Preprint
Article
Silver Nanoparticles Biosynthesized Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Degradation Studies
Altmetrics
Downloads
93
Views
31
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.zip (51.26KB )
This version is not peer-reviewed
Submitted:
17 June 2024
Posted:
18 June 2024
You are already at the latest version
Alerts
Abstract
In this work, silver nanoparticles (AgNPs) were biosynthesized from cashew nutshell liquid (CNSL) by varying the concentration of silver ions and pH of the CNSL extract. The synthesized AgNPs were further characterized to study their surface, structural, and morphological proper-ties, and tested for the degradation of methylene blue (MB) dye. The results of this study showed that depending on the conditions, particles of various sizes, ranging from 1 to 60 nm, and different degrees of stabilization and agglomeration were produced. The concentration of silver ions equal to 3 mM and the pH of the solution of ~ 4.5 (AgNP3) resulted in the most efficient biosynthesis, where particles appeared to be highly stabilized and homogeneously distributed on the surface, exhibiting a small average particle size and a narrow particle size distribution (6.7 ± 6.5 nm). Such particles further showed the highest degradation of MB, where up to 80 % degradation rates were recorded within the first 20 min. Higher concentrations of silver ions and higher pH of the extract resulted in substantial particle agglomeration and unstable synthesis, respectively, which further negatively affected the ability of particles to degrade MB. Finally, visible light showed no significant effect on the degradation of MB by AgNPs with the average degradation rates found to be about the same as under the dark conditions.

Keywords:
Subject: Chemistry and Materials Science - Nanotechnology
1. Introduction
Silver nanoparticles (AgNPs) are well known for their anti-bacterial, anti-fungal, anti-viral, and degradation properties attributed to their small size of less than 100 nm and their high surface area to volume ratio [1,2,3,4,5,6,7,8,9,10]. AgNPs are very effective and safe at low concentrations, as they have shown higher antibacterial activity than penicillin, biomycin, and other antibiotics with low cytotoxicity and immunological response, [3,4,7,8] as well as being capable of preventing the growth of bacteria like Bacillus cereus, Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Citrobacter koseri, and many more [1,4,7,11]. In addition, the functional groups on the surface of AgNPs provide electrostatic interactions with multiple pollutant molecules, assisting in their degradation [12,13,14,15]. Considering the above-mentioned properties of silver nanoparticles, some of their common applications include water purification, drug delivery, medical imaging, molecular diagnostics, therapeutics, food processing, and food packaging [4,5,7,9,10,12,16].
While silver nanoparticles have been proven beneficial, mass synthesizing them in a regulated manner with an efficient and eco-friendly process remains challenging [6,7,9,10]. Most metallic nanoparticles are made from a “bottom-up” approach which involves having atoms group up together to form the nanoparticles [1,7,10,17,18]. The benefit of this technique is that the reducing agent can control the particles’ structures and act as the catalyst for the synthesis [1,5,7,8,16]. Furthermore, biological methods are preferred over chemical and physical methods, as the former are more cost-effective, energy-efficient, simple, eco-friendly, and can be scaled up to produce high amounts of nanoparticles [1,5,6,7,8,9,16,18]. In biosynthesis, plants’ biomolecules and secondary metabolites such as flavonoids, ketones, aldehydes, tannins, and carboxylic acids act as reducing and stabilizing agents to convert silver ions to elemental silver [1,5,6,7,9,10,16,19]. First, the biomolecules’ antioxidant functional groups donate electrons to the silver ions to produce silver atoms that group up and nucleate to form nanoparticles of a specific size [7,10,17,19,20]. Lastly, the phytochemicals cap or stabilize the nanoparticles by binding to them, with the polar head of a phytomolecule coordinated towards the metal atom and the nonpolar tail encircling the medium, often enabling a synergistic effect between the AgNP and extract’s properties [7,10,17,19,20].
Different studies have shown that the concentration of the silver ions, temperature, pH of the solution, and reaction time influence the size, shape, and morphology of the AgNPs, which in turn impact their properties [3,5,6,7,16,17,20]. Thus, when changing the precursor concentration, often higher amounts will bring about a greater yield and smaller particles, but too much usually causes the size of the nanoparticles to increase [6,7,17]. Furthermore, higher temperatures have been found to produce more spherical nanoparticles and to boost the speed of the synthesis, [5,6,16,17] and a more basic media has been proven to be beneficial for the stability of nanoparticle synthesis due to the biomass’s electrical charge being more suitable for the binding, reduction, and stabilization of the metal ions [6,7,10,16,17]. Finally, optimizing the period of the biosynthesis is necessary for a stable synthesis, so that the nanoparticles would not cluster into a bulk metal due to agglomeration [6,7,16,17]. Close control of the above-mentioned parameters can be challenging at times as aggregation and other factors may have an unexpected effect on the physical properties of the nanoparticles [7,17].
In this work, a biosynthetic approach is applied to prepare silver nanoparticles, with cashew nutshell liquid (CNSL) as a reducing agent, and by varying the synthesis conditions to control the properties of AgNPs. In CNSL, secondary metabolites involved in nanoparticle formation are non-isoprenoid lipids including anacardic acids, cardols, cardanols, methylcardols, and polymeric materials [21,22,23]. Here cardanols and cardols are expected to play a pivotal role in the silver nanoparticle formation as they have been proven to strongly govern the antioxidant properties and the ability of CNSL to neutralize free radicals by giving up electrons [22,24].
The produced AgNPs are further tested for their dye degradation properties. For dye degradation, methylene blue – cationic azo dye – is chosen as a pollutant of interest. Dye pollution has become more abundant in our water systems due to discharge from paper, textile, leather, food, cosmetic, and pharmaceutical industries [2,25,26,27]. Out of the roughly 2.1 metric tons of dye produced worldwide, about 15% enter our waterways, effectively decreasing the water’s transparency which causes less light to reach aquatic plants [25]. This hinders photosynthesis, lowering the amount of dissolved oxygen in water, which is essential for aquatic ecosystems to support life [25,28]. Additionally, synthetic dyes are non-biodegradable and release possible carcinogenic products, [26,27,28] which makes their removal imperative for healthy water systems.
2. Results
2.1.1. Preparation of AgNPs
Figure 1 and Figure 2 demonstrate how varying the concentration of AgNO3 and pH of the extract altered the formation rates of the AgNPs, where a quicker color change from yellow to black indicates an increased reaction rate. The formation of AgNPs took place within 60–90 min for different concentrations of AgNO3 (Figure 1) and within ~ 30 min at the higher pH values of the extract (Figure 2); the increase in the formation rate was observed with the increase in the concentration of AgNO3 and pH of the extract.
Figure 3 shows the resulting surface plasmon resonance curves for AgNPs biosynthesized from different concentrations of AgNO3. For each concentration, a single SPR peak was produced and according to Mie’s theory, this indicates that the AgNPs are spherical rather than anisotropic in shape [6,11]. The curves became more intense as the biosynthesis proceeded and as the concentration of AgNO3 increased. More so, at higher concentrations of AgNO3, the curves got sharper and more intense during a shorter interval of time. These results indicate that solutions with more available silver cations made the reduction process more favorable [2,6,7,8,9,29,30]. The change in AgNP properties was most noticeable when increasing the AgNO3 concentration from 2 mM to 3 mM but was much less prominent from 3 mM to 4 mM. Additionally, a red shift occurred from 424 nm for AgNP2 to 442 nm for AgNP3 and AgNP4, likely from electron delocalization, suggesting a change in AgNP size [2,6,8,11,31,32].
For AgNPs synthesized at a higher pH of the extract, the formation rate of nanoparticles increased substantially at pH 6.5 and was instant at pH 8.5 (Figure 4). These results agree with the color observations shown in Figure 2. The faster reaction rate of the biosynthesis in a more basic medium could be attributed to the increased negative electrical charge of the biomolecules’ functional groups to reduce, cap, and stabilize the AgNPs [6,7,10,11].
