Preprint
Review
Gastropod Allergy: A Comprehensive Narrative Review
Altmetrics
Downloads
112
Views
43
Comments
0
A peer-reviewed article of this preprint also exists.
Submitted:
24 May 2024
Posted:
27 May 2024
You are already at the latest version
Alerts
Abstract
Food allergies have increased significantly in recent decades, with shellfish being a leading cause of food allergy and anaphylaxis worldwide, affecting both children and adults. The prevalence of shellfish allergies is estimated to be approximately 0.5-2.5% of the general population, varying significantly by geographical location, age, and consumption habits. Although mollusk consumption has risen, the prevalence of mollusk allergies remains unknown. While extensive research has focused on crustacean allergies, mollusk allergies, particularly those related to gastropods, have received comparatively less attention. Clinical manifestations of shellfish allergy range from localized symptoms to life-threatening systemic reactions, such as anaphylaxis. Notably, severe bronchospasm is a predominant clinical feature in cases involving gastropods. Several allergens have been identified in mollusks, including paramyosin, tropomyosin, and sarcoplasmic calcium-binding protein. In gastropods, documented allergens include tropomyosin, paramyosin, the heavy chain of myosin, and Der p 4 amylase. Diagnosis typically involves a thorough clinical history, skin testing, in vitro quantification of immunoglobulin (Ig) E, and confirmation through oral challenge, although the latter is reserved for selected cases. This review highlights the limited research on gastropod allergy, the identified allergens to date, and the necessity for further investigations to better understand the implications of shellfish allergy within this class.
Keywords:
Subject: Medicine and Pharmacology - Immunology and Allergy
1. Introduction
Seafood, including fish and shellfish, is a rich source of nutrients and antioxidants, making it a key component of the Mediterranean diet [1,2]. Known for providing essential protein and omega-3 fatty acids, seafood offers numerous cardiovascular health benefits [3,4,5]. As a result, its consumption has surged in recent years, paralleling an increase in allergic reactions to seafood [1,2]. While shellfish includes both crustaceans and mollusks, research has traditionally focused on crustaceans, leaving studies on mollusk allergies, particularly gastropod allergies, notably sparse. The aim of this paper is to collate the limited scientific literature on gastropod allergy and examine the current available both clinical and immunological data.
2. Epidemiology of Shellfish Allergy
Food allergy (FA) refers to an adverse immune system reaction to certain foods [6]. In recent years, the prevalence of FA has significantly increased, affecting an estimated 3.5%-4% of the global population [10,12]. The rising consumption of shellfish in recent years has heightened the risk of allergic and toxic reactions, presenting with a variety of symptoms that can be challenging to define. Shellfish are a leading cause of FA and anaphylaxis worldwide, with prevalence estimated at approximately 0.5-2.5% of the general population. This prevalence varies based on geographical location, age, and consumption habits [10]. Recent research by Gelis and coworkers et al. (2020) suggests that prevalence of shellfish allergies ranges from less than 1% to 10.3%, depending on geographical area [7]. For instance, in Spain, shellfish is the third most common cause of FA in adults over 15 years old, with cases increasingly reported at younger ages, following milk, egg, fruit, and fish allergies [8]. Moreover, shellfish allergy is one of the leading causes of FA in many Asian countries, such as Thailand, Taiwan, Hong Kong, Vietnam, and Singapore, where shellfish is frequently consumed [9]. Coastal regions of Asia are prominent consumers of mollusks, while Southern Europe, particularly Spain, favors cephalopods and other shellfish. Japanese diets feature higher quantities of squid, whereas Italians, French, Portuguese, and Spaniards consume significant amounts of terrestrial snails [10]. Consequently, awareness of mollusk allergy is growing, although its prevalence remains uncertain [11]. Additionally, allergy to mollusks, particularly gastropods, has received limited study.
3. Classification of Shellfish
The term shellfish is used for both crustaceans and mollusks. Mollusks represent the largest marine phylum, with around 85000 described species [10]. Shellfish belong to the Invertebrate Kingdom Eumatozoa, which is divided into three phyla: Mollusca, Athropoda y Echinodermata. Athropoda contains the class Crustacea. The Mollusca phylum is divided into eight classes, but only three are significant for human consumption: cephalopods (cuttlefish, squid, octopus), bivalves (clams, cockles, mussels, blue mussels, scallop, oyster), and gastropods (limpets, conchs, periwinkles, sea slugs, whelks, snails, and abalone) [12] (Figure 1).
4. Clinical Symptoms of Shellfish Allergy
FA can be categorized into immunoglobulin (Ig) E-mediated, non-IgE mediated, and mixed IgE and non-IgE mediated reactions, depending on the involvement of IgE in the immune response mechanism. These reactions can manifest as type I hypersensitivity (IgE-mediated), type III or type IV hypersensitivity (non-IgE-mediated), or a combination of IgE and cellular mechanisms (mixed IgE and non-IgE mediated) [6]. Typical IgE-mediated reactions usually occur within about two hours after ingestion and can manifest as urticaria, angioedema, abdominal pain, nausea, vomiting, or respiratory symptoms like bronchospasm, laryngeal edema, and/or anaphylaxis [6]. Additionally, patients may experience self-limited clinical manifestations localized in the oropharyngeal mucosa due to shellfish cross-reactivity with inhalant allergens such as house dust mite (HDM) and tropomyosin (TPM), known as mite-shellfish oral allergy syndrome [14].
Non-IgE-mediated reactions, increasingly recognized in children, typically occur several hours or days after exposure to the allergen and include food protein-induced enterocolitis syndrome (FPIES), food protein-induced enteropathy (FPE), and food protein-induced allergic proctocolitis (FPIAP) [1,15,16,17]. The main features of acute fish and shellfish FPIES, compared to other foods such as cow's milk or soybean FPIES, include a later onset, longer persistence, and the possibility of tolerating fish species other than the offending fish [1]. Also, contaminating toxins, viral and bacterial contamination or parasites can cause adverse symptoms such as vomiting, fever and diarrhea after ingestion of shellfish. These clinical manifestations typically emerge several hours after ingestion [18] (Figure 2).
Symptoms of FA are triggered by food proteins that activate the immune system, leading to an increase in IgE levels. Typical symptoms include itching and swelling of the mouth and throat (allergic oral syndrome), as well as potentially life-threatening anaphylaxis [10]. The clinical manifestations of shellfish allergy can vary widely and differ among individuals. It typically results in moderate to severe reactions, characterized by sensitization that often persists throughout life, with avoidance being the only effective treatment. Exposure to shellfish allergens can occur through ingestion, inhalation, or skin contact. Symptoms may include itching, hives (urticaria), swelling (angioedema), respiratory symptoms (shortness of breath, coughing, wheezing, rhinitis), gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain), and cardiovascular symptoms (hypotension). In severe cases, life-threatening reactions can occur. Allergic reactions to shellfish can be unpredictable. While type I reactions typically occur within the first hour, there have been reported cases where symptoms appeared up to 8 hours after ingestion of limpet and abalone [19].
