Preprint
Article
Viscoelastic Water-Based Lubricants with Nopal Cactus Mucilage as Green Metalworking Fluids
Altmetrics
Downloads
78
Views
29
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
26 January 2024
Posted:
29 January 2024
You are already at the latest version
Alerts
Abstract
The recent green manufacturing demands have boosted the development of new biodegradable lubricants to replace petroleum-based lubricants. In this regard, water-based lubricants have been at the forefront of recent research for a wide range of industrial applications, including metalworking fluids (MWFs). In this work, we present an experimental investigation on the performance of novel green MWFs based on aqueous nopal mucilage solutions. For this, fully biodegradable solutions with different mucilage concentrations were evaluated in terms of rheological, tribological, thermal stability and turning (minimum quantity lubrication) performance, and compared to a commercial semisynthetic oil-based MWF (Cimstar 60). Mucilage solutions exhibited viscoelastic shear-thinning behavior, which was enhanced along with mucilage concentration. The solution with the highest mucilage content studied resulted in the lowest wear, friction and temperature in comparison to the other solutions and neat water in extreme pressure four-ball tests, and a similar level of lubricity as compared to the commercial MWF in cutting tests. This performance is associated to the enhanced viscosity and elasticity of the solution, as well as to the contents of lipids with fatty acids in the mucilage. Overall, the present results reveal the relevance of the viscoelastic behavior of the lubricant, elasticity in particular, in lubrication processes, and points to nopal mucilage as an effective green additive to produce innocuous MWFs.
Keywords:
Subject: Engineering - Mechanical Engineering
1. Introduction
Water-based lubricants have been at the forefront of recent research for a wide range of industrial applications due to the abundance and total ecofriendlyness of water [1,2]. The idea of using water-based lubricants emerged from the global industry interest in replacing fossil oil-based lubricants with renewable sources (i.e., water or bio-derived oils). Water-based lubricants are blends of water with different chemical species (additives, polymers, stabilizers, nanoparticles, etc.) at low concentrations, either in dispersion or dissolution, to enhance viscosity and lubricity. Water on its own has poor lubricating performance compared to most oil-based lubricants (either fossil- or bio- based), this is due to its low viscosity and propensity to accelerate corrosion. However, considering that water is more sustainable than bio-derived oils, many researchers have explored the inclusion of a wide variety of additives (antioxidants, anti-foaming agents, corrosion inhibitors, detergents, friction modifiers, wear improvers, metal deactivators, and viscosity index improvers) to enhance the performance of water-based lubricants in the last decades [3,4,5]. Unfortunately, most additives employed in industry are noxious to the environment or human health. More recently, looking for better performance and environmental care, other research groups have studied the addition of different nanoparticles (i.e., TiO2 [6], polyethyleneimine-reduced graphene oxide nanosheets [7], etc.), ionic liquids [8,9] and bio-derived oils to produce water-based lubricants and green emulsions [10,11,12]. The use of bio-derived oils as additives has received increased attention since water-based lubricants in the form of green emulsions are extensively used in the global manufacturing industry as metalworking fluids (MWFs) for lubricating machining/cutting/removal processes.
Metalworking processes are high energy consumers and have significant environmental effects, as well as severe human occupational health disorders (i.e., cancers, dermatitis, lung disorders, etc.), mainly because of the use of low-performance and highly toxic water-based MWFs [13,14]. Commercial or typical MWFs are emulsions of a fossil or vegetable oil (2-20 wt%) and a sodium petroleum sulphonate emulsifier (10–15 wt%) in water. The oil provides the main lubricity properties, the emulsifier allows oil dispersion in water, and water cools down the machining interfaces. It is noteworthy that conventional MWFs are not readily biodegradable and have been reported as one of the most prevalent harmful effluents disposed to the environment [15]. According to the state-of-the-art of MWFs, the most novel and potential green formulations are those based either on water blended with ionic liquids [16,17], eutectic solvents [18] or bio-oils and green emulsifiers [12]. These three trending technologies are effective, but still present different challenges in terms of biodegradability, production costs, and usage conditions.
On the other hand, modern lubricants contain certain amounts of high-molecular weight polymers as thickeners or viscosity index modifiers, which make them to exhibit a non-Newtonian rheological behavior [19]. Such a behavior can be simply shear-thinning or more complex viscoelastic. The classical theory of fluid film lubrication is based on Newtonian flow behavior, however, researchers have largely studied the effects of non-Newtonian lubrication (see [20] for an early review on the use of different rheological models). Markedly, in a series of papers Bair and co-workers have analyzed the influence of viscous shear-thinning on film thickness in elastohydrodynamic lubrication (EHL) [21,22,23], with an emphasis on the importance of measuring the piezoviscous behavior of the fluids [24]; this is because EHL occurs at high pressures and shear rates, which can significantly affect lubricant’s viscous behavior. Fluid elasticity, on its side, has received limited attention, despite it is known that lubricants with sufficiently high elasticity may carry greater loads than Newtonian lubricants of similar viscosity [25]. Hutton et al. demonstrated the extra-load carrying capacity for an elastic lubricant in a journal bearing rig, and that this was greater than that predicted by existing theories. Also, the authors showed that the coefficient of friction (CoF) was lower with the viscoelastic lubricant than with a similar Newtonian one [25].
Elasticity of liquid lubricants has been an area of interest to tribologists, but there is yet no consensus on its effect on the thickness of a lubricating film. The reduction in the thickness of a film produced by shear thinning may mask the effects of elongational viscosity and normal stresses [21]. In fact, in a recent study on hydrodynamic lubrication with shear-thinning and viscoelastic polymer solutions, Veltkamp et al. reported that fluid’s elasticity does not change friction significantly; meanwhile shear-thinning affected the lubricant film thickness and the dependence of friction on relative velocity in contrast to Newtonian fluids [26]. Thus, in this work we follow a phenomenological approach to test novel viscoelastic water-based lubricants, namely, aqueous solutions of nopal mucilage, as a MWF. The purpose of this work is two-fold, the first one is to evaluate the influence of elasticity of the mucilage solutions on the turning performance, and the second is to introduce this novel bio-additive (nopal mucilage) as a natural thickener and friction modifier for water to form bio-MWFs.
