Preprint
Review
Neuroinflammatory Approach to Surgical Trauma: Biomarkers and the Mechanisms of the Immune and Neuroendocrine Response
Altmetrics
Downloads
148
Views
74
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.pdf (610.03KB )
Submitted:
26 June 2024
Posted:
28 June 2024
You are already at the latest version
Alerts
Abstract
The severity and invasiveness of clinical outcomes from organic responses to trauma are influenced by individual, surgical and anesthetic factors. A stress response elicits neuroendocrine and immune reactions that may lead to multi-organ dysfunction. The degree of neuroinflammatory reflex activation from trauma can increase pro-inflammatory cytokine production, leading to endothelial dysfunction, glycocalyx damage, neutrophil activation and multisystem tissue destruction. A shift in patient treatment towards a neuroinflammatory perspective has prompted a new evaluation protocol for surgical patients, required to understand surgical pathogenesis and its link to chosen anesthesia-surgical methods. This study aims to provide a description of the mechanisms involved in the immune and neuroendocrine response and below is a summary of the main mediators, focusing on video laparoscopic surgeries, by examining its frequency usage by researchers. We performed a narrative review of data from databases like PubMed, MEDLINE, EMBASE, high-impact journals, and studies from the past decade that used inflammatory mediator analysis to compare anesthetic and surgical strategies. Results showed preference for certain biomarkers more sensitive to tissue trauma. These markers display distinct behaviors, temporal fluctuations and their dosages serve as a tool to measure and validate therapeutic approaches in perioperative systemic inflammation.
Keywords:
Subject: Medicine and Pharmacology - Anesthesiology and Pain Medicine
Introduction
Video laparoscopic cholecystectomies represent the most common elective gastrointestinal video surgery. Gallstones are frequent in the adult population affecting over 10–15% of the Caucasian adult population. However, in certain ethnicities, such as North American Indians including Pima, Chippewa, Canadian Micmac and other American Indian tribes in Arizona and Dakotas, prevalence can be as high as 60–70% of the population [1].
Video laparoscopy is known to induce hemodynamic changes due to a combination of factors like pneumoperitoneum, hypercapnia, patient positioning and the anesthetic technique used. It’s increasingly recognized that anesthetic management can indirectly influence immunostimulatory and immunosuppressive mechanisms by modulating immune cells function or directly by mitigating the stress response through intravenous agents or regional anesthesia. This suggests that choosing an anesthetic technique could affect the balance between pro-inflammatory and anti-inflammatory responses, potentially altering clinical outcomes [2,3,4].
Surgical injury to tissue triggers a range of reactions crucial for restoring the organism’s homeostasis. An appropriate inflammatory response is vital for tissue repair. The stress response includes elevated hormone levels (ACTH, cortisol, catecholamines), complement system activation, leukocyte migration to the injury site, and cytokine release (interleukins, tumor necrosis factor), along with other cellular products (superoxide radicals, proteases, growth factors). This stress response can alter the patient’s immunological status, and a weakened immune system may lead to a higher risk of postoperative infections, impaired wound healing, and potential organ failure [5].
Tissue damage is detected by peripheral and central nociceptors, which trigger an electrical response through the depolarization of the axonal membrane and stimulate the activation and interaction of the hypothalamic-pituitary sympathetic, endocrine, and immune systems. The electrical impulse produced by these nociceptors’ activation is conveyed via nerve fibers to the spinal cord’s dorsal horn, prompting the activation of the central nervous system and an immediate sympathetic neurological reaction. Subsequently, a range of endocrine, tissue, and hemodynamic systemic responses ensue at varying intervals [6].
IL-6 serves as an indicator of the inflammatory response following surgical trauma. It triggers the liver to produce acute phase reactants, such as C-reactive protein, stimulates neutrophil production in the bone marrow, and aids in the differentiation of T helper cells that produce IL-17. It’s produced by macrophages, dendritic cells, endothelial cells, fibroblasts, and other cells in response to pathogen-associated molecular patterns, as well as IL-1 and TNF. The serum levels of these substances rise proportionally to the stress level, as seen in sepsis, leading to mitochondrial dysfunction, glycocalyx disruption, and endothelial dysfunction, all of which are associated with increased morbidity and mortality. CRP levels usually start to increase about four to six hours after surgical injury, peaking within 48 hours. After uncomplicated surgeries, CRP levels generally decrease, returning to normal within 72 to 168 hours. Consequently, both IL-6 and CRP levels in plasma are indicative of the extent of surgical trauma, reflecting their relationship and plasma kinetics [7,8,9].
The surgical stress response is mediated by neural afferents and the sympathetic nervous system through both pro-inflammatory and anti-inflammatory mediators, such as cytokines, catecholamines, cortisol, glucagon, insulin, growth hormone, aldosterone, and antidiuretic hormone. Normally, there’s a balance between pro-inflammatory and anti-inflammatory cytokines. However, under stress conditions like trauma, sepsis, and cancer, an excess of pro-inflammatory cytokines disrupts this balance, leading to increased morbidity and mortality [10]. Controlling stress-induced activation of the sympathetic nervous system is crucial to prevent hemodynamic compromise, hyperinflammation, coagulopathy, immune dysfunction, metabolic imbalances, and hypothermia [5].
Identifying the markers most sensitive to injury during laparoscopy could greatly advance the field. This narrative review aims to assess the inflammatory response and emphasize the markers most frequently utilized in clinical-surgical practice.
