Preprint
Article
The SGLT2 Inhibitors Empagliflozin Alleviate Renal Fibrosis by Inhibiting CTGF-Mediated Epithelial-Mesenchymal Transition in Diabetic Sprague-Dawley (SD) Rats
Altmetrics
Downloads
185
Views
69
Comments
0
This version is not peer-reviewed
Submitted:
23 February 2024
Posted:
23 February 2024
You are already at the latest version
Alerts
Abstract
Objective: Aims to investigate the effects of empagliflozin (EMPA), a sodium-glucose co-transporter 2 (SGLT2) inhibitor, on renal fibrosis in male diabetic Sprague-Dawley (SD) rat model. Methods: Streptozotocin(STZ) were injected to establish diabetes model, EMPA (10 mg/kg·d) or normal saline (NS) were administered for four weeks. The changes in renal fibrosis-related biomarkers, include vimentin (VIM), collagen types I(Col-I), collagen types III(Col-III), fibroblast-specific protein 1(FSP-1), connective tissue growth factor (CTGF), fibronectin (FN) were evaluated. Renal pathology, serum glucose levels and urinary protein excretion were also assessed. Results: Biomarkers of renal fibrosis were significantly reduced in the EMPA group compared to those in the NS group after 4-week treatment, including VIM (519.84±83.37 vs. 663.27±91.84 ng/ml, P<0.05), Col-I (21.27±1.46 vs. 25.71±2.82 ng/ml , P<0.05) and Col-III (7.06±1.12 vs. 9.43±1.36 ng/ml, P<0.05), FSP-1 (4.38±0.81 vs. 5.70±0.78 ng/ml, P<0.05), and CTGF (1147±217 vs. 1556±235 pg/ml, P<0.05),respectively. Nevertheless, compared to normal control (NC) group, the levels of renal fibrosis-related biomarkers in the EMPA group showed no significant differences except for VIM, which was VIM (336.66±28.87 vs. 519.84±83.37 ng/mL, P<0.001). NS group rats showed notable renal tubular dilation and casts, EMPA group exhibited only mild tubular dilation. Conclusion: These findings suggest that empagliflozin reverse renal fibrosis potentially through inhibiting CTGF-mediated epithelial-mesenchymal transition.
Keywords:
Subject: Medicine and Pharmacology - Endocrinology and Metabolism
Introduction
Diabetes is metabolic disorder characterized by abnormal insulin incretion or insulin resistance, leading to elevated blood glucose levels. As per the IDF Diabetes Atlas statistics in 2021, the global prevalence of diabetes among the population aged 20-79 was 10.5%, affecting approximately 536.6 million people. China, being the country with the highest number of diabetes patients worldwide, is projected to surpass 140 million cases in 2021 and is expected to exceed 174 million by the year 2045 [1]. According to the Chinese Kidney Disease Network Survey, since 2011, Diabetic Kidney Disease (DKD) has replaced glomerulonephritis as the leading cause of newly diagnosed chronic kidney disease in China [2].
Sodium-Glucose Co-Transporter 2 Inhibitors (SGLT2i) are emerging hypoglycemic drugs, which has been demonstrated to selectively inhibit SGLT2 in renal proximal tubules, reducing glucose reabsorption in the renal tubules, resulting in a hypoglycemic effect [3]. Furthermore, the renoprotective effects of various SGLT2i on diabetic kidneys have also been demonstrated in animal experiments and human studies [4,5,6]. The efficacy of empagliflozin in retarding the progression of diabetic nephropathy has been substantiated in prior studies [7].
Renal fibrosis is the ultimate outcome of diabetic nephropathy, characterized by disruption of normal kidney architecture, glomerulosclerosis, tubulointerstitial fibrosis, and tubular atrophy [8]. The main pathophysiological mechanism of diabetes nephropathy is that the increase of filtration membrane permeability leads to elevated proteinuria, the disrupted fluid equilibrium leads to hypertension, the decrease of erythropoiesis leads to anemia, and the increase of cardiovascular disease vulnerability. Our previous studies have confirmed that SGLT2i attenuate nephrin loss and enhance TGF-β1 excretion in type 2 diabetes patients with albuminuria [9], yet their underlying mechanism remains to be elucidated. We attempted to examine the effect of empagliflozin on the biomarkers of renal fibrosis in the Streptozotocin (STZ)-induced diabetes SD rats model.
Methods and Materials
Instruments and Reagents
The utilized instruments include the BS-350E Automatic biochemistry analyzer (Shenzhen Mindray Biomedical Electronics Co., Ltd., China), RT-6100 Fully Automatic Microplate Reader (Shenzhen LeiDu Life Science Co., Ltd., China), TS-10 Microplate Washer (Beijing Tianshi Tianxing Technology Co., Ltd., China), Electric Thermostatic Incubator (Wuhan Yiheng Sujing Scientific Instrument Co., Ltd., China), SCILOGEX D3024R Centrifuge (USA), and Pipettor (Thermo Scientific Finnpipette, USA). STZ was obtained from MP Biomedicals LLC (RRID MP_210055701). Vimentin (VIM)(RRID MM_71770R1), Fibronectin (FN)(RRID MM_0203R1), Collagen Type I (Col-I)(RRID MM_70241R1), Collagen Type III (Col-III)(RRID MM-70514R1), Connective Tissue Growth Factor (CTGF)(RRID MM_0084R1) Fibroblast-specific protein 1 (FSP-1)(1RRID MM_7836R1) were measured by enzyme-linked immunosorbent assay (ELISA) Kit (Jiangsu Meimian Industrial Co., Ltd., China). High-fat and high-sugar diets were purchased from Biotech Co., Ltd. (Beijing, China) (RRID HD_001). The SGLT2 inhibitors empaglifozin were purchased from Eli Lilly pharmaceuticals, Inc (USA). Pipette tips and experimental consumables are pre-sterilized prior to usage.