Nopal cactus or prickly pear (Opuntia ficus-indica (L.) Mill.) is one of the 1500 species of cactus in the dicotyledonous angiosperm Cactaceae family. It is a tropical and subtropical plant that can grow, either cultivated or wild, in arid and semi-arid climates in different regions of the world. México, Brazil, Peru, Tunisia and Morocco [27] are among the largest producers of nopal cactus. Nopal has been overall used as human feedstock and due to its significant contents of nutrients, antioxidants, pectin, polysaccharides and fibers, it is employed to make health and cosmetic products as well [28,29]. In addition, nopal mucilage is known for its film and threads forming capability, which arises from its high molecular weight resulting in high elasticity. Also, nopal contains lipids or fatty acids [30] with lubricant characteristics, which may make it useful for lubricating purposes.
Briefly, nopal pads or cladodes have multiple channels or conduits in their structure, which are filled with a naturally produced branched high-molecular weight polysaccharide known as mucilage [31]. This polysaccharide is often considered as a hydrocolloid, its structure is proposed as two distinctive water-soluble fractions (pectin with gelling properties with Ca2+ and mucilage without gelling characteristics) [32], so it can be easily dissolved in water. Depending on its molecular weight and concentration, nopal mucilage has the capacity to form aqueous viscoelastic solutions and gels [33], which allows the production of biodegradable, non-toxic, odorless, colorless and tasteless gels, emulsions, stabilizers, etc. [34]. These characteristics of nopal mucilage, along with its lipid contents, make it a potential thickening agent/additive for producing water-based lubricants. Notably, using nopal mucilage as an additive for producing green MWFs has not been reported before.
In this work, solutions with different mucilage concentrations were characterized in terms of the most critical MWFs´ properties such as rheological and tribological properties, thermal stability, and turning performance, and compared to a commercial MWF. Overall, the results in this work indicate that elasticity of nopal mucilage solutions and lipids contained in the mucilage resulted in reduced friction and wear, being the performance of the solution with the highest mucilage concentration similar to the commercial MWF. Finally, the results in this work suggest that viscoelastic aqueous nopal mucilage-based solutions can be safely used as green MWFs for turning operations under minimum quantity lubrication (MQL) conditions.
2. Materials and Methods
2.1. Preparation of fluid samples
The nopal mucilage utilized in this work was obtained from nopal flour (Droguería Cosmopolita) by the separation process described next. The nopal flour was used as received, and according to the supplier, it was obtained by lyophilization following the procedure reported elsewhere [35]. First, 50 g of nopal flour were dispersed into 900 mL of tridistilled water (Reactivos Química Meyer) by stirring at 500 rpm with a marine-type propeller coupled to a mechanical stirrer (BDC 2002, Caframo). During dispersion, the temperature was maintained at 45±2 °C using a stirrer hot plate mixer (PC320, Corning Life Sciences). After one hour of stirring, 0.1 g of antioxidant, ascorbic acid (Droguería Cosmopolita), was added into the suspension, while keeping the agitation for additional 30 minutes. Next, 0.6 g of benzoic acid (Droguería Cosmopolita) were added as a preservative to inhibit the growth of micro-organisms. After continuous agitation for 2 hours, the as obtained suspension was filtered using nylon meshes to remove insoluble compounds such as fibers and cellulose particles. The filtration system consisted of a ceramic Büchner funnel with the nylon meshes of apertures 600, 300, 150, 104, 80, and 48 μm, respectively, a 1000 mL Kitasato flask, and a vacuum pump (Siemens), whose maximum vacuum pressure was 65 cm-Hg. The obtained filtered solution contains mainly mucilage, and small amounts of fine particles of cellulose and water-soluble compounds, being mucilage the responsible for the viscoelastic behavior of this type of fluids [33].
The filtered nopal solution was placed in a volumetric flask, and the volume was adjusted to 1000 mL. This primal nopal mucilage solution was identified as “S3”, and dilutions were prepared with 1:2 (solution “S2”) and 2:1 (solution “S1”) parts of water and primal solution in a volumetric flask of 250 mL. The concentration of mucilage in each solution was determined by freeze-drying (lyophilization). For this 100 mL of the S1 solution were placed in a lyophilization vessel and frozen at -10 °C, then introduced into a LABCONCO FreeZone 6 lyophilizer. Freeze drying was carried out eight hours/day for three days at a vacuum pressure of 7x10-3 mBar and temperature of -25 °C. At the end of each day, the vessel was placed in a freezer to continue the process the next day. It was determined that 100 mL of S1 contained 0.229 g of solids, giving a concentration of 2.29 mg/mL of mucilage. From this, the concentrations of the S2 and S3 dispersions were 4.58 and 6.85 mg/mL, respectively.
For comparison, a commercial cutting fluid (Cimstar 60 (CIMCOOL® Industrial Products LLC)) was prepared as typically used in industry, namely, at 10 wt.% concentration in tap water. The Cimstar 60 is a semisynthetic oil-based cutting fluid, specially formulated with extreme pressure lubricating agents, used in the manufacturing process as cutting fluid, and grinding or metal forming fluid. In addition, due to its complex formulation, it confers lubricity properties and corrosion protection, while reducing foaming. The mucilage solutions (S1, S2, and S3) and the commercial cutting fluid (Cimstar 60) were characterized and compared by rheometry, tribology, thermogravimetric analysis, and turning tests, as described below.
2.2. Determination of the weight-average molecular weight (MW) of the mucilage
The molecular weight (MW) of macromolecules is one of the main parameters determining the rheological behavior of their solutions; the higher MW the more viscoelastic solutions. The MW of the mucilage utilized in this work was determined with the S3 solution by static light scattering (SLS) using an Abbemat 550 refractometer (Anton Paar) and a LiteSizer 500 device (Anton Paar). Full details of the procedure for MW determination may be found elsewhere [33]. The measurements resulted in MW =3.618x106 g/mol, which is consistent with the molecular weight measured for mucilage from young nopal pads (~ 100 days old) [33].
2.3. Rheological testing of the fluids
The three aforementioned nopal mucilage solutions and the Cimstar 60 cutting fluid were characterized by rheometrical experiments using a stress-controlled rotational rheometer (MCR 302, Anton Paar), by shear rate ramps. These were conducted in the range from 0.5 to 500 s-1 for the nopal mucilage solutions using a standard concentric cylinder flow geometry (Rinner= 13.33 mm, Router= 14.563 mm, and L=40 mm). In addition, a cone and plate geometry with D=50 mm and an angle of 2° was used to measure the first normal stress difference (N1) of the solutions. Due to its lower viscosity as compared to mucilage solutions, the Cimstar 60 fluid was characterized in the range from 1-100 s-1, using a double-gap flow geometry (R1=11.914 mm, R2=12.324 mm, R3=13.331mm, R4=13.795, and L=40 mm), which is intended to test low-viscosity samples. In each case, the flow geometry and fluid temperature were controlled at 25±0.01°C by using a Peltier system with a Platinum thermocouple (type S). Flow experiments were performed in triplicate to assure reproducibility.