Systemic Inflammation and Tissue Damage
Regardless of the surgical access route, the direct and indirect effects of surgery result in cell damage. Direct surgical injury occurs due to surgical manipulation, tissue mobilization, excision, and dissection, which can lead to the release of high levels of inflammatory mediators and cytokines that drive immunological, metabolic, and hormonal processes, known as the surgical stress response. Indirect injury may be caused by blood loss, changes in perfusion, microvascular alterations, and anesthetic techniques, which can affect hemoglobin concentration, cardiac output, and arterial oxygen saturation. This may result in a decrease in the supply of oxygen to the tissues, predisposing them to the development of organ dysfunction and SIRS [11].
Surgical stress is defined as an acute response to the impairment of body barrier functions due to sterile injury (incision, excision, manipulation, and pain), pathogen invasion (intestinal bacterial translocation or postoperative wound infection), and/or anesthesia. In trauma, the initial response is an increase in sympathetic discharge. If this is not contained, it has multiple effects on homeostasis, including the stimulation of inflammation, alterations in coagulation, modifications to immune competence and T-cell mobilization. This process is mediated by β2-adrenergic receptors, which increases susceptibility to infection and reduces tissue oxygenation [12].
The understanding that different drugs can act on receptors with specific mechanisms of action, modulating these reactions, drives the anesthesiologist to pursue more effective alternatives within favorable postoperative clinical outcomes.
Immune Response
Evolutionarily conserved biochemical mechanisms play a key role during exposure to surgical stress, leading to the engagement of signaling pathways in the innate and cell-mediated adaptive immune systems. The impact of surgery on the immune and neuroendocrine - metabolic systems is influenced by several factors, including the intensity of surgical trauma, malnutrition, infection and cancer [13].
Initially, there is a complex integration between cytokines, alarmins and physiological signals generating the recruitment of inflammatory cells, particularly macrophages, neutrophils and dendritic cells, activation of Th1, Th2 and Th17 effector CD4+ helper T cells of innate immunity, as well as natural killer (NK) cells, which are analogous to CD8+ cytotoxic T cells. These cells phagocytize the offending agent and produce additional doses of cytokines that lead to positive feedback for the activation of specific transcription factors for innate immunity cells and a possible adaptive immune response. Norepinephrine (NE) is released by the SNS and signals via β1- and β2-adrenergic receptors. The cytotoxicity of NK cells and the expression of IFNγ, granzyme B and perforin are reduced when the signaling of these receptors is activated. NE signaling exerts its inhibitory effect on the proliferation of Th2 lymphocytes by binding to beta-adrenergic receptors. For example, administration of the β2-adrenergic agonist clenbuterol during N. brasiliensis infection inhibits Th2 effector functions, leading to reduced recruitment of eosinophils, goblet cell hyperplasia and predisposition to infection [14].
Cytokines play a fundamental role as mediators of metabolic, hormonal, immunological and hematological responses. The main pro-inflammatory cytokines involved in innate immunity are: Tumor Necrosis Factor alpha and beta, Interleukins (ILs) 1-α and 1-β, IL-6 and IL-12, whose main cellular sources include macrophages, endothelial cells, dendritic cells and T lymphocytes. Their biological effects include activation of inflammation and coagulation, fever, catabolism, synthesis of acute phase proteins and differentiation of CD4+ Th1 and Th17 T lymphocytes. On the other hand, among the anti-inflammatories, IL-10 stands out, synthesized by macrophages and regulatory T cells, with actions related to inhibiting the synthesis of ILs 1, 12 and Tumor Necrosis Factor by macrophages and dendritic cells by negative feedback. There is therapeutic potential in blocking the cellular expression of these cytokines. However, there are many questions about this immunomodulation, and it is becoming increasingly necessary to elucidate the challenges that cytokines have left, not only for experimentation, but also for clinical practice [15,16].
The protective innate immune responses during trauma produce a series of immediate responses to eliminate damaged tissues, followed by the activation of repair mechanisms, with the ultimate aim of restoring cells and tissues to their pre-lesion state. Pathogen-associated molecular patterns (PAMPs) of infectious agents (bacteria, viruses and fungi) or the release of damage-associated molecular patterns (DAMPs) such as ATP, HMGB-1, histones and mitochondrial DNA can be detected by fluid-phase inflammatory pathways that contain proteins or lipids and participate in the so-called “first line of defense”. In particular, the serine protease system, which participates in the kinin, coagulation and complement cascades, can detect DAMPs and PAMPs, be rapidly activated after trauma and be reinforced in acidic (e.g. hypoxic) microenvironments. Directly or via such activated systems, DAMPs and PAMPs can transmit their signals to leukocytes via pattern recognition receptors (PRRs) such as TLRs, NLRs, RAGE, purinergic receptors or complement receptors. Cell translation and its interaction with the mechanisms described above result in broadly balanced pro-inflammatory and anti-inflammatory protective effects mediated by targeted chemotaxis and cytokine release that contribute to tissue repair and healing, such as the reprogramming of macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype [17].
The impact of analgesic and surgical methods on the tissue levels of two representative cytokines, TNF and IL-10, which span the pro- and anti-inflammatory spectra, was demonstrated in an experimental study in which laparoscopic surgery for colorectal cancer surgery was linked to lower values of IL-10, TNF, and mast cells in the mucosa of the tissue surrounding the tumor, regardless of the method of perioperative analgesia. The number of IL-10-producing Th2 lymphocytes was found to be higher in patients undergoing open surgery compared to those undergoing laparoscopic surgery. Additionally, the number of TNF-producing cells was found to be higher in the open surgery group that received patient-controlled intravenous analgesia compared to the group that received epidural analgesia. The combination of laparoscopic surgery with epidural analgesia has been demonstrated to result in a reduction in the expression of TNF and IL-10, as well as mast cells. This approach may therefore represent a promising avenue for achieving anti-inflammatory effects in cancer-related surgical inflammation [18].
