Preprint
Article
Predicting the Potential Distribution of Endangered Changnienia amoena S. S. Chien in China Based on Ensemble Modeling
Altmetrics
Downloads
107
Views
35
Comments
0
This version is not peer-reviewed
Submitted:
05 August 2024
Posted:
05 August 2024
You are already at the latest version
Alerts
Abstract
Climate change has significant impacts on the distribution of orchids. The endemic and endan-gered orchids are more susceptible to climate change than widely distributed orchids. To date, lit-tle is known concerning the response of endangered Changnienia amoena, endemic to China, to different climate scenarios. Here, we chose RF, MaxEnt, and GBM to build an ensemble model af-ter using ten models from Biomod2 to project its potential distribution in China. The outcomes showed that the four key environmental factors influencing its distribution were mean diurnal temperature range, minimum temperature of the coldest month, temperature seasonality, and precipitation of the warmest quarter. This orchid was mainly distributed in western Hubei, the junction between Shaanxi and Sichuan, eastern Guizhou, northwestern Hunan, and the junction among Anhui, Henan, and Hubei provinces in the current. The total suitable area of C. amoena was 58.33 × 104 km2, only accounting for 6.08% of China's total territory, which is larger than known. However, only 4.48% of the suitable area is located within national nature reserves, and 3.33% within provincial nature reserves, respectively. During the Last Inter Glacial and mid-Holocene, its suitable areas were larger than the current. Under six future climate scenarios, its suitable areas may averagely decrease by 2.26% relative to the current, with severe habitat fragmentation. Col-lectively, the centroid of C. amoena is expected to migrate towards the southeast in the future. Therefore, our findings demonstrate that climate change takes an adverse effect on its potential distribution. We recommend expanding protected areas or establishing new conservation sites for C. amoena. Furthermore, our study can help to inform the development of conservation manage-ment strategies for other endangered orchids in China under climate change.
Keywords:
Subject: Biology and Life Sciences - Ecology, Evolution, Behavior and Systematics
1. Introduction
Climate change may alter the geographical distribution of plants, especially for endemic plant species at the regional scale (Kelly and Goulden, 2008; Huang et al., 2024). The sixth assessment report published by the Intergovernmental Panel on Climate Change (IPCC) in 2023 stated that the global surface temperature had increased by 1.1°C from 2011 to 2020 compared to the period from 1850 to 1900, and with the global warming, the temperature is expected to increase by above 1.5°C in the coming future (2021-2040) (Gao et al., 2023). With the rising of global temperature, there will be more pronounced changes in hydrothermal conditions than ever, which will cause corresponding shift in the potential distribution of plants (Aitken et al., 2008).
Orchidaceae is ranked as the second largest family in angiosperms worldwide. It has 814 genera and over 27,500 species, and is a herbaceous taxon with many rare and endangered species (Chen et al., 2020). All orchidaceous species within this family are listed in the Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, https://cites.org/eng/app/appendices.php), accounting for over 90% of the total plant species of CITES (Jin et al., 2019). In fact, some endangered orchids have been protected by law in many countries. For example, there are 296 ones listed in the Chinese updated checklist, namely the List of National Key Protected Wild Plants issued in September of 2021, accounting for approximately 30% of the total species (https://www.forestry.gov.cn/c/www/lczc/10746.jhtml, accessed on 5 March 2024).
These herbaceous orchids, whether terrestrial or epiphytic, are more susceptible to the impacts of climate change compared with woody plants (Wotavova et al., 2004; Tsiftsis and Tsiripidis, 2020). Indeed, most orchid species possess unique flower in shape, which render them valuable in ornament (e.g., Phalaenopsis and Cymbidium). Simultaneously, many orchids have medicinal value (e.g., Gastrodia elata and Dendrobium spp.) (Jin et al., 2019). Moreover, orchids often rely on specialized pollinators for fruit set (Ren et al., 2012; Chen et al., 2021), making them particularly vulnerable to climate change. Although there are a large number of species in Orchidaceae, much little is concerned about their response to climate change at present. For instance, Qiu et al. (2023) found that the suitable habitat area for most of the eight Habenaria species in China would expand, while the suitable habitat area for ten Calanthe species would shrink dramatically. Xu et al. (2021) predicted that the suitable habitat of Cypripedium japonicum would shift towards higher elevations and latitudes in northwestern China in the context of climate warming. This suggests that future climate change has an adverse effect on its geographical distribution. The existing studies have shown that orchids are at the leading line of extinction because Orchidaceae has more threatened species than any other plant family in the world (Swarts and Dixon, 2009). As a result, orchids are much more sensitive to climate change than non-orchids. Even so, currently there are few researches on endemic and endangered orchids in response to climate change because of their limited range and specialized needs. Wang et al. (2015) discovered that Spiranthes parksii, an endangered terrestrial orchid endemic to central Texas in America, had high requirements for soil resources which were able to provide specific mycorrhizal fungi for such an orchid. They further pointed out that future climate change may make the orchid habitat more fragmented by affecting the growth and distribution of soil mycorrhiza.
Changnienia amoena S. S. Chien belongs to the genus Changnienia in the Orchidaceae family, which is a monotypic genus endemic to China (Wu and Peter, 2009). It is a perennial terrestrial herb, and often grows in the understory of broad-leaved or needle and broad-leaved mixed forests in the mountains of eastern and central China (Figure 1a) (Wu and Raven, 1999). This orchid has an elliptical or broadly ovoid fleshy pseudobulb with two or three nodes (Figure 1b). C. amoena has a solitary leaf at the apex of its pseudobulb, and its blade is spreading, recurved, adaxially dark green, abaxially purplish red. Its leaf is broadly ovate to broadly elliptic (Figure 1c). This orchid usually has a solitary inflorescence, which produces only a spreading and large flower, white or pink, with purplish red spots in white lip (Figure 1d). In general, it begins to bloom from April to May, and bears long ellipsoid capsules from October to November. It has high ornamental value because of its unique flower shape and bright color. Secondly, the whole plant or its pseudobulbs can be utilized as an important Chinese herb medicine. In addition, it can be used as an important material for systematic evolution of Orchidaceae as it is considered as a primitive taxon (Li, 2021).
C. amoena presents small populations in distribution, most of which are scattered and discontinuous in China (Li and Ge, 2006). Li et al. (2002) reported that C. amoena had low genetic diversity using RAPD technique. Due to its few flowers with defective floral structure, C. amoena fails to provide rewards for pollinators. Such a deceptive pollination strategy results in a very low fruit set rate in the field (Sun et al., 2006). We thereby speculate that this orchid heavily depends on asexual reproduction, thus making it difficult to regenerate its wild populations. Due to its biological reasons, in tandem with global warming and over-exploitation by humans, C. amoena is gradually decreasing in population size, and increasingly shrinking in distribution area. Consequently, as early as 1992 C. amoena was listed as a rare and endangered species in the China Plant Red Data Book -Rare and Endangered Plants (Vol. I) (Fu, 1992). It was classified as the second-grade species in the List of National Key Protected Wild Plants in 2021. Furthermore, it has been listed as "Endangered" (EN) species in the International Union for Conservation of Nature (IUCN) Red List (https://www.iucnredlist.org/, last accessed on 20 March 2024).
According to the "Flora of China", C. amoena was found in Anhui, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, and Zhejiang provinces in China (Wu and Raven, 1999), primarily concentrated in central Hunan and Hubei provinces. In recent years, Zhang (1996) discovered C. amoena at a moist forest edge in Wen County, Gansu Province. Chen et al. (2013) found two individuals of C. amoena in a subtropical forest at Shuanghe Village, Chengkou County, Chongqing City. Qin et al. (2018) identified a wild population of C. amoena in a bamboo forest with an altitude of 690 m at Mao'er Mountain National Nature Reserve, Guangxi Province. According to recent investigations and related reports, we think that C. amoena occurs in more than 13 provinces in China, including Anhui, Chongqing, Gansu, Guangxi, Guizhou, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, and Zhejiang provinces. Therefore, it remains unclear concerning its actual distribution range in China.
Species Distribution Models (SDMs) are powerful tools for examining the relationships between species' potential habitats and environmental factors (Fleishman et al. 2002). SDMs primarily rely on the geographical distribution information of species and environmental data to estimate their distribution (Elith and Leathwick, 2009). In view of modeling algorithm as the most importance source of uncertainty in performance from SDMs, it is generally recognized that the integration of multiple algorithms may provide more accurate predictions (Watling et al., 2015). The Biomod2 model is currently a reliable multi-model ensemble platform that utilizes different types of statistical methods to improve the predictive accuracy of single model and increase the reliability of projecting results (Thuiller et al., 2003; Fang et al., 2021). Each single model has its own advantages and disadvantages. Therefore, it seems a good choice to develop an ensemble model by combining different individual models. Such an ensemble model usually performs much better than single one in terms of accuracy (Araújo and New, 2007; Gong et al., 2022). The Biomod2 package includes ten species distribution models. Namely, they are Maximum Entropy model (MaxEnt), Generalized Linear Model (GLM), Generalized Additive Model (GAM), Multiple Adaptive Regression Spline (MARS), Surface Range Envelope (SRE), Flexible Discriminant Analysis (FDA), Categorical regression Tree Analysis (CTA), Gradient Boosting Model (GBM), Random Forest model (RF), and Artificial Neural Network (ANN).