2.4. Tribological testing
The extreme pressure tribological/lubricity properties of the mucilage solutions were obtained by conducting ASTM D4172 standard four-ball tests [36] under the conditions presented in Table 1. A schematic view of the test set-up can be seen in Figure 1. The four-ball test consists of rotating an AISI 52100 steel ball against other three similar stationary balls immersed in the fluid sample under a specified load, speed, temperature, and time. The coefficient of friction (CoF) and fluid temperature changes are measured by the tester. To compare the performance of mucilage solutions as cutting fluids, these and pure water (type 1), as well as the Cimstar 60 were tested under the same conditions. All the tests were initiated at room temperature (25±1°C). Since fluid temperature rises due to friction during the tribological test, each test was stopped either till it reached a maximum test time of 35 minutes or once the fluid temperature reached 100°C; this to avoid fluid evaporation and balls welding. The CoF was monitored during the whole test and then averaged for further analysis. After finishing each test, the wear scar diameter (WSD) generated in the three lower balls was measured by optical microscopy and then averaged. In order to compare the wear performance of each fluid, the WSD per unit time was calculated by dividing the mean WSD by each corresponding test time.
2.5. Turning test
The performance of the mucilage solutions as cutting fluid was assessed in actual turning processes under minimum quantity lubrication (MQL) conditions, following the methodology schematically represented in Figure 2. Dry cutting tests as well as lubricated cutting tests with pure water (type 1), Cimstar 60 and the mucilage solutions were also conducted for comparison purposes. The reason for selecting MQL is that it is nowadays considered as one of the greenest lubrication and cooling techniques for cutting processes [37]. MQL consists of applying the minimum dose of MWF required to lubricate and cool down the workpiece and cutting tool interface along with an air-assisted jet (tiny droplets of the MWF are dispersed in the air jet at high speed and applied to the cutting interface) [12]. The cutting tests were performed in a 5.5 HP Lathe (Pinacho SP 200) under the lubrication and cutting parameters given in Table 2. These MQL parameters were set on the basis of the cutting tool supplier recommendations for steel finishing operations. Steel (AISI 1018) was selected to prepare the workpieces since it is a relatively hard (116 HB) and common material. For each test, a new coated carbide insert was used as a cutting tool. The cutting performance of the fluids was determined by analyzing cutting forces (Fz), which can be related to energy consumption, workpiece finishing (in terms of surface roughness, Ra) and cutting interface temperature. Fz values were measured during the machining operation using a piezo-electric dynamometer (Kistler Type 9121), a dual-mode amplifier (Kistler type 5814B1), and a data acquisition device (NI-USB 6008). The resulting surface finishing of the workpieces was evaluated by measuring roughness (Ra) using a contact profilometer (Surfcom 130A) by taking the average of three roughness readings along the longitudinal direction with a 120° offset. The cutting interface temperature was monitored using a thermal image camera (FLIR TG 165) during the entire test. The temperature change was determined as the difference between the cutting interface temperature before and after the test.
2.6. Thermal stability analysis
The thermal stability of nopal mucilage solutions and the Cimstar 60 was investigated by thermogravimetric analysis, TGA (STAi 1000, Instruments Specialists Incorporated), from 30 to 200 °C using a temperature ramp of 10 °C/min and nitrogen atmosphere. Thermal stability indicates the thermal resistance and thermal degradation during the use of MWFs. For this, the samples were placed in platinum pans, and the weight change was determined using a balance with 0.1 μg of resolution. At the same time, the temperature was measured with a type R thermocouple with 0.1 °C of resolution.
3. Results and Discussion
3.1. Rheological behavior of nopal mucilage solutions and cutting fluid
The shear viscosity as a function of the shear rate for the mucilage solutions and Cimstar 60 fluid is presented in Figure 3, which also includes the first normal stress difference measured as a function of the shear rate for the S3 solution. First, it can be seen that mucilage solutions behave as non-Newtonian shear-thinning fluids, and that their viscosity increases with increasing mucilage concentration. Also, the shear-thinning behavior is more marked for higher mucilage concentrations, and can be well fitted by the Cross model (continuous lines in Figure 3) given by [33]:
where and are the viscosity values at zero and infinite shear rate, , respectively, and and are the other fitting parameters of the Cross model reported in Table 3. Another shear-tinning model that covers similar flow regimes, i. e., Newtonian plateaus in the low and high shear rate regions with a shear-tinning intermediate one, is the Carreau model, which has been used for calculations of the film thickness in different lubrication situations [22,38]. It is noteworthy that both mentioned rheological model are purely shear-tinning, so they do not consider the elastic behavior of the fluids. In this regard, only solution S3 exhibited measurable elasticity or normal stresses (N1), which scale in a power-law fashion with the shear rate:
Even though the dependence of on the shear rate is not very strong, Eq. 3 indicates that the elasticity of S3, that may be seen as a lifting effect [39,40], increases with the shear rate and may become significant at the very high shear rates (>106) found in lubrication. For the studied system both, the thickening effect of mucilage macromolecules and the elasticity of their solutions depend on their MW and concentration in solution; the greater these values the more pronounced the effects. On its side, MW depends on the age of the cladodes, the older the cladode the higher MW [33]. The MW calculated in this work for the obtained mucilage was 3.618x106 g/mol, which is consistent with that of young cladodes (~ 100 days old). In this respect, older nopal cladodes (> one year old), which in general have less practical use because of their high content of fiber and minerals [30], could be exploited to obtain highly elastic and thickening mucilage.
On the other hand, the Cimstar 60 fluid (Figure 3) exhibits a purely viscous Newtonian behavior (i.e., constant viscosity without elasticity), as is the case for most conventional and commercial MWFs, due to their main content of mineral or synthetic oil. It also exhibits lower viscosity than the three mucilage solutions for similar shear rates.
