Preprint
Review
One Health Ethics and the Ethics of Zoonoses: A Silent Call for Global Action
Altmetrics
Downloads
207
Views
66
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
10 August 2024
Posted:
12 August 2024
You are already at the latest version
Alerts
Abstract
This paper presents a critical review of key issues related to the emergence of new networks for the spread of zoonotic diseases amid the mass extinction of species. Zoonotic and infectious diseases account for approximately 70% of new and existing diseases affecting humans and animals. The initial section argues that the term "zoonoses" should not be confined to single-cause events within veterinary medicine. Instead, zoonoses should be viewed as complex, systemic phenomena shaped by interrelated factors, including environmental, sociocultural, and economic elements, influenced by anthropogenic climate change. The second section presents bioethical principles and potential strategies for those engaged in zoonotic disease prevention. The third section uses the slaughter of animals in disaster settings as a case study to illustrate the need for further clarification of normative and interspecies justice conflicts in One Health ethics. This section concludes with an outlook on "zoonoethics." Section four develops the analysis of the interlinked elements that trigger zoonoses and examines antimicrobial resistance (AMR) from an ethical and political standpoint, concluding with policy recommendations for addressing AMR. Section five offers a critical reflection, integrating contributions from zoonoethics, human ecology, and the ecotheological turn. Finally, section six concludes with a call to action and policy recommendations for an inclusive, intercultural, and gender-sensitive One Health approach.

Keywords:
Subject: Public Health and Healthcare - Public, Environmental and Occupational Health
1. Introduction: Incremental Risks of Zoonotic Diseases
Zoonotic pathogens can spread to humans through contact with domestic, farm or wild vertebrate animals [1]. However, invertebrates and other intermediate hosts can intervene in the complex network of emergence and transmission of zoonotic diseases [2,3,4,5] Some zoonotic diseases of animal origin are severe acute respiratory syndrome (SARS), Ebola and, recently, SARS-CoV-2. As shown by Jones et al. [6] (p. 990), most EIDs are zoonoses (60.3% of EIDs), and more than 70% originate from wild animal trafficking [7,8,9,10,11,12,13], increasingly close contact with farm and wild animals, intensive agriculture, unsustainable global food systems, and crimes against biodiversity [14,15,16,17]
The objective of this article is twofold. On the one hand, I want to join the recent call to action to accelerate the operationalization and implementation of the One Health approach (OH), made by the Quadripartite organizations working on One Health (FAO; UNEP; WHO; WOAH, 2022). On the other hand, the article emphasizes that the tools and methods of multispecies justice (MSJ) and ethics of zoonoses can help resolve conflicts between competing moral claims that arise in relation to both human and non-human life. This approach can help address the challenges facing the OH framework. In this article, I address the more established definition of One Health offered by the One Health High-Level Expert Panel (OHHLEP) and the Quadripartite: “One Health is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals, and ecosystems. It recognizes the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely linked and interdependent. The approach mobilizes multiple sectors, disciplines, and communities at varying levels of society to work together to foster well-being and tackle threats to health and ecosystems, while addressing the collective need for healthy food, water, energy, and air, taking action on climate change and contributing to sustainable development.” [18] (p. 2)
Today, there is ample evidence that working transdisciplinary and collaboratively across sectors to accelerate and implement the One Health approach will help build long-term capacities for ‘preparedness, resilience, mitigation, and effective prevention of future epidemics including zoonotic and (re-)emerging infectious diseases and non-communicable diseases linked to environmental risk factors, antimicrobial resistance (AMR) and climate adaptation’ [19,20,21] (p. 3). In this article, I suggest that the emergence and re-emergence of epidemic outbreaks is strongly related to the drivers of anthropogenic climate change, such as abrupt changes in land use, deforestation, ecological racism, ecocide, environmental crimes, and accelerated loss of biodiversity. Although there is still a strong debate between the scope, relevance, and epistemic and normative potential of the One Health and Planetary Health approaches, I emphasize that the best way forward is to articulate these approaches and bring them into dialogue to work together. [22,23,24]
According to the above, in this work I would like to highlight two primary objectives for accelerating and implementing the OH approach. The first priority can be stated as follow: It is imperative that governments, institutions, and civil society take immediate action to halt the systematic destruction of wildlife and put an end to the ongoing ecocide on the planet. Only through precautionary and holistic measures can we hope to see a reduction in the incidence of emerging and re-emerging zoonotic diseases [25,26,27,28,29,30]. As evidenced by a substantial body of scholarly research, there is a robust correlation between the loss of animal species, environmental crime, and ethnocide, and the mechanisms involved in the emergence and spread of zoonotic diseases. Secondly, in order to mitigate the risks associated with emerging infectious diseases (EIDs) and zoonoses in the context of mass species extinction, it is imperative to improve interepistemic and transdisciplinary communication in order to develop a holistic and non-anthropocentric perspective of EIDs and zoonoses [31,32,33,34].
Zoonoses and emerging infectious diseases should not be understood as single-cause events reduced to the world of veterinary medicine but as systemic ecosocial phenomena of increasing complexity that are produced by the confluence of environmental, sociocultural and economic factors linked to the anthropogenic climate change [23,35,36,37,38,39], and the complex nexus between virus flows, global capitalism and ecological racism [40,41,42,43,44,45]. It is evident that infectious diseases cannot be considered as a mere “biological phenomenon”; rather, they are intricately intertwined with the dynamics of modern technoscientific societies and the systematic annihilation of biocultural diversity [40,46,47,48,49]. To understand the new infectious diseases, it is necessary to critically examine the dynamics and flows of the global economy, which creates a socio-political climate of structural pathogenesis [50,51,52,53]. Figure 1 illustrates the network of factors associated with the emergence and spread of zoonoses.
Figure 1 illustrates some of the interdependencies and linkages that stress the health of planet Earth, including but not limited to (i) the widespread use of antimicrobials in the various stages of intensive production of plants and animals for human consumption; (ii) the advance of agricultural intensification is linked to the dynamics of global population growth, abrupt changes in land use, and ongoing crimes against biodiversity; and (iii) the progressive loss of wildlife and anthropogenic deforestation are linked to political and social phenomena such as ethnocide, ecocide, and environmental crimes. This model is intended to convey the idea that the health of human animals is not only inseparable from the environment in which they exist, but is intimately interconnected to the health of animals, plants, and the environment (natural and built). The dotted lines within the diagram allude to the deep interdependence and interconnectedness that affects all elements and layers of the sub-level in question. Finally, the outer lines emphasize that the interdependence and interconnectedness that govern the entire system are driven by dynamics of high complexity.
Zoonotic diseases can emerge and spread rapidly among populations, overwhelming the capacity of institutions responsible for epidemiological surveillance, due to globalized flows of biological agents, human, animal and plant. Accelerated population growth and increased international travel and trade also play a key role in the emergence of zoonotic risks ([3,22,54,55,56,57,58,59,60]. Other anthropogenic drivers that increase the risks of zoonoses are pollution of the seas, overfishing, excessive use of antibiotics in the global food production chain and the advance of “green extractivism” [61,62,63]. Recent studies even show a strong link between plastic pollution and infectious diseases. This link is particularly clear for arthropod-borne diseases (e.g., dengue, chikungunya, Zika, Japanese encephalitis, leishmaniasis, Chagas disease) [64,65]
Industrial poultry, swine, and livestock farms use excessive doses of antimicrobials at various stages of animal production. This practice puts them at risk of generating antimicrobial resistance chains and becoming hotspots for the spread of emerging and re-emerging zoonotic diseases. In fact, the call to “Curb the silent pandemic of AMR” is one of the main red traffic lights that we find in the One Health Joint Plan of Action, 2022–2026 [66]. In fact, the ethical and prudential use of bactericidal and antimicrobial preparations has ceased to be a question for experts and is becoming one of the great ethical imperatives for the construction of viable and sustainable health systems [67,68,69,70,71,72,73,74,75,76,77]
The widespread and prolonged use of antimicrobials in the different stages of intensive animal agriculture represents one of the main drivers for the development of resistant bacterial populations. The ethical use of antimicrobials has strong implications for both human ethics and animal ethics, although this difference is becoming increasingly blurred, and it is noted that the prudent management of zoonoses requires transdisciplinary approaches and interdisciplinary discussions including socio-political and bioethical issues [78,79,80]
Another factor that increases the risk of zoonotic diseases is the unsustainable transport of livestock and poultry, since these farm animals are often transported in unsanitary conditions over long distances, which deepens both animal abuse and the risks of spreading zoonotic diseases [81,82,83]. In other words, the crisis of the global food system, which has led to a decline in the intake of healthy foods with high nutritional value, overlaps with other crises, such as the loss of biodiversity, the water crisis, floods, lack of water treatment plants, lack of hygiene and sanitation, and the contextualized biodiversity crisis [84,85,86,87,88,89,90]
As Vercauteren et al., [91] (p. 3) show: “The livestock compartment and its interfaces with humans and wildlife appeared after domestication. These epidemiological interfaces have constituted opportunities for horizontal transmission between species and a new space for evolution, emergence, and maintenance of pathogens.” Due to anthropogenic stressors and accelerated changes driven by intensive agriculture, new chains of pathogen transmission have emerged that can range from human host epidemics with zoonotic genetic source contribution to animal host epidemics with human genetic source contribution. These chains of pathogen spread can involve primary reservoirs (wildlife), domestic animals as potential reservoirs, intermediate hosts, vector-borne, foodborne, and human hosts [92,93,94,95,96]
The close relationships that humans develop with both companion and wild animals are evident in various instances. Beekeepers form deep connections with insects, while individuals in indigenous communities, as well as divers, filmmakers, and researchers, cultivate friendships and empathy with mammals like whales and sharks, as well as cephalopods, primates, and elephants. Several factors are involved in the spread of pathogens, including wildlife, human and domestic animals, food systems, vectors, bacteria, parasites, fungi, and viruses, among other. While there are situations where nonhuman animals and humans coexist in relative harmony, the increased activity in biotechnology, science, and capitalist economy coupled with the rapid loss of wildlife and biodiversity, has led to close relationships between nonhuman animals and humans becoming a contributing factor to increased vulnerability and zoonotic risks. Zoonotic diseases such as avian influenza, rabies, Ebola, Rift Valley fever, Nile virus, food-borne diseases and antimicrobial resistance pose a threat to the future survival and sustainability of human and non-human communities [20,66,97]
The type of care, relationships, and inter-connections established with companion, farm, and wild animals result in incremental dependency and susceptibility to zoonotic diseases. The entangled communities of human, animal, plant, and microbial agents is a concept that can be supported by critical animal studies, ecofeminism, and symbiotic biology [98,99,100,101,102]. From the perspective of bodily ecology, it is evident that our practices of care and conviviality are deeply embedded in dynamic and complex exchanges with other species, as well as with the genes, memories, practices, and knowledge of our ancestors. Therefore, initiatives to prevent and mitigate infectious diseases must be contextualized and linked to the knowledge and culturally rooted practices of entangled communities.
As I will show in this article, pathogen transmission networks are diverse and complex, involving interconnected relationships and complex interdependencies between human and nonhuman animals and the environment. Influenza and coronaviruses are two typical cases of reverse zoonoses (zooanthroponoses) [103,104,105]. The chains of spread of zoonotic diseases can be direct: by being in contact with saliva, blood, urine or mucous membranes of an infected domestic or wild animal, or indirect, by coming into contact with areas, surfaces or objects that have been contaminated by germs [106] The increasing number of people who choose to live with and keep wild animals as pets has made it more complex and increased the risk of producing zoonoses in various ways [107,108,109]
As illustrated by El-Sayed and Kamel (2020): Endemic diseases can re-emerge in areas where they are endemic, leading to epidemics or increased prevalence. Re-emerging diseases are infections caused by previously known or newly introduced pathogens in new geographic regions. Löscher et al., [4] shows that the factors that explain changes in the dynamics of the spread of emerging and re-emerging pathogens are complex and range from macro-level factors such as population growth and climate change to micro-level factors linked to diet, hygiene, sanitation, and personal disease risks. Major emerging and re-emerging infectious agents in the past decade include Ebola virus in Africa, Middle East respiratory syndrome coronavirus in the Middle East, and Zika, chikungunya, yellow fever, and dengue viruses in the Americas. Another hot topic in One Health is infectious neglected diseases (NTDs). According to WHO (2024), NTDs “are a diverse group of conditions caused by a variety of pathogens (including viruses, bacteria, parasites, fungi and toxins) and associated with devastating health, social and economic consequences”. These diseases have traditionally been associated with poor countries and regions with wide health disparities. However, this dynamic is changing. “Europe is already seeing how climate change is creating more favourable conditions for invasive mosquitos to spread into previously unaffected areas and infect more people with diseases such as dengue” [110] Climate change affects vectors differently; for example, mosquitoes and ticks’ geographical expansion depends on temperature, wind, humidity, and rainfall [111]. Reports indicate increased malaria prevalence due to climate change in Zimbabwe, Brazil, Colombia, among others countries [112,113,114]
In Europe, ‘the main concern is mosquito-borne viruses. These include West Nile, dengue and chikungunya [115] Fortunately, these countries have powerful tools, robust health systems, and the resources to develop AI tools such as The Surveillance Atlas of Infectious Diseases, which can analyze large amounts of data on the risks of mosquito-borne viruses and provide early warnings. As the dynamics related to abrupt changes in land use, tourism, biodiversity loss, and wildlife trafficking deepen, vector-borne diseases increase due to the confluence of environmental, socio-cultural, and abiotic changes in temperature, air, and humidity, among other factors. The phenomenon of global warming, a consequence of climate change, provides an environment conducive to the proliferation of disease vectors that transmit and spread malaria and dengue.
Nevertheless, vector-borne zoonotic disease outbreaks may occur in regions and countries where communities and governments lack the necessary resources and capacity to conduct comprehensive epidemiological surveillance, thereby hindering the ability to trace and control outbreaks in a timely manner.
Figure 2 shows the interrelationship of events that trigger the onset of infectious diseases. There is strong evidence of an interdependence between the global impacts of climate change and increases in local and regional phenomena such as flooding, increased humidity and temperature. As shown by Gibb et al., [116], zoonotic host diversity increases as ecological relationships are disrupted by a wide range of economic and human activities. They "show that land use has global and systematic effects on local zoonotic host communities”. As Mahon et al., [117] (p. 234) states: “The fact that many global change drivers increase zoonotic parasites in non-human animals and increase all parasites in wild animals suggests that anthropogenic change might increase the occurrence of parasite spillover from animals to humans and, therefore, also pandemic risk.”
To understand the complexity of the interdependencies and linkages behind the long chains of zoonotic disease spread, we must continue to deepen the holistic perspective of One Health, which seeks to build bridges to address all risks and vulnerabilities that arise in overlapping communities of life [91,118,119,120,121]. In the context of ongoing technological change is urgent rethink the connections between ethics, climate change, technologies, and agricultural practices [122]. The relationships between endemic and re-emerging zoonotic diseases and the societal and anthropogenic drivers of climate change are becoming increasingly complex [123,124]
Moreover, international transportation and global trade of vast quantities of food, animals, and people are key factors in pathogen transmission networks. Viruses, microorganisms and pathogens spread as quickly as people do in a hyper-connected and interdependent world. As [124] (p. 195): “In modern times, air travel resulted in the importation of severe acute respiratory syndrome (SARS) coronavirus to 27 countries before transmission was halted.” And indeed, advances in health systems, sanitation, and tools for disease detection and tracking have led to reductions in overall mortality and morbidity from infectious diseases, particularly in high-income countries. As usual, however, scientific progress has not been evenly distributed around the world. As Baker et al., (Ibid., p. 193) caution: “infectious disease burden remains substantial in countries with low and lower-middle incomes, while mortality and morbidity associated with neglected tropical diseases, HIV infection, tuberculosis and malaria remain high. […] This points to a possible new era of infectious disease, defined by outbreaks of emerging, re-emerging and endemic pathogens that spread quickly, aided by global connectivity and shifted ranges owing to climate change”.
The nonlinear and increasingly complex events that trigger infectious diseases in the Age of Extinction are such that they cannot be understood from a single discipline or with narrow methods. While there is evidence that poverty, poor sanitation, and inadequate sewage and water systems provide a breeding ground for the emergence and spread of infectious diseases such as malaria and cholera, other diseases like those caused by SARS-CoV-2 may be related to increased affluence, population growth, and wildlife trade chains in megacities. The socioeconomic factors affecting the spatial distribution of potential risk zones must continue to be carefully studied [125,126,127,128,129]
2. Zoonoethics, Global Bioethics and One Health: We need “One Bioethics”?
In this section, I introduce a set ethical principles and potential strategies that zoonoethicists concerned with preventing emerging zoonotic diseases should consider. These guidelines aim to inform public policy and guide decision-making for communities, citizens, decision makers, and stakeholders. On the one hand, Zoonoethics, understood as a field of research in applied ethics and veterinary medicine, can promote rational, evidence-based discussions to exercise prudent judgment and public deliberation based on constructive conflict to resolve normative disputes about “conflicting value claims” linked to the health of humans, animals and environments. On the other hand, Zoonoethics must be rooted in the precautionary principle and provide normative and descriptive knowledge to inform decision makers and interested parties in the construction of public policies aimed at preventing EIDs. While not a definitive solution, the precautionary principle can provide a framework for navigating disputes over competing values and assets and for developing proactive measures to anticipate and assess the risks associated with ERIDs in uncertain contexts [130,131,132,133,134,135] The precautionary principle, when considered in conjunction with epistemic deliberation and prudent judgment, can inform the theory of change that guides epidemiological judgments and decisions at the One Health initiative [30,136,137,138,139]
In light of the statements above, it is possible to sustain a third claim: Zoonoethics can serve as a “bridge” to unite diverse epistemic fields and establish transdisciplinary and inter-paradigmatic debates between scientific, religious, cultural and ecosocial pluriverses [140,141]. One Health initiatives against infectious diseases should not underestimate the impact of ethnic, religious and cultural diversity on public health strategies and campaigns to reduce the spread of zoonoses. [142,143,144,145].
