Preprint
Article
Comparison of the Efficiency of Deep Eutectic and Organic Solvents on the Extraction of Phytochemicals from Cannabis sativa L.
Altmetrics
Downloads
123
Views
50
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Alerts
Abstract
Industrial hemp (Cannabis sativa L.) is an attractive candidate for sustainable pest management due to its abundance of bioactive compounds with potential pesticidal properties. Solvent choice has a significant impact on the extraction efficiency of bioactive compounds. Deep Eutectic Solvents (DESs) are gaining popularity in extraction because they are safe and environmentally friendly, making them viable alternatives to organic solvents (OSs). This research first compared the extraction efficiency of OSs on the extraction of phytochemicals from the infloresences of two hemp varieties, Citrus and Cherry Dwarf. Inflorescences were extracted using three OSs, ethanol, ethyl acetate, and hexane. The highest level of cannabidiol (CBD; 0.69%) was extracted from Cherry Dwarf using ethanol while the level of delta-9 tetrahydrocannabinol THC (0.19%) was essentially the same in both. Therefore, Cherry Dwarf was selected to compare the extraction efficiency of DESs to the OSs. The DESs were choline chloride/ethylene glycol, citric acid/ethylene glycol, menthol/lauric acid, choline chloride/urea and choline chloride/glycerol. In the targeted analysis, choline chloride/ethylene glycol extracted the highest amount of CBD (0.87%) followed by choline chloride/urea (0.78%). As some DESs outperformed ethanol, the popular solvent for extracting cannabinoids, DESs are viable candidates to replace organic solvents.
Keywords:
Subject: Chemistry and Materials Science - Applied Chemistry
1. Introduction
Industrial hemp (Cannabis sativa L.) is found in the Cannabaceae family, which contains only one genus (Cannabis) and one highly variable species, C. sativa [1]. The genus is one of the oldest crops cultivated for food, fiber, and medicinal purposes. Cannabis sativa L. plants have been used around the world since antiquity due to their multifunctional properties, including their use for oil and protein, food, and feed production; fiber, paper, and textiles, and resins, [1,2,3]. The genus contains more than 500 chemical constituents, 125 of which are classified as cannabinoids [1]. Cannabinoids, a class of terpenophenolic compounds, are the most psychoactive, accumulating primarily in the trichome cavity of female flowers along with non-cannabinoids that include phenols, flavonoids, and alkaloids[1,4]. Delta 9-tetrahydrocannabinol (THC), which is naturally present in the form of an acid (delta 9-tetrahydrocannabinolic acid, THCA), is the psychoactive cannabinoid component of cannabis with the highest concentration. To form the pharmacologically active THCA, the acid must be decarboxylated with time or heat [4]. Cannabidiol (CBD), another cannabinoid of interest, is the most promising compound from a pharmaceutical perspective that exhibits antioxidant, anti-inflammatory, antibacterial, antiproliferative, and neuroprotective properties. C. sativa contains four major cannabinoids in addition to delta THC and CBD: tetrahydrocannabivarin (THCV), cannabinol (CBN), cannabigerol (CBG) and cannabichromene (CBC), which exhibit remarkable antibacterial, anti-inflammatory, and antiproliferation properties [1].
Different extraction methods and solvents have been used to recover hemp phytochemicals and are grouped as conventional and modern techniques; some use organic solvents and some are solventless. Conventional techniques include liquid-liquid, Soxlet and reflux extraction, as well as maceration followed by extraction [5]. These techniques employ organic solvents, the selection of which depends on the polarity of the target compounds. These traditional methods are effective, but time consuming and require a large quantity of organic solvents that are toxic, flammable, and unfriendly to the environment [6].
Modern techniques include microwave-assisted extraction and ultrasound-assisted extraction [5]. Ultrasound-assisted extraction uses ultrasonic waves, which are mechanical vibrations that pass through the extraction medium. Waves induce acoustic cavitation by generating cycles of expansion and compression, causing the formation of expanding and collapsing bubbles [7]. These effects destroy the cell walls, releasing the contents of the cell. This technique is mostly performed in an ultrasonic bath containing organic solvents or their aqueous mixture. Microwave-assisted extraction, pressurized liquid extraction, extrusion, and rapid solid–liquid dynamic extraction are other modern green extraction techniques. Microwaves are electromagnetic radiations that can interact with polar molecules and penetrate plant biomass. The water in the biomass absorbs the microwave energy and rapidly heats the cells, causing their disruption and the release of the desired substances. [5,6,7,8].
Supercritical fluid extraction, an environmentally friendly technique that uses liquid CO2, has many benefits, including chemical stability, low toxicity, inflammability, and affordability. Two other benefits are supercritical-Carbon dioxide low critical temperature(310C) and the pressure (73.8 bar) for safe extraction of thermolabile components, which makes it an effective solvent for extracting volatile compounds such as terpenes from plant sources [6,8] and its simple separation from the extract.