2.1.2. MB Degradation Studies
The synthesized AgNPs were tested for MB degradation in the dark and under green light to study the effect of visible light on the extent of degradation. In the case of the latter, the light energy could promote the AgNP electrons from the valance to the conduction band, resulting in the formation of electron-hole pairs that are highly reactive and could trigger electron transfers with the MB molecules, causing their photodegradation [11,28,32,33,34]. The results of this MB degradation study are shown in Figure 5A. Here, only a slight increase in the average degradation rate is observed under the green light, suggesting that photodegradation takes place only to a negligible extent with no major improvement in the performance of AgNPs. Thus, the average percentage degradation across five or more different batches is about 3% higher under the green light than in the dark (Figure 5D). Furthermore, AgNPs synthesized from different concentrations of AgNO3 were tested and compared under the green light. Figures 5B,E show that the extent of degradation was on average the highest for AgNP3, followed by AgNP4 (decrease in % degradation of ~ 6%), and AgNP2 (decrease in degradation of ~ 16%). These results indicate that varying the concentration of AgNO3 produced nanoparticles of different properties, which in turn affected their performance for the degradation of MB. As per AgNPs synthesized at a higher pH of the extract, the average % degradation decreased by ~ 15% for AgNP3-6.5 and by ~ 17% for AgNP3-8.5 (Figure 5C,F) in comparison to AgNP3 synthesized at pH 4.5. This indicates that although increasing the pH of the extract should be beneficial for the stability of nanoparticle synthesis due to the enhanced electrostatic interactions between CNSL and silver ions, samples produced at higher pH did not show any improvement in MB degradation.
2.1.3. XRD Analysis
The crystalline structure of AgNPs was studied by XRD in the range of 2θ Bragg angles of 5 to 90°. The XRD diffractograms of AgNPs are presented in Figure 6. Here the clear reflections at 38.1° (111), 44.4° (200), 64.6° (220), and 77.5° (311) are characteristic of the face-centered cubic silver [JCPDF Card No-087–0720]. The additional peaks at 27.9°, 32.3°, 46.3°, 54.9° and 57.7° denoted by (*) in Figure 6B are observed for AgNP3-6.5 and AgNP3-8.5 and could be attributed to either amorphous organic phase [35] or silver chloride [36]. The latter is likely due to the pH adjustments of AgNP3-6.5 and AgNP3-8.5 with hydrochloric acid.
The average crystalline size of silver nanoparticles was calculated from the Debye-Sherrer equation using the reflections at 38.1°, 44.4°, 64.6°, and 77.5° which were found to be ~ 16 nm for AgNP2, ~12 nm for AgNP3, ~ 17 nm AgNP4, and ~ 11 nm for AgNP3-6.5. The intensity of reflections for AgNP3-8.5 were too low to estimate the particle size.
Figure 6.
XRD patterns of (A) AgNP2, AgNP3, and AgNP4 at the pH of the extract of 4.5 and (B) AgNP3-6.5 and AgNP3-8.5 at the concentration of AgNO3 of 3 mM.
Figure 6.
XRD patterns of (A) AgNP2, AgNP3, and AgNP4 at the pH of the extract of 4.5 and (B) AgNP3-6.5 and AgNP3-8.5 at the concentration of AgNO3 of 3 mM.

2.1.4. XPS Analysis
XPS analysis studied the surface chemical composition, and the results of this study are presented in Figure 7 and Table 1. The deconvolution of the C 1s core-level spectrum revealed the presence of C–C, C–O, and O–C=O functionalities. Here, C–C linkages related to carbon sp3 hybridization could be associated with the nonpolar hydrocarbon ends of CNSL extract components, such as anacardic acids, cardols, cardanols, and methylcardols [21,22,23,37,38]. On the other hand, C–O and O–C=O functionalities, related to phenol and carboxylic groups respectively, [22,37,38] could be linked to the polar ends. In addition, the O 1s peak at 532.6 eV further suggests most of the polar surface functionalities are of O–C type. [39] Noteworthily, AgNP3 contains the highest amount of C–C bonds (C 1s peak at 284.8 eV), and the lowest amount of surface oxygen-containing groups; no carboxylic functionalities (anacardic acid) were detected, and the C 1s peak at 286.5 eV (for AgNP2 and AgNP4) has shifted to 286.1 eV (Figure 7A,C and Table 1). These findings suggest that the capping and stabilization process for AgNP3 was the most successful, with the polar heads (C–O and O–C=O groups) of CNSL extract coordinated towards the bulk and facilitating the reduction of Ag+ to Ag0, while the nonpolar tails (C–C) encircling and stabilizing the nanoparticle medium. Furthermore, the analysis of the Ag 3d core-level spectrum reveals the presence of two main contributions ascribed to the doublet Ag 3d5/2 and Ag 3d3/2 which respectively appear at 368.5 and 374.5 eV and are attributed to metallic silver [40,41].
2.1.5. HRTEM/STEM-EDX Analysis
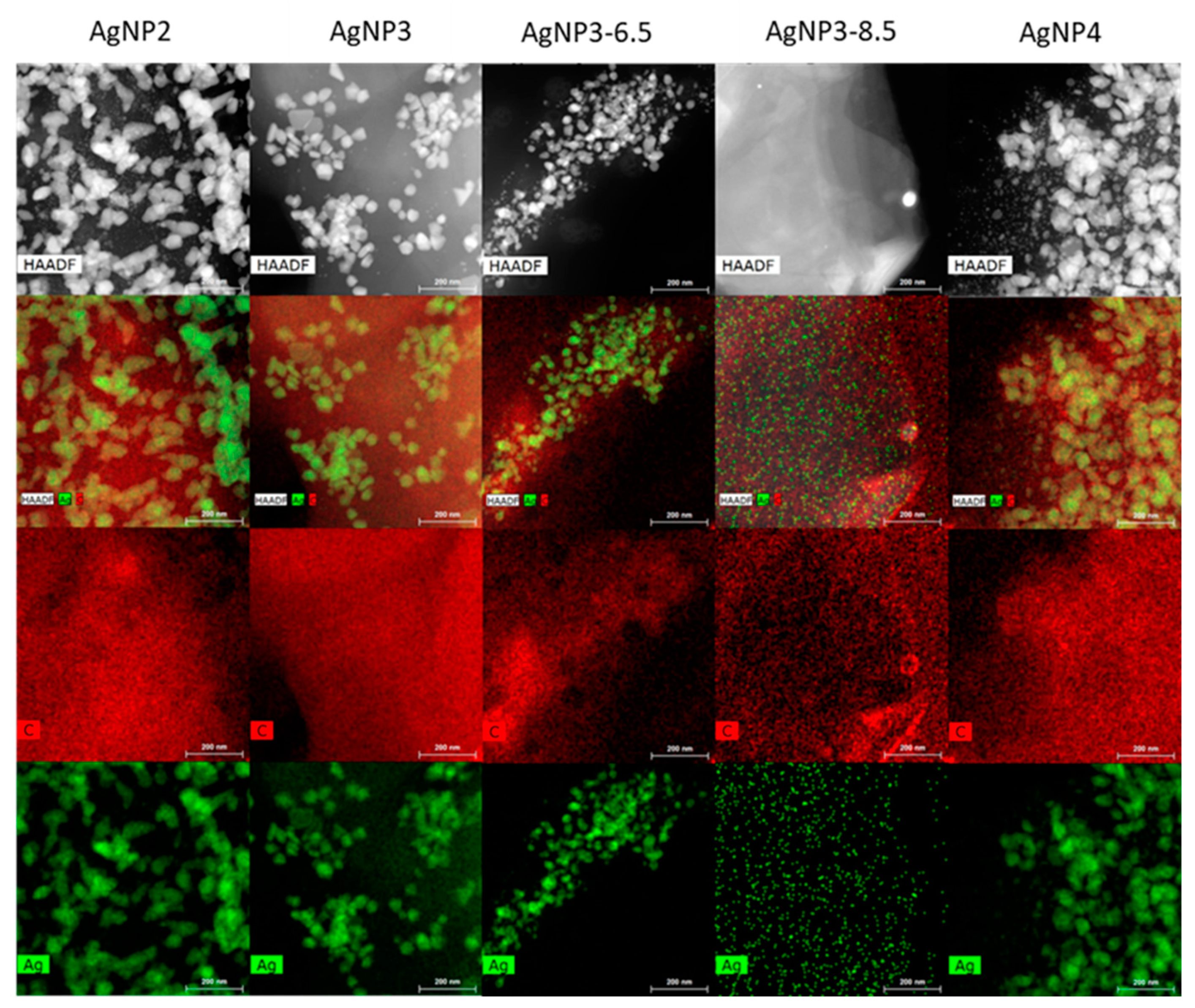
The morphology of the biosynthesized AgNPs was studied by HRTEM and STEM-EDX topography. The micrographic analysis of the AgNP samples revealed that they contain silver particles of different sizes, with particles of a considerable size found in all the samples except AgNP3-8.5 (Table 2, Figure 8). More so, it is observed that the AgNP3 samples have the smallest silver particle sizes compared to the AgNP2 and AgNP4 samples. The AgNP3 samples also have silver particles with a more uniform size, particularly AgNP3-8.5, which has the narrowest silver particle size range.