3.2. Tribological properties
Figs. 4a-c show the tribological behavior of the mucilage solutions in terms of WSD per unit time, CoF, and the final fluid temperature reached in the four-ball test. The error bars represent the standard deviation obtained from four test repeats. The temperature of the fluid rises because of friction through the test and the fluid thermal properties. According to Figure 4c, Cimstar 60 was the only fluid that resisted for 35 minutes without reaching 100 °C. The rest of the fluids reached 100 °C before 35 minutes of testing. Water (type 1) reached 100 °C after only 3 minutes, while S1, S2 and S3 reached this temperature after 10, 19 and 25 minutes of testing, respectively. This behavior is ascribed to the CoF behavior produced by each fluid, as shown in Figs. 4b and 5; the larger the CoF, the higher the temperature and the less testing time. Following tribological tests, the mean WSD was measured and divided by each corresponding test time in order to obtain the WSD per unit time, which is reported in Figure 4a. This is useful to compare the wear amount caused by the different fluids. Water (type 1) exhibited the largest WSD per unit time, while Cimstar 60 exhibited the lowest. According to the WSD per unit time results of the mucilage-based fluids, S1 exhibited the largest WSD while S3 exhibited the lowest. This means that increasing the content of mucilage in the solutions helps to reduce CoF and wear, which is associated with the increase in viscosity and elasticity, as well as the thermal stability that the mucilage provides to water for longer times. Also, it is noteworthy that the CoF of Cimstar 60 and S3 are comparable, being overall lower for S3, meaning that this concentration of mucilage is able to produce a similar level of lubricity as the commercial semisynthetic oil-based cutting fluid under extreme pressure conditions.
On the other hand, although it is well known that the viscosity of the lubricant is the main contributor to maintain the lubricating film thickness, the elasticity [38] and the presence of lipids with saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) of the mucilage in solution [41,42] may also contribute to improve lubricity. Depending on the age of the cladodes, the extracted mucilage can contain between 2.3-4.4 g 100 g-1 of lipids (with different fatty acids including SFAs and MUFAs), these decreasing with increasing cladodes age [43]. For similar young cladodes (~ 100 days old) the lipids content has been measured as ~4% wt., so these lipids may be acting synergistically with the viscoelastic properties of the mucilage solutions to produce good lubricating properties.
3.3. Turning performance
Figure 6 shows the cutting forces for the dry and MQL conditions using all the fluid samples. The cutting force represents the force required for cutting the workpiece (metal bar) in turning; the lowest cutting force, the best MWF performance and the lowest energy consumption. As expected, the highest and lowest cutting forces were measured under dry conditions (force about 155N) and when Cimstar 60 (force about 113N), respectively. In the case of the mucilage solutions, the cutting force shows a slight but systematic decrease with the mucilage content as compared to Water (type 1); S3 exhibiting the lowest force (about 120N). This result is ascribed to the higher viscosity and shear-thinning behavior of S3 (see Figure 3), which allows it to remain longer in the cutting region, as well as to the low CoF produced due to the fatty acids content and elasticity of this solution (see Figure 4b).
The obtained quality of the surface finish (surface roughness, Ra) after the cutting process and the increase in temperature (ΔT) at the cutting interface are shown in Figs. 7a and 7b, respectively. It was found that all the fluids tested generated similar workpiece surface quality/roughness (around 4µm, Ra) and it was about 35% lower than that produced under dry conditions, as shown in Figure 8a. Moreover, Cimstar 60, S2 and S3 presented the lowest reduction (about -1.8°C) in cutting interface temperature, as shown in Figure 7b, which means that these fluids promoted similar cooling performance.
3.4. Thermal stability
The thermogravimetric analysis results of the nopal mucilage solutions and Cimstar 60 fluid are shown in Figure 8. It can be seen that, in the analyzed temperature range, the most stable fluid was Cimstar 60, followed by the nopal mucilage solutions and water, this last completely evaporating at 95 °C. The Cimstar 60 fluid exhibited a weight loss of 44% at 107 °C while the same value was observed for S3 at 97 °C, S2 at 96 °C and S1 at 94 °C. For water, the same weight loss was observed at 76 °C. On the other hand, the Cimstar 60 fluid exhibited a thermal instability in the range of 107 to 126 °C, evidenced by oscillations of the weight loss and a rapid change from 44 to 95%. This thermal instability may be attributed to the breakage of the emulsion induced by the thermal degradation of the lipophilic phase and rapid evaporation of water. On its side, the nopal mucilage solutions evaporate entirely in the range from 113 to 119 °C, with the higher evaporation temperature for the most concentrated solution, S3, being this temperature 23 °C higher than pure water and 7 °C lower than Cimstar 60 fluid. Thus, the thermogravimetric analysis indicates that if the working temperature of the metalworking application is below 100 °C, the nopal mucilage solutions could be used as MWF safely since its thermal performance is comparable to the commercial cutting fluid (Cimstar 60) in this temperature range. Should it be necessary to enhance even more the thermal stability of nopal solutions, this may be achieved, for example, by adding nanoparticles, as functionalized graphene nanoplatelets [44], nano ZrO2 [45] or other additives for synthetic base oils.
In summary, the results obtained from rheology, tribology, thermal stability and turning tests suggest that mucilage-based viscoelastic solutions exhibit good characteristics to be used as conventional cutting fluid for MQL operations. In particular, S3 (the solution with the highest mucilage content) showed the most pronounced viscoelastic behavior and similar performance to the commercial MWF (Cimstar 60), which reveals the influence of the fatty acids composition of the mucilage and the elastic behavior of the solution in this lubrication process. In addition, considering the facts that mucilage solutions were produced by green chemistry methods using sustainable raw materials (water and nopal mucilage) and are innocuous and totally biodegradable, mucilage viscoelastic solutions represent potential alternative MWFs for industrial applications meeting the current global stringent demands of green manufacturing [12,15]. Notwithstanding, apart from the cutting/turning processes analyzed in this work, this kind of solutions need to be further evaluated to demonstrate their effectiveness in other types of cutting operations such as drilling, milling, etc. Finally, a more systematic and mechanistic evaluation of the isolated effect of elasticity of viscoelastic fluids on the tribological and metal cutting performance is necessary, this is topic of ongoing research in our group.
4. Conclusions
The main conclusions derived from this work can be summarized as follows:
- Three solutions of water with different concentrations (2.29, 4.58 and 6.85 mg/mL) of nopal mucilage and 0.6 g of benzoic acid, as a preservative to inhibit the growth of microorganisms, were effectively produced through green chemistry techniques.
