Preprint
Review
Integrated Management of Pathogens and Microbes on Cannabis sativa L. (Cannabis) under Greenhouse Conditions
Altmetrics
Downloads
172
Views
95
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
05 February 2024
Posted:
06 February 2024
You are already at the latest version
Alerts
Abstract
The increased cultivation of high THC-containing Cannabis sativa L. (cannabis), particularly in greenhouses, has resulted in a greater incidence of diseases and molds that can negatively affect the growth and quality of the crop. Among them, the most important diseases are root rots (Fusarium and Pythium spp.), Botrytis bud rot (Botrytis cinerea), powdery mildew (Golovinomyces ambrosiae), cannabis stunt disease (caused by Hop latent viroid), and a range of microbes that reduce post-harvest quality. An integrated management approach to reduce the impact of these diseases/microbes requires combining different approaches that target the reproduction, spread and survival of the associated pathogens, many of which can occur on the same plant simultaneously. These approaches will be discussed in the context of developing an integrated plan to manage the important pathogens of greenhouse-grown cannabis at different stages of plant development. These stages include maintenance of stock plants, propagation through cuttings, vegetative growth of plants, and flowering. The cultivation of cannabis genotypes with tolerance or resistance to various pathogens is a very important approach, followed by the maintenance of pathogen-free stock plants. When combined with cultural approaches (sanitation, management of irrigation, and monitoring for diseases) and environmental approaches (greenhouse climate modification), a significant reduction in pathogen development and spread can be achieved. The use of preventive applications of microbial biological control agents and reduced risk biorational products can also reduce disease development at all stages of production in jurisdictions where they are registered for use. The combined use of promising strategies for integrated disease management on cannabis plants during greenhouse production will be reviewed. Future areas for research are identified.
Keywords:
Subject: Biology and Life Sciences - Plant Sciences
1. Introduction
Integrated disease management (IDM) incorporates the coordinated use of multiple approaches to reduce the impact of disease-causing agents (pathogens) on agricultural crops [1]. When applied in parallel or consecutively, these tactics can achieve control of multiple pathogens using different and sometimes synergistic suppression tactics. IDM builds upon the concept of Integrated Pest Management (IPM), which has been widely utilized for decades to target and manage insect pests on agricultural crops, and requires different strategies to be employed in a coordinated manner, often with resounding success [2,3]. When IDM approaches are considered for cannabis (Cannabis sativa L., high THC-containing genotypes) grown under greenhouse conditions, several aspects need to be modified from traditional IDM programs. First and foremost is the fact that there are no synthetic fungicides available for use on cannabis crops, thus eliminating a widely-used disease management strategy. Instead, only reduced risk “biological” and “biorational” products are permitted. These products are mostly protective in action i.e. non-fungicidal, so they are best suited for preventative applications, although some products can also be deployed as sanitizers. While claims of product efficacy and applications for disease reduction on cannabis are often made, not all are supported by data from replicated research trials or third-party evaluations. This adds to the difficulty in identifying the specific IDM approaches that are best suited for each pathogen. The recent expansion of hemp cultivation (C. sativa, low THC-containing cultivars) in the USA following federal government approval should provide useful information on disease and pest management approaches which could be extended to cannabis [4]. The lack of synthetic fungicides for cannabis production has prompted the registration of several biological control products which can be used at different stages of production [5,6]. However, efficacy data for these products are often not available, and the modes of action of the biocontrol agents are not often fully understood, in the context of cannabis IDM, highlighting the need for further research in this area [6,7]. Fortunately, efficacy data may exist for many of these products on other crops e.g., for organic production, and can likely be extrapolated to cannabis crops [8]. A second challenge for IDM development in cannabis is that highly-bred cultivars containing specific resistance genes against important pathogens are lacking. Instead, genetic selections (genotypes) that target higher yields of inflorescences and THC content, and which display unique morphological traits, have been made a priority [9]. In most instances, these efforts have excluded the specific incorporation of disease resistance traits. Consequently, some high-yielding genotypes frequently show high susceptibility to various pathogens, as will be illustrated in this review. Fortunately, the broad genetic variation that currently exists among cannabis genotypes has led to the identification of resistance in various genotypes to specific pathogens, such as powdery mildew [6,10,11,12]. The mechanisms underlying this resistance are currently under investigation [13].
A third challenge is that when cannabis is compared to other widely-grown greenhouse crops, such as tomatoes, cucumbers, and peppers, the optimal cultural and environmental conditions for cultivation have not yet been fully established. Since different cannabis greenhouse operations can experience variable growing conditions, standardized research trials are needed to establish these parameters. Recent research has identified integral aspects of controlled environment cultivation practices that can be used as a baseline reference [14,15]. The prevalent pathogens affecting cannabis crops in greenhouses have been recently characterized and described [7], providing diagnostic information that is required for IDM implementation. Accurate diagnosis of the pathogen(s) involved in a disease syndrome is an important component of IDM and several diagnostic methods have been described [4,7,16,17,18,19]. In this review article, we describe the most important pathogens of cannabis crops cultivated under greenhouse conditions and highlight the various growth stages at which IDM approaches can be implemented during the crop production cycle, which generally occurs over 12-15 weeks (Figure 1).
The first stage of production of a cannabis crop is stock (mother) plant cultivation (Figure 2a), which provides a source of vegetative cuttings (Figure 2b). Once rooted, these are transferred to greenhouse growing conditions for 2-3 weeks (Figure 2c). The developing vegetative plants are then transferred to flowering rooms for 8 weeks (Figure 2 d, e), after which time the inflorescences are harvested (Figure 2f).
During each crop production year, up to 4-5 cropping cycles may take place per greenhouse compartment. The IDM approaches that can be developed include selection of disease-tolerant genotypes, implementation of cultural practices, modification of environmental climate settings, and application of reduced risk products (Figure 3).
We also discuss aspects of the microbial colonization of cannabis inflorescences by yeasts and molds and propose IDM strategies to reduce the total microflora present. Monitoring of microbial colonization of inflorescences is an important quality aspect for cannabis which is under strict regulatory control and presents a unique and challenging component of crop management that is not found in most other crops [19,20] This review should aid in the design or refinement of further IDM programs in greenhouse-cultivated cannabis operations. Detailed descriptions of the symptoms caused by various pathogens at different stages of cannabis growth during commercial production and the approaches that can be taken to manage them are described below.
2. Cannabis Pathogens: Symptoms and Management Approaches at Different Stages of Growth
2.1. Stock Cultivation Stage
Stock (mother) plants provide a source of vegetative cuttings which are commonly used in commercial cannabis production. These plants generally constitute a range of genotypes that are chosen for their desired phenotypic characteristics and biochemical profiles. They are grown in designated areas within the greenhouse or in separate indoor rooms. Physical separation of stock plants from larger-scale commercial production is important to prevent the spread of pathogens. The ages of these stock plants can vary, and typically range from 3 to 12 months, depending on the facility. In the context of disease development, older plants often exhibit signs of declining growth, such as reduced shoot growth, leaf yellowing, and poor root development (Figure 4a). These symptoms may be indicative of sub-lethal infections by Fusarium and Pythium spp. or Hop latent viroid (Figure 4 and Figure 5).
A closer inspection of the stems of diseased plants will often reveal internal discoloration in the pith and xylem tissues (Figure 4 b, c), a symptom of Fusarium infection, and/or root browning that can be caused by Fusarium or Pythium species [21-24]. Excessive waterlogging may also cause root browning on cannabis plants. Accurate pathogen diagnosis at this stage is critical to determine the most effective IDM strategies to implement. Stock plants are also susceptible to powdery mildew, visible as white colonies on the upper surfaces of leaves (Figure 4 g). A significant challenge in maintaining healthy stock plants is the recent emergence of hop latent viroid (HLVd) [25-27], which is mostly asymptomatic on stock plants but may cause occasional curling or mottling on the youngest leaves (Figure 4 h,i). The impact of HLVd infection on stock plants is seen when rooting frequency and vigor of cuttings derived from them are examined (Figure 5). HLVd infection leads to poor root growth (Figure 5 b) that continues to impact plant growth at the vegetative stage (Figure 5 c, d) and can also impact flowering (Figure 5 e). HLVd-infected flowering plants derived from infected stock plants display reduced inflorescence growth as well as lower levels of cannabinoid production [26]. This emphasizes the importance of maintaining pathogen-free stock plants during commercial production. Routine scouting for the presence of disease symptoms and testing of stock plants for the presence of HLVd, Fusarium and Pythium species is recommended.
Figure 5.
Symptoms of Hop latent viroid infection during propagation, vegetative growth and flowering stages of the cannabis crop cycle. (a) Infected stock plants may show unthrifty growth and smaller leaves. (b) Comparison of root development on cuttings derived from an HLVd-infected stock plant (left) and a healthy plant (right). (c) Vegetative plants may show curling and distortion of the youngest leaves. (d) Lateral branching may be seen on HLVd-infected vegetative plants. (e) Stunted growth of HLVd-infected flowering plant (left) compared to a healthy plant (right). (f, g) HLVd-infected inflorescence with yellowing compared to a healthy one, respectively. (h, i, j) Reduced inflorescence development in three different genotypes of cannabis resulting from HLVd infection. In all photos, the infected plant is shown on the left. (k) Dried inflorescences from an HLVd-infected plant (left) compared to a healthy plant (right).
Figure 5.
Symptoms of Hop latent viroid infection during propagation, vegetative growth and flowering stages of the cannabis crop cycle. (a) Infected stock plants may show unthrifty growth and smaller leaves. (b) Comparison of root development on cuttings derived from an HLVd-infected stock plant (left) and a healthy plant (right). (c) Vegetative plants may show curling and distortion of the youngest leaves. (d) Lateral branching may be seen on HLVd-infected vegetative plants. (e) Stunted growth of HLVd-infected flowering plant (left) compared to a healthy plant (right). (f, g) HLVd-infected inflorescence with yellowing compared to a healthy one, respectively. (h, i, j) Reduced inflorescence development in three different genotypes of cannabis resulting from HLVd infection. In all photos, the infected plant is shown on the left. (k) Dried inflorescences from an HLVd-infected plant (left) compared to a healthy plant (right).