Furthermore, micrographs obtained by HRTEM (Figure 9) and the mapping from EDX microanalysis (Figure 10) revealed that silver particles are homogeneously distributed on the carbonaceous support of all the samples, with the most uniform and homogeneous distribution of particles found for AgNP3-8.5. All the other samples exhibited a degree of particle agglomeration, with the most pronounced found in the AgNP2 and AgNP4 samples, where some particles reached up to 50 nm in size.
Table 2.
Lower and upper ranges of silver particle size for each sample.
| Sample | AgNP2 | AgNP3 | AgNP4 | AgNP3-6.5 | AgNP3-8.5 |
| Particle size range (nm) | 6.2–25.6 | 0.2–13.2 | 4.6–27.2 | 2.1–17.7 | 3.8–9.8 |
Figure 8.
Particle size distribution for AgNP samples obtained from EDX-mapping.
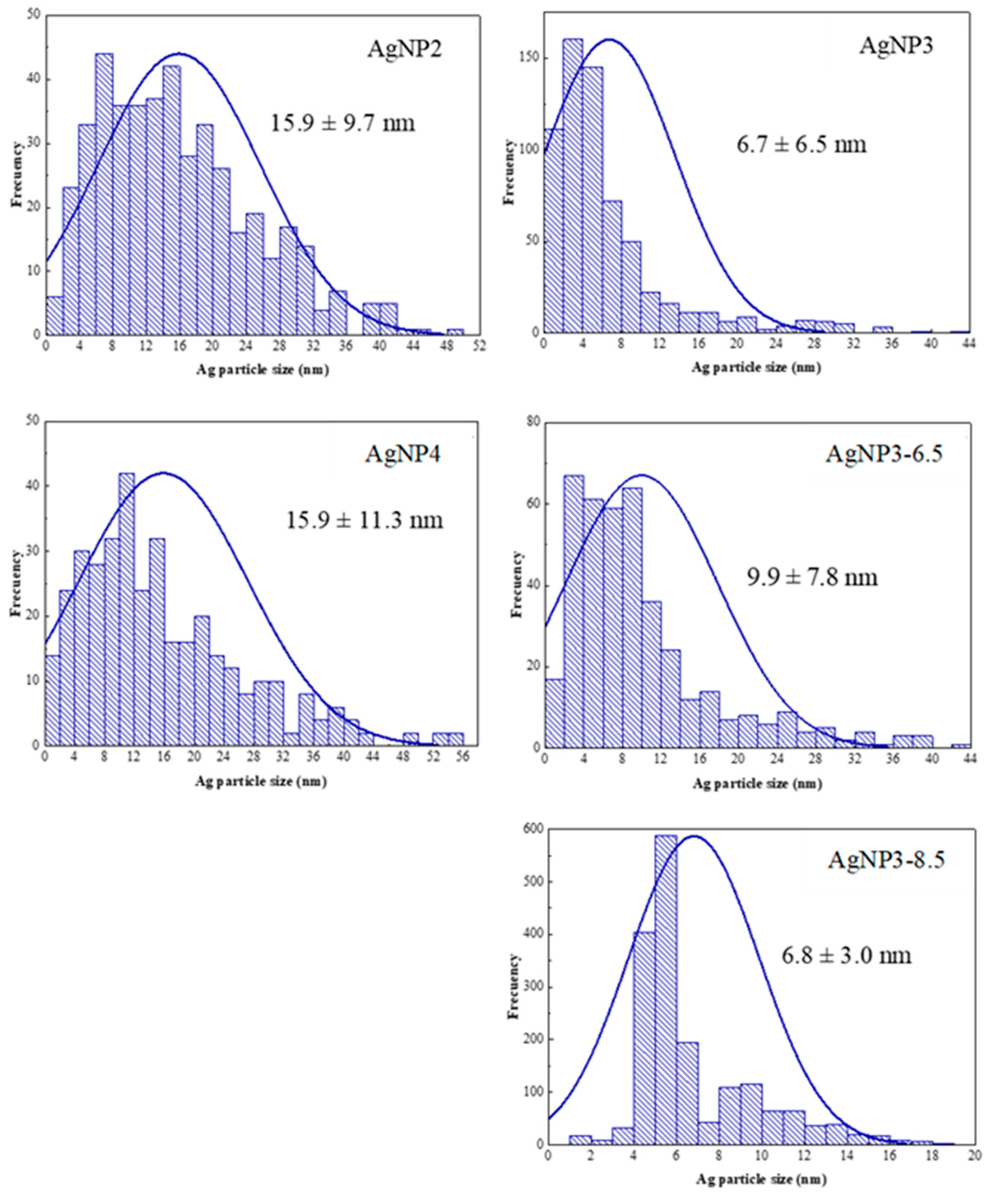
Figure 9.
Micrographs of AgNPs obtained by HRTEM.

Figure 10.
Maps of AgNPs obtained from STEM-EDX analysis.
3. Discussion
The degradation studies of MB showed that the AgNP3 sample had on average the best performance among the AgNPs studied, suggesting that the properties of AgNP3 nanoparticles were somewhat superior for the degradation of MB. Referring to the results of XPS analysis, the capping and stabilization process for AgNP3 was the most successful, where there seemed to be enough readily available silver cations to be reduced along with the necessary number of biomolecules to cap and stabilize the AgNPs effectively. In addition, its homogeneous and narrow particle size distribution with a minor degree of agglomeration, small average particle size, and the highest number of particles less than 8 nm in size (Figure 8) further proved to be beneficial for the enhanced degradation of MB. In the case of AgNP4, although the amount of silver on the surface was found to be the highest (Table 1), which should be favorable for the surface degradation of MB, the nanoparticle formation rate may have been too high, resulting in a quick formation and agglomeration of small nuclei. As previously noted, (Figure 1 and Figure 3), the formation of silver nanoparticles for AgNP4 was faster than for either AgNP2 or AgNP3, which likely led to quick nuclei agglomeration; substantial agglomeration of particles, large average particle size, and wide particle size distribution for AgNP4 were further confirmed by the results of HRTEM/STEM-EDX. As per AgNP2, although its detected amount of silver on the surface is the same as for AgNP3 (Table 1), the XPS data showed that nanoparticles seem to be less stabilized as indicated by the lower amount of C–C bonds and a higher number of oxygen-containing groups than that for AgNP3. More so, just like AgNP4, AgNP2 exhibited a high degree of agglomeration, a wide particle size distribution, and a large average particle size, all of which negatively affected its ability to degrade MB.
