Preprint
Review
The Use of Heterocyclic Azo Dyes on Different Textile Materials: A Review
Altmetrics
Downloads
101
Views
44
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
02 July 2024
Posted:
03 July 2024
You are already at the latest version
Alerts
Abstract
The art of dyeing textiles has a long history, as natural dyes have been used since prehis-toric times. With the development of synthetic dyes in the 19th century, the focus shifted from natural to synthetic dyes due to their superior properties. Recently, however, interest in natural dyes has increased again due to environmental and health concerns. Among industrial dyes, heterocyclic dyes, especially azo dyes, are of great importance due to their color brilliance and fastness. This review examines the synthesis, application and analysis of azo dyes, especially heterocyclic dyes. It deals with monoazo, diazo and polyazo dyes and highlights their structures, synthesis methods and fastness properties. In addition, the ecological impact of azo dyes and practical solutions for their synthesis and application are discussed.
Keywords:
Subject: Chemistry and Materials Science - Organic Chemistry
1. Introduction
The art of textile dyeing is an activity as old as mankind, as colored textiles have been found in archeological excavations in the oldest civilizations. However, it should be emphasized that heterocyclic dyes have also been in use since prehistoric times. At that time, only natural dyes obtained from natural resources of plant, animal, mineral and microbial origin were used [1]; many of them were in fact natural heterocyclic dyes such as indigo (obtained from the leaves of Indigofera tinctorial) and Tyrian purple (obtained from the shells of mollusks of the Muricidae family), which were used to dye textiles in blue and purple, respectively (Figure 1).
Since the second half of the 19th century, more precisely since the invention of synthetic dyes, the use of natural dyes has been almost completely replaced by synthetic dyes due to their ease of use and better fastness properties, although there has been a return to the use of natural dyes in recent decades, not only because they are environmentally friendly, but also due to the fact that many artificial textile dyes are hazardous to human health [2]. Nevertheless, the search for synthetic dyes is still very active, as the requirements of customers around the world are changing rapidly. In the field of industrial dyes, heterocyclic dyes have been increasingly used recently as they have higher color brilliance and strength as well as better color fastness compared to benzene analogous dyes. Heterocyclic dyes can be divided into different technical sections according to the type of electronic character of the dye molecule [3]. This overview reports on the most important group among them: the azo dyes. In particular, the synthesis, the applications on different textile materials and the analyzes carried out to verify the fastness properties are reported.
2. The Azo Dyes
Azo dyes are characterized by the functional group -N=N-, which connects two symmetrical or asymmetrical radicals, of which at least one, but usually both, are aromatic [4].
They are usually synthesized in a two-step reaction: synthesis of an aromatic diazonium ion from an aniline derivative (diazotization) and coupling of the diazonium salt with an aromatic compound (Figure 2) [5], but also by reduction of nitro aromatic compounds in an alkaline medium, oxidation of aromatic amine with various oxidizing agents such as lead tetraacetate, permanganate and haloxy acid [6].
If both radicals are aromatic radicals, the dyes are referred to as carbocyclic, whereas if they contain one or more heterocyclic radicals, the dyes are referred to as heterocyclic azo dyes.
All azo compounds are classified by Griffiths [7] as donor-acceptor chromogens (D-π-A) (Figure 2), and studies have shown that replacing the benzene ring of the acceptor with a less aromatic heterocycle in these chromogens leads to a significant bathochromic shift (red shift) of the visible absorption band, which results in greater color brilliance and higher color intensity, especially in synthetic materials [8].
Figure 3.
Scheme of D-π-A azo compounds.

Due to the structure of azo compounds they exhibit photochromic properties.
Photochromism is a phenomenon that occurs when two molecules are in chemical equilibrium with each other (simple two-way reaction). The two isomers have different absorption spectra and can therefore be distinguished. In general, a stable isomer A can transform into a less stable, higher energy isomer B when excited by light. The latter can spontaneously transform into the former by a thermal reaction overcoming an energy barrier ΔE. While the photochemical reaction A→B is usually very fast, the reverse (thermal) reaction B→A can be fast or slow depending on the system. In some cases, however, the photoproduct B is kinetically inert and the process can only be reversed with the aid of irradiance (hν’) (Figure 4), available via license: Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International). It follows that photochromic systems can be divided into two classes: thermally reversible photochromes (T-type) and photochemically reversible photochromes (P-type) [9].
The case of azobenzene isomerization has been widely studied and analyzed. In general, they belong to the T-type class and the azobenzene in the E configuration is more stable than that in the Z form. The two stereoisomeric forms have marked difference in the structure and properties (Figure 10a, available via license: CC BY-NC-ND 4.0), but also different UV–visible absorption spectra: the spectral position of the bands not only indicates the wavelength of light needed to induce the reaction but also the color of the molecule itself. The spectra of E- and Z-azobenzene in acetonitrile at room temperatures are reported in Figure 10b (available via license: CC BY-NC-ND 4.0) [9].
Changes in the absorption spectra between the two forms are generally not very pronounced due to a small difference in electron delocalization between the two isomers, and the color change is invisible by the naked eye. In contrast, the photochromic reaction induces very significant change of the free volume [10] but also density, viscosity, electrical conductivity, stiffness, and porosity of the molecule [ 9].
As early as 1990, Gregory reported that heterocyclic azo dyes are able to “combine the brightness and high fastness properties of anthraquinone dyes with the strength and economy of azo dyes” [11]. It is therefore not surprising that numerous attempts have since been made to synthesize ever more efficient heterocyclic azo dyes.
Figure 6.
a) The E and Z isomers of azobenzene and their photo-and thermally induced interconversion. b) UV-visible absorption spectra of E- (full line) and Z-azobenzene (dashed line) in acetonitrile at room temperature [9].
Figure 6.
a) The E and Z isomers of azobenzene and their photo-and thermally induced interconversion. b) UV-visible absorption spectra of E- (full line) and Z-azobenzene (dashed line) in acetonitrile at room temperature [9].