- Mucilage solutions were found to behave as non-Newtonian shear-thinning fluids, whose viscosity increases with increasing mucilage concentration. On the other hand, the reference MWF (Cimstar 60) exhibited a Newtonian viscous behavior with lower viscosity than the mucilage solutions.
- The solution with the highest mucilage content exhibited, in addition, viscoelastic behavior, which resulted in the lowest wear, friction and temperature in comparison to the other solutions and neat water in the extreme pressure four-ball tests. This is associated to the viscosity and elasticity of mucilage solutions. Besides, the solution with the highest concentration of mucilage was able to produce a similar level of lubricity as compared to the commercial semisynthetic oil-based MWF, which was ascribed to the viscoelastic behavior and content of lipids with fatty acids in the mucilage. These results provide insight into the relevance of the viscoelastic behavior of the lubricant, elasticity in particular, in the lubrication process, an influence that deserves further attention.
- According to the metal cutting tests, the cutting force decreased with increasing the mucilage content, which is associated with both the increase in viscosity and decrease of CoF promoted by the increase in mucilage content and viscoelastic behavior in the solutions. Also, it was found that the three solutions generated similar workpiece surface quality/roughness (around 4µm, Ra) and interface cool down than that produced by Cimstar 60.
- The thermogravimetric analysis indicated that the nopal mucilage solutions could be safely used as MWF below 100 °C since its thermal performance is comparable to the commercial cutting fluid (Cimstar 60) at these temperatures.
- Finally, it was demonstrated that the solution with the highest mucilage content presented similar lubricating effects to those of a commercial semisynthetic oil-based cutting fluid (Cimstar 60), making it a potential innocuous green cutting fluid for MQL operations.
Author Contributions
L.I.F.-C.: Conceptualization; investigation; writing—original draft preparation; writing—review and editing; formal analysis; project administration. O.A.A.-R: investigation; writing—original draft preparation; data curation. J.P.-G.: conceptualization; investigation; writing—review and editing; formal analysis; project administration. B.M.M.-S.: investigation; writing—review and editing; data curation. F.R.-G.: investigation; writing—review and editing; data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Oscar A. Aguilar-Rosas thanks the Mexican National Council of Humanities, Science, and Technology (CONAHCYT) and Tecnologico de Monterrey for the scholarships received for conducting part of this research project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, S.-S., Song, J.-J., Xu, P., He, M., Xu, M.-B., & You, F.-C. (2023). A Review on Tribology Characterization and Lubricants for Water-Based Drilling Fluids. [CrossRef]
- Rahman, M. H., Warneke, H., Webbert, H., Rodriguez, J., Austin, E., Tokunaga, K., Rajak, D. K., & Menezes, P. L. (2021). Water-based lubricants: Development, properties, and performances. Lubricants, 9(8), 73. [CrossRef]
- Menezes, P.L., Ingole, S.P., Nosonovsky, M., Kailas, S.V., Lovell, M.R. (2013). Tribology for Scientists and Engineers; Springer: New York, NY, USA.
- Lu, Q., Zhang, T., He, B., Xu, F., Liu, S., Ye, Q., & Zhou, F. (2022). Enhanced Lubricity and anti-wear performance of zwitterionic polymer-modified N-enriched porous carbon nanosheets as water-based lubricant additive. Tribology International, 167, 107421. [CrossRef]
- Yang, X., Jiang, G., Liu, F., He, Y., Liu, R., & Dong, T. (2023). Lubricity and mechanism of Catechol-based biomimetic lubricant in water-based drilling fluid. Tribology International, 188, 108862. [CrossRef]
- Wu, H., Zhao, J., Xia, W., Cheng, X., He, A., Yun, J. H., Wang, L., Huang, H., Jiao, S., Huang, L., Zhang, S., & Jiang, Z. (2017). A study of the tribological behaviour of tio2 nano-additive water-based lubricants. Tribology International, 109, 398–408. [CrossRef]
- Liu, C., Guo, Y., & Wang, D. (2019). Pei-RGO nanosheets as a nanoadditive for enhancing the tribological properties of water-based lubricants. Tribology International, 140, 105851. [CrossRef]
- Sagraloff, N., Dobler, A., Tobie, T., Stahl, K., & Ostrowski, J. (2019). Development of an oil free water-based lubricant for Gear Applications. Lubricants, 7(4), 33. [CrossRef]
- Wijanarko, W., Khanmohammadi, H., & Espallargas, N. (2022). Ionic liquid additives in water-based lubricants for bearing steel – effect of electrical conductivity and ph on surface chemistry, friction and Wear. Frontiers in Mechanical Engineering, 7. [CrossRef]
- Aguilar-Rosas, O., Blanco, S., Flores, M., Shirai, K., & Farfan-Cabrera, L. I. (2022). Partially deacetylated and fibrillated shrimp waste-derived chitin as biopolymer emulsifier for green cutting fluids—towards a cleaner production. Polymers, 14(3), 525. [CrossRef]
- Katna, R., Suhaib, M., & Agrawal, N. (2019). Nonedible vegetable oil-based cutting fluids for machining processes–A Review. Materials and Manufacturing Processes, 35(1), 1–32. [CrossRef]
- Farfan-Cabrera, L. I., Rojo-Valerio, A., Calderon-Najera, J. de, Coronado-Apodaca, K. G., Iqbal, H. M. N., Parra-Saldivar, R., Franco-Morgado, M., & Elias-Zuñiga, A. (2023). Microalgae oil-based metal working fluids for sustainable minimum quantity lubrication (MQL) operations—A perspective. Lubricants, 11(5), 215. [CrossRef]
- Nee, A.C., (2015). Handbook of Manufacturing Engineering and Technology. Springer Reference.