Recently, the Biomod2 model has been employed to predict the habitat suitability of endangered orchids (Dormann et al., 2018; Wang et al., 2023). For instance, Yu et al. (2020) used the Biomod2 to analyze the impact of climate change on the suitable habitats of both Calanthe sieboldii and its three pollinators in China, indicating that its distribution may be affected by future climate change and the distribution reduction of these pollinators as well. To date, there has been only one research on the potential suitable distribution of C. amoena, in which a single model (i.e. MaxEnt) was employed to forecast the population distribution of C. amoena. Unfortunately, this study only used the data of one province, i.e. Jiangxi Province, China (Chen, 2019). In fact, C. amoena is distributed in more than ten provinces of China. it seems unlikely to predict its geographical distribution based on data from only one province. Therefore, it remains unclear about the potential geographical distribution of C. amoena in China.
Here, we first build an ensemble model generated by Biomod2 to link species distribution records of C. amoena with environmental variables (climate, terrain, and soil). In conjunction with ArcGIS spatial analysis, we then to determine the suitable area of C. amoena in China under different climatic scenarios. Specifically, the objectives of this study are: (1) to identify the key environmental factors influencing the distribution of C. amoena; (2) to forecast the current potential suitable habitats for C. amoena in China; (3) to predict its suitable habitats under past and future climate scenarios, and magnitude and direction of centroid migration; (4) to analyze the conservation gap of C. amoena based on the distribution data of nature reserves in China. This study will provide scientific references for the conservation management for C. amoena.
2. Materials and Methods
2.1. Species Occurrence Data
In our study, we collected occurrence data of C. amoena mainly from field survey, related websites and published literatures. Firstly, we obtained its presence points based on our field investigation for C. amoena wild populations in Jiangsu, Anhui, Jiangxi, Hubei, and Zhejiang provinces in eastern and central China during the period 2021- 2023. For example, in the mid-April of 2023 we found a wild population of C. amoena nearby a creek in a subtropical mountainous area, which is situated in Longtan, Liyang City, Jiangsu Province, eastern China.
Secondly, we searched, collected, and compiled original specimen records containing latitude and longitude or detailed locality information through the Chinese National Specimen Information Infrastructure (NSII, http://www.nsii.org.cn, last accessed on 16 January, 2024) and the Global Biodiversity Information Facility (GBIF, https://www.gbif.org/, last accessed on 16 January, 2024). We simultaneously searched for species name or Latin name in the Plant Photo Bank of China (PPBC, http://ppbc.iplant.cn, last accessed on 16 January, 2024) obtained detailed locality information of the images through image library, and then converted into latitude and longitude.
Thirdly, we used both the species name and Latin name of C. amoena as keywords for searching related literatures, which included the Flora of China, local floras, published papers, and investigation reports. For a small amount of data with detailed collection locations but without corresponding coordinates, we searched on Google Earth or Baidu Maps to obtain the corresponding latitude and longitude coordinates, and refined these data to two decimal places. Next, we removed duplicates, artificially introduced cultivars (such as botanical gardens), and specimen records lacking latitude and longitude information or with unidentifiable fonts.
By doing so, we obtained 108 distribution point data of C. amoena. We set the Resolution to Rarefy Data to 1 km using the Spatially Rarefy Occurrence Data for SDMs tool in SDMtoolbox 2.0 to reduce sampling bias and decrease spatial autocorrelation (Brown, 2014; Kong et al., 2019). This process eliminated duplicate, erroneous, and ambiguous records of C. amoena within a 1 km × 1 km spatial range of the selected points. Finally, we retained 93 valid distribution records of C. amoena and saved these records in “.csv” format. The collected latitude and longitude of C. amoena distribution points are shown in Appendix 1, and the distribution of C. amoena in China is shown in Figure 2.
2.2. Environmental Variables
This study involved three types of environmental data. Firstly, the topographic data involved elevation data downloaded from the WorldClim v2.1 database (https://www.worldclim.org, last accessed on 16 January, 2024), as well as slope and elevation data obtained from the national DEM elevation data (http://www.tuxingis.com, last accessed on 16 January, 2024).
Secondly, the 19 bioclimatic variables (Bio1-Bio19) were downloaded from the World Climate Database, including past, current, and future climate data. The WorldClim 1.4 dataset (Hijmans et al., 2005), based on the Coupled Model Inter-comparison Project Phase 5 (CMIP5), was selected for paleoclimatic data, including the Last Inter Glacial (LIG; 120,000-140,000 years ago) and the Mid-Holocene (MH; about 6000 years ago). Global Climate Models (GCMs) used for the past period were derived from the Community Climate System Model version 4 (CCSM4), developed by the National Center for Atmospheric Research (NCAR). Current (the average between 1970 and 2000) and future (2050s and 2070s) climate data were derived from calculating the equally-weighted average values of three global climate models: the CCSM4, the Beijing Climate Center Climate System Model version 1.1 (BCC-CSM1-1), and an Earth system model based on the Model for Interdisciplinary Research on Climate (MIROC-ESM). (Fick & Hijmans, 2017). The representative concentration pathways (RCPs) consist of a series of greenhouse gas concentration scenarios that have been widely used to determine species' responses to climate change (Zhang et al., 2021). The three typical concentration pathways selected for this study represent different climate change scenarios ranging from the lowest to the highest emission scenario, including RCP 2.6 (representing the lowest emission scenario), RCP 4.5 (indicating a medium and stable emission scenario), and RCP 8.5 (representing the highest emission scenario). Each pathway includes two periods (i.e. 2050s and 2070s), using the average emissions for the years 2041-2060 and 2061-2080, respectively.
Thirdly, 17 types of topsoil (0-30 cm) data were downloaded from the National Tibetan Plateau Scientific Data Center based on the Harmonized World Soil Database v1.2 (HWSD, http://www.tpdc.ac.cn/zh-hans/). The environmental data were ultimately saved in ASCII format. The unified spatial resolution of the data is 30 arc seconds. Additionally, excessive environmental variables can increase the dimensionality of ecological space, which can lead to over-fitting or inaccurate modeling. We thereby performed a Pearson correlation coefficient test for reducing multicollinearity among environmental factors (Duan et al., 2009). For two environmental variables with a correlation coefficient | r | > 0.8, the larger contribution one was retained (Dormann et al., 2013; Jiang et al., 2018).
Ultimately, after screening, Table 1 shows the environmental variables of subsequent modeling in different periods and their corresponding contribution rates.
2.3. Species Distribution Modeling Methodology
Firstly, to evaluate the performance of SDMs, based on the occurrence data and environmental variables of C. amoena, we modeled the potential geographical distribution of C. amoena under climate change using the ten different model algorithms in the Biomod2 package. During the modeling process, R4.3.3 randomly generated 500 pseudo-presence points. 75% of the distribution points were randomly selected for model training, with the remaining 25% used to assess the accuracy of the model predictions. Moreover, to ensure the predictive accuracy of the models, this operation was repeated ten times to obtain the average value as the final modeling result, yielding the area under curve (AUC) and true skill statistics (TSS) values of each model. We used AUC and TSS to evaluate model performance because the combination of the two values can improve the reliability of model evaluation (Liu et al., 2013; Wang et al., 2019). The value of AUC ranges from 0 to 1. For each model, the larger the AUC value is, the stronger the correlation between the model and the environmental variables is, and accordingly the higher the accuracy of its prediction outcome is (Pavlovi et al., 2019; Wang et al., 2007). The AUC value is classified into five levels: (1) Excellent: 0.90–1.00. (2) Good: 0.80–0.90. (3) Fair: 0.70–0.80. (4) Poor: 0.60–0.70. (5) Failure: 0.50–0.60. (Phillips and Dudík, 2008; Jalaeian et al., 2018). The TSS is based on a method improved from Kappa, and it also takes into account the maximum specificity and sensitivity threshold. It not only retains the advantages of Kappa but also corrects the drawbacks of Kappa's susceptibility to the extent of species distribution (Allouche et al., 2006). The TSS index is calculated as: TSS = Sensitivity + Specificity − 1. Generally, the TSS value ranges from -1 to 1. If the TSS value is greater than 0.8, this indicates a good model. A value of 0.5 or less reflects that the predictive performance is worse than random prediction (Allouche et al., 2006; Chen et al., 2020). The top three models with AUC > 0.95 and TSS > 0.8 from the ten models were selected as the excellent predictive models to form an ensemble model. Finally, using the optimal combined model algorithm, we obtained the ensemble model results of nine climate scenarios (i.e. LIG, MH, current, and six future climates). Subsequently, these predictive results were output as maps in ArcGIS 10.8, showing the probability of presence of C. amoena at each grid in the study area.