3. Heterocyclic Azo Dyes
Heterocyclic azo dyes, like all azo dyes, can be divided into three categories according to the number of azo groups in the same molecule as monoazo, diazo and polyazo dyes.
3.1. Heterocyclic Monoazo Dyes
In 1999, Towns gave an overview of the progress made with heterocyclic diazo components over the past decade [12]. This paper reports on the applications to textiles of heteroazo dyes consisting of five-membered rings containing a sulfur heteroatom to which a diazotizable amino group is directly attached. These compounds are divided into four groups based on the heteroaromatic amine used for diazotization: Thiazoles 1 and Benzothiazoles 2, Isothiazoles 3 and Benzisothiazoles 4, Thiadiazoles 5a-5b and Thiophenes 6 (Figure 7).
Towns reported that most of the numerous patents filed in the last ten years have mostly been the result of modifications and improvements to existing products, with no real innovation in molecular structures. He also said that the search for brighter and more economical dyes was still ongoing and predicted that disperse dyes from heterocyclic components would be explored in the coming years.
A few years later, the synthesis and application of novel heterocyclic dyes based on a new fused heterocyclic compound, ll-amino-3-bromo-13h-acenaphtho[l,2-e]pyridazino[3,2-b]-quinazoli-ne-13-one, was reported [13]. A series of new azo dyes (7a-k) were synthesized by diazotization of this heterocyclic amino compound and coupling with various naphthols (a-k), leading to the general structure shown in Figure 8.
Specifically, these dyes were synthesized by diazotization with sodium nitrite and hydrochloric acid, and coupling was carried out in a moderately alkaline medium at 0-5 °C. After characterization, the dyes were applied as azo dyes to nylon 66 and polyester fibers, resulting in a variety of shades ranging from pink to red and brown. The differences in the shades of the dyed fibers are due to the type and position of the substituent.
The analysis showed that the dyes on both substrates had fairly good to good light fastness and very good to excellent fastness to washing, rubbing, perspiration and sublimation, while the percentage exhaustion of the dye bath was better on nylon 66 than on polyester, probably due to the better accessibility of the open structure.
In 2010, the same authors reported the synthesis, characterization and application of another series of heterocyclic mono-azo reactive dyes (8a-g), which were synthesized by coupling diazotized 2-phenyl-3{4’-[(4”-aminophenyl)sulfonyl]phenyl}-quinazolin-4(3H)-one-6-sulfonic acid with various 2-chloro-4-nitro-anilino-cyanurate coupling components [14], with the general structure shown in Figure 9.
The 2-chloro-4-nitro anilino cyanurated coupling components used are: H-acid (a), Gamma acid (b), J-acid (c), N-methyl-J-acid (d), N-phenyl-J-acid (e), Chicago acid (f), and Laurant acid (g).
When applied to silk, wool and cotton fabrics, the dyes obtained cover almost the entire visible range and generally produce yellow to violet shades. The differences in the color shades of the dyed fabric result from the change in the coupling components.
All dyes (8a-g) generally show moderate to good light fastness properties, while the fastness to washing and rubbing is very good to excellent. The presence of a quinazolinone structure in all dyes results in high color strength, as the quinazolinone structure exhibits intrinsic conjugation leading to excellent color strength. The heteroatoms in the dye structure lead to bathochromaticity and brightness of shades. The exhaustion and fixation properties are good for all dyes, although the introduction of a triazine group improves both properties.
The same authors have published many articles on this type of research, in which they have tested not only the fastness property but also the antimicrobial activity against bacteria and fungi [15,16], which is a new trend in this field, as people's lives have improved and consumers are aware of a hygienic lifestyle and ask for textile products that do not cause toxicity, allergy or irritation to the users [17-19].
For example, a recent paper reported on the synthesis of new antibacterial dyes for fabric printing [20]. In this work [21], three new azo dyes (9a-c) with sulfonamide chromophores were used to dye polyester fibers via azo coupling with nucleophilic precursors (Figure 10).
The analytical results showed that the fastness properties and color evaluation of the printed samples exhibited moderate to excellent results in washing, rubbing, perspiration, sublimation fastness and light fastness. Antibacterial activity was tested against four bacterial species (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Bacillus subtilis) for the new dyes, but also for the printed fabrics after washing, using ampicillin as a reference, which is normally considered a standard antibacterial agent [22]. The diameter of the inhibition zone (mm/mg sample) as a measure of antibacterial activities was measured for all samples and the results are shown in Table 1 and Table 2. From the results, it can be seen that the dyes 9a and 9b containing sulfate groups showed higher antibacterial activity against all types of bacteria. On the other hand, the dyed polyester fabrics showed moderate antibacterial activity, which in this case was also higher in the samples printed with 9a and 9b, showing that the antibacterial properties of the dyes were transferred to the corresponding dyed textile samples.
Figure 10.
The general structure of dyes 9a-c.