Animals
Thirty male SD rats (RRID: RRRC_00239) were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), aged 6-7 weeks and weighing 200-250 g, three SD rats were housed per cage. Animals were given free access to standard rodent chow and water and kept in a standard pathogen-free environment at 20°C - 25°C, relative humidity 40% - 70%, and alternating 12 hours of light-dark cycles (lights on at 8:00 and lights off at 20:00), and the experimental protocol received approval from the Medical Ethics Committee of Xiamen University School of Medicine (approval No. XMULAC20210043). All procedures were conducted following the Animal Experimental Ethics Commitment of the Xiamen University Laboratory Animal Center. At the end of the experiment, EMPA Group(n=11) and NS Group(n=10) rats survived, deceased rats in the NS group exhibited significant hematuria, suggesting a potential cause of death as acute renal failure. The cause of death in the EMPA group rat was suspected to be due to inter-fighting among the rats, resulting in fatal injuries. Cage separation was implemented accordingly.
Experimental Design
The thirty male SD rats were randomly allocated into three distinct groups using a random number table: The first group (Group 1) was consists of normal SD rats (n=6) receiving a normal diet and free drinking water for eight weeks, referred to as the Normal Control Group (NC group). The second group (Group 2) was consists of normal SD rats (n=12) receiving high-fat and high-sugar diets four weeks, followed by the administration of 35 mg/kg of STZ via intraperitoneal injection to establish diabetes models. At two or three days after the STZ injection, the fasting glucose levels were measured from the obtained tail vein blood samples. Rats with glucose level of 16.7 mmol/L or higher were considered diabetic models, and were selected for the experiments. Subsequently, they received daily intervention via oral gavage for four weeks at a dosage of 10 milliliters per kilogram of body weight with normal saline, referred to as the normal saline group (NS group). The third group (Group 3) was composed of normal SD rats (n=12) receiving the same diets and STZ administration as Group 2 to establish diabetic models. Upon the successful establishment of diabetes model, these rats received daily intervention of 10 mg/kg empagliflozin for four weeks via gavage (n=12), referred to as the empagliflozin group (EMPA group). Throughout the experiment, one rat from the EMPA Group and two rats from the NS Group experienced unforeseen fatalities. The experimenter could not be blinded to whether the rat was injected with empagliflozin or with normal saline Since all diabetes rats need to have their blood glucose monitored every 2-3 days.
During the feeding process, the body weight, blood glucose, urine output of the rats were meticulously monitored. The values of blood glucose were measured by Abbott FreeStyle blood glucose and ketone monitor system. Urinary protein excretion levels were quantified using a fully automated biochemical analyzer. If the blood glucose exceeds 20mmol/L, 1-3 units of insulin glargine (Sanofi-AnvanteCo., Ltd., 100U/ml) were administered according to the blood glucose levels to prevent diabetes ketoacidosis. At the end of experiment, all rats underwent euthanasia in a uniform manner to facilitate the assessment of relevant parameters, euthanasia was performed using sodium pentobarbital (150mg/kg, intraperitoneal injection), followed by cervical dislocation of the rats. Kidney specimens from rats were collected after euthanasia, rapidly frozen in liquid nitrogen and stored at -80 degrees Celsius.
Histology
Kidney samples were fixed in 10% buffered formalin solution and then embedded in paraffin. Sections of five microns thickness were prepared from the paraffin blocks and stained with hematoxylin and eosin.
ELISA Analysis of Kidney Tissue
After sectioning, the obtained samples were weighed and subsequently treated with a specified volume of phosphate-buffered saline. Rapid freezing using liquid nitrogen was carried out for preservation. Following sufficient thawing, the samples were maintained at a temperature of 2-8℃. A designated amount of PBS (pH 7.4) was added, and the samples were homogenized thoroughly using manual or mechanical homogenizers. Centrifugation was conducted for approximately 20 minutes (at 3000 rpm). The supernatant was meticulously collected. One portion was reserved for testing, while the remainder was frozen for future use, then proceed as follows: 1) Standard addition: Standard wells and sample wells were designated. Different concentrations of standard samples (50μL each) were added to the standard wells. (Note: The standard concentrations were 1000, 500, 250, 125, 62.5, and 0 ng/ml, respectively). 2) Sample addition: blank control wells were established, scant of both samples and enzyme reagents. Additionally, test sample wells were arranged. For the test sample wells on the ELISA plate, 40μL of sample diluent was added first, followed by 10μL of the test sample (achieving 5-fold final dilution of the sample). Samples were gently introduced into the bottom of the wells, minimizing contact with the well walls, and gently shaken for thorough mixing. 3) Enzyme addition: each well, except for the blank control wells, received 100μL of enzyme reagent. 4) Incubation: sealing the plate with a plate seal, incubation was carried out at 37℃ for 60 minutes. 5) Preparation of washing solution: the 20-fold concentrated washing solution was diluted 20 times with distilled water for subsequent use. 6) Washing: the plate seal was carefully removed, and liquid was discarded by inversion. Each well was filled with washing solution, left for 30 seconds, and then removed. This washing procedure was repeated 5 times, and the plate was gently tapped to dry. 7) Color development: in each well, 50μL of Color Developer A was added, followed by 50μL of Color Developer B. The plate was gently shaken for even mixing, then incubated at 37℃ in a light-protected environment for 15 minutes. 8) Termination: 50μL of Stop Solution was added to each well to halt the reaction (turning the blue color to yellow). 9) Measurement: the absorbance (OD values) of each well was measured sequentially at 450nm wavelength, with blank wells serving as the reference for zeroing. The measurement was conducted within 15 minutes after the addition of the stop solution.