Neuroendocrine and Humoral Response
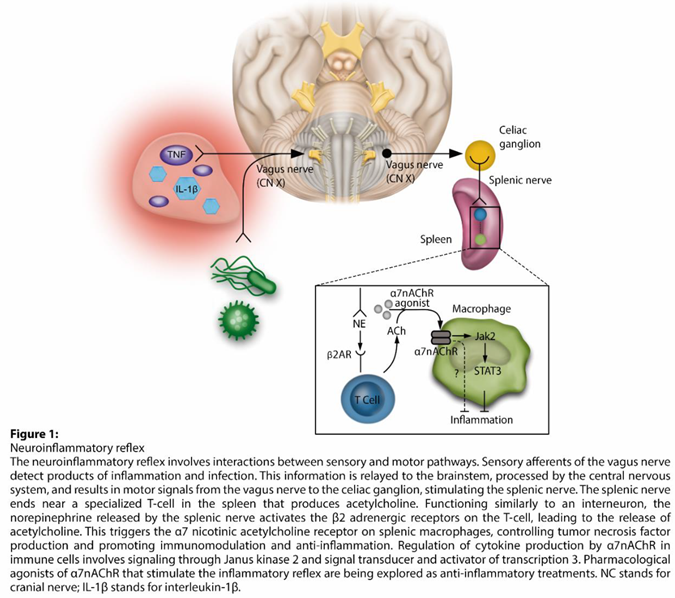
The neuro-inflammatory reflex is activated by the sensory afferents of the vagus nerve, which are stimulated by the products of inflammatory and infectious stimuli. This information is transmitted to the brainstem, where it is integrated by the central nervous system. The reflex is completed by the vagus motor signals being sent to the celiac ganglion and splenic nerve. The latter ends near a specialized acetylcholine-producing T-cell (ACh) in the spleen. The NE released by the splenic nerve activates the β2 adrenergic receptors (β2ARs) on the T-cell, which in turn releases ACh. ACh activates the α7 nicotinic acetylcholine receptor (α7nAChR) in splenic macrophages and negatively regulates their production of tumor necrosis factor (TNF). This mechanism appears to be mediated by the activation of Janus Kinase (JAK) 2 and transcriptional activator (STAT) 3 cell signaling, resulting in an anti-inflammatory effect (Figure 1). In this context, a series of experimental studies have demonstrated the therapeutic potential of modulating the neuro-inflammatory pathway. Pharmacological agonists of α7nAChR, which activate the inflammatory reflex, are being developed as potential anti-inflammatory therapies, with promising results in experimental models for sepsis and surgical injury [2,3,19].
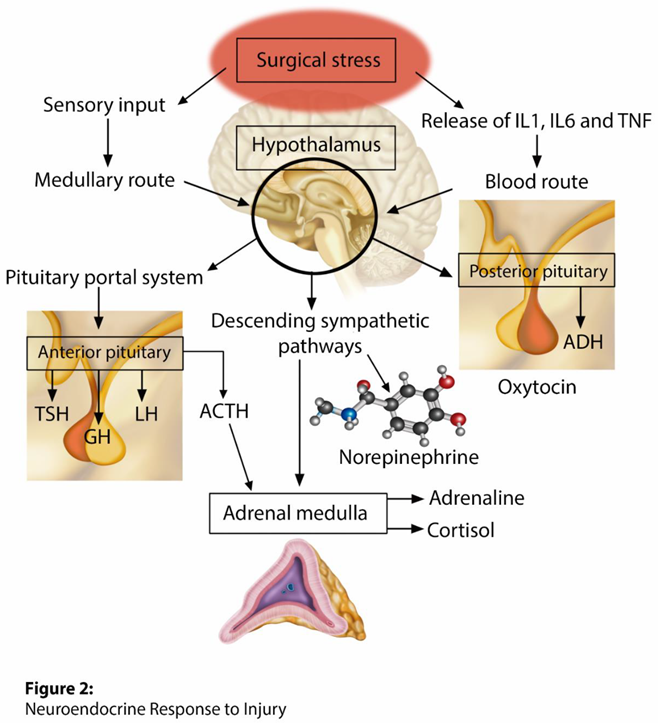
The parvocellular division of the hypothalamic paraventricular nucleus is the primary location of corticotrophin-releasing hormone (CRH) neurons and constitutes a key site for adaptive responses to stress. Sympathetic afferent stimuli arrive in the noradrenergic neurons of the brainstem located in the medulla oblongata (nucleus of the solitary tract - NTS), resulting in the activation and central regulation of the hypothalamic-pituitary-adrenal axis (Figure 2) [20]. CRH and arginine vasopressin are synthesized and released into the anterior pituitary gland by the pituitary portal vessels, which then promotes the synthesis of adrenocorticotropic hormone (ACTH). After release, ACTH binds to type 2 melanocortin receptors in the fasciculated zone of the adrenal cortex, triggering the synthesis and release of the primary stress hormones, glucocorticoids (cortisol). A neurological sympathetic response is initiated as an evolutionary form of survival, through a complex interaction between components of the central nervous system and peripheral systems, including the endocrine, immune, and cardiovascular systems [21].
Cortisol exerts its effects through its intranuclear, G-protein-coupled receptors, which are located in most organs, including the brain itself as part of negative feedback circuits. These receptors modulate and express numerous genes involved in mitochondrial metabolism, immune function, inflammation, growth, cognition, reproduction, and lung development. In the endothelium, for instance, the action of these receptors directly modulates endothelial physiology by regulating the expression of adhesion molecules (VCAM-1, ICAM-1, and E-selectin), the production of pro-inflammatory cytokines and chemokines. These include cytokines (IL-6, IL-17F, IL-8), vasodilators (nitric oxide) and vasoconstrictors (angiotensin II or endothelin-1), which are involved in maintaining endothelial morphology and reactivity [22].