Hydrodistillation and steam distillation are other methods commonly used to extract terpenes, including essential oils from plant sources [9]. These techniques reduce the boiling point of molecules by using water vapor pressure. The water vapor permeates the biomass and dissolves the volatile substances. Condensing the solvent and solutes causes their separation, with the essential oil occupying the upper phase of the liquid. In steam distillation, the plant material is brought directly into contact with steam, whereas in hydrodistillation, the plant material is soaked in water and brought to a boil prior to exposure to steam[8,9].Water is a precious resource and heating for extended periods consumes a lot of energy, so the environmental impact is reduced when these methods incorporate microwave heating [10].
Despite modern extraction techniques, organic solvents (chloroform, ethyl acetate, ethanol, and methanol) are frequently used to extract bioactive components from plant materials. Because organic solvents are expensive, flammable, toxic, and nonbiodegradable, and their excessive use not only harms the natural environment but also poses a health risk to humans, they do not adhere to the principles of green chemistry [11].
Therefore, it is critical to explore alternative methods for extracting phytochemicals. Deep eutectic solvents (DES) have emerged as a new class of environmentally friendly solvents to replace organic solvents [12]. As extraction solvents, DESs have the advantage of higher efficiency, shorter time, lower cost, lack of toxicity, biodegradability, and improved product purity, making them suitable for different applications[11,12]. DES combine a halide salt or another hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) (e.g., urea, carboxylic acid, sugar, amide, or another Lewis acid) at a specified molar ratio. Components are placed in a sealed bottle and heated at the specified temperature with magnetic stirring until a transparent liquid is produced[13,14].
Choline chloride (ChCl) is the most widely used HBA combined with an HBD, the most popular of which are urea, ethylene glycol, and glycerol; however, other alcohols, amino acids, carboxylic acids, and sugars have also been widely used[13,14,15]. These solvents have a well-defined composition, and they exhibit a unique, minimum melting point in the solid/liquid phase diagram, which is significantly lower than the melting points of the individual components, highlighting noncovalent molecular affinities. In most cases, DES can be used as a liquid at room temperature [15]. DESs are used to extract many secondary metabolites of plant materials, including phenolics, flavonoids, isoflavonoids, terpenoids, alkaloids, anthocyanins, anthraquinones and polysaccharides [13].
Although DESs have been reported to be environmentally friendly and have been used to extract various phytochemicals, little is known about the efficacy of DESs in the extraction of cannabinoids, THC, and terpenes from industrial hemp. Consequently, this study aims to compare the efficiency of different DESs and organic solvents in extracting phytochemicals from industrial hemp.
2. Materials and Methods
2.1. Plant Materials, Reagents, and Instruments
Industrial hemp flowers were harvested from the George Washington Carver Agricultural Experiment Station (GWCAES) at Tuskegee University in 2022, air dried, ground using a heavy-duty laboratory grinder, and stored in amber containers. Choline chloride (AR,98%), ethylene glycol (AR,99.5%), glycerin (AR,99%), citric acid (AR, >99.5), lauric acid (AR, 99%), menthol (AR, 99.9%) ethanol, ethyl acetate and hexane (Fischer Scientific, Roswell, GA USA), and reference standards (Sigma Aldrich, Burlington, MA USA) were used as received. The instruments used include a pH meter, viscosity measuring cup, shaker bath, ultrasonic bath, centrifuge, and magnetic stirring hot plate GC-MS and LC-HRMS instrument.
2.2. DES Preparation and Properties Determination
HBA and HBD reagents were mixed at an appropriate molar ratio and heated on the magnetic stirring hot plate at 800 C until a clear and homogeneous liquid was formed. On the basis of references from different literature, choline chloride, citric acid, and menthol were selected as HBAs and ethylene glycol, glycerol, urea, and lauric acid were selected as HBDs. The molar ratios of the five different DESs are given in Table 1.
The pH of each DES was measured at 250C; viscosity was measured using a viscosity determination cup according to the literature procedure. The density was calculated by using the mass and volume of the solvent, and the polarity was determined qualitatively using information from the literature. Each DES property was measured in triplicate.
2.3. Extraction using DES.
An accurate weight sample (1g) of industrial hemp powder was added to 20 ml of DES in a 50 ml centrifuge tube. The extraction was carried out with the help of a shaker bath set at 370C, 75 rpm overnight. The extract was centrifuged at 4000 g for ten minutes and the supernatant was collected [16]. The collected supernatant was stored at room temperature until further analysis. The extraction was carried out in triplicate to validate the results. The extracts were visually compared based on color to determine the qualitative efficiency of extracting colored compounds from industrial hemp.
2.4. Extraction using Organic Solvents.
Organic solvents used were ethanol, ethyl acetate, and hexane; the choice was based on the variation in polarity. The extraction procedure followed the method previously used with DESs detailed above with little modification. The extract was filtered using Whatman No.1 filter paper followed by a syringe filter of 0.22µm. The extraction was carried out in triplicate.
