Preprint
Review
Nitric Oxide in the Mechanisms of Cell Death and Cytoprotection
Altmetrics
Downloads
180
Views
64
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
26 March 2024
Posted:
27 March 2024
You are already at the latest version
Alerts
Abstract
The regulation of cell death is a fundamental issue in cell biology and continues to be intensely investigated worldwide. The significance of research in this field persists because understanding the determinants of cell lifespan directly impacts the maintenance of multicellular organism homeostasis and, consequently, is crucial for addressing biomedical challenges associated with various diseases. It is recognized that the initiation of cell death can be prompted by both specific signals (such as cytokines or hormones) and nonspecific stimuli, including radiation, temperature increase, or oxidants. When these factors affect a cell, numerous signaling pathways are triggered in the cell, leading to neutralization of the effects of their negative effects or, in case of irreparable damage, to cell elimination. This removal of damaged cells occurs through apoptosis or programmed cell death, a highly regulated process. Numerous signaling molecules participate in orchestrating the apoptotic process, many of which also govern other essential functions within the organism.
Physiological factors capable of triggering the apoptotic program in cells include nitric oxide (NO), which serves as one of the key signaling molecules regulating the functions of the cardiovascular, nervous, and immune systems of the organism. The unique chemical nature of NO, along with its numerous intracellular targets and physiologically active redox forms, raises questions about the specific mechanisms through which NO exerts its damaging effects on cells. Utilizing pharmacological preparations- such as sources (donors) of exogenous nitric oxide- represents an approach to investigating the initiation and execution of the apoptotic program in sensitive target cells. Furthermore, this approach holds promise as a direction for discovering new selective drugs.
Keywords:
Subject: Biology and Life Sciences - Cell and Developmental Biology
1. Introduction
Nitric oxide. Nitric oxide (NO) is a unique molecule that, despite its extremely simple structure, plays a pivotal role in various physiological processes within the body. Its multifunctionality has spurred intensive research over the past decade [1,2,3]. NO serves as a crucial element in the cardiovascular system, facilitating vasodilation and regulating blood pressure. Additionally, it participates in signaling within both the central and peripheral nervous systems [4,5,6].
It has been demonstrated that NO is essential for exerting cytotoxic effects on tumor cells and cells affected by viruses. In this context, the mechanism of action of nitric oxide does not involve the activation of guanylate cyclase but is primarily attributed to the direct effects of NO itself. It is noteworthy that the free-radical nature of the nitric oxide molecule, characterized by the presence of an unpaired electron at the nitrogen atom, renders it highly reactive. In vivo, NO has an average lifetime of 5-30 seconds, during which it rapidly interacts with its targets, primarily thiols and transition metals, or undergoes oxidation to form inactive nitrate and nitrite, for instance, via cytochrome C oxidase [7,8] or NO may generate reactive oxygen species. Hence, the action of NO can occur through direct or indirect mechanisms. Direct effects result from the reactions of NO itself with its targets, such as the stimulation of guanylate cyclase or the formation of nitrosyl complexes with metals, often leading to the inactivation of enzymes containing these metal ions. Indirect effects of NO are defined as chemical reactions mediated by active forms of nitric oxide, which are generated through interactions with superoxide (O2-) or oxygen (O2). The action of these active forms of NO leads to the development of nitrosylation stress (resulting in the formation of nitrosoamines, S-nitrosothiols, and deamination of DNA bases) or oxidative stress [9]. Owing to its high lipophilicity, NO efficiently penetrates membranes, enabling it to diffuse from its source to distances several times the size of the cell, thus affecting its targets within this extended range [10,11].
2. Basic Mechanisms of Nitric Oxide (NO) Regulation
2.1. NO Synthesis in the Body
The investigation into the origin of endogenous NO has revealed that L-arginine is essential for its production by active macrophages. Subsequently, it was discovered that a family of enzymes called nitric oxide synthases (NOS) is responsible for NO production. These enzymes catalyze the conversion of L-arginine into NO and L-citrulline, simultaneously utilizing NADPH and reducing oxygen to water [12,13,14]. NOS enzymes are ubiquitously present in cells of almost all tissue types and are categorized into constitutive (cNOS) and inducible (iNOS) forms based on their expression patterns. The cNOS group typically includes neuronal (ncNOS or NOS1) and endothelial (ecNOS or NOS3) isoforms, with primary localization in neurons and endothelial cells, respectively, although they are also found in other cell types. iNOS is predominantly associated with macrophages and plays a key role in the immune system [15,16,17,18,19,20]. Its expression increases in response to activation by cytokines (such as IFN-g, IL-1b, and TNF-a) and other agents like lipopolysaccharides (LPS). This isoform is also expressed in the liver upon stimulation, which is associated with the barrier function of this organ [21,22,23]. A comprehensive study of NOS has revealed that they are among the most intricately structured and regulated enzymes, boasting an unusually high number of cofactors. NOS exist in the cell as dimers and are active only in this state. Within each subunit of the dimer, distinct domains such as reductase, calmodulin-binding, and oxygenase can be identified. The reductase domain contains the flavins FAD and FMN: FAD serves as the primary electron acceptor from NADPH, while FMN transfers electrons from FAD to the heme of the oxygenase domain. The oxygenase domain contains heme, arginine (L-Arg), and tetrahydrobiopterin (BH4) binding sites. Calmodulin-Ca2+ is believed to confer the enzyme with the necessary conformation for internal electron transfer [24,25,26,27]. It is the variations in the binding affinity of calmodulin to the NOS dimer that underlie the catalytic discrepancies between the isoforms: the activity of nNOS and eNOS is highly reliant on Ca2+ concentration, whereas calmodulin binds to iNOS so tightly that Ca2+ supplementation is unnecessary. Although the specific activities of all NOS isoforms are comparable, in vivo, it seems that cNOS produces small amounts of NO over brief intervals, while iNOS generates much larger quantities of NO over extended periods (up to several days). Thus, the expression and activity of a specific isoform may dictate NO’s role as either a physiological modulator or a cytotoxic agent [28,29,30,31].
In vitro studies of NO-mediated macrophage cytotoxicity have unequivocally demonstrated that the addition of NOS inhibitors, such as the substrate analogue NG-monomethyl-L-arginine (L-NMMA), to the medium suppresses the cytotoxic effect of macrophages on tumour cells. This supports the prevailing role of NO in mediating the macrophage’s effect on target cells. However, it is important to acknowledge the well-known phenomenon of the respiratory burst, which also plays a crucial role in pathogen destruction by phagocytes. Additionally, recent data have emerged that complicate the understanding of macrophage cytotoxicity induced by nitric oxide. Specifically, it has been discovered that wound macrophages capable of NO production do not exhibit cytotoxicity towards NO-sensitive cells of the P815 line. Thus, the question arises regarding the necessity and sufficiency of NO for the cytotoxicity of macrophages [32,33,34,35,36]. It should also be noted that NO production can have significant negative effects on macrophages that produce it. Studies have demonstrated that phagocytosis and the production of reactive oxygen species are markedly inhibited in rat or peritoneal macrophages cultured under conditions that allow NO production. Macrophages expressing iNOS or treated with nitric oxide exhibit nuclear and cytoplasmic condensation. Therefore, the release of NO by activated macrophages leads to their functional suppression, eventually resulting in apoptosis. These phenomena are clearly attributed to NO, as they can be prevented by the addition of NOS inhibitors [37,38].
2.2. Mechanisms of NO Cytotoxicity
Nitric oxide targets are currently under active investigation to determine whether NO itself is sufficiently cytotoxic or if its derivatives are more potent [39]. NO in target cells is known to generate active intermediates such as nitrosonium (NO+), nitroxyl (NO-), and peroxynitrite (ONOO-). Some researchers posit that most of the cytotoxic effects attributed to NO actually stem from ONOO-, which forms through reaction with superoxide (O2-). Indeed, peroxynitrite is significantly more reactive; it extensively nitrosylates proteins and can serve as a source of highly toxic hydroxyl radicals (-OH) through reactions [39,40,41,42].
NO· + O2- а ONOO- + H+ а ONOOH а ONO· + · OH
OH causes lipid peroxidation and other phenomena associated with oxidative stress. Another challenge encountered in the study of the mechanisms of nitrogen cytotoxicity is related to the NO-donors utilized for its generation, as described above. Specifically, S-nitrosothiols (mainly GSNO and SNAP), frequently employed in many studies, have the capability to engage in transnitrosylation reactions. These reactions involve the transfer of the NO+ group to thiols (such as glutathione and SH-groups of proteins), thereby disrupting their cellular functions [43,44,45,46]. However, it remains unclear whether such reactions should be attributed solely to the effects of NO itself. Some possible mechanisms of the cytotoxic action of nitric oxide will be discussed below, and some reactions can be induced by its derivatives. It is demonstrated that NO (from macrophages or exogenously administered) primarily inhibits oxidative phosphorylation in the mitochondria of target cells [47,48]. This inhibition occurs because NO reversibly binds to cytochrome-C-oxidase of the mitochondrial electron transport chain. However, the inhibition of electron transport in the mitochondrion leads to the generation of superoxide and subsequently the formation of peroxynitrite. The conversion of nitric oxide to peroxynitrite involves a reaction between two radicals: O2- and NO, resulting in the formation of ONOO-, a potent oxidant in the mitochondrial matrix. Normally, ONOO is reduced by mitochondrial reducing agents such as NADH2, ubiquinol UQH2, and glutathione GSH. However, when produced in excess due to loss of control (e.g., during ischemia/reperfusion or inflammation), it leads to tyrosine nitration and mitochondrial dysfunction. Its cumulative effect contributes to tissue aging. Another radical widely produced by both enzymatic and non-enzymatic processes is nitric oxide (NO), which serves as an intra- and intercellular signaling molecule [49,50]. NO and superoxide react in a diffusion-limited manner. This reaction halts the chain reaction initiated by superoxide, although peroxynitrite is generally considered a harmful molecule [51]. Peroxynitrite is a short-lived and highly reactive oxidant, thus representing another mechanism that imparts indirect toxicity to O2-, particularly targeting DNA, proteins, and lipids. Additionally, peroxynitrite has the capability to nitrate tyrosine or tryptophan residues or oxidize methionine residues [52,53]. This suppression of mitochondrial respiration leads to a decrease in the mitochondrial membrane potential, which can initiate the apoptotic process [54]. Conversely, it’s known that in the absence of glucose or when glycolysis is blocked, NO-induced suppression of respiration leads to necrosis rather than apoptosis [55]. There is also evidence of direct activation of giant pore opening by nitric oxide (NO), leading to the release of cytochrome C and initiation of the caspase cascade. However, this is also controversial, as other investigators have shown that low doses of NO (close to physiological doses) slow giant pore opening and apoptosis, whereas peroxynitrite (ONOO-) and nitrosothiols promote them. NO and its derivatives can cause peroxidation of phospholipids and oxidation of thiol groups of mitochondrial membrane proteins, which also lead to the release of apoptogenic factors into the cytosol [56,57,58].
Nitrosylation of proteins at tyrosine residues by peroxynitrite (ONOO-) can have profound functional consequences, as it inhibits Tyr phosphorylation, thus disrupting vital signal transduction pathways within the cell. Recent studies have revealed that peroxynitrite can also nitrosylate cytochrome C within mitochondria, altering its function significantly. This modification renders cytochrome C incapable of supporting electron transport in the respiratory chain and resistant to reduction by ascorbate. Concurrently, nitrated cytochrome C is released into the cytoplasm, suggesting potential involvement in signaling processes [59,60]. Emerging hypotheses propose that selective protein nitrosylation may function as a regulatory mechanism akin to phosphorylation [61,62]. Peroxynitrite’s activity extends beyond protein modification to include guanine nitrosylation and DNA strand breaks, which can instigate mutations or trigger apoptosis pathways [63]. Additionally, nitric oxide (NO) exerts effects on DNA repair enzymes by inhibiting their activity. Notably, different NO donors impact various enzymes responsible for DNA repair, such as alkyltransferase, formamidopyrimidine-DNA-glycosylase, and ligase, suggesting a multifaceted role for NO in genomic integrity maintenance and cellular signaling processes [9,64].
It is well-established that nitric oxide (NO) can activate Poly(ADP-ribose) polymerase (PARP) and induce ADP-ribosylation, potentially as a response to DNA damage. However, this activation tends to lead to necrosis due to the depletion of the NAD and ATP pools rather than triggering apoptosis. Regarding NO and its derivatives’ impact on DNA, investigations into their effect on p53 expression are particularly intriguing. p53 is a crucial protein involved in tumor suppression, genome maintenance, and the regulation of cell cycle progression or apoptosis. It is known that p53 can upregulate the expression of pro-apoptotic proteins such as Bax, Fas, and p53AIP (apoptosis-inducing protein) and other apoptogenic proteins [65,66,67,68]. Additionally, during apoptosis, p53 translocates into the mitochondrion, which may be one of the reasons for the production of ROS [69,70].
Under normal conditions, the cellular concentration of p53 remains low as it undergoes rapid degradation. However, DNA damage triggers the accumulation of p53. Experiments conducted on macrophages and RINm5F insulinoma cells have demonstrated p53 accumulation in NO-induced cell death scenarios [71,72]. Further research revealed that L-NMMA, a nitric oxide synthase (NOS) inhibitor, suppresses p53 accumulation induced by cytokines or lipopolysaccharides (LPS), indicating an active involvement of NO in this process. Some evidence suggests that NO’s effect on p53 accumulation may be linked to its ability to inhibit proteasome function, thus interfering with p53 degradation pathways [73,74]. Nevertheless, further experiments have uncovered the operation of p53-independent pathways in NO-induced apoptosis [75,76]. Additional studies have elucidated a negative feedback mechanism between the levels of NO and p53 in various human cell types: the accumulation of NO leading to DNA damage triggers the expression of p53, which in turn suppresses the human inducible nitric oxide synthase (iNOS) gene [77]. Additionally, NO represses iNOS expression by dampening NFκB activity in hepatocytes. Through these intricate pathways, precise regulation of NO synthesis is achieved, thus mitigating its deleterious effects on the tissue [78,79,80].
Figure 1.
Nitric oxide synthesis.

2.3. Interaction of NO with Mitochondria
Since the involvement of mitochondria and nitric oxide (NO) in apoptosis has been extensively elucidated in this study, it is pertinent to describe their combined role in its regulation. In experiments involving the transfection of the RAW264.7 macrophage line with human Bcl-2, the transfected cells exhibited protection against death induced by inducible nitric oxide synthase (iNOS) activation [81,82,83,84]. This suggests that Bcl-2 operates by nullifying the NO-induced upregulation of Bax protein expression. Additional experiments demonstrated that P815 tumor line cells transfected with Bcl-2 showed resistance to the effects of the NO donor SNAP (S-nitroso-N-acetylpenicillin-amine) and to NO-associated cytotoxicity from activated murine macrophages [85,86]. Furthermore, L929 cells overexpressing Bcl-2 were shielded from apoptosis triggered by iNOS activation. A multitude of other instances showcasing the interaction between NO and Bcl-2 are provided in articles [87,88,89,90,91].
The interaction of nitric oxide (NO) with members of the Bcl-2 superfamily is further evidenced by the significant reduction in intracellular Bcl-2 protein levels upon exposure to NO within the cell [92]. This reduction may occur through caspase-induced cleavage or p53-dependent suppression of its expression, although conflicting evidence exists regarding this mechanism [93,94]. Additionally, the proapoptotic effect of nitric oxide manifests through its inducible increase in Bax expression [95]. Apart from the aforementioned mitochondrial functions, recent studies have shed light on the involvement of mitochondria not only in the reception of apoptotic signals from NO but also in the production of NO itself. Notably, a constitutive form of nitric oxide synthase (NOS) has been identified within mitochondria [96,97].
The initial detection of NO production in rat liver mitochondria prompted further investigation into the purification of mitochondrial NOS and elucidation of its enzymatic characteristics [98]. This isoform of NOS appears to be localized within the mitochondrial membrane, particularly in the inner membrane [99]. It has been revealed that mitochondrial nitric oxide synthase (mtNOS) bears a striking resemblance to macrophage inducible NOS (iNOS) but is expressed constitutively. However, the classification of mtNOS as a distinct isoform remains uncertain, as it remains unclear whether it represents a separate isoform or if it is iNOS undergoing post-translational modifications that dictate its distinct subcellular localization. Notably, mtNOS exhibits independence from calmodulin and calcium addition, indicating a strong association with calmodulin. Purified mtNOS, when subjected to suboptimal concentrations of L-Arginine, demonstrates the capability to generate O2-, albeit at a relatively modest rate. This observation aligns with the documented homology of the C-terminal domain of NOS to NADPH: cytochrome P450-oxidoreductase, which also possesses NADPH-oxidase activity and generates O2- albeit at a rate approximately tenfold faster than mtNOS [100,101,102,103,104,105,106,107].