At the epistemic level, zoonoethics could help weave networks of understanding between knowledge from veterinary medicine, biomedical sciences, animal agriculture, epidemiology, antimicrobial resistance, global bioethics, human ecology, conservation sciences, biocultural ethics, and environmental humanities, among other fields [3,22,23,43,46,47,54,57,137,146,147,148,149,150]
In this article, I introduce the term "zoonoethics" to highlight the necessity for an interdisciplinary field of work that integrates resources derived from normative ethics and veterinary medicine. The term "zoonoethics" can be understood as an interdisciplinary field that addresses ethical concerns related to the intersection of human, animal and environmental health. As demonstrated in another work [151], the term "zoonoethics" follows a similar path to "global bioethics" which strives to integrate insights from biological and human sciences for future sustainability [152,153,154]. Potter's vision facilitated the integration of public health concerns with broader issues pertaining to the sociocultural determinants of health and global challenges such as pollution, biodiversity loss, technological change, and the impact of accelerated population growth on future sustainability [141,155,156]. Zoonoethics can be understood as a subset of One Health ethics, encompassing a broader range of concerns for human, animal and ecosystem health, including concerns for future generations. These concerns include the ethics of antimicrobial resistance, ethics of species conservation, nonhuman ethics, wildlife ethics, and the ethics of global food. Therefore, the zoonoethics I am attempting to delineate can be regarded as a subfield of One Health ethics, rather than as another "silo" [150]. By situating zoonoethics within the One Health approach, it takes a more holistic view of the ethical challenges arising from the interaction between humans, animals and environments.
The zoonoethics should be focuses on the management and prevention of zoonotic diseases in three specific areas:
i) Primary prevention: zoonoethics, in close collaboration with the One Health ethics [29,30,139,157,158,159,160] can offers descriptive and epistemic resources for transforming our understanding of and interconnections with nonhuman beings and environments (both natural and built), as well as for clarifying our relationships with them. Primary prevention aims to anticipate risks before they become fully manifest. As Plowright et al., [161] (p. 2) show: “Primary pandemic prevention is the set of actions taken to reduce the risk of pathogen spillover from animals to humans, focusing on processes upstream of the spillover event”. The first preventive measure to reduce zoonotic risks and the spread of viruses with pandemic potential is to practice ecological wisdom related to the holistic protection of species and the care of the biodiversity of ecosystem hotspots. As highlighted by Vora et al.,[162] (p. 420), “tropical and subtropical forests must be protected”.
ii) Secondary prevention: Primary prevention is, by definition, anticipatory, while secondary prevention focuses on the implementation of specific measures, such as early detection, vaccines, improved health systems, drug therapy, but these are established in the ongoing process of preventing the outbreak from becoming an epidemic or pandemic [161,163,164]. At this stage, zoonoethics can provide a framework for setting standards and guiding policy makers on what actions are most effective, fair, and ethically relevant to prevent the escalation of the outbreak. One of the paradoxes of primary prevention is that it is largely undervalued as an effective strategy for responding to pandemic risks, while the great paradox of secondary prevention is that many interventions focus on implementing public health measures that may have adverse effects in order to contain the spread of spillover. However, there is sufficient evidence to support the idea that the most cost-effective, wise and politically relevant strategy for preventing future pandemics is to invest in prevention and capacity building in the context of future sustainability [2,20,165,166].
iii) Antimicrobial Stewardship (AMS): Zoonoethics can help to address the problems associated with antimicrobial resistance and to develop a more robust and comprehensive approach to AMS [167,168,169]. As Shallcross et al., [77] (p. 4) stresses: “If AMR is allowed to continue unchecked, we may enter a ‘post-antibiotic era’ of medicine, in which treatments from minor surgery to major transplants could become impossible, mortality will rise, and healthcare costs will spiral as we resort to newer, more expensive antibiotics and sustain a greater number of longer hospital admissions”. AMS is an ethical approach that takes very seriously the growing threat of entering a "post-antibiotic era". Curbing the emergence and spread of these resistant organisms and agents is a high priority not only in epidemiological surveillance and drug development programs, but also in inclusive public policies aimed at reducing inequities in access to safe and affordable medicines for all. As Dyar et al., [170] (p. 793) states: “Although antimicrobial stewardship originated within human healthcare, it is increasingly applied in broader contexts including animal health and One Health”. Experts in zoonoethics can significantly contribute to the efforts of antimicrobial stewardship (AMS) in addressing this issue. Zoonoethics can provide valuable insights and frameworks that can enhance the AMS strategies across different domains—animal health, human health, agriculture, and livestock. Firstly, zoonoethics emphasizes the interconnectedness of ecosystems and the ethical considerations that arise from this interconnectedness. By integrating zoonoethics principles, AMS experts can develop more holistic policies that consider the welfare of all species affected by antimicrobial use. This approach aligns with the One Health perspective, which recognizes the interdependence of human, animal, and environmental health. Moreover, zoonoethics can offer tools such as ethical risk assessments and value-based decision-making frameworks. These tools enable stakeholders to evaluate the implications of antimicrobial use and resistance not only from a scientific standpoint but also through an ethical lens, considering the long-term consequences for all species. For instance, ethical risk assessments can help identify practices that may inadvertently contribute to AMR and propose alternatives that are ethically and ecologically sound. My perspective on the problem of antimicrobial resistance is enriched by the multispecies justice approach, which seeks to integrate other species and wildlife into risk analyses [171,172,173].
Finally, one of the “heat issues” that zoonoethics must address is the fair, careful and ethical treatment of companion animals, farm and wild animals. In our techno-scientific society, it is generally assumed that the slaughter and killing of farm animals is justified in regard to ensuring food security for a voracious and exponentially growing human population. Nevertheless, killing animals without a “proper purpose” and sufficiently legitimate reasons is considered ethically incorrect by some scholars [174,175,176,177,178,179]. However, in many practices such as intensive agriculture, the culling of animals to mitigate biodiversity loss caused by invasive species, and medical research, among others, animal death is not the exception but the norm. There is still an open debate about whether the issue of death and the conditions in which animals are slaughtered, especially on farms, is properly an animal welfare issue [180,181,182,183,184,185,186]. On the other hand, the issue of euthanasia or "good death" of animals in zoos would be part of a zooethics rather than the field of Zoonoethics, which I am attempting to outline [187].
In this work, I will try to approach the question of the appropriate treatment of animals from an alternative approach, that of ecological and global bioethics [153,188,189,190,191,192]. Now, it is necessary to clarify that my vision of bioethics is opposite to the vision of Fiore [193] (p. 316) who maintains that the term environmental bioethics “seems qualified, derivative: a subgenre of biomedical ethics or environmental ethics rather than the ground for both.” My vision of global bioethics, grounded by the Potter’s works [194,195] is nearer to that of authors such as Gardiner [196]; Lee [146]; Macklin [144,197]; ten Have [145] and Valera & Rodriguez [154]. By rediscovering the normative significance and global appeal of bioethics and its interest in nonhuman ethics, global public health, and climate justice, we can build bridges and pathways between multispecies justice, ecological bioethics, and zoonoethics. [198]
Currently, there is renewed interest in rethinking and expanding the global agenda of bioethics, as it was conceived at the time by Van Rensselaer Potter [192,199,200,201,202]. I have shown that the One Health and Planetary Health approaches would gain much more traction and be easier to operationalize and put into practice if we reinvigorated the globality of bioethics in light of recent discoveries about the complex mechanisms of emergence, transmission and massive propagation of EIDs and zoonoses revealed during the pandemic [9,23,36,203,204,205,206,207,208,209].
If we recover the globality and multidimensionality of bioethics, we can imagine a bridging, comprehensive, and intersectional bioethics that serves to deepen dialog, transdisciplinary communication and cooperation between countries and regions for an early and prudent response to future outbreaks of EIDs and zoonoses[200,210,211].
This tension between One Bioethics and grassroots justice claims, or, in other words, between the globality of bioethics and the and context-dependent character of justice claims, is very interesting and complex, but it is beyond the scope of the present paper. Here I can only offer a few hints. First, One Bioethics, both in its epistemic roots and in its practical scope, should be both local (context-dependent) and global, because the problems it addresses − related to technological change, transhumanism, biomedicine, AI in health care, climate change, biodiversity loos, ERIDs, etc. − are both local and global, and require global action, but they also require deliberative approach and situated hermeneutics. The need to respond to the complexity and uncertainty of the problems we are dealing with is at the heart of the glocalization of bioethics [212]. Moreover, bioethics must be sufficiently dynamic and retain its deliberative and dialogical character to remain relevant across different cultural universes [212,213,214,215,216,217,218,219]. Once again, one of the most challenging issues in One Bioethics requires the elaboration of “bridges” and inter-epistemic dialogues between different epistemologies, value systems, cultures, religions, disciplines, worldviews, and social practices.
Second, whilst it is true that individuals assign varying values to species, nonhuman animals, ecological entities, and artificial objects (e.g., paintings, forests, violins, plants, rivers, lakes, landscapes), and that such values are contextual and culturally embedded, this should not diminish the globality of bioethics. My argument, therefore, is for a more humble and dialogical conception of One Bioethics, one that remains open to the uncertainties of a changing world [194,220,221,222,223] The globality of One bioethics is also an invitation to create a window for broad collaborative and interdisciplinary work, and there should be room for diverse symbolic universes, values and norms, and ultimate belief systems. Ethical evaluation and deliberation, in short, must be contextualized, but the global vision of vital problems can also serve as an impetus to tighten the thread of ethical reflection, which, as we know, can sometimes be transboundary, interspecies, transgenerational, and intercultural. Of course, the question of cross-cultural moral evaluation is closely related to the very possibility of engaging in inter-paradigmatic dialogues, and raises the very serious question of intercultural, interreligious dialogue and tolerance [202,224,225,226]. The question is to what extent we are willing to accept as legitimately valid ways of evaluating and assigning different kinds of moral, aesthetic, and religious value to different objects or entities from other cultures, and how willing we are to recognize and value non-Western symbolic universes and value and belief systems.
As Gardiner [196] (p. 571) emphasizes: “conventional bioethics has largely failed to engage, and so is left mainly to contribute to damage limitation, emergency management, and redress.” A way to overcome this limitation and avoid falling into the vices of a superficial and overly technical bioethics that only limits itself to acting when it is too late, but is incapable of providing normative, descriptive knowledge and practical wisdom to guide decision-making and anticipate risks, is to move toward what some authors have called “One Bioethics”[227,228] and One Health ethics [29,30,229]. One bioethics and One Health ethics could offer valuable normative and epistemic resources to prudently address the risks derived from the emergence and spread of EIDs and zoonoses in the Anthropocene Age.
Table 1 present some bioethical and zoonotic ethics guidelines to face zoonotic diseases. These guidelines aim to inform public policy and guide decision-making for communities, citizens, decision-makers, and stakeholders. The table below outlines practical guidelines for addressing infectious diseases, emphasizing surveillance, interdisciplinary research, public education, and sustainable policies. Effective crisis management, international cooperation, One Health initiatives, ethical research standards, and community empowerment are essential components to enhance prevention, detection, and control of zoonotic diseases, highlighting the interconnectedness of human, animal, and environmental health.
3. The Ethical Dilemmas of One Health: The Case of Animal Slaughter, Outline of One-Zoonoethics
In what follows, I briefly discuss the dilemma of mink culling in the pandemic scenario from the perspective of One Health. In 2020, the Prime Minister of Denmark, Mette Frederiksen, made the decision to sacrifice approximately 15 million minks due to a COVID-19 mutation identified on farms in the Nordic country. Denmark is the world's leading producer of mink fur. The main argument in support of this decision was that the mutation detected among minks could infect humans and put the effectiveness of a new vaccine at risk. As illustrated in this case, certain anthropocentric measures to control zoonoses could give rise to ethical dilemmas related to the improper treatment of animals when human health is at risk. In order to resolve ethical dilemmas between competing value claims, we need to deepen the debate on multispecies justice [230,231,232,233,234,235,236,237].
In addition, we need to clarify what we say when we want to include the value claims of animals, ecosystems, and ecological collectives within One Health. Additionally, we need to clarify what we mean by incorporating the value claims of animals, ecosystems, and ecological collectives within One Health. Ethical concerns about wildlife protection and the sources of ecological normativity and values are key elements of the ethics of One Health [47,238,239,240,241,242,243,244]. To advance the revitalization and acceleration of One Health, we need to develop a long-term vision of One Health policy that can positively value “entangled empathy” and relational vulnerability between different species [100,101,245]. In this part, I argue that in order to responsibly address ethical and political issues related to the slaughter of animals in epidemic or pandemic emergencies, reactive measures based on crude utilitarianism are not enough; rather, we need to deeply rethink the practices and imaginaries associated with the mass slaughter of animals and develop new political processes, programs, and management tools to ensure that justice can be extended to both human and nonhuman animals [246,247].
In the case of zoonotic diseases, the moral meaning that people attribute to animals is context dependent; the statements and emotions pro- and against animal culling change meaningfully depending on the uncertainty and risks involved: the decisions made in a “normal scenario” are not the ones the same as those made under catastrophe scenarios. As Van Herten et al. [248] (p. 30) state: “In cases where human health is at risk, most people justify the culling of healthy animals. In situations where there is no danger that humans become infected, culling is less accepted”. The effectiveness and impact of measures to address emerging and re-emerging zoonotic disease risks depend on the belief systems, values, and assumptions of individuals and communities [249]. Many times, unproven beliefs concerning wildlife and anthropo-centric value schemes can become strong agencies that hinder the adoption of holistic health measures from a comprehensive One Health perspective [24,54,148,250]
Since 2008, The American Veterinary Medical Association established the concept of One Health, envisioning the possibility of fighting for the maintenance of certain levels of “optimal health” and establishing win‒win solutions for all parties involved: human life, nonhuman life and environments: “One Health is the collaborative effort of multiple disciplines—working locally, nationally, and globally—to attain optimal health for people, animals and our environment”[251]. This definition has evolved over time, particularly to address critiques of a superficial and anthropocentric view of One Health[18]. However, controversies persist, especially from ethicists who advocate for a deeper and broader understanding of animal welfare and rights. The One Health approach recognizes the interdependence and complex interconnectedness among humans, animals, environments, and the entire planet's biosphere. Nevertheless, as Johnson et al., [252](p. 185) notes: “although OH approaches commonly mention animal welfare, they have given minimal attention to animal health for the sake of animals. The anthropocentrism for which OH has been criticized instrumentalizes animals by recognizing their value only insofar as it contributes to human flourishing, thereby reinforcing the very anthropocentrism that justifies exploitation of farmed animals, encroachment on animal habitats, and the wildlife trade that OH purports to address”.
One Health scholars must take these legitimate criticisms very seriously and strive to address them. Only the implementation of a comprehensive and non-anthropocentric One Health vision can accomplish the promise of discovering fair and equitable solutions considering the vital interests of all parties involved, including both humans and nonhuman entities. As highlighted by Meisner et al.,[253]: “Biomedical reductionism in One Health has resulted in a focus on human health threats from animals”. This demands a clarification of the concepts of interdependence, complexity, and interconnectedness that are at the core of One Health [157,158,254]. To build networks of care, solidarity, and kinship among nonhuman animals, communities, and environments, it is essential to reevaluate the normative and descriptive content of health and to consider ecological goods precisely as "common goods" rather than "natural resources," a term still used by some One Health scholars [255]. This involves recognizing and accepting as legitimate the value claims of various actors, both human and non-human, who are connected to an intergenerational network of interests [238]
As Nussbaum [237] and Pelluchon [256] recently emphasized, the unjustified suffering and harm inflicted on animals, such as in the mass abandonment of pets or large-scale animal slaughter, although often normalized, reflect the extreme violence, arrogance, and indifference toward both human and nonhuman life. And all these forms of violence “are also injustices: we grant ourselves absolute sovereignty over sentient beings whose ethological needs and subjectivity should limit our right to exploit them as we see fit”[257] (p. 21). In the case of mink, the decision in favor of animal culling could be justified under the “harm principle”, initially introduced by Mill [258], which authorizes, under exceptional circumstances in which certain human assets are at serious risk, the 'legitimate' use of violence. The crucial point here is to precisely determine what is truly at stake when a government attempts to take exceptional measures to protect economies and public health [259,260,261,262,263]. This decision, based on political exceptionalism, created a domino effect and spread to other countries such as Holland, Sweden, Greece and Spain[264,265]. This case illustrates the need for zoonoethics articulated to a comprehensive, multidimensional and nonanthropocentric vision of One-Health [3,29,30,147,229]. In the case of the animal culling during human pandemics, we must face two relevant ethical questions: first, is this an attitude of caution or an overreaction driven by panic? And, second: Who loses and who wins after the sacrifice of animals that are conceived as an “extra factor” in the global fur industry, with an annual value of more than 22 billion dollars a year? [266,267,268]
To address the issue, it is necessary to reactivate the ethics of care and the profound and genuine well-being of wild, farm, and companion animals. Living in a respectful and just manner with nonhuman animals is possible and represents the most cost-effective long-term solution. However, we need to consider a zoonoethics of sustainability and care by ensuring the well-being of farm and wild animals, which are reservoirs of zoonotic agents, and by immunizing them against pathogens whenever possible. Zoonoethics reminds us that to address the risks of infectious diseases, we need a healthy dose of prudence, caution, and common sense. As emphasized by Halabowsk and Rzymski [269] (p. 4): “The COVID-19 pandemic is the evidence that mink farming for fur represents a potential target of coronavirus spillovers and, consequently, a source of infection in humans who have contact with the animals. The ultimate solution to this is the development of an effective vaccine against SARS-CoV-2 in mink”. Finally, it is necessary to articulate a cautious perspective of the precautionary principle to zoonoethics to prevent control methods for managing zoonoses from bringing unforeseen consequences and unwanted harm to humans, animals and environments[135,270,271,272].
As warned by Resnik: “Some of the methods used to prevent mosquito-borne diseases, such as draining swamps and spraying pesticides (especially DDT), can have adverse environmental impacts, such as the destruction of habitats and species” [273](p. 2). Other cases that can illustrate the ethical dilemmas within One Health are the sacrifice of healthy surplus farm animals [244] and euthanasia or the “good death” of animals at zoos[187,274]. These cases, as seen by several scholars, are complex and go beyond the traditional tension between the positions of “welfarists” and “animal rights advocates”.
Table 2 presents policy recommendations aimed at overcoming ethical dilemmas associated with animal culling during pandemics. The first recommendation emphasizes the need to develop alternatives to culling, such as vaccination or quarantine measures, to reduce the need for mass culling and minimize harm to animal populations. Strategies to prevent the immoral slaughter of minks and other animals, such as phasing out the fur industry or implementing vaccination programs, are not always well received by all audiences. Sometimes, the economic argument is prioritized: "maintaining and investing in the well-being of farm animals can be very costly”[275], but failing to act or acting reactively can lead to increased costs and heightened risks at the complex human-nonhuman-environment interface[276]. The second recommendation focuses on strengthening ethical review processes to ensure decisions involving animal culling are evaluated through a comprehensive lens of multispecies justice and ecological impacts. This recommendation is backed by several scholars who stress the need for ethical and critical contextualized scrutiny in One Health interventions and policies [172,173].