Analysis of Physiological and Biochemical Indicators
Serum samples were analyzed using an enzyme marker (Labsystems Multiskan MS) and urine samples were performed using a fully automated biochemistry analyzer (MINDRAY). Samples stored at -80°C were removed from the freezer and thawed on ice for 10-30 minutes until completely dissolved. After thorough inversion to ensure uniform thawing, samples were inspected for the presence of particulate matter. If observed, centrifugation at 3000rpm, 4°C for 10 minutes was performed, followed by aspiration of the supernatant for further testing. Using a pipette, extract 200μl of the sample for analysis following the manufacturer’s instructions.
Statistical Analysis
SPSS version 26 (IBM Inc., USA) and GraphPad Prism version 9.1 (Inc., La Jolla, CA, USA) were used for data analysis and graph plotting. The Shapiro-Wilk test was used for normality testing, and Levene’s Test for Equality of Variances was employed for assessing the homogeneity of variances in the data. Normally distributed data were expressed as mean ± SD, while skewed distribution variables were presented as the median with the interquartile range. The comparison of serum glucose, VIM, FN, Col-I, Col-III, CTGF, and FSP-1 among multiple groups was conducted using one-way analysis of variance. In cases where the data did not meet the assumptions of normality or homogeneity of variance, the Kruskal-Wallis rank-sum test was employed for comparison. The pairwise comparisons between groups were conducted using the Bonferroni correction, P < 0.05 was considered a statistically significant difference.
Results
Renal Pathological Changes in SD Rats
As shown in Figure 1, renal tissue specimens from untreated NC group rats showed normal glomerular and tubular structures with no obvious signs of inflammation, necrosis or fibrotic changes. Slight renal tubular dilatation was observed in the renal tissues of SD diabetic rats treated with empagliflozin, whereas significant renal tubular dilatation and cast formation were observed in the renal tissues of treated with normal saline SD diabetic rats.
Alterations in Serum Glucose and Urinary Protein Excretion in SD Rats
Following the implementation of high-fat and high-sugar dietary regimens along with STZ injections, the serum glucose levels significantly increased in diabetic rats (26.48±3.60, 22.25±6.78 mmol/L) compare to the normal diet rats (6.20±0.40mmol/L) (Figure 2a,b; Table 1). In contrast, SD rats on the normal diet showed no significant changes in their fasting blood glucose levels throughout the entire experiment period. Furthermore, by the eighth week of the experiment, diabetic rats with empagliflozin intervention exhibited a significant decrease in fasting blood glucose levels compared to the NS group (18.62±4.54 vs. 28.26±4.34 mmol/L, p<0.001) (Table 1), while the fasting blood glucose levels of diabetic rats receiving NS intervention consistently elevated.
At the eighth week, compared to the NC group, the median urinary protein excretion levels increased in the NS group and the EMPA group diabetic rats, but not statistical significance, which were 1.55 (1.25, 2.50) g/L, 2.50 (1.80, 3.20) g/L, 1.70 (1.30, 3.10) g/L, (p=0.802), respectively. (Figure 2c).
Empagliflozin Mitigates Renal Fibrosis-Associated Biomarkers Compare with NS Treatment in SD Diabetic Rats
Compared to the NS group, the levels of renal fibrosis-associated biomarkers remarkably decreased in the empagliflozin group after four weeks of treatment, which were VIM(663.27±91.84 vs. 519.84±83.37 ng/mL, p=0.001), Col-I(25.71±2.82 vs. 21.27±1.46 ng/mL, p=0.002), Col-III(9.95(7.97, 10.58) vs. 6.51(6.22, 8.32) ng/mL p=0.008), CTGF(1555.81±234.80 vs. 1146.68±216.69 pg/mL, p=0.001) and FSP-1(5.70±0.78 vs. 4.38±0.81 ng/mL, p=0.012) respectively. (Figure 3a,c–f; Table 2). Notably, the difference in FN (85.11±11.99 vs. 74.17±6.71 ng/ml, p=0.055) was not statistically significant (Figure 3b; Table 2).
The Impact of Empagliflozin on Renal Fibrosis-Associated Biomarkers Compared to NC Group after 4 Weeks of Treatment
After 4 weeks of treatment, compared to the NC group, the levels of renal fibrosis-related biomarkers in the empagliflozin group showed no significant differences except for VIM, which were VIM(336.66±28.87 vs. 519.84±83.37ng/mL, p<0.001), FN(62.69±10.98 vs. 74.17±6.71ng/mL, p=0.094), Col-I(18.43±2.11 vs. 21.27±1.46ng/mL, p=0.111), Col-III(5.18(6.12, 8.53) vs. 6.51(6.22, 8.32)ng/mL, p=0.093), CTGF(885.16±166.10 vs. 1146.68±216.69pg/mL, p=0.073) and FSP-1(3.50±0.21 vs. 4.38±0.81 ng/mL, p=0.115), respectively (Figure 3a–f, Table 2).
Discussion
Our study had the following findings: 1. After 4 weeks of intervention, the mean blood glucose level of diabetic SD rats in the EMPA group significantly decreased, compared with that of the NS group. 2. At the end of the experiments, although the urinary protein excretion in the EMPA group was lower than that in the NS group, but there was no significant difference in urinary protein excretion between two groups of SD rats. 3. Histopathological examination of the kidneys in SD rats revealed that diabetic SD rats treated with normal saline exhibited marked tubular dilation and tubular casts, but no apparent extracellular matrix (ECM) accumulation or interstitial fibrosis were observed. In contrast, diabetic SD rats treated with empagliflozin showed only mild tubular dilation. 4 At the end of the experiment, the renal fibrosis biomarkers including VIM, Col-I, Col-III, CTGF, FSP-1 in the EMPA group dramatically decreased compared to the NS group, but the difference in the level of FN did not reach statistical significance. 5.Compared to the NC group, the levels of renal fibrosis-related biomarkers in the empagliflozin group showed no significant differences except for VIM after 4 weeks of intervention.