The identification of such a NOS within the mitochondrion raises numerous inquiries and suggests promising avenues for further investigation. Foremost among these is the inquiry into how the NO produced within mitochondria influences apoptosis. Considering the established role of non-mitochondrial NO, which acts directly on mitochondria, inducing a range of phenomena culminating in apoptosis, it is reasonable to speculate on the involvement of mtNOS in the regulation of apoptosis, although conclusive evidence is yet to be established [108,109]. Moreover, given its capacity to generate not only NO but also O2-, may be implicated in the production of reactive oxygen species (ROS), potentially contributing to various biological damages. In the context of apoptosis, intriguing insights have emerged from investigations into the release of cytochrome C from mitochondria upon mtNOS stimulation [110,111]. While elevated cytosolic Ca2+ levels have long been recognized as apoptosis inducers, recent findings have underscored the significant role of mtNOS in this process. It has been demonstrated that this form of apoptosis necessitates mitochondrial Ca2+ uptake, triggering mtNOS activation and subsequent cytochrome C release into the cytosol. Concurrently, there is an augmentation in lipid peroxidation (LPO). Notably, the release of cytochrome C and LPO are effectively inhibited by NOS inhibitors (such as L-NMMA), peroxynitrite scavengers (e.g., urate), and Bcl-2 expression. These findings suggest that upon Ca2+-induced activation of mtNOS, peroxynitrite is generated within the mitochondria, leading to LPO and cytochrome C release, ultimately leads to a pattern of typical apoptosis. Further elucidation of these mechanisms promises to enhance our comprehension of the roles played by mitochondria and NO in various pathways of cell death [112,113,114,115,116].
For instance, recent findings have demonstrated that inhibition of mitochondrial nitric oxide synthase (mtNOS) results in the accumulation of intramitochondrial Ca2+, indicating that NO produced by mtNOS impedes Ca2+ accumulation. Given that the elevation in matrix Ca2+ concentration is responsible for altering mitochondrial membrane permeability, it is deduced (contrary to the earlier assertion) that mitochondrial NO decelerates the opening of the mitochondrial permeability transition pore and the subsequent release of cytochrome C [117,118,119,120,121,122,123]. While there are indications that the synthesis of NO may be regulated through substrates of mtNOS (such as L-Arginine, NADPH) and its cofactors (including FMN, FAD, BH4), akin to other NOS isoforms, this aspect remains largely unexplored. Furthermore, this endogenous mitochondrial NO may play a crucial role in regulating mitochondrial activity by inhibiting cytochrome oxidase (Complex IV), as well as complexes I and II of the electron transport chain. Its reaction with oxygen could modulate mitochondrial respiration by altering the availability of oxygen for electron acceptance, thereby impacting cellular energy supply. Nevertheless, this facet of mitochondrial NO biology necessitates further investigation [124,125,126,127,128,129,130].
Summarizing all these data, we can say that the mitochondriа is the central link where many signaling pathways of apoptosis converge and are regulated. Within these pathways, mitochondria may assume a central role by initiating the pro-apoptotic cascade, particularly under stressors such as irradiation or NO action. Conversely, mitochondria can also amplify certain apoptogenic signaling cascades, such as those mediated by the Fas receptor or TNF receptor, through kinase-mediated mechanisms. Besides superoxide, both NO and its more aggressive derivative, peroxynitrite, are instrumental in the genesis of mitochondrial abnormalities and apoptosis [131,132,133,134,135,136]. Notably, neuronal mitochondria emerge as a significant source of NO, with evidence demonstrating the presence of a constitutive form of NOS localized in the inner mitochondrial membrane and NO production within the mitochondria of hippocampal neurons. Moreover, mitochondrial NOS is capable of generating superoxide at suboptimal concentrations of L-arginine. Importantly, mitochondrial NOS exhibits significant activation in response to the onset of glutamate excitotoxicity and mitochondrial calcium uptake. Additionally, cytokines such as IL-1β and TNF-α contribute to the activation of mitochondrial NOS [137,138,139,140,141,142].
This leads to the generation of peroxynitrite, which facilitates the opening of the mitochondrial permeability transition pore (mPTP). Additionally, peroxynitrite nitrosylates cytochrome C within mitochondria, inducing alterations in its functionality. Specifically, this modification renders cytochrome C incapable of supporting electron transfer within the respiratory chain and resistant to reduction by ascorbate. Concurrently, there is a concomitant release of cytochrome C, including nitrated forms, into the cytoplasm, suggesting the involvement of this nitrosylation process in various signaling pathways. Furthermore, peroxynitrite nitrosylates guanine, resulting in DNA strand breaks, mutations, or activation of apoptosis-related processes [138,143,144,145].
Excessive NO inhibits enzymes crucial for DNA repair, targeting alkyltransferase, formamidopyrimidine-DNA glycosylase, and ligase. Moreover, NO activates PARP and ADP-ribosylation, particularly under conditions of ATP depletion and accumulation of reduced pyridine nucleotides. Additionally, NO exerts a positive influence on the synthesis of the tumor suppressor protein p53. Enhanced p53 expression promotes the upregulation of pro-apoptotic proteins such as Bax, Fas, and p53AIP (apoptosis-inducing protein). Furthermore, NO translocates into the mitochondria during apoptosis, potentially contributing to the production of ROS and the reduction of transmembrane potential across the inner mitochondrial membrane [146,147,148,149,150,151]. Currently, there exists a widely recognized concept known as “mitochondrial dysfunction.” This represents a characteristic pathological process lacking etiological and nosological specificity.
The progression of mitochondrial dysfunction precipitates the disturbance of mediator reuptake (e.g., catecholamines, dopamine, serotonin), ion transport, impulse generation and conduction, de novo protein synthesis, as well as translation and transcription processes. Concurrently, “parasitic” energy-producing reactions are activated, resulting in a substantial depletion of neuronal cell energy reserves. Furthermore, the action of the hydroxyl radical triggers the opening of mitochondrial pores, leading to the expression and release of proapoptotic proteins into the cytosol. This pore opening occurs via the oxidation of thiol groups within the cysteine-dependent region of the mitochondrial inner membrane protein (specifically, the ATP/ADP antiporter), transforming it into a permeable nonspecific channel pore [152,153]. The opening of these pores transforms mitochondria from being “power plants” into “furnaces” for oxidation substrates, devoid of ATP production. Precise biochemical investigations have revealed that disturbances in tissue oxygenation, hyperproduction of excitotoxic amino acids, decreased “normal” calcium (Ca2+) accumulation by mitochondria, and damage to mitochondrial membranes ROS all contribute to the increased opening of these pores and subsequent release of apoptogenic proteins from damaged mitochondria. In this context, the pivotal role of the neurotrophic factor tumor necrosis factor-alpha (TNF-α) cannot be overstated, as it is intricately associated with the opening of mitochondrial pores, subsequent membrane disruption, and the onset of mitoptosis [138,154,155,156,157,158,159,160].
3. Effects of NO
3.1. Apoptosis and NO
Cu has been found to be repressed in neurons with evidence of apoptosis; while Zn-SOD is known to bind and facilitate the production of significant amounts of endogenous peroxynitrite (ONOO−) [161,162,163]. Low concentrations of NO donors have been shown to inhibit neuroapoptosis induced by growth factor deprivation or TNF-α addition, potentially through the activation of the heat shock protein HSP70 or the inhibition of caspase-3 [164,165]. Dinitrosyl iron complexes (DNICs) at concentrations ranging from 5 to 10 μM are believed to suppress IL-1β-induced neuroapoptosis by promoting NO synthesis. Conversely, elevating DNIC concentration (0.5 mM) has been associated with the induction of neuroapoptosis through the generation of ONOO− [166,167,168,169,170].
Conditions that lead to increased bioavailability of NO and reduced formation of ONOO- such as enhanced superoxide dismutase (SOD) activity and the presence of reduced thiol antioxidants render neurons resistant to Fas-induced apoptosis. Conversely, decreased bioavailability of NO and increased levels of cytotoxic forms of NO heighten cellular sensitivity to signals mediated through Fas receptors. Additionally, neuroapoptosis can be induced by the synergistic action of H2O2 and Fe2+, which convert NO to peroxynitrite, while depression of bcl-2 and induction of c-fos were simultaneously observed. In endothelial cells, H2O2 (125–1000 μM) stimulates the activity of NO synthase, contributing to oxidative cellular damage [171,172,173].
Enzymatic antioxidants such as catalase and glutathione peroxidase, along with α-tocopherol, exert inhibitory effects on apoptosis. However, ascorbic and gallic acids have been shown to enhance H2O2-induced neuroapoptosis. Notably, several antioxidants, including α-tocopherol, exhibit potent antiproliferative properties. In the presence of metal ions with varying valence states, ascorbic acid can display pro-oxidant characteristics and augment H2O2-induced neuroapoptosis [174,175,176]. Furthermore, it is noteworthy that NO production can have significant negative effects on macrophages producing it. Studies have demonstrated that phagocytosis and the production of reactive oxygen species are markedly inhibited in rat or peritoneal macrophages cultured under conditions conducive to NO production. Macrophages expressing inducible iNOS or treated with nitric oxide (NO) have condensed nucleus and cytoplasm. Consequently, the release of NO by activated macrophages results in their functional suppression and eventual apoptosis. These observations are directly linked to NO, as they are effectively prevented by the addition of NOS inhibitors. However, an additional challenge in investigating the mechanisms of NO cytotoxicity is related to the NO donors used for its generation, as described earlier. The utilization of S-nitrosothiols, particularly GSNO (S-nitrosoglutathione) and SNAP (S-nitroso-N-acetylpenicillamine), in numerous studies raises concerns regarding their potential involvement in transnitrosylation reactions. These reactions entail the transfer of NO⁺ groups to thiols, including glutathione and sulfhydryl groups of proteins, thereby disrupting their cellular functions. The attribution of such reactions to the effects of NO itself remains ambiguous [177,178,179,180,181,182,183] Importantly, studies have indicated that NO, whether produced endogenously by macrophages or administered exogenously, primarily inhibits oxidative phosphorylation in the mitochondria of neurocytes. This occurs because NO reversibly binds to cytochrome oxidase of the electron transport chain of mitochondria. Furthermore, there is evidence suggesting that NO can directly activate the opening of giant pores, resulting in the release of cytochrome C and the initiation of the caspase cascade, as previously described. Additionally, NO and its derivatives have the capacity to induce peroxidation of phospholipids and oxidation of thiol groups present on mitochondrial membrane proteins. These processes ultimately contribute to the release of apoptogenic factors into the cytosol [184,185,186,187,188].
3.2. Anti-Apoptotic Effects of NO
In addition to the extensively described cytotoxic effects of nitric oxide (NO), numerous studies in the literature have highlighted NO cytoprotective actions. However, upon comparing the evidence for the antiapoptotic effects of NO with the cytotoxic actions outlined earlier, it becomes evident that there are contradictions on many fronts [189,190]. This discrepancy underscores the highly ambiguous nature of NO’s actions, which are contingent upon various conditions.
The molecular mechanisms underlying the antiapoptotic effects of NO-mediated expression of HSP may involve two potential pathways [191]. The first possibility entails the direct suppression of apoptotic signal transduction pathways, involving the inhibition of caspase family protease activation. The second involves chaperone-mediated import of precursor proteins into mitochondria by HSP. This action controls mitochondrial function and membrane permeability, thereby preventing the release of cytochrome C, which is required for further activation of caspases. The relationship between Hsp70 and the induction of apoptosis in obstructive nephropathy was first discussed [192,193]. Other results have shown that Hsp70 can modulate the apoptosis cascade during renal obstruction [194]. Recently, we reported that nitric oxide prevents obstruction-induced cell death through the mitochondrial apoptotic pathway via the induction of heat shock protein 70 [195,196]. Our results demonstrated that the apoptotic effect induced by decreased levels of nitric oxide led to reduced expression of Hsp70. This was associated with a direct induction of apoptotic signal transduction involving caspase 3 activation by decreasing Bcl-2 stabilization. For some cell types, it has been shown that the protective effect of NO is mediated through the synthesis of cGMP [197,198]. Moreover, such an effect is produced by rather low doses of NO similar to those produced in vivo by NO synthases. It is assumed that cGMP generation can activate cGMP-dependent protein kinases, which in turn affect proteins of apoptotic cascades (e.g., caspases or Bcl-2) [199,200].
The protective effect of low doses of NO. Pretreatment of macrophage cells with low, nontoxic doses of GSNO (25-200 μM) induced resistance to higher doses of GSNO (1mM) upon repeated exposure [201,202]. Similarly, pretreatment of macrophages with LPS and IFN-γ in the presence of L-NMMA induced a comparable effect. Thus, the inducible defense mechanisms that suppress NO-induced apoptosis are activated by the action of NO-releasing substances as well as by pre-activation by lipopolysaccharides or cytokines. In other studies, low doses of NO were found to delay the opening of the giant pore and subsequent apoptotic events [203,204,205].
NO and defense proteins: Several studies have demonstrated enhanced expression of heat shock proteins (HSP) and Bcl-2 family proteins in response to NO. Heat shock proteins serve to protect the cell from various stressors, primarily temperature increases, but also oxidative stress and cytokine-induced cytotoxicity. The protective effect of NO was observed when hepatocytes were treated with tumor necrosis factor and when cells were deprived of serum [206,207,208]. In both cases, the anti-apoptotic action of NO correlated with an increase in Hsp70 synthesis. Hsp70 is characterized by its function as a molecular chaperone, assisting in protein folding and the removal of damaged proteins.
Hsp70 is known to play a crucial role in protecting the cell from ROS and mitochondrial damage by suppressing the interaction of proteins that transmit death signals to the mitochondria. Bcl-2, a proto-oncogene with anti-apoptotic properties, has already been described above. Increasing its expression upon NO treatment prevents apoptosis, most likely through the inhibition of giant pore opening [209,210,211]. A novel alternative anti-apoptotic mechanism of NO involves the induction of Hsp32 (hemoxygenase) and Hsp70 through NO-mediated modification of intracellular antioxidant levels [191,212]. The mechanism by which NO stimulates Hsp70 expression may involve the interaction of NO with thiol-containing molecules. There is ample evidence indicating that NO readily oxidizes low molecular weight thiols to form S-nitrosothiols and disulfides [213,214].
Among cellular low molecular weight thiols, glutathione is the most abundant and is also one of the intracellular targets of NO. NO can oxidize intracellular reduced glutathione, thereby altering antioxidant levels within the cell and leading to oxidative or nitrosative stress. This action stimulates the induction of the heat shock proteins Hsp32 (hemoxygenase) and Hsp70, which protect cells from apoptotic cell death induced by tumor necrosis factor (TNF) plus actinomycin D and oxidative or nitrosative stress . Pretreatment of hepatocytes with NO has been shown to alter the redox state accompanied by glutathione (GSH) oxidation and the formation of S-nitrosoglutathione [215,216,217]. GSH-oxidizing agents (diamide) and GSH-alkylating agents (N-ethylmaleimide) induced Hsp70 mRNA expression, whereas GSH synthesis inhibitor (buthionine sulfoximine) did not; this suggests that NO induces Hsp70 expression via GSH oxidation [218,219]. The aforementioned induction may occur via the activation of heat shock. Accumulation of misfolded proteins triggers the mobilization of HSP, leading to the formation of a free pool of Hsp70 and subsequent removal of the negative regulatory effect on HSF activation during heat shock or other stresses. The released HSF is phosphorylated and assembled into trimers, acquires DNA-binding activity, and leads to an increase in Hsp70 mRNA transcripts. During NO stimulation, multiple and complex pathophysiologic changes occur in the smooth muscle cells of blood vessels, including protein damage or modifications due to the cytotoxic action of NO [220,221].