5. Clinical Symptoms of Gastropod Allergy
Gastropod allergy is notable for its severe symptoms, particularly marked by pronounced bronchospasm, which serves as a hallmark of reactions within this category. While severe asthma can manifest in reactions to other shellfish, it is particularly distinctive in cases involving gastropods [19,20,21,22,23,24,25].
5.1. Terrestrial Snail
Snail hypersensitivity was initially reported by Palma Carlos et al. in 1985 [26]. Subsequent studies have further elucidated this phenomenon. Four years later, De la Cuesta and coworkers presented findings from 10 patients, 80% of whom reported respiratory symptoms, with two experiencing symptoms after consuming limpet and snail. Interestingly, despite all described patients tolerated the ingestion of both cephalopods and bivalves -belonging to different phylogenetic lines- there was a lack of data regarding concomitant tolerance to crustaceans and the prevalence of comorbid asthma [20]. In 1996, Van Ree et al. reported 28 subjects who experienced asthmatic episodes after consuming snails, with two cases resulting in anaphylactic reactions. Some of these patients also reported similar respiratory symptoms after ingesting limpets. Among the subjects, 23 out of those 28, presented symptoms within 5-60 minutes after snail ingestion, while the remaining experienced symptoms 1-5 hours post-ingestion. There was also a lack of data regarding tolerance to crustaceans or other mollusks. Notably, all subjects presented dust mite allergic rhinitis and asthma, therefore suggesting a potential co-sensitization [21]. Guilloux and Vuitton and co-workers reported in 1998 seven patients who experienced respiratory symptoms and anaphylaxis after consuming terrestrial snails. Diagnosis was established through skin tests and specific IgE against snail. Similar to previous findings, there was limited data concerning tolerance of other mollusks or crustaceans. All patients were allergic to Dermatophagoides pteronyssinus (D. pteronyssinus), with five of them being asthmatic [27]. In 2005, Lourenço Martins et al. also identified 60 allergic patients with specific IgE to H. aspersa. Among them, six developed asthma after consuming snails, with symptoms appearing 15 minutes to 3 hours post-ingestion. Once again, data regarding the tolerance of other mollusks or crustaceans are scarce. Of the 60 patients, 18 suffered from asthma, 36 from rhinitis and asthma, and 3 from rhinitis, 2 from atopic dermatitis, and 1 from irritative cough. Notably, 56 patients were allergic to D. pteronyssinus, suggesting a potential cross-reactivity between species [22].
5.2. Abalone
In 1990, Morikawa documented a case of anaphylaxis associated with abalones, highlighting specific IgE-mediated hypersensitivity to these shellfish confirmed through clinical history, prick skin tests, and RAST. Further analysis using radioallergosorbent (RAST) inhibition technique revealed cross-antigenicity between GKL, abalone, and keyhole limpet hemocyanin. However, data regarding the tolerance of other mollusks or crustaceans and the prevalence of asthmatics remained scarce [28]. Lopata et al. (1997) reported on 38 patients, with 66% experiencing symptoms within 2 hours and 34% between 2 and 7 hours after ingesting abalones. Respiratory and cutaneous reactions were predominant in this cohort. Diagnosis was confirmed trhough skin tests and positive RAST responses, with 58% of patients having atopic diseases. Yet, there is a lack of information concerning the tolerance of others shellfish and the prevalence of asthma within this group [19].
5.3. Limpet
In 1991, Carrillo and co-workers reported two cases of allergic reactions following limpet ingestion. One of the patients exhibited diffuse urticaria, angioedema, status asthmaticus, and severe hypotension 60 minutes post-ingestion, while the other experienced abdominal cramps, dysphagia, diffuse erythema, dysphonia, severe bronchospasm, loss of consciousness, and respiratory arrest 40-60 minutes following the ingestion of limpets. Both cases tested positive for cooked limpet extract on skin prick-prick tests and presented positive IgE against limpet. Remarkably, they tolerated other mollusks and crustaceans and presented rhinitis and asthma due to dust mite exposure [23]. Later in 1994, Carrillo and coworkers reported six subjects developing severe bronchospasm 30 to 120 minutes after consuming limpets. Diagnosis was confirmed by skin prick by prick test with limpet and specific IgE. While all patients were sensitized to D. pteronyssinus, the number of asthmatics was unspecified. Data regarding the tolerance of other mollusks or crustaceans were not available [29]. Azofra and Lombardero (2003) presented five cases of anaphylaxis following limpet ingestion, with symptoms occurring between 10- and 90-minutes post-ingestion, with bronchospasm being a prominent manifestation. Diagnosis was carried out by positive skin test to limpet and positive specific IgE against limpet. They tolerated crustaceans and other mollusks, and all had house-dust mite-related asthma [24]. In 2008, Gutiérrez-Fernández et al. reported one patient with urticaria and angioedema 30-45 minutes after ingestion of limpet, confirmed by skin test -prick by prick test with raw and cooked limpet- and specific IgE determinations for raw and cooked limpet. This patient tolerated crustaceans and other mollusks (cephalopods and bivalves) and only presented symptoms of rhinitis due to dust mite sensitization [30]. Azofra (2017) recruited 11 patients with gastropod allergy, diagnosis based on clear history of adverse reaction suggestive of IgE-mediated allergy after eating gastropods, along with positive skin test results with the same gastropod. Symptoms included systemic reactions such as urticaria, angioedema, bronchospasm, abdominal symptoms, and hypotension. While some patients showed positive results in skin tests to crustaceans, all tolerated crustaceans. These patients were predominantly dust mite-allergic asthmatics who frequently developed serious bronchospasm or anaphylaxis immediately after eating gastropods, as reported [31].
In 2023, our group reported 16 patients with confirmed limpet allergy, exhibiting good tolerance to other shellfish. Contrary to descriptions of other shellfish allergies, clinical symptoms typically appeared later (up to an average of 121 minutes) and were often severe, including anaphylaxis (62.5%) or asthma alone (31.25%). All patients also had a medical history of rhinoconjunctivitis, and 50% (8/16) had asthma due to dust mite allergy [25]. Upon analyzing the clinical presentations of allergic reactions following gastropod ingestion, the delayed onset of symptoms compared to other shellfish classes was remarkable.
6. Diagnostic Tools
6.1. In Vivo Diagnosis
Currently, the diagnostic tools for gastropod allergy are limited. The primary focus lies on conducting a thorough medical history, which includes understanding the patient's clinical background, the symptoms experienced, and the type of reaction observed, alongside a comprehensive physical examination.