These inexpensive, easy-to-synthesize biologically active dyes could be used to sterilize textiles for future applications.
3.2. Heterocyclic Diazo Dyes
Diazo dyes, which contain two groups –N=N-, are divided into three groups depending on the synthesis method used for their production [23]:
Primary diazo dyes
This type of dyes is synthesized by a coupling reaction of two moles of diazoic acid with the same coupling term. An example, a brown dye used for dyeing wool, is shown in Figure 11a).
Secondary symmetrical diazo dyes
This type of dye is derived from a diamine that is diazotized twice and copulated with the same or different terms. An example, a blue direct dye with a benzidine function, is shown in Figure 11b).
Secondary asymmetrical diazo dyes
This type of dye is synthesized by the coupling of an amino azoic acid with a phenolic coupler. Figure 11c) shows an example, an orange direct dye.
Although a patent [24] for the use of heterocyclic diazo dyes was granted as early as 1954, only a few studies [25-26] were conducted in the literature in comparison with the monoazo compounds untill 2005. In that year, Karci reported the synthesis of ten novel disazo dyes derived from various heterocyclic coupling components and aminomethylphenylazo pyrazoles [27]. The entire synthesis process begins with the preparation of nitrile 10, then pyrazoles 11a-b, which are finally used for the synthesis of disazo dyes 12 a-j (Figure 12).
All disazo compounds 12a-j may exhibit keto–enol tautomerism due to the functional groups highlighted in red in Figure 12. The tautomeric forms for compounds 12a-b are showen in Figure 13 as an example. In the particular case of dyes, tautomerism is important not only because the two tautomeric forms can have different color, but also different tinctorial strengths and properties [28,29,30]. From the data of the infrared spectra of compounds 12a-j it appears that some of them are predominantly in the hydrazone–keto form in the solid state, while other in the azo–enol form.
Instead, the 1H NMR spectra in dimethyl sulfoxide (DMSO) show that compounds 12a-d are present as a mixture of tautomeric forms, whereas in 12e-j the single tautomeric form predominates.
The effect of solvent and acid or base on the wavelength of the absorption maximum (λmax) was also investigated. It was found that the absorption spectra of dyes 12i-j show a maximum at a longer wavelength than those of dyes 12a-h in all solvents used. In addition, a bathochromic shift was observed for dyes 12c and 12e compared to the analogous dyes 12d and 12f in all solvents tested, while the other analogs showed very similar λmax, but only in DMSO and dimethylformamide (DMF). These results led to conclude that for the dyes 12a, 12c, 12e, 12g and 12i there could be a tautomeric equilibrium between the pyrazole ring and the phenylazo group at 1-H (Figure 14).
In conclusion, the authors report that all dyes 12a-j can be applied to polyester and/or polyamide fibers as disperse dyes.
Another important aspect studied over the years is the color yields obtainable by modifying the position of the substituents on the heteroatomic rings. In particular, for example, in a 2016 article a study on 6 novel pyrazole diazo disperse dyes (13a-c, 14a-c, Figure 15), substituted with methyl (-CH3) group at their o-, m-, p-position, is reported [31]. Their synthesis carried out according to the literature procedures [27, 32-33] (Figure 12 and Figure 15) and the studies of their application to 3 different synthetic fibers, at certain dyeing conditions are described.
All synthesized dyes were characterized by elemental analysis and spectral methods and then tested on different fibers. The fibers used for the evaluation were: Polylactic acid (PLA), polyethylene terephthalate (PET) and polyamide 6.6 (PA6.6) fibers.
The colorimetric properties, yield and fastness parameters of all fiber samples dyed with the novel synthesized disperse dyes were described and compared. Yellow and orange shades were obtained on PLA, PET and PA 6.6 fibers by applying 2% of all dyes. With the dyes 13a-c (K/S >10) predominantly darker shades were achieved and with the dyes 14a-c (K/S <10) medium-dark shades. In addition, due to the different molecular weights, the exhaustion and color yield of 13a-c dyes (lower molecular weight) are higher than those of 14a-c dyes. Differences between the dyes resulting from the position of the methyl group were also observed: The color yield of all tissues dyed with 13b and 14b dyes (meta-methyl auxochrome) had the darkest hue compared to the other dyes (ortho- and para-methyl auxochrome). The darkest shade was observed in PLA tissue dyed with 14a (p-CH3). The yield of all dyes was over 76 %, with the exception of the PET fiber fabric dyed with 14c, where the yield was 67 %.
The colorimetric properties, yield and fastness parameters of all fiber samples dyed with the novel synthesized disperse dyes were described and compared. Yellow and orange shades were obtained on PLA, PET and PA 6.6 fibers by applying 2% of all dyes. With the dyes 13a-c (K/S >10) predominantly darker shades were achieved and with the dyes 14a-c (K/S <10) medium-dark shades. In addition, due to the different molecular weights, the exhaustion and color yield of 13a-c dyes (lower molecular weight) are higher than those of 14a-c dyes. Differences between the dyes resulting from the position of the methyl group were also observed: The color yield of all tissues dyed with 13b and 14b dyes (meta-methyl auxochrome) had the darkest hue compared to the other dyes (ortho- and para-methyl auxochrome). The darkest shade was observed in PLA tissue dyed with 14a (p-CH3). The yield of all dyes was over 76 %, with the exception of the PET fiber fabric dyed with 14c, where the yield was 67 %.