The activation of the sympathetic nervous system (SNS) results in stimulation of glucagon and inhibition of insulin release. Secretion of the anabolic hormone insulin is reduced by the effect of the SNS on pancreatic alpha-2 adrenergic receptors, which subsequently leads to a decrease in insulin sensitivity in peripheral cells. The hormonal changes that ensue result in hyperglycemia and the release of fatty acids with unopposed catabolism of muscle tissue [23].
The activation of humoral systems, including the coagulation and complement systems, occurs early after injury. The severity of the trauma and its outcome appear to be associated with elevated levels of anaphylatoxins C3a, C4a and C5a. Acidic and hypoxic environments are known to activate the main complement factors, thus generating C3 and C5 activation products that can modulate intracellular mechanisms of lymphocytes. At lower concentrations, the metabolic effects of activated complement modulate CD4+ T cells in terms of glutamine utilization and increased T cell oxidative capacity. However, a higher concentration of complement results in cell death due to ATP depletion. Therefore, trauma alters the cellular responses of neutrophils, monocytes, and B cells, in addition to direct modulation of complement regulatory proteins in T cells and robust activation of C3a, C4a, and C5a [24].
Consequently, controlling the neuroendocrine and humoral response represents a pivotal strategy for regulating postoperative outcomes following trauma. Metabolic and hydroelectrolytic alterations resulting from the adrenergic response on the effector endocrine tissue can precipitate deleterious events in a susceptible organism. Therefore, multimodal anesthesia, which incorporates strategic drugs with specific mechanisms of action and regional blocks, is of paramount importance when this objective is pursued [25].
Modulation of the Inflammatory Response to Surgical Trauma—Therapeutic Interventions and Protective Strategies
The adrenergic tone on the immune system determines endocrine-metabolic changes and demonstrates the intercommunication between the neural, glandular effector, and immune systems. Therefore, there is a need to change the perspective of surgical injury treatment, which has previously been treated as pain, to a neuro-inflammatory perspective. Although immunosuppression in the perioperative period may increase the risk of infection, the anti-inflammatory effects of certain drugs may promote benefits in controlling SIRS and imply a favorable outcome in terms of the immediate postoperative period and early hospital discharge [26,27].
Anaesthetic Drugs
Dexmedetomidine
Dexmedetomidine exerts its effects on the locus ceruleus and spinal cord, inhibiting the pre-synaptic release of NE. This results in sedation, analgesia, and a central sympatholytic effect. It’s an excellent perioperative option as it produces beneficial effects in reducing the response to inflammatory and hemodynamic stress generated by surgical trauma, with minimal side effects that could be generated when other drugs are used (benzodiazepines and opioids) [2,3,27].
A single-blind randomized controlled clinical trial investigated the effect of dexmedetomidine on T helper 1 (Th1) and T helper 2 (Th2) cytokines and their ratio during and after laparoscopic cholecystectomy in ASA I and II patients. Th1 cells produce IFN-γ and favor cell-mediated immune responses. Th2 cells produce IL-4 and/or IL-10 and favor humoral immunity. Macrophages express alpha-2 adrenoreceptors on their surfaces, stimulating the production of IL-12, a potent inducer of Th1 cells. The study showed that intraoperative administration of dexmedetomidine attenuated the immune response induced by surgical stress by increasing the IFN-γ/IL-4 ratio (Th1/Th2 ratio).21 Another study also showed an increase in the Th17/Treg lymphocyte ratio, i.e. a decrease in the production of IL-4 and IL-10, which are Treg cell cytokines, and a shift in the balance towards Th17. Dexmedetomidine treatment also prevented an increase in CRP levels after surgery, indicating that the use of α-2 agonists can modulate the immune response during and after laparoscopy [28].
Studies have demonstrated the efficacy of dexmedetomidine in controlling postoperative pain. It is associated with improved quality of postoperative recovery and reduced opioid consumption in the immediate postoperative period. These factors make dexmedetomidine an attractive agent for enhanced recovery in surgery (ERAS) protocols and for patients with acute and chronic pain. Furthermore, it can be used in conjunction with other pharmaceutical agents to prolong the duration and the quality of analgesia in peripheral regional and neuroaxis blocks [29,30,31].
Ketamine
Ketamine exerts its effects at various levels of inflammation, including the recruitment of inflammatory cells, cytokine production and immunomodulation. Its analgesic properties are well-characterized and mainly attributed to non-competitive inhibition of glutamate and aspartate receptors. However, the pharmacological targets of ketamine are not limited to NMDARs. It has been demonstrated that ketamine interacts with other receptors and ion channels, including dopamine, serotonin, sigma, opioid and cholinergic receptors, as well as cyclic nucleotide gated (HCN) channels activated by hyperpolarization [32]. Patients with treatment-resistant depression and coexisting pain exhibited a higher antidepressant response and remission rate than patients without associated pain. In the aforementioned group, the levels of TNF-α and IL-6 on day 13 and IFN-γ, IL-10, IL-1β, IL-4, IL-6, TNF-α, and other chemokines on day 26 were found to be lower than at the commencement of the study. In the group without pain, the levels of TNF-α on day 13 and 26 were observed to be lower than at the commencement of the study. The observed changes were primarily in IL-6, which were associated with improved pain intensity and depressive symptoms following ketamine treatment [33]. In addition, studies on videolaparoscopic surgery have demonstrated that the intravenous administration of ketamine, administered preemptively and perioperatively, resulted in a reduction in pain scores immediately following surgery. Its analgesic effect is not sustained in the late postoperative period, and there is a positive impact on postoperative nausea and vomiting [34,35].