4. Nitric Oxide (NO) in Health and Disease: Interactions, Clinical Relevance, and Therapeutic Implications
4.1. NO and Superoxide Anion
Both NO and O2- are significant mediators of inflammation. Activated macrophages are known to release both NO and O2-. It is generally believed that the interaction of these radicals produces the even more cytotoxic peroxynitrite [222,223]. However, there is evidence suggesting that co-incubation of cells with NO and O2- results in a cross-protective effect, whereas separately both radicals cause apoptosis or necrosis [224,225]. It is thought that in this case, NO acts as a scavenger of O2-, neutralizing its negative effects. Probably, the protective effect requires a balanced presence of NO and O2- and a certain redox state of the cell, as it is necessary to neutralize the formation ONOO-, which is very likely in this situation [226]. Under normal physiological conditions, a balance between superoxide and nitric oxide exists in vivo. NO and superoxide react together at a diffusion-controlled rate to form peroxynitrite (ONOO-), which causes cellular damage by oxidizing many biological molecules. Additionally, ONOO- is involved in the inactivation of Mn and Fe superoxide dismutase [227,228]. NO can protect cells from cytotoxicity, ROS-mediated by removing superoxide anions, which are involved in toxicity through the formation of hydrogen peroxide or hydroxyl radicals [229]. Nitric oxide has been shown to inhibit the formation of superoxide anions. The mechanism of this inhibition is thought to be due to the inactivation of nicotinamide adenine dinucleotide phosphate oxidase because of the scavenging action of NO on superoxide [230]. Inhibition of caspases. Since cysteine is present in the active center of caspases, and reactive nitrogen species can nitrosylate SH-groups, the initial explanation for the suppression of the caspase cascade by nitric oxide was through such nitrosylation of functionally important Cys showed not only suppression of active caspases by nitric oxide but also interruption of caspase activation itself. The proteolytic activation of caspases 3 and 8 was found to be effectively inhibited by both endogenous and exogenous NO, and part of this inhibition was unrelated to S-nitrosylation [231,232].
4.2. NO and Arterial Hypertension
In studies on rats with spontaneous hypertension, it has been found that the central component of NO-ergic regulation of blood pressure involves neurons located in various regions of the brain, including the hypothalamus (such as the paraventricular and supraoptic nuclei, as well as the median eminence) and the medulla oblongata (including the nucleus of the solitary tract, dorsal nucleus, and ambiguous nucleus). Additionally, the peripheral component comprises NO-producing vascular endothelial cells and neurons in the adrenal medulla.
The development of arterial hypertension is accompanied by specific changes in the activity of NO-ergic neurons in the brain involved in blood pressure regulation. These changes include a decrease in the number of neurons positive for neuronal nitric oxide synthase (NOS) in the small cell zone of the paraventricular nucleus, fibers of the median eminence of the hypothalamus, and neurons of the nucleus of the solitary tract. Conversely, there is an increase in the number and activity of NOS-positive neurons in the endocrine nuclei of the hypothalamus, as well as the dorsal and ambiguous nuclei of the medulla oblongata. The systemic increase in blood pressure in spontaneous hypertension leads to the inhibition of NO-producing function in the endothelium of both muscular and elastic vessels. Additionally, the change in NO-ergic activity in adrenal medullary neurons exhibits a dynamic character. Our studies also revealed a depression of NO formation alongside a decrease in total nitric oxide synthase (NOS) activity, both in mitochondria and in the cytosol of the myocardium in all groups of SHR [116].
We observed a significant increase in the expression of inducible nitric oxide synthase (iNOS) in myocardial mitochondria of SHR rats compared to normotensive animals. The discoordination between the activity of total NOS in mitochondria and the formation of stable NO metabolites in the myocardium under conditions of experimental atherosclerosis alongside arterial hypertension, in our opinion, is associated with a surge of “parasitic” reactions. These reactions occur when NOS produces not only NO but also its cytotoxic derivatives, such as peroxynitrite and the nitrosonium ion, etc. Such reactions may occur in conditions of L-arginine deficiency, antioxidant deficiency, mitochondrial dysfunction, increased iNOS expression, and under the influence of proinflammatory factors [233]. Our assumption is confirmed by the detection of increased content of the nitrosative stress marker nitrotyrosine against the background of increased iNOS expression in the mitochondrial fraction of SHR heart homogenate. Additionally, in the myocardial cytosol of SHR rats, we observed a low level of stable NO metabolites (2.4-1.6 times lower) compared to normotensive rats, alongside inhibition of endothelial nitric oxide synthase (eNOS) activity. Analyzing the obtained results of studies on the NO system parameters and reduced intermediates of the thiol-disulfide system, we can conclude that in SHR rats with the most pronounced shifts of the myocardial thiol-disulfide system (including deficit of reduced equivalents, increased oxidation of intermediates, and deprivation of glutathione reductase activity), there were significant changes in the neurochemical profile of NO. It transitioned from a molecular messenger to an agent of nitrosative stress [138]. We have demonstrated that arterial hypertension is accompanied by the inhibition of NOS activity and NO deficiency. This deficiency, combined with the corresponding redox status of mitochondria, leads to protective effects that increase the cell’s resistance to adverse effects. In this context, the expression of inducible NOS increases in mitochondria, particularly when arterial hypertension is combined with diabetes and atherosclerosis. This increased expression has a compensatory value aimed at reducing blood pressure. However, under conditions of deficiency of reduced equivalents in the thiol-disulfide system of cardiac mitochondria, inducible NOS appears as an initiator of nitrosative stress. In this regard, it is important to determine the factor that determines whether NO exhibits cytoprotective or cytotoxic properties at a certain stage of the molecular-biochemical cascade. The thiol-disulfide system seems to play a special role in the development of mechanisms underlying NO cytotoxicity and target organ damage. Intermediates of the thiol-disulfide system possess transport properties with respect to NO, thereby increasing its bioavailability. Moreover, many thiols, such as glutathione, cysteine, and methionine, can significantly limit the cytotoxicity of NO and its derivatives, thus reducing the degree of damage to the target organ [138,183,234].
4.3. NO and the Thiol-Disulfide System
The addition of CDNB (80 μmol), a selective inhibitor of glutathione-S-transferase and a glutathione conjugate, to the incubation medium of neurons resulted in the depletion of the glutathione linkage of thiol-disulfide system (TDS), as evidenced by the deficiency of reduced forms of glutathione due to the inhibition of glutathione reductase (GR) and glutathione-S-transferase (G-S-T) activity. This depletion leads to uncontrolled production of reactive oxygen species, nitrogen and nitrosative stress, as indicated by the observed increase in the level of nitrotyrosine in the neuronal suspension [183,235].
Thus, the increase of nitrotyrosine in neurons treated with CDNB was found to be more than 2.2-fold. Concurrently, there was a shift of the TDS towards oxidized thiols, as evidenced by a decrease in the level of reduced glutathione by 6.6-fold and an increase in its oxidized form by 3-fold. Accumulation of glutathione disulfide proceeded against the background of decreased activity of key enzymes of TDS: glutathione-S-transferase (G-S-T) decreased by 2.7-fold and glutathione reductase (GR) decreased by 2.3-fold compared to intact neurons at 60 minutes of incubation. It is important to note that the described pathophysiological changes led to an increase in cellular damage in the neuron suspension, as evidenced by a statistically significant (p≤0.05) increase in the number of degenerately changed neurons in the test with Hoechst 33342. A possible mechanism of cell damage in neurons incubated with CDNB, in our opinion, may involve disruption of the TDS and the formation of mitochondrial dysfunction. It has been established that the deficit of glutathione not only occurs in conditions of accumulation of active derivatives of NO but also the decrease of its reduced form can be a triggering factor for the development of nitrosative stress. Restored thiols are intracellular NO scavengers. Nitric oxide interacts with cysteine to form S-nitrosocysteine and with glutathione to form S-nitroglutathione. S-nitroglutathione serves as the main transport molecule for NO transfer [138]. The deficiency of sulfhydryl (SH) groups inside the cell leads to a decrease in NO bioactivity and accumulation of reactive oxygen species (ROS). Additionally, uncontrolled growth of ROS leads to oxidation of the alkyl groups of the mitochondrial respiratory chain and inactivation of mitochondrial superoxide dismutase (SOD), further depleting the antioxidant system of the neuron. When modeling acute cerebral ischemia in Wistar rats, we observed marked differences in the concentrations of glutathione (GSH) and nitrotyrosine among the groups of animals with mild, moderate, and severe neurological disorders, as reported by P. McGraw. After conducting statistical analysis using Pearson’s coefficient, a negative correlation of -0.8289 was observed between neurological symptoms and reduced glutathione, while a positive correlation of 0.8272 was found with nitrotyrosine levels. The strong correlations suggest a clear dependence between the studied parameters. Consequently, it appears feasible to compute the ratio of nitrotyrosine level to reduced glutathione and utilize it for diagnosing neurological disorders. The calculated coefficients indicate that, under normal conditions, the ratio of nitrotyrosine to glutathione (Kn/GSH) is approximately 1.3.A mild degree of neurological deficit is characterized by a Kn/GSH close to 5.0; in severe neurological disorders, the Kn/GSH ratio increases substantially to about 138.5. Thus, the interaction within the “NO - reduced thiols” system plays a crucial role in the mechanisms of neurodegradation and endogenous neuroprotection, with its ratio determining the fate of neurons under conditions of central ischemia. The key factor of equilibrium in this system is the maintenance of the pool of reduced thiols and, especially, glutathione at a certain level. The reduced glutathione equivalents not only ensure the bioavailability of NO but also safeguard the proper functioning of the NO system within neurons, thereby preventing the formation of its neurotoxic derivatives [183]. A statistically significant linear correlation between the severity of neurological deficit and the functionality of the conjugated “NO - reduced thiols” system was identified. These findings provide experimental support for utilizing the nitrotyrosine/reduced glutathione coefficient as a diagnostic parameter for assessing the severity of cerebral stroke in clinical biochemistry. Testing its effectiveness in treating patients with cerebral blood flow disorders appears promising.
4.4. NO and Cerebral Ischemia
Numerous studies have demonstrated the direct involvement of nitric oxide (NO) in the neuronal destruction process during ischemia. This has been observed when selective inhibitors of neuronal and inducible NOS isoforms are administered to animals with acute cerebral circulatory disorders (ACBD), as well as in experiments involving animals with a deficiency in the gene encoding iNOS. Data also indicate an elevation in NO concentration in the brains of animals experiencing both focal and global ischemia [138]. The concentration of NO begins to rise within the first minutes of ischemia, peaking on the 1st to 3rd day. Measurement of NOS activity revealed a significant increase in enzyme activity both within the ischemic core and in the penumbra. However, this assessment did not differentiate between the various NOS isoforms. The involvement of NO in neuronal damage and death exhibits specificity determined by NOS isoforms and the type and stage of stroke development. In the initial phase of ischemia, expression of constitutive calcium-dependent NOS, triggered by transmitter autocoidosis. NO production during this phase is not directly responsible for neuronal death but contributes to indirect mechanisms such as the activation of phospholipases, augmentation of hydroxyl radical formation, and modulation of NMDA receptor activity. Subsequently, from 7-14 days in global ischemia and from 1-3 days in focal ischemia, during the delayed post-ischemic period, there is a surge in NO production involving inducible NOS activated within glia, macrophages, and neutrophils [138]. The delayed induction of inducible NOS expression correlates with the subsequent activation of astro- and microglia as well as inflammatory cells. In focal ischemia, these cells, known as NO producers, are localized within the penumbra, while in global ischemia, they are primarily found in structures most vulnerable to oxygen deficiency. Apart from NO synthases, nitrate/nitrite reductases in warm-blooded organisms serve as sources of NO, capable of reducing nitrate and nitrite. Gliocytes and thymocytes exhibit nitroreductase activity. Although xanthine oxidase has demonstrated the ability to convert nitrate and nitrite into NO, its role in neurodegeneration remains understudied. Currently, there is active research into the targets of nitric oxide and efforts to elucidate whether NO itself is sufficiently cytotoxic or if its derivatives are more active [236].
It is well-established that NO within target cells forms active derivatives such as nitrosonium (NO+), nitroxyl (NO-), and peroxynitrite (ONOO-). Recent studies have further emphasized the role of NO and its transformation products, including peroxynitrite (ONOO-), nitrosonium ion (NO+), nitroxyl (NO-), and diazotrioxide (N2O3), as primary factors in inducing nitrosative stress [183,237]. This stress arises from both the direct interaction of NO with metals, such as heme iron in hemoglobin and myoglobin, iron-containing enzymes, and non-heme iron in iron-sulfur proteins and DNA, as well as copper and zinc in enzyme active centers. Additionally, the indirect interaction of NO+ through S-, N-, and O-nitrosation with thiol, phenolic, hydroxyl, and amino groups of proteins and DNA further contributes to nitrosative stress. Such interactions lead to receptor desensitization, inhibition of mitochondrial enzyme activity, and nucleic acid fragmentation. Consequently, nitric oxide (NO), which reversibly binds to the Fe3+ active center of catalase, significantly inhibits its function both during the initial period of ischemia and in the post-ischemic phase of focal cerebral ischemia. Excessive NO levels depress heme enzymes within the mitochondrial electron transport chain. In the post-ischemic period, elevated NO concentrations can interact with heme iron and paired thiol groups to form dinitrosyl iron complex (DNIC) [183]. Unlike NO, DNIC serves as a potent nitrosylating agent, interacting with protein thiols, histidine, aspartate, glutamine, methionine, cysteine, and glutathione, forming N- and S-nitrosothiols. Under ischemic conditions, DNIC undergoes irreversible nitrosylation of iron-sulfur clusters in mitochondrial proteins (such as NADH-ubiquinone oxidoreductase, succinate-ubiquinone oxidoreductase, and aconitase), thereby contributing to mitochondrial dysfunction [183]. Our research has demonstrated that DNIC significantly inhibits the activity of superoxide dismutase (SOD), as well as enzymes involved in regulating thiol-disulfide equilibrium within cells, including glutathione reductase, glutathione-S-transferase, and glutathione peroxidase in neuronal suspensions.
Under ischemic conditions, the inhibition of these enzymes leads to oxidative modification of low-molecular-weight thiols, resulting in the formation of homocysteine and subsequent impairment of NO transport. This impairment leads to the generation of cytotoxic derivatives of NO, which further exacerbate thiol oxidation. Neurons equipped with a sufficiently active thiol antioxidant system capable of regulating NO transport exhibit resistance to nitrosative stress, which represents the earliest neurodegradative mechanism under ischemic conditions. It is well-documented that within the initial minutes of brain ischemia, NO (whether macrophage-derived or exogenous) inhibits oxidative phosphorylation in the mitochondria of target cells through reversible binding to mitochondrial cytochrome-C oxidase. Suppression of electron transport in mitochondria leads to the generation of superoxide, resulting in the formation of ОNOО–. Subsequently, peroxynitrite synthesis occurs in cells with high activity of NO synthase and enzymes producing ROS (such as xanthine oxidase, NADH- oxidoreductase, cyclooxygenase, lipoxygenase, and electron transport chain enzymes). Recent studies have revealed that during the initial stages of ischemia, peroxynitrite levels can be mitigated by mitochondrial nitroreductase, which reconverts it back to NO using NADPH and NADH as cofactors. The targets of oxidative and nitrosative attacks by peroxynitrite encompass thiols, CO2, metalloproteins, nucleic acids, transmitters, and lipids [183].
Peroxynitrite, being a relatively stable compound, undergoes rapid protonation to form its primary product, the nitrate anion, along with hydroxyl radicals and nitrogen dioxide, thereby determining its oxidative properties. Hence, during the initial stages of ischemia, peroxynitrite interacts with thiols via nitrosylation, leading to the formation of nitrosothiols. As the process progresses and lactate acidosis ensues, this interaction shifts towards oxidation, resulting in the formation of more persistent disulfides. These reactions significantly contribute to the mechanisms of neurodegradation by shifting the thiol disulfide system towards oxidized thiol compounds, thereby reducing the cell’s reductive potential. This oxidation process also disrupts gene expression by irreversibly oxidizing cysteine residues within redox-dependent domains and causing dissociation of the MAP kinase cascade. Moreover, peroxynitrite inhibits the activity of metabolic cycles involving methionine and cysteine, thereby impeding key enzymes regulating cysteine levels and promoting homocysteine formation. Additionally, peroxynitrite reacts with the metabolitotropic transmitter CO2 to form a potent nitrosylating agent, nitrosoperoxycarbonate. An essential mechanism of peroxynitrite’s neurotoxic action is its reaction with thiosin to form nitrotyrosine. Peroxynitrite significantly inhibits the activity of Cu-Zn-SOD and Mn-SOD by nitration of its 34th tyrosine residue and by binding to copper, altering its valence. Moreover, peroxynitrite serves as a specific agent that irreversibly depresses mitochondrial respiration during ischemia (Figure 2). Direct interaction with the iron of active centers of key enzymes and nitrosylation of thiol, phenol, hydroxyl, and amino groups of the protein component of these enzymes by S-, N-, O-elements, results in their irreversible oxidation under heightened nitrosative stress. Suppression of mitochondrial respiration leads to a decline in mitochondrial charge, which can trigger the apoptotic process and, in the absence of glucose, necrosis [238,239,240]. Evidence also suggests direct activation of the giant pore opening by nitric oxide, leading to the release of cytochrome C and triggering of the caspase cascade. These findings were obtained when mitochondria were exposed to cytotoxic derivatives of NO such as peroxynitrite and nitrosonium ion, whose mechanism is based on the modification of thiol proteins in the mitochondrial pore.