The fastness values (wash fastness, acid and alkaline perspiration fastness, wet and dry rubbing fastness, sublimation fastness, light fastness, water fastness and seawater fastness) were in the commercially acceptable range, which means that the dyeing of PLA, PET and PA 6.6 with the dyes 13a-c and 14a-c is suitable for the textile industry.
3.3. Heterocyclic Polyazo Dyes
Polyazo dyes are complex dyes characterized by the fact that the azo group is repeated three or more times in the same molecule. They are intended for dyeing leather in the colors red, brown and dark black [34]. The presence of multiple -N=N groups increases conjugation and enhances the delocalization of electrons. They also appear to be more stable, as the more azo groups present in the dye, the less likely it is to degrade; in general, monoazo or diazo dyes are more degradable than polyazo dyes [34].
There are few studies in the literature on the use of heterocyclic polyazo dyes in general, but especially with regard to their use for dyeing textiles.
In 2022, a paper was published on the synthesis and characterization of a novel heterocyclic polyazo dye, but the authors refer to its application as a universal acid-base indicator [35]. The authors also reported that the polyazo dyes can find numerous practical applications, but there is no mention of their use as dyes for tissues [36-39].
In any case, an article published in 2011 [40] reports on the synthesis of various natural trisazo dyes. Arylazopyridone dyes have been intensively synthesized as disperse dyes since the seventies of the 20th century. One of them, the trisazo pyridone dye shown in Figure 16, is used to dye polyamide materials such as nylon 6.
4. Ecological Impacts and Sustainable Solutions for Heterocyclic Azo Dyes
Approximately 70 % of the dyes used in the textile industry are azo dyes. During the dyeing process, 15-20% of these dyes are not absorbed and end up in wastewater, causing environmental pollution due to their toxicity. The industry is trying to mitigate these effects by improving dye binding or promoting degradation through biological or physico-chemical methods [41]. Wastewater also contains toxic substances such as heavy metals, which pose health risks such as cancer and chronic diseases [42] and damage ecosystems by interfering with aquatic life and plant growth [43]. Conventional treatment methods (filtration, adsorption, coagulation/flocculation) [44] have disadvantages such as secondary sludge formation, limited effectiveness and high costs [41]. Therefore, environmentally friendly approaches and methods are being researched not only for azo dyes in general, but also for those containing heterocycles.
In an article published in 2021 [45], for example, the authors report on an environmentally friendly approach for the synthesis of an azocoumarin dye using cation exchange resins. The azo dye with coumarin moieties 17 was prepared by diazotization of 4-nitroaniline 15 and subsequent coupling of the resulting diazonium salt with coumarin 16 (Figure 17). Amberlyst-15 was used as the acidic catalyst for the diazotization (Figure 18). Amberlyst-15, which consists of brown-gray granules, is a reticulated polystyrene-based ion exchange resin with strong acidic sulfonic groups. It is an effective heterogeneous acid catalyst for reactions in acidic media. It is safe to use and can be easily separated from the reaction mixture after completion of the reaction. Amberlyst-15 is also environmentally friendly as it can be renewed and reused up to four times.
The described synthesis has shown that it is an effective method for organic synthesis and that it could be suitable and economical for industrial and applied fields, possibly including textile dyeing as the application of some coumarin based azo dyes on polyester fibers [46] is already known.
At the same time, researches are being developed aimed at removing azo dyes from the environment as wastewater discharged from factories. The method using activated carbons has been widely studied [47]. However, commercially available activated carbon is not cost effective as it is produced from non-renewable sources such as coal and petroleum. Therefore, efforts are being made to use environmentally friendly, renewable and, most importantly, cost-effective feedstocks for the production of activated carbon. Biomass precursors have been introduced as a highly effective alternative. The performance of activated carbon in adsorbing azo dyes depends on several factors, including the type of biomass, activation agent, pH, activation temperature, contact time, initial dye concentration, and dosage of adsorbent. Future research could focus on optimizing the absorptive capacity of activated carbon for specific types of azo dyes [48].