Opioids
There’s a reciprocal interaction between the immune system and endogenous/exogenous opioids. These substances are among the most potent analgesics in the treatment of severe pain, despite the risk of induced hyperalgesia, respiratory depression, nausea and suppression of the immune response, which has the potential to increase vulnerability to infections. Exogenous opioids, including morphine, fentanyl and sufentanil have been demonstrated to impair the function of macrophages, natural killer cells, and T cells, as well as to weaken the intestinal barrier in vitro. Epidemiological studies have also indicated that high doses and the initiation of opioid therapy for non-malignant pain are correlated with an increased risk of infectious diseases such as pneumonia [36].
It’s postulated that there are centrally mediated mechanisms, as evidenced by the observation that opioids that cross the blood-brain barrier (BBB) exert more immunomodulatory effects than those that do not. While the effects of opioids are largely attributed to decreased outflow from the central sympathetic nervous system, opioids can also cause direct sympathetic nerve activation, which can suppress the proliferation and function of some immune cell populations and lymphoid tissues. Opioids attenuate the circadian rhythm of ACTH and cortisol, leading to consistent increases in circulating levels of these hormones, which may be sufficient to produce immunosuppression. Thus, long-term exposure to short-acting opioids (MOP-r agonists such as heroin or fentanyl) results in pathophysiological changes in neuroimmune and neuroinflammatory functions, affected in part by peripheral mechanisms (e.g. cytokines) and neuroendocrine systems such as the hypothalamic-pituitary-adrenal (HPA) stress axis [37]. However, study has indicated that opioid-sparing anesthesia has a lower incidence of postoperative complications than opioid-based anesthetic techniques in video-assisted surgery [38].
Peripheral Regional and Neuraxial Blocks
The capacity of neuronal blockade to modify the response to surgical trauma has been extensively investigated in recent years. For instance, the blockade of the Quadratus Lumborum Anterior has been demonstrated to attenuate the production of the cytokine Interleukin-6 and decrease the release of cortisol, accompanied by a significant attenuation of the surgical repercussions on pulmonary function and a reduction in postoperative pain scores and opioid consumption in laparoscopic cholecystectomy [39]. The use of perioperative epidural analgesia is potentially associated with fewer postoperative surgical complications. A retrospective, observational cohort study of patients who underwent pancreaticoduodenectomy showed reduction in post-operative surgical complications, such as the use of analgesics, antiemetics, antipyretics, blood transfusions and parenteral nutrition [40]. Thus, proposed benefits of regional techniques include an earlier return of gut function, reduced incidence of pulmonary dysfunction and lower inflammatory response to surgery.
Ischemic and Pharmacologic Preconditioning
The literature describes protective methods that can be employed to reduce unfavorable clinical outcomes related to excessive inflammatory response and organ damage. These interventions prepare cells for upcoming damage and prevent the activation of inflammatory cells / release of inflammatory mediators. Remote ischemic preconditioning (RIP) confers protection against renal ischemia-reperfusion injury in patients undergoing laparoscopic partial nephrectomy. This protection was observed in both the early and late phases, with the latter being more prominent. Serum neutrophil gelatinase-associated lipocalin (NGAL) and serum cystatin C (CysC) were lower after limb ischemic preconditioning [41]. This organ protection measure in patients undergoing laparoscopic colorectal cancer resection decreased the incidence of postoperative gastrointestinal dysfunction , lowered the IL-6, TNF-α and I-FABP levels, demonstrating protective effect on patients’ postoperative gastrointestinal function [42].
A cohort of men undergoing robot-assisted laparoscopic prostatectomy for localized prostate cancer associated with pelvic inflammation exhibited elevated levels of the IL-6, STAT3 and IFN genes, indicating a potential role in STAT3 gene signaling. Autologous primary prostate cells or cancer cell lines were inhibited by silencing STAT3 and IL-6 signaling using fludarabine, a STAT3 inhibitor, and tocilizumab, an IL-6 inhibitor. The study indicated that the development of inhibitors of the STAT-IL6 pathway signaling could help to mitigate the effects of inflammation-induced carcinogenesis [43]. In kidney transplantation, ischemia and reperfusion injury is a significant concern, with the potential to negatively impact the outcome of the graft and the patient. Commonly used volatile anesthetics, such as sevoflurane and isoflurane, have been shown to interfere with many of the pathophysiological processes involved in the reperfusion injury cascade. This cascade is characterized by dysfunction of the mitochondrial respiratory chain and uncontrolled formation of reactive oxygen species during reperfusion, leading to the opening of mitochondrial permeability transition pores and the release of danger-associated molecular patterns (DAMPs) into the intra- and extracellular space. Inhalants prevent the opening of mitochondrial pores, have protective effects on the glycocalyx, confer upregulation of Hypoxia Inducible Factors facilitating cellular adaptation to low oxygen conditions, and generate a positive effect on circulating immune cells [44].
It’s crucial to highlight that the selection of anesthetic regimen plays a pivotal role in reducing the size of ischemic heart lesions through pharmacological preconditioning. A study demonstrated that propofol effectively negated the preconditioning effect of milrinone and levosimendan, whereas sevoflurane exhibited no such effect. However, under the influence of dexmedetomidine, the outcomes were inconclusive [45].