6.1.1. Skin Testing
Diagnostic testing typically involves skin prick tests. Tests can be conducted using commercial whole allergen extracts or fresh allergens (prick-to-prick tests (PTP)). However, several factors need to be considered when interpreting the results. These include the potential for cross-reactivity among shellfish, house dust mites, and cockroaches, variations in test protocols, lack of standardization in allergen preparations, and the impact of shelf-life and reagent stability on the sensitivity and specificity of conventional tests. For example, in a study involving children and adults, five commercial shellfish SPT extracts showed a significant variability in IgE reactivity in immunoblotting [32], with a sensitivity range of 59–79%. The authors also noted a notable loss of protein bands in commercial extracts compared to freshly prepared in-house shrimp extract during sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) [32].
In the case of gastropods, given the lack of commercial extracts for limpet and abalone, additional prick-by-prick skin tests are often performed using the natural food items on the volar side of each subject's arm, with both raw and cooked presentations of the implicated gastropod. A skin test is considered positive if a wheal with a diameter equal to or greater than 3 mm appears, in comparison to a negative control (saline solution, 0 mm), with a positive response to histamine (10 mg/ml) [33]. Wheal diameters are measured 20 minutes after testing.
6.1.2. Oral Food Challenge (OFC)
Oral food challenges (OFCs) come in three types: open, single-blind, and double-blind, with the latter considered the gold standard for diagnosing shellfish allergy. However, OFCs are resource-intensive and carry a risk of severe, potentially life-threatening allergic reactions [39]. In clinical practice, if the medical history strongly suggests shellfish allergy based on reaction severity, and if skin tests and/or specific IgE tests are positive, an avoidance diet may be recommended [1,40]. Conversely, if skin and serological tests yield negative results, an open OFC is typically advised to confirm the diagnosis, with an emphasis on individualizing each case [1].
6.2. In Vitro Diagnosis
Since the discovery of IgE, technology has provided new laboratory tools to quantify IgE antibody levels in the serum of the allergic patient. Quantitative immunoassays for IgE antibodies can be an adjunct to skin tests, as well as an element of diagnostic accuracy [34].
Allergen-specific IgE antibodies are measured in the presence of other antibodies of the same isotype and of allergen-specific IgE antibodies and of different isotypes but specific for the same allergen. This requires specific recognition by allergen binding sites (Fab) and epitopes in the same assay. The allergenic extracts used in the assay should be well characterized so as to yield accurate and reproducible data in clinical allergy research.
The solid-phase (allergosorbent) reagent is the principal component of the assay that confers specificity on the IgE antibody assay. To improve on the antibody-binding capacity of the paper disc, a variety of carbohydrate-based allergosorbents (other than Sephadex and paper), such as microcrystalline cellulose and agarose, were historically used in research.
The most significant advance in clinical trials, however, was the development of an encapsulated hydrophilic polymer to which the allergen was attached. This polymer was configured in the form of a small cup and it was named CAP. In this way, immunoCAP was used to determinate the presence of IgE against common aeroallergens, shellfish allergens and against terrestrial snail (the only gastropod specific IgE available at this moment) in a singleplex configuration.
IgE antibody tests can be performed as singleplex or monoplex (single) assays, refer to laboratory methods in which one analyte is measured per analysis, multiallergen (<10) and multiplex (>100 allergen specificity) assays permit more than one analyte to be detected and quantified in a single assay analysis, all with design and performance characteristics, such as ISAC, ALEX2 and Euroline platforms [35,36,37]. In brief, ALEX® (MacroArray Diagnostics, Vienna, Austria) is a multiplex array containing 295 reagents -containing 178 molecules and 117 extracts of airborne allergens and cross-reactive food allergens- with the ability of simultaneously measuring the concentration of serum sIgE (test range 0.3-50 kUA/L) and total IgE (test range 1-2500 kU/L). The different allergens and components are coupled onto polystyrene nano-beads, and then the allergen beads are deposited onto a nitrocellulose membrane, as formerly published [38]. A total of 5 shellfish molecular allergens were investigated: Pen m 1, Pen m 2, Pen m 3, Pen m 4 and Cra c 6.
7. Overview of Mollusk Allergens
This section provides a brief overview of the biochemical properties and protein structure of the most relevant identified mollusk allergens.
7.1. Lepetellida
7.1.1. Haliotis Laevigata x Haliotis Rubra
Hal l 1 [41].
7.1.2. Haliotis Midae
Hal m 1. Abalone allergens are heat-stable proteins with molecular weights of 38 and 49 kDa, later designated as HalIn-1 according to International Union of Immunological Societies allergen nomenclature regulation. Former studies indicate a clear clinical and immunologic heterogeneity in patients reactive to abalone [19].
7.2. Neogastropoda
7.2.1. Rapana Venosa
Rap v 2: Paramyosin (PM) is an important structural protein in molluscan muscles. However, as an important allergen, there is a little information on PM in the molluscs. In this study, a 99 kDa molecular weight allergen protein was purified from Rapana venosa and confirmed as PM by mass spectrometry. The results of immunoglobulin E (IgE)-binding activity and physicochemical characterization showed that R. venosa PM could react with a specific IgE of the sera from sea snail-allergic patients, and the IgE binding activity could be reduced by thermal treatment. The full-length cDNA of R. venosa PM was cloned, which encodes 859 amino acid residues, and it has a higher homology among molluscan species. According to the circular dichroism results, Fourier transform infrared, and 2D and 3D structure analysis, both PM and tropomyosin are conserved proteins, which are mainly composed of the α-helix structure. These results are significant for better understanding the anaphylactic reactions in sea snail-allergic patients and allergy diagnosis [42].
7.3. Ostreida
7.3.1. Crassostrea Angulata
Cra a 1 [43].
Cra a 2 [44].
Cra a 4 A 20 kDa protein was purified from oyster and confirmed 27 to be sarcoplasmic-calcium-binding protein (SCP) by LC-MS/MS. A 537 bp open 28 reading frame was obtained from oyster SCP total RNA, which encoded 179 amino 29 acids, and was expressed in Escherichia coli. According to the circular dichroism 30 results, digestion assay, and inhibition ELISA, the recombinant SCP (rSCP) exhibited 31 similar physicochemical properties and IgG-binding activity to native SCP. rSCP 32 displayed stronger IgE-binding activity by immunological method. Moreover, a 33 different intensity of cross-reactivity and sequence homology were demonstrated 34 between shellfish species. Collectively, these findings provide novel insight into 35 shellfish allergens, which can be used to aid in the in vitro diagnosis of 36 oyster-sensitized patients (GenBank: QIJ32297.1).
7.3.2. Crassostrea Gigas
Cra g 1 [45].
7.3.3. Saccostrea Glomerata
Sac g 1. IgE-reactive Sydney rock oyster proteins were identified by mass spectrometry, and the novel major oyster tropomyosin allergen was cloned, sequenced, and designated Sac g 1 by the IUIS. Oyster extracts showed highest IgE cross-reactivity with other molluscs, while mussel cross-reactivity was weakest [46].