SGLT2 is primarily located in the proximal tubules of the kidney, responsible for more than 90% of glucose reabsorption. By blocking this physiological process, SGLT2 inhibitors contribute to increase glucose excretion, thereby exerting a hypoglycemic effect [10]. The hypoglycemic effect of SGLT2 inhibitor has been demonstrated in several animal and clinical studies [11,12,13]. Our study also confirmed the hypoglycemic effects of SGLT2 inhibitors. After four weeks treatment with empagliflozin, the blood glucose levels in SD rats from the EMPA group decreased by 27% compared to the levels observed at the successful modeling stage in the fourth week. In addition to ameliorate glucose homeostasis, SGLT2 inhibitors could slow the progression of overt diabetic nephropathy in animal models of type 2 diabetes mellitus [14,15]. In our study, regarding pathological changes, SD rats in the NS group exhibited mild renal injury, characterized by the observation of renal tubular casts and significant dilation of the renal tubular lumens. On the contrast, only slight tubular dilation was observed in renal tissue in the EMPA group of SD rats. Our results highlight the potential role of early application of empagliflozin in reversing renal pathological changes.
The precise mechanism behind SGLT2 inhibitors reverse renal fibrosis remains unclear, although several researches have been reported. Florian Gembardt et al. revealed that empagliflozin treatment significantly reduced glomerular hypertrophy, markedly decreased albuminuria, and increased creatinine clearance rate in diabetic BTBR ob/ob mice, which demonstrated its capacity to improve early features of diabetic nephropathy [16]. In our study, after 4 weeks of treatment, urinary protein excretion increased in the NS group compared with baseline. The urinary protein excretion in the EMPA group SD rats decreased compared to the NS group, but the difference did not reach statistical significance. This could be attributed to the following reasons: Firstly, the modeling period for our diabetes rats was relatively short, renal damage might not be sufficient to induce significant proteinuria, which was consistent with the renal pathology changes of SD rats in the NS group. Additionally, the duration of empagliflozin treatment was relatively short, which might not be enough to demonstrate that it could induce a significant reduction in urinary protein excretion.
A series of studies have demonstrated that SGLT2 inhibitors can prevent and slow down the progression of diabetic nephropathy through various mechanisms, including restoring tubuloglomerular feedback, inhibiting the activation of the RAAS system, suppressing oxidative stress and inflammation, as well as exerting anti-fibrotic effects [5,17,18,19]. Our research focused on the inhibitory effects of empagliflozin on the process of renal fibrosis. After the establishment of the diabetes model and the intervention of normal saline, we observed that the renal fibrosis markers of NS group rats were significantly higher than those of NC group. However, the levels of renal fibrosis biomarkers in diabetes SD rats significantly reduced under 4 weeks intervention of empagliflozin, which indicated that empagliflozin could reverse renal fibrosis in diabetes rats.
CTGF/CCN2 is a member of the matricellular protein family known as the CCN family, which was first was isolated and identified in 1991 [20]. CTGF plays a crucial role in promoting fibrosis and has been demonstrated to be involved in fibrotic processes in a variety of organs, including the kidneys, liver and skin. TGF-β is a key mediator in the process of renal fibrosis. CTGF acts on the downstream of TGF-β, participates in fibrotic signaling pathways such as Smad, ERK, and Wnt, mediates epithelial-mesenchymal transition (EMT), ultimately leading to excessive accumulation of extracellular matrix (ECM) in the kidneys [21,22]. Wang S. et al. indicated the increase in CTGF mRNA levels in the renal cortex of streptozotocin-induced diabetic rats [23]. After 8 weeks of induction of diabetes, male C57BL/6J mice exhibited marked CTGF expression in glomeruli, glomerular hypertrophy and mesangial matrix accumulation when compared with the control mice, which were ameliorated by the administration of CTGF antibody, CTGF antibody attenuated the deposition of glomerular mesangial matrix [24]. In our study, the expression of CTGF in the kidneys of diabetes SD rats was significantly upregulated in the NS group, suggesting the initiation of the renal fibrosis process. Compared to the NS group, the levels of CTGF remarkably decreased in the empagliflozin group after four weeks of treatment, which were close to those in the NC group. We speculate that early treatment with empagliflozin may reverse renal fibrosis.