Nitric oxide (NO) and its derivatives can induce peroxidation of phospholipids. Consequently, cytotoxic derivatives of NO, along with hydroxyl radicals, trigger the opening of mitochondrial pores and the expression and release of proapoptotic proteins into the cytosol. This pore opening occurs due to the oxidation or nitrosylation of thiol groups within the cysteine-dependent portion of the mitochondrial inner membrane protein, specifically the ATP/ADP-antiporter, transforming it into a permeable nonspecific channel-pore. This transformation converts mitochondria from “power plants” into “furnaces” of oxidation substrates without ATP formation [238,239,240]. Impaired tissue oxygenation, transmitter autocoidosis, disrupted calcium accumulation by mitochondria, and damage to the mitochondrial membrane by cytotoxic ROS and NO compounds further enhance pore opening, leading to the release of apoptogenic proteins from damaged mitochondria [242]. The mitochondrial pore is a channel spanning both mitochondrial membranes and comprises three proteins: an adenine nucleotide translocator, a potential-dependent anion channel (porin), and a benzodiazepine receptor. When this complex binds to Ca2+, substances with small molecular weight can traverse the membrane pore. This results in a reduction in membrane potential and swelling of the matrix, ultimately compromising the integrity of the outer membrane and leading to the release of apoptotic proteins from the intermembrane space into the cytoplasm.
Nitrosylation of proteins by tyrosine residues, facilitated by ONOO-, can have significant functional consequences, as it suppresses tyrosine phosphorylation and disrupts certain signal transduction pathways within the cell [183,243]. The balance between NO and the thiol-disulfide system is a critical factor determining the subsequent fate of neurons under ischemic conditions, particularly the mode of cell death. During ischemic brain injury, nitrosative stress emerges early, leading to thiol nitrosation and altering the thiol-disulfide equilibrium of mitochondrial pore proteins. At this juncture, mitochondrial NOS assumes a protective role by modulating cell death, favoring a transition towards apoptosis. Subsequently, oxidative and carbonyl stress ensue, resulting in a significant shift in the thiol-disulfide equilibrium towards oxidized thiols. This leads to persistent mitochondrial dysfunction, depletion of cellular energy reserves, onset of autocoidosis, perturbation of genomic responses, and ultimately, cell death via necrosis [244].
4.5. NO and Endothelial Dysfunction
The primary mechanism underlying endothelial dysfunction (ED) involves a reduction in the formation and bioavailability of nitric oxide (NO), accompanied by a concurrent increase in the level of superoxide ions and the production of active vasoconstrictors [245,246]. Consequently, ED manifests as an imbalance between mediators crucial for the optimal functioning of all endothelium-dependent processes under normal conditions [247]. Concurrently, disruptions in the production, interaction, and breakdown of endothelial vasoactive factors are observed, alongside abnormal vascular reactivity and alterations in the structure and growth of blood vessels, which are indicative of vascular diseases [248].
NO is synthesized from L-arginine under the influence of endothelial NO synthase (eNOS), a process involving the attachment of molecular oxygen to the terminal nitrogen atom of the guanidine group of L-arginine. The assessment of vascular wall integrity and the correction of ED in cardiovascular pathology represent one of the most promising fields of study, as they determine the likelihood of developing vascular diseases and their complications, thus contributing to the overall prognosis of the disease [249].
Therefore, the pursuit of targeted interventions for Endothelial Dysfunction (ED) and the development of a new class of effective drugs - endothelium protectors - represent critical clinical and experimental endeavors. ED is a systemic pathology linked to compromised microstructure and secretory function of endothelium-dependent cells, resulting in reduced endothelium-dependent vasodilation, hypercoagulability, increased thrombosis, heightened vascular permeability, and lipoprotein migration into the vascular intima, as well as smooth muscle cell proliferation, and myocardial and vascular remodeling [250,251,252]. The primary mechanism underlying the development of ED involves a reduction in the formation and bioavailability of NO, accompanied by the emergence of its cytotoxic forms amidst oxidative stress and a deficiency of reduced low molecular weight thiols [98,246]. Meanwhile, the primary causes of NO deficiency in endothelial cells may include a reduced content of its precursor, L-arginine, diminished expression or activity of endothelial nitric oxide synthase (eNOS), and a deficiency in NO synthesis cofactors, particularly tetrahydrobiopterin. Additionally, increased levels of endogenous eNOS inhibitors, such as asymmetric dimethylarginine and monomethyl-L-arginine, elevated formation of reactive oxygen species, notably superoxide anion, and the presence of low-density lipoproteins, especially their oxidized forms, contribute to NO depletion [253,254]. The molecular basis of vascular endothelial dysfunction remains complex and not entirely understood. However, the “eNOS - L-arginine - NO” system holds promise as a pivotal target for pharmacological correction of ED in the foreseeable future [98,255]. Numerous authors have highlighted the direct involvement of NO in cell death processes, including endothelial cells, under conditions such as ischemia, atherosclerosis, and alcohol intoxication. These findings were elucidated through the utilization of selective inhibitors targeting constitutive and inducible isoforms of nitric oxide synthases (NOS), alongside experiments conducted on animals with a deficiency in the gene encoding inducible NOS (iNOS). Investigations have demonstrated that NO transport occurs concomitantly with the formation of N2O3, subsequently leading to thiol nitrosylation. With the involvement of disulfide isomerase, NO is released [235]. Additionally, there exists a mechanism for NO release from S-nitrosoglutathione, facilitated by glutamyl transpeptidase, resulting in the formation of S-nitrosocysteinylglycine, which then liberates NO. Cystine plays a crucial role in the transportation of S-nitrosoglutathione, wherein it is reduced to cysteine. The latter, upon reacting with S-nitrosoglutathione, forms S-cysteine, thereby participating in the rapid conduction of neurons and facilitating the neuron’s adaptive responses to ischemia. These reactions are regulated by glutathione reductase and glutathione transferase.
Under ischemic conditions, inhibition of these enzymes leads to oxidative modification of low molecular weight thiols, homocysteine formation, and subsequent impairment of NO transport, resulting in the generation of cytotoxic NO derivatives that exacerbate thiol oxidation [256,257,258]. Given the current absence of specific drugs for correcting endothelial dysfunction (ED), insights into the effects of cardiovascular drugs from various pharmacological groups on endothelial functional characteristics hold significant value. A comprehensive approach to treating ED in conditions such as chronic cerebral ischemia, arterial hypertension, alcoholic myocardial and cerebral damage, and chronic heart failure may offer substantial practical benefits [259]. This approach involves combining fundamental cardioprotective and neuroprotective therapies with medications that optimize energy metabolism, thereby mitigating the adverse effects of oxidative and nitrosative stress on vascular endothelium and promoting nitric oxide formation. It is conceivable that the future lies with drugs possessing not only cardioprotective or neuroprotective effects but also indirect positive impacts on endothelial function. A particularly promising avenue is the comprehensive treatment of ED in cardiovascular pathology, where reperfusion, antithrombotic, and cardio- or neuroprotective therapies are integrated with medications targeting endothelial dysfunction correction [260].
In light of the above, it has become pertinent to investigate the endothelioprotective properties of drugs exhibiting diverse pathogenetic mechanisms of action. These drugs are known to enhance metabolism, possess antioxidant properties, serve as natural nitric oxide (NO) donors, and activate the NO synthase enzyme. They also contain “essential” phospholipids and affinity-purified antibodies to endothelial NO synthase, offering promising avenues for research in experimental models of cerebrovascular pathology [261].
Numerous drugs with distinct mechanisms of action exert varying degrees of influence on vascular endothelial function. For instance, nitrates replenish endogenous NO deficiency, ACE inhibitors not only reduce angiotensin-II (AT-II) synthesis but also prevent kinin degradation. Statins bolster endothelial cell barrier function against oxidized LDL, while calcium antagonists curb AT-II and endothelin activity in vascular smooth muscle, thereby amplifying NO’s vasodilatory effects. Angiotensin receptor blockers obstruct AT-II receptors, fostering NO accumulation, while endothelin-converting enzyme inhibitors and endothelin-1 receptor antagonists impede peptide activity [262].
Of particular interest are the “specific” effects directed at enhancing NO synthesis, such as replacement therapy involving L-arginine (the substrate eNOS) and tetrahydrobiopterin (eNOS cofactor), crucial for determining the enzyme’s activity [263].
4.6. Inhibitors of NOS Isoforms and Their Cytoprotective Effect
In experimental cerebral ischemia, the application of NOS inhibitors with varying selectivity leads to complex changes. For instance, L-NAME, a non-selective inhibitor, induces irreversible inhibition of constitutive and reversible inducible isoenzyme activity, demonstrating prooxidant properties during early stages. L-NAME’s inhibition of eNOS compromises local vasodilation, exacerbating the overall pathological process [264].
N-propyl-L-arginine during the first 12 hours of ischemia caused a significant decrease in NO level, but the effect on other parameters did not reach statistically significant differences. The mentioned inhibitor is selective in relation to nNOS, the activity of which is significantly increased during the first hours of ischemia. This is explained, firstly, by the observation period - by 12 hours the hyperactivation of nNOS caused by calcium ions begins to decrease with a parallel increase in the inducible form, and secondly, inhibition of nNOS leads to activation of the nuclear factor NF-kB, which causes induction of iNOS [265].
Treatment with the nNOS inhibitor - N-propyl-L-arginine on the 1st and 4th day did not significantly affect the studied parameters, since in more delayed periods the contribution of this isoenzyme to the formation of nitrosative stress was not significant. NO hyperproduction at these stages is caused by the participation of iNOS of glial cells, macrophages and neutrophils. The delayed nature of the iNOS increase is associated with later activation of astroglia. In contrast to nNOS and eNOS, iNOS remains active for a longer period of time and produces significant concentrations of NO [235]. This explains the beneficial effect of the inhibitors we found, which selectively suppress the activity of the inducible isoenzyme late in the observation period. At the end of 1 day after modeling of cerebral ischemia, administration of (S)-methylthiourea caused a significant decrease in the manifestations of nitrosative stress, its effect was more prolonged and persisted until the end of observation. On the 4th day of the experiment, the indicated drug reduced the level of protein oxidative modification products, nitrotyrosine and MDA [235].
The application of L-NAME caused similar but less pronounced changes, which is apparently associated with the inhibitory effect of this compound on eNOS. Our studies have noted the role of intermediates and enzymes of the thiol-disulfide system in the mechanisms of NO bioavailability both in in vitro experiments and in modeling cerebral ischemia in rats [235]. Decreased activity of enzymes of the glutathione system, primarily GPO, which ensures the cleavage of nitrosothiols with the release of NO, in conditions of oxidative stress is one of the reasons for the decrease in its bioavailability. Thus, the neurotoxic effect of NO depends on a certain NOS isoenzyme [235]. Analysis of the obtained data indicates a limited role of neuronal isoform in conditions of experimental нарушение мoзгoвoгo крoвooбращения. The most suitable target for pharmacological regulation of NO-dependent mechanisms of neurodegradation is iNOS, as its activity increases 12 hours after the development of ischemia, and its action is realized during the next few days.
4.7. Exogenous Nitric Oxide
Exogenous NO donors are of considerable help in studying the effect of NO on cells. These substances are widely used nowadays to create model systems in vitro, on which it is possible to study the effects of NO influence on cultures of different cells, or on separate compartments of cells (isolated mitochondria, nuclei) [266]. These models are very popular because they greatly simplify the system of interaction between NO and cells in the body. Since nitric oxide here comes from outside, the system appears to be independent of NO synthases and their regulation, which means that the results of NO action are easier to interpret. It is evident that in this context, the effects of other signaling substances potentially accompanying biogenic NO are largely eliminated. For instance, NOS can synthesize O2- under certain conditions, and the pathways activating NOS may also trigger the production of various additional mediators [117,267,268,269]. Exogenous donors, in contrast to L-Arg, incorporate NO within the structure of the molecule, facilitating the release of this molecule in its pure form [180].
Chemical classifications of NO donors typically include the following groups:
- -
- -
- Nitrites (amyl nitrite, NaNO2),
- -
- Nitrosothiols and substances that form various complexes with NO: S-nitrosoglutathione (GSNO), S-nitroso-N-acetylpenicillamine (SNAP), diethylamine-NO (DEA-NO).
NO donors can be classified based on their mechanism of action into those that spontaneously release NO (non-enzymatic) and those requiring enzymatic interaction to release nitric oxide. Currently, there are no “ideal” NO donors for research purposes. Firstly, NO donors vary in their efficiency of NO release and their ability to affect cells to different extents. Secondly, these substances may serve as sources of side compounds, some of which can be toxic (such as cyanide released by nitroprusside) [271,275,276].
Among the potential NO donors and drugs, there’s a promising original molecule called bromide 1-(β-phenylethyl)-4-amino-1,2,4-triazolium (Hypertril), a derivative of 1,2,2,4-triazole. Hypertril exhibits NO-mimetic properties, particularly when β1-receptor blockade is present. It enhances the expression and activity of endothelial NO-synthase, thus addressing NO deficiency. In the dose range of 7.5-20 mg/kg, “Hypertril” shows promising effects in mitigating disorders in the L-arginine-NO-synthase-NO system in spontaneous arterial hypertension. It achieves this by increasing NO production through enhanced expression of endothelial NOS, thereby reducing manifestations of nitrosative stress in the myocardium. Additionally, “Hypertril” reduces the expression of inducible NOS, leading to dose-dependent increases in cardiomyocyte nuclei density and cardiomyocyte area. Furthermore, it significantly increases RNA content in both nuclei and cytoplasm of cardiocytes, along with an increase in the nuclear-cytoplasmic index, indicative of decreased myocardial hypertrophy. Importantly, “Hypertril” also normalizes blood pressure [233,277]. Moreover, administration of “Hypertril” to animals with chronic heart failure (CHF) results in the prolongation of the depolarization phase (QRS complex) and repolarization phase of ventricles (T wave), as well as electrical diastole (TR interval). These findings suggest a crucial property of the drug in CHF therapy, specifically its ability to prevent the development of diastolic dysfunction [278]. The obtained results of experimental studies are the basis for authorization of the first phase of clinical trials of the new drug “Hypertril” as an antianginal and antihypertensive agent.
4. NO Scavengers
4.1. Xanthine Derivatives
The high efficacy of 8-benzylaminoxanthines in ROS inhibition assays stems from the NO radical’s high reactivity and the presence of a secondary amino group in these compounds. These substituents readily undergo nitrosation reactions to produce corresponding N-nitrosoamino derivatives. Pharmaceutical analysis often utilizes this interaction to qualitatively confirm the presence of such groups in drug structures [279].
It is known that these substituents easily undergo a nitrosation reaction to form the corresponding N-nitrosoamino derivatives. This interaction is used in pharmaceutical analysis to qualitatively confirm the presence of this group in the structure of drugs.
The NO radical is a potent nitrosating agent, demonstrating its detrimental impact on thiol and amino groups within protein molecules. 8-benzylaminooxanthines can function as NO scavengers, transforming into corresponding 8-N-nitrosobenzylaminooxanthines under NO radical exposure, thereby mitigating its adverse effects (Figure 3).
However, this interaction alone does not fully account for the high antioxidant activity observed with 8-benzylaminoxanthines in other in vitro methods. It’s important to note that the methylene group of the benzylamine fragment possesses considerable mobility due to the electron-accepting properties of the nitrogen atom and the benzene ring. Consequently, this site can undergo oxidation via dehydration reactions, functioning as a hydrogen atom donor. Thus, the heightened antioxidant activity of 8-benzylaminoxanthines may arise from their capability to oxidize and form corresponding imidazole derivatives or to undergo hydroxylation to produce corresponding hydroxy derivatives (Figure 4) [280,281].