2.5. Characterization of Extract using LC/MS
Analysis was performed on a Vanquish UHPLC system (Thermo Fisher, USA) coupled with a quadrupole orbitrap mass spectrometer (Orbitrap Exploris 120, Thermo) with electrospray ionization (H-ESI) in positive mode using Xcalibur software (V4.4.16.14). The samples were diluted to 0.1% (vol/vol) in 50% methanol 50% water containing 500 ng/mL of CBD-D3 and (−)-Δ9-THC-D3, each. Injection of 10 uL of the sample was made into a C18 column (ACQUITY UPLC® BEH C18, 1.7 µm, 2.1 × 50 mm, Waters) maintained at 40 ° C with a 400 μL/min flow rate of 400 L / min of the mobile phase solution A (20 mM ammonium carbonate pH 3.2) and the mobile phase B (100% acetonitrile) beginning at 40% B, held for 1 minute, increased to 85% B at 12 minutes, increased to 95% B and held for 2 minutes, followed by reequilibration to 40%B for 3 minutes for a total analysis time of 17 minutes. Samples were chilled to 10 ° C while the column was heated to 25 ° C. The MS scan range was 100-1000 m/z with resolution of 120,000, standard AGC target, 70% RF lens, maximum injection time of 100 ms, with EASY-IC run start on. The spray voltage was 3500 V in positive mode and 2500 V in negative mode, the ion transfer tube temperature was 320 ° C and the vaporizer temperature was 290 ° C, the sheath and aux gases were 30 and 7, respectively. There was a targeted fragmentation analysis of several compounds, including: CBDVA, CBDV, CBDA, CBGA, CBG, CBD, THCV, CBN, THCA and internal standards. The isolation window was 1.3, collision energy was normalized to 30%, the orbitrap resolution was 15000 with other parameters set to auto. Analysis of variance (ANOVA), Principal Component Analysis (PCA), and Partial Least Squares Discriminant Analysis (PLS-DA) were used to compare the metabolic differences among different solvents. The variable importance in the projection (VIP) was obtained from the first principal component of the PLS-DA model to evaluate the importance of metabolites using MetaboAnalyst 5.0
2.6. Characterization of Extracts Using GC/MS
Benzophenone (0.5 mg/mL) was added to the samples at 1% (vol/vol) and a terpene standard (Restek catalog No. 34095) was used for quantification. Terpenes were analyzed by gas chromatography-mass spectrometry on an Agilent 6890N GC and 5975 MS with a Restek Rxi-5Sil MS column with Integra-guard (15 m x 0.25 mm ID x 0.25 µm df). The injection port and MS transfer line temperature was set at 310 ° C and the flow was constant at 1.0 ml / min. 0.2 µL of the sample was injected in splitless mode. The temperature of the GC oven was programmed as follows: initial temperature of 40 ° C held for 3 min, ramp to 180 ° C at 10 °C/min, ramp to 320 ° C at 50 °C/min, with a hold of 5 minutes, thus requiring a total run time of 24.8 min. The EI was set to 70 eV and the mass range was from 35 to 350 m/z. The MS source was set to 230 and the quad was set to 150. Raw data were processed and analyzed using MS-DIAL. Statistical analysis was performed using GraphPad Prism 10.1 and MetaboAnalyst 5.0
3. Results
3.1. Properties of Deep Eutectic Solvents
Each DES was successfully prepared as a homogeneous liquid and remained liquid at room temperature (Figure 1). The properties of the DESs varied widely (Table 1), thus, density, acidity, alkalinity, viscosity, and polarity depended on the composition of each.. The ethylene glycol/citric acid DES had the highest acidity, while the menthol/lauric acid DES had the lowest viscosity and density. Choline chloride/urea DES possesses the highest pH and viscosity. Evaluating these properties will facilitate the efficient extraction of target compounds from natural materials.
3.2. Comparison of Extract Color
The color obtained using solvents for choline chloride/ethyl glycol, choline chloride/glycol, citric acid/ethyl glycol, and choline chloride / urea were comparable. In contrast, menthol/lauric acid produced a greenish color distinct from that of other solvents. Figure 2A. With organic solvents, the color of all solvents was comparable (green), except for the hexane extract, which cannot extract chlorophyll Figure 2B. The color of the hemp extract extracted with DES and organic solvents was distinct; therefore, quantitative results were used to demonstrate their distinctions.