It is well recognized that EMT plays a role in the development of renal fibrosis, which is the gradual transformation of epithelial cells into mesenchymal cells and loss of epithelial functions and properties [25]. Under physiological conditions, myofibroblasts disappear through apoptosis upon completion of epithelialization in a wound [26]. However, under the influence of fibrotic factors such as TGF-β and CTGF, the initiation of EMT leads to the abundant generation of myofibroblasts [27], resulting in the extensive accumulation of ECM. The initial phase of EMT is characterized by a loss of epithelial cell markers, with a particular reduction in the expression of E-cadherin, a transmembrane protein that forms adhesion junctions between epithelial cells, and E-cadherin is also important for the maintenance of apical-basal polarity [28]. The downregulation of E-cadherin expression weakens the tight connections and adhesion between epithelial cells, leading to a gradual transition from tight adhesion to loose adhesion and enhancing migratory capabilities of mesenchymal cells [29], which also occurs through the upregulation of Vimentin (VIM). VIM, a type III intermediate filament (IF) protein, is a widely studied member of the IF protein family. Numerous studies have indicated that VIM plays a crucial role in the assembly of cellular scaffolds in mesenchymal cells and outer embryonic cells [30]. VIM serves as a key regulatory role in the process of fibrosis and is an essential factor for the proliferation of mesenchymal cells. During the transition from epithelial cells to mesenchymal cells, epithelial cells that typically only express the IF of the keratin cytoskeleton begin to express VIM, making it a typical biomarker for EMT [31]. FSP-1, also known as fibroblast-specific protein 1, a gene encoding a fibroblast-specific protein associated with mesenchymal cell morphology and motility, is widely expressed in various fibroblast cells and plays a role in kidney inflammation and fibrosis processes. The expression of FSP-1 has been demonstrated to play a crucial early role in the EMT process and is commonly used as a biomarker for EMT [32,33]. Our study observed a significant increase in VIM and FSP-1 levels in diabetic SD rats with normal saline treatment, suggesting the initiation of the EMT process. Compared to the NS group, the levels of VIM and FSP-1 dramatically declined in the empagliflozin group after four weeks of treatment.
ECM which leads to tissue scarring, is characteristic of renal fibrosis [34]. The core components of the ECM include collagen, elastin, fibronectin, laminin, proteoglycans, hyaluronic acid, and various glycoproteins. Interacting with each other, they form a complex mesh-like structure capable of accommodating various types of cells [35]. Under the abnormal activation state of myofibroblasts, there is an excessive deposition of ECM, leading to the replacement of tissues with a stiff and disorganized ECM, ultimately resulting in the destruction of normal renal structure and loss of renal function [36,37]. In our study, elevated levels of core ECM components such as Col-I, Col-III and FN were observed in kidney tissues in the saline treatment group compared to those in the normal control group, indicating an increased secretion of ECM in diabetes SD rats. Compared with the NS group, the empagliflozin group demonstrated a significant decrease in Col-I and Col-III levels after four weeks of treatment. Nevertheless, there was no significant difference in Col-I and Col-III levels between the empagliflozin group and the normal control group. Based on our results, we speculate that empagliflozin can suppress excessive extracellular matrix secretion in the process of renal fibrosis.
The strengths of this study are that it is the first to reveal that empagliflozin may exert anti-renal fibrosis effects by inhibiting CTGF levels, which were the key of the initiation of the fibrotic process. Furthermore, empagliflozin also dramatically decreased the levels of VIM and FSP-1, indicating the inhibition of epithelial-mesenchymal transition, which may be attributed to ability of empagliflozin on improving the EMT process mediated by CTGF. Additionally, ECM core components Col-I and Col-III remarkbly reduced after empagliflozin treatment, suggesting suppression of extracellular matrix secretion. However, this study has certain limitations. Firstly, the duration of treatment with empagliflozin was relative short, and thus the long-term protective effect on the rat kidney could not be observed. Secondly, further studies are needed to explore the underlying mechanisms of empagliflozin alleviating CTGF mediated the EMT process, and gain and loss of function tests will been conducted in the passway.
Conclusions
In this study, we observed that empagliflozin intervention led to reduction in renal fibrosis biomarkers in the diabetic SD rat model. This decline may be attributed to the ability of empagliflozin to potential inhibit CTGF mediated EMT and ECM accumulation process, thereby alleviating renal fibrosis in diabetic SD rats. Our study suggests the possibility that early empagliflozin treatment can reverse renal fibrosis, which occurs prior to significant renal pathological changes. Therefore, we speculate that empagliflozin should be considered one of the most promising drug to mitigate renal fibrosis.
Author Contributions
XC conceived the research and obtained the funding. KJ, XZ and XC designed the entire study. XZ was responsible for rat feeding and the construction of the diabetes model. KJ, XC drafted the manuscript and provided critical revisions to the manuscript. KJ, XZ, JW, LZ and MF conducted the experiments, collected and analyzed the data. LH provided analysis of renal pathology in SD rats. All authors contributed to the writing of the manuscript and revised the final manuscript.
Funding
This study was supported by the Science and Technology Benefit Fund from Xiamen Municipal Bureau of Science and Technology (3502Z2022ZD1043)
Data Availability
The data of this study are available from the corresponding author upon reasonable request.
Acknowledgements
We are very grateful to Lai-rong Huang (Fuzhou KingMed for Clinical Laboratory Co., Ltd., Fuzhou, P. R. China) for the help with the experimental analysis.