The hydrazide 8-benzylaminotheophyllinyl-7-acetic acid (C-3) exhibited the most pronounced antioxidant effect due to its ability to bind NO via the presence of a hydrazine group in its structure, thereby acting as a spin trap. Administration of C-3 to animals with intracerebral hemorrhage (ICH) resulted in a significant decrease in the expression of neuronal nitric oxide synthase (nNOS) mRNA in the CA1 zone of the hippocampus by 95.3% compared to control values, along with an increase in nNOS mRNA expression relative to sham-operated animals. Moreover, the expression of inducible nitric oxide synthase (iNOS) mRNA decreased % following C-3 administration relative to the control and was at levels comparable to those of sham-operated animals [282,283,284]. Furthermore, the course administration of compound C-3 to animals with ICH led to a significant decrease in the activity of NOS, nitrites, and nitrotyrosine in brain mitochondria on the 4th day of the experiment, respectively. Administration of C-3 also decreased the expression of iNOS in brain mitochondria. Additionally, C-3 increased the level of HSP70 in the brain cytoplasm and in mitochondria of animals with ICH [285].
4.2. 1,2,4-triazole Derivatives
The compound (S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-thioacetate (Angiolin) demonstrates NO scavenger properties. The NO activity of this compound is attributed to the reactivity of both its cationic and anionic parts. Specifically, lysine interacts with NO through its ε-amino group, forming the corresponding N-nitroso derivative. Concurrently, the anionic portion of Angiolin appears to generate S-nitro derivatives, similar to those described elsewhere. It is evident that the NO activity of both the anionic and cationic parts of Angiolin is synergistic, explaining its pronounced effectiveness. The mechanism of interaction between the Angiolin molecule and NO may involve electron transfer from the highest occupied molecular orbital of the “spin trap” to the lower unoccupied molecular orbital of the nitrogen monoxide radical, leading to the formation of a more stable complex compound. Angiolin may function as a NO transfer molecule, enhancing its bioavailability [286].
Administration of Angiolin (100 mg/kg) in chronic cerebral ischemia results in increased survival of endotheliocytes in the vessels of the cerebral cortex and the vascular wall of cerebral vessels. Additionally, it augments the number of proliferating endotheliocytes and enhances the expression of vascular endothelial growth factor (VEGF). Angiolin exhibits the ability to normalize the eNOS/iNOS ratio, as evidenced by a histoimmunohistochemical study of the CA1-hippocampus. Additionally, Angiolin reduces the intensity of nitrosative stress in the ischemic brain, as indicated by a decreased level of nitrotyrosine. Furthermore, it enhances the expression of endogenous neuroprotective agents, such as heat shock proteins (HSP70), observed in both the cytosol and mitochondria of neurocytes. Interestingly, Angiolin also appears to mitigate mitochondrial dysfunction, as evidenced by a decrease in the number of mitochondria exhibiting signs of ultrastructure disorders (Figure 5) [183].
5. Conclusions
Thus, despite intensive studies on apoptosis, a detailed understanding of the pathways regulating this process still requires further clarification. It is becoming increasingly apparent that various mechanisms regulating cell death are intricately intertwined, making it challenging to distinguish between pro- and anti-apoptotic components in the actions of signaling molecules. Nitric oxide serves as a prime example, with scientific literature presenting nearly equal evidence of both cytotoxic and protective effects of this compound. This complexity complicates the translation of theoretical advancements into practical applications of such substances, as it is challenging to predict their effects at the multicellular organism level based solely on in vitro studies. Therefore, a comprehensive understanding of the regulation of vital cell functions is constructed through focused investigations into the effects of specific compounds under particular conditions on the development of specific signaling pathways.
Author Contributions
Conceptualization, I.B., O.P., N.B., O.K.; validation, I.B., N.B., V.O., D.S.; writing—review and editing, I.B., O.P., N.B., O.K., V.O.; visualization, V.O., O.K., O.P., D.S.; supervision, I.B., O.P., N.B., V.O., O.K., D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data availability
All the data generated during this research are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
| BH4 | - | tetrahydrobiopterin |
| FAD | - | flavinadenine dinucleotide |
| HSP | - | Heat shock proteins |
| IFN | - | interferon |
| IL-1 | - | interleukin-1 |
| L-NMMA | - | NG-monomethyl-L-arginine |
| NOS | - | nitric oxide synthase |
| PARP | - | poly(ADP-ribose) polymerase |
| TNF | - | tumour necrosis factor |
| TNFR | - | tumour necrosis factor receptor |
| ROS | - | reactive oxygen species |
| PCD | - | programmed cell death |
| sGC- | - | soluble guanylate cyclase |
| cGMP | - | cyclic guanosine monophosphate |
References
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv Sci (Weinh). 2023, 10(30):e2303259. [CrossRef]
- Rosselli, M.; Keller, P.J.; Dubey, R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998, 4(1):3-24. [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B. et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol Cancer. 2023, Oct 9;22(1):169. [CrossRef]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br J Pharmacol. 2002, 135(5):1079-95. [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Goldman, D.; Stephenson, A.H.; Dietrich, H.H.; Sprague, R.S. Erythrocytes: Oxygen sensors and modulators of vascular tone. Physiology (Bethesda). 2009, 24: 107–16. [CrossRef]
- Belenichev, I.; Gorbachova, S.; Pavlov, S.; Bukhtiyarova, N.; Puzyrenko, A.; Brek, O. Neurochemical Status of Nitric Oxide in the Settings of the Norm, Ischemic Event of Central Nervous System, and Pharmacological BN Intervention. Georgian Med News. 2021, (315):169-176.
- Nicholls, P.; Hildebrandt, V. Binding of ligands and spectral shifts in cytochrome c oxidase. Biochem J. 1978, 173(1):65-72. [CrossRef]
- Ismail, A. G. Miniaturized devices for bioanalysis : Case of nitric oxide stored as S-nitrosothiols in biological fluids. Analytical chemistry. Université Pierre et Marie Curie. Paris VI, 2016. English. ffNNT : 2016PA066357ff.
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int J Mol Sci. 2020, 21(24):9393. [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding Cell Penetration of Cyclic Peptides. Chem Rev. 2019. Sep 11;119(17):10241-10287. [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics. 2022, 14(3):543. [CrossRef]
- Campos, K.L.; Giovanelli, J.; Kaufman, S. Characteristics of the nitric oxide synthase-catalyzed conversion of arginine to N-hydroxyarginine, the first oxygenation step in the enzymic synthesis of nitric oxide. J Biol Chem. 1995, 270(4):1721-8. [CrossRef]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006, 147 Suppl 1(Suppl 1):S193-201. [CrossRef]
- Alkaitis, M.S.; Crabtree, M.J. Recoupling the cardiac nitric oxide synthases: Tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012, 9(3):200-10. [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Snyder, S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990, 347(6295):768-70. [CrossRef]
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990, 87(2):682-5. [CrossRef]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17(1):7-23. [CrossRef]
- Villanueva, C.; Giulivi, C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med. 2010, 49(3):307-16. [CrossRef]
- Gilchrist, M.; McCauley, S.D.; Befus, A. Dean Expression, localization, and regulation of NOS in human mast cell lines: Effects on leukotriene production. Blood. 2004, 104(2):462-9. [CrossRef]
- Mattila, J.T.; Thomas, A.C. Nitric oxide synthase: Non-canonical expression patterns. Front Immunol. 2014, 5:478. [CrossRef]
- Kany, S; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019, 20(23):6008. [CrossRef]
- Lotz, M.; König, T.; Ménard, S.; Gütle, D.; Bogdan, C.; Hornef, M.W. Cytokine-mediated control of lipopolysaccharide-induced activation of small intestinal epithelial cells. Immunology. 2007, 122(3):306-15. [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of iNOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE. 2016, 11(6):e0153289. [CrossRef]
- Ghosh, S.; Wolan, D.; Adak, S.; Crane, B.R.; Kwon, N.S.; Tainer, J.A.; Getzoff, E.D.; Stuehr, D.J. Mutational Analysis of the Tetrahydrobiopterin-binding Site in Inducible Nitric-oxide Synthase. J Biol Chem. 1999, 274(34):24100-12. [CrossRef]
- Iyanagi, T.; Xia, C.; Kim, J.J. NADPH-cytochrome P450 oxidoreductase: Prototypic member of the diflavin reductase family. Arch Biochem Biophys. 2012, 528(1):72-89. [CrossRef]
- Tengan, C.H.; Rodrigues, G.S.; Godinho, R.O. Nitric oxide in skeletal muscle: Role on mitochondrial biogenesis and function. Int J Mol Sci. 2012, 13(12):17160-84. [CrossRef]
- Gonçalves, D.A.; Jasiulionis, M.G.; Melo, F.H.M.d. The Role of the BH4 Cofactor in Nitric Oxide Synthase Activity and Cancer Progression: Two Sides of the Same Coin. Int. J. Mol. Sci. 2021, 22(17):9546. [CrossRef]
- McMurry, J.L.; Chrestensen, C.A.; Scott, I.M.; Lee, E.W.; Rahn, A.M.; Johansen, A.M.; Forsberg, B.J.; Harris, K.D.; Salerno, J.C. Rate, affinity and calcium dependence of nitric oxide synthase isoform binding to the primary physiological regulator calmodulin. FEBS J. 2011, 278(24):4943-54. [CrossRef]
- Aoyagi, M.; Arvai, A.S.; Tainer, J.A.; Getzoff, E.D. Structural basis for endothelial nitric oxide synthase binding to calmodulin. EMBO J. 2003, 22(4):766-75. [CrossRef]
- Janakiram, N.B.; Rao, C.V. iNOS-selective inhibitors for cancer prevention: Promise and progress. Future Med Chem. 2012, 4(17):2193-204. [CrossRef]
- Kolodziejski, P.J.; Koo, J.S.; Eissa, N.T. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc Natl Acad Sci U S A. 2004, 101(52):18141-6. [CrossRef]
- Albina, J.E.; Reichner, J.S. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998, 17(1):39-53. [CrossRef]
- Liew, F.Y.; Li, Y.; Millott, S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from L-arginine. Immunology. 1990, 71(4):556-9.
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites. 2020, 10(11):429. [CrossRef]
- Bajgar, A.; Krejčová, G. On the origin of the functional versatility of macrophages. Front Physiol. 2023, 14:1128984. [CrossRef]
- Cavinato, L.; Genise, E.; Luly, F.R.; Di Domenico, E.G.; Del Porto, P.; Ascenzioni, F. Escaping the Phagocytic Oxidative Burst: The Role of SODB in the Survival of Pseudomonas aeruginosa Within Macrophages. Front. Microbiol. 2020, 11:326. [CrossRef]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; Wink, D.A. Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid. Redox Signal. 2019, 30(8):1124–1143. [CrossRef]
- Young, D.; Worrell, A.; McDevitt, E.; Henein, L.; Howell, G.E.3rd. Alterations in macrophage phagocytosis and inflammatory tone following exposure to the organochlorine compounds oxychlordane and trans-nonachlor. Toxicol In Vitro. 2020, 65:104791. [CrossRef]
- Wink, D.A.; Hanbauer, I.; Krishna, M.C.; DeGraff, W.; Gamson, J.; Mitchell, J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993, 90(21):9813-7. [CrossRef]
- Stojanović, S.; Stanić, D.; Nikolić, M.; Spasić, M.; Niketić, Vesna. Iron catalyzed conversion of NO into nitrosonium (NO+) and nitroxyl (HNO/NO−) species. Nitric Oxide. 2004, 11(3):256-62. [CrossRef]
- Sharma, A.; Arambula, J.F.; Koo, S.; Kumar, R.; Singh, H.; Sessler, J.L.; Kim, J.S. Hypoxia-targeted drug delivery. Chem Soc Rev. 2019, 48(3):771-813. [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007, 87(1):315-424. [CrossRef]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012:137289. [CrossRef]
- Reis, A.K.C.A.; Stern, A.; Monteiro, H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox Biol. 2019, 27:101190. [CrossRef]
- Ali, A.A.; Coulter, J.A.; Ogle, C.H.; Migaud, M.M.; Hirst, D.G.; Robson, T.; McCarthy, H.O. The contribution of N2O3 to the cytotoxicity of the nitric oxide donor DETA/NO: An emerging role for S-nitrosylation. Biosci Rep. 2013, 33(2):e00031. [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules. 2023, 28(3):1447. [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011, 89(6):873-91. [CrossRef]
- Kobayashi, S.; Homma, T.; Fujii, J. Nitric oxide produced by NOS2 copes with the cytotoxic effects of superoxide in macrophages. Biochem Biophys Rep. 2021, 26:100942. [CrossRef]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci U S A. 2006, 103(3):708-13. [CrossRef]
- Szabó, C.; Módis, K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010, 34 Suppl 1(0 1):4-14. [CrossRef]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem. 2001, 276(31):28799-805. [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem. 2013, 288(37):26464-72. [CrossRef]
- Crack, J.C.; Balasiny, B.K.; Bennett, S.P.; Rolfe, M.D.; Froes, A.; MacMillan, F.; Green, J.; Cole, J.A.; Le Brun, N.E. The Di-Iron Protein YtfE Is a Nitric Oxide-Generating Nitrite Reductase Involved in the Management of Nitrosative Stress. J Am Chem Soc. 2022, 144 (16), 7129-7145. [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009, 43:95-118. [CrossRef]
- MacFarlane, M.; Robinson, G.L.; Cain, K. Glucose--a sweet way to die: Metabolic switching modulates tumor cell death. Cell Cycle. 2012, 11(21):3919-25. [CrossRef]
- Kawano, T.; Zoga, V.; Kimura, M.; Liang, M.Y.; Wu, H.E.; Gemes, G.; McCallum, J.B.; Kwok, W.M.; Hogan, Q.H.; Sarantopoulos, C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol Pain. 2009, 5:12. [CrossRef]
- Brüne, B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003, 10(8):864-9. [CrossRef]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin, Ali, A. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J Clin Biochem. 2015, 30(4):368-85. [CrossRef]
- Abdelmegeed, M.A.; Song, B.J. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014, 2014:149627. [CrossRef]
- Romero-Puertas, M.C.; Laxa, M.; Mattè, A.; Zaninotto, F.; Finkemeier, I.; Jones, A.M.; Perazzolli, M.; Vandelle, E.; Dietz, K.J.; Delledonne, M. S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell. 2007, 19(12):4120-30. [CrossRef]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants (Basel). 2019, 8, 404. [CrossRef]
- Jia, J.; Arif, A.; Terenzi, F.; Willard, B.; Plow, E.F.; Hazen, S.L.; Fox, P.L. Target-selective protein S-nitrosylation by sequence motif recognition. Cell. 2014, 159(3):623-34. [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst). 2019, 83:102673. [CrossRef]
- Parrish, M.C.; Chaim, I.A.; Nagel, Z.D.; Tannenbaum, S.R.; Samson, L.D.; Engelward, B.P. Nitric oxide induced S-nitrosation causes base excision repair imbalance. DNA Repair (Amst). 2018, 68:25-33. [CrossRef]
- Brunyanszki, A.; Szczesny, B.; Virág, L.; Szabo, C. Mitochondrial poly(ADP-ribose) polymerase: The Wizard of Oz at work. Free Radic Biol Med. 2016, 100:257-270. [CrossRef]
- Szabo, C.; Martins, V.; Liaudet, L. Poly(ADP- ribose) polymerase inhibition in acute lung injury: A re- emerging concept. Am. J. Respir. Cell. Mol. Biol. 2020, 63(5): 571-590. [CrossRef]
- Marei, H.E.; Althan,i A.; Afifi, N.; Hasan, A.; Caceci, T.;, Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21(1):703. [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp Mol Med. 2022, 54(10):1658-1669. [CrossRef]
- Dai, C.Q.; Luo, T.T.; Luo. S.C.; Wang. J.Q.; Wang, S.M.; Bai, Y.H.; Yang, Y.L.; Wang, Y.Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J Bioenerg Biomembr. 2016, 48(4):337-47. [CrossRef]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and p53: A versatile partnership. Free Radic Biol Med. 2008, 44(8):1529-35. [CrossRef]
- Borsos, B.N.; Pantazi, V.; Páhi, Z.G.; Majoros, H.; Ujfaludi, Z.; Berzsenyi, I.; Pankotai, T. The role of p53 in the DNA damage-related ubiquitylation of S2P RNAPII. PLoS ONE. 2022, 17(5):e0267615. [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014, 94(3):909-950. [CrossRef]
- Reddy, T.P.; Glynn, S.A.; Billiar, T.R.; Wink, D.A.; Chang, J.C. Targeting Nitric Oxide: Say NO to Metastasis. Clin Cancer Res. 2023, 29(10):1855-1868. [CrossRef]
- Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Connection between Osteoarthritis and Nitric Oxide: From Pathophysiology to Therapeutic Target. Molecules. 2023, 28(4):1683. [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life (Basel). 2022, 12(2):256. [CrossRef]
- Etti, I.C.; Rasedee, A.; Hashim, N.M.; Abdul, A.B.; Kadir, A.; Yeap, S.K.; Waziri, P.; Malami, I.; Lim, K.L.; Etti, C.J. Artonin E induces p53-independent G1 cell cycle arrest and apoptosis through ROS-mediated mitochondrial pathway and livin suppression in MCF-7 cells. Drug Des Devel Ther. 2017, 11:865-879. [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines (Basel). 2021, 9(2):94. [CrossRef]
- Dat, N.T.; Binh, P.T.; Quynh le, T.P.; Huong, H.T.; Minh, C.V. Sanggenon C and O inhibit NO production, iNOS expression and NF-κB activation in LPS-induced RAW264.7 cells. Immunopharmacol Immunotoxicol. 2012, 34(1):84-8. [CrossRef]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Talebi, M.; Kopustinskiene, D.M.; Samarghandian, S.; Bernatoniene, J. An Overview of NO Signaling Pathways in Aging. Molecules. 2021, 26(15):4533. [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Sig Transduct Target Ther. 2023, 8(1):267. [CrossRef]
- Lin, H.-Y.; Shen, S.-C.; Lin, C.-W.; Yang, L.-Y.; Chen, Y.-C. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys Acta. 2007, 1773(7):1073-86. [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016, 2016:1245049. [CrossRef]
- Gao, Q.; Zhou, Z.-Y.; He, Y.-N.; Dong, M.-H.; Wang, Z.-N.; Chen, H.-M. BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules. 2023, 28(5):2036. [CrossRef]
- Zielinska, E.; Tukaj, C.; Radomski, M.W.; Inkielewicz-Stepniak, I. Molecular Mechanism of Silver Nanoparticles-Induced Human Osteoblast Cell Death: Protective Effect of Inducible Nitric Oxide Synthase Inhibitor. PLoS ONE. 2016, 11(10):e0164137. [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15:55. [CrossRef]
- Salvucci, O.; Carsana, M.; Bersani, I.; Tragni, G.; Anichini, A. Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res. 2001, 61(1):318-26.