3.3. Targeted Analysis of Industrial Hemp Extract
3.3.1. Analysis of Cannabinoids
Two varieties of industrial hemp Cherry Dwarf (CD) and Citrus (CT) were analyzed for tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN) targeted using their corresponding reference standards. The two varieties were extracted using ethanol, hexane, ethyl acetate, and the combination of three solvents in a ratio of 1: 1. Overall, there was a significant difference between CD and CT in all compounds (P=0.004) and there was a significant difference between solvents (P< 0.0001) . The highest percentage of CBD was detected in the Cherry Dwarf (0.69) extracted with ethanol, followed by Citrus (0.61) and Cherry Dwarf extracted by ethyl acetate (0.59). The lowest percentage of CBD was obtained from Citrus samples extracted by hexane (0.04) while it was 0.3 in Cherry Dwarf (Figure 3a). All solvents extracted a similar percentage of CBN (0.08) from Cherry Dwarf while Citrus ethanol extracted a similar amount (0.08). The combination and ethyl acetate extracted very low amounts of 0.01 and 0.03, respectively, while hexane did not extract any detectable amount (Figure 3c). The highest percentage of THC was extracted by ethanol and was similar for both varieties (0.19), followed by Cherry Dwarf extracted with ethyl acetate (0.18). The remaining solvents in both varieties extracted very low percentages of THC and the least was hexane in Citrus (Figure 3b). Based on the results, the Cherry Dwarf was shown to contain the highest percentage of CBD, CBN, and THC compared to Citrus.
Cherry Dwarf had the highest amount of extracted cannabinoids and therefore was selected for further extraction using OS and DES solvents. The organic solvents used were ethanol, ethyl acetate, hexane, and the combinations thereof, while the DESs used were menthol / lactate, citric acid/ethylene glycol, choline chloride/ethylene glycol, choline chloride / urea, and choline chloride/glycerol. There was a significant interaction between compounds and solvents (P< 0.0001). DES showed a high ability to interact with CBD, resulting in the highest percentage of extract outperforming OS that is usually used to extract CBD. Choline chloride/ethylene glycol extracted the highest percentage of all solvents (0.87), followed by choline chloride/Urea (0.78), citric acid/ethylene glycol 0.48 per cent, choline chloride/urea 0.39 per cent and menthol/lauric acid 0.04 per cent. Ethanol extracted 0.69 percent, ethyl acetate 0.59 percent, hexane 0.32 percent, and combination 0.29 percent (Figure 4).
3.3.2. Analysis of Terpenes
A refence standard with 19 terpenes was used in the targeted analysis to determine the concentration of these terpenes in Cherry Dwarf and Citrus industrial hemp varieties. Nineteen terpenes were analyzed on both varieties, using organic and deep eutectic solvents on Gas Chromatography Mas spectrophotometry. Terpenes were detected in samples extracted using organic solvents; however, DES solvents produced large broad peaks in the gas chromatography; therefore, they were excluded from the determination process. After the initial analysis, two terpenes were screened early as they could not be detected in any of the varieties. Eight terpenes that had the significant concentration on both varieties were selected for further comparison between varieties and between solvents. There was a significant interaction between terpenes and solvents (P <0.001) in Citrus, while in Cherry Dwarf the terpenes differed significantly at (P< 0.001), while the solvents differed significantly differed (P=0.02). In general, there were no significant differences between varieties and solvents for all terpenes except for Beta caryophyllene in Citrus (P=0.03) with the concentration of 0.074 mg / ml. Although no statistically significant difference was detected between the solvents, Figure 5 shows that hexane has extracted the highest amount of terpenes.
3.4. Untargeted Analysis of Hemp Extracts
3.4.1. Univerity Statistical Analysis of Cannabinoids
Untargeted analysis of cannabinoids was performed using LC-MS, resulting in 90 compounds. The screening based on the 90% best match of the MS/MS fragmentation data compared to mzCloud resulted in nine compounds that were used for further comparison based on peak area. These included cannabichromene, cannabichromevarin, cannabicitran, cannabidiol, cannabidiolic acid, cannabidivarin, cannabinodiol, cannabinol, and THC. There was a significant interaction between varieties and solvents in all cannabinoids except cannabichromevarin and cannabinodiol which were not significant. Cherry Dwarf showed the highest peak areas in all compounds except cannabichromevarin which was similar in Citrus and Cherry Dwarf, however, Citrus has the highest peak area of cannabinodiol (Figure 6). These findings are similar to those obtained from targeted analysis, where Cherry Dwarf has the highest concentration of all selected compounds. Therefore, Cherry Dwarf was used to compare OS and DES on untargeted analysis.
3.4.2. Multivariate Statistical Analysis of Cannabinoids
PCA was carried out to better visualize, within the nine extraction solvents, the behaviors of the extracted compounds. The first PCA (Figure 7a, b and c) was performed to identify the correlation groups for solvents based on LC/MS results. The plots had 44.8% data variability on the first principal component (PC 1), and 39.6% on the second one (PC 2). The variance was caused mainly by M:LA (Menthol / Lauric acid), which contained a very low amount of all extracted compounds, and to some extent by CA:EG (Citric acid/ethylene glycol) which has the highest cannabinodiol in PC 1. The variation in the second principal component (PC 2) was mainly caused by the organic solvents ethanol, ethyl acetate which has the highest cannabicitran and minor from hexane and combination of solvents. These findings were further explained by the heat map (Figure 7d) which shows the constituents of each extracted compound in each solvent used for extraction.