2.4. Geospatial Analysis
To directly display the potential range changes of C. amoena under different climate scenarios, we utilized ArcMap 10.8 to visualize the data generated after running the models. The reclassification of model results was based on the "test sensitivity and specificity threshold" (0.20) when only presence data were available (Liu et al., 2013). The habitat suitability of C. amoena was divided into four levels: unsuitable area (0.00-0.20), low suitable area (0.20-0.46), moderately suitable area (0.46-0.73), and highly suitable area (0.73-1.00). The sum of the moderately and highly suitable area is considered the total suitable habitat (Guillera-Arroita et al., 2015). Finally, the reclassified maps of the potential suitable area of C. amoena were generated in ArcMap, and the SDM toolbox v2.5 (Brown et al., 2017) was employed to calculate distribution changes and centroid shifts of suitable area.
2.5. Conservation Gap Analysis
The dataset of nature reserves was derived from the most recent official list of the Ministry of Ecological Environment of China (http://www.mee.gov.cn, last accessed on 7 March, 2024) and the World Database on Protected Areas (http://www.protectedplanet.net/, last accessed on 7 March, 2024). After excluding marine protected areas, we developed a map layer of China's protected areas, which included 464 national and 806 provincial nature reserves, reflecting the current status of protected areas in China (Yu, 2023). The total area of the protected areas used in this study was 97.18 × 104 km2, accounting for approximately 10.12% of China's total land area. The practice of plant conservation is generally based on the actual distribution of a species, and its predicted geographical distribution under current climate scenario is considered to be the closest to its actual distribution. Therefore, we used ArcGIS v10.8 to overlay the identified current suitable grids for C. amoena with the layers of protected areas (i.e. national and provincial levels), to determine the C. amoena population range within the natural protected areas, to evaluate its current protective effectiveness, and to identify its conservation gaps in China (Yang et al., 2021; Xue et al., 2021). When a suitable grid of C. amoena falls within the Chinese natural protected area, this indicates that its population in this grid is protected; otherwise, it is considered a conservation gap (Chi et al., 2017). Then, we calculated the area of its suitable habitat within the protected areas and its corresponding proportion (i.e., the protection rate), respectively.
3. Results
3.1. Optimal Model and Model Evaluation
We performed ten individual models using Biomod2 for C. amoena with 93 occurrence records. The AUC and TSS values for the 10 individual models in Biomod2 were presented in Table 2 (the AUC and TSS values were described as the mean ± standard deviation (SD)). The ten models had the AUC values ranging from 0.7655 to 0.9680, and in terms of AUC the first three models were RF, GBM, and MaxEnt, respectively. Similarly, the ten models had the TSS values ranging from 0.5311 to 0.8596, and in terms of TSS the first three models were GBM, MaxEnt, and RF, respectively. This indicated that whether in terms of AUC or TSS, the three models were consistently ranked in the top three. However, the SRE model had the lowest values of AUC and TSS. This indicated that there were varying degrees of model reliability among the ten individual models in Biomod2 for C. amoena. Therefore, we chose RF, GBM, and MaxEnt to form a new ensemble model. Moreover, each of the three models had mean AUC > 0.95 and TSS > 0.8.
Such a new ensemble model generated a mean AUC of 0.9950 and TSS of 0.9330, respectively. It presented higher values of AUC and TSS than the default ensemble model of ten individual models, indicating that the former had a higher predictive accuracy. Moreover, the new ensemble model simultaneously displayed the lower SD than the default one, showing better stability. Therefore, the new ensemble model was markedly superior to the default one. Accordingly, we chose the three optimal individual models (i.e. RF, GBM, and MaxEnt) to build an ensemble model. The subsequent analysis was performed in the mixed effect model.
3.2. Key Environmental Variables
We calculated the contribution rates of each environmental variable using the ensemble model and selected these variables significantly affecting the distribution of C. amoena. The top four environmental variables with the highest contribution rates on the potential distribution of C. amoena were identified as the key environmental factors (Table 3). Namely, they were mean diurnal temperature range (Bio2, 43.7%), minimum temperature of the coldest month (Bio6, 14.8%), temperature seasonality (Bio4, 14.4%), and precipitation of the warmest quarter (Bio18, 13.2%). The total contribution value of temperature factors (i.e. Bio2, Bio6, Bio4) reached 72.9%. When the species existence probability was greater than 0.46, it indicated that the range of environmental variables was suitable for the growth of this orchid (Ren et al., 2020; Yan and Zhang, 2022).
According to the response curves, the optimal range of Bio2 for C. amoena growth was 6.8-8.9 °C, with the most suitable Bio2 being 7.4 °C (Figure 3a). The optimal growth range of Bio6 was -6.0-2.5 °C, and when the temperature increased, the existence probability of C. amoena gradually increased, peaking at -2.0 °C (0.84) (Figure 3b). When Bio4 was 720, it was suitable for the growth of C. amoena. The probability of its existence gradually increased as Bio4 increased, reaching the maximum at 780 (0.82). Then, the probability of existence decreased with the coefficient ranging from 780 to 910 (Figure 3c). When the precipitation of the warmest quarter (Bio18) exceeded 420 mm, the existence probability was over 0.46, and the maximum probability of existence was 0.84 at 625 mm. Within the range of 420-720 mm, the existence probability initially increased then decreased (Figure 3d).
3.3. Current Suitable Distribution
The highly suitable habitats for C. amoena were mostly located in the western Hubei, the junction area between Shaanxi and Sichuan, eastern Guizhou, northwest Hunan, and the junction between Anhui, Hubei, and Henan provinces, with some areas scattered in Zhejiang and southern Anhui provinces in China. The moderately suitable habitats were primarily on the edges of the highly suitable habitats, including northeast Sichuan, southeast Shaanxi, eastern Chongqing, eastern Guizhou and eastern Shandong, with few suitable areas scattered in the western Jiangsu, Zhejiang, Hunan, Jiangxi, and Anhui provinces. The low suitable habitats were mainly in Jiangsu, Guizhou, Hunan, Jiangxi, Zhejiang, Anhui, southeastern Sichuan, northern Fujian and Guangxi, the central part of Hubei and Taiwan, and southern Liaoning provinces (Figure 4).
We combined the highly and moderately suitable habitats into the suitable habitat area (Lu et al., 2022). For C. amoena, the current suitable area for C. amoena was 58.33 × 104 km2, accounting for 6.08% of China's total territory. The highly suitable area was 14.34 × 104 km2, only making up 24.58% of the total suitable area (Table 4), mainly concentrated in southwest Hubei province.
Under current climatic conditions the suitable area of C. amoena within national-level protected areas was 2.61 × 104 km². The coverage ratio of national protected areas for this species' suitable habitat was 4.48%, accounting for 2.69% of the total protected areas in China. These areas were mainly located in Shennongjia in Hubei province, Badagong Mountain in Hunan province, Anhui province, and the junction regions of Shaanxi and Sichuan provinces.
In addition, the suitable area of C. amoena within provincial protected areas was 1.95 × 104 km². The coverage ratio of provincial protected areas for this species' suitable habitat was 3.33%, accounting for 2.01% of the total protected areas in China. Therefore, the vast majority of C. amoena's suitable habitats are not effectively protected.
3.4. Past and Future Distribution Shift
In the past, the potential suitable habitat of C. amoena was larger than currently, and it mainly concentrated in Hubei, Hunan, Jiangxi, Zhejiang, Jiangsu, and Anhui provinces, especially during the MH period (Figure 5). During the LIG period, the suitable area was 64.51 × 104 km2, which had an increase of 10.59% compared to the present. Likewise, a significant expansion occurred during the MH period, with the largest suitable area of 75.25 × 104 km2, which had an increase of 29.01% relative to the current (Table 4).
As shown in Figure 6, under six future climate scenarios, the highly suitable area for C. amoena were expected to decrease in northeastern Hunan and at the junction between Shaanxi and Sichuan provinces, while it was anticipated to increase in central Hubei and at the junction among Anhui, Hubei, and Henan provinces. The moderately suitable area for C. amoena was mainly expected to reduce at the junction between Shaanxi and Sichuan provinces, the junction between Guizhou and Hunan provinces, and in eastern Shandong province (Figure 6).
In the future, the suitable area of C. amoena in different periods (2050s and 2070s) would respond distinctively to climate change. Overall, the future mean suitable area for C. amoena was predicted to be 57.01 × 104 km2, indicating a decreasing trend. It declined by 2.26% compared to the current area. In the 2050s, its suitable habitat decreased under both low and medium emission scenarios (i.e. RCP 2.6 and RCP 4.5), while it increased under high emission scenario (RCP 8.5). Under RCP 4.5, the suitable area is expected to be 48.49 × 104 km2, with a maximum decline of 16.87% compared to the current. In the 2070s, its suitable habitat increased under RCP 2.6, while it decreased under RCP 4.5 and RCP 8.5. Under RCP 2.6, the suitable area for C. amoena was expected to amount to 71.64 × 104 km2, with a largest increase of 22.82% relative to the current. The suitable area for C. amoena was predicted to decrease in the 2050s and 2070s under RCP 4.5 (Table 4).
In summary, from the past to the current and subsequently to future climate scenarios, the suitable area change for C. amoena showed a fluctuating type. The suitable area decreased in the current and future period compared to the two past periods, with severe habitat fragmentation.