Another group of researchers developed an economically viable bioprocess to convert various azo dyes into valuable compounds found in dye-containing wastewaters from textile industries, by utilizing the complementary catalytic properties of azoreductases (which reduce azo dyes to aromatic amines) and laccases (which facilitate oxidative coupling into valuable aromatic compounds and precursors of biologically active molecules). This process, using free and immobilized E. coli cells containing these enzymes, resulted in less contaminated reaction mixtures and final product yields of up to 90%. These optimized biocatalytic systems provide a sustainable and promising method for cleaning dye-containing wastewaters while producing valuable chemicals for various chemical industry applications [49].
Another alternative method recently reported consists in the biosorption of some azo dyes using activated biochar sewage sludge adsorbent, demonstrating high absorption percentages of the tested colors [50].
5. Conclusions
The field of heterocyclic azo dyes continues to be dynamic, driven by the need for brighter, more durable and environmentally friendly dyes. The synthesis and application of heterocyclic azo dyes, especially in textiles, offers significant advantages in terms of color brilliance, fastness and versatility. The development of novel dyes, including those with antimicrobial properties, is evidence of the constant innovation in this field. However, the impact of azo dyes on the environment, particularly in terms of wastewater pollution, remains a critical issue. Innovative and sustainable methods such as enzyme-based biocatalysis and biosorption using renewable materials are promising approaches to mitigate these effects. Future research should continue to focus on improving the environmental footprint of dye synthesis and application to ensure that advances in dye technology serve both industry needs and environmental sustainability.
References
- Saxena, S.; Raja, A.S.M. Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. In: Muthu, S. (eds) Roadmap to Sustainable Textiles and Clothing. Textile Science and Clothing Technology. Springer, Singapore, 2014, pp. 37–80. [CrossRef]
- Patel, B. H. Natural Dyes. In Handbook of textile and industrial dyeing: Principles, processes and types of dyes, Dr. M. Clark, Woodhead Publishing Ltd,Cambridge, UK, 2011, 1, pp. 395 – 421.
- Waring, D. R. Heterocyclic Dyes and Pigments. Comprehensive Heterocyclic Chemistry, 1984, 1– 7(1), pp. 317–346.
- Winkler, F. The colour science of dyes and pigments, Adam Hilger Ltd, Bristol (England), 1983.
- Decelles, C. The story of dyes and dyeing, J. Chem. Educ., 1949, 26, p. 583.
- Salman, M.; Jabbar, A.; Farooq, S.; Bashir, I.; Rafiq, M. S. New heterocyclic azo-disperse dyes; their synthesis, characterization, application, photo physical properties and solvatochromic studies J. Mol. Struct., 2023, 1287, pp. 135664-135664.
- Griffiths, J. Color and Constitution of Organic Molecules, Academic Press, London, 1976.
- Hallas, G., Choi, J.-H. Synthesis and properties of novel aziridinyl azo dyes from 2-aminothiophenes—Part 2: Application of some disperse dyes to polyester fibres. Dyes Pigm., 40, 1999, 2-3, pp.119–129.
- Baroncini, M.; Groppi, J.; S. Corra, S.; Silvi, S.; Credi, A. Light-Responsive (Supra)Molecular Architectures: Recent Advances. Adv. Opt. Mater. 2019, 7, 1900392.
- Wu, W., Yao, L., Yang, T., Yin, R., Li, F., and Yu, Y. (2011) NIR-light-induced deformation of cross-linked liquid crystalpolymers using upconversion nanophosphors. J. Am. Chem. Soc., 2011, 133 (40), pp.15810–15813.
- Gregory, P. (1990). Classification of Dyes by Chemical Structure. In The Chemistry and Application of Dyes: Topics in Applied Chemistry, D. R. Waring, & G. Hallas (Eds.), Boston, MA: Springer, 1990, pp. 17-47.
- Towns, A.D. Developments in azo disperse dyes derived from heterocyclic diazo components, Dyes Pigm., 1999, 42(1), pp. 3-28.
- Patel, V. J.; Patel, M. P.; Patel, R. G. Synthesis and application of novel heterocyclic dyes based on 1l-amino-3-bromo-13H-acenaphtho[l,2-e]pyridazino[3,2-b]- quinazoline-13-one. J. Serb. Chem. Soc., 2002, 67, 727–734. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis, characterization and application of quinazolinone based reactive dyes for various fibers. Fibers Polym. 2010, 11, 537–544. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis of Some New Thermally Stable Reactive Dyes Having 4(3H)-quinazolinone Molecule for the Dyeing of Silk, Wool, and Cotton Fibers. Fibers Polym. 2011, 12, 741–752. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis, characterization and in vitro antimicrobial screening of some new MCT reactive dyes bearing nitro quinazolinone moiety. J. Saudi Chem. Soc. 2015, 19 (4), pp. 347 – 359.
- Lellis, B.; Fávaro-Polonio, C. Z.; Pamphile, J. A.; Polonio, J. C. (2019). Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov., 2019, 3(2), pp. 275–290.
- Malinauskiene, L.; Bruze, M.; Ryberg, K.; Zimerson, E.;Isaksson, M. Contact allergy from disperse dyes in textiles: a review. Contact Dermatitis. 2013, 68(2), pp. 65-75.
- Moreau, L.; Goossens, A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005, 53(3) pp. 150-4.
- Miles, L.W.C. Textile printing, rev., 2nd ed.; Society of Dyers and Colourists: Bradford, England, 2003. [Google Scholar]