As per AgNP3 samples synthesized at a higher pH of the extract, the degradation performances of these samples were inferior to the sample synthesized at pH 4.5. Interestingly, although AgNP3-8.5 showed the most homogeneous and narrow particle size distribution with the smallest average particle size among the AgNPs studied (Figure 8, Figure 9 and Figure 10), its degradation performance was worse than that of AgNP3 synthesized at pH 4.5 (AgNP3). The most likely explanation for this, is that the synthesis-stabilization process for AgNP3-8.5 was less efficient than that at pH 4.5. As seen in Table 1, AgNP3-8.5 has a significantly lower number of C–C bonds and a higher number of oxygen-containing groups than AgNP3. Also, the amount of silver on the surface of AgNP3-8.5 is about twice as low as that of AgNP3 (Table 1). Most likely, the rate of nanoparticle formation at a higher pH was too fast, where the produced small nanoparticles got over-capped by biomolecules due to a fast and unstable synthesis, increasing the amount of the CNSL components compared to the elemental silver in those scenarios [42]. If the CNSL components were too reactive, they could have interfered with the stability of the AgNPs [19,42]. Similar results were obtained for AgNP3-6.5. Overall, altering the pH of the extract does not seem to be necessary for optimizing the biosynthesis of AgNPs and for improving their performance towards the degradation of MB.
The results of this study were compared to some literature studies (Table 3). In this work, on average 50% degradation of MB was achieved within the first 20 min for all the samples studied, but the kinetics of degradation differed thereafter depending on the sample. The highest percentage degradation was recorded for AgNP3 samples, where some batches showed up to 70-80% degradation within the first 20 min (Figure S1) and on average about 80% degradation within 3h of testing.
4. Materials and Methods
4.1.1. Preparation of AgNPs
Cashew nut shells were provided by the Sunshine Nut Company in Mozambique [44]. A mixture with a ratio of 1 g of ground shells to 12.5 mL of deionized water was prepared and boiled for 15 minutes in a round bottom flask on a heating mantle at 100 °C; the mixture was covered with aluminum foil to help prevent evaporation. After, the mixture was cooled to room temperature and filtered to separate the cashew nutshells and the cashew nutshell liquid (CNSL). The separated CNSL is referred to as the extract, and it was freshly prepared for each experiment.
Silver nitrate was purchased from Sigma-Aldrich®. First, 2-4 mM concentrations of silver nitrate were prepared in 100 mL amber volumetric flasks. Then, 10 mL of the extract and 90 mL of deionized water were pipetted into a 250 mL beaker. Next, 100 mL of the necessary concentration of silver nitrate was added. The beaker was covered and left overnight at room temperature so the biosynthesis reaction could proceed; the mixture changed from a golden yellow to dark gray, indicating nanoparticle formation. Afterward, the reaction mixture was centrifuged using Thermo Sorvall Legend XTR centrifuge at 12,000 rpm for 15 min, the solvent above the AgNPs was decanted, and the isolated AgNPs were dried in air. The resulting AgNPs were named AgNP2, AgNP3, and AgNP4 where the number following the letters corresponds to the millimolar concentrations of silver nitrate used during the biosynthesis.
Testing the biosynthesis at different pH values involved using sodium hydroxide and hydrochloric acid purchased from Sigma-Aldrich®. Before the addition of silver nitrate, the 250 mL beaker with 100 mL of diluted extract was placed on a hot plate with a stir bar at 300 rpm. Sodium hydroxide or hydrochloric acid was added dropwise until the pH value reached 6.5 and 8.5, monitored potentiometrically. When the pH reached the desired value, 100 mL of 3 mM silver nitrate was added, and the steps stated above were followed. The resulting AgNPs were named AgNP3-6.5 and AgNP3-8.5 where the numbers following the letters correspond to the millimolar concentration of silver nitrate and pH during the biosynthesis respectively.
4.1.2. Dye Degradation Studies
Methylene blue, MB (C16H18ClN3S·xH2O) was purchased from Sigma-Aldrich®. The solution of MB was prepared in a concentration of 20 mg/L. In a typical run, 10 mg of AgNPs were added to 20 mL of the 20 ppm MB solution on a compact digital mini rotator (Thermo Scientific) at 175 rpm. Aliquots of the mixture were collected every 30 minutes, and centrifuged, and the absorbance values were measured at 663 nm, using an Agilent 8453 UV-Visible Spectrophotometer (Agilent Technologies). The degradation studies were conducted in the dark and under the green light (Kessil KSPR 160L-525nm LED Photoredox Light), for different intervals until the equilibrium was reached. The percent degradation at any point in time was calculated using Equation (1):
where is the initial concentration of MB and is the concentration of MB at time t.
4.1.3. X-Ray photoelectron spectroscopy (XPS)
XPS studies were performed on a Physical Electronic spectrometer (PHI Versa Probe II) using monochromatic Al Kα radiation (25.1 W, 15 kV, 1486.6 eV) and a dual beam charge neutralizer for analyzing the core-level signals of the elements of interest with a hemispherical multichannel detector. The activated carbon sample spectra were recorded with a constant pass energy value of 29.35 eV and a beam diameter of 200 µm. Energy scale was calibrated using Cu 2p3/2, Ag 3d5/2, and Au 4f7/2 photoelectron lines at 932.7, 368.2, and 83.95 eV, respectively. The X-ray photoelectron spectra obtained were analyzed using PHI SmartSoft software and processed using MultiPak 9.6.0.15 package. The binding energy values were referenced to C 1s signal at 284.5 eV. Shirley-type background and Gauss-Lorentz curves were used to determine the binding energies. Atomic concentration percentages of the characteristic elements were determined considering the corresponding area sensitivity factor for the different measured spectral regions.
4.1.4. X-Ray diffraction (XRD)
Laboratory X-ray powder diffraction (XRPD) patterns were collected on a PANanalytical EMPYREAN automated diffractometer. Powder patterns were recorded in the Bragg-Brentano reflection configuration by using the PIXcel 3D detector with a step size of 0.017° (2θ). The powder patterns were recorded between 5 and 80 in 2θ with a total measuring time of 30 min.
4.1.5. High-Resolution Transmission Electron Microscopy (HRTEM)/Scanning Transmission Electron Microscopy (STEM)
The particle morphology and its distribution were obtained with HRTEM by a TALOS F200x instrument, which also operates in STEM mode. The microscope is equipped with a HAADF detector, working at 200 kV and 200 nA. The microanalysis was carried out with an EDX Super-X system provided with 4 X-ray detectors and an X-FEG beam. Particle size distribution was performed using Image J software, counting at least 400 particles for each sample.
5. Conclusions
To summarize, CNSL was successfully utilized to produce silver nanoparticles of different properties. The synthesis conditions where the concentration of silver ions was kept at 3 mM and the pH of the extract at 4.5, proved to be the most stable and efficient for biosynthesis, producing particles of small size and homogeneous and narrow particle size distribution. Such particles further displayed the best degradation properties for MB, with some batches achieving up to 70-80% degradation within the first 20 min. Higher concentrations of silver ions significantly increased the rate of nanoparticle formation and the amount of silver in nanoparticles but resulted in substantial particle agglomeration and wide particle size distribution. This in turn lowered the ability of these particles to degrade MB. Furthermore, the increase in the pH of the extract to 8.5 resulted in an instant nanoparticle formation and produced particles of small size and highly homogeneous and narrow particle size distribution with no observed agglomeration. However, the rate of biosynthesis in this case may have been too high leading to the unstable synthesis and a small amount of nanoparticle silver formed. As a result, nanoparticles synthesized at a higher pH of the extract did not show any improvement in the MB degradation properties in comparison to the particles synthesized at a lower pH. Finally, no significant difference was observed in the degradation of MB in the dark and under green light, indicating that the synthesized AgNPs exhibit negligible photodegradation properties under visible light conditions.