3.5. Centroid Migration of Suitable Area
From the Last Inter Glacial to the Mid-Holocene, the centroid (111.1392°E, 29.8431°N) of C. amoena shifted 38.47 km in a northwestward direction. From the MH to current period, the centroid (110.9099°E, 30.1266°N) migrated 24.30 km towards the north. Under six future climate scenarios, the centroid’s (110.9077°E, 30.3452°N) average migration distance for C. amoena was expected to be 19.72 km, with the majority of the movements directed to southeastward. Under RCP 2.6 scenario, the centroid migrated southwestward with the distance of 77.18 km in the 2070s, while it shifted 47.84 km to the southeast in the 2050s. Under RCP 4.5, it moved northeastward with the furthest distance of 249.89 km in the 2050s, and 72.79 km to the southeast in the 2070s. Under RCP 8.5, the centroid migrated 135.78 km southeastward in the 2050s, and 111.22 km to the southeast in the 2070s (Figure 7). Moreover, the past and future centroid shifts of C. amoena were expected in the junction area of Hubei, Hunan, and Chongqing provinces.
Collectively, the centroid migration pattern of C. amoena was circuitous, initially shifting northwestward from the LIG to the MH, and subsequently northward towards the current. From the current to future, the centroid generally migrated southeastward.
4. Discussion
4.1. Model Selection and Evaluation
Recent studies have shown that the accuracy of species distribution predictions can be considerably improved by applying ensemble model rather than a single model (Grenouillet et al., 2011). We first employed the Biomod2, which comprised ten individual models, to predict the potential geographical distribution of endangered C. amoena under different climate scenarios in China. Then we selected three of them (RF, MaxEnt, and GBM) in terms of AUC and TSS values. Finally, we developed an ensemble model whose AUC and TSS are > 0.9, respectively. This indicates that such an ensemble model presents a superior predictive performance relative to each of the ten individual models (Table 2). Furthermore, all known occurrence points of C. amoena falls within the forecasted distribution range under the current climate scenario (Figure 4). This further suggests that the prediction outcomes of the ensemble model used in this study are reliable. Therefore, we used the ensemble model to predict the potential distribution under past (i.e. LIG and MH), current, and six future climate scenarios, respectively.
4.2. The Key Influencing Factors of C. amoena
In this study, we take into accounts the effect of three types of environmental conditions (i.e. climate, soil, and topography) on the suitability of C. amoena. Our results have shown that the main factors influencing its potential distribution are mean diurnal range (Bio2), minimum temperature of the coldest month (Bio6), temperature seasonality (Bio4), and precipitation of the warmest quarter (Bio18), and that the sum of their percent contribution is up to 86.2% (Table 3). Hence, this indicates that bioclimate are more crucial factors affecting the potential distribution of C. amoena than soil and topography in China. Liu et al. (2010) contends that wild Orchids are exquisitely sensitive to alterations in temperature and moisture. C. amoena is currently distributed in more than 13 provinces in China, covering a wide range and spanning subtropical and warm temperate zones. Among the 19 bioclimatic factors, the top three are temperature-related (i.e. Bio2, Bio6, and Bio4), accounting for 72.9% of the total percent contribution for C. amoena. Especially, Bio2 contributes the maximum, which takes up 43.7%. This indicates that temperature-related variables may play a more important role in limiting the potential distribution of C. amoena than precipitation-related variables.
In light of the response curves of environmental factors, we determine the suitable range of Bio2 for C. amoena, which is between 6.8°C and 8.9°C, with the most suitable mean diurnal range of 7.4°C. Most Orchids have apparent pseudobulbs including C. amoena (Figure 1c). Pseudobulb is able to hold water and store nutrients in Orchidaceae, and such a bulblike enlargement of the stem can help orchids for survival and growth (Wu et al., 2013; Ng and Hew, 2000). C. amoena is a shade-tolerant herb, and it prefers to grow in soils with much rich humus. Therefore, we think that such a structure may be one of the major reasons for this trait.
In brief, we have identified for the first time the key environmental factors affecting the distribution of C. amoena and further determined their corresponding optimal ranges. Namely, C. amoena grows well in humid habitats with low variation in mean diurnal range and temperature seasonality.
4.3. Current Suitable Area of C. amoena
The predicted outcome from the Biomod2 shows that the potential suitable area for C. amoena in China is 58.33 × 104 km2 under the current climate scenario, accounting for only 6.08% of China's total territory (Table 4). The area is mainly distributed in Anhui, Henan, Hubei, Hunan, Guizhou, Shaanxi, and Sichuan provinces (Figure 4). The current suitable range of C. amoena involves 18 provinces in China, which is clearly more than eight provinces recorded in Flora of China. Therefore, the actual distribution of C. amoena is much larger than previously known, and it is also evidently greater than the predicted outcome (2.44 × 104 km2 ) within Jiangxi Province (Chen, 2019).
Furthermore, our predictive results also indicate that such provinces as Chongqing, Guangxi, Guizhou, Gansu, and Henan, which are not recorded in Flora of China (Wu and Raven, 1999), may indeed have a potential distribution of C. amoena in China. An example in point is Guizhou province, which has both moderately and highly suitable areas (Figure 4). Zhang and Yang (2010) reported the distribution of C. amoena under an evergreen broad-leaved forest at Fanjingshan National Nature Reserve, northeastern Guizhou Province. At the same time, we tend to think that some provinces like Fujian probably have wild populations of C. amoena although there has been no records in these areas so far.
In fact, there is an obvious habitat fragmentation for the majority of provinces with the suitability of C. amoena in China. This is consistent with the discontinuous distribution pattern of C. amoena in the five sampled provinces (Li and Ge, 2006). This orchid usually has only one flower per individual (Figure 1d). It does not secrete nectar and provide no reward for pollinators, falling into a deceptive pollination mode (Sun et al., 2006). Moreover, only one species of bumblebees, namely Bombus trifasciatus, is considered as its legitimate pollinator among the 15 candidate pollinating insects (Sun et al., 2003). All these may result in its low fruit set rate.
Therefore, we believe that climate change and biological characteristics are jointly responsible for the habitat fragmentation of C. amoena under the current climate scenario.
4.4. Suitable Area Change under Different Climate Scenarios
According to our analysis, the suitable habitat area for C. amoena during the LIG period was 64.51 × 104 km2, and it would expand to 75.25 × 104 km2 during the mid-Holocene. Compared to the current period, its suitable area increased by 10.59% and 29.01% during LIG and MH, respectively (Table 4). Therefore, the suitable areas in the past were significantly larger than currently one. The climate during the LIG period was warm and dry with less precipitation (Yan et al., 2022), which could limit the growth of C. amoena. The mid-Holocene was the last major warm period, characterized by a warm and moist climate with more precipitation (He et al., 2022). The overall warm and humid climatic conditions during this period may be more conducive to the growth and reproduction of C. amoena, resulting in a significant increase and concentration of its suitable area.
Conversely, under six future climate scenarios, the average suitable habitat area for C. amoena is 57.01 × 104 km2, decreased by 2.26% of the current distribution (Table 4). In different periods, climate change has different effects on its suitable areas. The average suitable area is 53.58 × 104 km2 in the 2050s and 60.44 × 104 km2 in the 2070s. Under different emission conditions, there is a slight fluctuation in the suitable area of C. amoena. Under the RCP2.6 climate scenario in the 2070s, the suitable area for C. amoena is expected to increase the most in the six future climate scenarios. Collectively, except 2050s-RCP8.5 and 2070s-RCP2.6, there is a moderate decreasing trend for each of the other four future scenarios in the suitable area.
Overall, from past to current and till the future, the suitable area for C. amoena shows a gradually decreasing trend, with increasing habitat fragmentation.
Under future climate scenarios, the centroid of C. amoena largely shows a southeastward migration (Figure 7). This is consistent with the migration directions of Lilium polyphyllum (Dhyani et al., 2021) and Osmanthus fragrans (Kong et al., 2021) in China in the future. Therefore, the migration of C. amoena towards low latitude in China may be related to its preference for growing in warm and humid region.
4.5. Conservation implications for C. amoena
Our model projects that the current suitable area of 58.33 × 104 km2 for C. amoena is significantly larger than the known range, across more than 10 provinces in China. Moreover, its suitable area will slightly decrease under future scenarios. At present, C. amoena is largely concentrated in Anhui, Chongqing, Hubei, Hunan, and other provinces where there are complex and diverse climatic conditions and vegetation types. Accordingly, it is most likely to have its wild populations in these regions. Therefore, we recommend conducting supplemental survey concerning C. amoena wild populations in China, including the whole Hubei, northern Hunan, southeastern Jiangsu, northern Jiangxi, and southern Shaanxi, especially in northern Fujian where no C. amoena population has been reported so far.
In this study, by overlaying the potential distribution area of C. amoena with national and provincial nature reserves, we find that only 4.48% of the suitable area for C. amoena is located within national nature reserves, and 3.33% within provincial nature reserves, respectively. This indicates that over 90% of the suitable habitat for C. amoena is in a zero-protection status, which may be a primary reason for the "endangered" category of wild C. amoena. This orchid is a national second-grade protected plant species. Nature reserves are the most effective way for in situ conservation of wild orchids (Qin et al., 2012; Jin, 2012; Zhang et al., 2015). Therefore, we propose to enlarge the nature reserve around which C. amoena is relatively concentrated in distribution, or to establish plant natural mini-reserves / protected sites.