Endothelial Glycocalyx Protection
The maintenance of endothelial glycocalyx integrity has the potential to modulate systemic inflammation. The glycocalyx functions as a sensor of mechanotransduction, physical protection barrier, signal transduction, and a source of interaction between cellular products and pro-inflammatory mediators (Figure 3). It has been linked to several crucial physiological processes within the microcirculation, including blood coagulation, immunity, antioxidation, and interactions with serum proteins and sodium. In sepsis management, the approach to glycocalyx protection include colloid substitution, preferably albumin and fresh frozen plasma; catecholamine restriction; restrictive fluid therapy; corticosteroids; anticoagulants; glycemic control and vitamin C [46,47].

Study was conducted to investigate the association between immediate postoperative serum levels of syndecan-1, a representative marker of endothelial degradation, and severe postoperative morbidity and mortality in patients undergoing robot-assisted esophagectomy. A high level of syndecan-1 in the immediate postoperative period (≥48 ng/mL) was found to be associated with an increased risk of morbidity and mortality within 30 days of surgery. Patients with syndecan-1 levels ≥48 ng/mL exhibited a higher incidence of unexpected returns to the operating room and anastomotic leaks, as well as longer hospital and ICU stays than patients with syndecan-1 levels <48 ng/mL [48]. In addition to the modality and magnitude of the surgical trauma, acutely injured patients can develop secondary injury, mainly caused by continuous tissue trauma during surgical preparation, inflammatory reaction, hypovolemia due to hemorrhage and other causes. While some interventions have been identified as having the potential to protect the glycocalyx, such as plasma transfusion, human serum albumin, hydrocortisone, and sevoflurane, there is currently no specific treatment for the protection and recovery of this barrier in clinical medicine that can be used during the perioperative period. However, the literature highlights strategies to minimize its impairment in the surgical environment. These include performing damage control surgery to remove potential sources of sepsis, minimizing surgical time, restoring and maintaining hemodynamic stability, and avoiding water overload [49]. The current experimental and clinical evidence indicates a potential for clinical interventions that can modulate endothelial dysfunction, such as the administration of its structural components, including sphingosine-1-phosphate, hyaluronan, and sulodeoxide; and the combination of medium-long chain heparan sulfate and dermatan sulfate. However, there is a need for properly designed and robust clinical trials to support routine use in acutely compromised patients [50].
Biomarkers
The studies investigated potential blood biomarkers to determine their correlation with the extent of surgical injury, anesthetic intervention, administered drugs, and the inflammatory state. The review indicated that therapeutic approaches could affect immunomodulatory processes either indirectly, by regulating the activity of immune cells, or directly, by mitigating the effects of stress. The extent of this influence varies depending on the clinical or surgical situation under consideration. The main biomarkers used are listed in Table 1.
There’s a trend to use some biomarkers that are more sensitive to tissue injury as they alter the balance between pro and anti-inflammatory responses to a greater extent. Each marker presents an individual behavior with fluctuations over time.
Conclusion
Several sustainable therapeutic approaches, when combined, can significantly reduce inflammation in response to infection or surgery/trauma, potentially improving patient outcomes and decreasing healthcare-related costs. Protective approaches in medical treatment, such as dexmedetomidine, ketamine, opioid-sparing techniques, interventions for ischemic and pharmacologic preconditioning, strategies to maintain the integrity of the endothelial glycocalyx, peripheral and neuroaxis blocks represent therapeutic avenues in the capable hands of anesthesiologists and critical care physicians. Biofluid biomarkers have proven to be clinically valuable in both diagnosis and prognosis. Their systematic categorization serves as a roadmap for investigating their behavior following interventions in cases of injury, making them an indispensable tool. The effects of immunomodulating inflammation approaches are multifaceted. While perioperative immunosuppression can heighten the risk of tumor metastasis and infection, the anti-inflammatory effects of various drugs and strategies may still contribute positively to conditions linked with systemic inflammation. In the future, researchs on advanced gene expression analysis may eventually pinpoint population groups with increased inflammatory vulnerability in the perioperative context, leading to improved clinical outcomes.
Author Contributions
Conceptualization, G.N.S. and V.G.B.; methodology, R.K.A.F.; software, M.V.P.; validation, G.N.S., K.-U.L. and M.V.P.; formal analysis, G.N.S. and R.K.A.F.; investigation, G.N.S. and M.V.P.; resources, G.N.S. and V.G.B.; data curation, M.V.P.; writing—original draft preparation, G.N.S., R.K.A.F. and K.-U.L.; writing—review and editing, G.N.S., K.-U.L. and V.G.B.; visualization, V.G.B.; supervision, R.K.A.F. and K.-U.L.; project administration, G.N.S. and V.G.B.; funding acquisition, G.N.S., V.G.B. and R.K.A.F. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors have no conflicts of interest regarding this study.
References
- Cao AM, Eslick GD. Epidemiology and pathogenesis of gallstones. In The Management of Gallstone Disease: A Practical and Evidence-Based Approach, p. 53-66, 2018.
