perfusion.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Prognostic Factors Associated with Tumor Recurrence and Overall Survival in Soft Tissue Sarcomas of the Extremities in a Colombian Reference Cancer Center
Sandra Díaz-Casas
et al.
,
2024
Effect of Neoadjuvant Therapies on Soft Tissue Sarcomas with Tail-Like Lesions: A Multicenter Retrospective Study
Hisaki Aiba
et al.
,
2021
Survival Outcomes of Patients with Head and Neck Sarcomas Submitted to Multimodal Treatment at a Single Cancer Center: A Retrospective Study
Wilber Edison Bernaola-Paredes
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