- Li, K., Aghazadeh, F., Hatipkarasulu, S., & Ray, T. G. (2003). Health risks from exposure to metal-working fluids in machining and grinding operations. International Journal of Occupational Safety and Ergonomics, 9(1), 75–95. [CrossRef]
- Wickramasinghe, K. C., Sasahara, H., Rahim, E. A., & Perera, G. I. P. (2020). Green metalworking fluids for sustainable machining applications: A Review. Journal of Cleaner Production, 257, 120552. [CrossRef]
- Bambam, A. K., Dhanola, A., & Gajrani, K. K. (2023). A critical review on halogen-free ionic liquids as potential metalworking fluid additives. Journal of Molecular Liquids, 380, 121727. [CrossRef]
- Amiril, S. A. S., Rahim, E. A., Embong, Z., & Syahrullail, S. (2018). Tribological investigations on the application of oil-miscible ionic liquids additives in modified jatropha-based metalworking fluid. Tribology International, 120, 520–534. [CrossRef]
- Nessakh, F. Z., Mutelet, F., & Negadi, A. (2022). Efficiency of two working fluids constituted of a deep eutectic solvent and water in absorption heat transformer. International Journal of Energy Research, 46(15), 23578–23594. [CrossRef]
- HAYASHI, H. (1991). Recent studies on fluid film lubrication with non-newtonian lubricants. JSME International Journal. Ser. 3, Vibration, Control Engineering, Engineering for Industry, 34(1), 1–11. [CrossRef]
- Marx, N., Fernández, L., Barceló, F., & Spikes, H. (2018). Shear thinning and hydrodynamic friction of viscosity modifier-containing oils. part I: Shear thinning behaviour. Tribology Letters, 66(3). [CrossRef]
- Bair, S. (1998). Elastohydrodynamic film forming with shear thinning liquids. Journal of Tribology, 120(2), 173–178. [CrossRef]
- Bair, S., Vergne, P., & Marchetti, M. (2002). The effect of shear-thinning on film thickness for space lubricants. Tribology Transactions, 45(3), 330–333. [CrossRef]
- Bair, S., Habchi, W., Sperka, P., & Hartl, M. (2015). Quantitative elastohydrodynamic film forming for a gear oil with complex shear-thinning. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 230(3), 289–299. [CrossRef]
- Bair, S., Khonsari, M., & Winer, W. O. (1998). High-pressure rheology of lubricants and limitations of the Reynolds equation. Tribology International, 31(10), 573–586. [CrossRef]
- Hutton, J. F., Jackson, K. P., & Williamson, B. P. (1986). The effects of lubricant rheology on the performance of journal Bearings. A S L E Transactions, 29(1), 52–60. [CrossRef]
- Veltkamp, B., Jagielka, J., Velikov, K. P., & Bonn, D. (2023). Lubrication with non-newtonian fluids. Physical Review Applied, 19(1). [CrossRef]
- Albuquerque, T.G., Pereira, P., Silva, M.A., Vicente, F., Ramalho, R., Costa, H.S. (2020). Chapter 44 - Prickly pear. A.K. Jaiswal (Ed.), Nutritional Composition and Antioxidant Properties of Fruits and Vegetables, Academic Press, pp. 709-728. [CrossRef]
- El-Mostafa, K., El Kharrassi, Y., Badreddine, A., Andreoletti, P., Vamecq, J., El Kebbaj, M., Latruffe, N., Lizard, G., Nasser, B., & Cherkaoui-Malki, M. (2014). Nopal cactus (opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules, 19(9), 14879–14901. [CrossRef]
- Stintzing, F. C., Schieber, A., & Carle, R. (2001). Phytochemical and nutritional significance of Cactus Pear. European Food Research and Technology, 212(4), 396–407. [CrossRef]
- Vargas-Solano, S.V., Rodríguez-González, F., Martínez-Velarde, R., Campos-Mendiola, R., Hurtado-Salgado, M.A., Muthuswamy Ponniah, J. (2022a). Chemical composition of pear cactus mucilage at different maturity stages. Agrociencia. [CrossRef]
- Trachtenberg, S., & Mayer, A. M. (1981). Composition and properties of Opuntia ficus-indica mucilage. Phytochemistry, 20, 2665–2668. [CrossRef]
- Goycoolea, M., Cárdenas, A. (2003). Pectins from Opuntia spp.: a short review. Journal of the professional association for cactus development, 5, 17-29. [CrossRef]
- Rodríguez-González, F., Pérez-González, J., Muñoz-López, C. N., Vargas-Solano, S.V. & Marín-Santibáñez, B.M. (2021). Influence of age on molecular characteristics and rheological behavior of nopal mucilage. Food Science & Nutrition, 9(12), 6776-6785. [CrossRef]
- Tosif, M. M., Najda, A., Bains, A., Kaushik, R., Dhull, S. B., Chawla, P., & Walasek-Janusz, M. (2021). A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for Nanocarrier Fabrication. Polymers, 13(7), 1066. [CrossRef]
- Maki-Díaz, G., Peña-Valdivia, C. B., García-Nava, R., Arévalo-Galarza, M. L., Calderón-Zavala, G., & Anaya-Rosales, S. (2015). Características físicas y químicas de nopal verdura (Opuntia ficus-indica) para exportación y consumo nacional. Agrociencia, 49(1), 31-51.
- ASTM. (2021). Standard D4172-21, Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid (Four-Ball Method), ASTM International, West Conshohocken, PA, 2021. www.astm.org.
- Singh, G., Aggarwal, V., & Singh, S. (2020). Critical review on ecological, economical and technological aspects of minimum quantity lubrication towards sustainable machining. Journal of Cleaner Production, 271, 122185. [CrossRef]
- Bair, S., & Qureshi, F. (2003). Ordinary shear thinning behavior and its effect upon EHL film thickness. Tribology Series, 693–699. [CrossRef]
- Wang, Y., Azam, A., Zhang, G., Dorgham, A., Liu, Y., Wilson, M. C., & Neville, A. (2022). Understanding the mechanism of load-carrying capacity between parallel rough surfaces through a deterministic mixed lubrication model. Lubricants, 10(1), 12. [CrossRef]
- Hu, S., Meng, F., & Doi, M. (2023). Effect of fluid viscoelasticity, shear stress, and interface tension on the lift force in lubricated contacts. The Journal of Chemical Physics, 159(16). [CrossRef]
- Farfan-Cabrera, L. I., Franco-Morgado, M., González-Sánchez, A., Pérez-González, J., & Marín-Santibáñez, B. M. (2022). Microalgae biomass as a new potential source of sustainable green lubricants. Molecules, 27(4), 1205. [CrossRef]
- Zainal, N. A., Zulkifli, N. W. M., Gulzar, M., & Masjuki, H. H. (2018). A review on the chemistry, production, and technological potential of bio-based lubricants. Renewable and Sustainable Energy Reviews, 82, 80–102. [CrossRef]
- Ramadan, M. F., & Mörsel, J. T. (2003). Recovered lipids from prickly pear [opuntia ficus-indica (L.) mill] peel: A good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chemistry, 83(3), 447–456. [CrossRef]
- Aguilar-Rosas, O.A., Alvis-Sánchez, J.A., Tormos, B., Marín-Santibáñez, B.M., Pérez-González, J., & Farfan-Cabrera, L.I. (2023). Enhancement of low-viscosity synthetic oil using graphene nanoparticles as additives for enduring electrified tribological environments. Tribology International, 188, 108848. [CrossRef]
- Nagabhooshanam, N., Baskar, S., Prabhu, T. R., & Arumugam, S. (2020). Evaluation of tribological characteristics of nano zirconia dispersed biodegradable canola oil methyl ester metalworking fluid. Tribology International, 151, 106510. [CrossRef]
Figure 1.