- Abdel Zaher, K. S.; Shaban, E.; Nawwar, G. A. M. Antibacterial Azo Dyes Containing Sulfa Drug Moieties and Their Colour Assessment on Printing Polyester Fabric ChemistrySelect, 2023, 8.
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 6249, Ampicillin" PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ampicillin (accessed 22 February, 2024).
- Benkhaya, S.; M'rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon, 2020 ,6(1).
- Gunst, R. (1953). HETEROCYCLIC DISAZO DYESTUFFS (U.S. Patent No 2,686,178), U.S. Patent and Trademark Office.
- Fabian, W.M.F.; Timofei, S. Comparative molecular field analysis (CoMFA) of dye-fibre affinities. Part 2. Symmetrical bisazo dyes. J. Mol. Struct.: THEOCHEM, 1996, 362(2), pp.155-162.
- Matsui, M., et al. Fluorine-containing benzothiazolyl bisazo dyes-their application to guest-host liquid crystal displays, Liq. Cryst. 25(2), 1998, pp. 235-240.
- Karcı, F. Synthesis of disazo dyes derived from heterocyclic components. Color. Technol., 2005, 121(5), pp. 275-280.
- Hadjoudis, E., Mavridis, I. M. Photochromism and thermochromism of Schiff bases in the solid state: structural aspects. Chem Soc Rev, 2004, 33(9), pp. 579-588.
- Raczyńska E.D., Kosińska W., Ośmiałowski B., Gawinecki R. Tautomeric equilibria in relation to pi-electron delocalization. Chem. Rev., 2005, 105, pp. 3561–3612.
- Bártová, K., Císařová, I., Lyčka, A., Dračínský, M. Tautomerism of azo dyes in the solid state studied by 15N, 14N, 13C and 1H NMR spectroscopy, X-ray diffraction and quantum-chemical calculations, Dyes Pigm., 2020, 178, 108342.
- Bakan, E., Karci, F., Avinc, O. Synthetic Fiber Dyeing with Synthesized Novel Disperse Disazo Dyes Containing Methyl (-CH3) Group as an Auxochrome and Their Color Properties IJEAST, 2016, 10(1).
- Elnagdi, M. H.; Sallam, M. M. M.; Fahmy, H. M.; Ibrahim, S. A. M.; Elias, M. A. M. Reactions with the Arylhydrazones of α-Cyanoketones: The Structure of 2-Arylhydrazono-3-ketimino-nitriles. Helv. Chim. Acta, 1976, 59(2), pp.551-557.
- Elnagdi, M. H.; Elgemeie, G. E.; Abd-elaal, F. A. E. Recent developments in the synthesis of pyrazole derivatives. Heterocycles (Sendai), 1985, 23(12), pp. 3121-3153.
- Benkhaya, S.; M'rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon, 2020, 6(1), 03271.
- Naime, J.; Al Mamun, M. S.; Aly, M. A. S.; Maniruzzaman, M.; Badal, M. M. R.; Karim, K. M. R. Synthesis, characterization and application of a novel polyazo dye as a universal acid–base indicator. RSC Adv., ,2022, 12(43), pp. 28034-28042.
- Çanakçı, D. Synthesis, characterisation, solvatochromic behaviour and thermal decomposition kinetics of novel polyazo dyes containing amide group and their transition metal complexes. J. Mol. Struct., 2019, 1181, 493–506. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J., Abednatanzi, S.; Van Der Voort, P. A ‘Defective’Conjugated Porous Poly-Azo as Dual Photocatalyst. Catalysts, 2021, 11(9), pp. 1064.
- Zhang, J.; Khayatnezhad, M.; Ghadimi, N. Optimal model evaluation of the proton-exchange membrane fuel cells based on deep learning and modified African Vulture Optimization Algorithm. Energy Sources, Part A, 2022, 44(1), pp. 287-305.
- Bo, G.; Cheng, P.; Dezhi, K.; Xiping, W.; Chaodong, L.; Mingming, G.; Ghadimi, N. (2022). Optimum structure of a combined wind/photovoltaic/fuel cell-based on amended Dragon Fly optimization algorithm: a case study. Energy Sources, Part A, 2022, 44(3), pp. 7109-7131.
- Mijin, D. Ž.; Ušćumlić, G. S., Valentić, N. V.; Marinković, A. D. Synthesis of azo pyridone dyes. Hem. Ind., 2011, 65(5), pp. 517-532.
- Zouari-Mechichi, H.; Benali, J.; Alessa, A.H.; Hadrich, B.; Mechichi, T. Efficient Decolorization of the Poly-Azo Dye Sirius Grey by Coriolopsis gallica Laccase-Mediator System: Process Optimization and Toxicity Assessment. 2024, Molecules, 29, pp. 477.
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C, 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. 2019, Biotechnol. Res. Innov., 3, pp. 275–290.
- Pereira, L., & Alves, M. Dyes—environmental impact and remediation. Environmental protection strategies for sustainable development, 2012, pp. 111-162.
- Hussien, F. A. H. An eco-friendly methodology for the synthesis of azocoumarin dye using cation exchange resins. 2021, Heliyon, 7(11).
- Amjad, R.; Munawar, M. A.; Khan, S. R.; Naeem, M. Synthesis and Spectral Studies of Some Novel Coumarin Based Disperse Azo Dyes: Studies of Coumarin Based Azo Dyes. 2009, Pak. J. Sci. Ind. Res., 52(3), pp. 117-121.
- Al-Harby, N. F.; Albahly, E. F.; Mohamed, N. A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo Red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. 2021, Polymers, 13(24), 4446.
- Ali, A.E.; Chowdhury, Z.Z.; Devnath, R.; Ahmed, M.M.; Rahman, M.M.; Khalid, K.;Wahab, Y.A.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; et al. Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact. 2023, Separations, 10, 506.
- Fernandes, A.; Pinto, B., Bonardo, L.; Royo, B.; Robalo, M. P.; Martins, L. O. Wasteful Azo dyes as a source of biologically active building blocks. 2021, Front. bioeng. biotechnol., 9, 672436.
- Ravindiran, G., Sundaram, H., Rajendran, E. M., Ramasamy, S., Nabil, A. Z., & Ahmed, B. (2023). Removal of azo dyes from synthetic wastewater using biochar derived from sewage sludge to prevent groundwater contamination. Urban Climate, 49, 101502.
Figure 1.
Structure of indigo and Tyrian purple.