7.4. Stylommatophora
7.4.1. Helix Aspersa
Hel as 1 Cloned brown garden snail tropomyosin shares high homology with other edible mollusk tropomyosins (84-69% identity) as well as with those from arthropods (65-62%), and less homology with vertebrate ones (56% identity). Tropomyosin reacted with 18% of the sera from patients with snail allergy. Inhibition experiments, using natural and recombinant tropomyosins, showed different degrees of cross-reactivity between invertebrate tropomyosins. Sera from snail-allergic subjects recognized tropomyosins in both mollusks and crustacean extracts [47].
7.5. Teuthida
7.5.1. Todarodes Pacificus
Tod p 1 The isolated squid allergen is a 38 kd, heat-stable protein. IgE antibody binding to the purified squid allergen was demonstrated by immunoblotting. Cross-reactivity between major squid and shrimp allergens was demonstrated with sera from patients allergic to squid or shrimp or with allergen-specific monoclonal antibodies. The amino acid sequence analysis of the major squid allergen showed a marked homology with tropomyosin from blood fluke planorbid (Biomphalaria glabrata), which is a common vector snail of Schistosoma mansoni. This 38 kDa protein is a major allergen of the squid, Todarodes pacificus, and is believed to be squid muscle protein tropomyosin which is known a Tod p 1 according to International Union of Immunological Societies allergen nomenclature regulation (WHO/IUIS) [48].
8. Focus on Gastropod Allergens: State of the Art
Among the various subgroups within shellfish allergy, crustacean allergy stands out as the most prevalent and extensively studied. Consequently, much of the research in this field has been focused on crustaceans. Tropomyosin (TM) was the first allergen identified in Penaeus indicus (shrimp), and it has long been recognized as the primary allergen associated with shellfish allergy. Interestingly, this panallergen has also been found in various invertebrate species such as cockroaches, Anisakis simplex, and dust mites, suggesting potential cross-reactivity between shellfish and other invertebrates [7].
However, subsequent investigations have revealed the complexity and diversity of the allergenic composition of shellfish. Several proteins shared between mollusks and crustaceans have been identified, potentially contributing to cross-reactivity. These include arginine kinase (AK), myosin light chain, sarcoplasmic calcium-binding protein (SCBP), troponin C, hemocyanin, triose phosphate isomerase, and others [7].
Within the Gastropoda class, allergy to terrestrial snails has emerged as a significant focus in scientific literature, drawing extensive study. Research by Guilloux et al. [27] and Van Ree et al. [21] has underscored the notable cross-reactivity between dust mites and terrestrial snails. Notably, several allergens implicated in this cross-reactivity, including Der p 4, p 5, p 7, and hemocyanin, have been identified [27]. Interestingly, while tropomyosin does not appear to play a role in this cross-reactivity, RAST assays conducted by these researchers have revealed compelling evidence. They found that the reactivity of snail IgE is inhibited by dust mite extract, suggesting that dust mites may serve as the primary sensitizing agent. This finding adds depth to our understanding of allergenic interactions between dust mites and terrestrial snails, shedding light on potential mechanisms underlying snail allergy [27].
In 2005, Lourenço Martins et al. conducted a study involving 60 allergic patients with specific IgE to H. aspersa. Interestingly, they found that specific IgE concentrations did not correlate with the number of recognized allergens or allergic responsiveness among the patients studied. Notably, only one individual out of 21 recognized a 37 kDa protein from H. aspersa extract. The study identified the heavy chain of myosin (225 kDa) as one of the two major allergens, found in 13 and 18 out of 21 patients, respectively. Additionally, five patients who experienced clinical symptoms after snail ingestion recognized at least one major allergen from H. aspersa extract > 208 kDa. This suggests that the protein domains involved in the allergic response may be present in the three-dimensional structure of snail myosin [22].
In 2016, Misnan et al. conducted a study investigating the effects of thermal treatments on major and minor allergens of sea snails (Cerithidea obtusa). They found that fried snails exhibited the most significant reduction in both the number of bands and their intensities compared to other cooking methods. The study revealed the presence of thermolabile proteins within a wide range of molecular weights, including those ranging from 10 to 17 kDa, 25 to 30 kDa, 40 to 74 kDa, and some high molecular weight bands (124-250 kDa) in all cooked extracts. Interestingly, the majority of snail proteins were sensitive to heat, with the exception of a few bands at 17, 18, 20, 33, 42, and 124 kDa, which demonstrated resistance to heat denaturation. Notably, the 33 kDa protein was identified as the most significant major allergen in C. obtusa, believed to be tropomyosin [49]. This study sheds light on the impact of thermal processing on the allergenicity of sea snails, providing valuable insights for food safety and allergen management practices.
In 1997, Lopata et al. documented 13 subjects who experienced symptoms up to 7 hours after consuming abalones [19]. Contrary to the findings of Guilloux et al., a RAST inhibition study conducted by Lopata et al. did not reveal cross-reactivity between abalone and dust mites. Interestingly, they identified a single 49 kDa protein, recognized by the serum IgE of 5 patients, but it was not related to abalone tropomyosin (38 kDa) [19]. This study highlights the complexity of allergenic proteins in abalones and suggests the presence of unique allergens unrelated to tropomyosin.
In the Canary Islands, limpet consumption is prevalent in the local diet. In 1991, Carrillo et al. [23] were the first to document two cases of anaphylaxis following the ingestion of limpets. Subsequently, in 1994, Carrillo et al. expanded their study to include six patients, concluding that limpets could pose a potentially serious allergen for individuals sensitized to D. pteronyssinus [29]. Conversely, in 2003, Azofra et al. described five patients with a history of limpet allergy and identified a 75 kDa protein in their cases that could be related to Der p 4 amylase [24]. This highlights the variability in allergenic proteins associated with limpet allergy and underscores the importance of continued research in this area for accurate diagnosis and management of allergic reactions.
In our local area, over a 12-month period, we recruited a total of 16 patients with a confirmed diagnosis of limpet allergy [25]. Among them, only four patients tested positive for several shellfish allergens using ALEX, including Cra c 6 (Troponin C), and one patient tested positive for Pen m 1, Pen m 3, and Pen m 4. Western blot analysis revealed that the pooled sera from these patients recognized a couple of bands between 36 and 40 kDa in both raw and cooked limpet extracts, as well as a 37 kDa band in cooked shrimp extract, consistent with tropomyosin. Individually, some patients also recognized bands between 25-40 kDa and 50-200 kDa, with these bands being more evident in the raw extracts, contrasting with findings in shrimp extracts, where bands are typically more evident in the cooked extract [25]. This observation could be attributed to the effects of thermal treatments on major and minor allergens, as described by Misnan et al. [49] (Table 2).
9. Limitations
Currently, the diagnosis of gastropod allergy faces significant challenges due to a lack of diagnostic tools. Additionally, scientific literature concerning gastropod allergy is sparse, with only a few reported cases documented. Furthermore, an important consideration is the emergence of novel foods such as insects, which are increasingly incorporated into certain diets. These novel food sources may pose a risk of cross-reactivity due to shared proteins with other invertebrates [52].