Table 2.
Ethical guidelines for addressing ERIDs and avoiding unnecessary animal culling. Source: Author 2023.
Table 2.
Ethical guidelines for addressing ERIDs and avoiding unnecessary animal culling. Source: Author 2023.
| Guideline | Description | Problem/Challenge | Authors |
|---|---|---|---|
| 1. Develop Alternatives to Culling | Invest in research and development of alternatives to animal culling, such as vaccination or quarantine measures. | Reducing the need for mass culling and minimizing harm to animal populations. | [276,277] |
| 2. Strengthen Ethical Review Processes | Implement stringent ethical review processes for animal culling decisions, ensuring consideration of multispecies justice and ecological impacts. | Ensuring ethical considerations are thoroughly evaluated before making culling decisions. | [135,248,278] |
| 3. Surveillance and Monitoring | Establish systems for early detection and tracking of zoonotic diseases to respond quickly and effectively. | Timely detection and response to emerging zoonotic diseases. | [18,111,163,279] |
| 4. Research and Development | Promote interdisciplinary research and develop new vaccines and treatments to combat zoonotic diseases. | Addressing gaps in knowledge and developing effective interventions. | [164,280,281] |
| 5. One Health Education and Ecological Awareness | Incorporate OH Core competencies and strenght educational programs including zoonoethics and AMR ethics for children, youth, and future professionals across curriculum. | Increasing public understanding and engagement in zoonosis prevention. | [282,283,284,285,286] |
| 6. Policies and Regulations | Create and enforce regulations to manage human-animal interactions and support sustainable practices. | Implementing bioetical and legal frameworks to prevent zoonotic disease transmission. | [19,20,287,288] |
| 7. Ethical Disaster Management and Global Cooperation | Develop and implement ethical guidelines and rapid response plans for health crises and natural disasters, protecting animal welfare and preventing zoonotic outbreaks. | Ensure preparedness, minimize harm, and respond ethically to zoonotic outbreaks during crises. | [150,289,290,291,292] |
| 8. Open Science for future Pandemic Resilience | Develop future-oriented OH policies that enhance data analysis capabilities to understand disease dynamics and ensure the availability, quality, and management of accurate data for evidence-based decision-making. | Open science can greatly enhance OH pandemic responses by enabling rapid data sharing and collaboration. | [293,294] |
| 9. Engage Local and Indigenous Communities | Engage local communities in zoonosis prevention and control, while fostering intercultural dialogue by integrating indigenous perspectives into One Health. | Ensuring local communities have a voice and active role in zoonosis prevention efforts in all levels. | [33,295,296,297] |
|
10. Sustainable and Resilient Health Systems (SRHS) |
Health systems need to develop new capacities and build synergies with other sectors and organizations to address risks of ERIDs. | Build better, more climate-resilient and environmentally sustainable health systems. | [10,298,299] |
| 11. Enhance Biosecurity Measures | Implement comprehensive biosecurity protocols in intensive farming operations, including regular health monitoring and rapid response plans. | Preventing the spread of infectious diseases within and between animal populations to avoid large-scale outbreaks. | [55,300] |
| 12. Promote Sustainable and One Welfare Farming Practices | Encourage sustainable and farming practices guided by interspecies ethics, reducing animal density and improving living conditions to lower disease risk. | Mitigating the conditions that facilitate the spread of zoonotic diseases in high-density farming and livestock. | [301,302,303] |
Table 2 includes additional policy recommendations aimed at preventing the repetition of mass culling in intensive animal farming. The final recommendation (11) advocates for enhancing biosecurity measures, which involve implementing rigorous health monitoring and rapid response plans in farming operations. By preventing the spread of infectious diseases within and between animal populations, this approach aims to avoid large-scale outbreaks, as highlighted by several studies [20,21,165,209]. The end recommendation focuses on promoting sustainable farming practices, such as reducing animal density and improving living conditions. This strategy aims to mitigate the conditions that facilitate the spread of zoonotic diseases in high-density farming environments, as discussed by [304,305,306]
4. Antimicrobial Resistance at the Human-Animal-Environment Interfaces: A Call for Global Action to Face the Antimicrobial Resistance (AMR)
This section is a silent but urgent call for global action and inter-epistemic dialogue on antimicrobial resistance (AMR). First, the best way forward in implementing a OH approach is to act preventively under three core principles: precaution, eco-wisdom and responsibility from a comprehensive One Health for all, including value claims and the interests of humans, animals and environments [135,271] As noted early by Potter [195,307], human and animal healthcare practices generate a large amount of waste and pollution that can harm the environment and have long-term and systemic effects on planetary health. The environmental impact of hospitals—due to plastics, syringes, waste, and high energy consumption—is increasingly problematic for managing zoonotic and ERIDs. This situation is further exacerbated by the challenge of antimicrobial resistance[308,309], as the high demand for medical supplies and energy-intensive practices contributes to pollution and creates conditions that can fuel the spread of resistant pathogens. Poultry production and intensive livestock farming are clearly linked to the spread of zoonotic diseases [310,311]. These practices not only create favorable conditions for the transmission of pathogens between animals and humans but also accelerate the emergence of antimicrobial resistance [312]. The intensive use of antibiotics in these production systems contributes to the evolution of resistant strains, further complicating the control of zoonotic diseases and posing a significant threat to global public health.
Second, today, we have solid evidence about the incredible complexity of the mechanisms of spread of ERIDs and zoonoses that originate largely from the same drivers of global climate change. The nexus between wildlife trafficking, biodiversity loss, and environmental crimes lies at the root of the increasing zoonotic outbreaks driven by land use changes, wildlife exploitation, and hunting [8,11,313]. Therefore, it is essential to incorporate the systematic study of environmental crimes within One Health environmentalism and to design multisectoral strategies to address ecocide [313]. From the One-Health approach, it has become commonplace to say that addressing zoonoses and ERIDs requires greater communication, collaboration and interdisciplinary work between zoologists, virologists, veterinarians, foresters and environmental humanities scholars. However, in practice, collaboration and inter-epistemic dialogue between experts in veterinary science, epidemiology, wildlife, and biological sciences on one side, and bioethicists, environmentalists, policymakers, and interested communities on the other, remain quite low. Therefore, we must continue strengthening efforts in One Health education and interdisciplinary training for various professionals and practitioners [254,284,314,315,316].
Another problem is that very few public policy experts understand the high complexity of the issues involved in zoonoses and the challenges of One-Health from a complex and multispecies justice approach. As noted by Waltner-Toews [254] (p. 7): “However, few animal science researchers, standing in their hazard suits and masks in a conventional chicken broiler barn, have made the mental connections between poultry rearing and ducks flying overhead, or between agribusiness and viruses and bacteria for which the ducks are a quiet, accessible vehicle for long-distance air travel”.
At a meeting in March 2023, the Quadripartite made an emphatic Call to action for One Health for a safer world. It calls for enhanced collaboration among sectors like human, animal, plant, and environmental health to create a safer, more resilient world. The call also highlights the need for integrated policy frameworks and investments to address health challenges at the human-animal-environment interface. And the latest World Health Assembly held in Geneva this year concluded without a clear agreement on the conditions and scope of the Pandemic Accord [317]. This brings me to my third and final call. Perhaps one of the problems that best illustrates the importance of zoonoethics is the problem of AMR. Until approximately fifty years ago, the problem of AMR was basically understood as a problem of interest to microbiologists, virologists and a small group of economists interested in the development of antibiotics and new drugs. Over the past two decades, significant efforts and transdisciplinary collaboration have highlighted that the indiscriminate use of antimicrobials is a major driver of antimicrobial resistance (AMR). This issue has emerged as one of the top ten ethical and political challenges facing humanity. AMR is closely linked to broader global crises, including food insecurity, poverty, lack of clean water and sanitation, inadequate primary healthcare, and failures in waste management and public health policies aimed at infection control [308,312,318,319]
The WHO has corroborated that all of these factors promote the spread of microbes, some of which may be resistant to antimicrobial treatment. This explains why the concept of antimicrobial stewardship has gained so much worth in recent years [170,320]. Reducing the AMR problem to a hospital issue would be irrational and could perpetuate injustices. The widespread use of bactericides in the care of humans, animals and agriculture means that we are in the middle of a malignant circle of bioaccumulation of bacteria and microorganisms resistant to antibiotics. As pointed out by several authors, antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs) are found beyond hospital settings, often originating from natural environments like soil and water. Environmental strains can transfer ARGs to human pathogens[320]. Therefore, identifying sources, distribution, and anthropogenic factors of is crucial for developing strategies to combat antibiotic resistance[71,73,80,171,321]. Table 3 presents a set of policy guidelines for addressing AMR comprehensively and with interspecies equity.
Finally, is important make a call to the Quadripartite and WHO to reinforce educational campaigns to inform the public and healthcare providers about the responsible use of antibiotics [340,341]. Increasing awareness about the consequences of antibiotic misuse and promoting proper prescription practices can reduce unnecessary antibiotic consumption and slow the spread of resistance [69,74,75,76]. An ethical approach to AMR must address the gaps and injustices that persist in equitable access to medications and medical treatments: [341] “Many people around the world still do not have access to antimicrobials. Ensuring equitable and affordable access to quality antimicrobial agents and their responsible and sustainable use is an essential component of the global response to antimicrobial resistance”. These recommendations provide a comprehensive framework to address AMR through an ethical and policy lens, incorporating the One Health approach and emphasizing the interconnectedness of human, animal, and environmental health.
5. Zoonoethics, Intercultural Dialogue and Entangled Empathy: A Silent Call for Interspecies Solidarity
A careful, transdisciplinary study of the intricate interdependence among all forms of life can lead to better management of zoonotic diseases and guide us in acting prudently to prevent the next pandemic. In closing, I reiterate my earnest call to urgently undertake prudent and systematic actions to halt the destruction of rainforests and counteract the effects of climate change, which are causing significant damage to Arctic and Antarctic ecosystems. We all share this planet, and all forms of life, from the smallest organisms to the largest mammals, face the threat of extinction. The One Health approach can be grounded in the profound vision of interdependence and interconnectedness embedded in the intercultural wisdom of indigenous peoples and the ethnomedical practices of diverse local communities [31,34]. As the Biological Biodiversity Convention has recently emphasized: [34] (P. 6) “The relationship between biodiversity loss, the emergence and spread of communicable and non-communicable diseases and increasing health inequalities is well known, as is the role of conservation, restoration and sustainable use of biodiversity in prevention, reduction and proactive management of communicable and non-communicable disease risks”. But there are still reasons for hope and joy. For instance, initiatives from southern Chile are advancing intercultural and interspecies encounters that broaden our traditional view of human agency. The Biocultural Education Program of the Omora Ethnobotanical Park in the Cape Horn Biosphere Reserve in Chile exemplifies life consortia between and beyond species and demonstrates ways to inhabit and cohabit environments responsibly and attentively [342].
By incorporating environmental philosophy [343,344], intercultural bioethics, One Health social sciences, and zoonoethics, we can broaden our concerns for planetary health from veterinary, epidemiology, and earth sciences to one of interconnectedness, entanglement, and complexity. The issue of epistemic injustice in bioethics is crucial for effectively addressing the risks of epidemics and pandemics. The question of who speaks, how they speak, and from where they speak— in other words, the question of testimony and the value of the voices, narratives, and struggles of Indigenous peoples, women, and local communities— is essential for a comprehensive response to zoonotic diseases. One Health approaches and policies cannot be imposed on local communities through draconian measures but must arise from consensus and constructive conflict. One Health researchers still have much to learn from the intercultural health practices of many Indigenous peoples in the Global South. Perhaps the most critical and challenging issue for One Health is addressing multispecies injustices, violence against women and girls, and ecological racism. Therefore, one of my final calls is to decolonize and gender the theory and practice of One Health. This is the only viable path to overcoming these injustices. My quiet plea is to advance along the path of interculturality and to envision ways of reparative and restorative justice for the life consortia between humans and non-humans [345,346,347].
As Waltner-Toews [254] states: “It is a grievous mistake to imagine that pandemics can be understood and managed by studying the pieces separately (viruses, birds, pigs, people). To understand the challenges of learning to live with diverse microbial populations, we need to re-imagine the world in deeper, more complex, more evidence-based ecosystem terms. It is one thing to document in detail the cellular and biochemical structure of dead ducks in a marsh in Saskatchewan, as well as, more recently, the microbiomes they carry, or those of dead chickens in a barn in British Columbia. It is quite another thing to understand the relationships among multiple species at multiple scales”. To deal ethically and prudently with emerging infectious diseases, we need to improve the intercultural collaboration between veterinary medicine, cutting-edge Western knowledge and the ancestral knowledge of traditional systems of indigenous peoples [57]. All of these efforts would lead us to a more modest vision of ourselves and would help us move toward policies of solidarity, care, and interspecies cooperation [100,101,230], Implementing the One Health approach necessitates integrating various diverse biocultural and symbolic universes and values, while also instigating constructive conflict and establishing the groundwork for intercultural and interreligious dialogue. This will allow us to craft fresh "interwoven narratives" that foster sustainable ways of inhabiting the future [231,233,234,348].
In order to tell and pass on stories of resilience and planetary health to future generations, it then becomes imperative to recognize the ignored interests and perspectives of value, the differences in political and economic power and how gender impacts policies to confront AMR, climate change and zoonoses. All this has a lot to do with multispecies justice. As stated by [254] (p. 7): “the real costs of producing low-cost chicken are being paid in economic subsidies to fossil fuels and corn, in lost biodiversity in Brazil and in the oceans, and in urgent adaptations to dramatic, unstable climate change.” As I previously showed in the case of mink, the cost of the utilitarian management model to prevent the spread of a potentially pandemic virus was borne by defenseless animals. Clearly, this is unfair, painful and immoral. The costs of developing new products for the cosmetic industry and drugs to stop aging are being paid by animals that are used in laboratories as “test objects” that at some point become “disposable.” The costs of so-called development and postponing solutions to climate change are being paid by millions of animals that are slaughtered annually on farms to feed a growing population and by impoverished farmers and food industry workers. The costs of our immense loneliness and dementia are being paid for by the suffering we cause to animal species and populations that we are pushing to the brink of extinction [349,350,351]. Terrestrial and aquatic ecosystems, large rivers, seas and lakes are also paying a high price for our immoral lifestyles and our excessive consumption of raw materials. It is absurd and shameful to see that the cost of wars, techno-scientific development and testing of unconventional weapons in the oceans can be paid at a very high price by our children, grandchildren and future generations of human and non-human animals. And thus, each generation can take part, almost unsuspected, in what Gardiner calls a “severe intergenerational tyranny” [352] . In order not to end with such a discouraging panorama,
From the heart of southern Chile, near the Cape Horn Biosphere Reserve, I make a quiet appeal: Environmentalists and One Health advocates, let’s unite to forge new synergies, enhance cross-cultural dialogue, and drive the changes needed to halt the systematic destruction of wildlife. We urgently need a new form of global ecological cooperation and solidarity to tackle the immense challenges of the Sixth Mass Extinction and address health issues, inequity, structural poverty, and conflicts that are closing the window of opportunity to reverse the course of extinction. But it is still within our reach to make a difference.
If “everything is related to everything else”, as the American biologist and environmentalist Barry Commoner also thought[154], then we still have the possibility of building what Lori Gruen [101] calls “the entangled empathy”, which is not only the impulse to go out to meet the other or try to “put oneself in the shoes of the other who suffers” but also requires a motivational effort. conscious to change our perspectives (p. 80). Entangled empathy has an emotional, cognitive, and psychological component, perhaps in the Aristotelian sense of cultivating a set of virtues and emotional responses such as solidarity, respect, and understanding, especially when we lack relevant information about the intentions, values, and belief systems at the table. Thus, entangled empathy has to do with our dispositions or habits; it also implies “moral courage” and practical-motivational components. Gruen adds: “Entangled empathy requires a certain amount of perspective taking. In this sense, it is more akin to some of the other ways of understanding empathy. Perspective taking means trying to get outside of your own sensibility even though of course we are limited, in some ways, by our own perspectives on the world” (Ibid, p. 81). In the case of prudent management of zoonoses, contextual observations are relevant because they help establish guidelines for action: for example, in the case of the COVID-19 pandemic, great panic and certain feelings of anger, fear and hatred towards bats and pangolins, which were considered scapegoats at the beginning of the pandemic; many media outlets and some politicians also inoculated their audiences with feelings of retaliation and revenge toward “the Chinese” and even toward wet markets and wildlife as a whole. Gruen (Ibid., p. 81) adds: “With some animals, dangerous wild animals, for example, it is not going to be possible or wise to get close to those individuals. However, there are usually trained ethologists and zoologists who study these individuals.” When dealing with certain wild animals, such as lions, whales, bears, bats, and others, empathetic treatment means staying as far away from their environment as possible and learning to appreciate the network of interspecies assemblages of their ecological communities from a healthy distance. To do this, it is not needed do a thorough analysis, but rather exercise our common sense and follow our “best judgment.” Experts in wildlife management could tell us storytelling about how they learned to live with them, and the narratives also become a powerful resource to enhance entangled empathy and prudent treatment toward wildlife.
Finally, Gruen hits the bullseye when she says (Ibid., p. 81): “The process of entangled empathy then involves emotion, imagination, cognition, justice and care. it focuses on flourishing there is also room in the process for emotions like outrage and indignance and anger and shame. When we recognize that other human beings and other animals are not flourishing, when we recognize that other animals and other human beings are thought to be disposable and killable.” In short, a zoonoethics that aims to provide guidelines for prudently managing zoonoses and EIDs must recover the idea that all forms of life are valuable in themselves and are finely intertwined. No form of human or nonhuman life on planet Earth should become something “sacrificeable”, something “disposable”, or something “superfluous”. I believe that this type of ethical stance and this mood are what should accompany and encourage future developments in zoonoethics.
6. A Call for Urgent Action: Policy Recommendations for an Inclusive, Intercultural and Gender-sensitive One Health Approach
In order to support a comprehensive and multidimensional approach to One Health, I present in this section a set of policy guidelines and ethical recommendations:
1. Advance international legislation to recognize the international crime of ecocide and crimes against biodiversity, not only as circumscribed and peripheral damages that affect human health, but as damages that affect the health of humans, animals and environments and constitute an attack against future generations.