Supplementary Materials
The following supporting information can be downloaded at website of this manuscript posted on Preprints.org, Figure S1: The MB degradation curves shown are for all the batches of the AgNP samples studied.
Author Contributions
Conceptualization, S.B.; methodology, S.B. and J.C.; software, E.R.-A. and D.B.P.; validation, J.C., E.R.-A., D.B.P., and S.B.; formal analysis, J.C.., E.R.-A., D.B.P., and S.B.; investigation, J.C., E.R.-A., D.B.P., and S.B.; resources, S.B., E.R.-A., and D.B.P.; data curation, J.C., S.B., D.B.P., and E.R.-A.; writing—original draft preparation, J.C. and S.B.; writing—review and editing, J.C., S.B., E.R.-A., and D.B.P.; visualization, S.B., E.R.-A., and D.B.P.; supervision, S.B.; project administration, S.B., E.R.-A., and D.B.P.; funding acquisition, S.B., E.R.-A., and D.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Spanish Ministry of Science and Innovation, project TED2021-130756B-C31 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe” by the European Union NextGeneration EU/PRTR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Grant-In-Aid Program and the Department of Chemistry, Biochemistry and Physics at Fairleigh Dickinson University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siddiqi, K. S.; Husen, A.; Rao, R. A. K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J Nanobiotechnol 2018, 16 (1), 14. [CrossRef]
- Sumi, M. B.; Devadiga, A.; Shetty K, V.; M. B., S. Solar Photocatalytically Active, Engineered Silver Nanoparticle Synthesis Using Aqueous Extract of Mesocarp of Cocos Nucifera (Red Spicata Dwarf). Journal of Experimental Nanoscience 2017, 12 (1), 14–32. [CrossRef]
- Mikhailova, E. O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. JFB 2020, 11 (4), 84. [CrossRef]
- Yin, I. X.; Zhang, J.; Zhao, I. S.; Mei, M. L.; Li, Q.; Chu, C. H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. IJN 2020, Volume 15, 2555–2562. [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S. V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res Pharm Sci 2014, 9 (6), 385–406.
- Liaqat, N.; Jahan, N.; Khalil-Ur-Rahman, null; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front Chem 2022, 10, 952006. [CrossRef]
- Habeeb Rahuman, H. B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal Plants Mediated the Green Synthesis of Silver Nanoparticles and Their Biomedical Applications. IET Nanobiotechnology 2022, 16 (4), 115–144. [CrossRef]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K. C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5 (22), 13123–13129. [CrossRef]
- Mohanta, Y. K.; Panda, S. K.; Bastia, A. K.; Mohanta, T. K. Biosynthesis of Silver Nanoparticles from Protium Serratum and Investigation of Their Potential Impacts on Food Safety and Control. Front. Microbiol. 2017, 8. [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicology and Environmental Safety 2023, 253, 114636. [CrossRef]
- Vanaja, M.; Paulkumar, K.; Baburaja, M.; Rajeshkumar, S.; Gnanajobitha, G.; Malarkodi, C.; Sivakavinesan, M.; Annadurai, G. Degradation of Methylene Blue Using Biologically Synthesized Silver Nanoparticles. Bioinorganic Chemistry and Applications 2014, 2014, 1–8. [CrossRef]
- Devi, T. A.; Ananthi, N.; Amaladhas, T. P. Photobiological Synthesis of Noble Metal Nanoparticles Using Hydrocotyle Asiatica and Application as Catalyst for the Photodegradation of Cationic Dyes. J Nanostruct Chem 2016, 6 (1), 75–92. [CrossRef]
- Lugaresi, O.; Perales-Rondón, J. V.; Minguzzi, A.; Solla-Gullón, J.; Rondinini, S.; Feliu, J. M.; Sánchez-Sánchez, C. M. Rapid Screening of Silver Nanoparticles for the Catalytic Degradation of Chlorinated Pollutants in Water. Applied Catalysis B: Environmental 2015, 163, 554–563. [CrossRef]
- Gola, D.; Kriti, A.; Bhatt, N.; Bajpai, M.; Singh, A.; Arya, A.; Chauhan, N.; Srivastava, S. K.; Tyagi, P. K.; Agrawal, Y. Silver Nanoparticles for Enhanced Dye Degradation. Current Research in Green and Sustainable Chemistry 2021, 4, 100132. [CrossRef]
- Yu, J.; Yang, Y.; Sun, F.; Chen, J. Research Status and Prospect of Nano Silver (Ag)-Modified Photocatalytic Materials for Degradation of Organic Pollutants. Environ Sci Pollut Res 2023. [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sciences 2020, 15 (1), 819–839. [CrossRef]
- Chugh, D.; Viswamalya, V. S.; Das, B. Green Synthesis of Silver Nanoparticles with Algae and the Importance of Capping Agents in the Process. J Genet Eng Biotechnol 2021, 19 (1), 126. [CrossRef]
- Khan, Y.; Nasar, M.; Numan, M.; Ullah, I.; Shinwari, Z. Biomimetic Synthesis of Silver Nanoparticles for Breast Cancer Therapeutics and Its Mechanism. International Journal of Nanotechnology and Nanomedicine 2018, 3.
- Talabani, R. F.; Hamad, S. M.; Barzinjy, A. A.; Demir, U. Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials 2021, 11 (9), 2421. [CrossRef]
- De Melo, A. P. Z.; De Oliveira Brisola Maciel, M. V.; Sganzerla, W. G.; Da Rosa Almeida, A.; De Armas, R. D.; Machado, M. H.; Da Rosa, C. G.; Nunes, M. R.; Bertoldi, F. C.; Barreto, P. L. M. Antibacterial Activity, Morphology, and Physicochemical Stability of Biosynthesized Silver Nanoparticles Using Thyme (Thymus Vulgaris) Essential Oil. Mater. Res. Express 2020, 7 (1), 015087. [CrossRef]
- Velmurugan, P.; Iydroose, M.; Lee, S.-M.; Cho, M.; Park, J.-H.; Balachandar, V.; Oh, B.-T. Synthesis of Silver and Gold Nanoparticles Using Cashew Nut Shell Liquid and Its Antibacterial Activity Against Fish Pathogens. Indian J Microbiol 2014, 54 (2), 196–202. [CrossRef]
- Gaitán-Jiménez, S.-Y.; Restrepo-Sánchez, L.-P.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Cashew (Anacardium Occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules 2022, 27 (24), 8733. [CrossRef]