2.2. IDM Approaches at Stock Cultivation Stage
During the stock plant cultivation stage, various IDM strategies can be implemented to minimise the development of plant pathogens. The following are examples of some commonly used practices.
2.2.1. Biosecurity and Quarantine Inspection
Biosecurity practices which include foot baths, wearing protective clothing, and removing pruned leaves and diseased plants are standard in most horticultural greenhouse operations [28]; these practices should be implemented for cannabis growing operations. In addition, it is important to establish a quarantine protocol in cases where plant materials, such as unrooted cuttings or whole plants, are brought in from an external source [2]. Such precautionary measures can prevent pathogen introduction and are standard biosecurity protocols in commercial crop production [29]. When applied to cannabis, this necessitates an isolation period for 3-4 weeks during which plants are monitored for disease symptoms and tested for the presence of potential viruses and other pathogens [6,7]. After the plants are confirmed to be free of detectable pathogens, they can be used for commercial propagation.
2.2.2. Cultural and Environmental Management
Environmental management is a component of IDM across all stages of cannabis growth since climatic conditions can influence both plant growth as well as pathogen growth. Standard cannabis cultivation environmental setpoints, which are established for baseline pathogen management during low disease pressure periods, have been described [15,30]. Conditions that are unfavorable for disease development while at the same time supporting optimal plant growth are required. This often necessitates maintaining lower humidity levels by enhancing venting, heating, and air circulation. Seasonal adjustments may also need to be made, as warmer temperatures with higher humidity in the summer months may increase the incidence of root-infecting pathogens, such as Fusarium and Pythium species. Similarly, cooler and more humid conditions during winter seasons may enhance the development of powdery mildew infections.
2.2.3. Testing for Pathogen Presence and Eradication
Early detection of disease symptoms on stock plants is important to prevent pathogens from spreading within the growing environment. There are several diagnostic approaches that have been described to detect cannabis pathogens and a number of commercial laboratories provide testing services for a range of pathogens [4,7,19,26]. The practice of culling and replenishing stock plants is a standard component of IDM programs when diseased plants are detected [31]. Stock plants should be replaced after several months of production with new, pathogen-free plants, which is key to maintaining a healthy and vigorous stock plant population. Plants infected with Fusarium, Pythium or HLVd should be promptly removed from a facility. Eradication of diseased plants, particularly those infected with HLVd, is essential. When regular (weekly) pathogen testing is followed by destruction of those plants infected by HLVd, a gradual decline in the occurrence of diseased stock plants can be achieved (Figure 6). After many rounds of testing performed over a 6-month period, this strategy was shown to reduce HLVd frequency on stock plants from 22% to 1% (Figure 6). Peaks of infection can still be seen which are attributed to the re-introduction of diseased plant material that went undetected initially and were inadvertently used as a source of cuttings. Removing them upon detection resulted in the continued downward trend of infection.
2.2.4. Sanitary Practices
Thorough sanitation of the growing environment before planting a new crop is important to reduce residual pathogen inoculum, which can be spread by water, air, on tools, and potentially on clothing, gloves, or shoes. This is a common practice used on most greenhouse crops, especially where viruses are of concern [6,28]. To reduce pathogen transmission, all surfaces and equipment, as well as gutters, tables, floors, drip emitters, and pots should be cleaned by using reduced risk sanitary products. These products include hydrogen peroxide with peracetic acid (Sanidate® or Zerotol®), dodecyl dimethyl ammonium chloride (Chemprocide® or KleenGrow®), isopropyl alcohol, and bleach [6]. The efficacy of these products in inhibiting pathogen growth can vary, depending on the pathogen, product and concentrations used. A comparison of two products used at four concentrations against growth of two pathogens is shown in Figure 7. At increasing concentrations, both Zerotol® and hypochlorous acid reduced pathogen growth but Pythium showed a greater sensitvity compared to Fusarium (Figure 7c). These products can potentially also negatively affect the growth of beneficial Trichoderma species when applied as biocontrol agents (Figure 7d). Therefore, care must be taken to consider the potential impact of applying reduced risk products in conjunction with biocontrol products. These types of evaluations are important to conduct for any reduced risk product targeted for the cannabis market to demonstrate efficacy and possible non-target effects.
2.2.5. Utilizing Disease Tolerant Genotypes
The utility of disease-tolerant genotypes that may have been developed through selective breeding and genotype screening is an important aspect of IDM for stock plants. Disease-tolerant genotypes of cannabis to a number of pathogens have been identified, including to Fusarium oxysporum [23], powdery mildew [10,11,12,32], Alternaria leaf blight [33] and Botrytis bud rot (34,35) (Figure 8). Recent research suggests that specific defense genes may play a role in certain host-pathogen interactions, leading to a resistant phenotype [11,12,13,36]. The impact of cannabis genotype on disease development at the flowering stage will be discussed later in this review.
2.3. Propagation Stage
Cannabis plants can be initiated from seeds or from vegetative cuttings which originate from stock plants, but the latter is more commonly used in commercial production, since large-scale propagation from seeds is less common. Routine testing for pathogens that may be present on seeds is not currently a standard practice in the cannabis industry, which can result in the spread of seed-borne pathogens. Cannabis and hemp seeds are known to harbor species of Alternaria, Fusarium, and several post-harvest molds [33,37], as well as HLVd [26,27]. Testing for pathogen presence and sanitation are important IDM approaches during plant propagation in greenhouse crops, including cannabis [38,39]. These steps can minimize the subsequent spread of fungal, bacterial, and viral/viroid pathogens. Vegetative cuttings used for propagation are required to be rooted under high-humidity conditions over a two-week period. This environment is conducive for the spread of pathogens such as Fusarium spp. and Botrytis cinerea (Figure 9), as well as a number of bacteria that may be spread by water or in the air. Testing conducted in the rooting environment by swabbing of surfaces, sampling of water and air, or plating of surface sterilised plant material can be used to assess total microbes that may be present [21-23]. Cuttings may unknowingly harbor inoculum of Fusarium spp., and infection by powdery mildew or HLVd are likely to be present if the original stock plants were infected [6,7,23,26]. Cuttings taken from stock plants infected internally by Fusarium species can result in spread of the pathogen, resulting in damping-off symptoms, particularly in susceptible genotypes (Figure 9). The infection causes the pith and xylem tissues to collapse, resulting in death of the cuttings. Powdery mildew symptoms may also appear on cuttings, from inoculum either carried over from the stock plants or introduced at the propagation stage. The most significant pathogen affecting root development and growth of cuttings is HLVd that originates from infected stock plants [26]. Additionally, under high humidity conditions, vegetative cuttings may be affected by gray mold (B. cinerea) and common saprophytic fungi, including Penicillium spp., which can potentially reduce the appearance and quality of the cuttings [7,23]. Many of these fungal pathogens that affect cannabis cuttings as well as other stages of plant development produce large numbers of spores, which can be spread by water, air and on tools throughout the growing facility (Figure 10). These spores can serve as sources of initial inoculum and can be challenging to manage. The inclusion of HEPA filters and HVAC systems is advisable to reduce the total counts of air-borne fungal propagules.
2.4. Propagation Stage IDM Approaches
2.4.1. Cultural and Environmental Management
During the propagation stage, ensuring that cuttings are obtained from healthy stock plants reduces the probability of pathogens being transferred through these cuttings. In particular, the incidence of F. oxysporum is reported to be greater in cuttings taken from the base of the plant compared to locations higher up the plant [7,23]. Therefore, cuttings from the uppermost part of stock plants may limit transmission of this pathogen and possibly of HLVd, although sufficient data is lacking at the present time for this pathogen. Ensuring that cuttings are acclimatized in a transitional environment prior to resuming vegetative growth reduces stress on the rooted plants [38,39].
2.4.2. Application of Biological Control Agents
Several biological control products containing Trichoderma spp. or Gliocladium cantenulatum are registered for use on cannabis in Canada [5]. These products are classified as “reduced risk” and provide an alternative disease management option in the absence of registered synthetic fungicides. They can be used at all stages of cannabis crop growth but are particularly useful for managing damping-off caused by Fusarium spp. on cuttings. When applied at the vegetative stage of plant growth, they can reduce mortality due to Fusarium and Pythium species [40]. Several weeks after application, the biocontrol agents can be recovered from cannabis tissues, indicating they are able to survive for a period of time (Figure 11). Their effectiveness is based on protection of tissues and therefore they should be applied before pathogen infection occurs, ideally as a drench or as a dip when cuttings are being rooted, or as a drench at later stages of crop growth. The biocontrol agents protect susceptible root tissues from infection by these pathogens and can colonize cuttings internally, possibly functioning as endophytes; they can potentially enhance root and shoot growth in addition to providing protection against pathogens [40]. Trichoderma sp. also exhibit direct antagonism to F. oxysporum in dual culture (Figure 12). Therefore, several registered biocontrol agents have been demonstrated to be effective against root-infecting pathogens when applied preventatively to cannabis plants.
2.5. Vegetative Growth Stage
Following the establishment of rooted plants from cuttings, the plants are allowed to continue vegetative growth for an additional 2-3 weeks before being transferred to flowering rooms. During this growth stage, root-infecting pathogens, including Fusarium and Pythium species, as well as HLVd, may continue to develop and spread. Development of powdery mildew may also become more severe at this stage of production. Internal stem infections by Fusarium spp. in rooted cuttings can significantly reduce the growth and development of vegetative plants. Symptoms such as yellowing, stunted growth, browning of roots, and plant death are often linked to infection by Fusarium and Pythium species (Figure 13). The development of these pathogens can be exacerbated by root damage and excessive watering or flooding, which can also spread pathogen inoculum.
In addition, HLVd infection of rooted cuttings can adversely affect root development and plant growth at the vegetative stage, leading to reduced plant size, particularly in susceptible genotypes (Figure 5). Molecular diagnostic methods should be used to ensure that vegetative plants are not infected by this viroid [4,26]. In greenhouse environments where recycling of nutrient solution is practiced, monitoring for the presence of Fusarium and Pythium inoculum is necessary since both are known to be present in hydroponic nutrient solutions [21]. Regular testing of electrical conductivity (EC) and potential hydrogen (pH), coupled with testing of drip and drain nutrient ratios, will ensure that the nutrient profiles remain within the optimal range for crop development, preventing nutrient deficiencies that could lead to predisposition to pathogen infection [41,42]. In addition, monitoring of water temperature and oxygen levels can reduce extremes which can enhance root infection by pathogens [42]. Treatment of recirculated water with reduced risk products, such as those indicated in Section 2.2.4, can reduce the incidence of root pathogens (Figure 7).
2.6. Vegetative Growth Stage IDM Approaches
2.6.1. Cultural and Environmental Management
Root pathogen development on vegetative plants can be minimized by increasing the interval between watering events, leading to fewer and shorter irrigation events as long as adequate moisture is provided for optimal root development. This strategy has been used to reduce root pathogen development on various crops [2]. On the foliage, exposure of plants to ultraviolet radiation, especially UV-C light, can suppress powdery mildew development [43]. Night-time exposure enhances pathogen susceptibility by limiting light-activated DNA repair mechanisms [44]. UV-C may also enhance plant defense responses including accumulation of reactive oxygen species [45]. To avoid phytotoxicity, exposure of plants to UV-C should be made gradually over several weeks, according to manufacturer’s guidelines. Treated plants may show a reduction in plant height and increased lateral branching as has been observed in some ornamental plant species [46, 47].
2.6.2. Application of Biological Control Agents
Biological control agents can also be applied as drenches to vegetative plants to reduce the severity of root pathogens similar to treatments made at the propagation stage [40]. The extent to which these agents can survive following application at this stage has not been determined.
2.7. Flowering Stage
After vegetative plants have been transferred to greenhouse compartments where the photoperiod is reduced from 18:6 hr light:dark to 12:12 hr or other iterations of light:dark [48,49]; the onset of inflorescence development is triggered within 1-2 weeks. At this stage of crop development, symptoms of root infection by Fusarium or Pythium spp. originating from the propagation/vegetative stage may rapidly become apparent. These symptoms include leaf yellowing, plant wilting, crown and root rot, and stunted growth (Figure 14). There is no evidence that new infections from residual inoculum is occurring on flowering plants if all sanitary practices have been followed and recirculated water is not being used. Symptoms attributed to HLVd infection, which may have been previously undetected on vegetative plants, will typically manifest within 1-3 weeks after transfer to the flowering room. These symptoms are distinct, appearing as reduced inflorescence size, yellowing of the bract leaves, and stunted plant growth [26] (Figure 5). The environmental conditions during inflorescence development, which includes higher humidity due to increased plant biomass, may also promote the development of powdery mildew, particularly in more susceptible genotypes. Closer towards the harvest period when inflorescences begin to mature, bud rot caused by B. cinerea is likely to become visible, depending on environmental conditions and the genotype. This can lead to significant reductions in inflorescence quality and yield (Figure 14).
In addition to the above pathogens that infect the crop during plant growth, colonization of inflorescences by yeasts and molds prior to harvest is common and generally remains undetected until after harvest when quality tests are performed. On the inflorescence tissues, the most common fungal genera recovered include Penicillium, Alternaria, Cladosporium, and Fusarium (Figure 15). These microbes can be detected by conducting bud swab tests as described in recent studies [19,20,50]. This buildup of yeasts and molds can lead to the final dried product failing to meet quality standards [20,50]. Various factors influence the levels of yeast and mold contamination, which are discussed in the following sections.
2.8. Flowering stage IDM approaches
2.8.1. Cultural and Environmental Management
The increased plant biomass resulting from plant development during the flowering stage creates challenges for maintenance of consistent environmental conditions, particularly with regard to ambient humidity. Reducing plant densities can significantly lower humidity levels in the greenhouse and also allows for better light penetration and ease of application of disease control products. However, lower plant densities can decrease overall yield per unit area of production [35,51]. A lower ambient relative humidity can also be achieved by increasing air circulation with circulating fans placed near the plants in the weeks leading up to harvest. Maintaining air movement at 0.5-1.0 m/s appears to be an optimal target for microbial suppression in cannabis [51]. Under experimental conditions, enhanced air flow around maturing inflorescences was demonstrated to significantly reduce the populations of various microbes within the tissues of genotype ‘PH’ (Figure 16). This reduction in humidity, combined with appropriate climate control settings, can mitigate the severity of diseases such as bud rot (B. cinerea) and powdery mildew during high-risk periods.
In relation to seasonal effects on disease development in the greenhouse, bud rot development was shown to be influenced by external vapour pressure deficits that impacted moisture levels in the air and hence ambient humidity [35]. To avoid periods of high disease pressure brought on by external environmental conditions, one IDM strategy is to alter the time of seasonal plantings. By scheduling planting and harvest times to avoid periods of high disease pressure, particularly with desirable but susceptible cannabis genotypes, producers can reduce the impact of seasonal pathogens such as B. cinerea [35], as well as reduce the build-up of total inflorescence microbes that are also impacted by seasonal fluctuations (Figure 17).
An alternate approach is to harvest inflorescences after a shorter crop development period to avoid prolonged exposure to environmental conditions that favour disease development at the maturation stage. For example, harvesting at 6 weeks of inflorescence development instead of 8 weeks can reduce bud rot incidence but could result in compromised yield and potency in certain genotypes unless they are close to maturity [35,52]. Areas within a greenhouse that have localized disease or “hot spots” should be identified followed by the eradication of the affected plants to minimize pathogen spread. The diseased plants should be recorded, and if the causal pathogen is unclear, diagnostic testing should be performed, typically through submissions of samples to a diagnostic laboratory [4,7]. In addition to visualization of these areas with the naked eye, the utility of infra-red (IR) and artificial intelligence (AI) powered scouting technologies could be of value as they have been used in a range of other crops [53-57] but further evaluation of how these technologies could be modified for application to cannabis is needed.
2.8.2. Utility of Disease Tolerant Genotypes
The cannabis genotype being grown can have a profound impact on the development of certain pathogens especially under disease-conducive conditions. The impact of genotypes on disease development at the stock cultivation and propagation stages were described previously. A similar effect of genotypes on pathogen infection can also be demonstrated at the flowering stage. A comparison of the response of six genotypes to four pathogens is shown in Figure 18. The genotype ‘LO’ showed high susceptibility to powdery mildew but low susceptibility to HLVd, Botrytis bud rot and root pathogens. A second genotype ‘LB’ showed high tolerance to all four pathogens while the remaining genotypes varied in their response to these specific diseases. These data were collected from observation trails under natural infection and not from replicated trials. They demonstrate, however, that cannabis producers have an option to select those genotypes that show tolerance to pathogens under the specific cultivation conditions of greenhouse production. While the genetic basis for this level of tolerance has not been determined, it indicates there is a basis on which to establish breeding programs that can lead to the development of disease-tolerant cannabis cultivars.
2.8.3. Application of Biological Control Agents.
As described for cuttings during propagation and during vegetative growth of plants, biological control agents also show promise in reducing specific diseases at the flowering stage of cannabis plants. The diseases of importance that can be targeted are Botrytis bud rot and powdery mildew. Application of several biological control products and reduced risk chemicals at weekly intervals as a fine spray, at full label rates, onto developing inflorescences of the genotype ‘PH’, was observed to reduce the development of Botrytis bud rot under both low and high disease pressure resulting from natural infection during the Fall growing season (Figure 19).
The most effective product was Rootshield HC® (containing Trichoderma harzianum) followed by Regalia® (Reynoutria sachalinensis), Double Nickel® (Bacillus amyloliquefaciens), Lifegard® (Bacillus mycoides) and Prestop® (Gliocladium catenulatum). Zerotol® (hydrogen peroxide) did not show an effect (Figure 19). The efficacy of the various biocontrol agents likely stems from their ability to pre-emptively colonize the inflorescence tissues and exert competition against the pathogen, a mode of action also reported on other crops [58,59]. The application of T. harzianum was also found to suppress the development of other microbes naturally present within the inflorescences, including Penicillium spp., and this was reflected by a reduction in all three categories of microbial counts (Figure 20).
Following this spray trial, a second trail demonstrated that applying T. harzianum (Rootshield HC®) thrice to the foliage of flowering cannabis plants also reduced the development of powdery mildew compared to untreated plants, as shown in Figure 21. These results indicate that a single biological control agent may target two important diseases affecting cannabis, namely Botrytis bud rot and powdery mildew. Trichoderma applications have been shown to suppress powdery mildew in several crops [60,61,62].
2.8.4. Application of Reduced-Risk Products
A number of reduced risk products are available for use on cannabis plants at the flowering stage. During this phase of crop development, care must be taken to avoid damage to inflorescence tissues and to avoid visual quality changes. Products including Agrotek vaporized sulfur®, Regalia Maxx®, Suffoil-X® and Milstop® are registered to reduce powdery mildew development [43]. Sulfur is applied via vaporizing pots, a method that ensures uniform dispersal and is commonly used on many other greenhouse crops [31] while the remainder are applied as sprays. In a comparative study to evaluate these and other products for powdery mildew control on flowering cannabis plants, nine products were applied thrice at days 0, 7 and 14 of the flowering period (~ 60 mL per plant) during the spring season on ‘MP’, a susceptible cannabis genotype, prior to disease appearance. Subsequently, disease severity was rated visually using a leaf infection coverage scale, as follows: 0 = 0%, 1 = 1-33% = 2 = 34-66%, 3 =67-100% (Figure 22a). Results showed that Suffoil-X® applied at a rate of 10 mL/L and Regalia Maxx® applied at a rate of 2.5 mL/L were the best preventative products (Figure 22b). In a subsequent trial with same genotype, flowering plants visibly infected with powdery mildew (disease rating of 1) received one application of seven products at their maximum label rates made at day 42 of the flowering period to evaluate their curative potential. The findings showed that Milstop® applied at a rate of 3 g/L and Cyclone® applied at a rate of 12 mL/L were the best for curative treatments (Figure 22c). The remaining products provided varying levels of disease reduction. No phytotoxicity was observed in any of the treatments. The active ingredients in Milstop® (potassium bicarbonate), Cyclone® (citric and lactic acid) and Suffoil-X® (mineral oil) are all considered to be ‘physical’ in their mode of action, altering leaf surface pH and osmotic pressure or desiccating/coating mycelium and spores [6]. The active ingredient in Regalia Maxx® is an extract from the giant knotweed Reynoutria sachalinensis and was shown to be effective against pathogens such as B. cinerea and powdery mildew on cannabis as well as on various other crops [63-66]. This product enhances plant defense responses through the salicylic acid (SA)-dependent pathway by inducing the accumulation of plant defence chemicals such as hydrogen peroxide and formation of mechanical plant defences such as callose papillae [66,67]. Additional research is needed to explore the breadth to which Regalia Maxx® can control other pathogens and the duration of the protection offered following application.
A summary of the IDM approaches that can be used against four important pathogens of cannabis is provided in Table 1.
2.9. Post-Harvest IDM Approaches