2. Stop the illegal wildlife trade, the slaughter of wild animals in wet markets, and the illegal timber trade in tropical forests, especially in areas of high biodiversity in Asia, Africa, and the Americas. This requires deepening the partnership against wildlife crime and developing new intercultural capacities to reconnect human and non-human animals, places and the planet.
3. We need closer transdisciplinary collaboration, including gender and intercultural perspectives, to study the socioeconomic, cultural and environmental determinants and drivers of zoonotic diseases: “Developing a multi-sectoral preparedness and response plans for control of zoonotic diseases through a comprehensive risk assessment, improving laboratory diagnostic capacities, joint surveillance activities at the animal-human interface” [111,353].
4. Strengthen political commitment, national planning and regional coordination mechanisms, this requires working towards a One Health approach based on principles of intersectionality, interculturality and global solidarity. These plans and long-term strategies should be evaluated from a complexity approach at the local, regional and global levels [66,354].
5. Promote equitable and long-term synergies between Western health systems and local and indigenous community health knowledge systems and practices. In addition, we need to develop novel strategies and regional and global information networks to share knowledge and work collaboratively and cross-sectorally to address the various One Health interfaces. In particular, the wildlife-livestock-human interface is one of the areas of greatest risk and vulnerability.
6. Promoting a One Digital Health approach: Europe, the United States, and other high-income countries have strong epidemiological surveillance systems that provide access to comprehensive data, tables, and maps on infectious diseases, but low- and middle-income countries in regions such as South and Central Asia, Africa, and Central and South America do not yet have robust surveillance systems to develop systemic preparedness, mitigation, and prevention plans and strategies for zoonotic diseases.
7. Given the growing importance of AI and accelerating digitalization in health systems, countries and regions should develop synergies and share resources to overcome gaps in access to resources through a One Digital Health equity approach [279,355,356].
8. One of the challenges highlighted by the Covid-19 pandemic was the need to work together across sectors and regions to develop greater North-South synergies of cooperation, equity and multispecies justice to lay the foundations for a sustainable One Health system based on a broad vision of health, common goods, and eco-solidarity.
7. Conclusions
In examining the historical development of infectious diseases, scholars have demonstrated that the impact of these illnesses extends well beyond the morbidity and mortality indicators associated with epidemics [126,357,358,359,360,361]. The history of emerging and re-emerging infectious diseases frequently coincides with the history of social inequities and the social construction of specific communities, groups, and regions as "vulnerable" and primary sources of infectious risk. As Rushton [334] states (p. 121): “The impacts of disease have included inducing political and social instability (in extreme cases even contributing to the collapse of entire civilisations, as occurred when Amerindian societies were devastated by smallpox), causing migration as people attempt to ‘get out of harm’s way’, undermining economies, and playing a part in determining the course of armed conflicts. Globalisation, however, is generally understood to have fundamentally changed the nature of the cross-border disease threat as a result of the more extensive, and more rapid, international movement of people, animals, food, and other goods”. It is evident that globalization, economic growth, extractivism, and the acceleration of large flows of humans, animals, and goods due to global transportation in the capitalist economy are significant contributors to the emergence and spread of infectious diseases. “But whilst globalisation-related changes have no doubt sped up the geographical movement of pathogens, such spread was always a reality of life on earth. As a result, fear of the importation of pathogens from a dangerous outside world has been a topic of political discussion and action for centuries” [334] (p. 121). Today, the fears and new biophobias raised by Covid-19 and the climate of economic and geopolitical instability have undermined the efforts of many organizations to move toward One Health Diplomacy [333] and advance the construction of a global Pandemic Agreement to prevent, control, and respond to emerging zoonotic disease risks [288,317,362].
In conclusion, it is imperative to address the pervasive phenomenon of fear, disinformation, and parsimony that has become an obstacle to achieving viable and sustainable futures for human and non-human communities. To this end, it is crucial to integrate One Health initiatives with studies on power and security dynamics. As Price-Smith [126] emphasizes (p. 189): “The role of infectious disease in modern security studies originates in the historical accounts of Thucydides, Machiavelli, and Rousseau, all regarded as republican progenitors of the political paradigm known as Classical Realism”. With relative ease and in a domino effect, public health policies during times of fear, economic decline, and disaster scenarios can be guided by political exceptionalism leading to draconian measures based on political realism rather than a One Health Ethics approach [363,364]. As highlighted by the COVID-19 pandemic, policy decisions for managing infectious diseases in disaster scenarios are not neutral; they are based on visions, values, and imaginaries of health and disease that emerged in modernity. Therefore, it is crucial to continue clarifying what is the ethics and policies that underpin political decision-making and to promote One Health initiatives for the vulnerable planet we inhabit.
Funding
This work has been founded by Project ANID/BASAL- FB210018 —Cape Horn International Center for Global Change Studies and Biocultural Conservation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The author has no conflicts of interest to declare that are relevant to the content of this article.
References
- Horefti, E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Konda, M.; Dodda, B.; Konala, V.M.; Naramala, S.; Adapa, S. Potential Zoonotic Origins of SARS-CoV-2 and Insights for Preventing Future Pandemics Through One Health Approach. Cureus 2020, 12, e8932. [Google Scholar] [CrossRef] [PubMed]
- Integrated approaches to health; Brill: PA Leiden, Netherlands, 2018.
- T. Löscher and L. Prüfer-Krämer, “Emerging and Re-emerging Infectious Diseases,” in Modern Infectious Disease Epidemiology. Concepts, Methods, Mathematical Models, and Public Health, Alexander Krämer, Mirjam Kretzschmar, and Klaus Krickeberg, Eds., New York: Springer, 2009, ch. 3, pp. 39–67. [CrossRef]
- WHO, “A health perspective on the role of the environment in One Health,” Copenhagen, 2022. Accessed: Jul. 25, 2023. [Online]. Available: http://apps.who.
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Mendoza-Roldan, J.A.; Thompson, R.A.; Dantas-Torres, F.; Otranto, D. Illegal Wildlife Trade: A Gateway to Zoonotic Infectious Diseases. Trends Parasitol. 2021, 37, 181–184. [Google Scholar] [CrossRef] [PubMed]
- T. Wyatt, Wildlife Trafficking. A Deconstruction of the Crime, Victims and Offenders. Cham: Palgrave Macmillan, 2021.
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; A Patz, J.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Heal. 2021, 5, e237–e245. [Google Scholar] [CrossRef] [PubMed]
- Reaser, J.K.; Tabor, G.M.; Becker, D.J.; Muruthi, P.; Witt, A.; Woodley, S.J.; Ruiz-Aravena, M.; Patz, J.A.; Hickey, V.; Hudson, P.J.; et al. Land use-induced spillover: priority actions for protected and conserved area managers. PARKS 2021, 161–178. [Google Scholar] [CrossRef]
- J. Maher and R. Sollund, “Wildlife Trafficking: Harms and Victimization,” in Fighting Environmental Crime in Europe and Beyond, R., Sollund, C., Stefes, and A. Germani, Eds., London: Palgrave Macmillan, 2016, pp. 99–128. [CrossRef]
- Rush, E.R.; Dale, E.; Aguirre, A.A. Illegal Wildlife Trade and Emerging Infectious Diseases: Pervasive Impacts to Species, Ecosystems and Human Health. Animals 2021, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- R. Sollund, “The Use and Abuse of Animals in Wildlife Trafficking in Colombia: Practices and Injustice,” in Environmental Crime in Latin America. The Theft of Nature and the Poisoning of the Land, David Rodríguez Goyes, Hanneke Mol, Avi Brisman, and Nigel South, Eds., London: Palgrave Macmillan, 2017, ch. 10, pp. 215–243. [CrossRef]
- Sollund, R. Green Criminology: Its Foundation in Critical Criminology and the Way Forward. Howard J. Crime Justice 2021, 60, 304–322. [Google Scholar] [CrossRef]
- C. Gibbs and R. Boratto, “Environmental Crime,” in Oxford Research Encyclopedia of Criminology and Criminal Justice, H. Pontell, Ed., New York: Oxford University Press, 2017, pp. 1–33. [CrossRef]
- R. W. Y. Wong, “The Harms and Crimes Against Terrestrial Wildlife (Nonhuman Animals),” in Oxford Research Encyclopedia of Criminology and Criminal Justice, H. Pontell, Ed., New York: Oxford University Press, 2023, pp. 1–19. [CrossRef]
- E. Gladkova, “The Harms and Crimes of Farming/Food,” in Oxford Research Encyclopedia of Criminology and Criminal Justice, H. Pontell, Ed., New York: Oxford University Press, 2023. [CrossRef]
- One Health High-Level Expert Panel (OHHLEP); Adisasmito, W. B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Becerra, N.C.; Charron, D.F.; Chaudhary, A.; et al. One Health: A new definition for a sustainable and healthy future. PLOS Pathog. 2022, 18, e1010537. [Google Scholar] [CrossRef]
- European Union Agencies, “Cross-agency knowledge for One Health action Joint statement by European Union Agencies,” 2023. Accessed: Mar. 27, 2024. [Online]. Available: https://www.efsa.europa.eu/sites/default/files/2023-11/one-health-2023-joint-statement.
- UNEP, Preventing the next Pandemic. Zoonotic diseases and how to break the chain of transmission. 2020. Accessed: Aug. 04, 2024. [Online]. Available: https://www.unep.
- F. Van Langevelde et al., “The link between biodiversity loss and the increasing spread of zoonotic diseases,” Luxembourg, 2020.
- de Castañeda, R.R.; Villers, J.; Guzmán, C.A.F.; Eslanloo, T.; de Paula, N.; Machalaba, C.; Zinsstag, J.; Utzinger, J.; Flahault, A.; Bolon, I. One Health and planetary health research: leveraging differences to grow together. Lancet Planet. Heal. 2023, 7, e109–e111. [Google Scholar] [CrossRef]
- Zinsstag, J.; Kaiser-Grolimund, A.; Heitz-Tokpa, K.; Sreedharan, R.; Lubroth, J.; Caya, F.; Stone, M.; Brown, H.; Bonfoh, B.; Dobell, E.; et al. Advancing One human–animal–environment Health for global health security: what does the evidence say? Lancet 2023, 401, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Lerner, H.; Berg, C. A Comparison of Three Holistic Approaches to Health: One Health, EcoHealth, and Planetary Health. Front. Veter- Sci. 2017, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Jer, S.B. Precedentes conceptuales para una ecosuicidología. Top. De Filos. 2023, 453–477. [Google Scholar] [CrossRef]
- R. White, “Loving the Planet to Death: Tourism and Ecocide,” in Deviant Leisure. Palgrave Studies in Crime, Media and Culture., S. O. Raymen T., Ed., Cham: Palgrave Macmillan, 2019. https://doi-org.ezproxy2.acu.edu.au/10.1007/978-3-030-17736-2_13.
- Crook, M.; Short, D.; South, N. Ecocide, genocide, capitalism and colonialism: Consequences for indigenous peoples and glocal ecosystems environments. Theor. Criminol. 2018, 22, 298–317. [Google Scholar] [CrossRef]
- Schwegler, V. The Disposable Nature: The Case of Ecocide and Corporate Accountability. Amst. Law Forum 2017, 9, 71. [Google Scholar] [CrossRef]
- B. Capps, One Health Environmentalism. Cambridge: Cambridge University Press, 2024.
- Capps, B. One health ethics. Bioethics 2021, 36, 348–355. [Google Scholar] [CrossRef]
- Pollowitz, M.; Allick, C.; Campbell, K.B.; Ellison, N.L.K.; Perez-Aguilar, G.; Vera, M.; Ramirez, V.; Nadal, D.; Meisner, J. One Health, many perspectives: Exploring Indigenous and Western epistemologies. CABI One Heal. 2024. [Google Scholar] [CrossRef]
- Redvers, N.; Celidwen, Y.; Schultz, C.; Horn, O.; Githaiga, C.; Vera, M.; Perdrisat, M.; Plume, L.M.; Kobei, D.; Kain, M.C.; et al. The determinants of planetary health: an Indigenous consensus perspective. Lancet Planet. Heal. 2022, 6, e156–e163. [Google Scholar] [CrossRef]
- Riley, T.; Anderson, N.E.; Lovett, R.; Meredith, A.; Cumming, B.; Thandrayen, J. One Health in Indigenous Communities: A Critical Review of the Evidence. Int. J. Environ. Res. Public Heal. 2021, 18, 11303. [Google Scholar] [CrossRef]
- CBD, “Biodiversity and health,” Nairobi, May 2024. Accessed: Jun. 11, 2024. [Online]. Available: https://www.cbd.int/cop.
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; de Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation—Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Romanello, M.; Romanello, M.; di Napoli, C.; di Napoli, C.; Green, C.; Green, C.; Kennard, H.; Kennard, H.; Lampard, P.; Lampard, P.; et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet 2023, 402, 2346–2394. [Google Scholar] [CrossRef]
- The Lancet Planetary Health Can the Paris Agreement save us from a climate catastrophe? Lancet Planet. Heal. 2018, 2, e140–E140. [CrossRef] [PubMed]
- IPCC, “Climate Change 2022 - Impacts, Adaptation and Vulnerability - Summary for Policymakers,” Switzerland, 2022. [Online]. Available: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_SummaryForPolicymakers.pdf.
- IPCC, “Climate Change 2023. Synthesis Report.,” in Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, H. Lee and J. Romero, Eds., Geneva, 2023, pp. 1–34. [CrossRef]
- Correia, T.; Daniel-Ribeiro, C.T.; Ferrinho, P. Calling for a planetary and one health vision for global health. One Heal. 2021, 13, 100342. [Google Scholar] [CrossRef] [PubMed]
- J. Fernando and J. L. Fernando, “The Virocene Epoch: the Vulnerability Nexus of Viruses, Capitalism and Racism,” J Polit Ecol, vol. 27, pp. 635–684, 2020, Accessed: Aug. 05, 2024. [Online]. Available: https://commons.clarku.edu/faculty_idce/145.
- Correia, T. The precariousness of political management of the SARS-CoV-2 pandemic in the search for scientific answers: Calling for prudence in public health emergencies. Int. J. Heal. Plan. Manag. 2021, 36, 1387–1391. [Google Scholar] [CrossRef]
- Correia, T.; Ricciardi, W.; McKee, M. Preparing for the ‘next pandemic’: Why we need to escape from our silos. Int. J. Heal. Plan. Manag. 2024, 39, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Correia, T. SARS-CoV-2 pandemics: The lack of critical reflection addressing short- and long-term challenges. Int. J. Heal. Plan. Manag. 2020, 35, 669–672. [Google Scholar] [CrossRef]
- Kaplan, S.; Lefler, J.; Zilberman, D. The political economy of COVID-19. Appl. Econ. Perspect. Policy 2021, 44, 477–488. [Google Scholar] [CrossRef]
- R. Rozzi, “Earth Stewardship and the Biocultural Ethic: Latin American Perspectives.,” in Earth Stewardship. Ecology and Ethics, vol 2., Rozzi et al., Ed., Cham: Springer, 2015, pp. 87–112.
- R. Rozzi, “Inter-species and Inter-cultural Encounters: The Biocultural Education Program of the Omora Ethnobotanical Park,” in Field Environmental Philosophy, Ecology and Ethics 5, Ricardo Rozzi, Alejandra Tauro, Noa Avriel-Avni, T. Wright, and Roy H. May Jr., Eds., Cham: Springer, 2023, pp. 153–174. [CrossRef]
- Sonne, C.; Langebæk, R.; Dietz, R.; Andersen-Ranberg, E.; Houser, G.; Hansen, A.J.; Sinding, M.-H.S.; Olsen, M.T.; Egevang, C.; Gilbert, M.T.P.; et al. Greenland sled dogs at risk of extinction. Science 2018, 360, 1080–1080. [Google Scholar] [CrossRef]
- Dirzo, R.; Ceballos, G.; Ehrlich, P.R. Circling the drain: the extinction crisis and the future of humanity. Philos. Trans. R. Soc. B: Biol. Sci. 2022, 377, 20210378. [Google Scholar] [CrossRef]
- Sell, S.K.; Williams, O.D. Health under capitalism: a global political economy of structural pathogenesis. Rev. Int. Politi- Econ. 2019, 27, 1–25. [Google Scholar] [CrossRef]
- Gill, S.R.; Benatar, S.R. Reflections on the political economy of planetary health. Rev. Int. Politi- Econ. 2019, 27, 167–190. [Google Scholar] [CrossRef]
- Baer, H.A.; Singer, M. Planetary Health: Capitalism, Ecology and Eco-Socialism. Capital. Nat. Social. 2023, 34, 20–38. [Google Scholar] [CrossRef]
- Sathyamala, C. COVID-19: The Political Economy of a Global Pandemic. Dev. Chang. 2022. [Google Scholar] [CrossRef] [PubMed]
- Antoine-Moussiaux, N.; de Bisthoven, L.J.; Leyens, S.; Assmuth, T.; Keune, H.; Jakob, Z.; Hugé, J.; Vanhove, M.P.M. The good, the bad and the ugly: framing debates on nature in a One Health community. Sustain. Sci. 2019, 14, 1729–1738. [Google Scholar] [CrossRef]
- Layton, D.S.; Choudhary, A.; Bean, A.G. Breaking the chain of zoonoses through biosecurity in livestock. Vaccine 2017, 35, 5967–5973. [Google Scholar] [CrossRef]
- Rahman, T.; Sobur, A.; Islam, S.; Ievy, S.; Hossain, J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Zinsstag, J.; Hediger, K.; Osman, Y.M.; Abukhattab, S.; Crump, L.; Kaiser-Grolimund, A.; Mauti, S.; Ahmed, A.; Hattendorf, J.; Bonfoh, B.; et al. The Promotion and Development of One Health at Swiss TPH and Its Greater Potential. Diseases 2022, 10, 65. [Google Scholar] [CrossRef]
- R. Ghai and C. Behravesh, “Zoonoses − The One Health Approach,” in CDC yellow book 2024 : health information for international travel, J. Nemhauser, Ed., New York: Oxford University Press, 2023, pp. 222–224.
- Nemhauser, *!!! REPLACE !!!*; et al. , CDC yellow book 2024 : health information for international travel. New York: Oxford University Press, 2023.
- A. Walker and R. LaRocque, “Introduction. Disease Patterns in Travels,” in CDC yellow book 2024: health information for international travel, J. Nemhauser, Ed., New York: Oxford University Press, 2023, pp. 1–12. [CrossRef]
- J. Allouche, “Ebola and Extractive Industry,” Feb. 2015. Accessed: Jul. 28, 2023. [Online]. Available: https://opendocs.ids.ac.uk/opendocs/handle/20.500.12413/5854.