In view of its high ornamental and medicinal value, together with the adverse effect of climate change on its distribution, we recommend selecting appropriate sites for C. amoena introduction and cultivation in light of its projected suitable habitats. Simultaneously, we also recommend considering the key climatic factors identified by the ensemble model in this study (i.e. Bio2, Bio6, Bio4, Bio18).
5. Conclusions
We first developed an ensemble model comprising three individual models (i.e. RF, MaxEnt, and GBM) from the Biomod2 in this study. Then, we combined occurrence records of the endangered and endemic C. amoena in China with climate, terrain and soil variables to successfully project its potential distribution under various climate scenarios. Our results show that mean diurnal range (Bio2), minimum temperature of the coldest month (Bio6), temperature seasonality (Bio4), and precipitation of the warmest quarter (Bio18) are the key environmental factors limiting its geographical distribution. For the first time we have determined its current suitable area of 58.33 × 104 km2, accounting for only 6.08% of China's total territory. The orchid is mainly distributed in central and eastern China, with larger range than known. Its suitable area is considerably larger during the LIG and MH periods than currently. Under future climate scenarios, its suitable area will averagely decrease by 2.26%. Our findings reveal a declining trend in the suitable area of C. amoena from past to current till future. This demonstrates that climate change has an adverse impact on this orchid, with increasing habitat fragmentation. Our analysis further shows that over 90% of the suitable area of C. amoena is outside national and provincial nature reserves in China, suggesting significant conservation gaps. Therefore, we recommend expanding protected areas or establishing new conservation sites for C. amoena. Furthermore, our study can also provide reference for conservation and management for other endangered orchids in China under climate change. In addition, only natural factors are used in the current study, and other factors such as human disturbance and land use change can be taken into account in the future.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Latitude and Longitude Coordinates of 93 Occurrence Records of the Endangered C. amoena in China.
Author Contributions
Data curation, formal analysis, and writing—original draft, T.L.; investigation, data curation, H.C.; investigation, conceiving the study and leading the writing., G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research This research was funded by investigation and assessment of key protected wild plants in Jiangsu Province (No. 2023053SMnull0162). Check carefully that the details given are accurate and use the standard spelling of funding agency names at https://search.crossref.org/funding. Any errors may affect your future funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Y.R. Zhou and H.R. Wang for their valuable advice on an earlier draft of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Chen, Y.; Yu, S. Climate factors drive plant distributions at higher taxonomic scales and larger spatial scales. Front. Ecol. Evol. 2024, 11, 1233936. [Google Scholar] [CrossRef]
- Gao, Q.H.; Qin, Y.Y.; Liang, M.C.; Gao, X. Interpretation of the main conclusions and suggestions of IPCC AR6 synthesis report. Environ. Prot. 2023, 51, 82–84. [Google Scholar]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; et al. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.D.; Lu, A.M.; Liu, B.; Ye, J.F. Tree of Life for Chinese Vascular Plants; Science Press: Beijing, China, 2020; ISBN 9787030635600. [Google Scholar]
- Jin, X.H.; Li, J.W.; Ye, D.P. Atlas of Native Orchids in China; Henan Science and Technology Press: Zhengzhou, China, 2019; ISBN 9787534991066. [Google Scholar]
- Wotavová, K.; Balounová, Z.; Kindlmann, P. Factors affecting persistence of terrestrial orchids in wet meadows and implications for their conservation in a changing agricultural landscape. Biol. Conserv. 2004, 118, 271–279. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I. Temporal and spatial patterns of orchid species distribution in Greece: Implications for conservation. Biodivers. Conserv. 2020, 29, 3461–3489. [Google Scholar] [CrossRef]
- Ren, Z.X.; Wang, H.; Luo, Y.B. Deceptive pollination of orchids. Biodivers. Sci. 2012, 20, 270. [Google Scholar]
- Chen, X.H.; Tan, S.L.; Liang, Y.L.; Huang, L.; Xiao, H.W.; Luo, H.L.; Xiong, D.J.; Yang, B.Y.; Ren, Z.X. The pollination of Habenaria rhodocheila (Orchidaceae) in South China: When butterflies take sides. Ecol. Evol. 2021, 11, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Jacquemyn, H.; Burgess, K.S.; Zhang, L.G.; Zhou, Y.D.; Yang, B.Y.; Tan, S.L. Contrasting range changes of terrestrial orchids under future climate change in China. Sci. Total Environ. 2023, 895, 165128. [Google Scholar]
- Xu, Y.D.; Huang, Y.; Zhao, H.R.; Yang, M.; Zhuang, Y.Q.; Ye, X.P. Modelling the effects of climate change on the distribution of endangered Cypripedium japonicum in China. Forests 2021, 12, 429. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Species distribution modelling for conservation of an endangered endemic orchid. AOB Plants 2015, 7, plv039. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Peter, H.R. Flora of China; Science Press: Beijing, China, 2009; Volume 25, ISBN 9787030255334. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China, 1999; Volume 18, ISBN 9787030073228. [Google Scholar]
- Liu, G.Y. Flora of Zhejiang: The second edition; Zhejiang Science and Technology Press: Hangzhou, China, 2021; Volume 10, ISBN 9787534191763. [Google Scholar]
- Li, A.; Ge, S. Genetic variation and conservation of Changnienia amoena, an endangered orchid endemic to China. Plant Syst. Evol. 2006, 258, 251–260. [Google Scholar] [CrossRef]
- Li, A.; Luo, Y.B.; Xiong, Z.T.; Ge, S. A preliminary study on conservation genetics of three endangered orchid species. Acta Bot. Sin. 2002, 44, 250–252. [Google Scholar]
- Fu, L.G. China plant red data book -rare and endangered plants; Science Press: Beijing, China, 1992; Volume I, ISBN 703000485X. [Google Scholar]
- Sun, H.Q.; Luo, Y.B.; Alexandersson, R.; Ge, S. Pollination biology of the deceptive orchid Changnienia amoena. Bot. J. Linn. Soc. 2006, 150, 165–175. [Google Scholar] [CrossRef]
- Zhang, H.S. Distribution of Changnienia amoena S. S. Chien in Wenxian County, Gansu Province. J. Plants 1996, 29. [Google Scholar]
- Chen, S.; Ma, Y.; Wang, X.B. A rare and endangered flower, Changnienia amonena S. S. Chien, was found in Chengkou County. South China Agriculture 2013, 7, 7–12. [Google Scholar]
- Qin, Y.; Zou, C.Y.; Meng, T. Two newly recorded genera of Orchidaceae from Guangxi, China. Guihaia 2018, 38, 1475–1479. [Google Scholar]
- Fleishman, E.; Ray, C.; Sjögren-Gulve, P.; Boggs, C.L.; Murphy, D.D. Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv. Biol. 2002, 16, 706–716. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Watling, J.I.; Brandt, L.A.; Bucklin, D.N.; Fujisaki, I.; Mazzotti, F.J.; Romanach, S.S.; Speroterra, C. Performance metrics and variance partitioning reveal sources of uncertainty in species distribution models. Ecol. Modell. 2015, 309, 48–59. [Google Scholar] [CrossRef]
- Thuiller, W.; Araújo, M.B.; Lavorel, S. Generalized models vs. classification tree analysis: predicting spatial distributions of plant species at different scales. J. Veg. Sci. 2003, 14, 669–680. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Zhang, X.H.; Wei, H.Y.; Wang, D.J.; Chen, R.D.; Wang, L.K.; Gu, W. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.F.; Hu, X.K.; Hao, Y.W.; Luo, Z.W.; Feng, J.X.; Xue, J.B.; Guo, Z.Y.; Li, Y.L.; Zhang, L.J.; Xia, S.; et al. Projecting the proliferation risk of Oncomelania hupensis in China driven by SSPs: A multi-scenario comparison and integrated modeling study. Adv. Clim. Change Res. 2022, 13, 258–265. [Google Scholar] [CrossRef]
- Dormann, C.F.; Calabrese, J.M.; Guillera-Arroita, G.; Matechou, E.; Bahn, V.; Bartoń, K.; Beale, C.M.; Ciuti, S.; Elith, J.; Gerstner, K.; et al. Model averaging in ecology: A review of Bayesian, information-theoretic, and tactical approaches for predictive inference. Ecol. Monogr. 2018, 88, 485–504. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yin, J.; Wang, X.; Chen, Y.; Mao, Z.K.; Lin, F.; Gong, Z.Q.; Wang, X.G. Habitat suitability evaluation of invasive plant species Datura stramonium in Liaoning Province: Based on Biomod2 combination model. J. Appl. Ecol. 2023, 34, 1272–1280. [Google Scholar]