Following harvest of cannabis inflorescences, they undergo a phase of drying to reduce moisture content to levels that would minimize the development of microbes [19,20,52], following which they are trimmed and prepared for packaging and stored prior to shipment. During each of the post-harvest processing stages, there is the potential for microbial contamination to be increased, primarily consisting of total yeasts and molds (TYM), total aerobic microbial count (TAMC), and bile-tolerant Gram-negative count (BTGN). Some of these microbes likely originated from the original fresh harvested inflorescences in the greenhouse or otherwise may have been picked up through contamination during harvesting and post-harvest processing stages. Detailed studies are lacking regarding at which specific stages the levels of microbes may build-up to cause the final product to potentially fail to meet regulatory standards. However, pre-harvest, it has been shown that cannabis genotype and growing conditions can significantly influence TYM build-up; in addition, post-harvest drying methods and handling practices can affect TYM levels [19,20,52]. A number of commonly encountered fungi have been identified on dried cannabis products pre- and post-harvest (Figure 15) and they contribute to TYM levels [19].
The implementation of Integrated Disease Management (IDM) approaches to reduce total yeast and mold (TYM) is complicated by several pre-harvest variables. For example, TYM levels tend to be higher in the summer season than in winter, certain cannabis genotypes accumulate much higher TYM than others, and post-harvest handling practices influence TYM levels (hang-dried inflorescences have lower TYM than those that are rack-dried) [19]. Managing these factors to minimize microbial build-up depends on the appropriate application of IDM strategies that were previously outlined for stock plants, and during propagation, vegetative growth, and flowering. Post-harvest processing practices, such as reducing moisture by hang-drying plants at a high vapor pressure deficit (VPD) and trimming only after inflorescences are dry, along with thorough cleaning of post-harvest processing equipment using sanitizing agents, can significantly reduce microbial load on inflorescences. Additionally, conducting detailed inspections at each stage of post-harvest processing to detect presence of molds is critical. This may involve various standard practices, including predefined in-process Acceptable Quality Level (AQL) checks, to ensure that any quality issues are identified and addressed prior to shipment, as is commonly done in many food processing plants [68].
Irradiation of cannabis products with gamma and electron beam irradiation has been shown to be an effective option for producers; they can be used to sterilize commercial batches of inflorescences without major changes in quality, but they are costly [69-71]. Irradiation is typically used in cases where microbial levels have exceeded regulatory limits or where a zero tolerance is recommended i.e. for medical patients with immunocompromised immune systems that rely on cannabis [20]. Other approaches have been described that require more in-depth studies to demonstrate their commercial utility [72,73]A summary of the various approaches that can be implemented as a part of an IDM program for greenhouse-cultivated cannabis is presented in Figure 23. These are organized according to growth stages of the cannabis crop. These approaches can be readily implemented, and examples of their successful use have been included in this review. Additional potential IDM approaches for cannabis that require further research, but which have shown potential in other crops, are described below.
2.10. Future Potential Areas for IDM Development for Cannabis
2.10.1. Evaluation of Endophytes and Microbial Antagonists in Cannabis
Endophytes, consisting primarily of fungal and bacterial species, are present within various tissues and organs of cannabis and hemp plants and vary in species composition, depending on the tissue source, such as roots, stems, petioles, leaves, flowers and seeds [37,40,74-76]. For example, endophytes which are consistently present in stems of cannabis plants include species of Penicillium and Chaetomium, as well as others (Figure 24).
Various plant growth-promoting rhizobacteria, including species of Bacillus and Pseudomonas, have also been reported to be present in roots and can inhibit the growth of root pathogens [40,77]. These endophytes can potentially improve plant growth and development [78,79] although research evaluating their growth benefits to cannabis and hemp plants are currently lacking. Dumigan and Deyholos [37] reported that seed-borne bacterial endophytes, including Bacillus subtilis and B. inaquosorum, showed inhibitory activity in dual culture assays against fungal pathogens, including Alternaria and Fusarium species. These endophytes were also present in hemp seeds and included Bacillus velezensis and Paenibacillus polymyxa, which were also inhibitory to growth of Alternaria, Aspergillus, Fusarium and Penicillium species. Pseudomonas species have also shown growth inhibition of Fusarium species in vitro [80]. In previous research, antagonism to B. cinerea in dual culture assays was demonstrated for several cannabis-derived endophytes (Paecilomyces lilacinus and Penicillium spp.) [75] and for several hemp-derived endophytes (Pseudomonas fulva and P. orientalis ) [80]. In a study conducted by Gabriele et al. [81] on investigating the endophytes present in the seeds and young plants of a cannabis cultivar, a unique resistance to the plant's own antimicrobial compounds was discovered, along with an enhancement of nutraceutical aspects such as polyphenol content and antioxidant activity in the plants. This finding suggests the potential for introducing these endophytes as natural biostimulants and biological control agents against pathogenic microbes, unhindered by the plant’s inherent antimicrobial properties. Such symbiotic relationships underscore the significant potential of endophytes in cannabis cultivation but further research is needed to establish their potential applications. The antagonistic properties of endophytic bacteria have been attributed to antibiotic production, host defense response induction, growth promotion, competition, parasitism and quorum signal interference [82-85]. Despite these promising studies, however, whole plant assays demonstrating the benefits of these bacteria and other fungal endophytes are presently lacking for cannabis. It should be noted that fungal endophytes can also be present in stem tissues of mother plants, including those shown in Figure 24, and they could negatively impact the health of these plants over time and complicate attempts to initiate tissue cultures using explants from these plants [86].
Another aspect of potential microbial antagonism against fungal pathogens infecting cannabis that requires research is the diverse microflora that can be present in organic soils compared to conventional hydroponic cultivation. Punja and Scott [87] reported that a diverse range of microbes were recovered from cannabis inflorescences grown in organic soil compared to coco-fibre medium commonly used in hydroponic cannabis production. These communities were comprised of pathogenic, saprophytic, and beneficial microbes. Of the beneficial microbes detected, Trichoderma harzianum and Metharzium anisopliae are currently used as biological control agents for root disease suppression and for insect suppression, respectively. M. anisopliae may hold some potential for cannabis pathogen suppression as well [86]. In the context of disease management, similar microbes which originate from organic soils that exhibit general antagonistic properties such as mycoparasitism, host defense response induction, competition, and antibiotic production are worthy of evaluation [85,89,90]. Cannabis plants grown in ‘living soil’ or growing media amended with ‘compost teas’ may foster greater colonization of roots by these beneficial endophytes although more research is needed to demonstrate their utility in an IDM program. Caution should be exercised in ensuring these microbes do not colonize the inflorescences internally or externally, potentially leading to a failure of the product due to excessive levels of TYM.
2.10.2. Tissue Culture Applications for Cannabis
Tissue culture of cannabis has received considerable recent interest in efforts to obtain a source of clean plant materials that can be free of pathogenic microbes, including fungi, viruses and viroids. Detailed methods have been described from several laboratories and various techniques have been developed [38, 86,91,92]. The interest among cannabis producers in utilizing tissue culture methods is to obtain pathogen-free plants and minimize pathogen re-introduction into commercial production facilities. This is particularly relevant in the context of Hop latent viroid, which is known to be spread through vegetative cuttings taken from infected mother plants [26]. Meristem tip culture technology has been used for many decades to eliminate the potential for virus introduction in other vegetatively propagated crops, such as potatoes, bananas, and strawberries [93-95]. Meristem and shoot tip culture techniques have been utilized not only for virus elimination but also for rapid clonal multiplication and germplasm preservation of many vegetatively propagated crops [96,97]. In some cases, these methods are augmented with cryotherapy (cold treatment), thermotherapy (heat treatment), chemotherapy (anti-viral chemical treatment), electrotherapy (electrical current treatment) and shoot-tip grafting (micrografting technique) to enhance the chances of obtaining pathogen-free planting materials [98-102]. Research to evaluate the applicability of these methods to obtain pathogen-free planting materials of cannabis, particularly for HLVd, are still in the early stages of evaluation and development. Tissue culture-derived plants can be obtained from meristems and nodal explants of cannabis, resulting in shoot growth of a number of genotypes in vitro (Figure 25). However, confirmation of the eradication of pathogens of importance requires additional research. Hence, while tissue culture approaches hold promise for potential inclusion in an IDM program for cannabis, more effort to generate high frequencies of plants confirmed to be pathogen-free on an economically feasible scale is needed. The confirmation of pathogen-free planting materials could be utilized for certification programs for cannabis, similar to many agriculturally important crops.
2.10.3. Pathogen Control Product Registration for Cannabis
To evaluate new products aimed at managing fungal pathogens in agricultural crops, screening for pathogen growth inhibition is an initial step. For example, effective concentration (EC50) values are determined for the product to identify the fungicide levels needed to inhibit 50% of the pathogen's growth in vitro. However, such studies are less commonly reported for products intended for cannabis. EC50 studies, which are relatively straight-forward to conduct as demonstrated in Figure 7, are informative about the potential of new products to inhibit the growth of specific pathogens affecting cannabis, especially when followed by whole plant assays. These studies can also determine if there are any secondary effects on biocontrol fungi, such as Trichoderma spp. (Figure 7). Recent evaluations of products for powdery mildew control in organic hemp production [103] serve to identify products that may be acceptable for registration in cannabis. Such products could be utilized for pathogen control at the stock plant and propagation stages, which are critical to ensure that the subsequent vegetative and flowering stages do not carry over pathogen inoculum and to reduce concerns about product residues on the finished flower. Currently, the majority of products registered for use on cannabis can be applied up until harvest, as outlined by Scott et al. [5]. Several commercial products have since been added to the registered list that are not included in [5]. For soil fumigation: Pic Plus Fumigant® (Chloropicrin – 85.1%), Chloropicrin 100 Liquid Soil Fumigant® (Chloropicrin – 85.1%), and Mustgrow Crop Biofumigant® (Oriental Mustard Seed Meal – 100%) can be used pre-plant. For powdery mildew suppression: Vegol Crop Oil® (Canola Oil – 96%), Suffoil-X® (Mineral Oil – 80%), Purespray FX (Mineral Oil - 80%), and General Hydroponics Suffocoat (Canola Oil – 96%) can be applied as foliar sprays. For suppression of Botrytis cinerea, powdery mildew, and Sclerotinia sclerotiorum: Timorex Gold® (Tea Tree Oil – 23.8%) can be used. For Phytophthora spp. and Verticillium dahlia suppression: Foretryx® (Trichoderma asperellum strain ICC 012 and Trichoderma gamsii strain ICC 080) can be used. While many of these products are different formulations of the same active ingredient, unique products have been added each year since the legalization of cannabis production in Canada. A current list of registered products can be found at Health Canada - Pesticide Label Search (hc-sc.gc.ca).
2.10.4. Nutrient Supplements for Cannabis Disease Suppression
Nutrient amendments have been shown to impact plant susceptibility to infection by a range of pathogens, often reducing disease development through various mechanisms. These changes involve a wide range of macronutrients and micronutrients. In hydroponic greenhouse cultivation, nutrient levels are carefully monitored to prevent deficiencies; thus, additional nutrient supplements must be approached cautiously to avoid phytotoxicity. Formulation and rates are critical factors when considering these nutrients for disease management [104,105]. The use of nutrient supplements containing copper, silicon, and calcium show particular promise for cannabis and can be applied via the roots or foliage.
Copper has a long history of use as a bactericide and fungicide on various crops against numerous pathogens since the discovery of 'Bordeaux mixture' in 1885. It disrupts fungal cell membrane integrity and interferes with key enzyme activities, thereby inhibiting pathogen growth and survival [106,107]. Copper can be applied to cannabis as rootzone drenches, foliar sprays, or seed treatments. For instance, Mayton et al. [108] assessed different seed treatments to manage damping-off caused by Pythium and Fusarium species on industrial hemp. Seeds treated with a copper-containing product, Ultim® at 0.05 mg Cu/seed, showed efficacy comparable to fungicide treatments. Moreover, copper nanoparticles have been successfully applied as dips and foliar treatments on tomatoes and watermelons to reduce Fusarium infection [109,110]. A copper formulation, Copper CropTM, reduced powdery mildew on melons [111]. This suppression aligns with the conventional use of copper sulfate pentahydrate as a foliar fungicide on plant species such as roses and dogwood [112,113]. On grapevines, copper citrate effectively reduced Botrytis cinerea infections [114]. The diverse range of pathogens suppressed by copper formulations suggests its potential for use on cannabis; however, copper is not currently registered for this purpose. Silicon is effective against various bacterial, fungal, and viral pathogens and can strengthen cell walls via silicon deposits and also induce plant defense responses [115,116]. Scott and Punja [43] reported that multiple weekly sprays of potassium silicate, Silamol®, on vegetative cannabis plants significantly reduced powdery mildew development. In contrast, a single application showed no effect in the current study. Akinrinlola et al. [103] reported that Sil-Matrix®, a fungicide with potassium silicate, significantly reduced hemp powdery mildew by 88%. Dixon et al. [117] demonstrated that root-applied silicon at a rate of 600 kg/ha significantly reduced powdery mildew severity in hemp. Similar benefits of silicon supplementation have been observed in crops such as cucumbers, roses, and strawberries [118-123].Calcium application has been shown to reduce pathogen infection by strengthening plant cell walls, thereby providing greater structural integrity against fungal and bacterial infections [124]. However, its effectiveness in reducing pathogens affecting cannabis has not been studied. There are no reports of a direct toxic effect of calcium-containing compounds on fungal pathogens affecting cannabis, suggesting that its activity may stem from reducing host susceptibility or through other mechanisms. In some crops, root-zone supplementation of calcium nitrate was reported to reduce B. cinerea severity on beans and tomatoes, although higher doses increased disease on beans [125]. Supplementing roses with calcium nitrate and adding calcium chloride or calcium sulfate to solutions for harvested flowers reduced B. cinerea incidence under conducive disease conditions [126]. Similarly, increasing calcium and reducing nitrogen levels in the irrigation water for sweet basil plants reduced both sporulation and infection severity of B. cinerea [127]. Whether enhanced calcium supplementation on cannabis plants can influence development of B. cinerea remains to be determined.
2.10.5. Alternative Technologies for Cannabis Disease Detection
The use of recently developed robotic and imaging technologies for scouting for diseases has garnered interest from cannabis producers. Various options are available with pros and cons, depending on greenhouse scale, layout and operations. For small-scale greenhouses, fixed crop monitoring cameras or AI-powered phone scouting apps are often utilized. Several cannabis-focused scouting apps include Koppert’s Natutec Scout app (https://www.koppert.com/natutec-scout/), BioBest’s Crop-scannerTM app (https://www.crop-scanner.com/), GrowDoc AI’s app (https://growdoc.ai/), and the IPM ScoutekTM app (https://ipmscoutek.com/). In contrast, larger greenhouse operations, with a more consistent layout, have trialed autonomous robotic scouting carts or booms with cameras attached to crop carts, such as IUNU’s LUNA AI scouting above-crop cameras or scouting carts (https://iunu.com/luna-ai), Ecoation’s OKO or ROYA scouting carts (https://www.ecoation.com/integrated-pest-management), and Budscout AI’s Budscout above-crop cameras (https://budscout.ai/budscout/). The challenge for AI and imaging solutions currently is that they may not reliably distinguish symptoms caused by various pathogens from nutrient deficiencies and other environmental stressors. Supplemental and tailored training are likely needed to achieve accurate results. In the broader agricultural sector, significant progress is being made in the robot AI-assisted vision space [56]. An example of the training process and customizability of AI scouting technology is demonstrated in the following two studies. Anagnostis et al. [55] aimed to build a fast and accurate object detection system to identify anthracnose-infected leaves (by Colletotrichum spp.) in a commercial walnut orchard. The study involved segmenting high-resolution images into smaller sub-images and training an object detector to recognize disease-specific features. The deep learning approach achieved high accuracy under real-field conditions. Similarly, Mahmud et al. [54] focused on developing an innovative machine vision system to accurately detect powdery mildew in strawberry fields. This system utilized real-time image processing and artificial neural networks (ANNs) to distinguish diseased leaves from healthy ones. The study demonstrated the system's adaptability to field conditions and showed high accuracy in detecting powdery mildew. These examples point to the potential for applications in integrated disease management and early disease intervention in different agricultural settings.
Infrared imaging (IR), a spectrum used in some remote sensing technologies, identifies variations in crop or leaf temperature to reflect reduced transportational activity or metabolic functions, signalling the potential presence of stressors including disease [53,57,128]. In cannabis, detection of leaf surface temperature changes due to root diseases or poor root development when tested at different developmental stages can be captured with a handheld device such as a FLIR E8 ProTM infrared camera (https://www.flir.ca/products/e8-pro/?vertical=condition+monitoring&segment=solutions) (Figure 26). This method showed definitively that poorly developed root systems on affected plants was correlated directly with a reduced rate of transpiration and hence a build-up of leaf surface temperature that was detectable with the IR camera. However, when powdery mildew-infected plants or cannabis plants affected by Hop latent viroid were similarly compared to healthy plants, these plants did not show a corresponding reduced transpirational activity pattern, suggesting that the IR camera was unable to detect physiological changes in these diseased plants. It is unknown whether infrared or other spectrums could be used to effectively detect hop latent viroid; limited research has been done on virus detection with infrared but there may be potential applications [129,130]. Vagelas et al. [131] utilized a low-cost infrared camera and a standard RGB web camera to analyze vine, chrysanthemum, and rose leaves that had been infected with various fungi. Results showed that infected leaves exhibited temperature deviations from uninfected ones, which occurred before visible symptoms developed. Specifically, infected vine and rose leaves showed a decrease in temperature, while chrysanthemum and another set of rose leaves demonstrated an increase, compared to healthy tissue. Lindenthal et al. [132] used infrared thermography to detect downy mildew infection on cucumbers. In controlled environments, the study showed that the maximum temperature difference in a leaf could be used to distinguish between healthy and infected tissues. Under natural environments, while leaf temperatures and transpiration rates were similar in both healthy and infected plants, diseased leaves showed more varied transpiration rates depending on the severity of the symptoms. Liaghat et al. [133] utilized Fourier Transform Infrared (FT-IR) spectroscopy to detect Ganoderma infections in oil palm trees. This method involved analyzing leaf samples from both healthy and infected trees, examining the infrared spectra of these samples, and using a statistical model for classification. The researchers successfully identified differences linked to the disease, accurately identifying infected trees at early, symptomless stages. Therefore, there is a growing body of evidence to demonstrate that IR approaches could be applied to cannabis for early detection of infection by foliar pathogens but additional studies are required to validate this approach.
2.10.6. Induction of Plant Defence Responses in Cannabis
The potential for inducing plant defense responses before pathogen infection has not yet been developed to a practical level for cannabis. However, as discussed earlier, Regalia Maxx (Reynoutria sachalinensis) when applied to cannabis plants can reduce pathogens such as Botrytis cinerea and powdery mildew, confirming reports in the literature of the efficacy through presumed induction of defence responses and pathogen reduction [63-67]. Weekly applications are recommended to ensure on-going protection on cannabis plants. The role of endophytic microbes in the growing medium in promoting plant health and reducing pathogen infection on cannabis awaits confirmation. Previous reports demonstrate the defence-boosting properties of endophytic organisms on various plant species [82-85,134]. The utility of a biological control product containing Trichoderma spp. appears to be promising when applied preventatively to the rootzone or to inflorescences; however, additional research is necessary to establish whether induction of defence responses in cannabis can be confirmed as it has in previous reports on numerous plants [135,136].
3. Conclusions
This comprehensive review of integrated disease management (IDM) approaches for greenhouse-cultivated cannabis underscores the significance of developing a multifaceted approach to control the various pathogens of economic concern. The review highlights the importance of pre-emptive measures, including selection of disease-tolerant genotypes and use of stringent sanitation practices, in minimizing pathogen incidence. The utilisation of biological control agents and reduced-risk products, and the modification of cultural and environmental conditions, have shown promising results in suppressing Botrytis bud rot, powdery mildew, Pythium and Fusarium root diseases and hop latent viroid causing stunt disease. Moreover, the exploration into alternative strategies including utility of endophytes, tissue culture, nutrient supplementation and technology-aided scouting, offers potential new avenues for enhancing plant health. This review underscores the dynamic nature of IDM in cannabis cultivation, and emphasizes the continuing need for research and adoption of sustainable strategies to meet the evolving challenges in disease management within the greenhouse cannabis industry. Such strategies should receive support from governmental regulatory agencies to ensure they meet the criteria set forth by the appropriate jurisdictions.
Author Contributions
Conceptualization, L.B, Z.K.P.; methodology, L.B.; formal analysis, L.B., Z.K.P.; investigation, L.B.; resources, L.B., Z.K.P.; writing—original draft preparation, L.B.; writing—review and editing, L.B., Z.K.P.; visualization, L.B., Z.K.P.; supervision, Z.K.P.; project administration, Z.K.P.; funding acquisition, Z.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an Alliance Grant (ALLRP 571270-21) from the Natural Sciences and Engineering Research Council of Canada (NSERC) with matching funding from Pure Sunfarms Corp.
Data Availability Statement
There was no new data created in this study.
Acknowledgments