- Fulda, S.; Meyer, E.; Debatin, K.M. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002, 21(15):2283-94. [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol. 2022, 12:985363. [CrossRef]
- Kurschat, C.; Metz, A.; Kirschnek, S.; Häcker, G. Importance of Bcl-2-family proteins in murine hematopoietic progenitor and early B cells. Cell Death Dis. 2021, 12, 784. [CrossRef]
- Ivanova, H.; Vervliet, T.; Monaco, G.; Terry, L.E.; Rosa, N.; Baker, M.R.; Parys, J.B.; Serysheva, I.I.; Yule, D.I.; Bultynck, G. Bcl-2-Protein Family as Modulators of IP3 Receptors and Other Organellar Ca2+ Channels. Cold Spring Harb Perspect Biol. 2020, 12(4):a035089. [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30(6):492–506. [CrossRef]
- Snyder, C.M.; Shroff, E.H.; Liu, J.; Chandel, N.S. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE. 2009, 4(9):e7059. [CrossRef]
- Dorstyn, L.; Kumar, S. The p53-caspase-2 axis in the cell cycle and DNA damage response. Exp Mol Med. 2021, 53(4):517-527. [CrossRef]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18(9):1441-9. [CrossRef]
- Tarr, J.M.; Eggleton, P.; Winyard, P.G. Nitric oxide and the regulation of apoptosis in tumour cells. Curr Pharm Des. 2006, 12(34):4445-68. [CrossRef]
- Barbosa, A.D.; Osório, H.; Sims, K.J.; Almeida, T.; Alves, M.; Bielawski, J.; Amorim, M.A.; Moradas-Ferreira, P.; Hannun, Y.A.; Costa, V. Role for Sit4p-dependent mitochondrial dysfunction in mediating the shortened chronological lifespan and oxidative stress sensitivity of Isc1p-deficient cells. Mol Microbiol. 2011, 81(2):515-27. [CrossRef]
- Lacza, Z.; Pankotai, E.; Busija, D.W. Mitochondrial nitric oxide synthase: Current concepts and controversies. Front Biosci (Landmark Ed). 2009, 14(12):4436-43. [CrossRef]
- Belenichev, I.F.; Bak, P.G.; Popazova, O.O.; Bukhtiyarova, N.V.; Yadlovsky, O.E. Nitric oxide-dependent mechanism of endothelial dysfunction formation is a promising target link for pharmacological management. Biopolym. Cell. 2022, 38(3):145-157. [CrossRef]
- Yuryev, A.; Ono, M.; Goff, S.A.; Macaluso, F.; Wennogle, L.P. Isoform-specific localization of A-RAF in mitochondria. Mol Cell Biol. 2000, 20(13):4870-8. [CrossRef]
- Lacza, Z.; Snipes, J.A.; Zhang, J.; Horváth, E.M.; Figueroa, J.P.; Szabó, C.; Busija, D.W. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003, 35(10):1217-28. [CrossRef]
- Aitken, A. Post-translational modification of 14-3-3 isoforms and regulation of cellular function. Semin Cell Dev Biol. 2011, 22(7):673-80. [CrossRef]
- Gregorich, Z.R.; Cai, W.; Lin, Z.; Chen, A.J.; Peng, Y.; Kohmoto, T.; Ge, Y. Distinct sequences and post-translational modifications in cardiac atrial and ventricular myosin light chains revealed by top-down mass spectrometry. J Mol Cell Cardiol. 2017, 107:13-21. [CrossRef]
- Huang, M.; Zhu, L.; Feng, L.; Zhan, L.; Zhao, Y.; Chen, X. Reforming Nitrate Metabolism for Enhancing L-Arginine Production in Corynebacterium crenatum Under Oxygen Limitation. Front Microbiol. 2022, 13:834311. [CrossRef]
- Meirelles, C.M.; Matsuura, C.; Silva, R.S.Jr.; Guimarães, F.F.; Gomes, P.S.C. Acute Effects of L-Arginine Supplementation on Oxygen Consumption Kinetics and Muscle Oxyhemoglobin and Deoxyhemoglobin during Treadmill Running in Male Adults. Int J Exerc Sci. 2019, 12(2):444-455.
- Hamdane, D.; Xia, C.; Im, S.C.; Zhang, H.; Kim, J.J.; Waskell, L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009, 284(17):11374-84. [CrossRef]
- Pi, X.; Xie, L.; Portbury, A.L.; Kumar, S.; Lockyer, P.; Li, X.; Patterson, C. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1α-stimulated angiogenesis. Arterioscler Thromb Vasc Biol. 2014, 34(9):2023-32. [CrossRef]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr Opin Immunol. 2019, 60:130-140. [CrossRef]
- O’Rourke, B. Mitochondrial ion channels. Annu Rev Physiol. 2007, 69:19-49. [CrossRef]
- Patwardhan, G.A.; Beverly, L.J.; Siskind, L.J. Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr. 2016, 48(2):153-68. [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [CrossRef]
- Graceffa, V. Therapeutic Potential of Reactive Oxygen Species: State of the Art and Recent Advances. SLAS Technology. 2021, 26(2):140-158. [CrossRef]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; Wieckowski, M.R.; Pinton, P. Mitochondrial Ca(2+) and apoptosis. Cell Calcium. 2012, 52(1):36-43. [CrossRef]
- Dubois, C.; Kondratskyi, A.; Bidaux, G.; Noyer, L.; Vancauwenberghe, E.; Farfariello, V.; Toillon, R.-A.; Roudbaraki, M.; Tierny, D.; Bonnal, J.-L.; Prevarskaya, N.; Abeele, F.V. Co-targeting Mitochondrial Ca2+ Homeostasis and Autophagy Enhances Cancer Cells’ Chemosensitivity. iScience. 2020, 23(7):101263. [CrossRef]
- Giorgi, C.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; Suski, J.M.; Wieckowski, M.R.; Pinton, P. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion. 2012, 12(1):77-85. [CrossRef]
- Pesaresi, M.G.; Amori, I.; Giorgi, C.; Ferri, A.; Fiorenzo, P.; Gabanella, F.; Salvatore, A.M.; Giorgio, M.; Pelicci, P.G.; Pinton, P.; Carrì, M.T.; Cozzolino, M. Mitochondrial redox signalling by p66Shc mediates ALS-like disease through Rac1 inactivation. Hum Mol Genet. 2011, 20(21):4196-208. [CrossRef]
- Belenichev, I.F.; Abramov, A.V.; Puzyrenko, A.; Bukhtiyarova, N.V.; Gorchakova, N.O.; Bak, P.G. Molecular mechanisms of myocardial damage in the hypertensive rats and hypertensive rats with metabolic disorders (diabetes mellitus, atherosclerosis). Research Results in Pharmacology. 2022, 8(4): 25–33. [CrossRef]
- Iova, O.M.; Marin, G.E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders-An Overview. Antioxidants (Basel). 2023, 12(3):753. [CrossRef]
- Wang, Y.; Hong, F.; Yang, S. Roles of Nitric Oxide in Brain Ischemia and Reperfusion. Int. J. Mol. Sci. 2022, 23(8):4243. [CrossRef]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246:2399–2406. [CrossRef]
- Hurst, S.; Hoek, J.; Sheu, S.-S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr. 2017, 49(1):27-47. [CrossRef]
- Popazova, O.; Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Oksenych, V.; Kamyshnyi, A. Cardioprotective Activity of Pharmacological Agents Affecting NO Production and Bioavailability in the Early Postnatal Period after Intrauterine Hypoxia in Rats. Biomedicines 2023, 11(10):2854. [CrossRef]
- O-Uchi, J.; Ryu, S.-Y.; Jhun, B.S.; Hurst, S.; Sheu, S.-S. Mitochondrial ion channels/transporters as sensors and regulators of cellular redox signaling. Antioxid Redox Signal. 2014, 21(6): 987–1006. [CrossRef]
- O-Uchi, J.; Jhun, B.S.; Xu, S.; et al. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid Redox Signal. 2014, 21(6): 863–879. [CrossRef]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. 2010, 13(1):97-104. [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012, 33(7):829-37, 837a-837d. [CrossRef]
- Paulo, M.; Costa, D.E.F.R.; Bonaventura, D.; Lunardi, C.N.; Bendhack, L.M. Nitric Oxide Donors as Potential Drugs for the Treatment of Vascular Diseases Due to Endothelium Dysfunction. Curr Pharm Des. 2020, 26(30):3748-3759. [CrossRef]
- Zhang, Y.; Janssens, S.P.; Wingler, K.; Schmidt, H.H.; Moens, A.L. Modulating endothelial nitric oxide synthase: A new cardiovascular therapeutic strategy. Am J Physiol Heart Circ Physiol. 2011, 301(3):H634-46. [CrossRef]
- Makinde, E.; Ma, L.; Mellick, G.D.; Feng, Y. Mitochondrial Modulators: The Defender. Biomolecules. 2023, 13(2):226. [CrossRef]
- Mimaki, M.; Wang, X.; McKenzie, M.; Thorburn, D.R.; Ryan, M.T. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012, 1817(6):851-62. [CrossRef]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306-6344. [CrossRef]
- Zhang, S.; Rao, S.; Yang, M.; Ma, C.; Hong, F.; Yang, S. Role of Mitochondrial Pathways in Cell Apoptosis during He-Patic Ischemia/Reperfusion Injury. Int J Mol Sci. 2022, 23(4):2357. [CrossRef]
- Long, R.T.; Peng, J.B.; Huang, L.L.; Jiang, G.P.; Liao, Y.J.; Sun, H.; Hu, Y.D.; Liao, X.H. Augmenter of Liver Regeneration Alleviates Renal Hypoxia-Reoxygenation Injury by Regulating Mitochondrial Dynamics in Renal Tubular Epithelial Cells. Mol Cells. 2019, 42(12):893-905. [CrossRef]
- Qajari, N.M.; Shafaroudi, M.M.; Gholami, M.; Khonakdar-Tarsi, A. Silibinin treatment results in reducing OPA1&MFN1 genes expression in a rat model hepatic ischemia-reperfusion. Mol Biol Rep. 2020, 47(5):3271-3280. [CrossRef]
- Qian, L.; Mehrabi Nasab, E.; Athari, S.M.; Athari, S.S. Mitochondria Signaling Pathways in Allergic Asthma. Journal of Investigative Medicine. 2022, 70(4):863-882. [CrossRef]
- Martinvalet, D. Mitochondrial Entry of Cytotoxic Proteases: A New Insight into the Granzyme B Cell Death Pathway. Oxid Med Cell Longev. 2019, 2019:9165214. [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Kashiwaya, Y.; Haskó, G.; Liaudet, L.; Szabó, C.; Pacher, P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009, 296(5):H1466-83. [CrossRef]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and disease. Dis Model Mech. 2021, 14(6):dmm048912. [CrossRef]
- Belenichev, I.F.; Cherniy, V.I.; Nagornaya, E.A.; Bukhtiyarova, N.V.; Kucherenko, V.I. Neuroprotection and neuroplasticity. Kiev: Logos, Publisher: K.: Polygraph Plus Ltd. 2015. 510.