- Andrade, D. Neoliberal extractivism: Brazil in the twenty-first century. J. Peasant. Stud. 2022, 49, 793–816. [Google Scholar] [CrossRef]
- Bruna, N. A climate-smart world and the rise of Green Extractivism. J. Peasant. Stud. 2022, 49, 839–864. [Google Scholar] [CrossRef]
- Ormsby, M.J.; Woodford, L.; Quilliam, R.S. Can plastic pollution drive the emergence and dissemination of novel zoonotic diseases? Environ. Res. 2024, 246, 118172. [Google Scholar] [CrossRef]
- Maquart, P.-O.; Froehlich, Y.; Boyer, S. Plastic pollution and infectious diseases. Lancet Planet. Heal. 2022, 6, e842–e845. [Google Scholar] [CrossRef]
- One Health Joint Plan of Action, 2022–2026; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022.
- J. Littmann, “Antimicrobial resistance and Distributive Justice,” UCL Discovery, no. February, pp. 1–211, 2014.
- Rollin, B. Ethics, Science, and Antimicrobial Resistance. J. Agric. Environ. Ethic- 2001, 14, 29–37. [Google Scholar] [CrossRef]
- WHO, “Global Action Plan on Antimicrobial Resistance,” Geneva, 2015. [Online]. Available: https://www.amcra.be/swfiles/files/WHO actieplan_90.pdf.
- Littmann, J.; Viens, A.M. The Ethical Significance of Antimicrobial Resistance. Public Heal. Ethic- 2015, 8, 209–224. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Kahn, L.H. Antimicrobial resistance: a One Health perspective. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 255–260. [Google Scholar] [CrossRef]
- Parsonage, B.; Hagglund, P.K.; Keogh, L.; Wheelhouse, N.; Brown, R.E.; Dancer, S.J. Control of Antimicrobial Resistance Requires an Ethical Approach. Front. Microbiol. 2017, 8, 2124–2124. [Google Scholar] [CrossRef]
- Kahn, L. One Health and the Politics of Antimicrobial Resistance; Project MUSE: Baltimore, MD, United States, 2016. [Google Scholar]
- European Commission, “A European One Health Action Plan against Antimicrobial Resistance (AMR),” 2017. Accessed: Jul. 16, 2024. [Online]. Available: https://health.ec.europa.eu/document/download/353f40d1-f114-4c41-9755-c7e3f1da5378_en?filename=amr_2017_action-plan.pdf.
- Shallcross, L.J.; Howard, S.J.; Fowler, T.; Davies, S.C. Tackling the threat of antimicrobial resistance: from policy to sustainable action. Philos. Trans. R. Soc. B: Biol. Sci. 2015, 370, 20140082–20140082. [Google Scholar] [CrossRef]
- Anthony, R.; Vieira, A.D.P. One Health Animal Disaster Management: An Ethics of Care Approach. J. Appl. Anim. Welf. Sci. 2022, 25, 180–194. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Lammie, S.L.; Hughes, J.M. Antimicrobial Resistance, Food Safety, and One Health: The Need for Convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef]
- Belay, E.D.; Kile, J.C.; Hall, A.J.; Barton-Behravesh, C.; Parsons, M.B.; Salyer, S.; Walke, H. Zoonotic Disease Programs for Enhancing Global Health Security. Emerg. Infect. Dis. 2017, 23, S65–S70. [Google Scholar] [CrossRef]
- Marchese, A.; Hovorka, A. Zoonoses Transfer, Factory Farms and Unsustainable Human–Animal Relations. Sustainability 2022, 14, 12806. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Venkatachalam-Vaz, J.; Drake, J.M. Spillover of zoonotic pathogens: A review of reviews. Zoonoses Public Heal. 2021, 68, 563–577. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare complementing or conflicting with other sustainability issues. Appl. Anim. Behav. Sci. 2019, 219, 104829. [Google Scholar] [CrossRef]
- Steenson, S.; Buttriss, J.L. The challenges of defining a healthy and ‘sustainable’ diet. Nutr. Bull. 2020, 45, 206–222. [Google Scholar] [CrossRef]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Flórez, M.G.; Karanis, P. The impact of water crises and climate changes on the transmission of protozoan parasites in Africa. Ann. Trop. Med. Parasitol. 2018, 112, 281–293. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef]
- Mardones, F.O.; Rich, K.M.; Boden, L.A.; Moreno-Switt, A.I.; Caipo, M.L.; Zimin-Veselkoff, N.; Alateeqi, A.M.; Baltenweck, I. The COVID-19 Pandemic and Global Food Security. Front. Veter- Sci. 2020, 7, 578508. [Google Scholar] [CrossRef]
- Brooks, D.R.; Hoberg, E.P.; Boeger, W.A.; Trivellone, V. Emerging infectious disease: An underappreciated area of strategic concern for food security. Transbound. Emerg. Dis. 2021, 69, 254–267. [Google Scholar] [CrossRef]
- K. C. Vercauteren, C. K. C. Vercauteren, C. Gortázar, D. Beltrán-Alcrudo, and J. Vicente, “Host Community Interfaces: The Wildlife-Livestock,” in Diseases at the Wildlife - Livestock Interface. Wildlife Research Monographs, vol. 3, J., Vicente, K. C., Vercauteren, and C. Gortázar, Eds., Cham: Springer, 2021, pp. 3–32. [CrossRef]
- Schlundt, J.; Toyofuku, H.; Fisher, J.; Artois, M.; Morner, T.; Tate, C. The role of wildlife in emerging and re-emerging zoonoses. Rev. Sci. et Tech. de l'OIE 2004, 23, 485–496. [Google Scholar] [CrossRef]
- P. W. Daniels, · K Halpin, · A Hyatt, and · D Middleton, “Infection and Disease in Reservoir and Spillover Hosts: Determinants of Pathogen Emergence,” in Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Current Topics in Microbiology and Immunology, vol 315., J. E., M. J. S., R. J. A. Childs, Ed., Berlin: Springer, 2007. [CrossRef]
- Escudero-Pérez, B.; Lalande, A.; Mathieu, C.; Lawrence, P. Host–Pathogen Interactions Influencing Zoonotic Spillover Potential and Transmission in Humans. Viruses 2023, 15, 599. [Google Scholar] [CrossRef]
- Marie, V.; Gordon, M.L. The (Re-)Emergence and Spread of Viral Zoonotic Disease: A Perfect Storm of Human Ingenuity and Stupidity. Viruses 2023, 15, 1638. [Google Scholar] [CrossRef]
- Tomori, O.; Oluwayelu, D.O. Domestic Animals as Potential Reservoirs of Zoonotic Viral Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 33–55. [Google Scholar] [CrossRef]
- F. W. WHO, “A Tripartite Guide to Addressing Zoonotic Diseases in Countries Taking a Multisectoral, One Health Approach,” 2019. Accessed: Apr. 04, 2024. [Online]. Available: https://www.woah.org/fileadmin/Home/eng/Media_Center/docs/EN_TripartiteZoonosesGuide_webversion.pdf.
- G. Gaard, “Ecofeminism,” in The Routledge Companion to Gender and Animals, Chloë Taylor, Ed., New York: Routledge, 2024, pp. 49–70. [Online]. Available: www.routledge.com/Routledge-.
- T. Laird, “Zoonoses,” in The Routledge Companion to Gender and Animals, Chloë Taylor, Ed., New York: Routledge, 2024, pp. 680–696.
- L. Gruen, Critical Terms for Animal Studies. Chicago and London: The University of Chicago Press, 2018.
- L. Gruen, “Entangled Empathy: Politics and Practice,” in Animal Encounters Kontakt, Interaktion und Relationalität, Alexandra Böhm and Jessica Ullrich, Eds., Chicago and London: The University of Chicago Press, 2018, pp. 77–83.
- L. Gruen, Entangled Empathy: An Alternative Ethic for our Relationships with Animals. Brooklyn: Lantern Press, 2015.
- Yoo, H.S.; Yoo, D. COVID-19 and veterinarians for one health, zoonotic- and reverse-zoonotic transmissions. J. Veter- Sci. 2020, 21, e51. [Google Scholar] [CrossRef]
- Messenger, A.M.; Barnes, A.N.; Gray, G.C. Reverse Zoonotic Disease Transmission (Zooanthroponosis): A Systematic Review of Seldom-Documented Human Biological Threats to Animals. PLOS ONE 2014, 9, e89055. [Google Scholar] [CrossRef]
- Glud, H.A.; George, S.; Skovgaard, K.; Larsen, L.E. Zoonotic and reverse zoonotic transmission of viruses between humans and pigs. APMIS 2021, 129, 675–693. [Google Scholar] [CrossRef]
- CDC, “Centers for Diseases Control and Prevention.,” Zoonotic diseases. Accessed: Apr. 22, 2022. [Online]. Available: https://www.cdc.gov/one-health/about/about-zoonotic-diseases.html.
- Eskew, E.A.; Olival, K.J. De-urbanization and Zoonotic Disease Risk. Ecohealth 2018, 15, 707–712. [Google Scholar] [CrossRef]
- Olival, K.J.; Cryan, P.M.; Amman, B.R.; Baric, R.S.; Blehert, D.S.; Brook, C.E.; Calisher, C.H.; Castle, K.T.; Coleman, J.T.H.; Daszak, P.; et al. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: A case study of bats. PLOS Pathog. 2020, 16, e1008758. [Google Scholar] [CrossRef]
- Zoonoses: Infections Affecting Humans and Animals; Springer Nature: Dordrecht, GX, Netherlands, 2023.
- ECDC, “Worsening spread of mosquito-borne disease outbreaks in EU/EEA, according to latest ECDC figures,” European Centre for Disease Prevention and Control.
- WHO, “World Health Statistics 2023. Monitoring health for the SDGs Sustainable Development Goals,” Geneva, 2023. Accessed: Jul. 17, 2024. [Online]. Available: https://reliefweb.int/report/world/world-health-statistics-2023-monitoring-health-sdgs-sustainable-development-goals.
- Augusto, L.G.d.S.; Gurgel, A.M.; Costa, A.M.; Diderichsen, F.; A Lacaz, F.; Parra-Henao, G.; Rigotto, R.M.; Nodari, R.; Santos, S.L. Aedes aegypti control in Brazil. Lancet 2016, 387, 1052–1053. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America – Public health and travel medicine importance. Travel Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef]
- Chapungu, L.; Nhamo, G. Interfacing vector-borne disease dynamics with climate change: Implications for the attainment of SDGs in Masvingo city, Zimbabwe. Jamba-Journal Disaster Risk Stud. 2021, 13, 12. [Google Scholar] [CrossRef]
- C. Ainsworth, “Tropical diseases move north,” Nature Outlook: Neglected tropical diseases, Nov. 2023.
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Mahon, M.B.; Sack, A.; Aleuy, O.A.; Barbera, C.; Brown, E.; Buelow, H.; Civitello, D.J.; Cohen, J.M.; de Wit, L.A.; Forstchen, M.; et al. A meta-analysis on global change drivers and the risk of infectious disease. Nature 2024, 629, 830–836. [Google Scholar] [CrossRef]
- K. A. Alexander et al., “The Ecology of Pathogen Spillover and Disease Emergence at the Human-Wildlife-Environment Interface,” 2018, pp. 267–298. [CrossRef]
- M. E. Benbow, J. L. M. E. Benbow, J. L. Pechal, J. K. Tomberlin, and H. R. Jordan, “Interkingdom Community Interactions in Disease Ecology,” in The Connections Between Ecology and Infectious Disease, Advances in Environmental Microbiology, vol. 5, C. J. Hurst, Ed., Cham: Springer, 2018, ch. 1, pp. 3–38. [CrossRef]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2018, 4, 10–19. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- L. Valera, New Technologies. Rethinking Ethics and the Environment. Cham: Springer, 2020. [CrossRef]
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- A. Price-Smith, Contagion and Chaos: Disease, Ecology, and National Security in the Era of Globalization. Cambridge: MIT Press, 2009. [Online]. Available: http://ebookcentral.proquest.com/lib/acu/detail.action?docID=3338979.
- Price-Smith, “The Plagues of Affluence: Human Ecology and the Case of the,” Environ Hist Durh N C, vol. 20, no. 4, pp. 765–778, 2015, Accessed: Jul. 16, 2024. [Online]. Available: https://www.jstor.org/stable/24690826.
- Ren, H.; Zhao, L.; Zhang, A.; Song, L.; Liao, Y.; Lu, W.; Cui, C. Early forecasting of the potential risk zones of COVID-19 in China's megacities. Sci. Total. Environ. 2020, 729, 138995–138995. [Google Scholar] [CrossRef]
- Sridhar, K.S. Urbanization and COVID-19 Prevalence in India. Reg. Sci. Policy Pr. 2021, 15, 493–506. [Google Scholar] [CrossRef]
- S. H. Ali, C. S. H. Ali, C. Connolly, and R. Keil, Pandemic Urbanism: Infectious Diseases on a Planet of Cities. Polity Press: Cambridge, 2023.
- H. Jonas, The Imperative of Responsability. In Search of an Ethics for the Technological Age. Chicago and London: The University of Chicago Press, 1984.
- Cummings, L. Emerging Infectious Diseases: Coping with Uncertainty. Argumentation 2008, 23, 171–188. [Google Scholar] [CrossRef]
- Baekkeskov, E. Explaining science-led policy-making: pandemic deaths, epistemic deliberation and ideational trajectories. Policy Sci. 2016, 49, 395–419. [Google Scholar] [CrossRef]
- Tulodziecki, D. How (not) to think about theory-change in epidemiology. Synthese 2019, 198, 2569–2588. [Google Scholar] [CrossRef]
- Alter, H.J. Pathogen Reduction: A Precautionary Principle Paradigm. Transfus. Med. Rev. 2008, 22, 97–102. [Google Scholar] [CrossRef]
- van Herten, J.; Bovenkerk, B. The Precautionary Principle in Zoonotic Disease Control. Public Heal. Ethic- 2021, 14, 180–190. [Google Scholar] [CrossRef]
- Nichol, A.; Magnus, D. The One Health Approach to Zoonotic Emerging Infectious Diseases. Am. J. Bioeth. 2018, 18, 1–2. [Google Scholar] [CrossRef]
- Coghlan, S.; Coghlan, B. One Health, Bioethics, and Nonhuman Ethics. Am. J. Bioeth. 2018, 18, 3–5. [Google Scholar] [CrossRef]
- Lindenmayer, J.M.; Kaufman, G.E.; Baker, L.; Coghlan, S.; Koontz, F.W.; Nieuwland, J.; Stewart, K.L.; Lynn, W.S. One health ethics: “What then must we do?”. CABI One Heal. 2022. [Google Scholar] [CrossRef]
- Capps, B.; Lederman, Z. One Health, Vaccines and Ebola: The Opportunities for Shared Benefits. J. Agric. Environ. Ethic- 2015, 28, 1011–1032. [Google Scholar] [CrossRef]
- W. Mignolo and C. Walsh, On Decoloniality. Concepts, Analitics, Praxis. Durham and London: Duke University Press, 2018.
- Baños, J.R. Un puente hacia el enfoque de One Health. 2021; 13. [Google Scholar] [CrossRef]
- Manguvo, A.; Mafuvadze, B. The impact of traditional and religious practices on the spread of Ebola in West Africa: time for a strategic shift. 2015, 22. [CrossRef]
- Oliveira, D.V.B.; da Silva, J.F.; Araújo, T.A.d.S.; Albuquerque, U.P. Influence of Religiosity and Spirituality on the Adoption of Behaviors of Epidemiological Relevance in Emerging and Re-Emerging Diseases: The Case of Dengue Fever. J. Relig. Heal. 2021, 61, 564–585. [Google Scholar] [CrossRef] [PubMed]
- R. Macklin, “Respect for cultural diversity and pluralism,” in Handbook of Global Bioethics, Henk A.M.J. ten Have and Bert Gordijn, Eds., Cham: Springer, 2014, pp. 153–167. [CrossRef]
- H. ten Have, “Globalizing Bioethics Through, Beyond and Despite Governments,” in Global Bioethics: The Impact of the UNESCO International Bioethics Committee, vol. 5, A., M. J., S. S. Bagheri, Ed., Cham: Springer, 2016, ch. 1, pp. 1–11. [CrossRef]
- Lee, L.M. A Bridge Back to the Future: Public Health Ethics, Bioethics, and Environmental Ethics. Am. J. Bioeth. 2017, 17, 5–12. [Google Scholar] [CrossRef]
- Rüegg, S.R.; Nielsen, L.R.; Buttigieg, S.C.; Santa, M.; Aragrande, M.; Canali, M.; Ehlinger, T.; Chantziaras, I.; Boriani, E.; Radeski, M.; et al. A Systems Approach to Evaluate One Health Initiatives. Front. Veter- Sci. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag et al., One Health. The Theory and Practice of Integrated Health Approaches. London: CAB International , 2021.
- A. Wilcox and J. A. Steele, “One Health and Emerging Zoonotic Diseases,” in Handbook of Global Health, I., Kickbusch, D., Ganten, and M. Moeti, Eds., Cham: Springer, 2021, pp. 1–49.
- Pettan-Brewer, C.; Penn, G.; Biondo, A.W.; Jaenisch, T.; Grützmacher, K.; Kahn, L.H. Who coined the term “One Health”? Cooperation amid the siloization. One Heal. 2024, 18, 100678. [Google Scholar] [CrossRef] [PubMed]
- J. Rodriguez, “Making zoonoethics and becoming a One Health ethicist from the Global South: Do we need a Zoonoethics and One Health Ethic to address Pandemic Risks?,” in A Companion to Doing Ethics, A. Preti and T. Weidel, Eds., New Jersey: Wiley-Blackwell (In press), 2024.
- Have, H.T. The challenges of global bioethics. Glob. Bioeth. 2022, 33, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Global Bioethics; Springer Nature: Dordrecht, GX, Netherlands, 2014.
- J. Valera, L. & Rodriguez, “Technology, Ecology and Survival Science: A Look at Barry Commoner’s Thought for the Future,” in Techonological Society and Human Future. Vol. 2, H. Velázquez, Ed., Editorial Tirant Lo Blanch S.L, 2021.