4. Discussion
The physical properties of DES, viscosity, polarity, and pH may influence the extraction efficiency and, consequently, the type and amount of phytochemical compound obtained. In this study, no water was added to the DES, so its properties were determined in its pure form. Menthol/lauric acid DES was the least viscous and slightly acidic of all DESs. Similar properties were determined by [16,17] and therefore identified as hydrophobic DES [18]. Since the color of the pure solvent was transparent and the color of the hemp extract was greenish, a greenish compound was likely extracted from hemp (possibly chlorophyll). Despite M: With LA being less viscous, the extraction efficiency of cannabinoids (CBD, THC, and CBN) was very low. M: LA was unable to extract THC or CBN and could only extract 0.04% of CBD. A study conducted in Portugal [16,17]M: LA was able to extract the highest concentration of CBD, although it extracted the leaves, flowers, and seeds of the Futura 75 variety of hemp. The density, molar ratio, and viscosity of the solvent are identical to those of our data. However, they also report fewer chlorophyll and wax impurities in their extractions. These findings indicate that the interactions between DESs and target compounds influence the extraction efficiency of DESs. This effect could be explained by several factors such as polarity, water content, and extraction temperature, key indicators of DES dissolution capacity[17,19]. Growth conditions, location, and harvest time also alter the content of cannabinoids in hemp [20].
Comparison of ethanol, ethyl acetate, hexane, and their combination on two industrial hemp varieties revealed that ethanol extracted the highest amount of all test compounds in Cherry Dwarf (0.69%) and Citrus (0.61%). The concentration of CBD, CBN, and THC varied depending on the variety. These findings are comparable to those of Wongwailikhit et al. [21] who showed that ethanol outperformed isopropanol in extracting CBD and THC. Furthermore, the findings relate to De Vita et al. [21], who showed that percentages of CBD and THC concentrations increased with increasing ethanol concentration. Since 99.99% purity ethanol was used, our data could be the optimum concentration that could be obtained from both varieties. Therefore, this finding is consistent with other reports [22,23] that ethanol is preferred to the extraction of CBD than nonpolar solvents.
A further comparison of OS with DES was performed on the extraction of CBD, CBN, and THC from both varieties. The OS solvents were ethanol, ethyl acetate, hexane, and their combination while the deep eutectic solvents used were menthol/lauric acid, citric acid/ethylene glycol, choline chloride/ethylene glycol, choline chloride/urea, and choline chloride/glycerol. The results revealed that deep eutectic solvents based on choline chloride (choline chloride/ethylene glycol and choline chloride/urea) extracted the highest amount of CBD 0.87% and 0.78% respectively, than ethanol (0.69%) the organic solvent most used in hemp extraction. These findings are consistent with those of Liu et al. [24] in which deep eutectic solvents with choline chloride / urea extracted the highest amount of flavonoids from lotus leaves. Contrary to the findings of Tiago et al., in which they observed a large amount of CBD extracted using M:LA [19] deep eutectic solvent M:LA in this study did not extract any CBN and THC and extracted very small amount of CBD. One reason for the difference could be the addition of some water (30%) that increased the polarity of the solvent, thus improving the extraction efficiency [19,25] while in our study water was not added to any solvent.
Nine terpenes were found in a high amount in both varieties, which are alpha bisabolol, alpha caryophyllene, beta bisabolene, beta caryophyllene, d-limonene, nerolidol 1 nerolidol 2, and Nero acetate. Some similar terpene findings agree with the hemp line CS12 studied by Namdar et al., in which hexane extracted more beta caryophyllene, and ethanol extracted more beta bisabolene and Nerolidol [26]. The study by Benelli et al. found similar terpenes as our study, including other that were found in very small amounts in the varieties we used in our studies [25].
5. Conclusions
This study aimed to evaluate the extraction efficiency of deep eutectic solvents and organic solvents on cannabinoids and terpenes from industrial hemp inflorescence. Ethanol among the organic solvents extracted the highest amount of CBD and THC with more extracted from the Cherry Dwarf variety than the Citrus variety. There was no significant difference between the CBN extracted by all organic solvents in both varieties. The comparison of deep eutectic solvents and organic solvents on the extraction of cannabinoids indicated that choline chloride/ethylene glycol extracted the highest amount of CBD followed by choline chloride / urea, and these DES outperformed even ethanol, the solvent popular for the extraction of cannabinoids. There was no significant difference between solvents when extracting CBN, and citric acid/ethylene glycol DES extracted a comparable amount of THC with ethanol and ethyl acetate. Eight terpenes were identified in the two hemp varieties, these terpenes were also identified in other varieties in various studies. DESs are known to be safe, cheap, and environmentally friendly compared to organic solvents, the findings suggest that deep eutectic solvents can be used to extract cannabinoids replacing organic solvents. However, there is a need for further research on the effective and efficient means of separating deep eutectic solvents from the extract to obtain pure crude extracts. In addition, analysis of terpenes extracted with deep eutectic solvents needs to be optimized and validated. Since identified cannabinoids and terpenes are known to be antimicrobial, antifungal, and with some pesticide properties, the findings will be the basis for continued research on the use of hemp as a biopesticide.