Tribological (four-ball) test set-up (ASTM D4172).
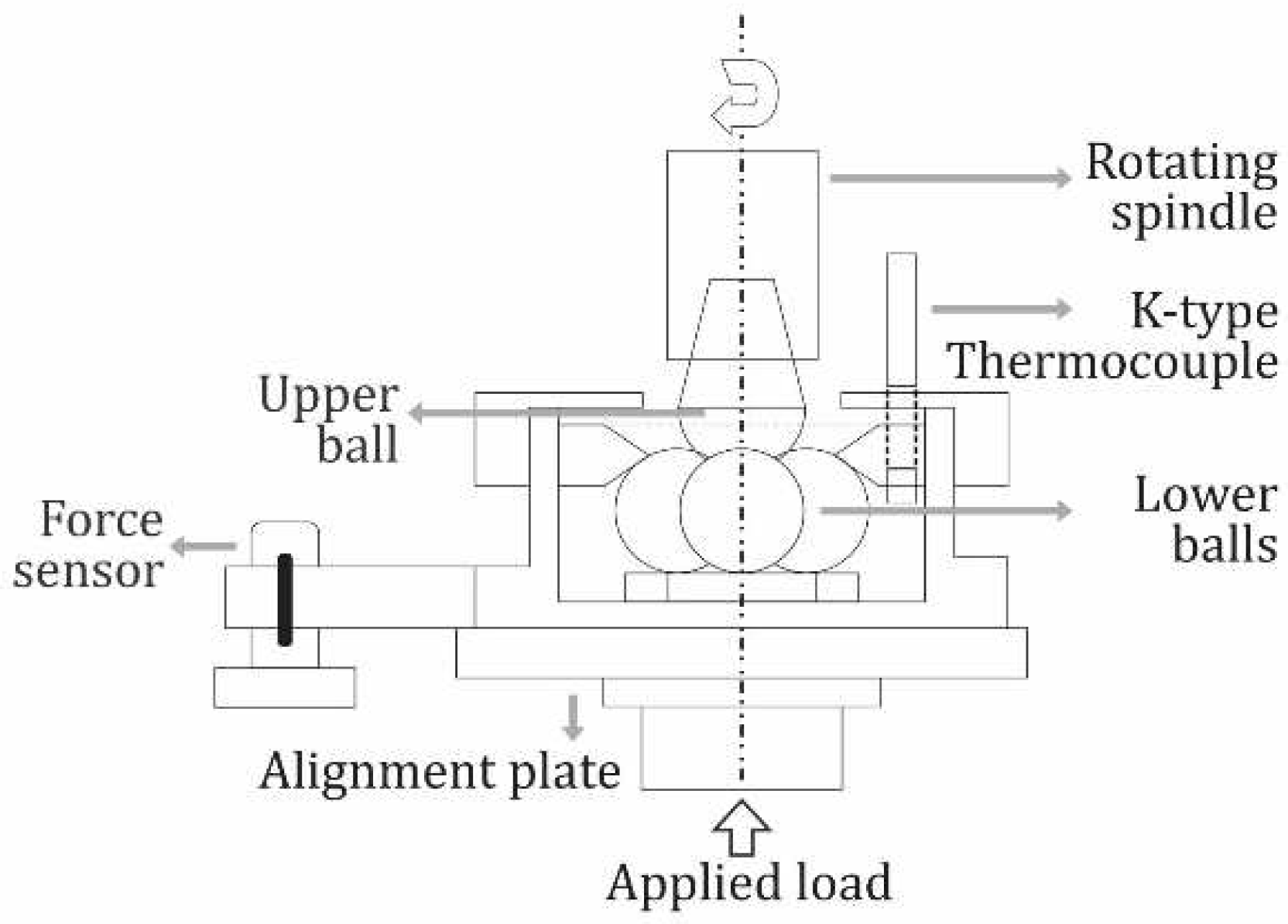
Figure 2.
Schematic representation of the cutting performance test methodology.

Figure 3.
Shear viscosity of nopal mucilage solutions and the cutting fluid as a function of shear rate, and N1 versus shear rate for S3. Continuous lines indicate the fittings of the shear viscosity of nopal mucilage solutions to the Cross model, while the dotted line indicates the fitting of cutting fluid to the Newtonian model.
Figure 3.
Shear viscosity of nopal mucilage solutions and the cutting fluid as a function of shear rate, and N1 versus shear rate for S3. Continuous lines indicate the fittings of the shear viscosity of nopal mucilage solutions to the Cross model, while the dotted line indicates the fitting of cutting fluid to the Newtonian model.