10. Future Perspective and Conclusions
Allergy to gastropods is inadequately documented in the scientific literature, with only a limited number of reported cases. This scarcity of documentation may be attributed to the localized consumption of this type of shellfish, primarily in regions such as Spain, France, Italy, and Portugal. Additionally, coastal regions of Asia are known for their significant consumption of mollusks (10), contributing to the prevalence of gastropod allergy in these areas [10].
Unlike other shellfish allergies, reactions to gastropods often manifest later and tend to be more severe, frequently involving severe respiratory symptoms. Due to the potential severity of these reactions, it is advisable for individuals experiencing suggestive symptoms to avoid not only ingesting gastropods but also inhaling cooking vapors or coming into contact with these shellfish.
At present, our diagnostic capabilities for gastropod allergy are limited. We rely on commercial snail extract available for conducting skin prick tests and specific IgE testing against snail allergens. Unfortunately, there are no commercial extracts or specific IgE avalaible for limpet and/or abalone, necessitating the use of fresh raw and cooked food for skin prick tests. Additionally, we have access to the ALEX technique, which includes a panel of five shellfish allergens (Pen m 1, Pen m 2, Pen m 3, Pen m 4 y Cra c 6). However, further studies are required to ascertain the reliability of these allergens for diagnosing gastropod allergy in our patients. It's worth noting that our group has presented preliminary findings at the EAACI 2023, indicating some degree of allergen recognition among a subset of patients using the ALEX technique [25].
While the gold standard for diagnosing food allergies remains the oral tolerance test, in many cases, a comprehensive medical history combined with positive results from a skin prick test or specific IgE testing may provide sufficient confirmation. This approach is particularly applicable given the often-severe reactions experienced by patients following ingestion of certain types of shellfish. Additionally, in mild cases, many patients may opt out of undergoing the oral tolerance test.
The available studies on limpets are scarce, which poses significant limitations in the diagnosis of limpet allergy. Currently, the lack of both a specific molecular diagnosis for this gastropod and commercial extract restricts the diagnostic procedure, particularly in regions like ours, the Canary Islands, where limpet consumption is prevalent compared to other geographical areas. There is an urgent need to optimize the study and diagnosis of limpet allergy to enhance the performance of allergy studies and improve the accuracy of diagnosis. By doing so, we aim to reduce the unnecessary avoidance of limpets and provide better management options for our patient
Despite numerous proteins being described, tropomyosin and Der p 4 amylase are often mentioned in the context of gastropod allergy and potential cross-reactivity with dust mites, based on their approximate molecular weights. However, further studies are essential to precisely identify the specific proteins involved, ascertain their allergenic properties, and determine their clinical significance. Many studies have indicated cross-reactivity with dust mites, as evidenced by the presence of proteins in both extracts with similar molecular weights and positive results in inhibition RAST, where D. pteronyssinus appears to be the sensitizing agent [27,53]. However, in populations like ours, it is conceivable that proteins shared between dust mites and gastropods may exist, suggesting the possibility of co-sensitization. Nevertheless, comprehensive series and molecular studies are required to fully elucidate this complex matter and provide a better understanding of the mechanisms underlying cross-reactivity and allergenicity between dust mites and gastropods.
Cross-reactivity among different gastropods or between gastropods and other shellfish remains understudied. Additionally, recent research suggests that O-glycosylation may play a role in patients experiencing anaphylaxis due to snails and allergy to Artemisia vulgaris [54]. This finding highlights the complexity of allergenic mechanisms and underscores the importance of further investigation into the role of glycosylation and its implications for shellfish allergy management and diagnosis.
In 2008, the first IgE-mediated anaphylactic reaction to the therapeutic monoclonal antibody Cetuximab was identified in a patient with meat allergy [55,56]. This IgE antibody is specific to alpha-Gal, as demonstrated by the analysis of neoglycoprotein (e.g., human serum albumin-alpha-Gal) conjugates [57]. It is known that allergens from various sources, such as invertebrates and parasites like helminths, present common carbohydrate structures. These glycans, known as classical carbohydrate determinants (CCDs), have well-established IgE-binding properties. The non-human IgE-binding monosaccharide unit of classical CCDs is xylose, while fucose linked in CCDs is tipically a human monosaccharide. Both fucose and xylose residues have been identified as responsible for IgE binding and cross-reactivity [58,59]. Based on this new understanding, two main groups of subjects with IgE are defined: group A with antiglycan IgE and group B with IgE against the peptide fraction of an allergen. While patients in group B are relatively well-understood in clinical practice and can often be diagnosed using available testing systems, group A presents a diagnostic challenge due to IgE cross-reactivity against cross-reactive carbohydrate determinants (CCDs). Within this group A, two subgroups are distinguished: group A1 comprises patients with antiglycan IgE and clinically relevant allergy symptoms, such as IgE against galactose-alpha-(1,3)-galactose (alpha-Gal), while group A2 includes patients with antiglycan IgE but either no allergy symptoms or minor symptoms, primarily directed against CCDs. For antiglycan IgE diagnosis, in addition to CCD diagnosis, recent advancements include commercial systems for detecting antiglycan IgE against alpha-Gal (57). Therefore, considering carbohydrates and glycosylation could be pivotal in allergy diagnosis and management.
Therefore, it is imperative to acquire commercial extracts with enhanced sensitivity to effectively detect patients allergic to gastropods. Additionally, efforts should focus on identifying allergenic proteins from various consumable gastropods to incorporate them into diagnostic tests. This approach aims to ascertain whether the coexistence of dust mite allergy and gastropod shellfish allergy, as well as allergy to other shellfish groups, arises from common proteins (cross-reactivity) or mere co-sensitization, thus providing insight into the actual probability of cross-reactivity between these groups. Allergic reactions to gastropods tend to be severe, posing potential life-threatening risks to affected individuals. Consequently, it is crucial to offer comprehensive health education, prescribe, and provide guidance on the use of epinephrine auto-injectors and other necessary medications. However, addressing these challenges consumes significant time and resources. Advancements in the characterization of gastropod allergens are paramount. These advancements not only facilitate the development of accurate diagnostic methods but also contribute to a deeper understanding of this condition, thereby improving overall knowledge and management of gastropod allergies.
Author Contributions
Conceptualization, P.P-G. and R.G-P.; methodology, E.M-L. and R.G-P.; software, F.P.; validation, P.P-G. and I.S-M.; formal analysis, P.P-G. and I.S-M.; investigation, E.M-L., F.P. and R.G-P; resources, F.P. and R.G-P; data curation, E.M-L. and R.G-P.; writing—original draft preparation, E.M-L. and R.G-P; writing—review and editing, P.P-G., F.P. and I.S-M.; supervision, P.P-G. and R.G-P; project administration, R.G-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. It should be noted that this article is derived from reviews of existing medical literature and does not involve any studies regarding human participants or animals.