- Yu, Y.J.; Luo, H.L.; Liu, N.N.; Xiong, D.J.; Luo, Y.B.; Yang, B.Y. Influence of the climate change on suitable areas of Calanthe sieboldii and its pollinators in China. Biodivers. Sci. 2020, 28, 769. [Google Scholar] [CrossRef]
- Chen, Y.R. Phylogentic Diversity and its Structure of Wild Orchid Plants in Jiangxi Province and the Change Trend of the Suitable Areas; Nanchang University: Nanchang, China, 2019. [Google Scholar]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Zou, H. Optimization of Maximum Entropy Model in species distribution prediction. J. Appl. Ecol. 2019, 30, 2116. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Fan, X.; Miao, C.; Duan, Q.; Shen, C.; Wu, Y. The performance of CMIP6 versus CMIP5 in simulating temperature extremes over the global land surface. J. Geophys. Res. Atmos. 2020, 125, e2020JD033031. [Google Scholar] [CrossRef]
- Gao, T.; Xu, Q.; Liu, Y.; Zhao, J.; Shi, J. Predicting the potential geographic distribution of Sirex nitobei in China under climate change using Maximum entropy model. Forests 2021, 12, 151. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, Z.; Li, L. Projection of climate extremes in China, an incremental exercise from CMIP5 to CMIP6. Sci. Bull. 2021, 66, 2528–2537. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, K.J.; Liu, X.F.; Yang, L.; Shen, S.K. Interspecific variance of suitable habitat changes for four alpine Rhododendron species under climate change: Implications for their reintroductions. Forests 2021, 12, 1520. [Google Scholar] [CrossRef]
- Duan, Y.Z.; Yu, H.; Wang, H.T.; Du, Z.Y. Geographical distribution and prediction of potentially suitable regions of endangered relict plant Tetraena mongolica. Plant Sci. J. 2019, 37, 337–347. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, G.J.R.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T.; Wu, Y.; Hu, R.; Huang, K.; Shao, X. Past distribution of epiphyllous liverworts in China: The usability of historical data. Ecol. Evol. 2018, 8, 7436–7450. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Wang, D.; Cui, B.; Duan, S.; Chen, J.; Fan, H.; Lu, B.; Zheng, J. Moving north in China: The habitat of Pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar] [CrossRef]
- Pavlović, L.; Stojanović, D.; Mladenović, E.; Lakićević, M.; Orlović, S. Potential elevation shift of the European beech stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 436609. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, B.; Wan, F.; Xiao, Q.; Dai, L. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Jalaeian, M.; Golizadeh, A.; Sarafrazi, A.; Naimi, B. Inferring climatic controls of rice stem borers’ spatial distributions using maximum entropy modelling. J. Appl. Entomol. 2018, 142, 388–396. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Chen, Q.H.; Yin, Y.J.; Zhao, R.; Yang, Y.; Teixeira da Silva, J.A.; Yu, X.N. Incorporating local adaptation into species distribution modeling of Paeonia mairei, an endemic plant to China. Front. Plant Sci. 2020, 10, 1717. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Global Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Yu, J.H.; Qin, F.; Xue, T.T.; Zhang, W.D.; Liu, Q.; AN, M.T.; Yu, S.X. Conservation status and prediction analysis of potential distribution of National Key Protected Wild Plants. Guihaia 2023, 43, 1404–1413. [Google Scholar]
- Yang, X.; Liu, B.; Bussmann, R.W.; Guan, X.; Xu, W.B.; Xue, T.T.; Xia, C.Y.; Li, J.; Jiang, H.; Wu, L.; et al. Integrated plant diversity hotspots and long-term stable conservation strategies in the unique karst area of southern China under global climate change. Forest Ecol. Manage. 2021, 498, 119540. [Google Scholar] [CrossRef]
- Xue, T.T.; Gadagkar, S.R.; Albright, T.P.; Yang, X.D.; Li, J.; Xia, C.Y.; Wu, J.Y.; Yu, S.X. Prioritizing conservation of biodiversity in an alpine region: Distribution pattern and conservation status of seed plants in the Qinghai-Tibetan Plateau. Global Ecol. Conserv. 2021, 32, e01885. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Z.; Xu, X.; Zhang, X.; Zhao, Z.; Liu, Y.; Wang, Q.G.; Wang, H.; Li, Y.; Yang, G.; et al. Threatened medicinal plants in China: Distributions and conservation priorities. Biol. Conserv. 2017, 210, 89–95. [Google Scholar] [CrossRef]
- Ren, Z.; Zagortchev, L.; Ma, J.; Yan, M.; Li, J. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, G. Predicting the potential distribution of endangered Parrotia subaequalis in China. Forests 2022, 13, 1595. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, R.; Zhang, G. Predicting the potential distribution of four endangered holoparasites and their primary hosts in China under climate change. Front. Plant Sci. 2022, 13, 942448. [Google Scholar] [CrossRef] [PubMed]
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble modelling of species distribution: the effects of geographical and environmental ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Liu, H.; Feng, C.L.; Luo, Y.B.; Chen, B.S.; Wang, Z.S.; Gu, H.Y. Potential challenges of climate change to orchid conservation in a wild orchid hotspot in southwestern China. Bot. Rev. 2010, 76, 174–192. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Yang, C.D. A new taxa record genus and species of Orchidaceae in Guizhou Province (Changnienia S. S. Chien: Changnienia amoena S. S. Chien). Seed 2010, 29, 65–66. [Google Scholar]
- Sun, H.Q.; Luo, Y.B.; Ge, S. A preliminary study on pollination biology of an endangered orchid, Changnienia amoena, in Shennongjia. Acta Bot. Sin. 2003, 45, 1019–1023. [Google Scholar]
- Wu, H.Z.; Song, X.Q.; Yang, F.S.; Zhu, G.P. Pseudobulbs on the Survival and Mating System of Dendrobium sinense (Orchidaceae) in Hainan Island. Chin. J. Trop. Crops 2013, 34, 625–629. [Google Scholar]
- Ng, C.K.Y.; Hew, C.S. Orchid pseudobulbs–false'bulbs with a genuine importance in orchid growth and survival! Sci. Hortic. 2000, 83, 165–172. [Google Scholar] [CrossRef]
- Yan, H.; Ma, S.M.; Wei, B.; Zhang, H.X.; Zhang, D. Historical distribution patterns and environmental drivers of relict shrub Amygdalus pedunculata. Chin. J. Plant Ecol. 2022, 46, 766. [Google Scholar] [CrossRef]
- He, X.; Ma, W.X.; Zhao, T.T.; Ma, Q.H.; Liang, L.S.; Wang, G.X.; Yang, Z. Prediction of potential distribution of endangered species Corylus chinensis Franch in climate change context. Forest Res. 2022, 35, 104–114. [Google Scholar]
- Dhyani, A.; Kadaverugu, R.; Nautiyal, B.P.; Nautiyal, M.C. Predicting the potential distribution of a critically endangered medicinal plant Lilium polyphyllum in Indian Western Himalayan Region. Reg. Environ. Change 2021, 21, 30. [Google Scholar] [CrossRef]
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.H.; Jiang, M.K.; Xu, W.G.; He, Z.H. Assessment of in situ conservation of 1,334 native orchids in China. Biodivers. Sci. 2012, 20, 177. [Google Scholar]
- Jin, X.H. A comment on “Assessment of in situ conservation of 1,334 native orchids in China”. Biodivers. Sci. 2012, 20, 235. [Google Scholar]
- Zhang, Y.B.; Du, H.D.; Jin, X.H.; Ma, K.P. Species diversity and geographic distribution of wild Orchidaceae in China. Chin. Sci. Bull. 2015, 60, 179–188. [Google Scholar]
Figure 1.
Photos of C. amoena. (a) C. amoena community habitat preferences; (b) Subellipsoid to broadly ovoid pseudobulbs (red arrow); (c) A single leaf at apex of pseudobulb, abaxially purplish red (left), adaxially dark green (right); (d) A solitary inflorescence usually with a pink or white flower. The photos were taken by Guangfu Zhang.
Figure 1.
Photos of C. amoena. (a) C. amoena community habitat preferences; (b) Subellipsoid to broadly ovoid pseudobulbs (red arrow); (c) A single leaf at apex of pseudobulb, abaxially purplish red (left), adaxially dark green (right); (d) A solitary inflorescence usually with a pink or white flower. The photos were taken by Guangfu Zhang.

Figure 2.
Current occurrence records of C. amoena in China.

Figure 3.
Response curves of C. amoena to key environmental variables.

Figure 4.
Predicted current distribution of C. amoena in China.

Figure 5.
Predicted Last Inter Glacial (LIG) (a) and Mid-Holocene (MH) (b) distribution of C. amoena in China.
Figure 5.
Predicted Last Inter Glacial (LIG) (a) and Mid-Holocene (MH) (b) distribution of C. amoena in China.