We appreciate the assistance and insights provided by several licenced cannabis producers who have shared their knowledge of disease managements practices and approaches that were included in this review. We acknowledge the resources, staff and cultivation expertise provided by Pure Sunfarms Corp., a licensed cannabis facility in Delta, BC, during the experimental trials. Technical help was provided by S. Lung and C. Scott.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Clercq: P. Integrated pest and disease management in greenhouse crops. In Developments in Plant Pathology. R. Albajes, M.L. Gullino, J.C. van Lenteren and Y. Elad, Eds.; Kluwer Academic Publishers, Dordrecht, The Netherlands, 2000.
- Razdan, V.K.; Sabitha, M. Integrated disease management: Concepts and practices. In Integrated Pest Management. R. Peshin and A.K. Dhawan, Eds.; Springer: Netherlands, 2009; pp. 369–389.
- Nicot, P.C.; Gullino, M.L.; Albajes, R. Integrated pest and disease management in greenhouse crops. Springer International Publishing, 2020.
- Wang, S. Diagnosing hemp and cannabis crop diseases. Oxfordshire, UK; Boston, MA: CABI, CAB International, 2021.
- Scott, C.; Punja, Z.K.; Sabaratnam, S. Diseases of cannabis in British Columbia. BC Ministry of Agriculture. 2021. Retrieved from https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/plant-health/diseases_of_cannabis_in_british_columbia.pdf.
- Punja, Z.K.; Scott, C. Management of diseases on cannabis in controlled environment production. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 216–252.
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag. Sci. 2021. 77, 3857–3870. [CrossRef]
- Awasthi, L.P. Biopesticides in organic farming: recent advances. CRC Press: Taylor & Francis, Boca Raton. FL, USA, 2021.
- Grof, C.P.L. Cannabis, from plant to pill. Brit. J. Clin. Pharmacol. 2018. 84, 2463–2467. [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy. 2021. 13, 546–561.
- Mihalyov, P.D.; Garfinkel, A.R. Discovery and genetic mapping of PM1, a powdery mildew resistance gene in Cannabis sativa L. Front. Agron. 2021. 3, 720215. [CrossRef]
- Stack, G.M.; Cala, A.R.; Quade, M.A.; Toth, J.A.; Monserrate, L.A.; Wilkerson, D.G.; Carlson, C.H.; Mamerto, A.; Michael, T.P.; Crawford, S.; Smart, C.; Smart, L.B. Genetic mapping, identification, and characterization of a candidate susceptibility gene for powdery mildew in Cannabis sativa L. Mol. Plant-Microbe Interact. 2024. 37(1). [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Molecular mechanisms underlying potential pathogen resistance in Cannabis sativa. Plants (Basel). 2023. 12, 2764. [CrossRef]
- Zheng, Y. Handbook of Cannabis Production in Controlled Environments. CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022.
- Fleming, H.; Chamberlain, Z.; Zager, J.J.; Lange, B.M. Controlled environments for cannabis cultivation to support "omics" research studies and production. Methods Enzym. 2023. 680, 353–380.
- Punja, Z.K. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018. 40, 514–527. [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019. 10, 1120. [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Fungal pathogens affecting the production and quality of medical cannabis in Israel. Plants (Basel) 2020. 9, 882. [CrossRef]
- Punja, Z.K.; Ni, L.; Lung, S.; Buirs, L. Total yeast and mold levels in high THC-containing cannabis (Cannabis sativa L.) inflorescences are influenced by genotype, environment, and pre-and post-harvest handling practices. Front. Microbiol. 2023. 14, 1192035. [CrossRef]
- Gwinn, K.D.; Leung, M.C.K.; Stephens, A.B.; Punja, Z.K. Fungal and mycotoxin contaminants in cannabis and hemp flowers: implications for consumer health and directions for further research. Front. Microbiol. 2023. 14, 1278189. [CrossRef]
- Punja, Z.K.; Rodriguez, G. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018. 40, 498–513. [CrossRef]
- Punja, Z.K.; Ni, L.; Roberts, A. The Fusarium solani species complex infecting cannabis (Cannabis sativa L., marijuana) plants and a first report of Fusarium (Cylindrocarpon) lichenicola causing root and crown rot. Can. J. Plant Pathol. 2021. 43, 567–581. [CrossRef]
- Punja, Z.K. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L.) plants in commercial greenhouse production. Can. J. Plant Pathol. 2021. 43(2), 216–235. [CrossRef]
- Punja, Z.K.; Scott, C.; Lung, S. Several Pythium species cause crown and root rot on cannabis (Cannabis sativa L.) plants grown under commercial greenhouse conditions. Can. J. Plant Pathol. 2022. 44(1), 66–81. [CrossRef]
- Adkar-Purushothama, C.R.; Sano, T.; Perreault, J.-P. Hop latent viroid: A hidden threat to the cannabis industry. Viruses 2023. 15(3), 681. [CrossRef]
- Punja, Z.K.; Wang, K.; Lung, S.; Buirs, L. Symptomology, prevalence, and impact of hop latent viroid on greenhouse-grown cannabis (Cannabis sativa L.) plants in Canada. Can. J. Plant Pathol. 2024. 19(1), 1-24. [CrossRef]
- Atallah, O.O.; Yassin, S.M.; Verchot, J. New insights into hop latent viroid detection, infectivity, host range, and transmission. Viruses. 2024. 16, 30. [CrossRef]
- Kruidhof, H.M.; Elmer, W.H. Cultural methods for greenhouse pest and disease management. In Integrated Pest and Disease Management in Greenhouse Crops. M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 285–330.
- Van Lenteren, J.C.; Nicot, P.C. Integrated pest management methods and considerations concerning implementation in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops. M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 177–193.
- Stasiak, M.; Dixon, M. Growing facilities and environmental control. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 14-40.
- Konstantinidou, P.C.; Gilardi, G.; Gard, B.; Gullino, M.L. Vegetable and herb disease management in protected culture. In Handbook of Vegetable and Herb Diseases. Springer, Cham: Edinburgh, Scotland. 2022; pp. 1-50.
- Sirangelo, T.M. Nlr- and mlo-based resistance mechanisms against powdery mildew in Cannabis sativa. Plants (Basel). 2023. 13(1), 105. [CrossRef]
- Roberts, A.J.; Punja, Z.K. Pathogenicity of seedborne Alternaria and Stemphylium species and stem-infecting Neofusicoccum and Lasiodiplodia species to cannabis. Can. J. Plant Pathol. 2022. 44(2), 250–269. [CrossRef]
- Punja, Z.K.; Ni, L. The bud rot pathogens infecting cannabis (Cannabis sativa L., marijuana) inflorescences: symptomology, species identification, pathogenicity and biological control. Can. J. Plant Pathol. 2021. 43(6), 827–854. [CrossRef]
- Mahmoud, M.; BenRejeb, I.; Punja, Z.K.; Buirs, L.; Jabaji, S. Understanding bud rot development, caused by Botrytis cinerea, on cannabis (Cannabis sativa L.) plants grown under greenhouse conditions. Botany. 2023, 101(7), 200–231. [CrossRef]
- Balthazar, C.; Cantin, G.; Novinscak, A.; Joly, D.L.; Filion, M. Expression of putative defense responses in cannabis primed by Pseudomonas and/or Bacillus strains and infected by Botrytis cinerea. Front. Plant Sci. 2020, 11, 572112. [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Cannabis seedlings inherit seed-borne bioactive and anti-fungal endophytic Bacilli. Plants (Basel). 2022, 11(16), 2127. [CrossRef]
- Jones, M.; Monthony, A.S. Cannabis propagation. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 41–90.
- Munkvold, G.P.; Gullino, M.L. Seed and propagative material. In Integrated Pest and Disease Management in Greenhouse Crops; M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 331–354.
- Scott, C.; Punja, Z.K. Biological control of Fusarium oxysporum causing damping-off and Pythium myriotylum causing root and crown rot on cannabis (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2023. 45(3), 238–252. [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient management in substrate systems. In Plant Nutrition of Greenhouse Crops; C. Sonneveld and W. Voogt, Eds.; Springer: Netherlands: 2009; pp. 277–312.
- Zheng, Y. Rootzone management in cannabis production. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 163–188.
- Scott, C.; Punja, Z.K. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Can. J. Plant Pathol. 2020. 43(3), 394–412. [CrossRef]
- Janisiewicz, W.J.; Takeda, F.; Nichols, B.; Glenn, D.M.; Jurick II, W.M.; Camp, M.J. Use of low-dose UV-C irradiation to control powdery mildew caused by Podosphaera aphanis on strawberry plants. Can. J. Plant Pathol. 2016. 38(4), 430–439. [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016. 105, 1–11. [CrossRef]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Tiniakou, C. Photomorphogenic reactions in geranium stimulated by brief exposures of ultraviolet-C irradiation. Plant Growth Regul. 2012. 68(3), 343–350.
- Bridgen, M.P. Using ultraviolet-c (UV-C) irradiation on greenhouse ornamental plants for growth regulation. Acta Hortic. 2016. 1134, 49-56. [CrossRef]
- Zheng, Y.; Llewellyn, D. Lighting and CO2 in cannabis production. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 163-186.
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Is twelve hours really the optimum photoperiod for promoting flowering in indoor-grown cultivars of Cannabis sativa ? Plants (Basel). 2023. 12(14), 2605. [CrossRef]
- Punja, Z.K. The diverse mycoflora present on dried cannabis (Cannabis sativa L., marijuana) inflorescences in commercial production. Can. J. Plant Pathol. 2021. 43(1), 88–100. [CrossRef]
- Matzneller, P.; Gutierrez, J.D.; Caplan, D. Canopy management. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 109-125.
- Caplan, D.; Matzneller, P.; Gutierrez, J.D. Harvest and post-harvest. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 292-310.
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010. 72(1), 1–13. [CrossRef]
- Mahmud, M.S.; Zaman, Q.U.; Esau, T.J.; Chang, Y.K.; Price, G.W.; Prithiviraj, B. Real-time detection of strawberry powdery mildew disease using a mobile machine vision system. Agronomy (Basel). 2020. 10(7), 1027. [CrossRef]
- Anagnostis, A.; Tagarakis, A.C.; Asiminari, G.; Papageorgiou, E.; Kateris, D.; Moshou, D.; Bochtis, D. A deep learning approach for anthracnose infected trees classification in walnut orchards. Comput. Electron. Agric. 2021, 182, 105998. [CrossRef]
- Fountas, S.; Malounas, I.; Athanasakos, L.; Avgoustakis, I.; Espejo-Garcia, B. AI-assisted vision for agricultural robots. AgriEngineering. 2022. 4(3), 674–694. [CrossRef]
- Shakeel, Q.; Bajwa, R.T.; Rashid, I.; Aslam, H.U.; Iftikhar, Y.; Mubeen, M.; Li, G.; & Wu, M. Concepts and applications of infrared thermography for plant disease measurement. In Trends in Plant Disease Assessment. I. Ul Haq and S. Ijaz, Eds.; Springer: Singapore, 2022; pp. 109–125.
- Elad, Y. Mechanisms involved in the biological control of Botrytis cinerea incited diseases. Eur. J. Plant Pathol. 1996. 102(8), 719–732. [CrossRef]
- Vos, C.M.F.; De Cremer, K.; Cammue, B.P.A.; De Coninck, B. Toolbox of Trichoderma spp. in the biocontrol of Botrytis cinerea disease. Mol. Plant Pathol. 2015. 16(4), 400–412. [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000. 19(8), 709–714. [CrossRef]
- Ahmed, M.F.A. Evaluation of some biocontrol agents to control Thompson seedless grapevine powdery mildew disease. Egypt. J. Biol. Pest Control 2018. 28(1), 1–7. [CrossRef]
- Esawy, A.A.; Elsharkawy, M.M.; Omara, R.I.; Khalifa, M.A.F.; Fadel, F.M.; El-Naggar, M.M. Biological control of Golovinomyces cichoracearum, the causal pathogen of sunflower powdery mildew. Egypt. J. Biol. Pest Control 2021. 31(1), 1–10. [CrossRef]
- Konstantinidou-Doltsinis, S.; Schmit, A. Impact of treatment with plant extracts from Reynoutria sachalinensis (F. Schmidt) Nakai on intensity of powdery mildew severity and yield in cucumber under high disease pressure. Crop Prot. 1998. 17(8), 649–656. [CrossRef]
- Avila-Adame, C.; Tan, E.; Campbell, B.; Huang, H.; Fernandez, L.; Koivunen, M.; Marrone, P. MOI-106: A new alternative for controlling fungal plant pathogens in ornamentals and edible crops. Phytopathology 2008. 98(6).
- Esquivel-Cervantes, L.F.; Tlapal-Bolaños, B.; Tovar-Pedraza, J.M.; Pérez-Hernández, O.; Leyva-Mir, S.G.; Camacho-Tapia, M. Efficacy of biorational products for managing diseases of tomato in greenhouse production. Plants (Basel) 2022. 11(13), 1638. [CrossRef]
- Margaritopoulou, T.; Toufexi, E.; Kizis, D.; Balayiannis, G.; Anagnostopoulos, C.; Theocharis, A.; Rempelos, L.; Troyanos, Y.; Leifert, C.; Markellou, E. Reynoutria sachalinensis extract elicits SA-dependent defense responses in courgette genotypes against powdery mildew caused by Podosphaera xanthii. Sci. Rep. 2020. 10(1), 3354. [CrossRef]
- Abdu-Allah, G.A.M.; Abo-Elyousr, K.A.M. Effect of certain plant extracts and fungicides against powdery mildew disease of grapevines in upper Egypt. Arch. Phytopathol. Plant Prot. 2017. 50(19-20), 957–969.
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. 2nd ed. 2018; Springer, Cham: Edinburgh, Scotland.
- Hazekamp, A. Evaluating the effects of gamma-irradiation for decontamination of medicinal cannabis. Front. Pharmacol. 2016. 7(108). [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Effects of cold plasma, gamma and e-beam irradiations on reduction of fungal colony forming unit levels in medical cannabis inflorescences. J. Cannabis Res. 2020. 2(1), 12. [CrossRef]
- Majumdar, C.G.; ElSohly, M.A.; Ibrahim, E.A.; Elhendawy, M.A.; Stanford, D.; Chandra, S.; Wanas, A.S.; Radwan, M.M. Effect of gamma irradiation on cannabinoid, terpene, and moisture content of cannabis biomass. Molecules (Basel) 2023. 28(23), 7710. [CrossRef]
- Dhillon, G.S.; Hukkeri, S.; Nightingale, D.; Callaway, J. Evaluation of different techniques to decontaminate medical cannabis and their effect on cannabinoid content. ACS Agric. Sci. Technol. 2022. 2(6), 1126–1133. [CrossRef]
- Frink, S.; Marjanovic, O.; Tran, P.; Wang, Y.; Guo, W.; Encarnacion, N.; Alcantara, D.; Moezzi, B.; Vrdoljak, G. Use of X-ray irradiation for inactivation of Aspergillus in cannabis flower. PloS One 2022, 17(11), 277649-277649. [CrossRef]
- Gautam, A.K.; Kant, M.; Thakur, Y. Isolation of endophytic fungi from Cannabis sativa and study their antifungal potential. Arch. Phytopathol. 2013. 46(6), 627–635. [CrossRef]
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013. 60(1), 137–151. [CrossRef]
- Taghinasab, M.; Jabaji, S. Cannabis microbiome and the role of endophytes in modulating the production of secondary metabolites: An overview. Microorganisms (Basel). 2020. 8(3), 355. [CrossRef]
- Balthazar, C.; Novinscak, A.; Cantin, G.; Joly, D.L.; Filion, M. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other cannabis fungal pathogens. Phytopathology 2022. 112(3), 549–560. [CrossRef]
- Lyu, D.; Backer, R.; Robinson, W.G.; Smith, D.L. Plant growth-promoting rhizobacteria for cannabis production: Yield, cannabinoid profile and disease resistance. Front Microbiol. 2019. 10(1761). [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions between Bacillus spp., Pseudomonas spp. and Cannabis sativa promote plant growth. Front. Microbiol. 2021. 12, 715758. [CrossRef]
- Scott, M.; Rani, M.; Samsatly, J.; Charron, J.-B.; Jabaji, S. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: Identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can. J. Microbiol. 2018. 64(10), 664–680. [CrossRef]
- Gabriele, M.; Vitali, F.; Chelucci, E.; Chiellini, C. Characterization of the cultivable endophytic bacterial community of seeds and sprouts of Cannabis sativa L. and perspectives for the application as biostimulants. Microorganisms (Basel). 2022. 10(9), 1742. [CrossRef]
- Kusari, P.; Kusari, S.; Lamshöft, M.; Sezgin, S.; Spiteller, M.; Kayser, O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl. Microbiol. Biotechnol. 2014. 98(16), 7173–7183. [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases – Review and future prospects. Biol. Control. 2016. 103, 62–68. [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001. 52, 487–511. [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27(12), 3622–3633. [CrossRef]
- Holmes, J.E.; Lung, S.; Collyer, D.; Punja, Z.K. Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front. Plant Sci. 2021. 12, 732344. [CrossRef]
- Punja, Z.K.; Scott, C. Organically grown cannabis (Cannabis sativa L.) plants contain a diverse range of culturable epiphytic and endophytic fungi in inflorescences and stem tissues. Botany. 2023. 101(7), 255–269. [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The entomopathogenic fungi Metarhizium brunneum and Beauveria bassiana promote systemic immunity and confer resistance to a broad range of pests and pathogens in tomato. Phytopathology. 2022. 112(4), 784–793. [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: modifiers of plant disease. Plant Mol. Biol. 2016. 90, 645–655. [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K. Use of endophytes as biocontrol agents. Fung. Biol. Reviews. 2019. 33(2), 133-148. [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; Bhowmik, P. Medical cannabis and industrial hemp tissue culture: present status and future potential. Front. Plant Sci. 2021. 12, 627240–627240. [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants. 2021. 10(1), 185. [CrossRef]
- Slack, S.A.; Tufford, L.A. Meristem culture for virus elimination. In Plant Cell, Tissue and Organ Culture. O.L. Gamborg and G.C. Phillips, Eds.; Springer: Berlin, Germany, 1995; pp. 117-128.
- Rao, C. K. Adoption of tissue culture in horticulture : A study of banana-growing farmers from a South-Indian state. Cambridge Scholars Publishing. 2014.
- Sharma, N.; Sharma, L.; Singh, B.P. Propagation. In Strawberries. R.M. Sharma, R. Yamdagni, A.K. Dubey, and V. Pandey, Eds.; CRC Press: United Kingdom, 2019; pp. 179–192.
- Nehra, N.S.; Kartha, K.K. Meristem and shoot tip culture: requirements and applications. In Plant Cell and Tissue Culture; I.K. Vasil and T.A. Thorpe, Eds.; Springer: Dordrecht, Netherlands, 1994; pp. 37-70.
- De Jesús Romo-Paz, F.; Folgado, R.; Delgado-Aceves, L.; Zamora-Natera, J.F.; Portillo, L. Tissue culture of Physalis angulata L. (Solanaceae): techniques for micropropagation and germplasm long-term preservation. Plant Cell Tissue Organ Cult. 2021. 144(1), 73–78. [CrossRef]
- Zapata, C. J.; Miller, C. J.; Smith, R. H. An in vitro procedure to eradicate potato viruses X, Y, and S from Russet Norkotah and two of its strains. In Vitro Cell. Dev. Biol. Plant 1995. 31(3), pp. 153–159. [CrossRef]
- Bhojwani, S. S.; Dantu, P. K. Production of virus-free plants. In Plant Tissue Culture: An Introductory Text. Springer India, 2013; pp. 227–243.
- Wang, Q.; Cuellar, W. J.; Rajamäki, M.-L.; Hirata, Y.; Valkonen, J. P. Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol. Plant Pathol. 2008. 9(2), 237–250. [CrossRef]
- Singh, V.; Adil, S.; Quraishi, A. Elimination of BBTV via a systemic in vitro electrotherapy approach. J. Virol. Methods 2022. 300, 114367–114367. [CrossRef]
- Kanwar, J.; Kaul, M. K.; Naruka, I. S.; Singh, P. P. In-vitro micrografting technique in sweet orange (Citrus sinensis) cv. Blood Red to produce virus free plants. Indian J. Agric. Sci. 2019. 89(3), 494-499. [CrossRef]
- Akinrinlola, R. J.; Hansen, Z. R. Efficacy of organic fungicides against hemp powdery mildew caused by Golovinomyces ambrosiae in a greenhouse in Tennessee. Plant Dis. 2023. 107(6), 1867–1873. [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K. P.; Keswani, C.; Minkina, T.; Srivastava, A. K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022. 13, 883970–883970. [CrossRef]
- Datnoff, E. L.; Elmer, W. H.; Rodrigues, F. A. Mineral Nutrition and Plant Disease. Second Edition. Amer. Phytopathol. Soc. St. Paul, Minnesota, 2023.
- Lamichhane, J. R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J. B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018. 38(3), 1–18. [CrossRef]
- Flemming, C. A.; Trevors, J. T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989. 44(1), 143–158. [CrossRef]
- Mayton, H.; Amirkhani, M.; Loos, M.; Johnson, B.; Fike, J.; Johnson, C.; Myers, K.; Starr, J.; Bergstrom, G. C.; Taylor, A. Evaluation of industrial hemp seed treatments for management of damping-off for enhanced stand establishment. Agric. (Basel). 2022. 12(5), 591. [CrossRef]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Plaza Pérez, C. D.; De La Torre-Roche, R.; Zuverza-Mena, N.; Haynes, C. L.; White, J. C.; Hamers, R. J. Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): Role of particle morphology, composition and dissolution behavior. ACS Sustain. Chem. Eng. 2018. 6(11), 14847–14856. [CrossRef]
- Shen, Y.; Borgatta, J.; Ma, C.; Elmer, W.; Hamers, R. J.; White, J. C. Copper nanomaterial morphology and composition control foliar transfer through the cuticle and mediate resistance to root fungal disease in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2020. 68(41), 11327–11338. [CrossRef]
- Freires, A.L.A.; Figueiredo, F.R.A.; Alves, T.R.C.; Barroso, K.A.; da Silva, I.V.P.; Silva, J. L.S.; de Almeida Nogueira, G.; Melo, N.J.A.; Júnior, R.S.; Negreiros, A.M.P.; de Queiroz Ambrósio, M.M. Alternative products in the management of powdery mildew (Podosphaera xanthii) in melon. Trop. Plant Pathol. 2022. 47(5), 608–617. [CrossRef]
- Newman, S.; Roll, M.; Harkrader, R. Naturally occurring compounds for controlling powdery mildew of greenhouse roses. HortScience. 1999. 34(4), 686–689. [CrossRef]
- Mmbaga, M. T.; Sauve, R. J. Management of powdery mildew in flowering dogwood in the field with biorational and conventional fungicides. Can. J. Plant Sci. 2004, 84(3), 837–844. [CrossRef]
- Aleksic, G.; Milicevic, Z.; Kuzmanovic, S.; Starovic, M.; Stevanovic, M.; Delibasic, G.; Zivkovic, S. Efficacy of copper citrate in grapevine disease control. Pestic. Fitomed. 2019. 34(2), 103–109. [CrossRef]
- Sakr, N. Silicon control of bacterial and viral diseases in plants. J. Plant Prot. Res. 2016. 56(4), 331–336. [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H. Y. H. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020. 168, 104641. [CrossRef]
- Dixon, E.; Leonberger, K.; Amsden, B.; Szarka, D.; Munir, M.; Payee, W.; Datnoff, L.; Tubana, B.; Gauthier, N. Suppression of hemp powdery mildew using root-applied silicon. Plant Health Prog. 2022. 23(3), 260–264. [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Ehret, D.L.; Menzies, J.G. Distribution of silicon in cucumber leaves during infection by the powdery mildew fungus (Sphaerotheca fuliginea). Can. J. Bot. 1991. 69(1), 140–146. [CrossRef]
- Liang, Y.; Sun, W.; Si, J.; Romheld, V. Effects of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005. 54(5), 678–685. [CrossRef]
- Shetty, R.; Jensen, B.; Shelton, D.; Jørgensen, K.; Pedas, P.; Jørgensen, H.J.L. Site-specific, silicon-induced structural and molecular defence responses against powdery mildew infection in roses. Pest Manag. Sci. 2021. 77(10). [CrossRef]
- Shetty, R.; Jensen, B.; Shetty, N.P.; Hansen, M.; Hansen, C.W.; Starkey, K.R.; Jørgensen, H.J.L. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 2012. 61(1), 120-131. [CrossRef]
- Kanto, T.; Miyoshi, A.; Ogawa, T.; Maekawa, K.; Aino, M. Suppressive effect of potassium silicate on powdery mildew of strawberry in hydroponics. J. Gen. Plant Pathol. 2004. 70(4), 207. [CrossRef]
- Liu, B.; Davies, K.; Hall, A. Silicon builds resilience in strawberry plants against both strawberry powdery mildew Podosphaera aphanis and two-spotted spider mites Tetranychus urticae. PloS One. 2020. 15(12). [CrossRef]
- Reekie, H.; Punja, Z.K. Calcium and plant disease. In Mineral Nutrition and Plant Disease – Second Edition. Datnoff, E.L., Elmer, W.H., Rodrigues, F.A., Eds.; Amer. Phytopathol. Soc., St. Paul, Minnesota, 2023.
- Elad, Y.; Volpin, H. Reduced development of grey mould (Botrytis cinerea) in bean and tomato plants by calcium nutrition. J. Phytopathol. 1993, 139(2), 146–156. [CrossRef]
- Volpin, H.; Elad, Y. Influence of calcium nutrition on susceptibility of rose flowers to Botrytis blight. Phytopathology 1991. 81(11), 1390. [CrossRef]
- Yermiyahu, U.; Shamai, I.; Peleg, R.; Dudai, N.; Shtienberg, D. Reduction of Botrytis cinerea sporulation in sweet basil by altering the concentrations of nitrogen and calcium in the irrigation solution. Plant Pathol. 2006. 55(4), 544-552. [CrossRef]
- Traversari, S.; Cacini, S.; Galieni, A.; Nesi, B.; Nicastro, N.; Pane, C. Precision agriculture digital technologies for sustainable fungal disease management of ornamental plants. Sustainability. 2021, 13(7), 3707. [CrossRef]
- Berdugo, C.A.; Zito, R.; Paulus, S.; Mahlein, A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2014. 63(6), 1344-1356. [CrossRef]
- Xu, H.; Zhu, S.; Ying, Y.; Jiang, H. Early detection of plant disease using infrared thermal imaging. SPIE Conference Proceedings 2006. 6381(1), 638110-638117.
- Vagelas, I.; Papadimos, A.; Lykas, C. Pre-symptomatic disease detection in the vine, chrysanthemum, and rose leaves with a low-cost infrared sensor. Agronomy. 2021. 11(9), 1682. [CrossRef]
- Lindenthal, M.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology. 2005. 95(3), 233-240. [CrossRef]
- Liaghat, S.; Mansor, S.; Ehsani, R.; Shafri, H.Z.M.; Meon, S.; Sankaran, S. Mid-infrared spectroscopy for early detection of basal stem rot disease in oil palm. Comput. Electron. Agric. 2014. 101, 48-54. [CrossRef]
- Morelli, M.; Bahar, O.; Papadopoulou, K.K.; Hopkins, D.L.; Obradović, A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020. 11(1312). [CrossRef]
- Sharma, A.K.; Sharma, P. Eds. Trichoderma – Host-pathogen interactions and applications. Springer Nature, Singapore Pte. Limited. 79 pp.
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: our best fungal allies in the biocontrol of plant diseases –A review. Plants (Basel). 2023 12(3):432. [CrossRef]
Figure 1.
The different stages of cannabis production under greenhouse conditions. Each crop cultivation cycle from propagation to harvest spans ~12-15 weeks. This is followed by a final stage of post-harvest processing that includes drying, trimming, curing and storage.
Figure 1.
The different stages of cannabis production under greenhouse conditions. Each crop cultivation cycle from propagation to harvest spans ~12-15 weeks. This is followed by a final stage of post-harvest processing that includes drying, trimming, curing and storage.