Informed Consent Statement
Not applicable. It should be noted that this article is derived from reviews of existing medical literature and does not involve any studies regarding human participants or animals.
Data Availability Statement
The data that support the findings of this study are available from Servicio Canario de la Salud, however, restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with the permission of Servicio Canario de la Salud.
Conflicts of Interest
The authors declare no conflicts of interest in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F.; et al. IgE-Mediated Shellfish Allergy in Children. Nutrients 2023, 15, 2714. [CrossRef]
- Xu, L; Cai, J; Gao, T; Ma, A. Shellfish consumption and health: A comprehensive review of human studies and recommendations for enhanced public policy. Crit Rev Food Sci Nutr. 2022, 62, 4656-4668. [CrossRef]
- Venter, C.; Smith, P.K.; Arshad, H. Dietary strategies for the prevention of asthma in children. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 123–131. [CrossRef]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of dietary fiber in promoting immune health-An EAACI position paper. Allergy 2022, 77, 3185–3198. [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [CrossRef]
- Alexandra F. Santos, Carmen Riggioni, Ioana Agache, Cezmi A. Akdis, Mubeccel Akdis, Alberto Álvarez-Perea, Montserrat Alvaro-Lozano, Barbara Ballmer-Weber et al. EAACI guidelines on the diagnosis of IgE-mediated food allergy. Allergy First Published: 10 October 2023. [CrossRef]
- Gelis S, Rueda M, Valero A, Fernández EA, Moran M, Fernández-Caldas E. Shellfish allergy: Unmet needs in diagnosis and treatment. J Investig Allergol Clin Immunol 2020; Vol 30 (6): 409-420. [CrossRef]
- Alergológica 2015, SEAIC. https://www.seaic.org/inicio/noticias-general/alergologica-2015.html.
- Wai, C. Y., Leung, N. Y., Leung, A. S., Wong, G. W., & Leung, T. F. (2021). Seafood allergy in Asia: Geographical specificity and beyond. Frontiers in Allergy, 2, 676903. [CrossRef]
- Khora SS. Seafood-Associated Shellfish Allergy: A Comprehensive Review. Immunol Invest. 2016 Aug;45(6):504-30. [CrossRef]
- Azofra J, Echechipia S, Irazábal B, Muñoz D, Bernedo N, Gacía BE, Gastaminza G,Goikoetxea MJ, Joral A, Lasa E, Gamboa P, Díaz C, Beristain A, Quiñones D, Bernaloa G, Echenagusia MA, Liarte I, García E, Cuesta J, Martínez MD, Velasco M, Longo N, Pastro-Vargas C. Heterogenecity in allergy to mollusks: A clinical-immunological study in a population from the north of Spain. J Investig Allergol Clin Immunol 2017; Vol.27(4):252-260. [CrossRef]
- Steve L Taylor. Molluscan Shellfish Allergy. Advances in food and nutrition research. 2008. Vol 54. Chapter 4: 139-177. [CrossRef]
- Ruethers, T., Taki, A. C., Johnston, E. B., Nugraha, R., Le, T. T., Kalic, T., ... & Lopata, A. L. (2018). Seafood allergy: A comprehensive review of fish and shellfish allergens. Molecular immunology, 100, 28-57. [CrossRef]
- Tuano, K.T.S.; Davis, C.M. Oral allergy syndrome in shrimp and house dust mite allergies. J. Allergy Clin. Immunol. Pract. 2018, 6, 2163–2164. [CrossRef]
- Wang, H.T.; Warren, C.M.; Gupta, R.S.; Davis, C.M. Prevalence and Characteristics of Shellfish Allergy in the Pediatric Population of the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 1359–1370. [CrossRef]
- Sopo, S.M.; Monaco, S.; Badina, L.; Barni, S.; Longo, G.; Novembre, E.; Viola, S.; Monti, G. Food protein-induced enterocolitis syndrome caused by fish and/or shellfish in Italy. Pediatr. Allergy Immunol. 2015, 26, 731–736. [CrossRef]
- Ayuso, R.; Sánchez-Garcia, S.; Lin, J.; Fu, Z.; Ibáñez, M.D.; Carrillo, T.; Blanco, C.; Goldis, M.; Bardina, L.; Sastre, J.; et al. Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J. Allergy Clin. Immunol. 2010, 125, 1286–1293. [CrossRef]
- La Bella, G, V Martella, M G Basanisi, G Nobili, V Terio, G La Salandra. Food-Borne viruses in shellfish: Investigation on novovirus and HAV presence in Apulia (SE Italy). Food Environ. Virol. 2017, 9, 179-186. [CrossRef]
- Lopata AL, Zinn C, Potter PC. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J Allergy Clin Immunol 1997; Vol 100 (5). [CrossRef]
- C G de la Cuesta, B E García, H Córdoba, I Diéguez, A Oehling. Food allergy to Helix terrestre (snail). Allergol Immunopathol (Madr) 1989 Nov-Dec;17(6):337-9.
- Van Ree R, Antonicelli L, Akkerdaas JH, Pajno GB, Barberio G, Corbetta L, Ferro G, Zambito M, Garritani MS, Aalberse RC, Bonifazi F. Asthma after consumption of snails in house-dust-miteallergic patients: A case of IgE cross-reactivity. Allergy 1996; 51: 387-393.
- Martins, L. M. L., Peltre, G., da Costa Faro, C. J. F., Vieira Pires, E. M., & da Cruz Inácio, F. F. (2005). The Helix aspersa (brown garden snail) allergen repertoire. International Archives of Allergy and Immunology, 136(1), 7-15. [CrossRef]
- Carrillo T, De Castro FR, Cuevas M, Caminero J, Cabrera P. Allergy to limpet. Allergy 1991; 46: 515-519. [CrossRef]
- Azofra J, Lombardero M. Limpet anaphylaxis: Cross-reactivity between limpet and house dust mite Dermatophagoides pteronyssinus. Allergy 2003; 58:146-149. [CrossRef]
- Mederos-Luis E, Poza-Guedes P, Martínez MJ, González-Pérez R, Galán T, Sánchez-Machín I. Limpet molecular profile: Tropomyosin or not tropomyosin, that is the question. (2023), Thematic poster session (TPS). Allergy, 78: 283-682. [CrossRef]
- Palma Carlos. Asthme per ingestión d’escasrgots. Allergie Immunol 1985; 17:5-6.
- Guilloux L, Vuitton DA, Delbourg M, Lagier A, Adessi B, Marchand CR, Ville G. Cross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy 1998; 53:151-158. [CrossRef]
- Morikawa A, Kato M, Tokuyama K, Kuroume T, Minoshima M, Iwata S. Anaphylaxis to grand keyhole limpet (abalone-like shellfish) and abalone. Ann Allergy.1990 Nov; 65(5):415- 417.