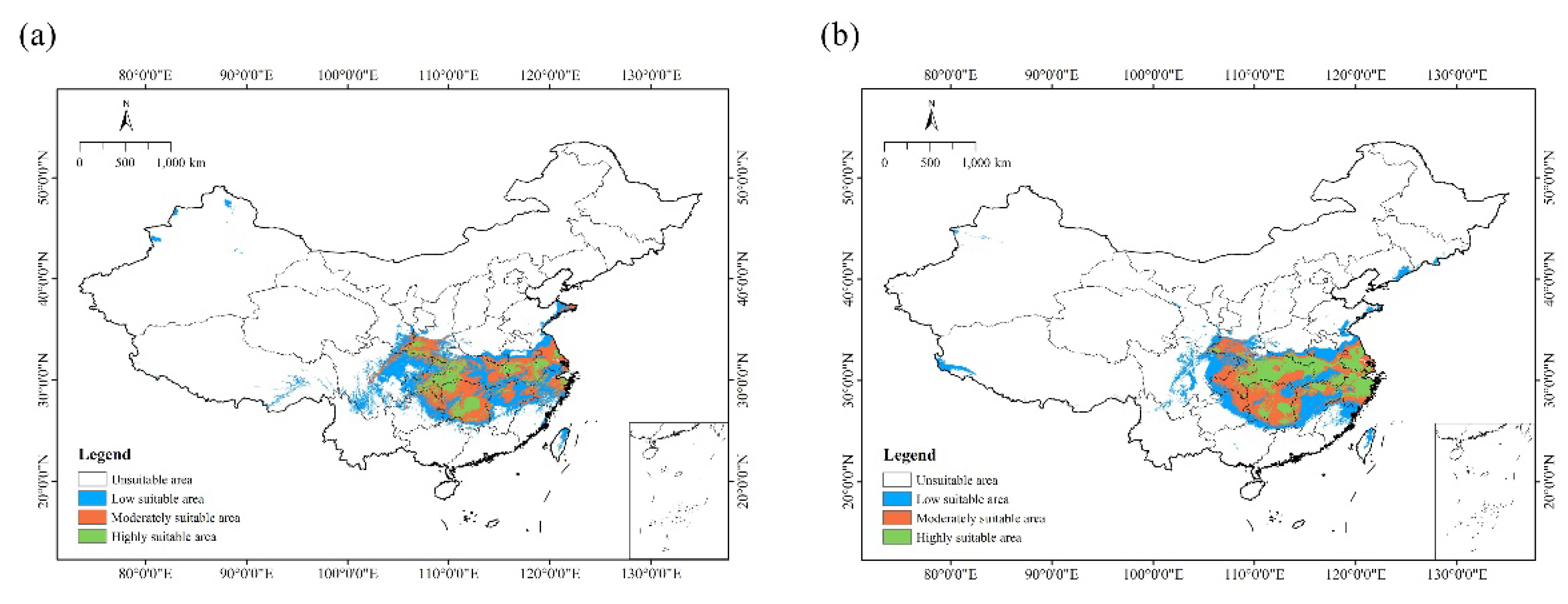
Figure 6.
Potential suitable distribution of C. amoena in China under different future climatic scenarios (RCP 2.6, RCP 4.5, and RCP 8.5) in the 2050s and 2070s using the ensemble model.
Figure 6.
Potential suitable distribution of C. amoena in China under different future climatic scenarios (RCP 2.6, RCP 4.5, and RCP 8.5) in the 2050s and 2070s using the ensemble model.
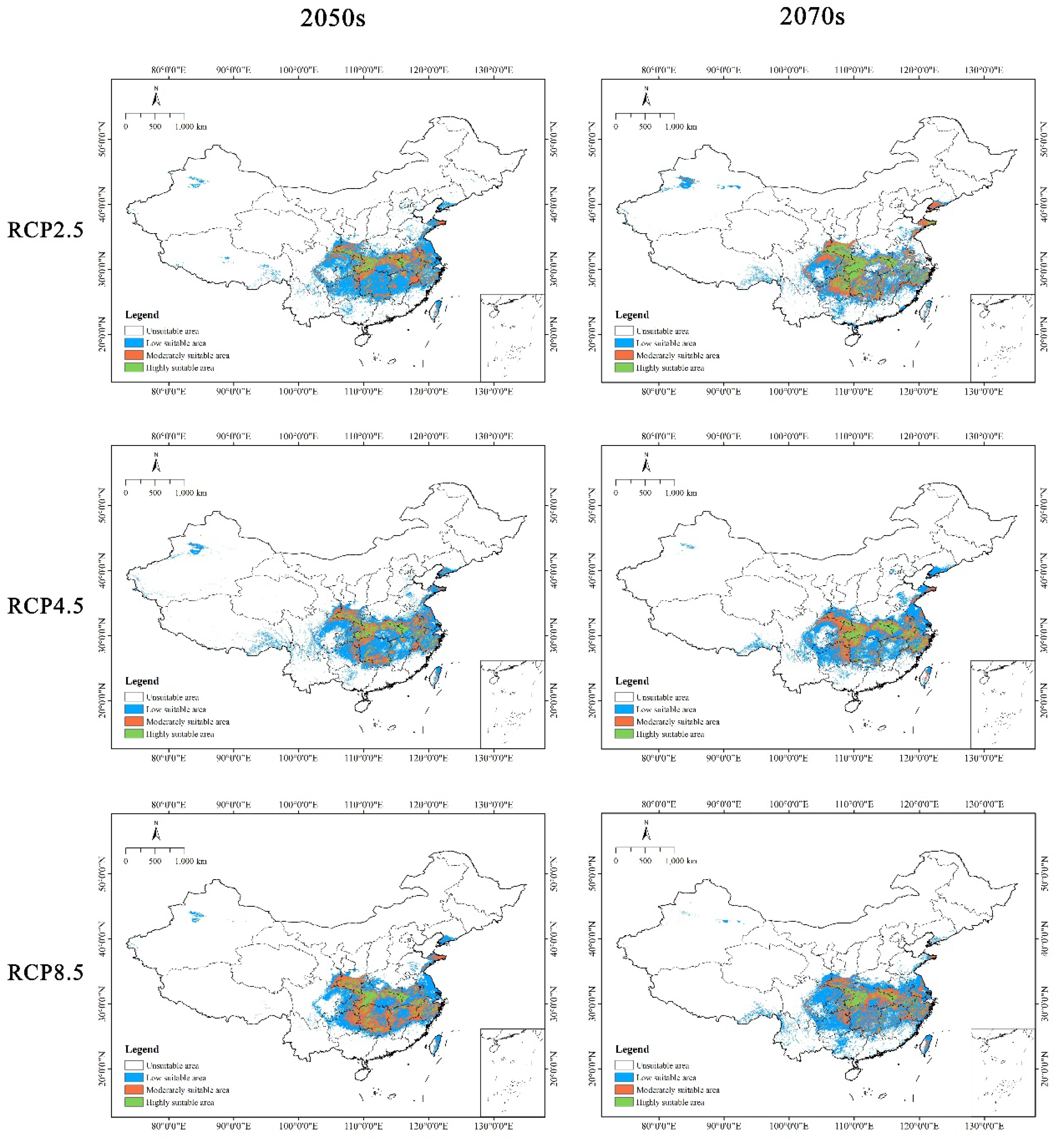
Figure 7.
Migration of the core distribution in suitable areas of C. amoena in China.
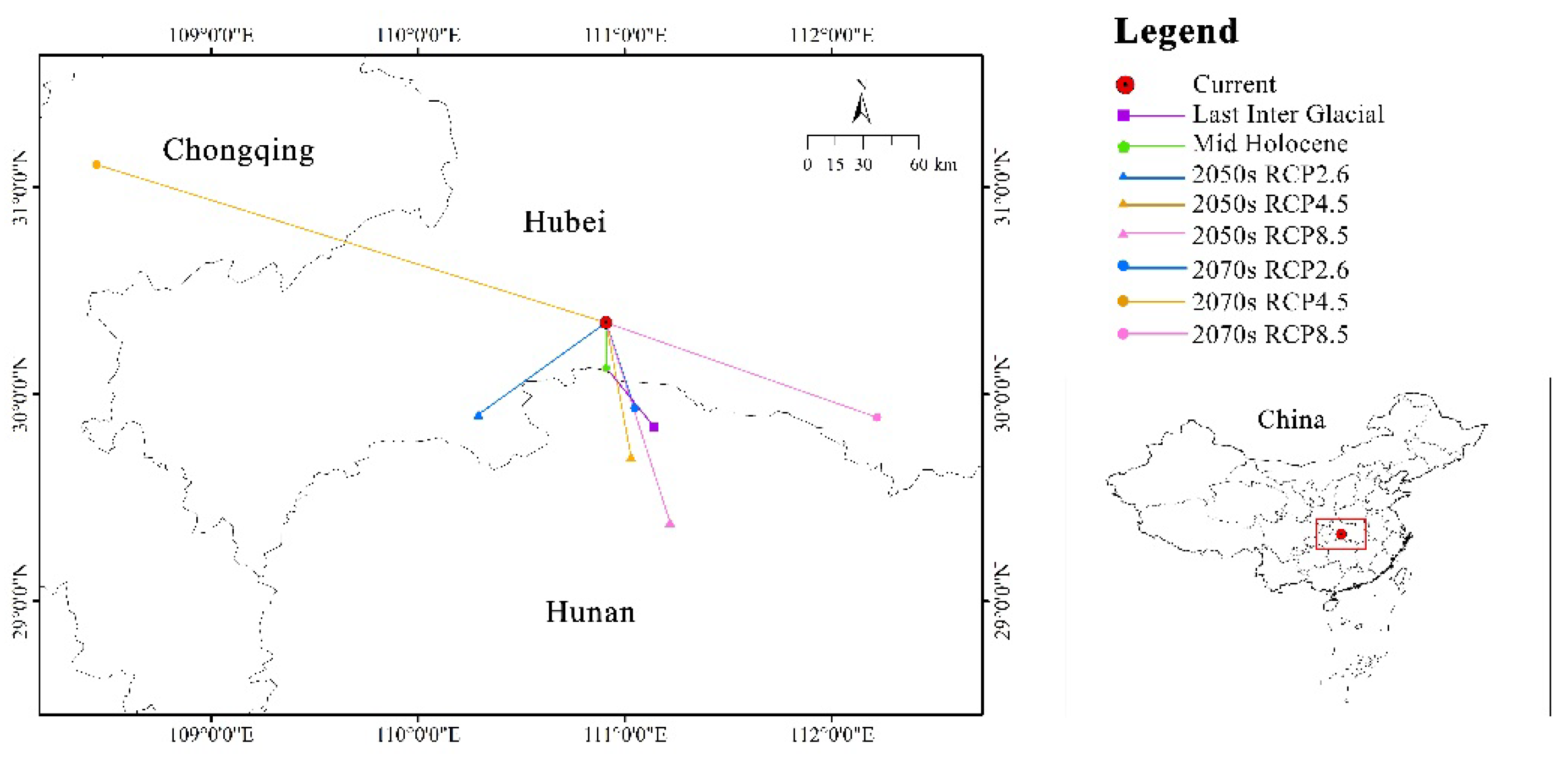
Table 1.