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008, 13:5323-44. [CrossRef]
- Belenichev, I.; Aliyeva, O.; Popazova, O.; Bukhtiyarova, N. Molecular and biochemical mechanisms of diabetic encephalopathy. Acta Biochim Pol. 2023, 70(4):751-760. [CrossRef]
- Provenzano, F.; Torazza, C.; Bonifacino, T.; Bonanno, G.; Milanese, M. The Key Role of Astrocytes in Amyotrophic Lateral Sclerosis and Their Commitment to Glutamate Excitotoxicity. Int J Mol Sci. 2023, 24(20):15430. [CrossRef]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L; Estrela, J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants (Basel). 2020, 9(9):901. [CrossRef]
- Perrelli, M.G.; Pagliaro, P.; Penna, C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011, 3(6):186-200. [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells. 2023, 12(9):1273. [CrossRef]
- De Zio, D.; Cianfanelli, V.; Cecconi, F. New insights into the link between DNA damage and apoptosis. Antioxid Redox Signal. 2013, 19(6):559-71. [CrossRef]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb Perspect Biol. 2013, 5(4):a012583. [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol Biol Cell. 2019, 30(20):2584-2597. [CrossRef]
- Kahraman, S.; Siegel, A.; Polster, B.M.; Fiskum, G. Permeability transition pore-dependent and PARP-mediated depletion of neuronal pyridine nucleotides during anoxia and glucose deprivation. J Bioenerg Biomembr. 2015, 47(1-2):53-61. [CrossRef]
- Pflaum, J.; Schlosser, S.; Müller, M. p53 Family and Cellular Stress Responses in Cancer. Front Oncol. 2014, 4:285. [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; Sollott, S.J.; Zorov, D.B. Mitochondrial membrane potential. Anal Biochem. 2018, 552:50-59. [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014, 94(3):909-50. [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Grechko, A.V.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. The Role of Mitochondrial Dysfunction in Vascular Disease, Tumorigenesis, and Diabetes. Front Mol Biosci. 2021, 8:671908. [CrossRef]
- Armstrong, D. Diagnosis and nosology in primary care. Soc Sci Med. 2011, 73(6):801-7. [CrossRef]
- Skulachev, V.P.; Vyssokikh, M.Y.; Chernyak, B.V.; Mulkidjanian, A.Y.; Skulachev, M.V.; Shilovsky, G.A.; Lyamzaev, K.G.; Borisov, V.B.; Severin, F.F.; Sadovnichii, V.A. Six Functions of Respiration: Isn’t It Time to Take Control over ROS Production in Mitochondria, and Aging Along with It? Int J Mol Sci. 2023, 24(16):12540. [CrossRef]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract--A Novel Platform of Mitochondria-Targeted Antioxidants With Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. Am J Ther. 2016, 23(1):e98-117. [CrossRef]
- Тitova, E.; Shagieva, G.; Ivanova, O.; Domnina, L.; Domninskaya, M.; Strelkova, O.; Khromova, N.; Kopnin, P.; Chernyak, B.; Skulachev, V.; Dugina, V. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell Cycle. 2018, 17(14):1797-1811. [CrossRef]
- Bielenichev, I.F.; Gorchakova, N.A.; Doroshenko, E.Yu.; Samura, I.B.; Ryzhenko, V.P.; Bukhtiiarova, N.V. Use of metabolites, metabolithotropic agents and nutritional supplements in sports and sports medicine: A modern view on the problem. Modern medical technologies. 2023,- 4(59):76-88. (In Ukranian). [CrossRef]
- Shemarova, I.; Nesterov, V.; Emelyanova, L.; Korotkov, S. Mitochondrial mechanisms by which gasotransmitters (H2S, NO and CO) protect cardiovascular system against hypoxia. Front. Biosci. (Schol Ed). 2021, 13(2), 105–130. [CrossRef]
- Maeda, A.; Fadeel, B. Mitochondria released by cells undergoing TNF-α-induced necroptosis act as danger signals. Cell Death Dis. 2014, 5(7):e1312. [CrossRef]
- Osei, D.; Baumgart-Vogt, E.; Ahlemeyer, B.; Herden, C. Tumor Necrosis Factor-α Receptor 1 Mediates Borna Disease Virus 1-Induced Changes in Peroxisomal and Mitochondrial Dynamics in Neurons. Int J Mol Sci. 2024, 25(3):1849. [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018, 217(6):1915-1928. [CrossRef]
- Jensen, L.T.; Culotta, V.C. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J Biol Chem. 2005, 280(50):41373-9. [CrossRef]
- Lob, H.E.; Vinh, A.; Li, L.; Blinder, Y.; Offermanns, S.; Harrison, D.G. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension. 2011, 58(2):232-9. [CrossRef]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: The prospect of using HSP70 modulators. Front Cell Neurosci. 2023, 17:1131683. [CrossRef]
- Zhang, K.; Zhai, R.; Xue, T.; Xu, X.; Ren, Y.; Ma, M.; Shi, F.; Wang, H.; Wang, N.; Zhou, F. HSP70 regulates cell proliferation and apoptosis in actinomycin-D-treated lung cancer cells. Transl Cancer Res. 2020, 9(2):1167-1173. [CrossRef]
- Trujillo, M.; Alvarez, B.; Radi, R. One- and two-electron oxidation of thiols: Mechanisms, kinetics and biological fates. Free Radic Res. 2016, 50(2):150-71. [CrossRef]
- Vanin, A.F. Physico-Chemistry of Dinitrosyl Iron Complexes as a Determinant of Their Biological Activity. Int J Mol Sci. 2021, 22(19):10356. [CrossRef]
- Chen, Y.-C.; Chen, Y.-H.; Chiu, H.; Ko, Y.-H.; Wang, R.-T.; Wang, W.-P.; Chuang, Y.-J.; Huang, C.-C.; Lu, T.-T. Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications. Int. J. Mol. Sci. 2021, 22(18):10101. [CrossRef]
- Jandy, M.; Noor, A.; Nelson, P.; Dennys, C.N.; Karabinas, I.M.; Pestoni, J.C.; Singh, G.D.; Luc, L.; Devyldere, R.; Perdomo, N.; Mitchell, C.E.; Adams, L.; Fuse, M.A.; Mendoza, F.A.; Marean-Reardon, C.L.; Mehl, R.A.; Estevez, A.G.; Franco, M.C. Peroxynitrite nitration of Tyr 56 in Hsp90 induces PC12 cell death through P2X7R-dependent PTEN activation. Redox Biol. 2022, 50:102247. [CrossRef]
- Kucharczyk, M.W.; Valiente, D.; Bannister, K. Developments in Understanding Diffuse Noxious Inhibitory Controls: Pharmacological Evidence from Pre-Clinical Research. J Pain Res. 2021, 14:1083-1095. [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014, 20(7):1126-67. [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants (Basel). 2023, 12(9):1675. [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int J Mol Sci. 2020, 21(19):7152. [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr J. 2016, 15(1):71. [CrossRef]
- Sakagami, H.; Satoh, K. Prooxidant action of two antioxidants: Ascorbic acid and gallic acid. Anticancer Res. 1997, 17(1A):221-4.
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23(3):1247. [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants. 2021, 10(2):313. [CrossRef]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells. 2021, 10(11):3194. [CrossRef]
- Pigott, B.; Bartus, K.; Garthwaite, J. On the selectivity of neuronal NOS inhibitors. Br J Pharmacol. 2013, 168(5):1255-65. [CrossRef]
- Poh, W.H.; Rice, S.A. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules. 2022, 27(3):674. [CrossRef]
- Melvin, A.C.; Jones, W.M.; Lutzke, A.; Allison, C.L.; Reynolds, M.M. S-Nitrosoglutathione exhibits greater stability than S-nitroso-N-acetylpenicillamine under common laboratory conditions: A comparative stability study. Nitric Oxide. 2019, 92:18-25. [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic Biol Med. 2019, 140:14-27. [CrossRef]
- Belenichev, I.F.; Shah, F.; Chekman, I.S.; Nagornaya, E.A.; Gorbacheva, S.V.; Gorchakova, N.A. Thiol-disulfide system: Role in endogenous cyto-and organoprotection, pathways of pharmacological modulation. LLC “Vydavnytstvo” Yuston “, Kyiv. 2020.
- Wang, Y.; Tang, B.; Long, L.; Luo, P.; Xiang, W.; Li, X.; Wang, H.; Jiang, Q.; Tan, X.; Luo, S.; Li, H.; Wang, Z.; Chen, Z.; Leng, Y.; Jiang, Z.; Wang, Y.; Ma, L.; Wang, R.; Zeng, C.; Liu, Z.; Wang, Y.; Miao, H.; Shi, C. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat Commun. 2021, 12(1):102. [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. 2019, 88:61-72. [CrossRef]
- Presley, T.; Vedam, K.; Liu, X.; Zweier, J.L.; Ilangovan, G. Activation of Hsp90/NOS and increased NO generation does not impair mitochondrial respiratory chain by competitive binding at cytochrome c oxidase in low oxygen concentrations. Cell Stress Chaperones. 2009, 14(6):611-27. [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; Prakash Mishra, A.; Nigam, M.; El Rayess, Y.; Beyrouthy, M.E.; Polito, L.; Iriti, M.; Martins, N.; Martorell, M.; Docea, A.O.; Setzer, W.N.; Calina, D.; Cho, W.C.; Sharifi-Rad, J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020, 11:694. [CrossRef]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins. Cold Spring Harb Perspect Biol. 2020, 12(4):a036319. [CrossRef]
- Brockhaus, F.; Brüne, B. Overexpression of CuZn superoxide dismutase protects RAW 264.7 macrophages against nitric oxide cytotoxicity. Biochem J. 1999, 338(Pt2)(Pt2):295-303.
- Lanzarin, G.A.B.; Félix, L.M.; Monteiro, S.M.; Ferreira, J.M.; Oliveira, P.A.; Venâncio, C. Anti-Inflammatory, Anti-Oxidative and Anti-Apoptotic Effects of Thymol and 24-Epibrassinolide in Zebrafish Larvae. Antioxidants. 2023, 12(6):1297. [CrossRef]
- Mazzei, L.; Docherty, N.G.; Manucha, W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress and Chaperones. 2015, 20(6):893-906. [CrossRef]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia. 2022, 2(4):1624–1636. [CrossRef]
- Wali, G.; Kumar, K.R.; Liyanage, E.; Davis, R.L.; Mackay-Sim, A.; Sue, C.M. Mitochondrial Function in Hereditary Spastic Paraplegia: Deficits in SPG7 but Not SPAST Patient-Derived Stem Cells. Front Neurosci. 2020, 14:820. [CrossRef]
- Dudeja, V.; Mujumdar, N.; Phillips, P.; Chugh, R.; Borja-Cacho, D.; Dawra, R.K.; Vickers, S.M.; Saluja, A.K. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009, 136(5):1772-82. [CrossRef]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science. 2008, 319(5865):916-9. [CrossRef]
- Wei, Y.; Zhuang, Y.; Zhang, Y.; Luo, L.; Yu, B.; Zeng, J. Role of heat shock protein 70 in silibinin-induced apoptosis in bladder cancer. J Cancer. 2024, 15(1):79-89. [CrossRef]
- Zhai, C.; Lv, J.; Wang, K.; Li, Q.; Qu, Y. HSP70 silencing aggravates apoptosis induced by hypoxia/reoxygenation in vitro. Exp Ther Med. 2019, 18(2):1013-1020. [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol. 2022, 221(6):e202201159. [CrossRef]
- Gantner, B.N.; LaFond, K.M.; Bonini, M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020, 34:101550. [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010, 62(3):525-63. [CrossRef]
- Methela, N.J.; Islam, M.S.; Lee, D.-S.; Yun, B.-W.; Mun, B.-G. S-Nitrosoglutathione (GSNO)-Mediated Lead Detoxification in Soybean through the Regulation of ROS and Metal-Related Transcripts. Int. J. Mol. Sci. 2023, 24(12):9901. [CrossRef]
- Yang, R.; Gao, Y.; Li, H.; Huang, W.; Tu, D.; Yang, M.; Liu, X.; Hong, J.-S.; Gao, H.-M. Posttranslational S-nitrosylation modification regulates HMGB1 secretion and promotes its proinflammatory and neurodegenerative effects. Cell Reports. 2022, 40(11):111330. [CrossRef]
- He, M.T.; Park, H.S.; Kim, Y.S.; Lee, A.Y.; Cho, E.J. Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2021, 22(13):6894. [CrossRef]
- Wu, C.H.; Chen, T.L.; Chen, T.G.; Ho, W.P.; Chiu, W.T.; Chen, R.M. Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages. J Trauma. 2003, 55(3):540-5. [CrossRef]
- Sangaran, P.G.; Ibrahim, Z.A.; Chik, Z.; Mohamed, Z.; Ahmadiani, A. Lipopolysaccharide Pre-conditioning Attenuates Pro-inflammatory Responses and Promotes Cytoprotective Effect in Differentiated PC12 Cell Lines via Pre-activation of Toll-Like Receptor-4 Signaling Pathway Leading to the Inhibition of Caspase-3/Nuclear Factor-κappa B Pathway. Front Cell Neurosci. 2021, 14:598453. [CrossRef]
- Кim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells. 2020, 9(9):2020. [CrossRef]
- Szyller, J.; Bil-Lula, I. Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxid Med Cell Longev. 2021, 2021:6678457. [CrossRef]
- Kim, J.S.; Ohshima, S.; Pediaditakis, P.; Lemasters, J.J. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004, 39(6):1533-43. [CrossRef]
- Guedes, T.A.; Moreira-de-Sousa, C.; Lima, H.M.S.; Grella, T.C.; Socolowski, P.C.; Fontanetti, C.S. Cytoprotective and anti-apoptotic action of HSP70 stress protein in Oreochromis niloticus exposed to residual dilutions of insecticides with fipronil and ethiprole. J Environ Sci Health B. 2020, 55(8):687-693. [CrossRef]
- Li, X.; Yu, Y.; Gorshkov, B.; Haigh, S.; Bordan, Z.; Weintraub, D.; Rudic, R.D.; Chakraborty, T.; Barman, S.A.; Verin, A.D.; Su, Y.; Lucas, R.; Stepp, D.W.; Chen, F.; Fulton, D.J.R. Hsp70 Suppresses Mitochondrial Reactive Oxygen Species and Preserves Pulmonary Microvascular Barrier Integrity Following Exposure to Bacterial Toxins. Front Immunol. 2018, 9:1309. [CrossRef]
- Kaloni, D., Diepstraten, S.T., Strasser, A. et al. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis. 2023, 28, 20–38. [CrossRef]
- Li, L.; Li, C.M.; Wu, J.; Huang, S.; Wang, G.L. Heat shock protein 32/heme oxygenase-1 protects mouse Sertoli cells from hyperthermia-induced apoptosis by CO activation of sGC signalling pathways. Cell Biol Int. 2014, 38(1):64-71. [CrossRef]
- Mouawad, N.; Capasso, G.; Ruggeri, E.; Martinello, L.; Severin, F.; Visentin, A.; Facco, M.; Trentin, L.; Frezzato, F. Is It Still Possible to Think about HSP70 as a Therapeutic Target in Onco-Hematological Diseases? Biomolecules. 2023, 13(4):604. [CrossRef]
- Broniowska, K.A.; Hogg, N. The chemical biology of S-nitrosothiols. Antioxid Redox Signal. 2012, 17(7):969-80. [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients. 2019, 11(8):1926. [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6:183-197. [CrossRef]
- Baldelli, S.; Ciccarone, F.; Limongi, D.; Checconi, P.; Palamara, A.T.; Ciriolo, M.R. Glutathione and Nitric Oxide: Key Team Players in Use and Disuse of Skeletal Muscle. Nutrients. 2019, 11(10):2318. [CrossRef]
- Girard, P.M.; Peynot, N.; Lelièvre, J.M. Differential correlations between changes to glutathione redox state, protein ubiquitination, and stress-inducible HSPA chaperone expression after different types of oxidative stress. Cell Stress Chaperones. 2018, 23(5):985-1002. [CrossRef]
- Zhang, H.; Gong, W.; Wu, S.; Perrett, S. Hsp70 in Redox Homeostasis. Cells. 2022, 11(5)829. [CrossRef]
- Collins, C.B.; Nguyen, T.T.; Leddy, R.S.; Alula, K.M.; Yeckes, A.R.; Strassheim, D.; Aherne, C.M.; Luck, M.E.; Karoor, V.; Jedlicka, P.; Pierce, A.; de Zoeten, E.F. Heat shock factor 1 drives regulatory T-cell induction to limit murine intestinal inflammation, Mucosal Immunology. 2024, 17(1):94-110,. [CrossRef]
- Belenichev I.; Bila Yu. The effect of the heat shock protein HSP70 modulators on the energy metabolism of the rats brain in acute cerebral ischemia. Biological Markers and Guided. 2019. 6(1):51-62. [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10:1462. [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y. et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 2003, 97(10):2499–2574 (2023). [CrossRef]
- Grossini, E.; Bellofatto, K.; Farruggio, S.; Sigaudo, L.; Marotta, P.; Raina, G.; De Giuli, V.; Mary, D.; Pollesello, P.; Minisini, R.; Pirisi, M.; Vacca, G. Levosimendan inhibits peroxidation in hepatocytes by modulating apoptosis/autophagy interplay. PLoS ONE. 2015, 10(4):e0124742. [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019, 15(1):4-33. [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants. 2024, 13, 312. [CrossRef]
- Dikalov, S.I.; Mayorov, V.I.; Panov, A.V. Physiological Levels of Nitric Oxide Diminish Mitochondrial Superoxide. Potential Role of Mitochondrial Dinitrosyl Iron Complexes and Nitrosothiols. Front. Physiol. 2017, 8:907. [CrossRef]
- Carballal, S.; Bartesaghi, S.; Radi, R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochimica et Biophysica Acta. 2014, 1840(2):768-780. [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid Med Cell Longev. 2022, 2022:1225578. [CrossRef]
- Fragoso-Morales, L.G.; Correa-Basurto, J.; Rosales-Hernández, M.C. Implication of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and Its Inhibitors in Alzheimer’s Disease Murine Models. Antioxidants. 2021, 10(2):218. [CrossRef]
- Shahani, N.; Sawa, A. Protein S-nitrosylation: Role for nitric oxide signaling in neuronal death. Biochim Biophys Acta. 2012, 1820(6):736-42. [CrossRef]
- Shi, X.; Qiu, H. Post-Translational S-Nitrosylation of Proteins in Regulating Cardiac Oxidative Stress. Antioxidants. 2020, 9(10):1051. [CrossRef]
- Mazur, I.; Belenichev, I.; Kucherenko, L.; Bukhtiyarova, N.; Puzyrenko, A.; Khromylova, O.; Bidnenko, O.; Gorchakova, N. Antihypertensive and cardioprotective effects of new compound 1-(β-phenylethyl)-4-amino-1,2,4-triazolium bromide (Hypertril). European Journal of Pharmacology. 2019, 853:336–344. [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants. 2021, 10(2):201. [CrossRef]
- Belenichev, I.F.; Gorbacheva, S.V.; Demchenko, A.V. et al. The thiol-disulfide balance and the nitric oxide system in the brain tissue of rats subjected to experimental acute impairment of cerebral blood flow: The therapeutic effects of nootropic drugs. Neurochem. J. 2014, 8, 24–27. [CrossRef]
- Belenichev, I.F.; Burlaka, B.S.; Bukhtiyarova, N.V. et al. Pharmacological Correction of Thiol-Disulphide Imbalance in the Rat Brain by Intranasal Form of Il-1b Antagonist in a Model of Chronic Cerebral Ischemia. Neurochem. J. 2021, 15, 30–36. [CrossRef]
- Di Giacomo, G.; Rizza, S.; Montagna, C.; Filomeni, G. Established Principles and Emerging Concepts on the Interplay between Mitochondrial Physiology and S-(De)nitrosylation: Implications in Cancer and Neurodegeneration. Int J Cell Biol. 2012, 2012:361872. [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012, 16(11):1323-67. [CrossRef]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002, 33(11):1451-64. [CrossRef]
- Belenichev, I.; Gorbachova, S.; Pavlov, S.; Bukhtiyarova, N.; Puzyrenko, A.; Brek, O. Neurochemical status of nitric oxide in the settings of the norm, ischemic event of central nervous system, and pharmacological intervention. Georgian Med News. 2021, (315):169-176.