- De Lima, S. G.; Feitosa, C. M.; Cito, A. M. G. L.; Moita Neto, J. M.; Lopes, J. A. D.; Leite, A. S.; Brito, M. C.; Dantas, S. M. M.; Melo Cavalcante, A. A. C. Effects of Immature Cashew Nut-Shell Liquid (Anacardium Occidentale) against Oxidative Damage in Saccharomyces Cerevisiae and Inhibition of Acetylcholinesterase Activity. Genet. Mol. Res. 2008, 7 (3), 806–818. [CrossRef]
- Oliveira, M. S. C.; Morais, S. M. de; Magalhães, D. V.; Batista, W. P.; Vieira, Í. G. P.; Craveiro, A. A.; de Manezes, J. E. S. A.; Carvalho, A. F. U.; de Lima, G. P. G. Antioxidant, Larvicidal and Antiacetylcholinesterase Activities of Cashew Nut Shell Liquid Constituents. Acta Tropica 2011, 117 (3), 165–170. [CrossRef]
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; IntechOpen, 2020. [CrossRef]
- Spagnoli, A. A.; Giannakoudakis, D. A.; Bashkova, S. Adsorption of Methylene Blue on Cashew Nut Shell Based Carbons Activated with Zinc Chloride: The Role of Surface and Structural Parameters. Journal of Molecular Liquids 2017, 229, 465–471. [CrossRef]
- Bello, R.; Rodríguez-Aguado, E.; Smith, V. A.; Grachev, D.; Castellón, E. R.; Bashkova, S. Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2022, 12 (10), 1625. [CrossRef]
- Elbadawy, H. A.; Elhusseiny, A. F.; Hussein, S. M.; Sadik, W. A. Sustainable and Energy-Efficient Photocatalytic Degradation of Textile Dye Assisted by Ecofriendly Synthesized Silver Nanoparticles. Sci Rep 2023, 13 (1), 2302. [CrossRef]
- Al-Zaban, M. I.; Mahmoud, M. A.; AlHarbi, M. A. Catalytic Degradation of Methylene Blue Using Silver Nanoparticles Synthesized by Honey. Saudi Journal of Biological Sciences 2021, 28 (3), 2007–2013. [CrossRef]
- Jaast, S.; Grewal, A. Green Synthesis of Silver Nanoparticles, Characterization and Evaluation of Their Photocatalytic Dye Degradation Activity. Current Research in Green and Sustainable Chemistry 2021, 4, 100195. [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2 (4), 738. [CrossRef]
- Tam, K. T.; Thanh, D. V.; Van, H. T.; Mai, N. T. P.; Hai, C. T.; Phuong, T. M.; Xuan, N. T.; Nguyen, V.-T. Green Synthesis of Silver Nanoparticles Using Extract of Disporopsis Longifolia for Photocatalytic Degradation of Methylene Blue. American Journal of Environmental Sciences 2022, 18 (5), 116–124. [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; Bharti, R. K. Synthesis of Silver Nanoparticles Utilizing Various Biological Systems: Mechanisms and Applications—a Review. Prog Biomater 2020, 9 (3), 81–95. [CrossRef]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S. P.; Chaudhry, V.; Mudiam, M. K. R.; Shukla, S.; Kakkar, P.; Nautiyal, C. S. Physico-Chemical Condition Optimization during Biosynthesis Lead to Development of Improved and Catalytically Efficient Gold Nano Particles. Sci Rep 2016, 6 (1), 27575. [CrossRef]
- Awwad, A. M.; Salem, N. M.; Abdeen, A. O. Green Synthesis of Silver Nanoparticles Using Carob Leaf Extract and Its Antibacterial Activity. Int J Ind Chem 2013, 4 (1), 29. [CrossRef]
- Wibowo, A.; Tajalla, G. U. N.; Marsudi, M. A.; Cooper, G.; Asri, L. A. T. W.; Liu, F.; Ardy, H.; Bartolo, P. J. D. S. Green Synthesis of Silver Nanoparticles Using Extract of Cilembu Sweet Potatoes (Ipomoea Batatas L Var. Rancing) as Potential Filler for 3D Printed Electroactive and Anti-Infection Scaffolds. Molecules 2021, 26 (7), 2042. [CrossRef]
- Telascrêa, M.; Leão, A. L.; Ferreira, M. Z.; Pupo, H. F. F.; Cherian, B. M.; Narine, S. Use of a Cashew Nut Shell Liquid Resin as a Potential Replacement for Phenolic Resins in the Preparation of Panels – A Review. Molecular Crystals and Liquid Crystals 2014, 604 (1), 222–232. [CrossRef]
- Kishore, S. C.; Perumal, S.; Atchudan, R.; Edison, T. N. J. I.; Sundramoorthy, A. K.; Alagan, M.; Sangaraju, S.; Lee, Y. R. Eco-Friendly Synthesis of Functionalized Carbon Nanodots from Cashew Nut Skin Waste for Bioimaging. Catalysts 2023, 13 (3), 547. [CrossRef]
- Rojas, J. V.; Toro-Gonzalez, M.; Molina-Higgins, M. C.; Castano, C. E. Facile Radiolytic Synthesis of Ruthenium Nanoparticles on Graphene Oxide and Carbon Nanotubes. Materials Science and Engineering: B 2016, 205, 28–35. [CrossRef]
- Carmona, E. R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green Synthesis of Silver Nanoparticles by Using Leaf Extracts from the Endemic Buddleja Globosa Hope. Green Chemistry Letters and Reviews 2017, 10 (4), 250–256. [CrossRef]
- Ruíz-Baltazar, Á. D. J.; Reyes-López, S. Y.; Mondragón-Sánchez, M. D. L.; Estevez, M.; Hernández-Martinez, A. R.; Pérez, R. Biosynthesis of Ag Nanoparticles Using Cynara Cardunculus Leaf Extract: Evaluation of Their Antibacterial and Electrochemical Activity. Results in Physics 2018, 11, 1142–1149. [CrossRef]
- Rakib-Uz-Zaman, S. M.; Hoque Apu, E.; Muntasir, M. N.; Mowna, S. A.; Khanom, M. G.; Jahan, S. S.; Akter, N.; R. Khan, M. A.; Shuborna, N. S.; Shams, S. M.; Khan, K. Biosynthesis of Silver Nanoparticles from Cymbopogon Citratus Leaf Extract and Evaluation of Their Antimicrobial Properties. Challenges 2022, 13 (1), 18. [CrossRef]
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic Degradation of Methyl Orange Dye Using Silver (Ag) Nanoparticles Synthesized from Ulva Lactuca. Colloids and Surfaces B: Biointerfaces 2013, 103, 658–661. [CrossRef]
- Sunshine Nut Co. https://sunshinenuts.com/pages/who-we-are.
Figure 1.
The change in solution color during the AgNP biosynthesis at different concentrations of AgNO3 and pH 4.5.
Figure 1.
The change in solution color during the AgNP biosynthesis at different concentrations of AgNO3 and pH 4.5.

Figure 2.
The change in solution color during the AgNP biosynthesis at different pH values of the extract and AgNO3 concentration of 3 mM.
Figure 2.
The change in solution color during the AgNP biosynthesis at different pH values of the extract and AgNO3 concentration of 3 mM.