- Silva GN, Brandão VG, Perez MV, Lewandrowski K-U, Fiorelli RKA. Effects of Dexmedetomidine on Immunomodulation and Pain Control in Videolaparoscopic Cholecystectomies: A Randomized, Two-Arm, Double-Blinded, Placebo-Controlled Trial. J Pers Med. 2023, 13, 622. [Google Scholar] [CrossRef]
- Silva GN, Brandão VG, Fiorelli R, et al. Outcomes of dexmedetomidine as adjuvant drug in patients undergoing videolaparoscopic cholecystectomy: A randomized and prospective clinical trial. Int J Immunopathol Pharmacol. 2023, 37, 03946320231196977. [CrossRef]
- Brandão VGA, Silva GN, Perez MV, Lewandrowski K-U, Fiorelli RKA. Effect of Quadratus Lumborum Block on Pain and Stress Response after Video Laparoscopic Surgeries: A Randomized Clinical Trial. J Pers Med. 2023, 13, 586. [Google Scholar] [CrossRef]
- Dobson, GP. Trauma of major surgery: a global problem that is not going away. Int J Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef]
- Burford NG, Webster NA, Cruz-Topete D. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int J Molec Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Silva GN, Brandão VG, Perez MV, et al. Immunotherapeutic Properties of Dexmedetomidine on Pain Management and Cardiovascular Function in Videolaparoscopic Cholecystectomies: a randomized, two-arm, double-blinded, placebo-controlled trial. Surg Innov. 2024, 31, 137–147. [Google Scholar] [CrossRef]
- Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surg. 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Floras T, Phillippou A, Bardakostas D, Mantas D, Koutsilieris M. The growth endocrine axis and inflammatory responses after laparoscopic cholecystectomy. Horm. 2016, 15, 73–80. [Google Scholar] [CrossRef]
- Page AJ, Ejaz A, Spolverato G, Zavadsky T, Grant MC, Galante DJ, Wick EC, Weiss M, Makary MA, Wu CL, Pawlik TM. Enhanced recovery after surgery protocols for open hepatectomy--physiology, immunomodulation, and implementation. J Gastrointest Surg. 2015, 19, 387–399. [Google Scholar] [CrossRef]
- Curry N, Brohi K. Surgery in Traumatic Injury and Perioperative Considerations. Semin Thromb Hemost. 2020, 46, 73–82. [Google Scholar] [CrossRef]
- Hotamisligil GS, Davis RJ. Cell Signaling and Stress Responses. Cold Spring Harb Perspect Biol. 2016, 8, a006072. [Google Scholar] [CrossRef]
- Thurairajah K, Briggs GD, Balogh ZJ. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg. 2018, 44, 325–334. [Google Scholar] [CrossRef]
- Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Op Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef]
- Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science, 6566. [CrossRef]
- Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nature Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Faisal M, Schäfer CN, Myrelid P, Winberg ME, Söderholm JD, Keita ÅV, Eintrei C. Effects of analgesic and surgical modality on immune response in colorectal cancer surgery. Surg Oncol. 2021, 38, 101602. [Google Scholar] [CrossRef]
- Steinberg BE, Sundman E, Terrando N, Eriksson LI, Olofsson PS. Neural Control of Inflammation: Implications for Perioperative and Critical Care. Anesthesiol. 2016, 124, 1174–1189. [Google Scholar] [CrossRef]
- Barman, SM. 2019 Ludwig Lecture: Rhythms in sympathetic nerve activity are a key to understanding neural control of the cardiovascular system. Am J Physiol Regul Integr Comp Physiol. 2020, 318, R191–R205. [Google Scholar] [CrossRef]
- Burford NG, Webster NA, Cruz-Topete D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int J Mol Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Zielińska KA, Van Moortel L, Opdenakker G, De Bosscher K, Van den Steen PE. Endothelial Response to Glucocorticoids in Inflammatory Diseases. Front Immunol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Cusack B, Buggy DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef]
- Chakraborty S, Karasu E, Huber-Lang M. Complement After Trauma: Suturing Innate and Adaptive Immunity. Front Immunol, 2018; 9, 2050. [CrossRef]
- Cruz FF, Rocco PRM, Pelosi P. Anti-inflammatory properties of anesthetic agents. Critical Care, 2050; 21, 1–7. [CrossRef]
- Kaye, A.D. , Chernobylsky, D.J., Thakur, P. et al. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) Protocols for Postoperative Pain. Curr Pain Headache Rep. 2020, 24, 21. [Google Scholar] [CrossRef]
- Silva GN, Brandão VG, Perez MV, et al. Immunotherapeutic Properties of Dexmedetomidine on Pain Management and Cardiovascular Function in Videolaparoscopic Cholecystectomies: A Randomized, Two-Arm, Double-Blinded, Placebo-Controlled Trial. Surg Innov. 2024, 31, 137–147. [Google Scholar] [CrossRef]
- Lee JM, Han HJ, Choi WK, Yoo S, Baek S, Lee J. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: a prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017, 118, 167–181. [Google Scholar] [CrossRef]
- Modir H, Yazdi B, Piri M, Almasi-Hashiani A. An investigation of the effects of dexmedetomidine and fentanyl as an adjuvant to ropivacaine on pain scores and hemodynamic changes following laparoscopic cholecystectomy. Med Gas Res. 2021, 11, 88–93. [Google Scholar] [CrossRef]