Conflicts of Interest
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Glossary
| Col-I | Collagen Type I |
| Col-III | Collagen Type III |
| CTGF | Connective Tissue Growth Factor |
| DKD | Diabetic Kidney Disease |
| ELISA | Enzyme-linked immunosorbent assay |
| EMPA | Empagliflozin |
| FN | Fibronectin |
| FSP-1 | Fibroblast-specific protein 1 |
| NC | Normal Control |
| NS | Normal Saline |
| SD | Sprague-Dawley |
| STZ | Streptozotocin |
| SGLT2i | Sodium-Glucose Co-Transporter 2 Inhibitors |
| VIM | Vimentin |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, J.; Jiang, W.; Shi, Y.; He, X.; Zhou, Z.; Li, Y.; Yeung, R.O.; Wang, J.; Matsushita, K.; et al. Trends in Chronic Kidney Disease in China. N. Engl. J. Med. 2016, 375, 905–906. [Google Scholar] [CrossRef]
- Ravindran, S.; Munusamy, S. Renoprotective mechanisms of sodium-glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J. Cell Physiol. 2022, 237, 1182–1205. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Ward, M.S.; Fotheringham, A.K.; Zhuang, A.; Borg, D.J.; Flemming, N.B.; Harvie, B.M.; Kinneally, T.L.; Yeh, S.M.; McCarthy, D.A.; et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 2016, 6, 26428. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhao, Y.; Wang, Q.; Hillebrands, J.L.; van den Born, J.; Ji, L.; An, T.; Qin, G. Dapagliflozin Attenuates Renal Tubulointerstitial Fibrosis Associated With Type 1 Diabetes by Regulating STAT1/TGFβ1 Signaling. Front. Endocrinol. (Lausanne) 2019, 10, 441. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, H.; Lu, H.; Xiang, H.; Chen, S. Research progress of endothelial-mesenchymal transition in diabetic kidney disease. J. Cell Mol. Med. 2022, 26, 3313–3322. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, X.M.; Liang, X.M.; Wu, X.B.; Yao, C.M. SGLT2 inhibitors attenuate nephrin loss and enhance TGF-β(1) secretion in type 2 diabetes patients with albuminuria: A randomized clinical trial. Sci. Rep. 2022, 12, 15695. [Google Scholar] [CrossRef]
- Saisho, Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases 2020, 8. [Google Scholar] [CrossRef]
- Bailey, C.J.; Iqbal, N.; T’Joen, C.; List, J.F. Dapagliflozin monotherapy in drug-naïve patients with diabetes: A randomized-controlled trial of low-dose range. Diabetes Obes. Metab. 2012, 14, 951–959. [Google Scholar] [CrossRef]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.A.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Arakawa, K.; Ueta, K.; Matsushita, Y.; Kuriyama, C.; Martin, T.; Du, F.; Liu, Y.; Xu, J.; Conway, B.; et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE 2012, 7, e30555. [Google Scholar] [CrossRef] [PubMed]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T. Effects of the SGLT2 inhibitor ipragliflozin on various diabetic symptoms and progression of overt nephropathy in type 2 diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Gembardt, F.; Bartaun, C.; Jarzebska, N.; Mayoux, E.; Todorov, V.T.; Hohenstein, B.; Hugo, C. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am. J. Physiol. Renal Physiol. 2014, 307, F317–F325. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Chung, S.; Kim, S.J.; Lee, E.M.; Yoo, Y.H.; Kim, J.W.; Ahn, Y.B.; Kim, E.S.; Moon, S.D.; Kim, M.J.; et al. Effect of Sodium-Glucose Co-Transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes. PLoS ONE 2016, 11, e0165703. [Google Scholar] [CrossRef]
- Hatanaka, T.; Ogawa, D.; Tachibana, H.; Eguchi, J.; Inoue, T.; Yamada, H.; Takei, K.; Makino, H.; Wada, J. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol. Res. Perspect. 2016, 4, e00239. [Google Scholar] [CrossRef]
- Bradham, D.M.; Igarashi, A.; Potter, R.L.; Grotendorst, G.R. Connective tissue growth factor: A cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J. Cell Biol. 1991, 114, 1285–1294. [Google Scholar] [CrossRef]
- Wang, W.; Koka, V.; Lan, H.Y. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 2005, 10, 48–56. [Google Scholar] [CrossRef]
- Toda, N.; Mukoyama, M.; Yanagita, M.; Yokoi, H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 2018, 38, 14. [Google Scholar] [CrossRef]
- Wang, S.; Denichilo, M.; Brubaker, C.; Hirschberg, R. Connective tissue growth factor in tubulointerstitial injury of diabetic nephropathy. Kidney Int. 2001, 60, 96–105. [Google Scholar] [CrossRef]
- Dai, H.Y.; Ma, L.N.; Cao, Y.; Chen, X.L.; Shi, H.; Fan, Y.P.; Yang, B. Protection of CTGF Antibody Against Diabetic Nephropathy in Mice Via Reducing Glomerular β-Catenin Expression and Podocyte Epithelial-Mesenchymal Transition. J. Cell Biochem. 2017, 118, 3706–3712. [Google Scholar] [CrossRef]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Capaldo, C.; Gumbiner, B.M.; Macara, I.G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005, 171, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of vimentin in health and disease. Genes. Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. Faseb J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, M.; Huang, F.; Cheng, O.H.; Xiao, X.; Lee, T.H.; Truong, L.; Cheng, J. Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Danoff, T.M.; Kalluri, R.; Neilson, E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 1997, 273, F563–F574. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, H.; Liu, Y. The fibrogenic niche in kidney fibrosis: Components and mechanisms. Nat. Rev. Nephrol. 2022, 18, 545–557. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. Febs J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis--a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Histopathological examination of the kidneys of SD rats in each group (H&E staining, ×100). (a) Normal control group, normal glomeruli and tubules can be seen (n=6). (b) Empagliflozin group, minimal dilation of renal tubules can be seen (n=11). (c) Normal saline group, renal tubular casts can be seen (n=10). (d) Normal saline group, significant renal tubular dilation can be seen (n=10).
Figure 1.
Histopathological examination of the kidneys of SD rats in each group (H&E staining, ×100). (a) Normal control group, normal glomeruli and tubules can be seen (n=6). (b) Empagliflozin group, minimal dilation of renal tubules can be seen (n=11). (c) Normal saline group, renal tubular casts can be seen (n=10). (d) Normal saline group, significant renal tubular dilation can be seen (n=10).