Figure 2.
The stages of cannabis crop development. (a) Stock plants. (b) Rooting of cuttings. (c) Vegetative plants. (d, e) Flowering plants. (f) Harvested inflorescences.
Figure 2.
The stages of cannabis crop development. (a) Stock plants. (b) Rooting of cuttings. (c) Vegetative plants. (d, e) Flowering plants. (f) Harvested inflorescences.

Figure 3.
Integrated disease management strategies (left, in brown) are developed according to the crop development stage (top). The hexagons (in green) illustrate the specific diseases being targeted, which are discussed in more detail below. HLVd = Hop latent viroid, PM = powdery mildew, Botrytis = bud rot.
Figure 3.
Integrated disease management strategies (left, in brown) are developed according to the crop development stage (top). The hexagons (in green) illustrate the specific diseases being targeted, which are discussed in more detail below. HLVd = Hop latent viroid, PM = powdery mildew, Botrytis = bud rot.

Figure 4.
Symptoms of infection by a range of pathogens commonly observed on cannabis stock plants. (a) Declining growth with reduced vigour. (b, c) Internal stem discoloration due to F. oxysporum infection. (d) Isolation of colonies of F. oxysporum from diseased tissues. (e) Browning of roots due to Pythium infection. (f) Isolation of Pythium colonies from diseased roots. (g) Powdery mildew infection on leaves. (h, i,) Infection by Hop latent viroid may cause reduced vigor and curling of young leaves.
Figure 4.
Symptoms of infection by a range of pathogens commonly observed on cannabis stock plants. (a) Declining growth with reduced vigour. (b, c) Internal stem discoloration due to F. oxysporum infection. (d) Isolation of colonies of F. oxysporum from diseased tissues. (e) Browning of roots due to Pythium infection. (f) Isolation of Pythium colonies from diseased roots. (g) Powdery mildew infection on leaves. (h, i,) Infection by Hop latent viroid may cause reduced vigor and curling of young leaves.