Author Contributions
Conceptualization, G.G.K., D.G.M. and W.E.C.; methodology, G.G.K., D.G.M., W.E.C. and M.B.C.; validation, G.G.K., D.G.M., W.E.C. and M.B.; data curation, G.G.K. and M.B.; formal analysis, G.G.K. and M.B.; writing—original draft preparation G.G.K and W.E.C.; writing—review and editing, G.G.K., D.G.M., W.E.C. and M.B.C.; funding acquisition, D.G.M. and W.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Contribution of the George Washington Carver Agricultural Experiment Station and USDA/NIFA Evans Allen Program (Grant no. ALX-FVC18)”
Data Availability Statement
Data are contained within the manuscript.
Acknowledgments
Schlumberger Foundation Faculty for the Future for fellowship support of G.G.K
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis Sativa, L.: A Comprehensive Review on the Analytical Methodologies for Cannabinoids and Terpenes Characterization. J Chromatogr A 2021, 1637. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis Sativa: A Comprehensive Ethnopharmacological Review of a Medicinal Plant with a Long History. J Ethnopharmacol 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Shaikh, |; Uddin, J.; Alam, | Md Ashraful; Satyajit, |; Sarker, D. Extraction of Naturally Occurring Cannabinoids: An Update.
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis Sativa l. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Nahar, L.; Shaikh, |; Uddin, J.; Alam, | Md Ashraful; Satyajit, |; Sarker, D. Extraction of Naturally Occurring Cannabinoids: An Update;
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis Sativa l. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Mazzara, E.; Torresi, J.; Fico, G.; Papini, A.; Kulbaka, N.; Dall’acqua, S.; Sut, S.; Garzoli, S.; Mustafa, A.M.; Cappellacci, L.; et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, and Micromorphological Characterization of 9 Commercial Varieties of Cannabis Sativa L. Plants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Mcpartland, J.; Mcpartland, J.M.; Sheikh, Z. A Review of Cannabis Sativa-Based Insecticides, Miticides, and Repellents. J Entomol Zool Stud 2018, 6. [Google Scholar]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a Green Solvent Combined with Different Techniques for Extraction of Essential Oil from Lavender Flowers. Comptes Rendus Chimie 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Bakar, M.F.A.; Alomar, M.; Sabran, S.F.; Hanafi, A.F.M.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8. [Google Scholar] [CrossRef]
- Azizi, N.; Dezfooli, S.; Hashemi, M.M. A Sustainable Approach to the Ugi Reaction in Deep Eutectic Solvent. Comptes Rendus Chimie 2013, 16, 1098–1102. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J Agric Food Chem 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Xinyu Zhang; Jianqing Su; Xiuling Chu; Xiaoya Wang A Green Method of Extracting and Recovering Flavonoids from Acanthopanax Senticosus Using Deep Eutectic Solvents. Molecules 2022, 27. [CrossRef]
- Gao, M.Z.; Cui, Q.; Wang, L.T.; Meng, Y.; Yu, L.; Li, Y.Y.; Fu, Y.J. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus Alba L.) Leaves by Deep Eutectic Solvent. Microchemical Journal 2020, 154. [Google Scholar] [CrossRef]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds From Cannabis Sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front Nutr 2022, 9. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain Chem Eng 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Naik, P.K.; Kundu, D.; Bairagya, P.; Banerjee, T. Phase Behavior of Water-Menthol Based Deep Eutectic Solvent-Dodecane System. Chemical Thermodynamics and Thermal Analysis 2021, 3, 100011. [Google Scholar] [CrossRef]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds From Cannabis Sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front Nutr 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and Wild Hemp: How Different Are Their Essential Oil Profile and Antimicrobial Activity? Molecules 2020, 25. [Google Scholar] [CrossRef]
- Wongwailikhit, K.; Ruangdech, J. Comparison of the Two Common Solvents for THC and CBD Extractions. In Proceedings of the Proceedings of the World Congress on Mechanical, Chemical, and Material Engineering; Avestia Publishing; p. 2021.
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis Sativa L. (Hemp). J Pharm Biomed Anal 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Sagili, S.U.K.R.; Addo, P.W.; Macpherson, S.; Shearer, M.; Taylor, N.; Paris, M.; Lefsrud, M.; Orsat, V. Effects of Particle Size, Solvent Type, and Extraction Temperature on the Extraction of Crude Cannabis Oil, Cannabinoids, and Terpenes. ACS Food Science and Technology 2023. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, A.; Zhang, Y.; Duan, S. Efficient Extraction of Flavonoids from Lotus Leaves by Ultrasonic-Assisted Deep Eutectic Solvent Extraction and Its Evaluation on Antioxidant Activities. Separations 2023, 10. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The Essential Oil from Industrial Hemp (Cannabis Sativa L.) by-Products as an Effective Tool for Insect Pest Management in Organic Crops. Ind Crops Prod 2018, 122, 308–315. [Google Scholar] [CrossRef]
Figure 1.