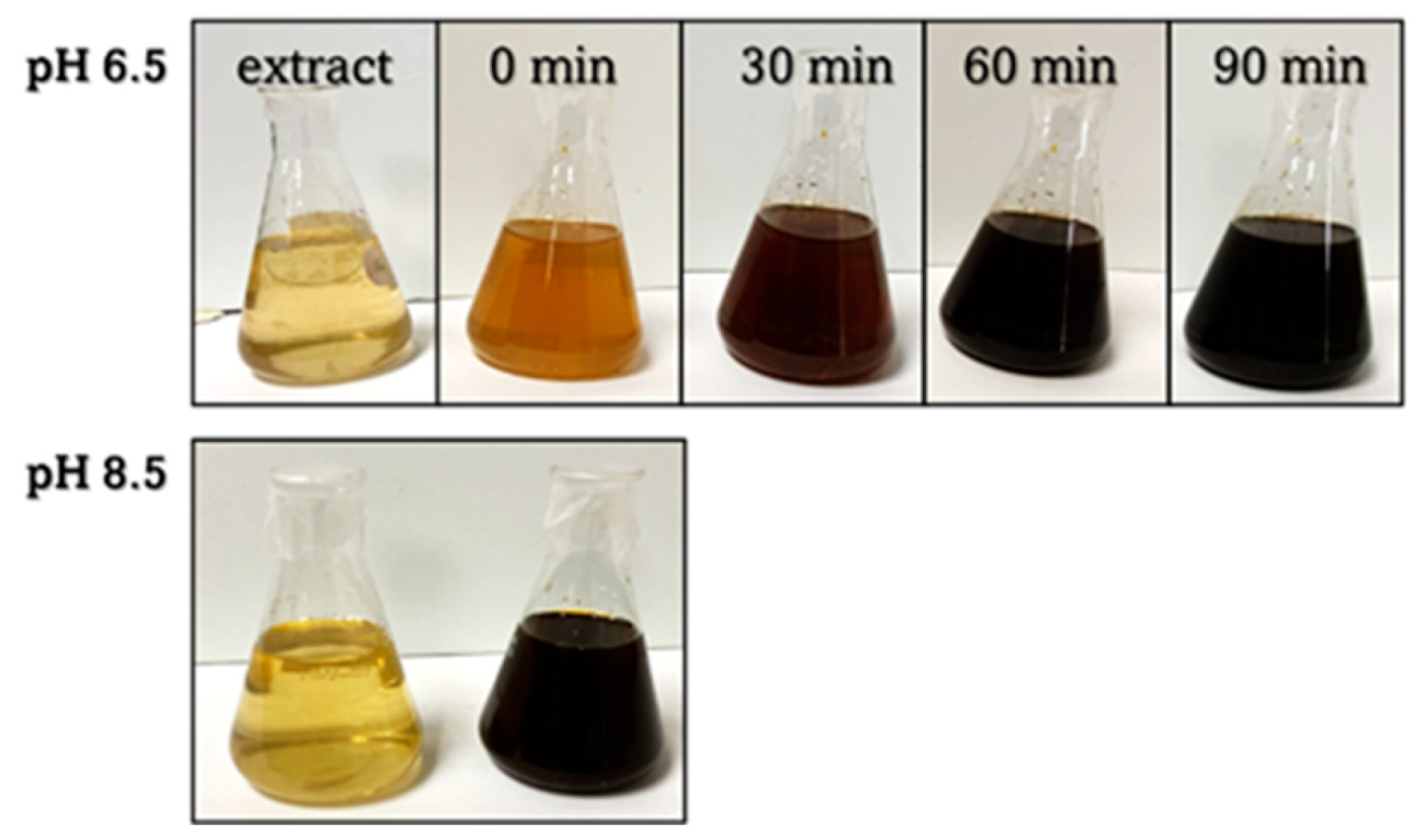
Figure 3.
The AgNP surface plasmon resonance curves during the biosynthesis with 2–4 mM AgNO3 and pH of the extract of 4.5.
Figure 3.
The AgNP surface plasmon resonance curves during the biosynthesis with 2–4 mM AgNO3 and pH of the extract of 4.5.
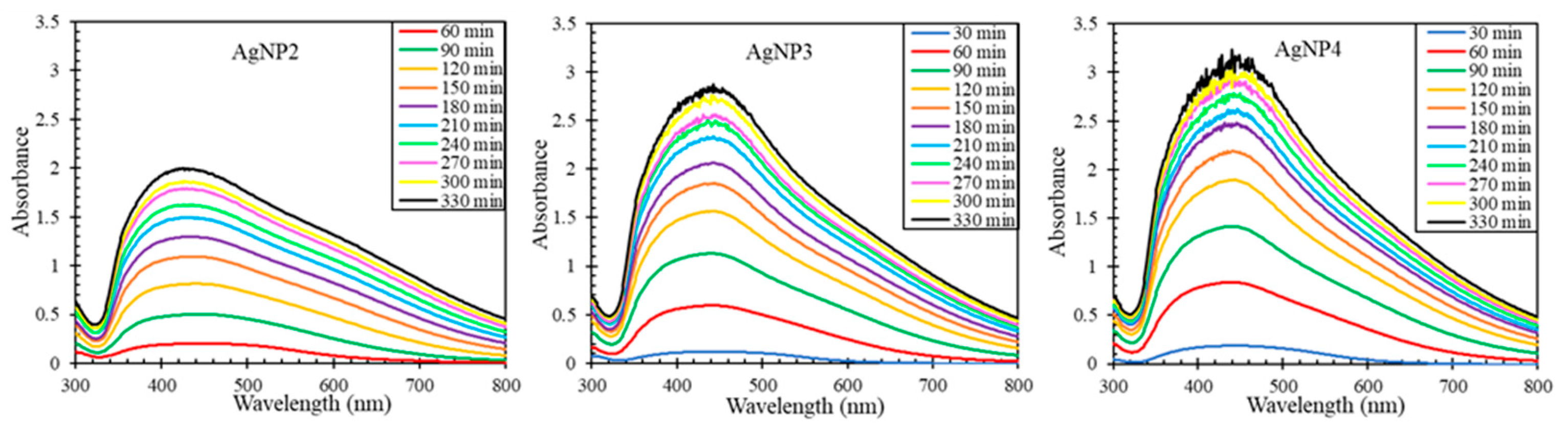
Figure 4.
The AgNP surface plasmon resonance curves during the biosynthesis with pH 6.5 and 8.5 extract and AgNO3 concentration of 3 mM.
Figure 4.
The AgNP surface plasmon resonance curves during the biosynthesis with pH 6.5 and 8.5 extract and AgNO3 concentration of 3 mM.
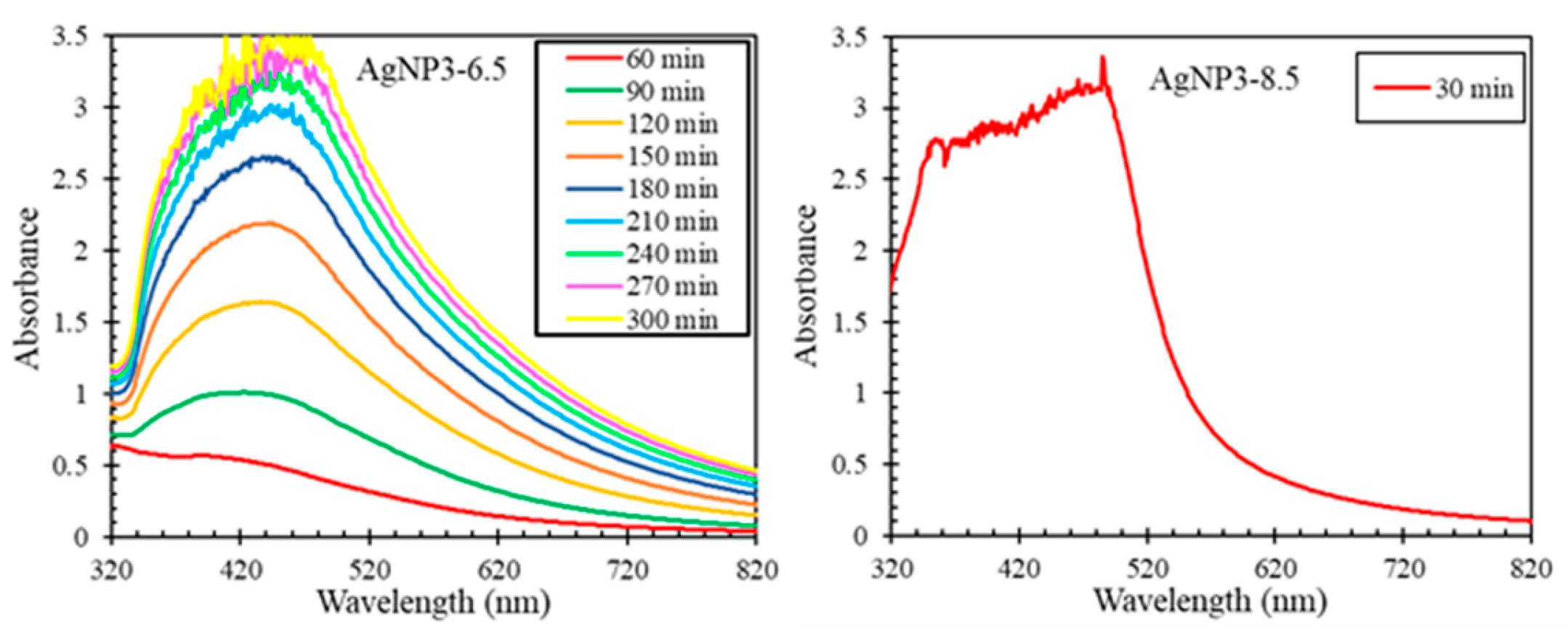
Figure 5.