Figure 6.
The impact of eradication of HLVd-infected stock plants on the frequency of positively infected plants over a 6-month duration. The blue line shows the actual incidence of infected plants, which fluctuates over time. The solid green line is the general trend that shows a decline in numbers of infected plants.
Figure 6.
The impact of eradication of HLVd-infected stock plants on the frequency of positively infected plants over a 6-month duration. The blue line shows the actual incidence of infected plants, which fluctuates over time. The solid green line is the general trend that shows a decline in numbers of infected plants.
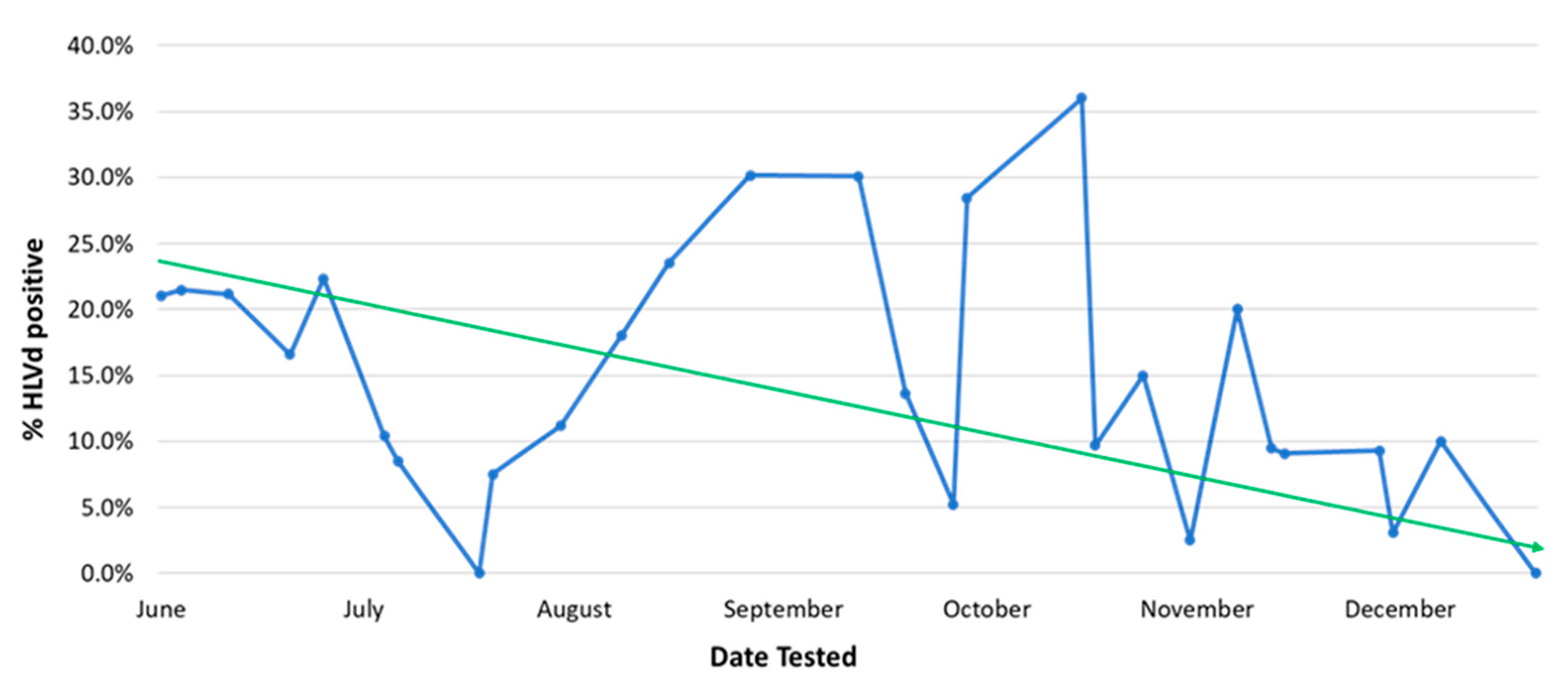
Figure 7.
The effect of reduced risk products on pathogen growth can be evaluated under laboratory conditions by testing a range of concentrations in liquid culture medium. (a) Growth in potato dextrose broth containing a range of concentrations of individual products is measured by obtaining mycelium dry weights after a 7-day exposure. (b) The effect of Zerotol® and hypochlorous acid on growth of two pathogens at increasing concentrations is shown. Both Fusarium and Pythium are reduced at higher concentrations but growth of Pythium shows greater sensitivity compared to Fusarium. (c) Growth of Trichoderma can also be reduced by the presence of specific compounds.
Figure 7.
The effect of reduced risk products on pathogen growth can be evaluated under laboratory conditions by testing a range of concentrations in liquid culture medium. (a) Growth in potato dextrose broth containing a range of concentrations of individual products is measured by obtaining mycelium dry weights after a 7-day exposure. (b) The effect of Zerotol® and hypochlorous acid on growth of two pathogens at increasing concentrations is shown. Both Fusarium and Pythium are reduced at higher concentrations but growth of Pythium shows greater sensitivity compared to Fusarium. (c) Growth of Trichoderma can also be reduced by the presence of specific compounds.