Figure 2.
Synthesis of azo compounds.

Figure 4.
Simplified energy profile for the interconversion between the two isomers of a photochromic system [9].
Figure 4.
Simplified energy profile for the interconversion between the two isomers of a photochromic system [9].

Figure 5.
Isomerization E-Z for azo compounds.

Figure 7.
Structures of heteroaromatic amines.

Figure 8.
General structure of dyes 7a-k.

Figure 9.
The general structure of dyes 8a-g.

Figure 11.
Structure of: a) a brown dye, b) a blue dye, c) an orange dye.

Figure 12.
Synthesis process of disazo dyes 12a-j.

Figure 13.
Tautomeric forms ketone-enol for disazo compounds 12a-b.

Figure 14.
Tautomeric forms enamine–imine for disazo compounds 12a, 12c, 12e, 12g, 12i.

Figure 15.
Synthesis and molecular structures of pyrazole disperse dyes 13a-c and 14a-c.

Figure 16.
Strucure of a trisazo pyridone dye.

Figure 17.
Synthesis of the azocoumarin 17.
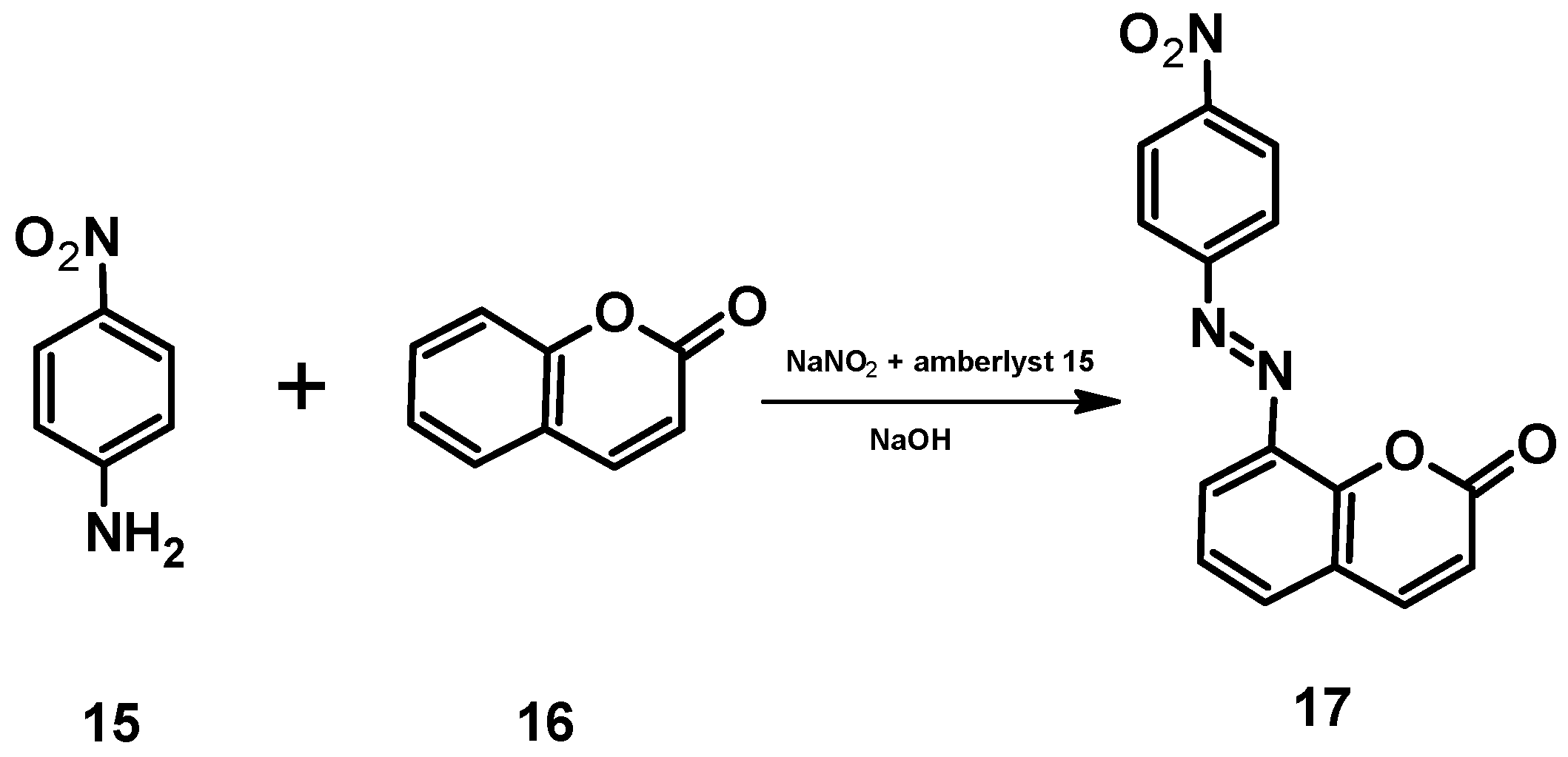
Figure 18.
Structure of amberlyst -15.