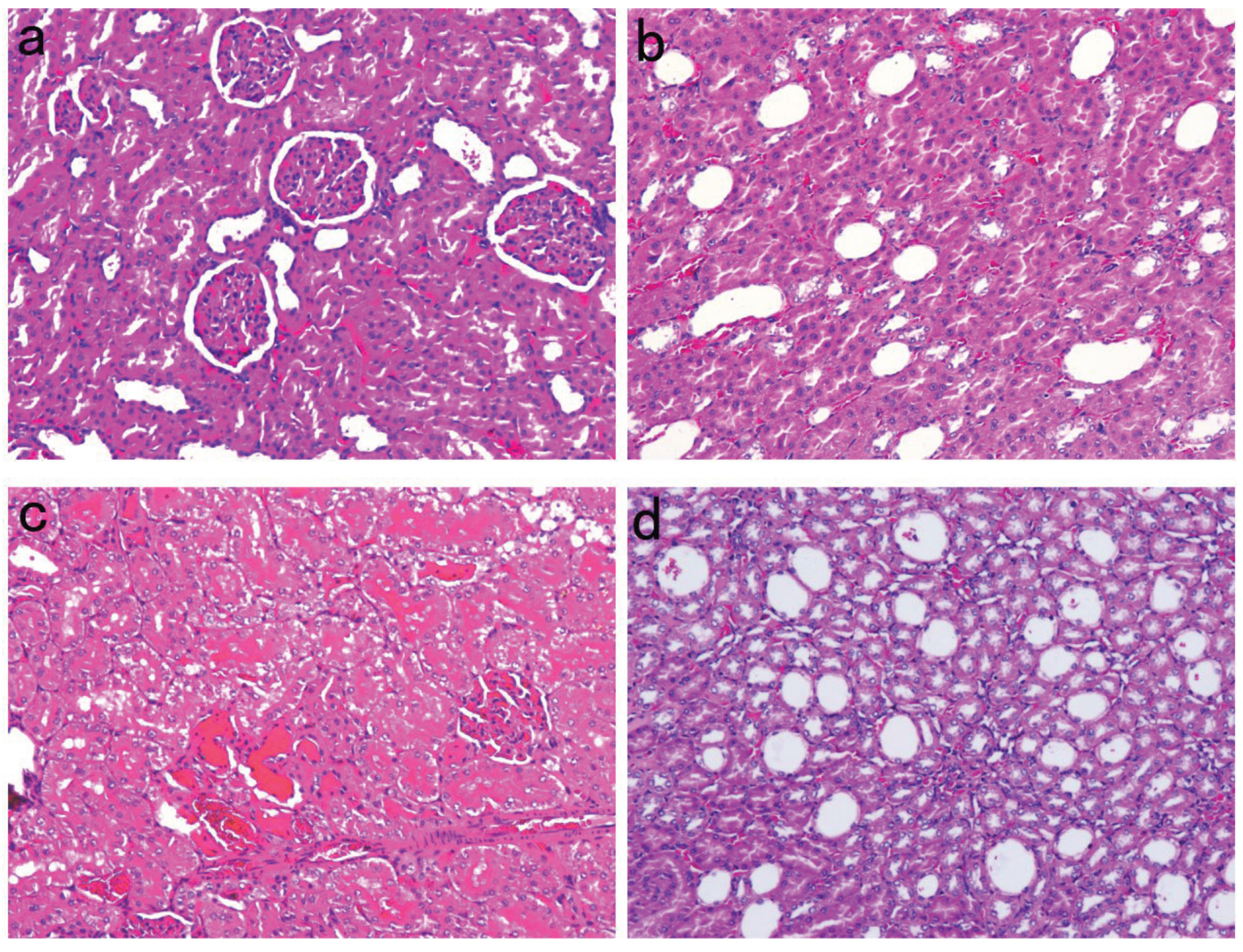
Figure 2.
The changes in blood glucose levels and urinary protein excretion throughout the study. (a) The change of blood glucose in different time line (n=6~11). (b) At the eighth week, the mean blood glucose levels in each group (n=6~11). (c) At the eighth week, the mean urinary protein excretion levels in each group (n=6~11). Data are expressed as the mean ± SD or median (interquartile range, IQR) in each group. ††: P>0.05.
Figure 2.
The changes in blood glucose levels and urinary protein excretion throughout the study. (a) The change of blood glucose in different time line (n=6~11). (b) At the eighth week, the mean blood glucose levels in each group (n=6~11). (c) At the eighth week, the mean urinary protein excretion levels in each group (n=6~11). Data are expressed as the mean ± SD or median (interquartile range, IQR) in each group. ††: P>0.05.