Figure 8.
Examples of cannabis genotypes that exhibit a level of disease tolerance to different pathogens. (a) Fusarium damping-off, with susceptible genotype on the left and tolerant genotype on the right. (b) Powdery mildew, with susceptible genotype on the left and tolerant one on the right. (c,d) Alternaria leaf blight, with tolerant genotype on the left and susceptible one on the right. (d) Botrytis bud rot, with tolerant genotype on the left and susceptible one on the right..
Figure 8.
Examples of cannabis genotypes that exhibit a level of disease tolerance to different pathogens. (a) Fusarium damping-off, with susceptible genotype on the left and tolerant genotype on the right. (b) Powdery mildew, with susceptible genotype on the left and tolerant one on the right. (c,d) Alternaria leaf blight, with tolerant genotype on the left and susceptible one on the right. (d) Botrytis bud rot, with tolerant genotype on the left and susceptible one on the right..

Figure 9.
Propagation of cannabis from vegetative cuttings and development of Fusarium damping-off. (a) A tray of healthy cuttings. (b) A tray of cuttings infected with Fusarium oxysporum. (c, d, e) Close-up views of damped-off cuttings. (f) A cross-sectional view of the stem a healthy cutting (left) compared to a diseased one (right) in which tissue browning can be seen. (g) A scanning electron microscopic view of a section through the stem of a healthy cutting. The central pith can be seen. (h) A collapsed stem of a diseased cutting viewed through the scanning electron microscope. The central pith has collapsed as well as surrounding cells.
Figure 9.
Propagation of cannabis from vegetative cuttings and development of Fusarium damping-off. (a) A tray of healthy cuttings. (b) A tray of cuttings infected with Fusarium oxysporum. (c, d, e) Close-up views of damped-off cuttings. (f) A cross-sectional view of the stem a healthy cutting (left) compared to a diseased one (right) in which tissue browning can be seen. (g) A scanning electron microscopic view of a section through the stem of a healthy cutting. The central pith can be seen. (h) A collapsed stem of a diseased cutting viewed through the scanning electron microscope. The central pith has collapsed as well as surrounding cells.

Figure 10.
Spores of a range of pathogens that can affect cannabis plants at various stages of crop growth. (a) Fusarium oxysporum micro-conidia. (b) Botrytis cinerea spores. (c) Large cluster of spores of Aspergillus sp. (d, e) Chains of spores of Penicillium sp. (f) Powdery mildew spores. .
Figure 10.
Spores of a range of pathogens that can affect cannabis plants at various stages of crop growth. (a) Fusarium oxysporum micro-conidia. (b) Botrytis cinerea spores. (c) Large cluster of spores of Aspergillus sp. (d, e) Chains of spores of Penicillium sp. (f) Powdery mildew spores. .

Figure 11.
Application of biological control agents provides protection to cannabis cuttings against Fusarium damping-off. (a) Rootshield-treated cuttings (left) show greater survival compared to pathogen-only (right). (b) Growth of Trichoderma harzianum from Rootshield-treated cuttings. (c) Asperello-treated cuttings (right) show greater survival compared to pathogen-only (left). (d) Growth of Trichoderma asperellum from Asperello-treated cuttings. (e) Prestop-treated cuttings (left) show greater survival compared to pathogen-only. (f) Growth of Gliocladium catenulatum from Prestop-treated cuttings.
Figure 11.
Application of biological control agents provides protection to cannabis cuttings against Fusarium damping-off. (a) Rootshield-treated cuttings (left) show greater survival compared to pathogen-only (right). (b) Growth of Trichoderma harzianum from Rootshield-treated cuttings. (c) Asperello-treated cuttings (right) show greater survival compared to pathogen-only (left). (d) Growth of Trichoderma asperellum from Asperello-treated cuttings. (e) Prestop-treated cuttings (left) show greater survival compared to pathogen-only. (f) Growth of Gliocladium catenulatum from Prestop-treated cuttings.

Figure 12.
Growth of T. asperellum (top) is observed to stop the growth of Fusarium oxysporum (bottom) when both are placed on a Petri dish. After a few days, the biocontrol agent continues to grow over and inhibit further growth of the pathogen..
Figure 12.
Growth of T. asperellum (top) is observed to stop the growth of Fusarium oxysporum (bottom) when both are placed on a Petri dish. After a few days, the biocontrol agent continues to grow over and inhibit further growth of the pathogen..

Figure 13.
Pythium and Fusarium infection on vegetative plants of cannabis. (a) Symptoms of yellowing of the foliage are indicative of root infection by these pathogens. (b) Death of rooted cuttings due to Fusarium infection. (c) Root development on healthy plant (left) compared to one infected by Fusarium (right). (d) Internal stem discoloration is indicative of infection by Fusarium. (e, f) Infection by Pythium can cause significant stunting of plant growth and death (right) compared to healthy plants (left).
Figure 13.
Pythium and Fusarium infection on vegetative plants of cannabis. (a) Symptoms of yellowing of the foliage are indicative of root infection by these pathogens. (b) Death of rooted cuttings due to Fusarium infection. (c) Root development on healthy plant (left) compared to one infected by Fusarium (right). (d) Internal stem discoloration is indicative of infection by Fusarium. (e, f) Infection by Pythium can cause significant stunting of plant growth and death (right) compared to healthy plants (left).

Figure 14.
Symptoms due to pathogen infection on flowering cannabis plants. (a) Yellowing of the foliage and stunted growth due to infection by Fusarium. (b) Wilting of plants and yellowing of foliage due to infection by Pythium. (c) Powdery mildew development on inflorescences and surrounding leaves. (d,e) Bud rot caused by B. cinerea destroys the inflorescence.
Figure 14.
Symptoms due to pathogen infection on flowering cannabis plants. (a) Yellowing of the foliage and stunted growth due to infection by Fusarium. (b) Wilting of plants and yellowing of foliage due to infection by Pythium. (c) Powdery mildew development on inflorescences and surrounding leaves. (d,e) Bud rot caused by B. cinerea destroys the inflorescence.

Figure 15.
The most common fungi recovered from inflorescences of cannabis plants. The Petri dishes show the results from swabbing of samples and plating onto an agar medium that allows growth of yeasts and molds to occur. On top row – (left to right) Penicillium, Cladosporium, Aspergillus. (On bottom row) Botrytis, Penicillium and Fusarium. Photos were taken after 7 days.
Figure 15.
The most common fungi recovered from inflorescences of cannabis plants. The Petri dishes show the results from swabbing of samples and plating onto an agar medium that allows growth of yeasts and molds to occur. On top row – (left to right) Penicillium, Cladosporium, Aspergillus. (On bottom row) Botrytis, Penicillium and Fusarium. Photos were taken after 7 days.

Figure 16.
(a) Effect of enhanced air flow around cannabis plants using circulating fans on total colony-forming units of microbes in these tissues. Vertical bars show total colony-forming units of total aerobic count (TAMC), bile-tolerant Gram-negative count (BTGN) and total yeast and mold count (TYMC) with and without air circulation. (b) Fans were positioned 35 cm above the crop to circulate air continuously at ~7 m/s over ~40 plants, beginning in week 2 of the flowering period until harvest. The trial was replicated three times in different greenhouse compartments. Inflorescences were dried prior to microbial analysis.
Figure 16.
(a) Effect of enhanced air flow around cannabis plants using circulating fans on total colony-forming units of microbes in these tissues. Vertical bars show total colony-forming units of total aerobic count (TAMC), bile-tolerant Gram-negative count (BTGN) and total yeast and mold count (TYMC) with and without air circulation. (b) Fans were positioned 35 cm above the crop to circulate air continuously at ~7 m/s over ~40 plants, beginning in week 2 of the flowering period until harvest. The trial was replicated three times in different greenhouse compartments. Inflorescences were dried prior to microbial analysis.
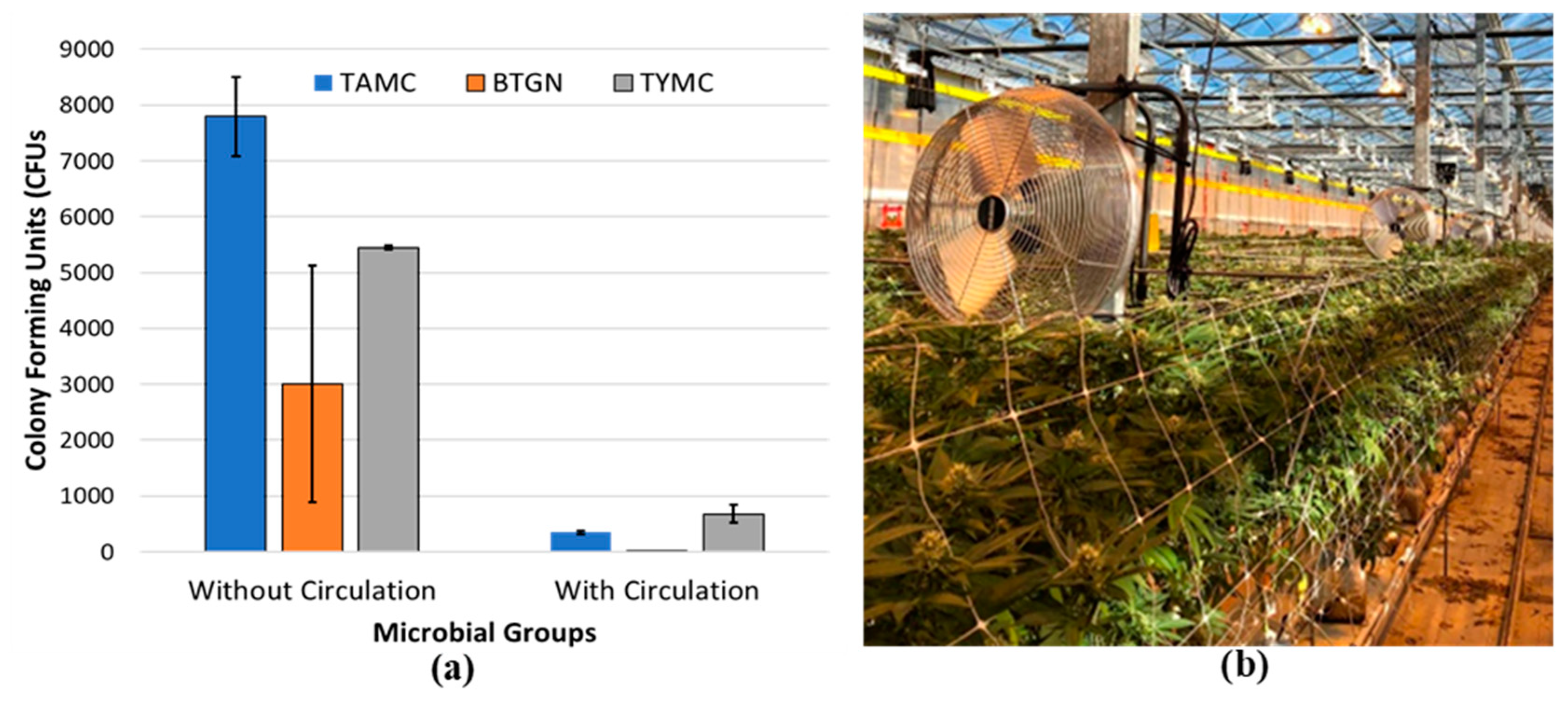
Figure 17.
Influence of cannabis genotype and time of year (season) on total microbes present in dried cannabis inflorescences. Vertical bars denote total aerobic microbial count (TAMC), bile-tolerant Gram-negative count (BTGN) and total yeast and mold count (TYMC). Samples were taken from three genotypes during three harvests in each season (fall, winter, summer season) of the same year. Highest microbial counts were observed in the September harvest period corresponding to late-summer production. The failure thresholds for each microbial group are shown by the horizontal lines. Genotype ‘PD’ contained the highest microbial levels.
Figure 17.
Influence of cannabis genotype and time of year (season) on total microbes present in dried cannabis inflorescences. Vertical bars denote total aerobic microbial count (TAMC), bile-tolerant Gram-negative count (BTGN) and total yeast and mold count (TYMC). Samples were taken from three genotypes during three harvests in each season (fall, winter, summer season) of the same year. Highest microbial counts were observed in the September harvest period corresponding to late-summer production. The failure thresholds for each microbial group are shown by the horizontal lines. Genotype ‘PD’ contained the highest microbial levels.