- Belenichev, I.F.; Litvinenko, E.S.; Kamishny, A.M. Character of mRNA HIF-1α and HIF-3α expression, level of nitrotyrosine, cGMP and interleukins in the brain homogenate of Mongolian sand rats with acute cerebral blood flow disturbance and against the background of therapy with modulators of the glutathione system. Visnyk problem biolohii i medytsyny. 2018, 1 (142).
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells. 2022, 11(4):706. [CrossRef]
- Griswold-Prenner, I.; Kashyap, A.K.; Mazhar, S.; Hall, Z.W.; Fazelinia, H.; Ischiropoulos, H. Unveiling the human nitroproteome: Protein tyrosine nitration in cell signaling and cancer. J Biol Chem. 2023, 299(8):105038. [CrossRef]
- Belenichev, I.F.; Bila, Yu.V. The relationship between the concentration of HSP 70 activity of the thiol-disulfide system and the degree of neurological disorders in the modeling of acute cerebral ischemia. Bulletin of Problems of Biology and Medicine. 2017, 1.135: 86-91.
- Ray, A.; Maharana, K.Ch.; Meenakshi, S.; Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update, Health Sciences Review. 2023, 7:100084,. [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc J Afr. 2012, 23(4):222-31. [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines. 2021, 9(7):781. [CrossRef]
- Matjuda, E.N.; Engwa, G.A.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. An Overview of Vascular Dysfunction and Determinants: The Case of Children of African Ancestry. Front Pediatr. 2021, 9:769589. [CrossRef]
- Hanssen, H.; Streese, L.; Vilser, W. Retinal vessel diameters and function in cardiovascular risk and disease. Progress in Retinal and Eye Research. 2022, 91:101095. [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22(8):3850. [CrossRef]
- Moschetti, L.; Piantoni, S; Vizzardi, E.; Sciatti, E.; Riccardi, M.; Franceschini, F.; Cavazzana, I. Endothelial Dysfunction in Systemic Lupus Erythematosus and Systemic Sclerosis: A Common Trigger for Different Microvascular Diseases. Front Med (Lausanne). 2022, 9:849086. [CrossRef]
- Steyers, C.M. 3rd; Miller, F.J. Jr. Endothelial Dysfunction in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2014, 15(7):11324-11349. [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell Mol Biol Lett. 2023, 28(1):21. [CrossRef]
- Lorin, J.; Zeller, M.; Guilland, J.-C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and nitric oxide synthase: Regulatory mechanisms and cardiovascular aspects. Mol Nutr Food Res. 2014, 58(1):101-16. [CrossRef]
- Belenichev, I.F.; Mazur, I.A.; Abramov, A.V.; Kucherenko, L.I.; Bukhtiyarova, N.V.; Egorov, A.A.; Belenicheva, O.I.; Polyakova, E.N. The endothelium-protective effect of 3-methyl-1,2,4-triazolyl-5-thioacetate (S)-2,6-diaminohexanic acid (lysinium): Effects on the expression of vascular endothelial growth factor (VEGF) and the characteristics of the endothelio cytes of the cerebral vessels of animals with cerebral ischemia. Neurochem J. 2013, 7:296–302. [CrossRef]
- Rajendran, S.; Shen, X.; Glawe, J.; Kolluru, G.K.; Kevil, C.G. Nitric Oxide and Hydrogen Sulfide Regulation of Ischemic Vascular Growth and Remodeling. Compr Physiol. 2019, 9(3):1213-1247. [CrossRef]
- Correia, M.J.; Pimpão, A.B.; Fernandes, D.G.F.; Morello, J.; Sequeira, C.O.; Calado, J.; Antunes, A.M.M.; Almeida, M.S.; Branco, P.; Monteiro, E.C.; et al. Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine. Molecules. 2022, 27(4):1416. [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J Amino Acids. 2012, 2012:736837. [CrossRef]
- Tiurenkov, I.N.; Voronkov, A.V.; Slietsans, A.A.; Volotova, E.V. Endothelial protection drugs--a new class of pharmacological agents. Vestn Ross Akad Med Nauk. 2012, (7):50-7. Russian. [CrossRef]
- De Leonardis, F.; Colalillo, G.; Finazzi Agrò, E.; Miano, R.; Fuschi, A.; Asimakopoulos, A.D. Endothelial Dysfunction, Erectile Deficit and Cardiovascular Disease: An Overview of the Pathogenetic Links. Biomedicines. 2022, 10(8):1848. [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010, 459(6):793-806. [CrossRef]
- Everett, A.D.; Stoops, T.D.; Nairn, A.C.; Brautigan, D. Angiotensin II regulates phosphorylation of translation elongation factor-2 in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001, 281(1):H161-7. [CrossRef]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. San Rafael (CA): Morgan & Claypool Life Sciences. 2011.
- Gorbacheva, S.V.; Belenichev, I.F. Indicators of thiol-disulfide system and nitrosative stress in neurons under conditions of modeling glutamate excitotoxicity in vitro and against the background of application of nos inhibitors of different selectivity. The world of medicine and biology. 2015, 11 4-2 (54):112-116. (In Russian).
- Gorbacheva, S.V.; Belenichev, I.F. Possible ways of interrupting NO-dependent pathways of neurodegeneration with the use of no-synthase inhibitors of different selectivity in conditions of experimental cerebral circulation disorder. Achievements of Biology and Medicine. 2015, 2:21-25. (In Ukranian).
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta. 2006, 224(6):1363-72. [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014, 12(4):207-18. [CrossRef]
- Sun, L.; Liu, H.; Ye, Y. et al. Smart nanoparticles for cancer therapy. Sig Transduct Target Ther. 2023, 8(1):418. [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol Rev. 2019, 99(1):311-379. [CrossRef]
- Zhao, N.; Xu, J.; Singh, B.; Yu, X.; Wu, T.; Huang, Y. Nitrates for the prevention of cardiac morbidity and mortality in patients undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2016. 2016(8):CD010726. [CrossRef]
- Hottinger, D.G.; Beebe, D.S.; Kozhimannil, T.; Prielipp, R.C.; Belani, K.G. Sodium nitroprusside in 2014: A clinical concepts review. J Anaesthesiol Clin Pharmacol. 2014, 30(4):462-71. [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim Biophys Acta. 2013, 1830(5):3173-81. [CrossRef]
- Goudie, M.J.; Brisbois, E.J.; Pant, J.; Thompson, A.; Potkay, J.A.; Handa, H. Characterization of an S-nitroso-N-acetylpenicillamine-based nitric oxide releasing polymer from a translational perspective. Int J Polym Mater. 2016, 65(15):769-778. [CrossRef]
- Sysel, A.M.; Dunphy, M.J.; Bauer, J.A. Antimicrobial properties of diethylamine NONOate, a nitric oxide donor, against Escherichia coli: A pilot study. J Antibiot (Tokyo). 2021, 74(4):260-265. [CrossRef]
- da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Lima Silva, E.M.; Gouvêa, F.F.F.; de França Lopes, L.G.; Araújo, A.V.; Ferraz Pereira, K.N.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology (Basel). 2021, 10(10):1041. [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl Med. 2019, 8(11):1135-1148. [CrossRef]
- Belenichev, I.; Bak, P.; Popazova, O.; Ryzhenko, V.; Bukhtiyarova, N.; Puzyrenko, A. Integrative and Biochemical Parameters in Rats in the Simulation of Doxorubicin Chronic Heart Failure and During the Use of β-Adrenergic Blockers. Journal of Faculty of Pharmacy of Ankara University. 2023, 47(1): 228–238. [CrossRef]
- Goncharov, O.; Belenichev, I.; Abramov, A.; Popazova, O.; Kucherenko, L.; Bukhtiyarova, N.; Pavliuk, I. Influence of experimental heart failure therapy with different generations of β-adrenergic blockers on Cardiac Electrical Activity (ECG) and Autonomic Regulation of Heart Rhythm (ARHR). Pharmacia. 2023, 70(4): 1157–1165. [CrossRef]
- Ryzhenko, V.P.; Belenichev, I.F.; Samura, I.B.; Ryzhov, O.A. Development of software for prediction and virtual screening of antioxidant activity of new synthesized azaheterocyclic compounds. Int J Basic Clin Pharmacol. 2019, 8(6):1292-1296. [CrossRef]
- Ryzhenko, V.; Ryzhov, O.; Belenichev, I.F.; Levich, S.V. Study of Dependence of Xanthine Derivatives NO-Scavenger Properties from Energy Descriptors. Biological Markers and Guided Therapy. 2018, 5(1)37-46. [CrossRef]
- Ryzhov, O.A.; Ryzhenko, V.P.; Levich, S.V.; Belenichev, I.F. Analysis of influence of quantum chemical descriptors on no-scavenger properties among xanthine derivatives. Biological Markers and Guided Therapy. 2017, 4(1):39-48. [CrossRef]
- Belenichev, I.F.; Nosach, S.G.; Samura, I.B.; Levich, S.V. Some Aspects of Neuroprotective Action of a New Derivative of 3-Methylxanthine (Compound C-3) Under Conditions of Acute Disorder of Cerebral Circulation (ADCC) Modeling by Ischemic Stroke Type. Biological Markers and Guided Therapy. 2018, 5(1):63-73. [CrossRef]
- Belenichev, I.; Aleksandrova, K.; Shkoda, A.; Levich, S.; Nosach, S. Influence of 3-methylxanthine derivative on the morphological and functional characteristics of neurons of sensorimotor cortex of rats with experimental intracerebral hemorrhage. In Journal of cerebral blood flow and metabolism. 2016, 36:787-787.
- Belenichev, I.F.; Aleksandrova, K.V.; Buhtiyarova, N.V., Levich, S.V., Nosach, S.G.; Sinchenko, D.N. Biological markers and guided therapy. 2016, 3(1):139-145.
- Ryzhenko, V.P. Optimization of purposeful search of NO scavengers in a number of xanthine derivatives. − The dissertation on competition of a scientific degree of the candidate of biological sciences on a specialty 14.03.05 “Pharmacology”. State Institution “Institute of Pharmacology and Toxicology of the National Academy of Medical Sciences of Ukraine”, Kyiv, 2020.
- Chekman, I.S.; Belenichev, I.F.; Syrova, A.O.; Gorchakova, N.A.; Bukhtiyarova, N.V.; Ryzhenko, V.P.; Chalenko, N.N. Aspects of creation of neuroprotective, anti-inflammatory drugs. Dopov. Nac. akad. nauk Ukr.2019, 9:88-98.
Figure 2.
NO and cerebral ischemia.

Figure 3.
Scheme of the potential interaction of 8-benzylaminoquinones with NO.

Figure 4.
Scheme of the potential oxidation of 8-benzylaminoxanthines.
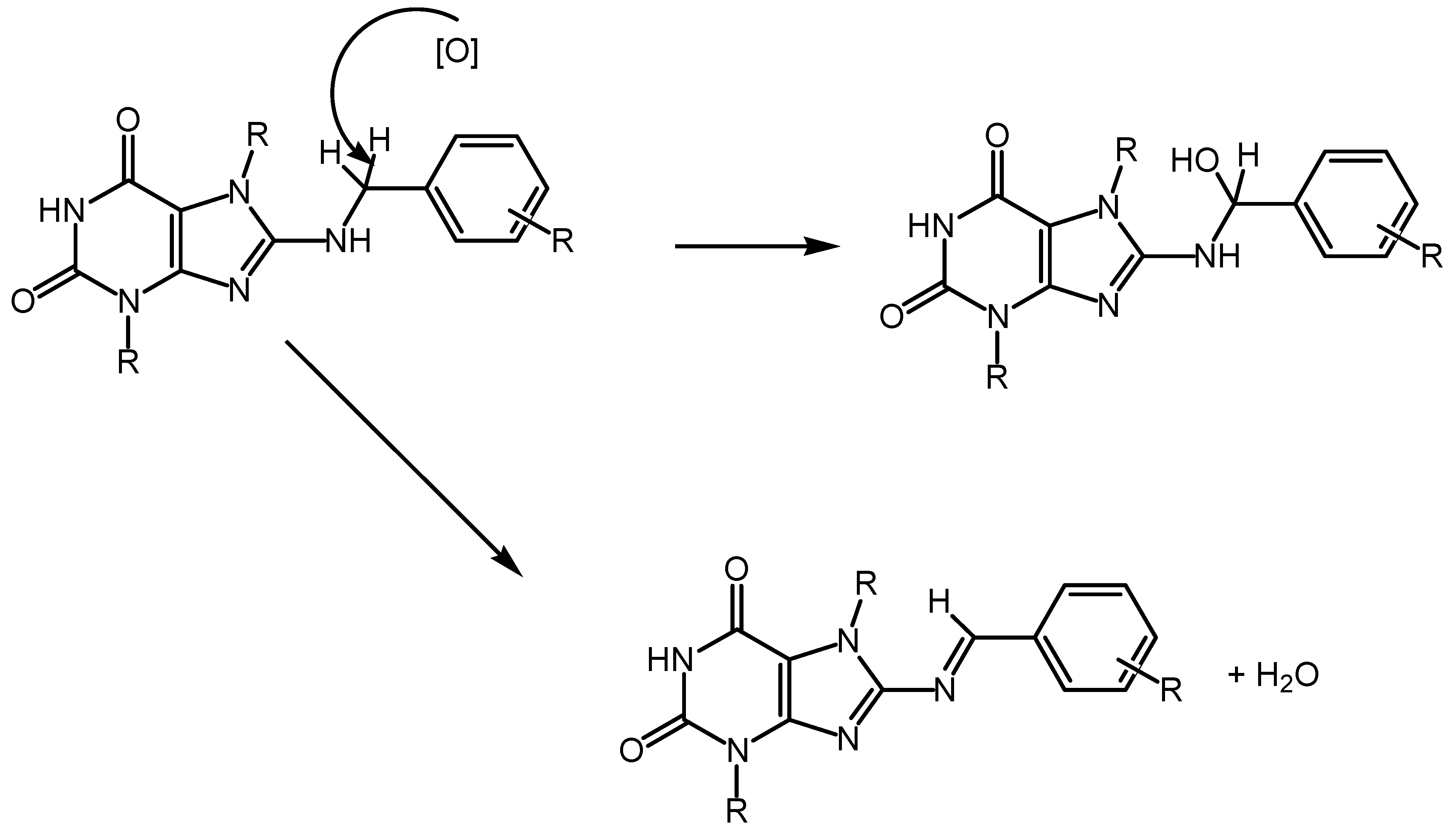
Figure 5.
Hypothetical mechanism of (S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-thioacetate (Angiolin) interaction with NO.
Figure 5.
Hypothetical mechanism of (S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-thioacetate (Angiolin) interaction with NO.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Redox Regulation of the Immune Response: Nitro-Oxidative Stress and Antioxidants Regulate Macrophage, Neutrophil, and T and B Lymphocyte, and Dendritic and Natural Killer Cell Functions
Gerwyn Morris
et al.
,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