- Potter, V.R. Bioethics, the Science of Survival. Perspect. Biol. Med. 1970, 14, 127–153. [Google Scholar] [CrossRef]
- V. R. Potter, Bioethics: bridge to the future. Englewood Cliffs: Prentice-Hall, 1971. Accessed: Mar. 18, 2018. [Online]. Available: http://todosibuc.uc.cl/primo_library/libweb/action/display.do?tabs=detailsTab&ct=display&fn=search&d oc=puc_alma2161019060003396&indx=1&recIds=puc_alma2161019060003396&recIdxs=0&elementId=0&re nderMode=poppedOut&displayMode=full&frbrVersion=&query=any%2Ccon.
- Beever, J.; Morar, N. The epistemic and ethical onus of ‘One Health’. Bioethics 2018, 33, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Beever, J.; Morar, N. Interconnectedness and Interdependence: Challenges for Public Health Ethics. Am. J. Bioeth. 2017, 17, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Capps, B.; Bailey, M.M.; Bickford, D.; Coker, R.; Lederman, Z.; Lover, A.; Lysaght, T.; Tambyah, P. Introducing One Health to the Ethical Debate About Zoonotic Diseases in Southeast Asia. Bioethics 2015, 29, 588–596. [Google Scholar] [CrossRef]
- Meagher, K.M. Can One Health Policy Help Us Expand an Ethics of Interconnection and Interdependence? AMA J. Ethic- 2024, 26, E162–170. [Google Scholar] [CrossRef]
- Plowright, R.K.; Ahmed, A.N.; Coulson, T.; Crowther, T.W.; Ejotre, I.; Faust, C.L.; Frick, W.F.; Hudson, P.J.; Kingston, T.; Nameer, P.O.; et al. Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nat. Commun. 2024, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.M.; Hannah, L.; Lieberman, S.; Vale, M.M.; Plowright, R.K.; Bernstein, A.S. Want to prevent pandemics? Stop spillovers. Nature 2022, 605, 419–422. [Google Scholar] [CrossRef] [PubMed]
- OHHLEP, “Prevention of Zoonotic Spilover. From Relying to Reducing the Risks at source.” Accessed: Aug. 06, 2024. [Online]. Available: https://www.who.int/publications/m/item/prevention-of-zoonotic-spillover.
- Vora, N.M.; Hannah, L.; Walzer, C.; Vale, M.M.; Lieberman, S.; Emerson, A.; Jennings, J.; Alders, R.; Bonds, M.H.; Evans, J.; et al. Interventions to Reduce Risk for Pathogen Spillover and Early Disease Spread to Prevent Outbreaks, Epidemics, and Pandemics. Emerg. Infect. Dis. 2023, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Catherina, R.; Frye, H.; Shelley, L. Illicit Wildlife Trade, Wet Markets, and COVID-19: Preventing Future Pandemics. World Med Heal. Policy 2020, 12, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A. Developing Global Capacity in Conservation Medicine: Predicting and Preventing the Next Epidemic from Wildlife. Glob. Bioeth. 2011, 24, 51–54. [Google Scholar] [CrossRef]
- Doron, S.; Davidson, L.E. Antimicrobial Stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.; Price, N. Antimicrobial stewardship – can we afford to do without it? Br. J. Clin. Pharmacol. 2015, 79, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T. Developing an Ethical Evaluation Framework for Coercive Antimicrobial Stewardship Policies. Public Heal. Ethic- 2024, 17, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESGAP (ESCMID Study Group for Antimicrobial stewardshiP). What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Martínez, J.-L.; Aracil-Gisbert, S.; Lanza, V.F. Gene Transmission in the One Health Microbiosphere and the Channels of Antimicrobial Resistance. Front. Microbiol. 2019, 10, 2892. [Google Scholar] [CrossRef]
- Malfrán, Y.I.M.; Baquero, O.S. Problematizing alterities for a feminist and decolonial understanding of One Health of Peripheries. Saude E Soc. 2023, 32, e220301pt. [Google Scholar] [CrossRef]
- Baquero, O.S.; Fernández, M.N.B.; Aguilar, M.A. From Modern Planetary Health to Decolonial Promotion of One Health of Peripheries. Front. Public Heal. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- David. DeGrazia, Animal Rights: A Very Short Introduction.. Oxford: Oxford University Press, 2002.
- M. Rowlands, “Making light of the ethical? The ethics and politics of animal rights,” in Ethical and Political Approaches to Nonhuman Animal Issues, Springer International Publishing, 2017, pp. 21–38. [CrossRef]
- M. Rowlands, Can Animals Be Moral? New York: Oxford University Press, 2013.
- G. Francione, “Animals—Property or Persons?,” in Animal rights : current debates and new directions, M. Sunstein, Cass & Nussbaum, Ed., Oxford: Oxford University Press, 2004, pp. 108–142. Accessed: Jun. 23, 2019. [Online]. Available: https://books.google.com.au/books?id=e7FME0btkH0C&printsec=frontcover&hl=es&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false.
- T. Regan, The Case for Animal Rights. Berkeley, Los Angeles: University of California Press, 1983.
- P. Singer, Animal Liberation: A New Ethics for Our Treatment of Animals., 40th ed. New York: Open Road Media, 2015.
- T. J. Kasperbauer and P. Sandøe, “Killing as a Welfare Issue,” in The Ethics of Killing Animals, Tatjana Višak and Robert Garner, Eds., Oxford: Oxford University Press, 2015, pp. 17–31. [CrossRef]
- Persson, K.; Selter, F.; Neitzke, G.; Kunzmann, P. Philosophy of a “Good Death” in Small Animals and Consequences for Euthanasia in Animal Law and Veterinary Practice. Animals 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- UlAin, Q.; Whiting, T.L. Is a “Good Death” at the Time of Animal Slaughter an Essentially Contested Concept? Animals 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Browning, H.; Veit, W. Is Humane Slaughter Possible? Animals 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Yeates, J.W. Death is a Welfare Issue. J. Agric. Environ. Ethic- 2009, 23, 229–241. [Google Scholar] [CrossRef]
- Browning, H.; Veit, W. Is Humane Slaughter Possible? Animals 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- M. Higgin, A. Evans, and M. Miele, “A Good Kill: Socio-Technical Organizations of Farm Animal Slaughter,” in Human and Other Animals, B., C. N. Carter, Ed., London: Palgrave Macmillan, 2011, ch. 9, pp. 173–194. [CrossRef]
- Browning, H. No Room at the Zoo: Management Euthanasia and Animal Welfare. J. Agric. Environ. Ethic- 2018, 31, 483–498. [Google Scholar] [CrossRef]
- J. A. Lecaros, “Ecological Ethics: The road of Responsability towards Global Bioethics,” Ramon Llull Journal of Applied Ethics, no. 4, pp. 201–215, 2013, Accessed: Nov. 22, 2022. [Online]. Available: https://raco.pre.csuc.cat/index.php/rljae/article/view/281043.
- Valdéz &, Lecaros; et al. , Handbook of Bioethical Decisions. Volume II Scientific Integrity and Institutional Ethics. Cham: Springer, 2023.
- Valdéz &, Lecaros; et al. , Handbook of Bioethical Decisions. Volume I Decisions at the Bench. Cham: Springer, 2023.
- Muzur, A.; Rinčić, I. Two kinds of globality: a comparison of Fritz Jahr and Van Rensselaer Potter's bioethics. Glob. Bioeth. 2015, 26, 23–27. [Google Scholar] [CrossRef]
- Ortiz-Millán, G. Bioethics, globalization and pandemics. Glob. Bioeth. 2022, 33, 32–37. [Google Scholar] [CrossRef]
- R. Fiore, “Bioethics: Evironmental,” in Encyclopedia of Global Bioethics, H. ten Have, Ed., Cham: Springer International Publishing, 2016, pp. 313–324. [CrossRef]
- Whitehouse, P.J. The Rebirth of Bioethics: Extending the Original Formulations of Van Rensselaer Potter. Am. J. Bioeth. 2003, 3, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Potter, V.R. Global Bioethics; Michigan State University Press: East Lansing, MI, United States, 2012. [Google Scholar]
- Gardiner, S.M. Environmentalizing Bioethics: Planetary Health in a Perfect Moral Storm. Perspect. Biol. Med. 2022, 65, 569–585. [Google Scholar] [CrossRef]
- Macklin, R. A new definition for global bioethics: COVID-19, a case study. Glob. Bioeth. 2022, 33, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Diller, E.R.; Williamson, L. Supporting One Health for Pandemic Prevention: The Need for Ethical Innovation. J. Bioethical Inq. 2023, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, C. Global bioethics: it’s past and future. Glob. Bioeth. 2022, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Tong, R. Towards a feminist global ethics. Glob. Bioeth. 2022, 33, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Missoni, E. Global health, planetary health, One Health: conceptual and ethical challenges and concerns. Theor. Med. Bioeth. 2024, 45, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Have, H.T. The challenges of global bioethics. Glob. Bioeth. 2022, 33, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Holguin-Rivera, Y.; Perez-Vargas, S.; Trejos-Mendoza, A.E.; Balbin-Ramon, G.J.; Dhama, K.; Barato, P.; Lujan-Vega, C.; Rodriguez-Morales, A.J. Importance of the One Health approach to study the SARS-CoV-2 in Latin America. One Heal. 2020, 10, 100147–100147. [Google Scholar] [CrossRef]
- Becerra, N.M.C.; Medellin, A.M.O.; Tomassone, L.; Chiesa, F.; De Meneghi, D. A Survey on One Health Approach in Colombia and Some Latin American Countries: From a Fragmented Health Organization to an Integrated Health Response to Global Challenges. Front. Public Heal. 2021, 9. [Google Scholar] [CrossRef]
- Cediel-Becerra, N.M.; Prieto-Quintero, S.; Garzon, A.D.M.; Villafañe-Izquierdo, M.; Rúa-Bustamante, C.V.; Jimenez, N.; Hernández-Niño, J.; Garnier, J. Woman-Sensitive One Health Perspective in Four Tribes of Indigenous People From Latin America: Arhuaco, Wayuú, Nahua, and Kamëntsá. Front. Public Heal. 2022, 10, 774713. [Google Scholar] [CrossRef]
- Hemida, M.G.; Abduallah, M.M.B. The SARS-CoV-2 outbreak from a one health perspective. One Heal. 2020, 10, 100127–100127. [Google Scholar] [CrossRef]
- Hayman, D.T.; Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Becerra, N.C.; Charron, D.F.; Chaudhary, A.; et al. Developing One Health surveillance systems. One Heal. 2023, 17, 100617. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Carlisle, K.; Anderson, D.; Baker, M.G.; Chipman, R.B.; Benschop, J.; French, N.P.; Greenhalgh, S.; McDougall, S.; Muellner, P.; et al. Steps towards operationalizing One Health approaches. One Heal. 2024, 18, 100740. [Google Scholar] [CrossRef]
- Arredondo-Rivera, M.; Barois, Z.; Monti, G.E.; Steketee, J.; Daburon, A. Bridging Food Systems and One Health: A key to preventing future pandemics? One Heal. 2024, 18, 100727. [Google Scholar] [CrossRef] [PubMed]
- J. Hattingh, “Protection of the environment, the biosphere and biodiversity,” in Handbook of Global Bioethics, Henk A.M.J. ten Have and Bert Gordijn, Eds., Cham: Springer, 2014, ch. 15, pp. 225–250. [CrossRef]
- Rawlinson, M.C. The Concept of a Feminist Bioethics: IJFAB at Ten. IJFAB: Int. J. Fem. Approaches Bioeth. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Bhakuni, H. Glocalization of bioethics. Glob. Bioeth. 2022, 33, 65–77. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.B.; Williams-Jones, B. Applying the ecosystem approach to global bioethics: building on the Leopold legacy. Glob. Bioeth. 2023, 34. [Google Scholar] [CrossRef]
- M. Parker, “A Deliberative Approach to Clinical Bioethics,” in Clinical Bioethics. International Library of Ethics, Law, and the New Medicine, vol 26., D. C., W. D. N., K. T. K., V. C. Thomasma, Ed., Dordrech: Springer, 2005, pp. 61–71.
- Finkler, K. Can Bioethics Be Global and Local, or Must It Be Both? J. Contemp. Ethnogr. 2008, 37, 155–179. [Google Scholar] [CrossRef]
- Dealing with Bioethical Issues in a Globalized World; Springer Nature: Dordrecht, GX, Netherlands, 2020.
- LeBlanc, A.B. At the confluence of ethics, laws and society: global working theory merging bio-ethics. SN Soc. Sci. 2023, 4, 1–21. [Google Scholar] [CrossRef]
- LeBlanc, A.B.; Williams-Jones, B.; Aenishaenslin, C. Bio-Ethics and One Health: A Case Study Approach to Building Reflexive Governance. Front. Public Heal. 2022, 10, 648593. [Google Scholar] [CrossRef]
- P. J. Whitehouse, “The Ecosystem of Bioethics: Building Bridges to Public Health,” 2017. [Online]. Available: https://commons.case.edu/facultyworks/47.
- Brescia, T. The rediscovery of Potterian bioethics. Glob. Bioeth. 2015, 26, 190–197. [Google Scholar] [CrossRef]
- Stepke, F.L. The hermeneutical dimension of the bioethical enterprise. Notes on the dialogical/narrative foundations of bioethics. Acta bioethica 2018, 24, 153–159. [Google Scholar] [CrossRef]
- Árnason, V. Toward Critical Bioethics. Camb. Q. Heal. Ethic- 2015, 24, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Ferrarello et al., Phenomenology of Bioethics: Technoethics and Lived-Experience. Cham: Springer, 2021. [Online]. Available: http://www.springer.com/series/16538.
- Have, H.T. Global health and global bioethics. 10, 65. [CrossRef]
- P. A. T. D. C. B. J., Marshall, “Intercultural Reasoning: The Challenge for International Bioethics,” Cambridge Quarterly of Healthcare Ethics, vol. 5, pp. 321–328, 1994.
- J. O. Griffiths, “Bioethics, the Ontology of Life, and the Hermeneutics of Biology,” in Phenomenology of Bioethics: Technoethics and Lived-Experience, S. Farrarello, Ed., Cham: Springer, 2021, ch. 1, pp. 1–21. [CrossRef]
- Thompson, P.B. From Synthetic Bioethics to One Bioethics: A Reply to Critics. Ethic- Policy Environ. 2015, 18, 215–224. [Google Scholar] [CrossRef]
- Thompson, P.B.; List, M. Ebola Needs One Bioethics. Ethic- Policy Environ. 2015, 18, 96–102. [Google Scholar] [CrossRef]
- Lederman, Z.; Capps, B. One health ethics: a response to pragmatism. J. Med Ethic- 2020, 46, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.; Schlosberg, D.; Wadiwel, D.; Winter, C. A Political Theory for a Multispecies, Climate-Challenged World: 2050. Politi- Theory 2023, 51, 39–53. [Google Scholar] [CrossRef]
- The Promise of Multispecies Justice; Walter de Gruyter GmbH: Berlin, Germany, 2022.
- Heise, U.K. Stories for a multispecies future. Dialog- Hum. Geogr. 2018, 8, 96–99. [Google Scholar] [CrossRef]
- P. Emel, J. P. Emel, J., & Nirmal, “A feminist research agenda for multispecies justice,” in A Research Agenda for Animal Geographies., and L. V. P. Alice Hovorka, Sandra McCubbin, Ed., Cheltenham: Edward Elgar Publishing, 2021, ch. 2. [CrossRef]
- Tschakert, P.; Schlosberg, D.; Celermajer, D.; Rickards, L.; Winter, C.; Thaler, M.; Stewart-Harawira, M.; Verlie, B. Multispecies justice: Climate-just futures with, for and beyond humans. WIREs Clim. Chang. 2020, 12. [Google Scholar] [CrossRef]
- Schlosberg, “Ecological Justice for the Anthropocene,” Political Animals and Animal Politics, pp. 75–89, 2014.
- Berkey, B. Prospects for an Inclusive Theory of Justice: The Case of Non-Human Animals. J. Appl. Philos. 2015, 34, 679–695. [Google Scholar] [CrossRef]
- M. Nussbaum, Justice for Animals: Our Collective Responsibility. New York: Simon & Schuster, 2022.
- R. Attfield, The Ethics of the Global Environment, 2nd ed. Edinburgh: Edinburgh University Press, 2015. [Online]. Available: http://ebookcentral.proquest.com/lib/acu/detail.action?docID=4306096.
- Callicott, J.B.; Rozzi, R.; Delgado, L.; Monticino, M.; Acevedo, M.; Harcombe, P. Biocomplexity and conservation of biodiversity hotspots: three case studies from the Americas. Philos. Trans. R. Soc. B: Biol. Sci. 2006, 362, 321–333. [Google Scholar] [CrossRef]
- Tauro, A.; Ojeda, J.; Caviness, T.; Moses, K.P.; Moreno-Terrazas, R.; Wright, T.; Zhu, D.; Poole, A.K.; Massardo, F.; Rozzi, R. Field Environmental Philosophy: A Biocultural Ethic Approach to Education and Ecotourism for Sustainability. Sustainability 2021, 13, 4526. [Google Scholar] [CrossRef]
- L. Valera, “Intrinsic Values, Pantheism, and Ecology: Where Does Value Come From?,” in Pantheism and Ecology. Cosmological, Philosophical, and Theological Perspectives, L. Valera, Ed., Cham: Springer, 2023, pp. 301–311.
- Palmer, “Does nature matter? The place of the nonhuman in the ethics of climate change,” in The Ethics of Global Climate Change, D. Arnold, Ed., Cambridge: Cambridge University Press, 2010, ch. 13, pp. 272–291. [CrossRef]
- Palmer, C. Living Individuals; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2016. [Google Scholar]
- B. C. Palmer, C. B. C. Palmer, C. Fischer, J. Gamborg, and P. S. Hampton, Wildlife Ethics: The Ethics of Wildlife Management and Conservation. Oxford: Wiley-Blackwell, 2023.
- Gruen, L. Expressing Entangled Empathy: A Reply. Hypatia 2017, 32, 452–462. [Google Scholar] [CrossRef]
- Cochrane, A.; Garner, R.; O’sullivan, S. Animal ethics and the political. Crit. Rev. Int. Soc. Politi- Philos. 2016, 21, 261–277. [Google Scholar] [CrossRef]
- Cochrane, A. Sentientist Politics; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2018. [Google Scholar]
- van Herten, J.; Bovenkerk, B.; Verweij, M. One Health as a moral dilemma: Towards a socially responsible zoonotic disease control. Zoonoses Public Heal. 2018, 66, 26–34. [Google Scholar] [CrossRef]
- Ryan, E.B.; Weary, D.M. Public attitudes toward the use of technology to create new types of animals and animal products. Anim. Welf. 2023, 32, e43. [Google Scholar] [CrossRef]
- Roger, F.; Caron, A.; Morand, S.; Pedrono, M.; de Garine-Wichatitsky, M.; Chevalier, V.; Tran, A.; Gaidet, N.; Figuié, M.; de Visscher, M.-N.; et al. One Health and EcoHealth: the same wine in different bottles? Infect. Ecol. Epidemiology 2016, 6, 30978. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. One Health InitiativeTask Force. “One Health: A New Professional Imperative”. 15 July 2008. Available online: https://www.avma.org/sites/default/files/resources/onehealth_final.pdf (accessed on 14 April 2020).