Deep Eutectic Solvents, which were used in the extraction. ChCl: EG (Choline Chloride: Ethylene Glycol), ChCl: G (Choline Chloride: Glycerol), M: LA (Menthol: Lauric Acid), CA:EG (Citric acid: Ethylene Glycol) ChCl: U (Choline Chloride: Urea).
Figure 1.
Deep Eutectic Solvents, which were used in the extraction. ChCl: EG (Choline Chloride: Ethylene Glycol), ChCl: G (Choline Chloride: Glycerol), M: LA (Menthol: Lauric Acid), CA:EG (Citric acid: Ethylene Glycol) ChCl: U (Choline Chloride: Urea).

Figure 2.
Industrial hemp extract showing the variation of color depending on the solvent used (a) DES extract ChCl: G (Choline Chloride: Glycerol), M: LA (Menthol: Lauric Acid), ChCl: EG (Choline Chloride: Ethylene Glycol), ChCl: U (Choline Chloride: Urea) CA: EG (Citric Acid: Ethylene Glycol) (b) organic solvent extracts H-Hexane, EA-Ethyl Acetate, E-Ethanol, and M-Methanol.
Figure 2.
Industrial hemp extract showing the variation of color depending on the solvent used (a) DES extract ChCl: G (Choline Chloride: Glycerol), M: LA (Menthol: Lauric Acid), ChCl: EG (Choline Chloride: Ethylene Glycol), ChCl: U (Choline Chloride: Urea) CA: EG (Citric Acid: Ethylene Glycol) (b) organic solvent extracts H-Hexane, EA-Ethyl Acetate, E-Ethanol, and M-Methanol.

Figure 3.
Comparison of the percentage concentration of (a) CBD, (b)THC and (c) CBN cannabinoids in the Cherry Dwarf (CD) and Citrus (CT) industrial hemp varieties extracted using organic solvents H-Hexane, ET-Ethanol, EA-Ethyl Acetate and C-Combination of three solvents at 1:1: 1 using targeted analysis.
Figure 3.
Comparison of the percentage concentration of (a) CBD, (b)THC and (c) CBN cannabinoids in the Cherry Dwarf (CD) and Citrus (CT) industrial hemp varieties extracted using organic solvents H-Hexane, ET-Ethanol, EA-Ethyl Acetate and C-Combination of three solvents at 1:1: 1 using targeted analysis.

Figure 4.
Percentage concentration of CBD, CBN and THC extracted by organic solvents (H-Hexane, ET-Ethanol, EA-Ethyl Acetate, and C-Combination of three solvents at 1:1:1) and Deep Eutectic Solvents (Menthol/Lauric Acid, Citric Acid/Ethylene Glycol, Choline Chloride/Ethylene Glycol, Choline Chloride/Urea and Choline Chloride/Glycerol.
Figure 4.
Percentage concentration of CBD, CBN and THC extracted by organic solvents (H-Hexane, ET-Ethanol, EA-Ethyl Acetate, and C-Combination of three solvents at 1:1:1) and Deep Eutectic Solvents (Menthol/Lauric Acid, Citric Acid/Ethylene Glycol, Choline Chloride/Ethylene Glycol, Choline Chloride/Urea and Choline Chloride/Glycerol.

Figure 5.
Comparison of the terpene concentration extracted using organic solvents hexane, ethanol, ethyl acetate and combination of the three solvents at 1:1:1 extracted from Cherry Dwarf and Citrus industrial hemp varieties.
Figure 5.
Comparison of the terpene concentration extracted using organic solvents hexane, ethanol, ethyl acetate and combination of the three solvents at 1:1:1 extracted from Cherry Dwarf and Citrus industrial hemp varieties.

Figure 6.
Comparison of cannabinoids peak area resulted from untargeted analysis. The Cherry Dwarf and Citrus industrial hemp varieties were extracted using organic solvents hexane, ethanol, ethyl acetate, and combination of the three solvents at 1: 1: 1.
Figure 6.
Comparison of cannabinoids peak area resulted from untargeted analysis. The Cherry Dwarf and Citrus industrial hemp varieties were extracted using organic solvents hexane, ethanol, ethyl acetate, and combination of the three solvents at 1: 1: 1.