Description of environmental variables and percent contribution of variables (in bold font) used in the final the ensemble model under different climate scenarios. Note: LIG and MH mean the Last Inter Glacial and the middle Holocene, respectively.
Table 1.
Description of environmental variables and percent contribution of variables (in bold font) used in the final the ensemble model under different climate scenarios. Note: LIG and MH mean the Last Inter Glacial and the middle Holocene, respectively.
| Environmental variables | Description | Unit | Percent Contribution (%) | ||
|---|---|---|---|---|---|
| LIG | MH | Current | |||
| Bio2 | Mean diurnal range (mean of monthly(max temp-min temp)) | °C | 38.6 | 37.8 | 43.7 |
| Bio3 | Isothermality ( (Bio2/Bio7)×100) | % | 6.6 | 2.4 | - |
| Bio4 | Temperature seasonality (standard deviation×100) | - | - | 7.1 | 14.4 |
| Bio5 | Max temperature of warmest month | °C | 0.4 | - | - |
| Bio6 | Min temperature of coldest month | °C | - | - | 14.8 |
| Bio7 | 16.2 | - | - | ||
| Bio8 | Mean temperature of wettest quarter | °C | - | 0.1 | 0.4 |
| Bio11 | - | 9.4 | - | - | |
| Bio12 | - | - | 6.4 | - | |
| Bio13 | Precipitation of wettest month | mm | 0.1 | 2.4 | - |
| Bio14 | Precipitation of driest month | mm | - | - | 0.6 |
| Bio15 | 0.4 | 12 | - | ||
| Bio18 | Precipitation of warmest quarter | mm | - | 3.7 | 13.2 |
| Bio19 | Precipitation of coldest quarter | mm | 1.3 | - | - |
| Elevation | - | m | - | - | 1.6 |
| Slope | - | ° | - | - | 2.6 |
| T-BS | Topsoil Base Saturation | % | - | - | 0.3 |
| T-CaCO3 | Topsoil Calcium Carbonate | % | - | - | 0.3 |
| T-CEC-CLAY | Topsoil CEC (clay) | - | - | - | 0.7 |
| T-CLAY | Topsoil Clay Fraction | % | - | - | 0.6 |
| T-ESP | Topsoil Sodicity | - | - | - | 0.6 |
| T-GRAVEL | Topsoil Gravel Content | % | - | - | 0.2 |
| T-OC | Topsoil Organic Carbon | % | - | - | 1.9 |
| T-SILT | Topsoil Silt Fraction | % | - | - | 0.1 |
| T-TEB | Topsoil Exchangeable Base | - | - | - | 0.1 |
Table 2.
Comparison of mean value (±SD) of the area under curve (AUC) and true skill statistic (TSS) of individual models and ensemble model.
Table 2.
Comparison of mean value (±SD) of the area under curve (AUC) and true skill statistic (TSS) of individual models and ensemble model.
| Models | AUC | TSS |
|---|---|---|
| ANN | 0.9082 ± 0.1260 | 0.7526 ± 0.1259 |
| CTA | 0.9098 ± 0.1259 | 0.8120 ± 0.1258 |
| FDA | 0.9393 ± 0.1269 | 0.7587 ± 0.1272 |
| GAM | 0.8127 ± 0.1202 | 0.6222 ± 0.1215 |
| GBM | 0.9653 ± 0.1217 | 0.8596 ± 0.1214 |
| GLM | 0.9301 ± 0.1214 | 0.8029 ±0 .1214 |
| MARS | 0.9358 ± 0.1269 | 0.8122 ± 0.1269 |
| RF | 0.9680 ± 0.1270 | 0.8430 ± 0.1267 |
| SRE | 0.7655 ± 0.1262 | 0.5311 ± 0.1267 |
| MaxEnt | 0.9550 ± 0.0010 | 0.8577 ± 0.0136 |
| Ensemble model | 0.9940 ± 0.1270 | 0.9160 ± 0.1269 |
| Ensemble model (GBM, RF, and MaxEnt) | 0.9950 ± 0.0056 | 0.9330 ± 0.0074 |
Table 3.
Key climatic factors influencing habitat distribution of C. amoena.
| Environmental variables | Percent Contribution (%) | Suitable range | Optimum | Maximum probability of existence |
|---|---|---|---|---|
| Bio2 (°C) | 43.7 | 6.8 -8.9 | 7.4 | 0.73 |
| Bio6 (°C) | 14.8 | -6.0 -2.5 | -2 | 0.85 |
| Bio4 | 14.4 | 7.2 -9.1(×100) | 7.8(×100) | 0.82(×100) |
| Bio18 (mm) | 13.2 | 420 -720 | 625 | 0.84 |
Table 4.
Potential suitable areas of C. amoena under different climate scenarios. Up arrow (↑) means increase; down arrow (↓) means decrease.
Table 4.
Potential suitable areas of C. amoena under different climate scenarios. Up arrow (↑) means increase; down arrow (↓) means decrease.
| Climate scenarios | Unsuitable area |
Low suitable area |
Moderately suitable area |
Highly suitable area |
Suitable area(moderately and highly) | ||||||
| Area(×104 km2) | Trend(%) | Area(×104 km2) | Trend(%) | Area(×104 km2) | Trend(%) | Area(×104 km2) | Trend(%) | Area(×104 km2) | Trend(%) | ||
| Last Inter Glacial (LIG) | 839.70 | ↑2.20 | 56.79 | ↓27.70 | 44.13 | ↑0.32 | 20.38 | ↑42.12 | 64.51 | ↑10.59 | |
| Mid-Holocene (MH) | 835.92 | ↑1.74 | 49.83 | ↓36.56 | 41.89 | ↓4.77 | 33.36 | ↑57.01 | 75.25 | ↑29.01 | |
| Current | 821.64 | - | 78.55 | - | 43.99 | - | 14.34 | - | 58.33 | - | |
| 2050s | RCP2.6 | 820.39 | ↓0.15 | 88.09 | ↑12.14 | 37.49 | ↓14.78 | 12.54 | ↓12.55 | 50.03 | ↓14.23 |
| RCP4.5 | 823.84 | ↑0.27 | 86.18 | ↑ 9.71 | 34.18 | ↓22.30 | 14.31 | ↓ 0.21 | 48.49 | ↓16.87 | |
| RCP8.5 | 827.99 | ↑0.77 | 68.29 | ↑13.06 | 44.88 | ↑ 2.02 | 17.35 | ↑20.99 | 62.23 | ↑ 6.69 | |
| 2070s | RCP2.6 | 817.76 | ↓0.47 | 69.11 | ↓12.02 | 43.90 | ↓ 0.20 | 27.74 | ↑93.44 | 71.64 | ↑22.82 |
| RCP4.5 | 823.45 | ↑0.22 | 80.46 | ↑ 2.43 | 37.67 | ↓14.37 | 16.92 | ↑17.99 | 54.59 | ↓ 6.41 | |
| RCP8.5 | 809.52 | ↓1.47 | 93.90 | ↑19.54 | 41.22 | ↓ 6.30 | 13.87 | ↓ 3.28 | 55.09 | ↓ 5.55 | |
| The mean value of six future climate scenarios | 820.49 | ↓0.14 | 81.01 | ↑3.13 | 39.89 | ↓9.32 | 17.12 | ↑19.39 | 57.01 | ↓2.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