- Johnson, L.S.M.; Ferdowsian, H.; Pierce, J. How One Health Instrumentalizes Nonhuman Animals. AMA J. Ethic- 2024, 26, E184–190. [Google Scholar] [CrossRef]
- Meisner, J.; McLeland-Wieser, H.; Traylor, E.E.; Hermesh, B.; Berg, T.; Roess, A.; Van Patter, L.; Rosenthal, A.; Davidovitch, N.; Rabinowitz, P.M. Relational One Health: A more-than-biomedical framework for more-than-human health, and lessons learned from Brazil, Ethiopia, and Israel. One Heal. 2024, 18, 100676. [Google Scholar] [CrossRef]
- Waltner-Toews, D. Zoonoses, One Health and complexity: wicked problems and constructive conflict. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160171. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Meyer, J.M.; Bonfoh, B.; Fink, G.; Dimov, A. One Health in human-environment systems: Linking health and the sustainable use of natural resources. CABI One Heal. 2024. [Google Scholar] [CrossRef]
- C. Pelluchon, Manifiesto animalista. Politizar la causa animal. Barcelona: Reservoir Books, 2018.
- Corine. Pelluchon, Reparemos el mundo: humanos, animales, naturaleza. Barcelona: Ned Ediciones, 2022.
- Turner, P.N. “Harm” and Mill’s Harm Principle. Ethics 2014, 124, 299–326. [Google Scholar] [CrossRef]
- Arato, J.; Claussen, K.; Heath, J.B. The Perils of Pandemic Exceptionalism. Am. J. Int. Law 2020, 114, 627–636. [Google Scholar] [CrossRef]
- Kirk, J.; McDonald, M. The Politics of Exceptionalism: Securitization and COVID-19. Glob. Stud. Q. 2021, 1. [Google Scholar] [CrossRef]
- Roth, E. “Bats Who Harm” and “Bats Who May Be Harmed”: The Interspecies Politics of Virus Sampling. Soc. Anim. 15. [CrossRef]
- Mysterud, A.; Rauset, G.R.; Van Moorter, B.; Andersen, R.; Strand, O.; Rivrud, I.M. The last moves: The effect of hunting and culling on the risk of disease spread from a population of reindeer. J. Appl. Ecol. 2020, 57, 2509–2518. [Google Scholar] [CrossRef]
- Lederman, Z.; Magalhães-Sant’ana, M.; Voo, T.C. Stamping Out Animal Culling: From Anthropocentrism to One Health Ethics. J. Agric. Environ. Ethic- 2021, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Frutos, R.; Devaux, C. Mass culling of minks to protect the COVID-19 vaccines: is it rational? New Microbes New Infect. 2020, 38, 100816–100816. [Google Scholar] [CrossRef] [PubMed]
- Lesté-Lasserre, C. Pandemic dooms Danish mink—and mink research. Science 2020, 370, 754–754. [Google Scholar] [CrossRef]
- Cripps, E. Climate Change and the Moral Agent; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2013. [Google Scholar]
- Degeling, C.; Lederman, Z.; Rock, M. Culling and the Common Good: Re-evaluating Harms and Benefits under the One Health Paradigm. Public Heal. Ethic- 2016, 9, 244–254. [Google Scholar] [CrossRef]
- Halteman, M.C. Varieties of Harm to Animals in Industrial Farming. J. Anim. Ethic- 2011, 1, 122–131. [Google Scholar] [CrossRef]
- Halabowski, D.; Rzymski, P. Taking a lesson from the COVID-19 pandemic: Preventing the future outbreaks of viral zoonoses through a multi-faceted approach. Sci. Total. Environ. 2020, 757, 143723–143723. [Google Scholar] [CrossRef] [PubMed]
- J. Sebo, A. E. J. Sebo, A. E. White, and T. Sims, “One Health and Multispecies Urban Infrastructure (peer reviewed),” in One Health and the Law: Existing Frameworks, Intersections and Future Pathways, vol. (PRE, Jane Kotzmann and Katie Woolaston, Eds., 2024, pp. 1–17. [CrossRef]
- Origgi, G. Fear of principles? A cautious defense of the Precautionary Principle. Mind Soc. 2014, 13, 215–225. [Google Scholar] [CrossRef]
- von Essen, E.; Redmalm, D. License to Cull: A Research Agenda for Investigating the Necropolitics of Countryside Culling and Urban Pest Control. Soc. Anim. 2023, -1, 1–16,. [CrossRef]
- Resnik, D.B. Human Health and the Environment: In Harmony or in Conflict? Heal. Care Anal. 2009, 17, 261–276. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, S.P.; Reiss, M.J. Bovine Tuberculosis and Badger Control in Britain: Science, Policy and Politics. J. Agric. Environ. Ethic- 2017, 30, 469–484. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Shi, Q.; Chen, C.; Wei, J.; Li, J.F.; Zheng, L.R.; Song, H.B. Proton transport enabled by a field-induced metallic state in a semiconductor heterostructure (Retraction of Vol 369, Pg 184, 2020) (Retraction of Vol 369, Pg 184, 2020). Science 2020, 370, 179–179. [Google Scholar] [CrossRef] [PubMed]
- Lederman, Z.; Magalhães-Sant’ana, M.; Voo, T.C. Stamping Out Animal Culling: From Anthropocentrism to One Health Ethics. J. Agric. Environ. Ethic- 2021, 34, 1–14. [Google Scholar] [CrossRef]
- Park, J.M.; Koh, J.H.; Kim, J.M. Consumer awareness of culling and animal welfare. Food Control. 2022, 133. [Google Scholar] [CrossRef]
- J. Van Herten, “Considerations for an ethic of One Health Towards a socially responsible zoonotic disease control,” Wageningen University, Wageningen, 2021.
- Benis, A.; Haghi, M.; Deserno, T.M.; Tamburis, O. One Digital Health Intervention for Monitoring Human and Animal Welfare in Smart Cities: Viewpoint and Use Case. JMIR Public Heal. Surveill. 2023, 11, e43871. [Google Scholar] [CrossRef]
- Farlow, A.; Torreele, E.; Gray, G.; Ruxrungtham, K.; Rees, H.; Prasad, S.; Gomez, C.; Sall, A.; Magalhães, J.; Olliaro, P.; et al. The Future of Epidemic and Pandemic Vaccines to Serve Global Public Health Needs. Vaccines 2023, 11, 690. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Matthies-Wiesler, F.; Bierne, N.; Binot, A.; Boissier, J.; Devouge, A.; Garric, J.; Gruetzmacher, K.; Grunau, C.; Guégan, J.-F.; et al. Getting out of crises: Environmental, social-ecological and evolutionary research is needed to avoid future risks of pandemics. Environ. Int. 2021, 158, 106915–106915. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, M.S.; Conrad, P.A.; Winer, J.N. One Health–One Education: Medical and Veterinary Inter-Professional Training. J. Veter- Med Educ. 2019, 46, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lerner, H.; Berg, C. The concept of health in One Health and some practical implications for research and education: What is One Health? Infect. Ecol. Epidemiol. 2015, 5, 25300. [Google Scholar] [CrossRef]
- Nieuwland, J.; Meijboom, F.L.B. One Health: How Interdependence Enriches Veterinary Ethics Education. Animals 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Togami, E.; Gardy, J.L.; Hansen, G.R.; Poste, G.H.; Rizzo, D.M.; Wilson, M.E.; Mazet, J.A.K.; University of British Columbia; Consulting, L. H.; Arizona State University Core Competencies in One Health Education: What Are We Missing? NAM Perspect. 2018, 8. [Google Scholar] [CrossRef]
- K. Spanjol and P. Zucca, “Biophilia, One Health, and Humane Education: Mitigating global risk through embracing humanity’s interconnection with the natural world,” in Socio-Political Risk Management. Assessing and Managing Global Insecurity, K. J. Engemann, C. F. Lavery, and J. M. Sheehan, Eds., Berlin, London: De Gruyter, 2023, ch. 7, pp. 109–132.
- (Ecdc), E.C.F.D.P.A.C. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Lehtimaki, S.; Hannon, E.; Hanbali, L.; Soltan, D.-F.; Peek, K.; Nassiri-Ansari, T.; Schwalbe, N. Where there is a will, there is a way: independent assessment of member state compliance with the pandemic agreement. Lancet Glob. Heal. 2024, 12, e18–e19. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate Change and Cascading Risks from Infectious Disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Agyarko, *!!! REPLACE !!!*; et al. , “The imperative for global cooperation to prevent and control pandemics,” in Modernizing Global Health Security to Prevent, Detect, and Respond, Scott J.N. McNabb, Affan T. Shaikh, and Carol J. Haley, Eds., London: Academic Press, 2024, ch. 4.
- Scully, J.L. COVID, Vulnerability, and the Death of Solidarity: “Who Do We Not Save?”. J. Bioethical Inq. 2023, 20, 601–606. [Google Scholar] [CrossRef]
- Tomasini, F. Solidarity in the Time of COVID-19? Camb. Q. Heal. Ethic- 2020, 30, 234–247. [Google Scholar] [CrossRef]
- Branda, F. Social impact: Trusting open science for future pandemic resilience. Soc. Impacts 2024, 3. [Google Scholar] [CrossRef]
- Besançon, L.; Peiffer-Smadja, N.; Segalas, C.; Jiang, H.; Masuzzo, P.; Smout, C.; Billy, E.; Deforet, M.; Leyrat, C. Open science saves lives: lessons from the COVID-19 pandemic. BMC Med Res. Methodol. 2021, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pettan-Brewer, C.; Martins, A.F.; de Abreu, D.P.B.; Brandão, A.P.D.; Barbosa, D.S.; Figueroa, D.P.; Cediel, N.; Kahn, L.H.; Brandespim, D.F.; Velásquez, J.C.C.; et al. From the Approach to the Concept: One Health in Latin America-Experiences and Perspectives in Brazil, Chile, and Colombia. Front. Public Heal. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- WHO, “Intergovernmental Negotiating Body to draft and negotiate a WHO convention, agreement or other international instrument on pandemic prevention, preparedness and response,” Geneva, May 2024. [Online]. Available: https://apps.who.int/gb/inb/.
- Rink, E.; Stotz, S.A.; Johnson-Jennings, M.; Huyser, K.; Collins, K.; Manson, S.M.; Berkowitz, S.A.; Hebert, L.; Shanks, C.B.; Begay, K.; et al. “We don’t separate out these things. Everything is related”: Partnerships with Indigenous Communities to Design, Implement, and Evaluate Multilevel Interventions to Reduce Health Disparities. Prev. Sci. 2024, 25, 474–485. [Google Scholar] [CrossRef] [PubMed]
- A Barrett, M.; A Bouley, T.; Stoertz, A.H.; Stoertz, R.W. Integrating a One Health approach in education to address global health and sustainability challenges. Front. Ecol. Environ. 2010, 9, 239–245. [Google Scholar] [CrossRef]
- Reaser, J.K.; Witt, A.; Tabor, G.M.; Hudson, P.J.; Plowright, R.K. Ecological countermeasures for preventing zoonotic disease outbreaks: when ecological restoration is a human health imperative. Restor. Ecol. 2021, 29, e13357. [Google Scholar] [CrossRef]
- FAO, “The Emergency Prevention System for Animal Health Enhancing the prevention and control of high-impact animal and zoonotic diseases through biosecurity and One Health Strategic Plan (2023-2026),” 2023. Accessed: Jun. 10, 2024. [Online]. Available: https://openknowledge.fao.org/server/api/core/bitstreams/cbea52a7-8b1f-46ff-a3d1-3d6acc72decd/content.
- C. Ritter and M. King, “One Health Une santé One Welfare: The role of veterinary professionals,” Can Vet J., vol. 64, no. 8, pp. 784–786, 2023, Accessed: Jun. 26, 2024. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10352033/.
- Mota-Rojas, D.; Monsalve, S.; Lezama-García, K.; Mora-Medina, P.; Domínguez-Oliva, A.; Ramírez-Necoechea, R.; Garcia, R.d.C.M. Animal Abuse as an Indicator of Domestic Violence: One Health, One Welfare Approach. Animals 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Marchant-Forde, J.N.; Boyle, L.A. COVID-19 Effects on Livestock Production: A One Welfare Issue. Front. Vet. Sci. 2020, 7, 585787. [Google Scholar] [CrossRef]
- Moreno-Madriñan, M.J.; Kontowicz, E. Stocking Density and Homogeneity, Considerations on Pandemic Potential. Zoonotic Dis. 2023, 3, 85–92. [Google Scholar] [CrossRef]
- Guo, Y.; Ryan, U.; Feng, Y.; Xiao, L. Association of Common Zoonotic Pathogens With Concentrated Animal Feeding Operations. Front. Microbiol. 2022, 12, 810142. [Google Scholar] [CrossRef]
- Stel, M.; Eggers, J.; Alonso, W.J. Mitigating Zoonotic Risks in Intensive Farming: Solutions for a Sustainable Change. Ecohealth 2022, 19, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.D. Bioethics, climate change, and civilization. J. Clim. Chang. Heal. 2024. [Google Scholar] [CrossRef]
- Okeke, *!!! REPLACE !!!*; et al. , “The scope of the antimicrobial resistance challenge,” The Lancet, 2024.
- Djordjevic, S.P.; Jarocki, V.M.; Seemann, T.; Cummins, M.L.; Watt, A.E.; Drigo, B.; Wyrsch, E.R.; Reid, C.J.; Donner, E.; Howden, B.P. Genomic surveillance for antimicrobial resistance — a One Health perspective. Nat. Rev. Genet. 2023, 25, 142–157. [Google Scholar] [CrossRef] [PubMed]
- A. J., Hernández, Chaves, and C., A. J. Hernández, “Poultry and Avian Diseases,” in Encyclopedia of Agriculture and Food Systems, 2nd ed., N. Van Alfen, Ed., San Diego: Elsevier, 2014.
- Alam, M.-U.; Rahman, M.; Masud, A.A.; Islam, M.A.; Asaduzzaman, M.; Sarker, S.; Rousham, E.; Unicomb, L. Human exposure to antimicrobial resistance from poultry production: Assessing hygiene and waste-disposal practices in Bangladesh. Int. J. Hyg. Environ. Heal. 2019, 222, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Heal. 2024, 2. [Google Scholar] [CrossRef]
- A. I. Alonso and N. South, “Incorporating a One Health Approach Into the Study of Environmental Crimes and Harms: Towards a ‘One Health Green Criminology,’” Br J Criminol, Jul. 2024. [CrossRef]
- S. Barrett, M. A. S. Barrett, M. A., & Osofsky, One Health: Interdependence of people, other species and the planet. Philadelphia: Saunders Elsevier, 2013.
- A. Leboeuf, Making Sense of One Health. Cooperating at the Human-Animal-Ecosystem Health Interface, no. April. 2011. [Online]. Available: http://www.academia.edu/633338/Making_sense_of_One_Health.
- Wilkes, M.S.; Conrad, P.A.; Winer, J.N. One Health–One Education: Medical and Veterinary Inter-Professional Training. J. Veter- Med Educ. 2019, 46, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. The Pandemic Treaty: shameful and unjust. Lancet 2024, 403, 781–781. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, N.; Muhindo, J.; Nyumu, J.; Enns, C.; Massé, F.; Bersaglio, B.; Cerutti, P.; Nasi, R. Understanding Factors that Shape Exposure to Zoonotic and Food-Borne Diseases Across Wild Meat Trade Chains. Hum. Ecol. 2022, 50, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Comission, “EU One Health Action Plan against AMR,” EU One Health Action Plan against AMR, p. 24, 2017, [Online]. Available: http://www.who.int/entity/drugresistance/documents/surveillancereport/en/index.html%0Ahttps://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf.
- Hibbard, R.; Mendelson, M.; Page, S.W.; Ferreira, J.P.; Pulcini, C.; Paul, M.C.; Faverjon, C. Antimicrobial stewardship: a definition with a One Health perspective. npj Antimicrob. Resist. 2024, 2, 1–9. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef]
- H. ten Have, “Equity, Justice and Responsibility in the Perspective of Global Health,” in Healing Mission: The Catholic Church in the Era of Global Public Health, Branka Gabric and Stefan Hofmann, Eds., Frankfurt: Verlag, 2023, pp. 173–198.
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- A., Lianou, J. N. Sofos, and K. P. Koutsoumanis, “Food Safety: Emerging Pathogens,” Encyclopedia of Agriculture and Food Systems . Elsevier, San Diego, 2014. Accessed: Jun. 09, 2024. [Online]. Available: https://search.credoreference.com/articles/Qm9va0FydGljbGU6MzE4MTc0NQ==?aid=257358.
- Nieuwland, J. & Meijboom, “One-Health as a normative concep: implications for food safety at the wildlife interface,” in Know your food: Food ethics and innovation, The Netherlands: Wageningen Academic Publishers, 2013, pp. 132–137. [Online]. Available: https://books.google.cl/books?hl=es&lr=&id=SBPTDwAAQBAJ&oi=fnd&pg=PA132&dq=health+as+normative+concept&ots=xXXraWU2bs&sig=u1QCeeqo6xyCnpCziY3wxs6R7Lg&redir_esc=y#v=onepage&q&f=true.