- Sen IM, Prashanth K, Bhatia N, Goel N, Kaman L. Paravertebral block using levobupivacaine or dexmedetomidine-levobupivacaine for analgesia after cholecystectomy: a randomized double-blind trial. Braz J Anesthesiol. 2021, 71, 358–366. [Google Scholar] [CrossRef]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Zhou Y, Wang C, Lan X, Li H, Chao Z, Ning Y. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: improvement by ketamine. J Neuroinflammation. 2021, 18, 200. [Google Scholar] [CrossRef]
- Hung KC, Wu SC, Chang PC, Chen IW, Hsing CH, Lin CM, Chen JY, Chu CC, Sun CK. Impact of Intraoperative Ketamine on Postoperative Analgesic Requirement Following Bariatric Surgery: a Meta-analysis of Randomized Controlled Trials. Obes Surg. 2021, 31, 5446–5457. [Google Scholar] [CrossRef]
- Jain S, Nazir N, Mustafi SM. Preemptive low-dose intravenous ketamine in the management of acute and chronic postoperative pain following laparoscopic cholecystectomy: a prospective randomized control study. Med Gas Res. 2022, 12, 141–145. [Google Scholar] [CrossRef]
- Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Butelman ER, Goldstein RZ, Nwaneshiudu CA, Girdhar K, Roussos P, Russo SJ, Alia-Klein N. Neuroimmune Mechanisms of Opioid Use Disorder and Recovery: Translatability to Human Studies, and Future Research Directions. Neuroscience. 2023, 528, 102–116. [Google Scholar] [CrossRef]
- Jiang YY, Li ZP, Yao M, Zhou QH. Standard opioid-containing versus opioid-sparing anesthesia on early postoperative recovery after video-assisted thoracic surgery: A propensity-weighted analysis. Front Surg. 2022, 9, 1015467. [Google Scholar] [CrossRef]
- Brandão VGA, Silva GN, Alvim Fiorelli RK, Perez MV. Outcome of Ultrasound Guided Anterior Quadratus Lumborum Block After Video Laparoscopic Cholecystectomies: A Prospective Randomized Clinical Trial. Surg Innov. 2023, 30, 283–296. [Google Scholar] [CrossRef]
- Negrini D, Ihsan M, Freitas K, Pollazzon C, Graaf J, Andre J, Linhares T, Brandao V, Silva G, Fiorelli R, Barone P. The clinical impact of the perioperative epidural anesthesia on surgical outcomes after pancreaticoduodenectomy: A retrospective cohort study. Surg Open Sci. 2022, 10, 91–96. [Google Scholar] [CrossRef]
- Hou YY, Li Y, He SF, Song J, Yu DX, Wong GTC, Zhang Y. Effects of differential-phase remote ischemic preconditioning intervention in laparoscopic partial nephrectomy: A single blinded, randomized controlled trial in a parallel group design. J Clin Anesth. 2017, 41, 21–28. [Google Scholar] [CrossRef]
- Yi M, Wu Y, Li M, Zhang T, Chen Y. Effect of remote ischemic preconditioning on postoperative gastrointestinal function in patients undergoing laparoscopic colorectal cancer resection. Int J Colorectal Dis. 2023, 38, 68. [Google Scholar] [CrossRef]
- Chakravarty D, Ratnani P, Huang L, Dovey Z, Sobotka S, Berryhill R, Merisaari H, Al Shaarani M, Rai R, Jambor I, Yadav KK, Mittan S, Parekh S, Kodysh J, Wagaskar V, Brody R, Cordon-Cardo C, Rykunov D, Reva B, Davicioni E, Wiklund P, Bhardwaj N, Nair SS, Tewari AK. Association between Incidental Pelvic Inflammation and Aggressive Prostate Cancer. Cancers (Basel). 2022, 14, 2734. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke GJ, Bosch DJ, Leuvenink HGD. Molecular Aspects of Volatile Anesthetic-Induced Organ Protection and Its Potential in Kidney Transplantation. Int J Mol Sci. 2021, 22, 2727. [Google Scholar] [CrossRef]
- Bunte S, Lill T, Falk M, Stroethoff M, Raupach A, Mathes A, Heinen A, Hollmann MW, Huhn R. Impact of Anesthetics on Cardioprotection Induced by Pharmacological Preconditioning. J Clin Med. 2019, 8, 396. [Google Scholar] [CrossRef]
- Astapenko D, Benes J, Pouska J, Lehmann C, Islam S, Cerny V. Endothelial glycocalyx in acute care surgery - what anaesthesiologists need to know for clinical practice. BMC Anesthesiol. 2019, 19, 238. [Google Scholar] [CrossRef]
- Iba T, Maier CL, Helms J, Ferrer R, Thachil J, Levy JH. Managing sepsis and septic shock in an endothelial glycocalyx-friendly way: from the viewpoint of surviving sepsis campaign guidelines. Ann Intensive Care. 2024, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim HJ, Choi YS, Park BJ, Shin HJ, Jeon SY, Kim DJ, Kim SY. Immediate Postoperative High Syndecan-1 is Associated with Short-Term Morbidity and Mortality After Robot-Assisted Esophagectomy: A Prospective Observational Study. Ann Surg Oncol. 2023, 30, 5870–5880. [Google Scholar] [CrossRef] [PubMed]
- Astapenko D, Benes J, Pouska J, Lehmann C, Islam S, Cerny V. Endothelial glycocalyx in acute care surgery - what anaesthesiologists need to know for clinical practice. BMC Anesthesiol. 2019, 19, 238. [Google Scholar] [CrossRef]
- Mathis S, Putzer G, Schneeberger S, Martini J. The Endothelial Glycocalyx and Organ Preservation-From Physiology to Possible Clinical Implications for Solid Organ Transplantation. Int J Mol Sci. 2021, 22, 4019. [Google Scholar] [CrossRef]
Table 1.
Biomarkers and main characteristics.
| INFLAMMATORY MARKERS | CHARACTERISTICS OF ITS SYSTEMIC EFFECTS |
|---|---|
| TNF-α |
|
| IL1-β |
|
| IL-2 |
|
| IL-6 |
|
| IL-10 |
|
| IFN-γ |
|
| CRP |
|
| Cortisol |
|
| TP/ TTP/ TAPT |
|
| Adrenaline/Norepinephrine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Serum Biomarkers of Inflammation in the Identification and Prognostication of Frail Patients Undergoing Emergency Laparotomy
Michael George
et al.
,
2023
Can Peri-Surgical Electroacupuncture Relieve Immunity Suppression? A Pilot Study in Dogs
Vanessa Rabbogliatti
et al.
,
2023
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated