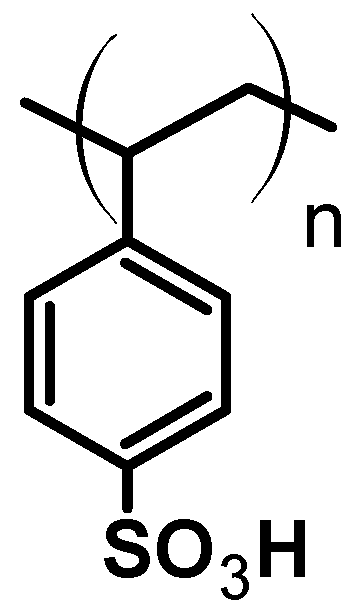
Table 1.
Inhibition zone of 9a-c as a measure of the antibacterial activities (mm/mg samples).
| 9a | 9b | 9c | Ampicillin | |
|---|---|---|---|---|
| Pseudomonas aeruginosa | 29 | 30 | 10 | 26 |
| Escherichia coli | 28 | 30 | 11 | 25 |
| Staphylococcus aureus | 29 | 30 | 9 | 21 |
| Bacillus subtilis | 26 | 20 | 9 | 26 |
Table 2.
Inhibition zone of the fabrics dyed with after 4 washes 9a-c as a measure of the antibacterial activities (mm/mg samples).
Table 2.
Inhibition zone of the fabrics dyed with after 4 washes 9a-c as a measure of the antibacterial activities (mm/mg samples).
| Polyester fabric printed with 9a |
Polyester fabric printed with 9b | Polyester fabric printed with 9c | Ampicillin | |
|---|---|---|---|---|
| Pseudomonas aeruginosa | 15 | 12 | 8 | 26 |
| Escherichia coli | 12 | 12 | 10 | 25 |
| Staphylococcus aureus | 12 | 14 | 9 | 21 |
| Bacillus subtilis | 16 | 12 | 8 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