- Berry, S.L.; Chipman, S.; Gregg, M.E.; Haffey, H.; Devenot, N.; McMullin, J. Justice, Equity, Diversity, Inclusion, and Belonging: A Health Humanities Consortium Initiative. J. Med Humanit. 2024, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Sharan, M.; Vijay, D.; Yadav, J.P.; Bedi, J.S.; Dhaka, P. Surveillance and response strategies for zoonotic diseases: a comprehensive review. Sci. One Heal. 2023, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Ballash, G.A.; Parker, E.M.; Mollenkopf, D.F.; Wittum, T.E. The One Health dissemination of antimicrobial resistance occurs in both natural and clinical environments. J. Am. Veter- Med Assoc. 8. [CrossRef]
- Vezeau, N.; Kahn, L. Spread and mitigation of antimicrobial resistance at the wildlife-urban and wildlife-livestock interfaces. J. Am. Veter- Med Assoc. 7. [CrossRef]
- Mitchell, J.; Cooke, P.; Ahorlu, C.; Arjyal, A.; Baral, S.; Carter, L.; Dasgupta, R.; Fieroze, F.; Fonseca-Braga, M.; Huque, R.; et al. Community engagement: The key to tackling Antimicrobial Resistance (AMR) across a One Health context? Glob. Public Heal. 2021, 17, 2647–2664. [Google Scholar] [CrossRef] [PubMed]
- Marvasi, M.; Casillas, L.; Vassallo, A.; Purchase, D. Educational Activities for Students and Citizens Supporting the One-Health Approach on Antimicrobial Resistance. Antibiotics 2021, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- The Oxford Handbook of Global Health Politics; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2018.
- The Oxford Handbook of Global Health Politics; Oxford University Press (OUP): Oxford, Oxfordshire, United Kingdom, 2018.
- A. O. Oyekan and W. A. Balogun, “Borders, Boundaries, and Identities: Navigating the Barriers to Solidarity and Cohesion in a Pandemic,” in Global Health, Humanity and the COVID-19 Pandemic, F. Egbokhare and A. Afolayan, Eds., Cham: Palgrave Macmillan, 2023, pp. 197–222. [CrossRef]
- World Health Organization. Rapid Communication on Forthcoming Changes to the Programmatic Management of Tuberculosis Preventive Treatment. Available online: http://apps.who.int/bookorders. (accessed on 21 March 2020).
- Waltner-Toews, D. One ecosystem, one food system: the social and ecological context of food safety strategies. J. Agric. Environ. Ethic- 1991, 4, 49–59. [Google Scholar] [CrossRef]
- LeBlanc, A.B.; Motulsky, A.; Moreault, M.-P.; Liang, M.Q.; Feze, I.N.; Côteaux, L.D. Building a Logic Model to Foster Engagement and Learning Using the Case of a Province-Wide Multispecies Antimicrobial Use Monitoring System. Evaluation Rev. 2023, 48, 736–765. [Google Scholar] [CrossRef] [PubMed]
- van Dam, A.; van Engelen, W.; Müller-Mahn, D.; Agha, S.; Junglen, S.; Borgemeister, C.; Bollig, M. Complexities of multispecies coexistence: Animal diseases and diverging modes of ordering at the wildlife–livestock interface in Southern Africa. Environ. Plan. E: Nat. Space 2023, 7, 353–374. [Google Scholar] [CrossRef]
- Quadripartite, “Quadripartite Key Recommendations and Priorities for the 2024 UNGA High-Level Meeting on Antimicrobial Resistance (AMR),” 2024. Accessed: Aug. 08, 2024. [Online]. Available: https://www.unep.org/resources/policy-and-strategy/quadripartite-key-recommendations-and-priorities-2024-unga-high-level.
- IACG-UN, “No time to wait: Securing the Future from Drug-resistant Infections Report to the Secretary- General of the United Nations,” 2019. Accessed: Aug. 08, 2024. [Online]. Available: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant- infections.
- R. Rozzi, “Inter-species and Inter-cultural Encounters: The Biocultural Education Program of the Omora Ethnobotanical Park,” 2023, pp. 153–174. [CrossRef]
- M. Hourdequin, Environmental Ethics, From theory and practice. London: Bloomsbury, 2015.
- Rozzi, *!!! REPLACE !!!*; et al. , Field Environmental Philosophy. Education for Biocultural Conservation. Cham: Springer, 2023.
- Boyd, K. Global health justice: epistemic theory and pandemic practice. J. Med Ethic- 2023, 49, 303–304. [Google Scholar] [CrossRef]
- de Vries, J.; Pratt, B. Epistemic justice in bioethics: interculturality and the possibility of reparations. J. Med Ethic- 2023, 49, 347–347. [Google Scholar] [CrossRef] [PubMed]
- A., N. L. A., N. L. Broadbent, “Intercultural Medical Disagreements: Ebola vs. Covid-19,” in The Politics of Knowledge in the Biomedical Sciences, J., Jansen and J. Auerbach, Eds., Cham: Springer.
- Chao, S.; Celermajer, D. Introduction. Cult. Politi- Int. J. 2023, 19, 1–17. [Google Scholar] [CrossRef]
- Sandler, R. De-extinction: Costs, benefits and ethics. Nat. Ecol. Evol. 2017, 1, 105. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R. De-extinction and Conservation Genetics in the Anthropocene. Häst. Cent. Rep. 2017, 47, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R. On the Massness of Mass Extinction. Philosophia 2022, 50, 2205–2220. [Google Scholar] [CrossRef]
- Gardiner, S.M. On the Scope of Institutions for Future Generations: Defending an Expansive Global Constitutional Convention That Protects against Squandering Generations. Ethic- Int. Aff. 2022, 36, 157–178. [Google Scholar] [CrossRef]
- WHO, “Compendium of WHO and other UN guidance on health and environment 2024 update,” 2023. Accessed: Jul. 14, 2024. [Online]. Available: https://reliefweb.int/report/world/compendium-who-and-other-un-guidance-health-and-environment-2024-update.
- U. W. W. FAO, “One Health Joint Plan of Action (2022–2026). Working together for the health of humans, animals, plants and the environment,” FAO; UNEP; WHO; World Organisation for Animal Health (WOAH) (founded as OIE);, Rome, 2022. Accessed: Apr. 02, 2024. [Online]. Available: https://www.who.int/publications/i/item/9789240059139.
- Benis, A.; Tamburis, O. The Need for Green and Responsible Medical Informatics and Digital Health: Looking Forward with One Digital Health. Yearb. Med Informatics 2023, 32, 007–009. [Google Scholar] [CrossRef]
- Tamburis, O.; Benis, A. One Digital Health for more FAIRness. Methods Inf. Med. 2022, 61, E116–E124. [Google Scholar] [CrossRef]
- Bankoff, “The Historical Geography of Disaster: ‘Vulnerability’ and ‘Local Knowledge’ in Western Discourse,” in Mapping Vulnerability : Disasters, Development and People, Greg Bankoff, Georg Frerks, and Dorothea Hilhorst, Eds., New York: Taylor and Francis , 2004, ch. 2, pp. 25–36. [Online]. Available: http://ebookcentral.proquest.com/lib/acu/detail.action?docID=981736.
- Bankoff, G. Rendering the World Unsafe: ‘Vulnerability’ as Western Discourse. Disasters 2001, 25, 19–35. [Google Scholar] [CrossRef]
- J. Lederberg, “Infectious History,” Science (1979), vol. 288, pp. 287–293, 2000. [CrossRef]
- A.Cassidy, “Humans, Other Animals and ‘One Health’ in the Early Twenty-First Century,” in Animals and the Shaping of Modern Medicine. One Health and its Histories, A. Woods, M. Bresalier, A. Cassidy, and R. M. Dentiger, Eds., Cham: Palgrave macmillan, 2018, ch. 6, pp. 193–224.
- Woods, A.; Bresalier, M.; Cassidy, A.; Dentinger, R.M. Animals and the Shaping of Modern Medicine; Springer Nature: Dordrecht, GX, Netherlands, 2018. [Google Scholar]
- Schwalbe, N.; Hannon, E.; Lehtimaki, S. The new pandemic treaty: Are we in safer hands? Probably not. BMJ 2024, 384, q477. [Google Scholar] [CrossRef]
- Bortolotti, L.; Murphy-Hollies, K. Exceptionalism at the Time of covid-19: Where Nationalism Meets Irrationality. Dan. Yearb. Philos. 2022, 55, 90–111. [Google Scholar] [CrossRef]
- Ricard, J.; Medeiros, J. Using misinformation as a political weapon: COVID-19 and Bolsonaro in Brazil. 2020. [Google Scholar] [CrossRef]
Figure 1.
Networks of emergence, amplification and transboundary transmission of zoonotic diseases.
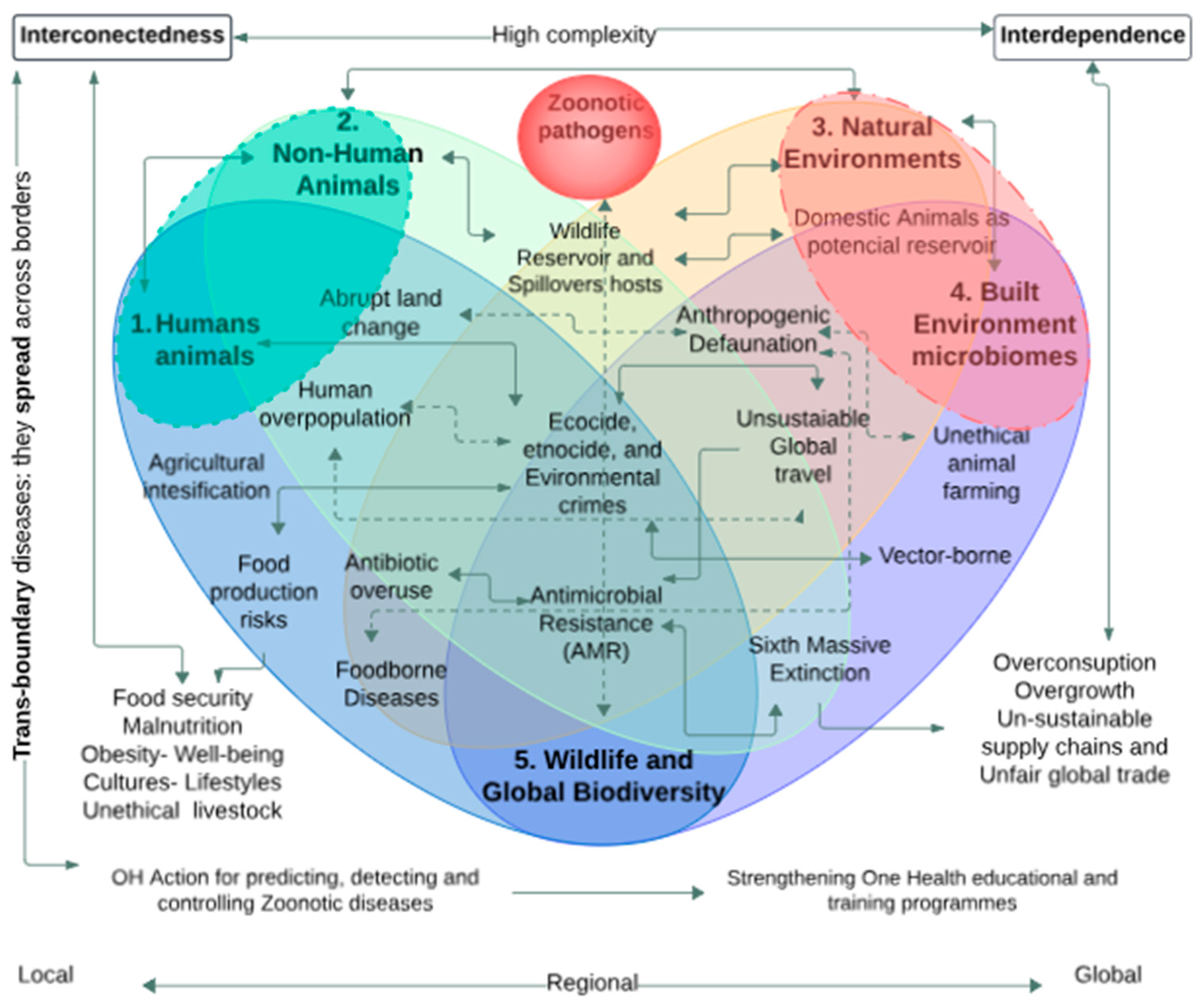
Figure 2.
The interrelationship of events that trigger the onset of infectious diseases. Source: Author 2024.
Figure 2.
The interrelationship of events that trigger the onset of infectious diseases. Source: Author 2024.

Table 1.
Bioethical Principles and Political Guidelines for Addressing Emerging and Re-emerging Infectious Diseases. Source: Author 2024.
Table 1.
Bioethical Principles and Political Guidelines for Addressing Emerging and Re-emerging Infectious Diseases. Source: Author 2024.
| Ethical Principles | Zoonotic Ethics Guidelines | Problem/Challenge |
|---|---|---|
| Autonomy | Respect the autonomy of communities and individuals in One Health decision-making. | Ensuring individual and community rights are upheld during public health interventions. |
| Beneficence | Implement measures that maximize well-being and minimize harm for both humans, animals and environments. | Balancing the benefits of interventions against potential risks and harms. |
| Non-maleficence | Avoid actions that cause unnecessary harm, such as indiscriminate culling of animals without comprehensive ethical analysis. | Preventing unethical practices that could cause harm to animals and ecosystems. |
| Justice | Ensure equitable distribution of resources and treatments to prevent and control zoonotic diseases. Ensuring universal access to health innovations and vaccines. | Addressing disparities in access to healthcare and resources for disease prevention and control. |
|
Ethical deliberation/ Constructive conflict |
Consider all relevant interests at stake and integrate the value and welfare of human animals when implementing management plans and measures to control zoonoses. Strategies: Multi-layered and multi-actor assessment. Context-dependent analysis | Mitigate the negative impacts and side-effects on non-human animals of strategies and plans to control zoonoses and epidemic risks. |
| Environmental Responsibility | It is imperative to adopt responsible practices in all sectors that safeguard ecosystems and biodiversity, acknowledging their integral role in One Health and the secure survival of future generations. | Mitigating the impact of human activities on ecosystems and preventing biodiversity loss. |
| Transparency and Communication | Maintain open and honest communication with the public about risks and measures taken to control zoonoses. | Building public trust and ensuring informed participation in public health measures. |
| Respect for Life | Value and protect the lives of all living beings, recognizing the interdependence between humans, animals and environments. | Promoting a holistic view of life that includes the well-being of all species. |
| Solidarity and Cooperation | Promote international collaboration and solidarity among nations to combat global zoonotic threats. | Fostering global cooperation to address transboundary zoonotic disease threats. |
| Precaution and Prudence | Adopt preventive and prudent measures in the face of scientific uncertainty and potential risks of new zoonoses. | Taking proactive steps to prevent outbreaks even when full scientific certainty is not available. |
| Intergenerational Equity | Make decisions that do not compromise the health and well-being of future generations. Avoid practices and policies that transfer risks and damages to future generations. Inter/trans-generational justices. | Ensuring sustainable practices that do not deplete resources or harm future generations. |
| Care | It is imperative that robust legislation be enacted to address the crime of ecocide, including the introduction of criminal laws to deter and punish the systematic destruction of biodiversity and the trafficking of wildlife. | Mitigation of large-scale species extinction and crimes against biodiversity |
Table 3.
Ethical and Policy Recommendations for Addressing Antimicrobial Resistance (AMR). Source: Author 2024.
Table 3.
Ethical and Policy Recommendations for Addressing Antimicrobial Resistance (AMR). Source: Author 2024.
| Category | Recommendation | Rational |
|---|---|---|
|
Preventive Action |
Accelerate the implementation of the One Health approach from a preventive and anticipatory lens. Shifting from reactive to proactive zoonotic risk mitigation model is essential. | Implement a preventive One Health strategy with a precautionary approach, helps to address the root causes of AMR and promotes sustainable practices [135,322] |
|
Biosecurity Processes |
Ensure that all medical and veterinary waste undergoes effective decontamination processes, such as autoclaving or chemical disinfection, to eliminate pathogens and reduce the risk of AMR. | Proper waste management and biosecurity tools minimizes the ecological footprint of animal and healthcare practices, reducing environmental pollution and the spread of AMR [320,323] |
|
Sustainable Food and Agricultural Practices |
Promote sustainable agriculture and livestock practices, reducing antimicrobial use in food production to prevent resistant pathogens. | Sustainable practices prevent AMR by reducing unnecessary antimicrobial use and promoting ethical treatment of animals and the environment [318,324,325,326] |
| Public Policy | Educate public policy experts on the complexity of zoonoses, EIDs, and AMR from a One Health and multispecies justice perspective. | Informed policymakers can develop more effective policies to address AMR, considering its broader impact on public health and the environment [253,327,328] |
|
Antimicrobial Stewardship |
Promote interdisciplinary and Antimicrobial Stewardship programs to ensure the responsible use of antimicrobials in human and animal healthcare, agriculture, and food-chains. | Antimicrobial Stewardship programs help mitigate the overuse and misuse of antimicrobials, reducing the development of resistant pathogens [320,329,330] |
|
Infrastructure Improvement |
Improve infrastructure for clean water, sanitation, and waste management to prevent the spread of resistant microbes. | Improving infrastructure tackles AMR’s root causes in low-resource settings by addressing inadequate sanitation and waste management [323,330] |
|
Ethical Reflection |
Encourage ethical reflection on healthcare practices, animal treatment, and environmental impact in decision-making processes. | Ethical considerations ensure that actions taken to address AMR are just, sustainable, and responsible, benefiting all stakeholders involved [67,68,70,72] |
|
One Health Education and Antibiotic Awareness |
Increase public awareness and education about the causes and consequences of AMR, and the importance of responsible antimicrobial use. | One Health Education promotes responsible behavior and support for AMR-reducing policies, making ethical reflection on AMR crucial for a sustainable and just future [77,331,332]. |
|
Global Cooperation |
Strengthen international cooperation and coordination in monitoring, research, and response to AMR. | Global cooperation is crucial for addressing AMR, as it is a transboundary issue that requires coordinated efforts across countries and regions. [333,334,335]. |
|
Food chains and Water Systems |
Strengthen and enforce regulations on the use of antibiotics in agriculture and aquaculture. | By regulating the use of antibiotics in food production, we can reduce the development of antibiotic-resistant bacteria in the environment, which can be transferred to humans through the food chain [324,325,336,337]. |
|
Multispecies Justice |
Implement comprehensive OH policies that prioritize the welfare and the intrinsic value of all including environments, human, and non-human animals affected by AMR. | Addressing AMR ethically requires considering the impact on diverse species and ecosystems, ensuring fair treatment and health outcomes for all [253,338,339]. |
| One Welfare | One Welfare by integrating animal, environmental, and public health in antimicrobial stewardship policies. | Integrating this approach into AMR strategies enables sustainable, equitable solutions across all sectors [279,301,302]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
One Health Landscape in Sub-Saharan African Countries
Folorunso Oludayo Fasina
et al.
,
2020
One Health Approach for combating Antimicrobial Resistance in Animals
Chung-Ming Chang
et al.
,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