- Carrillo T, Rodríguez de Castro F, Blanco C, Castillo R, Quiralte J, Cuevas M. Anaphylaxis due to limpet ingestión. Ann Allergy1994 Dec;73(6):504-8.
- Gutierrez-Fernandez, D., Fuentes-Vallejo, M. S., Zavala, B. B., Foncubierta-Fernandez, A., Lucas-Velarde, J., & Leon-Jimenez, A. Urticaria-angioedema due to limpet ingestion. Journal of investigational allergology & clinical immunology. 2009:19(1), 77-79.
- Azofra, J., Echechipía, S., Irazábal, B., Muñoz, D., Bernedo, N., García, B. E., ... & Pastor-Vargas, C. Heterogenecity in allergy to mollusks: A clinical-immunological study in a population from the north of Spain. J Investig Allergol Clin Immunol 2017; vol27 (4): 252-260. [CrossRef]
- Asero, R.; Scala, E.; Villalta, D.; Pravettoni, V.; Arena, A.; Billeri, L.; Colombo, G.; Cortellini, G.; Cucinelli, F.; De Cristofaro, M.L.; et al. Shrimp Allergy: Analysis of Commercially Available Extracts for In Vivo Diagnosis. J. Investig. Allergol. Clin. Immunol. 2017, 27, 175–182. [CrossRef]
- Heinzerling,L; Mari, A; Bergmann, K.C; Bresciani, M; Burbach, G; Darsow, U; Durham, S; Fokkens, W; Gjomarkaj, M; Haahtela, T; et al. The skin prick test- European standards. Clin. Transl. Allergy 2013, 3,3. [CrossRef]
- H J Chong Neto, N A Rosário. Studying specific IgE: In vivo or in vitro. Allergol et Immunopathol 2009;37(1):31-5. [CrossRef]
- Hiller R, Laffer S, Harwanegg C, Huber, M., Schmidt, W. M., Twardosz, A., ... & Valenta, R.. Microarrayed allergen molecules: Diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16(3):414-416. [CrossRef]
- Lupinek C, Wollmann E, Baar A, Banerjee, S., Breiteneder, H., Broecker, B. M., ... & Valenta, R. Advances in allergenmicroarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods. 2014;66(1):106-119. [CrossRef]
- Keshavarz B, Platts-Mills TAE, Wilson JM. The use of microarray and other multiplex technologies in the diagnosis of allergy. Ann Allergy Asthma Immunol. 2021;127(1):10-18. [CrossRef]
- Lis K, Bartuzi Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Curr Issues Mol Biol. 2023 Jun 29;45(7):5481-5493. [CrossRef]
- Gelis, S, Rueda, M., Pascal, M., Fernández-Caldas, E., Fernández, E. A., Araujo-Sánchez, G., ... & Valero, A. Usefulness of the nasal allergen provocation test in the diagnosis of shellfish allergy. J Investig Allergol Clin Immunol. 2022, 32, 460-470. [CrossRef]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [CrossRef]
- Liu T., Kamath S.D., Nugraha R., Waltenspiel B., Lopata A.L. Molecular cloning, expression, sequence analyses of abalone tropomyosin and its IgE-binding reactivity with shellfish allergic patients. GenBank: APG42675.1.
- Yu, C., Gao, X., Lin, H., Xu, L., Ahmed, I., Khan, M. U., ... & Li, Z. Purification, Characterization, and Three-Dimensional Structure Prediction of Paramyosin, a Novel Allergen of Rapana venosa. J Agric Food Chem. 2020;68(49):14632-14642. [CrossRef]
- Yun, X., Li, M. S., Chen, Y., Huan, F., Cao, M. J., Lai, D., ... & Liu, G. M. Characterization, Epitope Identification, and Cross-reactivity Analysis of Tropomyosin: An Important Allergen of Crassostrea angulata. J Agric Food Chem. 2022;70(29):9201-9213. [CrossRef]
- Huan, F., Han, T. J., Liu, M., Li, M. S., Yang, Y., Liu, Q. M., ... & Liu, G. M. Identification and characterization of Crassostrea angulata arginine kinase, a novel allergen that causes cross-reactivity among shellfish. Food Funct. 2021;12(20):9866-9879. Published 2021 Oct 19. [CrossRef]
- Han, T. J., Liu, M., Huan, F., Li, M. S., Xia, F., Chen, Y. Y., ... & Liu, G. M. Identification and Cross-reactivity Analysis of Sarcoplasmic-Calcium-Binding Protein: A Novel Allergen in Crassostrea angulata. J Agric Food Chem. 2020;68(18):5221-5231. [CrossRef]
- Rolland, J. M., Varese, N. P., Abramovitch, J. B., Anania, J., Nugraha, R., Kamath, S., ... & O'Hehir, R. E.. Effect of Heat Processing on IgE Reactivity and Cross-Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1. Mol Nutr Food Res. 2018;62(14):e1800148. [CrossRef]
- Asturias JA, Eraso E, Arilla MC, Gómez-Bayón N, Inácio F, Martínez A. Cloning, isolation, and IgE-binding properties of Helix aspersa (brown garden snail) tropomyosin. Int Arch Allergy Immunol. 2002;128(2):90-96. [CrossRef]
- Miyazawa, H., Fukamachi, H., Inagaki, Y., Reese, G., Daul, C. B., Lehrer, S. B., ... & Sakaguchi, M. Identification of the first major allergen of a squid (Todarodes pacificus). J Allergy Clin Immunol. 1996;98(5 Pt 1):948-953. [CrossRef]
- Misnan, R., Abd Aziz, N. S., Yadzir, Z. H. M., Bakhtiar, F., Abdullah, N., & Murad, S.. Impacts of thermal treatments on major and minor allergens of sea snail (Cerithidea obtuse). Iran J Allergy Asthma Immunol august 2016; 15(4):309-316.
- Varun Muddaluru, Rudolf Valenta, Susanne Vrtala, Thomas Schlederer, James Hindley, Pascal Hickey, Mark Larché, Elena Tonti. Comparison of house dust mite sensitization profiles in allergic adults from Canada, Europe, South Africa and USA. Allergy 2021 Jul;76(7):2177-2188. [CrossRef]
- Suzuki, M., Kobayashi, Y., Hiraki, Y., Nakata, H., & Shiomi, K. (2011). Paramyosin of the disc abalone Haliotis discus discus: Identification as a new allergen and cross-reactivity with tropomyosin. Food Chemistry, 124(3), 921-926.