The MB degradation curves for (A) AgNP3 in the dark and under the green light; (B) for AgNP2, AgNP3, and AgNP4 under the green light; (C) for AgNP3-6.5 and AgNP3-8.5 under the green light. The comparison in percent degradation for (D) AgNP3 in the dark and under the green light; (E) for AgNP2, AgNP3, and AgNP4 under the green light; (F) for AgNP3-6.5 and AgNP3-8.5 under the green light.
Figure 5.
The MB degradation curves for (A) AgNP3 in the dark and under the green light; (B) for AgNP2, AgNP3, and AgNP4 under the green light; (C) for AgNP3-6.5 and AgNP3-8.5 under the green light. The comparison in percent degradation for (D) AgNP3 in the dark and under the green light; (E) for AgNP2, AgNP3, and AgNP4 under the green light; (F) for AgNP3-6.5 and AgNP3-8.5 under the green light.
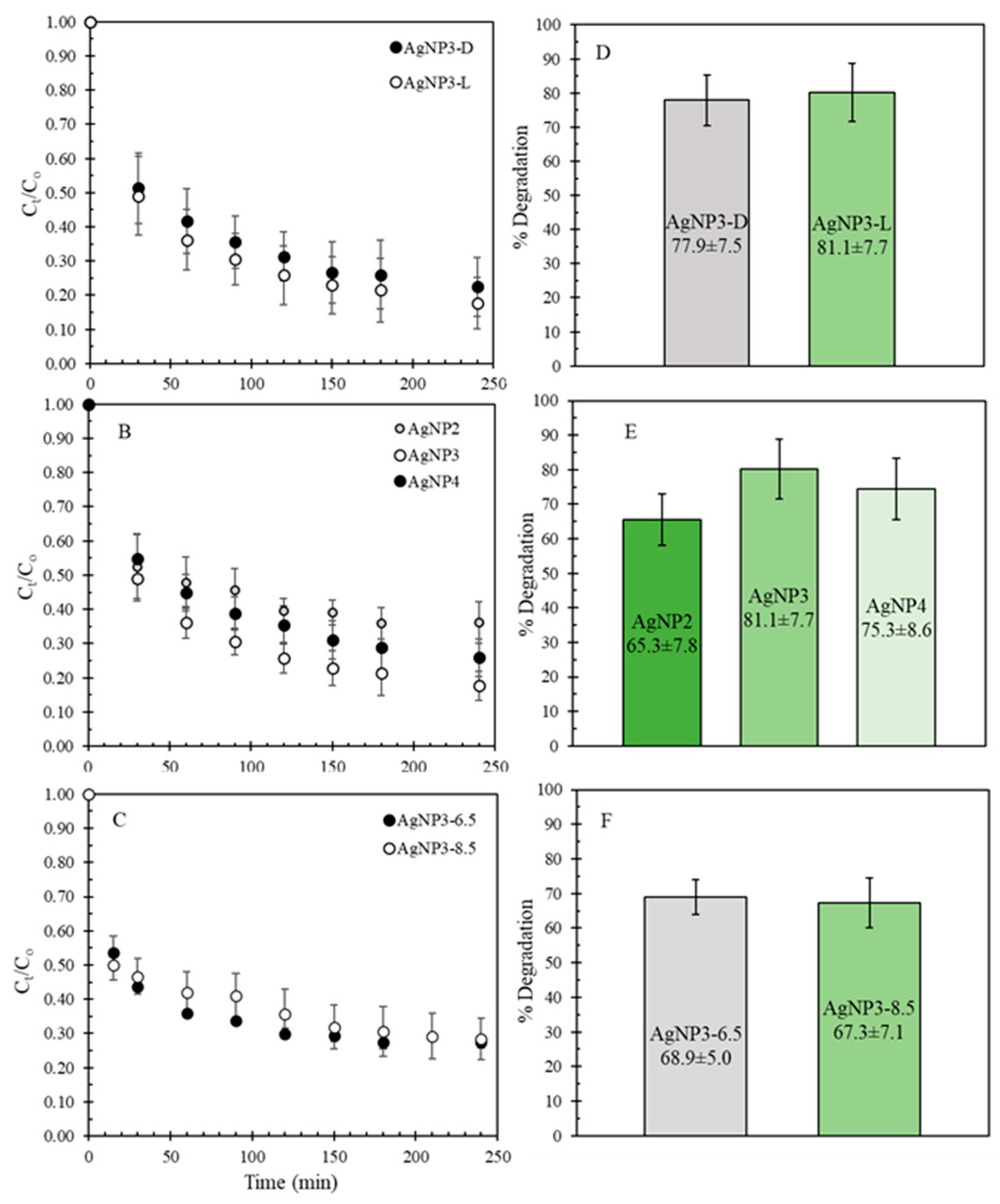
Figure 7.
(A) C 1s, (B) O 1s, (C) Ag 3d core-level XPS spectra of AgNP2, AgNP3, and AgNP4 at the pH of the extract of 4.5; (D) C 1s, (E) O 1s, (F) Ag 3d core-level XPS spectra of AgNP3-6.5 and AgNP3-8.5 at the concentration of AgNO3 of 3 mM.
Figure 7.
(A) C 1s, (B) O 1s, (C) Ag 3d core-level XPS spectra of AgNP2, AgNP3, and AgNP4 at the pH of the extract of 4.5; (D) C 1s, (E) O 1s, (F) Ag 3d core-level XPS spectra of AgNP3-6.5 and AgNP3-8.5 at the concentration of AgNO3 of 3 mM.

Table 1.
Results of the deconvolution of the XPS core-level spectra for C 1s, O 1s, and Ag 3d, and the element content in atomic percentage for AgNP samples.
Table 1.
Results of the deconvolution of the XPS core-level spectra for C 1s, O 1s, and Ag 3d, and the element content in atomic percentage for AgNP samples.
| C 1s (%) | O 1s (%) | Ag 3d (%) | |||
| BE, eV |
284.8C–C |
286.5C–O |
288.6O–C=O |
532.6O–C/O=C |
368.5/374.5 |
| AgNP2 | 86.6 | 10.1 | 2.3 | 19.4 | 1.6 |
| AgNP3 | 91.5 | 8.4 | N/A | 12.3 | 1.6 |
| AgNP4 | 78.7 | 16.3 | 5.0 | 20.4 | 2.9 |
| AgNP3-6.5 | 85.5 | 12.4 | 2.1 | 16.9 | 0.4 |
| AgNP3-8.5 | 78.6 | 17.1 | 4.3 | 19.0 | 0.8 |
Table 3.
Lower and upper ranges of silver particle size for each sample.
| Plant Extract | Type of Dye | Light Source | AgNP Size (nm) | Time (h) | Degrad. (%) | Ref. |
|---|---|---|---|---|---|---|
| Cashew Nutshell Liquid | Methylene Blue | Green | 0.2–13.2 | 3 | 81 | This study |
| Dark | 3 | 78 | ||||
| Camellia sinensis leaf | Methylene Blue | Solar | 25–40 | 72 | 95 | [36] |
| Morinda tinctoria leaf | Methylene Blue | Solar | 79–96 | 72 | 95 | [11] |
| Ulva lactuca seaweed | Methylene Blue | Solar | 49 | 12 | 85 | [43] |
| Kitchen vegetable waste | Methylene Blue | Solar | 10–100 | 3 | 88 | [38] |
| Cauliflower waste | Methylene Blue | Solar | 10 | 2.5 | 98 | [31] |
| Indian screw tree | Methylene Blue | Solar | 25–45 | 0.5 | 95 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