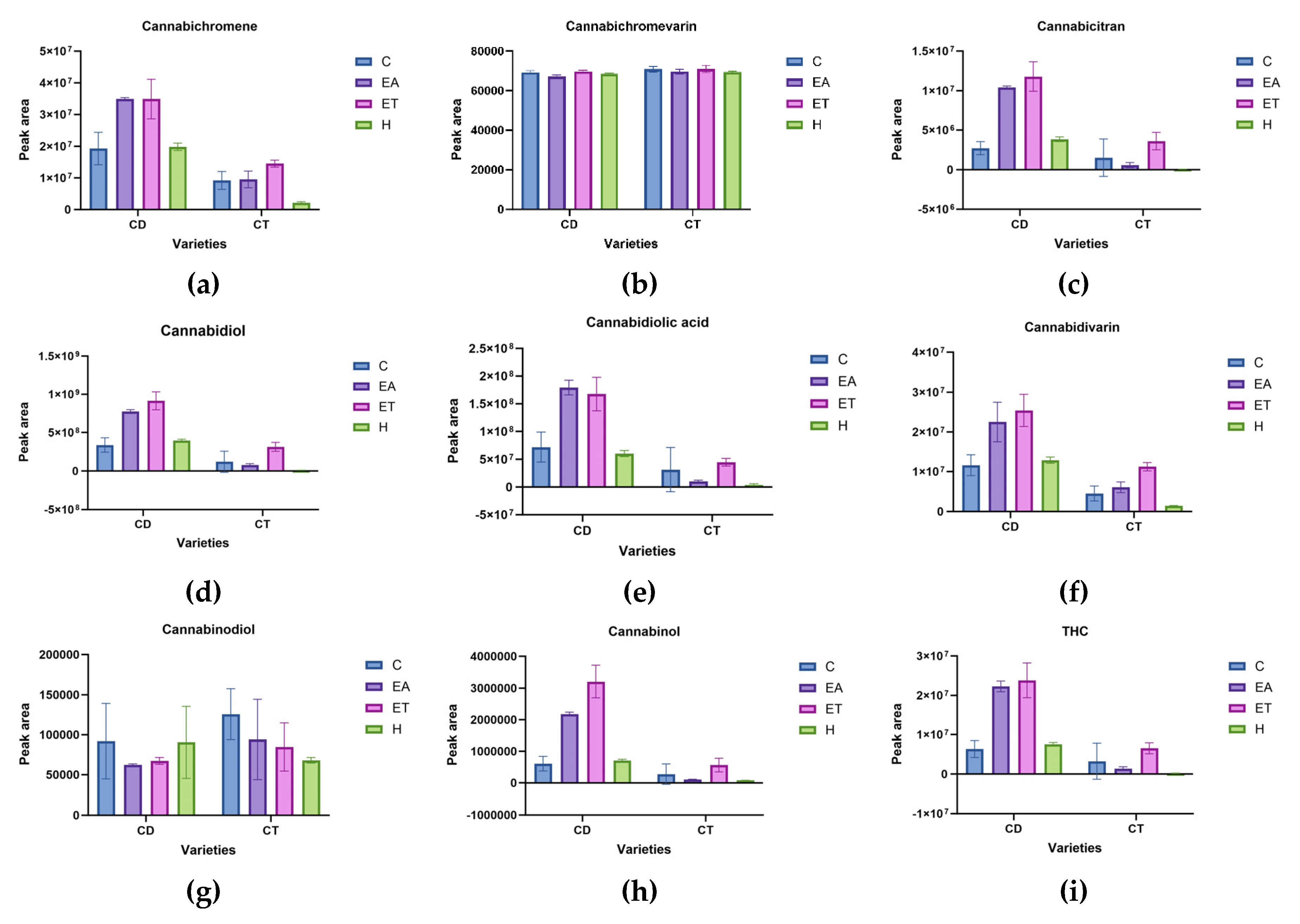
Figure 7.
(a) PCA score plot of solvents used in extraction and (b) loadings of compounds extracted, (c) Bi plot showing distribution of compounds and solvents, and (d) Heat map showing variability of organic solvents and deep eutectic solvents in extraction cannabinoids using untargeted analysis.
Figure 7.
(a) PCA score plot of solvents used in extraction and (b) loadings of compounds extracted, (c) Bi plot showing distribution of compounds and solvents, and (d) Heat map showing variability of organic solvents and deep eutectic solvents in extraction cannabinoids using untargeted analysis.

Table 1.
Properties of deep eutectic solvents, M (menthol), LA (Lauric Acid), CA (Citric Acid), EG (Ethylene Glycol, CCL (Choline Chloride), U (Urea) and G (Glycerol).
Table 1.
Properties of deep eutectic solvents, M (menthol), LA (Lauric Acid), CA (Citric Acid), EG (Ethylene Glycol, CCL (Choline Chloride), U (Urea) and G (Glycerol).
| HBA | HBD | Molar ratio | Color | pH | Density (g/ml) | Viscosity (cP) | Polarity | |
|---|---|---|---|---|---|---|---|---|
| M | LA | 2:1 | Transparent colorless | 5.61 | 0.85 | 10.24 | Nonpolar | |
| CA | EG | 1:4 | Transparent colorless | 1.23 | 1.22 | 102.39 | Polar | |
| CCL | EG | 1:2 | Yellowish | 5.05 | 1.07 | 20.09 | Medium polarity | |
| CCL | U | 1:2 | Transparent colorless | 8.95 | 1.13 | 250.07 | Medium polarity | |
| CCL | G | 1:2 | Yellowish | 5.03 | 1.11 | 196.45 | Medium Polarity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Comparison of the Efficiency of Deep Eutectic and Organic Solvents on the Extraction of Phytochemicals from Cannabis sativa L.
Getrude G Kanyairita
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