- Scala E, Abeni D, Villella V, Villalta D, Cecchi L, Caprini E, Asero R. Investigating Novel Food Sensitization: A Real-Life Prevalence Study of Cricket, Locust, and Mealworm IgE-Reactivity in Naïve allergic Individuals. J Investig Allergol Clin Immunol, 35 (3), 3. [CrossRef]
- De Maat- Bleeker F, Akkerdaas JH, van Ree R, Aalberse RC. Vineyard snail allergy possibly induced by sensitization to house-dust mite (Dermatophagoides pteronyssinus). Allergy 1995; 50:438-440. [CrossRef]
- Manuel Prados-Castaño, Stefan Cimbollek, Borja Bartolomé, Miriam Castillo, Joaquin Quiralte. Snail-induced anaphylaxis in patients with underlying Artemisia vulgaris pollinosis: The role of carbohydrates. Allergol Immunopathol (Madr). 2024 Jan 1;52(1):60-64. eCollection 2024. [CrossRef]
- Chung CH, Mirakhur B, Chan E, Le Q-T, Berlin J, Morse M; et al. Cetuximab induced anaphylaxis and IgE specific for galactose-a-1,3-galactose. N Engl J Med 2008;358:1109-17. [CrossRef]
- Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD; et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-a-1,3-galactose. J Allergy Clin Immunol 2009 Feb;123(2):426-33. [CrossRef]
- Arne Homann, Gabriele Schramm, Uta Jappe. Glycans andglycan-specific IgE in clinical and molecular allergology: Sensitization, diagnostics and clinical symptoms. J Allergy Clin Immunol 2017;140:356-368. [CrossRef]
- Aalberse RC, Akkerdaas J, Van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy 2001;56:478-90. [CrossRef]
- Van Ree R. Beta (1,2)-Xylose and alpha (1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J Biol Chem 2000;275:11451-8. [CrossRef]
Figure 1.
Taxonomic tree of edible shellfish species within Crustacea subphylum and the Mollusca phylum [adapted from 13].
Figure 1.
Taxonomic tree of edible shellfish species within Crustacea subphylum and the Mollusca phylum [adapted from 13].

Figure 2.
Classification of food reactions based on the mechanism involved.
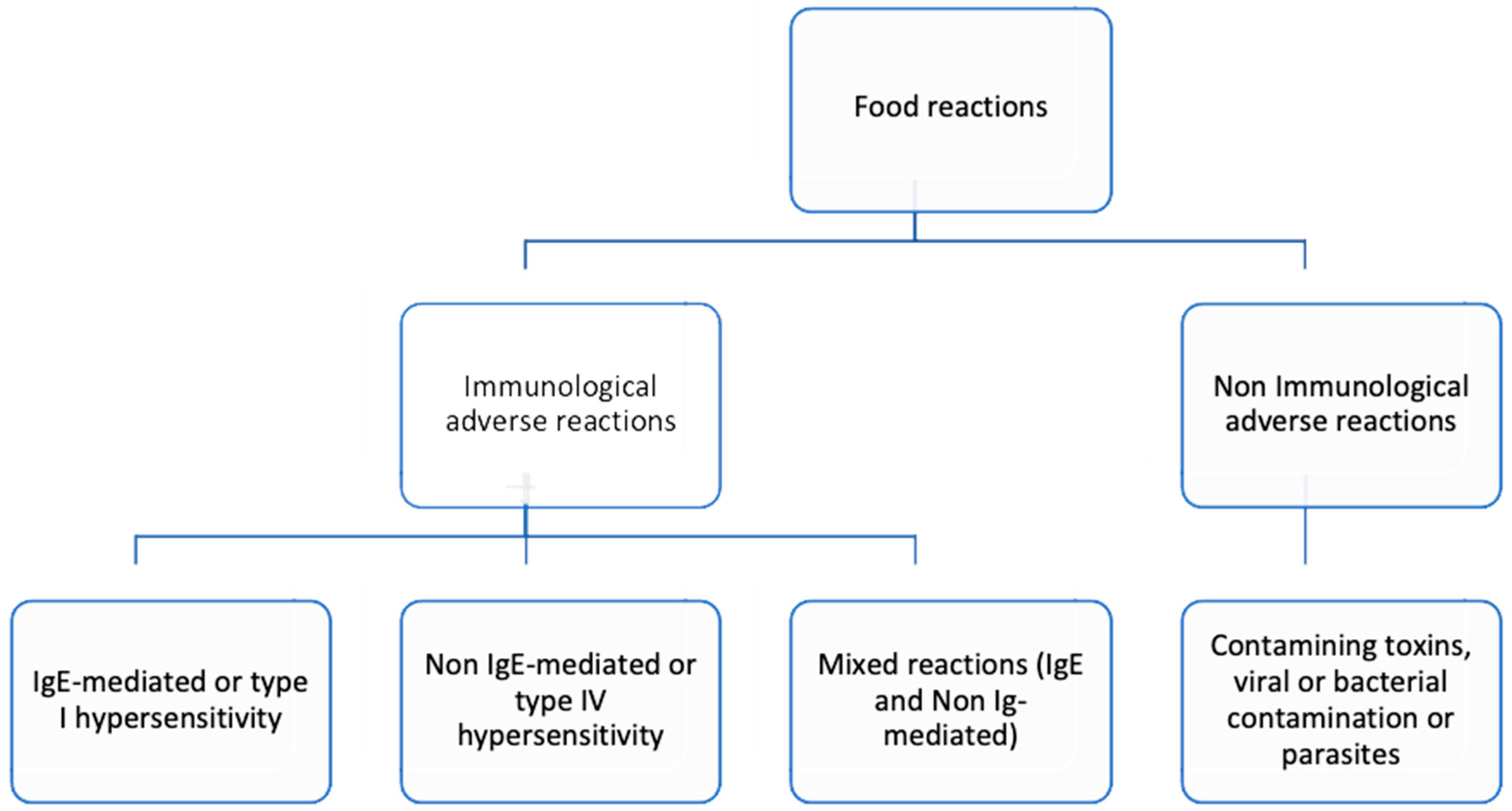
Table 2.
Literature Review: Studies on Gastropods. Unk: Unknown.
| Author | Year of publication | Gastropod | Allergen | Molecular Weight (kDa) | References |
|---|---|---|---|---|---|
| Morikawa et al. | 1990 | Abalone | Unk | Unk | [28] |
| Carrillo et. al | 1991; 1994 | limpet | Unk | Unk | [23,29] |
| Van Ree et al. | 1996 | Snail | Unk | Unk | [21] |
| Lopata et al. | 1997 | Abalone | Unk | 49 | [19] |
| Guilloux et al. | 1998 | Snail | Der p 4 Der p 5 Der p 7 Hemocyanin |
18 13 14 75-85 |
[27,50] |
| Azofra et. al | 2003 | limpet | Der p 4 amylase | 75 | [24] |
| Laurenço Martins et al. | 2005 | Snail | Tropomyosin Heavy chain of myosin 3-dimensional structure of snail myosin |
37 225 >208 |
[22] |
| Suzuki et al. | 2011 | Abalone | Paramyosin | 89 | [51] |
| Misnan et. al | 2016 | Snail | Tropomyosin | 33 | [49] |
| Azofra et al. | 2017 | Limpet | Actin | 46-47 | [11] |
| Mederos-Luis et al. | 2023 | limpet | Unk Unk |
36-40 50-200 |
[25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