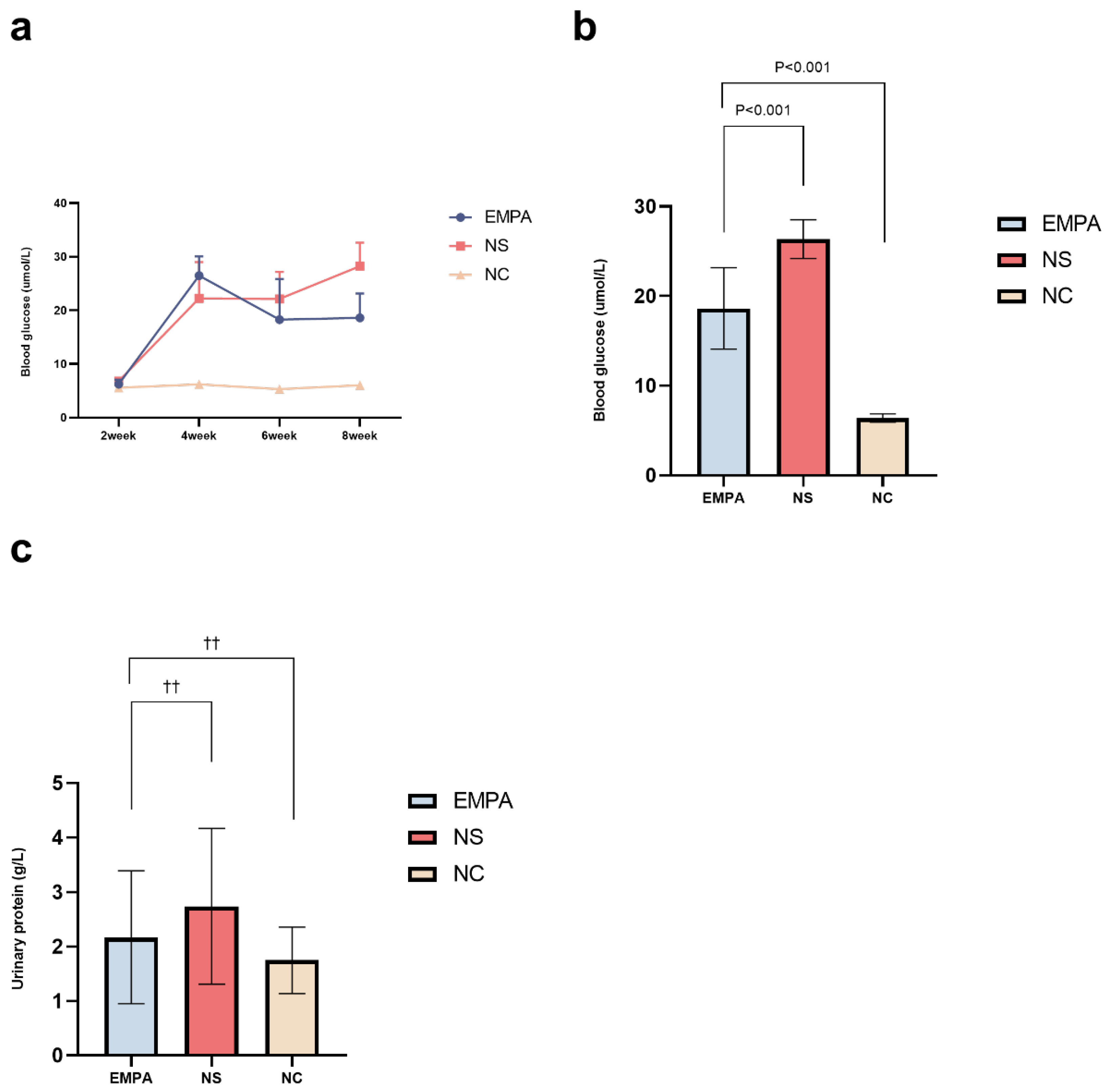
Figure 3.
The changes in the levels of fibrotic biomarkers in each group at the end of the study. (a) Vimentin (n=6~11). (b) Fibronectin (n=6~11). (c) Collagen Type I (n=6~11). (d) Collagen Type III (n=6~11). (e) Connective Tissue Growth Factor (n=6~11). (f) Fibroblast-specific protein 1 (n=6~11). Data are expressed as the mean ± SD or median (interquartile range, IQR) in each group.
Figure 3.
The changes in the levels of fibrotic biomarkers in each group at the end of the study. (a) Vimentin (n=6~11). (b) Fibronectin (n=6~11). (c) Collagen Type I (n=6~11). (d) Collagen Type III (n=6~11). (e) Connective Tissue Growth Factor (n=6~11). (f) Fibroblast-specific protein 1 (n=6~11). Data are expressed as the mean ± SD or median (interquartile range, IQR) in each group.

Table 1.
The change of blood glucose in different time line.
| Time line | NC n=6 | NS n=10 | EMPA n=11 | P value |
|---|---|---|---|---|
| 2-week | 5.58±0.20 | 6.81±0.64 | 6.25±0.82 | 0.004 |
| 4-week | 6.20±0.40 | 22.25±6.78* | 26.48±3.60* | <0.001 |
| 6-week | 5.33±0.23 | 22.15±5.03* | 18.27±7.58* | <0.001 |
| 8-week | 6.05±0.48 | 28.26±4.34* | 18.62±4.54*† | <0.001 |
NC: normal control; NS: normal saline; EMPA: empagliflozin. *: Versus NC group, P<0.05. †: Versus NS group, P<0.05.
Table 2.
Comparisons of renal fibrosis-related biomarkers after 4-week treatment in diabetic Sprague-Dawley rats.
Table 2.
Comparisons of renal fibrosis-related biomarkers after 4-week treatment in diabetic Sprague-Dawley rats.
| Markers |
NC n=6 |
NS n=10 |
EMPA n=11 |
P value |
|---|---|---|---|---|
| VIM (ng/mL) | 336.66±28.87 | 663.27±91.84* | 519.84±83.37*† | < 0.001 |
| FN (ng/mL) | 62.69±10.98 | 85.11±11.99* | 74.17±6.71 | 0.001 |
| Col-I (ng/mL) | 18.43±2.11 | 25.71±2.82* | 21.27±2.46† | < 0.001 |
| Col-III (ng/mL) | 5.18(6.12, 8.53) | 9.95(7.97, 10.58)* | 6.51(6.22, 8.32)† | < 0.001 |
| CTGF (pg/mL) | 885.16±166.10 | 1555.81±234.80* | 1146.68±216.69† | < 0.001 |
| FSP-1 (ng/mL) | 3.50±0.21 | 5.70±0.78* | 4.38±0.81† | 0.001 |
VIM: Vimentin; FN: Fibronectin; Col-I: Collagen Type I; Col-III: Collagen Type III; CTGF: Connective Tissue Growth Factor; FSP-1: Fibroblast-specific protein 1. *: Versus NC group, P<0.05. †: Versus NS group, P<0.05.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Sotagliflozin Promotes Anti-oxidant Properties in the Kidneys of Type 1 Diabetic Akimba Mice
Lakshini Y Herat
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