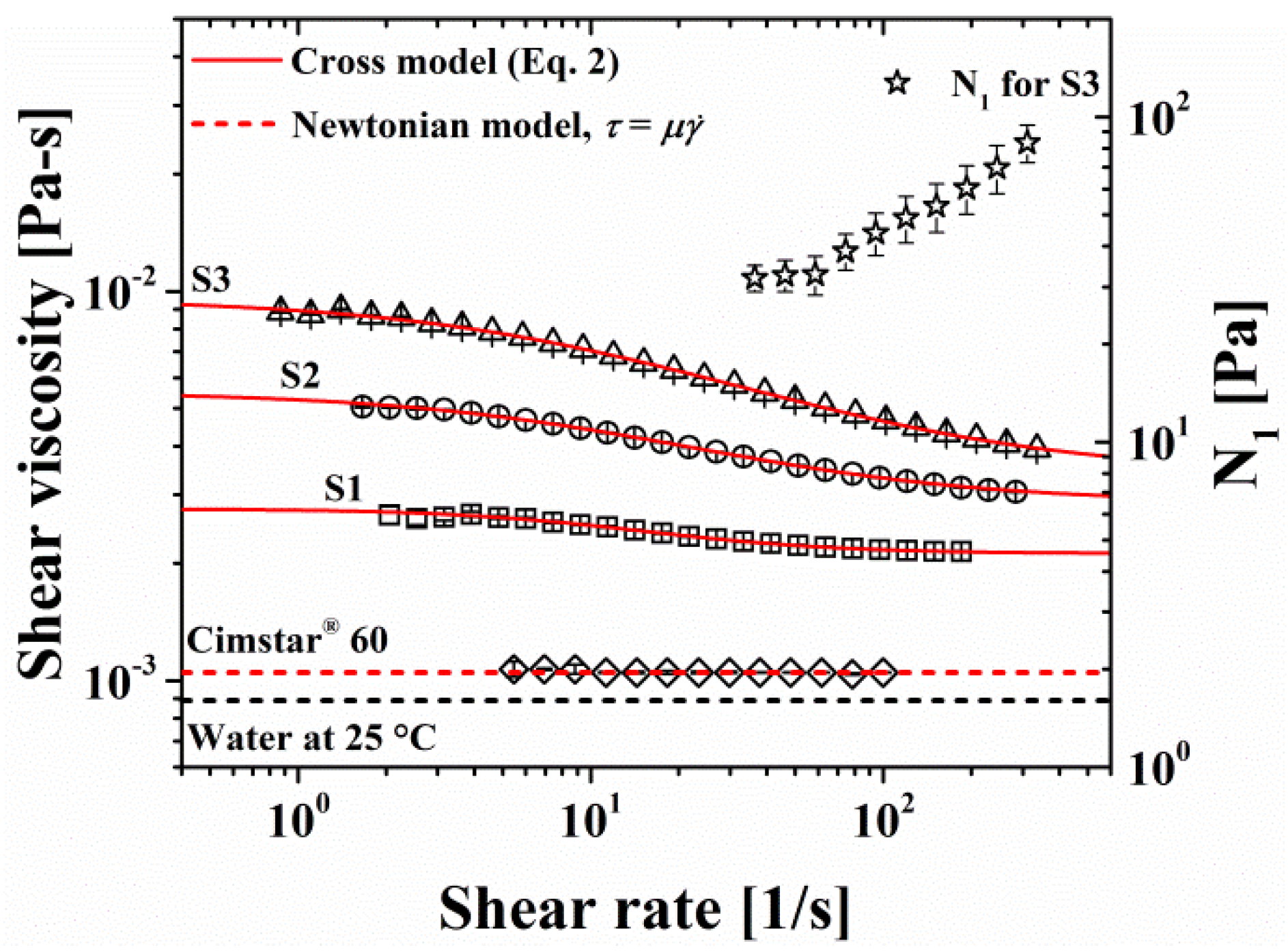
Figure 4.
Tribological results of the nopal mucilage solutions and the cutting fluid in terms of: (a) WSD per unit time (µm/s); (b) CoF; and (c) final fluid temperature (°C). t values in (c) represent the duration of the test for each fluid.
Figure 4.
Tribological results of the nopal mucilage solutions and the cutting fluid in terms of: (a) WSD per unit time (µm/s); (b) CoF; and (c) final fluid temperature (°C). t values in (c) represent the duration of the test for each fluid.

Figure 5.
CoF evolution during lubrication with nopal mucilage solutions and the cutting fluid.

Figure 6.
Cutting forces generated by the nopal mucilage solutions and the cutting fluid under dry and MQL conditions.
Figure 6.
Cutting forces generated by the nopal mucilage solutions and the cutting fluid under dry and MQL conditions.

Figure 7.
(a) Surface roughness (Ra, µm), and (b) temperature change (Δ °C) at the cutting interface obtained by using the nopal mucilage solutions and the cutting fluid.
Figure 7.
(a) Surface roughness (Ra, µm), and (b) temperature change (Δ °C) at the cutting interface obtained by using the nopal mucilage solutions and the cutting fluid.

Figure 8.
Thermogravimetric analysis of nopal mucilage solutions and the cutting fluid.

Table 1.
Tribological testing parameters.
| Parameter | Value | |
| Fluid sample quantity (mL) | 25 | |
| Starting temperature (°C) | 25±1 | |
| Relative humidity (%) | 36 | |
| Machine speed (RPM) | 1200 | |
| Load (kg) | 40 | |
| Length of lever arm (cm) | 5.275 | |
| Test duration (min) | Water (type 1) | 3 |
| Cimstar 60 in tap water | 35 | |
| S1 | 10 | |
| S2 | 19 | |
| S3 | 25 | |
| Test repeats | 3 | |
Table 2.
Metal cutting test parameters.
| Parameter | Type/value | |
| Lubrication | Lubrication type | MQL |
| Fluid feed (m3/h) | 4x10-5 | |
| Air pressure (MPa) | 0.4 | |
| Fluid nozzle diameter (mm) | 20 | |
| Nozzle-workpiece distance (mm) | 0.4 | |
| Cutting process | Workpiece material | AISI 1018 steel bars (25.4mm diameter) |
| Cutting tool | WNmG 080404e-Fm Grade T9325 (Pramet) coated carbide insert | |
| Surface Speed (m/min) | 70 | |
| Rotational Speed (RPM) | 860 | |
| Feed (mm/rev) | 0.15 | |
| Cutting time (s) | 39±2 | |
| Depth of Cut (mm) | 0.5 | |
| Room temperature (°C) | 25±1 | |
| Test repeats | 4 | |
Table 3.
Cross-model parameters for nopal mucilage solutions.
| Nopal mucilage solutions | Cross model parameters | |||
| η0 [mPa-s] | η∞ [mPa-s] | c [s] | p [1] | |
| S1 | 2.76±0.019 | 2.12±0.004 | 0.073±0.003 | 1.26±0.04 |
| S2 | 5.52±0.059 | 2.87±0.048 | 0.067±0.002 | 0.832±0.039 |
| S3 | 9.61±0.119 | 3.38±0.043 | 0.062±0.002 | 0.751±0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Viscoelastic Water-Based Lubricants with Nopal Cactus Mucilage as Green Metalworking Fluids
Leonardo Israel Farfan-Cabrera
et al.
,
2024
Thermo-Oxidative Stability and Tribological Characteristics of Bio-Based Lubricants Synthesized Using Isoamyl Alcohol
M. Marliete F. Melo Neta
et al.
,
2023
Lubricating Greases from Fried Vegetable Oils
Olga Valerica Săpunaru
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