Figure 18.
Comparison of disease incidence on six cannabis genotypes to four pathogens, demonstrating variation in susceptibility to Botrytis bud rot, powdery mildew, hop latent viroid and Pythium or Fusarium root diseases. Incidence data were obtained from scouting reports made during the cultivation of batches of genotypes in comparable greenhouse compartments over three production cycles in the summer season.
Figure 18.
Comparison of disease incidence on six cannabis genotypes to four pathogens, demonstrating variation in susceptibility to Botrytis bud rot, powdery mildew, hop latent viroid and Pythium or Fusarium root diseases. Incidence data were obtained from scouting reports made during the cultivation of batches of genotypes in comparable greenhouse compartments over three production cycles in the summer season.

Figure 19.
Comparative efficacy of six biological control products and reduced risk chemicals on Botrytisbud rot development on flowering cannabis plants. Three applications were made at weeks 2, 3 and 4 of the flowering period at maximum label rates. The sprays were applied to ca. 216 plants using a robotic pipe rail sprayer that delivered ~60 mL of product to each plant. Disease assessments were made at harvest (week 8) in a greenhouse compartment with low and high Botrytis bud rot pressure from natural inoculum. (a) Low disease pressure flower room; (b) High disease pressure flower room.
Figure 19.
Comparative efficacy of six biological control products and reduced risk chemicals on Botrytisbud rot development on flowering cannabis plants. Three applications were made at weeks 2, 3 and 4 of the flowering period at maximum label rates. The sprays were applied to ca. 216 plants using a robotic pipe rail sprayer that delivered ~60 mL of product to each plant. Disease assessments were made at harvest (week 8) in a greenhouse compartment with low and high Botrytis bud rot pressure from natural inoculum. (a) Low disease pressure flower room; (b) High disease pressure flower room.

Figure 20.
Effect of Rootshield HC® (T. harzianum) applications made at weeks 2, 3, and 4 of the flowering period on final microbial levels in harvested cannabis inflorescences. (a) Total counts of all microbes in both untreated and sprayed plants are shown. Total microbes were reduced following Rootshield applications. (b) The growthof microbial colonies after blending of the treated inflorescences in distilled water and subsequent plating onto agar medium. A comparison is shown of samples following applications of Rootshield made at weeks 2, 3 and 4 of the flowering period. Samples treated atweek 4 show maximum suppression of Penicillium growth compared to week 2 where there is no suppression and no colonies of Trichoderma were recovered.
Figure 20.
Effect of Rootshield HC® (T. harzianum) applications made at weeks 2, 3, and 4 of the flowering period on final microbial levels in harvested cannabis inflorescences. (a) Total counts of all microbes in both untreated and sprayed plants are shown. Total microbes were reduced following Rootshield applications. (b) The growthof microbial colonies after blending of the treated inflorescences in distilled water and subsequent plating onto agar medium. A comparison is shown of samples following applications of Rootshield made at weeks 2, 3 and 4 of the flowering period. Samples treated atweek 4 show maximum suppression of Penicillium growth compared to week 2 where there is no suppression and no colonies of Trichoderma were recovered.

Figure 21.
Effect of Rootshield HC® applications on development of powdery mildew. Three weekly applications were made to the foliage of flowering plants as preventative treatments and compared to an untreated control and a water control. (a) Untreated control leaves. (b) Rootshield HC® treated leaves. (c) Water treated leaves.
Figure 21.
Effect of Rootshield HC® applications on development of powdery mildew. Three weekly applications were made to the foliage of flowering plants as preventative treatments and compared to an untreated control and a water control. (a) Untreated control leaves. (b) Rootshield HC® treated leaves. (c) Water treated leaves.

Figure 22.
Comparative efficacy of reduced risk products at managing powdery mildew development on cannabis genotype ‘MP’. (a-d) Disease was rated according to the scale shown, from 0 (a) to 3 (d). (e) Products were applied as preventative treatments at days 0, 7, and 14 of the flowering period. (f) Products were applied as a curative treatment, once at day 42 of the flowering period, after the onset of disease development. The trials were conducted during the spring growing season.
Figure 22.
Comparative efficacy of reduced risk products at managing powdery mildew development on cannabis genotype ‘MP’. (a-d) Disease was rated according to the scale shown, from 0 (a) to 3 (d). (e) Products were applied as preventative treatments at days 0, 7, and 14 of the flowering period. (f) Products were applied as a curative treatment, once at day 42 of the flowering period, after the onset of disease development. The trials were conducted during the spring growing season.

Figure 23.
Operational flow chart for various IDM approaches that can be incorporated into an IDM program according to cannabis cultivation stage.
Figure 23.
Operational flow chart for various IDM approaches that can be incorporated into an IDM program according to cannabis cultivation stage.

Figure 24.
Examples of endophytic fungal and bacterial species recovered from cannabis stem segments following sterilization. On the left Petri dish are Penicillium species and on the right are Chaetomium and bacterial species. .
Figure 24.
Examples of endophytic fungal and bacterial species recovered from cannabis stem segments following sterilization. On the left Petri dish are Penicillium species and on the right are Chaetomium and bacterial species. .

Figure 25.
Tissue-culture derived plants of cannabis can be obtained from meristem tips (a) and nodal explants (b) resulting in growth of a number of genotypes (c). The feasibility to generate large-scale production of pathogen-free planting materials awaits further research and development.
Figure 25.
Tissue-culture derived plants of cannabis can be obtained from meristem tips (a) and nodal explants (b) resulting in growth of a number of genotypes (c). The feasibility to generate large-scale production of pathogen-free planting materials awaits further research and development.

Figure 26.
Infrared and digital image comparisons to illustrate changes in plant surface temperatures at different stages of cannabis propagation. (a, b) A stock plant exhibiting a low transpiration rate (and high temperature, in yellow) compared to an adjacent plant with high transpiration (and lower temperature, in purple) shows a difference in surface temperatures that was attributed to infection by a root pathogen. (c, d) A cutting in the centre of a tray (arrow) with low transpiration (in yellow) surrounded by cuttings with higher transpiration rates. While the former cutting showed no obvious visual symptoms (d), early signs of pathogen infection and reduced rooting were observed. (e, f) A vegetative plant (arrow) with low transpiration (seen in yellow) among other plants with higher transpiration rates, shows notable differences in root health (g).
Figure 26.
Infrared and digital image comparisons to illustrate changes in plant surface temperatures at different stages of cannabis propagation. (a, b) A stock plant exhibiting a low transpiration rate (and high temperature, in yellow) compared to an adjacent plant with high transpiration (and lower temperature, in purple) shows a difference in surface temperatures that was attributed to infection by a root pathogen. (c, d) A cutting in the centre of a tray (arrow) with low transpiration (in yellow) surrounded by cuttings with higher transpiration rates. While the former cutting showed no obvious visual symptoms (d), early signs of pathogen infection and reduced rooting were observed. (e, f) A vegetative plant (arrow) with low transpiration (seen in yellow) among other plants with higher transpiration rates, shows notable differences in root health (g).
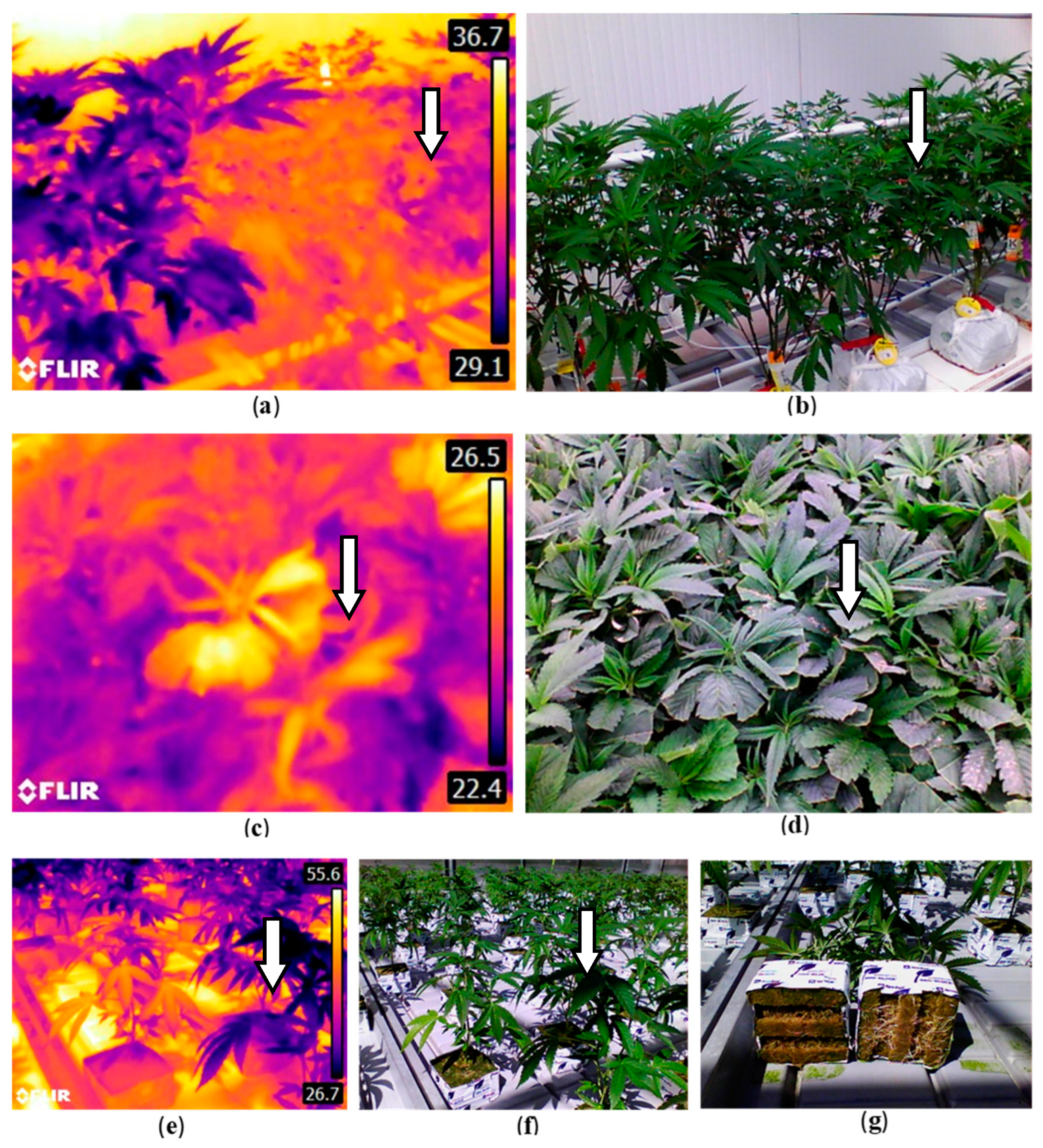
Table 1.
Summary of IDM strategies for four important pathogens affecting cannabis plants.
| HLVd Stunting Disease | Fusarium/Pythium Root & Crown Rot | Botrytis Bud Rot | Powdery Mildew | |
| Prevention | Test propagative materials and stock plants; utilize pathogen-free planting materials. | Test propagative materials and stock plants; utilize pathogen-free planting materials. | Reduce canopy humidity by adjusting planting density and enhancing air circulation. | Maintain an even climate, above 21°C, and vaporize sulfur nightly. |
| Sanitation | Clean equipment and bench surfaces; destroy diseased plants. | Clean equipment and bench surfaces; actively remove dead or diseased tissues. | Fog growing environment with reduced risk products prior to planting. | Fog growing environment with reduced risk products prior to planting. |
| Protection | Isolate propagative materials and stock plants in controlled access areas. | Apply Trichoderma harzianum and Gliocladium cantenulatum as a drench to rooted cuttings and plants. | Apply Rootshield HC® on developing inflorescences, from day 14 to day 28 of flowering. | Preventatively spray reduced risk products such as Suffoil-X, Regalia Maxx, on susceptible genotypes. |
| Monitoring | Scout regularly for symptoms; routinely sample water and suspect plants. | Scout regularly for symptoms; routinely sample water and suspect plants. | Conduct daily scouting for bud rot from the sixth week of flowering onwards. | Conduct weekly scouting at all plant development stages. |
| Eradication | Immediately remove and safely dispose of diseased plants at all stages of growth. | Immediately remove and safely dispose of diseased plants at all stages of growth. | Remove and dispose infected inflorescences; perform post-drying bud rot severity checks. | Remove infected leaves and dispose; spot spray reduced risk products. |
| Genotype Selection | Avoid highly susceptible genotypes; evaluate tolerant genotypes. | Avoid highly susceptible genotypes; evaluate tolerant genotypes. | Avoid planting highly susceptible genotypes during Botrytis-prone periods; evaluate tolerant genotypes. | Avoid highly susceptible genotypes; evaluate tolerant genotypes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









